Abstract
Winter low temperature tolerance is the result of genotype interaction with environmental cues influencing plant development and metabolic activity in preparation for sustained low temperatures and freezing conditions. In deciduous fruit trees the phenology of acclimation and dormancy processes are well documented and recent advances in functional genomic analyses are beginning to map the molecular mechanisms underlying the physiological and biochemical responses resulting from inductive short photoperiod and/or low temperatures. There are many commonalities between herbaceous annual plant acclimation and perennial plant acclimation; however, the ability to survive sustained periods of low temperature, extreme low temperatures and the presence of ice within tissues are not common in annual model plant systems. In addition, subzero temperature tolerance mechanisms vary with tissue type and age and seasonal and climatic conditions; thus, defining the phenotypes and underlying genetic control of these dynamic and complex quantitative traits is both time and land intensive. Rapidly increasing genomic sequences and databases of transcriptomic, proteomic and metabolomics data sets, coupled with existing mapping studies and new genotype by sequencing and single nucleotide polymorphism (SNP) marker analyses provide the opportunity to dissect winter survival and elucidate its genetic basis. This review explores recent genomic and functional genomic analyses that are contributing to a greater understanding of the molecular mechanisms regulating low temperature response and freezing tolerance in temperate woody plants. Developments are providing the opportunity to more quickly and precisely link responses to genes and gene variations underlying low temperature response and tolerance in temperate fruit crops.
I. WINTER SURVIVAL IN WOODY TEMPERATE FRUIT CROPS
Subzero freeze events in late spring and early fall and winter minimum temperatures limit the sustainable production of fruit tree crops. Orchard location, cultivar selection and cultural practices have been used extensively to minimize production losses; however, freezing injury is a continuing problem for perennial crop production in cold winter climate regions. Freezing tolerance is an active trait, responsive to environmental fluctuations and it is genotype dependent for the timing of acclimation and dormancy, genetic potential for mid-winter freezing tolerance, rate of deacclimation, ability to reacclimate and timing of budbreak (Stushnoff, Citation1972). Therefore, winter survival is a multi-trait phenomenon that is impacted by photoperiod, temperature and water and nutritional status; all of which can interact differentially with genotype and tissue developmental stages (Fennell, Citation2004; Gusta and Wisniewski, Citation2012; Palonen and Buszard, Citation1997; Stushnoff Citation1972). Progress in selecting sustainable cultivars requires identifying critical phenotypes and susceptible tissues as well as critical environmental events (early fall freeze, mid-winter ultimate low temperature, deacclimation events). Breeding and selection of adapted cultivars is hindered not only by the complexity of the trait, but also by long generation time, heterozygosity and inbreeding depression, large plant size, rootstock interactions as well as fruit quality traits; all of which confound precise phenotyping and genetic analysis. Therefore advances in identification of trait characters and their genomic basis are needed to promote marker identification and marker assisted selection for improved winter survival in temperate fruit trees.
Survival of subzero temperatures depends on the woody plants ability to acclimate in response to low temperature and or photoperiod; promoting physical and biochemical changes in multiple tissues, which will allow the plant to avoid ice nucleation in tissues or compartmentalize ice in locations where it does not cause injury (Ashworth, Citation1982; Citation1992; Gusta and Wisniewski, Citation2012; Pearce, Citation2001; Wisniewski et al., Citation2004). It should be noted that freezing tolerance and avoidance traits within a given species are not static, rather they are dynamically influenced by stage of development, intrinsic and extrinsic nucleators and environmental conditions (Gusta and Wisniewski, Citation2012; Pearce Citation2001). Tolerating the presence of ice in tissue apoplast and cellular dehydration as water moves from the cell to ice in the extracellular spaces allows many plant tissues to tolerate lower temperatures without injury (Levitt Citation1980). In apple (Malus), the bark and phloem tissue tolerate extracellular ice formation while the xylem parenchyma cells tolerate subzero temperatures by supercooling as freezing of the xylem parenchyma results in tissue death (Ashworth, Citation1992; Gusta et al., Citation2009; Quamme et al., Citation1973). Supercooling limits the strain of freeze dehydration, although progressive dehydration can occur with extended subzero temperatures. It also limits the range of plants using this mechanism to regions with winter low temperatures above the homogenous nucleation temperature or greater (Ashworth and Wisniewski, Citation1991; George et al., Citation1974; Quamme, Citation1976). Development of the ability to supercool depends on low temperature and/or photoperiod induced acclimation responses that promote reduction in water content, changes in cellular biochemistry and the development of barriers to ice propagation from the stem into the bud tissue (Ashworth, Citation1982; Ashworth and Wisniewski, Citation1991; Burke and Stushnoff, Citation1978; Jones et al., 2000; Gusta and Wisniewski, Citation2012, Levitt, Citation1980). In grapevine (Vitis species) and some Prunus species, the overwintering flower or compound buds supercool and adjacent stem tissue may tolerate extracellular freezing (Ashworth, Citation1992; Fennell, Citation2004; Mathers, Citation2004; Kader and Proebsting, Citation1992; Quamme et al., Citation1995). The ability to supercool depends on tissue water content, shoot morphology, the stage of bud development and absence of intrinsic and extrinsic nucleators. It is a dynamic characteristic and will change in response to climatic conditions and dormancy status of the plant. The factors that determine ice initiation and location are frequently overlooked in genomic analysis of woody plant systems and careful experimental planning is needed to ensure functional analysis of relevant freezing tolerance characteristics (Arora and Rowland, Citation2011; Ashworth, Citation1984; Gusta and Wisniewski, Citation2012). Therefore, to improve the understanding of the genomic mechanisms contributing to the physical and biochemical changes involved in winter survival requires temporal studies and systems biology approaches (from a holistic rather than a reductionist perspective) that address specific tissues in the context of the whole plant and its responses to natural environmental conditions (Dhanaraj et al., Citation2007).
Table 1 Fruit tree genome assemblies and databases
Table 2 Genome-wide gene family analyses
In spite of the complexity of woody plant systems, an atlas is emerging for some of the physiological and molecular responses to environmental cues that drive plant low temperature acclimation and deacclimation processes (Arora et al., Citation2003; Gusta and Wisniewski, Citation2012, Guy, Citation2003; Janska et al., Citation2010; Obata and Fernie, Citation2012; Theocharis et al., Citation2012; Welling and Palva, Citation2006). This review will focus specifically on genomic resources and recent functional genomic analyses that are furthering the understanding of winter survival of temperate tree fruit crops, with an emphasis on apple (Malus), blueberry (Vaccinium), grapevine (Vitis) and peach (Prunus).
II. GENOMIC RESOURCES
The number of flowering plant genome sequences and tools for functional genomics has increased dramatically in the decade since the first plant genome sequence assembly (Arabidopsis thaliana, 2001) was released. In 2007, genome assemblies of an inbred of Pinot Noir and the heterozygous Pinot Noir (V. vinifera) cultivar were published, making these two genomes the first fruit crop genomes and grapevine the fourth vascular plant species genome sequence to be released () (Jaillon et al., Citation2007; Velasco et al. Citation2007). Preceding the grapevine genome release, markers, expressed sequence tags (ESTs) and tools such as VitisGeneChip (Affymetrix) were made publically available thus providing resources to initiate systems biology research (Adam-Blondon, et al., Citation2004; Goes da Silva et al., Citation2005). This was quickly followed by the development of visualization tools for the large transcriptomic, proteomic or metabolomic (omic) datasets (Grimplet et al., Citation2009; 2011; http://PlexDB.org). Availability of the genome sequence assemblies, ESTs and proteomes has promoted rapid identification of gene families. Several gene families that have been associated with abiotic stress or growth and development have been described very recently (). Genome-wide analysis of gene families with expression analysis, in differential environmental conditions, for relevant tissues and stages of development have aided the functional annotation of genomes and provided phenotype information needed for discovery of genetic mechanisms.
In the five years since the first grapevine genome sequence assembly was released, 29 new vascular plant genomes have been published and many more assemblies are in progress (Michelmore, Citation2012). The vast majority of these genomes are crop plants and several are highly valuable fruit tree crop species making comparative genomic analyses of important crop traits more accessible (). The fruit crop or relative species are diploids with relatively small genomes making them more amenable to sequencing. However, the fruit trees are highly heterozygous and have a long generation time, which has made genetic analysis and advancement in selection for quantitative traits like cold tolerance onerous. DNA markers and linkage maps of apple, peach, grapevine and other fruit species have long been published, but availability of genomes has made it possible to more fully integrate genetic maps and identify gene variation between genotypes. Genome publications and international public and consortium databases provide links not only to genome sequences, but also for genomic and bioinformatics tools and transcriptomic, proteomic, metabolomics and microRNA data sets, thereby enhancing opportunities for genome annotation improvement and comparative and functional genomic analyses.
III. FUNCTIONAL GENOMICS OF ACCLIMATION RESPONSES FOR WINTER SURVIVAL
Acclimation for winter seasons are cued by short photoperiod and/or low temperature and over a century of observations and studies in natural temporal or controlled environment conditions have identified changes in plant biochemistry and physiology related to the ability of plants to tolerate low and freezing temperatures. Acclimation processes are marked by a reduction in water content, fluxes in calcium, modifications in membranes, metabolic reprogramming, accumulation of carbohydrates, amino acids, proteins and secondary metabolites and changes in cell wall structure (Ashworth, Citation1992; Fennell, Citation2004; Gusta and Wisniewski, Citation2012; Guy, Citation2003; Levitt, Citation1980; Smallwood and Bowles, Citation2002; Theocharis, 2012; Welling and Palva, Citation2006; Wisniewski et al., Citation2009). Correlation of these biochemical changes with freezing tolerance has provided many gene candidates for functional gene analysis; however the complexity of winter survival indicates that, rather than single gene analysis, targeted and holistic analysis are needed to precisely determine the genetic mechanism involved in winter survival. Functional genomic tools have been increasingly applied to acclimation and dormancy studies () in fruit trees in the last decade and characteristics specific to species and woody plant systems are emerging.
Table 3 Functional genomics studies of low temperature and short photoperiod response in fruit trees
A. Apple
Apple acclimates in response to low temperature, but not photoperiod, to induce acclimation and dormancy (Heide and Prestrud, Citation2005). The acclimated apple tree tolerates equilibrium freezing in phloem and bark tissues while the xylem parenchyma cells supercool (Ashworth and Wisniewski, Citation1991; Quamme et al., Citation1973). A comparison of gene expression in apple leaf, bark and xylem tissues during early acclimation to low temperature and water deficit identified about 1000 genes (∼65% known function) differentially expressed between tissues and low temperature and water deficit treatments (Wisniewski et al., Citation2008). In low temperature acclimated apple bark and xylem, genes related to energy, cell growth and development, protein metabolism and defense related were up-regulated relative to control; and photosynthesis, metabolism and transport were down-regulated relative to warm control. Most notable was a strong up-regulation of dehydrin genes at low temperatures in the bark and xylem but not in the leaves. A recent genome-wide survey identified 12 dehydrin genes in apple and four of these (MdDHN2, MdDHN4, MdDHN6 and MdDHN8) increased dramatically in leaf tissue upon exposure to low temperature () (Liang et al., Citation2012). The MdDHN2 showed a strong similarity to the dehydrin previously identified in apple bark under low temperature induction and bark and buds under midwinter conditions (Garcia-Bañuelos et al., Citation2009; Wisniewski et al,. 2008). Dehydrins have been shown to increase during low temperature acclimation and decreased water content in a variety of woody species and this associative data suggest a potential role in winter survival mechanisms (Artlip et al., Citation1997; Sarnighausen et al., Citation2004; Welling et al., Citation2004; Wisniewski et al., Citation1996, 2006).
B. Peach and Apricot
Peach (Prunus persica) responds to decreasing photoperiod and/or temperature to initiate dormancy and acclimation. A study in peach bark designed to separate low temperature acclimation and short photoperiod dormancy induction specific responses found dehydrins (PpDhn1 and PpDhn3), chitinase and thaumitin transcripts specifically upregulated in low temperature while heatshock protein transripts were up-regulated in short photoperiod treatments (Bassett et al., Citation2006). Suppression subtractive hybridization of cDNA libraries for seasonally induced endodormant and ecodormant buds from low and high chill requiring peach cultivars produced 364 ESTs, 101 contigs and identified genes specific to endodormancy, ecodormancy and the low chill genotype (Leida et al., Citation2010). Genes up-regulated in buds during dormancy included metabolism, stress and defense and signaling and transcription (Late embryo abundant (LEA), Dormancy associated MADs box (DAM), NAC domain and AP2/EREBP). Genes upregulated during the ecodormant phase included a large number of peroxidases. Thus, there was a distinct difference in transcriptomes corresponding to the dormancy dehydration and chilling fulfillment in the two cultivars with changes in transcription related to dehydration and protection being the most relevant during acclimation and winter phase of dormancy.
Discovery of an ever-growing peach genotype has advanced genetic analysis of peach tree dormancy and cold acclimation induction. The evergrowing peach (P. persica evg) genotype does not respond to short photoperiods for induction of dormancy and acclimation and is less freezing tolerant than the deciduous peach (Arora et al., Citation1992). Suppression subtractive hybridization of short photoperiod induced cDNA libraries from wild type (WT) and evg peach buds was used to identify 177 ESTs that produced 106 contigs (Jimenez et al., Citation2010b). Analysis of the expression of 23 of these genes in WT and evg, during short photoperiod treatments, indicated three patterns of expression, two of which appear to be associated with dormancy induction. A group of genes were upregulated quickly upon transition to short photoperiod and subsequently declined at two weeks of short photoperiod (signaling/transcription, transport and defense). Another group was upregulated later in short photoperiod, but continued to increase in expression for eight weeks (including: LEA, PR proteins, DAM and metallothioneins). These patterns indicate signaling responses resulting in reprogramming of the metabolism as well as sustained gene expression are associated with dormancy maintenance.
Proteomic analysis of peach bark from low temperature and/or short photoperiod treatments indicated that low temperature had the greatest effect on proteome expression with 25 peptide spots more abundant and six less abundant relative to 25°C treatment (Renaut et al., Citation2008). Short photoperiod specific expression included one peptide spot of higher abundance and seven peptide spots of lower abundance. It is noteworthy that short photoperiod and low temperature, which promote the best acclimation response, showed additive effects, resulting in 36 more abundant and three less abundant peptide spots. The low temperature specific increased abundance peptides were related to stress response (dehydrin and PR-protein), energy, amino acid, lignin metabolism, cytoskeleton organization and defense mechanisms. Dehydrins are suggested, but not conclusively shown, to have a protective role in cells under freeze dehydrated conditions. Similarly PR-proteins such as glucanases and thaumitin-like proteins have been reported to have antifreeze properties that impact the location and growth of ice crystals (Griffith et al., Citation2005). Changes in transcript and protein abundance of the dehydrins and PR-proteins during the acclimation of bark tissues, which tolerate equilibrium freezes, provide support for a potential role in mitigating freeze dehydration stresses; however their precise function remains to be determined (Bassett et al., 2002; Wisniewski et al., Citation1996, 1999; 2006; Welling and Palva, Citation2006).
Proteomic analysis of apricot (Prunus mume) flower buds showed an increased abundance of 32 protein spots in endodormant buds subjected to low temperature (Zhuang et al., Citation2012). Similarities between peach bark and apricot floral buds include stress response and defense, energy metabolism and cell structure related (Renaut et al., Citation2008). Further, analysis of microRNAs in low temperature acclimated peach buds and actively growing leaves identified several highly abundant microRNAs in buds that have been suggested to target floral development genes; however, this study did not include non-acclimated floral bud therefore it is difficult to determine whether there was a true abundance of these negative regulators (Barakat et al., Citation2012). The negative regulatory role of many microRNAs requires that studies include the same tissue exposed to differential temperature or photoperiod and for microRNA and transcriptome analysis to identify relevant microRNAs and their potential targets.
C. Blueberry
An analysis of 600 ESTs each from non-normalized cold acclimated and non-acclimated blueberry (Vaccinium corymbosum) floral buds showed a greater abundance of beta amylase, dehydrin, early light-inducible protein and senescence-associated protein transcripts in cold acclimated buds (Dhanaraj et al., Citation2004). A cDNA microarray containing 2500 elements was subsequently used to compare cold acclimation in field conditions with controlled environment conditions (Dhanaraj et al., Citation2007). Studies indicated that freezing tolerance of the blueberry floral bud was greater in the field after prolonged acclimation than in the controlled environment conditions (Rowland et al., Citation2008). While dehydrins, cell wall protein, LEAs and early-light inducible protein were abundant in both conditions, a greater number of genes were induced in controlled environment and it was noted that many of these were suppressed in the field conditions, perhaps in part due to fluctuating temperatures, temperature extremes or other environmental factors. Genes (in particular, auxin-repressed protein and protein kinase PINOID), which had not previously been identified as cold induced in Arabidopsis, were found to be cold induced in both field and controlled environment acclimated floral buds. Cold induced gene expression specific to the field or controlled environment acclimation were found, as were differences in gene expression between the non-acclimated plants in field or controlled environment. Genes related to light stress, photosystem IID1, photosystem II CP 47 and early light-inducible protein, were specifically up-regulated in the field in contrast to controlled environment. The greater abundance of gene expression between controlled environment and field environment are a caution and may result from shock-like conditions in controlled environment rather than the more gradual temperature reduction and diurnal cycling, however, it should also be noted that additional environmental cues may play a role in dampening gene expression in the field. Therefore, it is important to cross verify controlled environment systems biological studies with natural environmental conditions to address the holistic plant responses.
D. Grapevine
Grapevines acclimate in response to photoperiod and temperature and tolerate midwinter subzero temperatures by extracellular freezing in stem tissues and supercooling of bud tissue (Fennell, Citation2004; Victor et al., Citation2010). Under short photoperiod and cold acclimation transcripts related to responses to external stimuli and PR-proteins such as chitinases and thaumitin-like proteins were up-regulated during early cold acclimation (Mathiason et al., Citation2009). Sustained chilling resulted in down regulation of the bud transcriptome; however, many transcripts related to response to external stimuli and reactive oxygen species were maintained or increased during the transition to ecodormancy. A transcriptome analysis of temporal/seasonal bud development from paradormancy to ecodormancy showed the greatest differential gene expression during bud initiation phase, with moderate levels during endodormancy and transition to ecodormancy and lowest levels during mid-winter (∼1500, 800, 900 and 200 genes, respectively) (Díaz-Riquelme et al., Citation2012). While specific analysis in relation to low temperature response were not conducted, it was noted that during the endodormant phase of bud development transcripts related to temperature stress response, starch catabolism, ABA catabolism, CCAAT family transcription factor, HSP-mediated protein folding and stilbenoid biosynthesis were up-regulated and gene pathways related to photosynthesis, primary and secondary metabolism (fatty acid, carbohydrate, cell wall and flavonoid biosynthesis and cell cycle) were down regulated. Similarly, a tissue atlas of gene expression in fifty-two grapevine tissues identified gene expression related to seasonal programming of tissue development (Fasoli et al., Citation2012). When gene expression was clustered by organ, the transcriptomes of bud and woody stem tissues were most closely related. Genes related to these mature/woody tissues (stem and bud) were enriched in protein metabolic processes, translation, cellular processes and response to external stimuli, with a high level of expression of dehydrin and metallothioneins. Coexpression analysis indicated a small group of genes (105) that were related to tissue maturation in stem and bud. Bud specific expression identified 384 genes, predominately signaling proteins, transcription factors and transporters, with 29 specific to paradormant and endodormant buds and 92 that were expressed from paradormancy through budburst. Tissue specific genes are useful in functional analysis and it is well established that tissue maturation is a key component of grapevine freezing tolerance; however, acclimation requires coordinated reprogramming within the whole plant so caution should be exercised in using only tissue specific genes as candidates for freezing tolerance studies (Fennell and Hoover, Citation1991; Howell and Shaulis, Citation1980). The identification of coexpressed genes between bud and woody stem tissues does provide potential paths for elucidating plant responses to environmental stimuli that promote cold acclimation as well as development of barriers to ice nucleation of the grapevine bud.
IV. TRANSCRIPTIONAL REGULATION OF LOW TEMPERATURE RESPONSE
A. Calcium Signaling
Deciduous trees respond to low temperature and/or short photoperiod to induce cold acclimation and that short photoperiod and low temperature are synergistic in many species promoting a greater freezing tolerance than low temperature (Fennell, Citation2004; Arora et al., Citation2003; Tanino et al., Citation2010). Analyses of low temperature responses are well defined in the model herbaceous plant (Arabidopsis thaliana) and cold acclimation processes in woody fruit trees show many commonalities with Arabidopsis. In model systems, low temperature responses are mediated through calcium and reactive oxygen signaling, which results in a cascade of cold mediated transcription regulation that promotes development of protective proteins and metabolites () (Theocharis et al., Citation2012; Zhu et al.,2007).
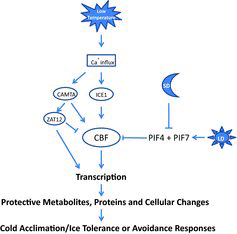
In mulberry buds, increased calcium levels were noted in the cytosol and nucleus in response to decreased day length and dormancy induction and theses levels declined in midwinter (Jian et al., Citation2000). Similarly, decreasing temperature promoted an influx of calcium into cytosol and nuclei in apricot buds (Wang et al., Citation2008). In grapevine, calcium signaling related transcripts were shown to be upregulated in leaves in response to a short-term low temperature exposure (Tattersall et al., Citation2007). In addition to calcium fluxes as signals, calcineurin B-like proteins (CBL) are suggested to interact with CBL-interacting protein kinases (CIPK) to “decode” calcium signaling and promote spatially specific cellular response (Batistič et al. Citation2010). Recently, an apple CBL- interacting protein kinase (MdCIPK6L) was shown to be induced by calcium and cold temperature (4°C) and promoted enhanced freezing tolerance in Arabidopsis, thus supporting the role of calcium and CIPK in low temperature signal transduction (Wang et al., Citation2012). Further emphasizing the role of calcium signaling, a calcium regulated calmodulin-binding transcription activator (CAMTA), that has been shown to bind to a C-repeat binding factor (CBF) gene promoter, and a calcium/calmodulin-regulated receptor-like kinase (CIPK) were shown to be involved in increased freezing tolerance of Arabidopsis (Doherty et al., Citation2009; Yang et al., Citation2010). Thus, a low temperature network is suggested to be regulated by calcium and/or kinase/phosphatases (Theocharis et al., Citation2012). Additionally, it is suggested that a calcium-calmodulin CAMTA complex may directly regulate members of the AP2 domain-containing transcription factors, specifically CBF1 and CBF2, also called dehydration-responsive element (DRE) binding proteins (DREB1) or low temperature response elements (LTREs) (Thomashow, Citation2010). The CBFs are generally accepted as a regulatory hub of cold responsive genes (COR) during herbaceous plant cold acclimation; however, the CAMTA role still needs to be established (Theocharis et al., Citation2012; Thomashow, Citation2010). In addition, there is substantial evidence in Arabidopsis for a parallel cold induction pathway through a zinc finger protein (ZAT12), which plays a role in high light and may play a role in the high light and low temperature responses through the ROS signaling pathway (Doherty et al., Citation2009; Davletova et al., Citation2005; Theocharis et al., Citation2012; Vogel et al., Citation2005).
B. Inducer of CBF Expression (ICE)
The transcription factor inducer of CBF expression (ICE) acts upstream of the CBFs and is a constitutively expressed basic helix-loop-helix transcription factor (bHLH) that is rapidly activated by low temperature, through sumoylation and phosphorylation (Theocharis et al., Citation2012; Thomashow, Citation2010). It has been shown that ICE1 is a major regulator of CBF3 and overexpression of ICE1 has been shown to increase freezing tolerance in Arabidopsis (Miura et al., Citation2011; Thomashow, Citation2010). An apple cold-induced bHLH gene (MdCIbHLH1), similar to AtICE1 and AtICE2, was shown to increase in expression within three hours of exposure to 4°C (Feng et al., Citation2012). Upregulation of MdCIbHLH1 was also followed by an increased expression of the five apple CBFs (MdCBF1, MdCBF2, MdCBF3, MdCBF4 and MdCBF5) during the low temperature exposure and the MdCIbHLH1was shown to bind to an AtCBF3 promoter MYC4 site. When MdCIbHLH1was constitutively expressed in Arabidopsis all three AtCBFs were upregulated. In apple, the MdCIbHLH1 bound to three MdCBF2 promoter MYC recognition regions, but did not bind to MdCBF1, MdCBF3, MdCBF4 or MdCBF5. Ectopic expression in apple callus and tissue cultures resulted in increased expression of MdCBF1, MdCBF2, MdCBF3, MdCBF4 and MdCBF5 and maintained viability of transgenic cells at low temperature, indicating that the MdCIbHLH1 plays a similar role to AtICE1 in mediating CBF regulation at low temperature.
C. CBF/DREB1 Expression
The CBF/DREB1 genes, a sub-family of the APETALA2/ETHYLENE RESPONSE Factor (AP2/ERF) transcription factors, have been characterized as a regulatory hub in freezing tolerance in herbaceous plant systems (Theocharis et al., Citation2012; Thomashow, Citation2010). CBF/DREB1 homologs have also been identified in fruit trees and show similar responses to environmental stimuli as the Arabidopsis CBFs.
Sour cherry (Prunus cerasus L., PcCBF1) and strawberry (Fragaria x ananassa Duchesne, FaCBF1) were up-regulated in their respective leaves at 4°C and ectopic expression of FaCBF1 in strawberry increased leaf freezing tolerance (Owens et al., Citation2002). Similarly, a sweet cherry (Prunus avium) CBF ortholog (PaCBF) increased freezing tolerance in Arabidopsis (Kitashiba et al. Citation2004). A CBF found in highbush blueberry (V. corymbosum, BB-CBF) was expressed at higher levels in a cold tolerant northern cultivar than in southern cold sensitive cultivar during early cold acclimation stage (Polashock et al., Citation2010). In addition, overexpression of BB-CBF in a southern cold sensitive cultivar resulted in increased leaf and floral bud freezing tolerance at nonacclimating temperatures. Two almond CBFs (PdCBF1 and PdCBF2) were shown to bind to DRE elements and to be rapidly induced by low temperature. Induction of PdCBF1 and PdCBF2 was higher during the early dark period of the photoperiod cycle suggesting both photoperiod and temperature regulation (Barros et al., Citation2012a, Citationb). In apple, which acclimates and enters dormancy solely in response to low temperatures, apple CBFs (MdCBF1, MdCBF2) increased between 30 minutes and 2 hours in response to low temperature (Heide and Prestrud, Citation2005; Wisniewski et al., Citation2011). In addition, an apple DREB/CBF gene, with similarity to the Arabidopsis DREB2B, was up-regulated by cold, drought, heat and ABA within 2 hours and increased expression for 8 to 24 hours () (Zhao et al., Citation2012). In apple rootstock (Malus baccata) DREB1/CBF (MbDREB1) was rapidly up-regulated by cold, drought, salinity and ABA in several tissues and was most strongly up-regulated in stem and leaves at 4°C (Yang et al., Citation2011). The MbDREB1 promoter region contained an ICE1-like and an ABA responsive element (ABRE) binding site. Cold, drought and ABA stimuli promoted induction of a chimeric GUS gene (MbDREB1 promoter). In Arabidopsis, expression was localized to the nucleus and overexpression of MbDREB1 increased freezing tolerance (Yang et al., Citation2011). Analysis of the CBF/DREB1 pathway in grapevines has identified four CBF genes in V. vinifera and V. riparia that are up-regulated in response to low temperature (Tillett et al., Citation2012; Xiao et al., Citation2006, 2008). Overexpression of VvCBF4, in the hybrid grapevine rootstock Freedom, increased freezing tolerance and reduced growth (Tillett, et al., Citation2012). In contrast to VrCBF1, overexpression of VrCBF4 resulted in sustained expression of all CBFs (days rather than hours) and a greater increase in freezing tolerance and greater drought tolerance (Siddiqua and Nassuth, Citation2011). In addition, overexpression of three V. vinifera CBFs and a zinc finger protein (VvZFPL) increased cold tolerance in Arabidopsis and promoted the expression of a CIPK7 (Takuhara et al., Citation2011; Kobayashi et al., Citation2012). These studies all indicate that CBFs are present in fruit trees and respond to low temperature in a similar way to what has been shown in the Arabidopsis model.
D. CBF/DREB1 Regulated Gene Expression
The CBF/DREB1 proteins bind to the C-repeat/dehydration-responsive element (CRT/DRE) of target COR genes and promote increased freezing tolerance (Theocharis et al., Citation2012; Thomashow, Citation2010).
In non-acclimated transgenic grapevines overexpressing VvCBF4, 59 transcripts (47 upregulated and 12 downregulated) were differentially expressed in relation to control plants (Tillett et al., Citation2012). These included transcripts for stress-responses, lipid metabolism and cell wall structure. A comparison of the non-acclimated grapevines overexpressing VvCBF4 with Arabidopsis overexpressing multiple CBF/DreB1 or Populus overexpressing AtCBF1 indicated 13 and 14 genes, respectively, with patterns of expression in common between grapevine, Populus and Arabidopsis. Of note are stress responsive, calcium-binding proteins and cell wall–related genes indicating that the CBF mediated responses are highly conserved across herbaceous and woody species. A test of VrCBF1 and VrCBF4 overexpression on endogenous Arabidopsis COR (AtCOR15a, AtRD29A, AtCOR6/KIN2 and AtCOR47) and development genes (AtGA2ox3, AtRGL3, AtFLC and AtICE1) indicated that both CBFs enhanced expression of COR genes and the AtRD29A (Siddiqua and Nassuth, Citation2011). Thus this suggests a role in both ABA-independent and ABA-dependent low temperature response pathways; however, the interaction of the CBF with a basic leucine zipper domain transcription factor (bZIP) remains to be shown to support its role in the ABA-dependent pathway. Further, differential response was noted among the development genes with greater expression of AtGAox7 in VrCBF1 lines and expression of AtRGL3 only detected in VrCBF4 lines. The flowering locus C gene (AtFLC) and At1ICE1 were up-regulated in both VrCBF1 and VrCBF4 transgenic lines. The ICE1 gene promotes CBF3 expression resulting in an increased freezing tolerance in several species. However, overexpression of VrCBF1 or VrCBF4 in Arabidopsis also enhanced ICE1 expression suggesting a potential CBF and ICE1 interaction involved in maintaining CBF3 expression (Siddiqua and Nassuth, Citation2011).
The most well documented cold and CBF regulated genes are the dehydrin gene family which contain the DRE/CRT/LTRE regulatory element in their promoter. Several studies have shown an increased abundance of dehydrin transcripts and proteins accumulating in woody plant buds and bark during induction of dormancy and cold acclimation (Arora et al., Citation1997; Arora and Wisniewski, Citation1994, Citation1996; Garcia-Bañuelos, et al., Citation2009; Muthalif and Rowland, Citation1994; Winiewski et al., Citation2006, 2008; Xiao et al., Citation2006; Yamane et al., Citation2006). In blueberry, constitutive expression of BB-CBF resulted in a high level of expression of two dehydrin genes and a galactinol synthase under non-acclimating conditions (Walworth et al., Citation2012). Further the almond PdCBF1 and PdCBF2 were shown to bind DRE elements and promoted a ≥ twofold increase in expression of an almond dehydrin gene PdDHN1 and expression of an ABA-dependent stress-induced gene (AtRD29A) (Barros et al., Citation2012b). The almond CBFs were induced more strongly at the end of the photoperiod. When low temperature occurred in the dark period peak expression occurred earlier than when cold temperature occurred in the light period. In apple, overexpression of MbDREB1 upregulated the expression of COR15a and rd29B, further supporting the suggestion that DREB1 may be active in both ABA-dependent and ABA-independent pathways (Yang et al., Citation2011). Insertion of a peach CBF (PpCBF1) in an apple rootstock increased freezing tolerance in non-acclimated and cold acclimated plants over the wild type and resulted in high level of expression of an apple dehydrin (MdDhn1) in stems and leaves of the transgenic plant (Wisniewski et al., Citation2011). In apple, dormancy and cold acclimation are normally induced by low temperature not photoperiod (Heide and Prestrud, Citation2005). However, overexpression of the PpCBF1 in apple plants increased freezing tolerance and promoted induction of dormancy under shortphotoperiods alone, without a low temperature inductive stimulus (Wisniewski et al., Citation2011). The induction of photoperiod sensitivity in apple by PpCBF1, is novel and suggests interplay between genes regulating cold acclimation and photoperiod induction of dormancy. This is very noteworthy as it has long been recognized that in many woody plants there is a synergistic effect of short photoperiods and low temperature in the development of cold acclimation (Fennell, Citation2004; Arora et al., Citation2003; Tanino et al., Citation2010).
Recently it has also been shown that freezing tolerance in Arabidopsis can be induced by short photoperiod (Dong et al., Citation2011; Harmer et al., Citation2000). Short photoperiod resulted in increased expression of CBF1, CBF2 and CBF3 at 8 hours after dawn (zeitgeber time 8, ZT8) and upregulated expression of cold regulons AtCOR15 and AtGOL3 (Lee and Thomashow, Citation2012). It was also noted that short photoperiod and low temperature resulted in greater freezing tolerance in Arabidopsis as has been shown for woody plant systems and that concordant expression of PIF4 and PIF7 were required to repress acclimation in long photoperiods. The role of short photoperiod was uncovered using a double mutant of the phytochrome-interacting factor, a bHLH transcription factor, (pif4 and pif7). The pif4 and pif7 mutant eliminated repression of CBF pathway under long photoperiods resulting in increased CBF transcription levels and an increased freezing tolerance, similar to freezing tolerance in wild type plants under short photoperiod (Lee and Thomashow, Citation2012). Therefore, the short photoperiod induction of freezing tolerance and synergistic responses of photoperiod with cold temperature that has long been observed in woody plants may be mediated in part through the CBF pathway (). Further analysis of the genes such as the peach CBF for an evening element and related DNA regulatory motifs should be conducted and may explain the photoperiod responsiveness observed by Winiewski et al., (Citation2011) in the overexpressing PpCBF1 apple rootstocks.
Both photoperiod and temperature are factors affecting the entrainment of the circadian clock. The circadian clock senses and resets the clock in response to diurnal light and temperature cycles. A change in photoperiod resets the clock and subsequently the metabolism and physiology of the plant promoting induction of dormancy and acclimation responses (Cook et al., Citation2012; Espinoza et al., Citation2010; Lindlőf, Citation2010). Circadian regulation through circadian clock associated 1 (CCA1) and LATE HYPOCOTYL (LHY) exert positive regulation on CBF cold induction resulting in maximum freezing tolerance (Dong et al., Citation2011). In chestnut (Castanea sativa), low temperature disrupts the circadian oscillations resulting in a high levels of constant expression of TIMING OF CHLOROPHYLL a/b binding protein expression 1 (TOC1/PRR1), LHY1, and LHY2 and PSUEDO-RESPONSE REGULATOR-genes (PRR5, PRR7, and PRR9) (Ibanez et al., Citation2008). These responses further emphasize that there is an interchange between photoperiod and cold temperature on circadian rhythms that impacts the CBF hub regulatory pathway.
E. Dormancy Associated MADS Box Gene Expression
Dormancy associated MADS Box genes (DAM) have been associated with the photoperiod and low temperature induction of dormancy in peach and apricot and it is noted that prolonged cold temperature suppresses their expression (Li et al., Citation2009; Sasaki et al., Citation2011). Thus the DAM genes are thought to play a role in the induction, maintenance as well as release from dormancy during the winter period. Growth cessation and leaf abscission do not occur in the evergreen peach mutant (evg) when exposed to short photoperiod and /or low temperatures (Rodriguez et al., Citation1994). Sequence analysis of the evg loci indicated the evg peach was missing 6 DAM genes (DAM1, DAM2, DAM3, DAM4, DAM5, and DAM6) (Bielenberg et al., Citation2008). Characterization of the DAM genes in field and controlled short photoperiod grown wild type peach indicated that five genes (DAM1, DAM2, DAM4, DAM5, and DAM6) were photoperiod responsive, with DAM6 expression showing the highest level of up-regulation upon exposure to short photoperiod. DAM1 and DAM2 were expressed at the time of dormancy induction in field conditions and DAM5 and DAM6 were most highly expressed in mid-winter, suggesting a role in dormancy maintenance. In addition, DAM5 and DAM6 expression was found to be higher in high chill requiring cultivars (Jiménez et al., 2010). The DAM3 appears to be low temperature regulated and decreases throughout the winter season and may play a role in dormancy release (Bielenberg et al., 2009). The role of DAM genes in dormancy release is further supported by work of Yamane et al. (Citation2011) which showed that prolonged exposure to low temperature decreased expression of DAM5 and DAM6 and the decreased expression correlated with increased budbreak potential. Thus differential responsiveness of the DAM genes under controlled photoperiod and warm temperatures, in contrast to field conditions, suggests that they differ in potential freezing tolerance induction and dormancy release roles.
F. Epigenetic Regulation
Epigenetic regulation is emerging as an important integrator of the environmental influences on plant growth and development. Vernalization, the cold induction of flower development, has been shown to be regulated by histone modification of chromatin (Ahmad et al., Citation2010; Pin and Nilsson, Citation2012). In chestnut, expression of two histone genes involved in chromatin remodeling, histone monoubiquitinase (HUB2) and histone acetyltransferase (GCN5L), was greater at dormancy induction than at dormancy release (Santamaria et al., Citation2011). In peach, decreasing levels of DAM6 expression are associated with chilling fulfillment and dormancy release (Jimenez et al., Citation2010a; Leida et al., Citation2011; Yamane et al., Citation2011). Investigation of histone modifications of DAM6 in low and high chill peach cultivars indicated specific chromatin modifications correlated with the down regulation of DAM6 and the progression from bud dormancy through dormancy release (Leida et al., Citation2011). This suggests that sustained low temperature represses DAM6 expression through histone modification of chromatin. This response is similar to the low temperature induced histone modifications that results in the repression of Flowering locus C, a MADS-box transcription factor, expression of which prevents flowering transitioning prior to vernalization (Ahmad et al., Citation2010). While epigenetic responses are apparent in low temperature mediated plant developmental processes their role in cold acclimation processes remains to be determined.
V. GENETIC ANALYSIS OF WINTER SURVIVAL
It is well established that acclimation and freezing tolerance are under genetic control and tissue and species specific (Ashworth, Citation1984; Gusta and Wisniewski, Citation2012; Kader and Proebsting, Citation1992; Owens, Citation2005). While the complexity of winter survival has limited detailed genetic analysis, early studies in apple, peach and blueberry, testing different components of the trait (photoperiod response, dormancy, acclimation, chilling requirement and timing of budbreak) indicate trait heritability and suggest additive variance (Cain and Anderson, Citation1980; Fear et al., Citation1985; Fejer, Citation1976; Mowry, 1964; Watkins and Sangelo, 1970).
Intraspecific hybridization of fruit crop species divergent in freezing tolerance and backcrossing to improve quality are frequently used to introgress winter survival traits and improve cultivar sustainability in adverse environments. Analyses of blueberry F1 and testcross populations, derived from blueberry species divergent in freezing tolerance (Vaccinium darrowi and V. caesariense, freezing sensitive (−13°C) and freezing tolerant (−20°C)) provided the opportunity to dissect inheritance patterns (Arora et al., Citation2000). Analysis of the F1 populations indicated that lower freezing tolerance characteristic is a partially dominant trait in blueberries with no significant maternal influence. Analysis of the generation means indicated additive gene effects of a small family of genes. Dehydrins have been observed to increase in buds during seasonal transition into dormancy and cold acclimation, therefore a candidate dehydrin (bbDHN1) was mapped in the testcross populations and compared with freezing tolerance of the populations (Panta et al., Citation2004; Rowland et al., Citation2004). Inheritance of freezing tolerance was not correlated with the bbDHN1 marker used; however, this represents the mapping of only one gene in a small population against a quantitative trait.
Chilling is required to transition bud and cambial tissues from endodormancy to ecodormancy in preparation for resumption of growth in spring. In Prunus species, chilling requirement plays a role in potential injury as a result of late winter and early spring freezing temperatures. Therefore, chilling fulfillment and budbreak are considered major factors in freezing tolerance. An F2 mapping population from a selfed F1 (generated from low and high chill requiring parents) was phenotyped for chilling requirement, heat requirement (growing degree hours) and bloom date (Fan et al., Citation2009). Twenty QTL were identified, including one major QTL for chilling requirement and two for bloom date. The remainder co-localized with other traits. One of the co-localized groups was identified in the region previously identified for the deciduous peach (EVG). Similar to peach, apricot is susceptible to freezing damage in late winter and early spring resulting in reduced yields (Olukolu et al., Citation2009). In apricot, chilling requirement segregated within a mapping population generated from low and high chill genotypes and 12 QTL were identified, with several localized to similar QTL as previously described for peach (Fan et al. Citation2009). Gene candidates associated with the QTL include the MADS-box transcription factors, a mitogen activated protein kinase 7 (MPK7), and abscisic acid insensitive 3 (ABI3) genes. The recent publication of the peach genome assembly will aid in further identification of genes and genetic variation underlying these QTL.
In grapevine, response to photoperiod promotes early growth cessation, dormancy and acclimation processes and promotes synergistic increase in freezing tolerance upon cold acclimation (Fennell, Citation2004; Fennell and Hoover, Citation1991; Schnabel and Wample, Citation1987). An F2 mapping population from a selfed F1 (generated from V. riparia, very freezing tolerant and highly photoperiod responsive and hybrid wine grape Seyval, moderate freezing tolerance and low photoperiod responsive) was monitored for growth cessation and lateral emergence in controlled environment and field conditions (Garris et al., Citation2009). Different QTL were observed for growth cessation and critical photoperiod response in the two environmental conditions. A major QTL on linkage group 13 explained 80–90% of phenotypic variation for controlled environment conditions with non-inducing low temperatures. In contrast, a QTL on linkage group 11 explaining 85 to 94% of phenotypic variance was observed in field conditions. The differences between environments suggest the presence of non-photoperiodic cues for induction of growth cessation in the field. These results are in sync with the observations that both photoperiod and low temperatures play a role in cold acclimation processes.
Changing climate with increased warming trends impacts the timing of budbreak and flowering which can increase potential for freezing injury. Analysis of budbreak and flowering time was conducted in 120 progeny of a Reisling x Gewurztraminer cross (V. vinifera) (Duchêne et al., Citation2012). Three phenotypes related to thermal time were examined and six QTL were identified for the traits of degree days for chilling requirement to budbreak, budbreak to flowering and flowering to veraison. Two main QTL explaining 11 and 12% of phenotypic variance were identified for budbreak on linkage group 4 and 19 and two main QTL explaining 16 and 27% of the phenotypic variance were found for flowering time. Candidate genes for the budbreak QTL were identified by comparison of genes within the loci with previous bud chilling transcriptomic analysis (Mathiason et al., Citation2009). These comparisons identified a glutathione S-transferase and a WRKY transcription factor underlying the QTL loci. Analysis of the QTL loci for flowering time indicated nine genes linked to flowering including known flowering time gene VvFT and a zinc finger Constans-like gene (VvCOL2). The availability of transcriptomic expression datasets and genome sequence provide the opportunity to quickly identify relevant candidate genes underlying QTL.
These studies indicate that genetic variation for traits related to winter survival exist in most fruit crops and/or related wild species. However, the need to select for freezing tolerance at the same time as other quantitative traits (fruit quality and production characteristics) intensifies the challenge for the breeder. Finally, decreased genotyping costs provide the opportunity for high density SNP discovery allowing the development of suites of SNP markers and increasing potential marker definition without the need to construct linkage maps (Elshire et al., Citation2011). Plant gene expression is also controlled by regulatory regions that fall outside of the coding DNA and genome-wide SNP discovery provides the ability to identify SNPs in all regulatory regions for marker assisted breeding. Thus the availability of genome, genotype by sequencing and SNP analysis and transcriptomic, metabolomics and proteomic expression profiles from relevant tissues and environmental conditions provide the enhanced opportunities for dissecting complex traits like winter survival.
VI. OPPORTUNITIES
Development of genomic and functional genomic resources is providing strong platforms to use in addressing, in a holistic fashion, the multifaceted trait of woody plant winter survival. The ability to use transcriptomic, proteomic and metabolomics expression data in conjunction with inheritance studies present new opportunities for determining the molecular and genetic basis of cold tolerance in fruit trees. Recent genetic analysis of metabolites in apple fruits identified a metabolite QTL (mQTL) for phenolic compounds (Khan et al., Citation2012). It is known that acclimation responses result in major shifts in metabolism (Janska et al., Citation2010) and through-put capacity in metabolite analysis is increasing steadily; therefore use of metabolite profile as phenotypes provide a similar opportunity for dissecting genetic mechanisms, as using transcriptome profiles guided candidate gene identification in budbreak QTL (Duchêne et al., Citation2012). Similarly proteogenomics, expressed protein tags from mass spectrometric analysis of proteins, can provide opportunities for protein QTL (pQTL) and greater depth in expression profiling as well as genome annotation. Finally any study of the genomic components of winter survival must consider that the ability of the plant to survive winter conditions, involves response to multiple environmental cues and that the presence or avoidance of ice includes physiological, morphological and biophysical factors. Thus studies must take into consideration water interactions with cellular components and molecules, intracellular communication, interactions between tissues and kinetics of ice formation and duration of freeze for complete systems biology analyses to dissect the underlying genomic components.
ACKNOWLEDGMENTS
Support was received from the South Dakota State University Agricultural Experiment Station.
REFERENCES
- Adam-Blondon, A-F., Roux, C., Claux, D., Butterlin, G., Merdinoglu, D., and This, P. 2004. Mapping 245 SSR markers on the Vitis vinifera genome: a tool for grape genetics. Theor. Appl. Genet. 109: 1017–1027.
- Agrout, X., Salse, J., Aury, J-M., Guiltinan, M.J., Droc, G., Gouzy, J., Allegre, M., Chaparro, C., Legavre, T., Maximova, S.N., Abrouk, M., Murat, F., Fouet, O., Poulain, J., Ruiz, M., et al. 2011. The genome of Theobroma cacao. Nature Genet. 43: 101–106.
- Ahmad, A., Zhang, Y., and Cao, X-F. 2010. Decoding the epigenetic language of plant development. Mol. Plant. 3: 719–728.
- Aquea, F., Vega, A., Timmermann, T., Poupin, M.J., and Arce-Johnson, P. 2011. Genome-wide analysis of the SET DOMAIN GROUP family in grapevine. Plant Cell Rep. 30: 1087–1097.
- Arora, R. and Rowland, L.J. 2011. Physiological research on winter-hardiness: deacclimation resistance, reacclimation ability, photoprotection strategies, and a cold acclimation protocol design. HortScience. 46: 1070–1078.
- Arora, R., Rowland, L.J., Lehman, J.S., Lim, C.C., Panta, G.R., and Vorsa, N. 2000. Genetic analysis of freezing tolerance in blueberry (Vaccinium section Cyanococcus). Theor. Appl. Genet. 100: 690–696.
- Arora, R., Rowland, L.J., and Panta, G.R. 1997. Chill-responsive dehydrins in blueberry: Are they associated with cold hardiness or dormancy transitions. Physiologia Plantarum 101: 8–16.
- Arora, R., Rowland, L.J., and Tanino, K. 2003. Induction and release of bud dormancy in woody perennials a science comes of age. HortScience. 38: 911–921.
- Arora, R. and Wisniewski, M.E. 1994. Cold acclimation in genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch). II. A 60 kD polypeptide in cold acclimated bark tissue of peach is heat-stable and related to dehydrin family of proteins. Plant Physiol. 105: 95–101.
- Arora, R. and Wisniewski, M.E. 1996. Accumulation of 60 kD dehydrin protein in peach xylem tissues and its relationship to cold acclimation. HortScience. 31: 923–925.
- Arora, R., Wisniewski, M.E., and Scorza, R. 1992. Cold acclimation in genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch). Plant Physiol. 99: 1562–1568.
- Artlip, T.S., Callahan, A.M., Basset C.L., and Wisniewski, E.N. 1997. Seasonal expression of a dehydrin gene in sibling deciduous and evergreen genotypes of peach (Prunus persica [L.] Batsch). Plant Mol. Biol. 33: 61–70.
- Arús, P., Verde, I., Sosinski, B., Zhebentyayeva, T., and Abbott, A.G. 2012. The peach genome. Tree Genet. Genomes. 8: 531–547.
- Ashworth, E.N. 1982. Properties of peach flower buds which facilitate supercooling. Plant Physiol. 70: 1475–1479.
- Ashworth, E.N. 1984. Xylem development in Prunus flower buds and the relationship to deep supercooling. Plant Physiol. 74: 862–865.
- Ashworth, E.N. 1992. Formation and spread of ice in plant tissues. Hortic. Rev. 13: 215–255.
- Ashworth, E.N. and Wisniewski, M.E. 1991. Response of fruit tree tissues to freezing temperatures. HortScience. 26: 501–504.
- Barakat, A., Sriram, A., Park, J., Zhebentyayeva, T., Main, D., and Abbott, A. 2012. Genome wide identification of chilling responsive microRNAs in Prunus persica. BMC Genomics 13: 481.
- Barros, P.M., Gonçalves, N., Saibo, N.J. M., and Oliveira, M.M. 2012a. Cold acclimation and floral development in almond bud break: insights into the regulatory pathways. J. Expt. Bot. 63: 4585–4596.
- Barros, P.M., Gonçalves, N., Saibo, N.J. M., and Oliveira, M.M. 2012b. Functional characterization of two almond C-repeat-binding factors involved in cold response. Tree Physiol. 32: 1113–1128.
- Bassett, C.L., Wisniewski, M.E., Artlip, T.S., and Norelli, J.L. 2006. Global analysis of genes regulated by low temperature and photoperiod in peach bark. J. Amer. Soc. Hort. Sci. 131: 551–563.
- Bassett, C.L., Wisniewski, M.E., Artlip, T.S., Richart, G., Norelli, J.L., and Farrell, R.E. 2009. Comparative expression and transcript initiation of three peach dehydrin genes. Planta 230: 107–118.
- Batistič, O., Waadt, R., Steinhorts, L., Held, K., and Kudla, J. 2010. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J. 61: 211–222.
- Bielenberg, D.G., Wang, Y., Li, Z., Zhebentyayeva, T., Fan, S., Reighard,G.L., Scorza, R., and Abbott, A.G. 2008. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet. Genomes. 4: 459–507.
- Burke, M.J. and Stushnoff, C. 1978. Frost hardiness: a discussion of molecular causes of injury with particular reference to deep supercooling of water. In: Mussel1, H. and Staples, R. eds. Stress Physiology in Crop Plants.pp. 197–225. Wiley, New York.
- Cain, C.W. and Anderson, R.L. 1980. Inheritance of wood hardiness among hybrids of commercial and wild asian peach genotypes. J. Amer. Soc. Hort. Sci. 105: 349–354.
- Çakir, B., Kiliçkaya, O., and Olcay, A.C. 2013. Genome-wide analysis of Aux/IAA genes in Vitis vinifera: cloning and expression profiling of a grape Aux/IAA gene in response. Acta Physiol Plant 35: 365–377.
- Cook, J.E., Eriksson, M.E., and Junttila, O. 2012. The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant Cell Environ. 35: 1707–1728.
- Davletova, S., Schlauch, K., Coutu, J., and Mittler, R. 2005. The zinc-finger protein ZAT12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 139: 847–856.
- Dhanaraj, A.L., Alkharouf, N.W, Beard, H.S., Choukha, I.B., Matthews, B.F., Wei, H., Arora, R., and Rowland, L.J. 2007. Major differences observed in transcript profiles of blueberry during cold acclimation under field and cold room conditions. Planta. 225: 735–751.
- Dhanaraj A.L., Slovin, J.P., and Rowland, L.J. 2004. Analysis of gene expression associated with cold acclimation in blueberry floral buds using expressed sequence tags. Plant Sci. 166: 863–872.
- Dhanaraj, A.L., Slovin, J.P, and Rowland, L.J. 2004. Analysis of gene expression associated with cold acclimation in blueberry floral buds using expressed sequence tags. Plant Sci. 166: 863–872.
- Díaz-Riquelme, J., Grimplet, J., Marinez-Zapater, J.M., and Carmona, J.J. 2012. Transcriptome variation along bud development in grapevine (Vitis vinifera L.). BMC Plant Biol. 12: 181.
- Díaz-Riquelme, J., Lijavetzky, D., Marinez-Zapater, J.M., and Carmona, J.J. 2009. Genome-wide analysis of MIKCC-type MADS box genes in grapevine. Plant Physiol. 149: 354–369.
- Doherty, C.J., Van Bukirk, H.A., Myers, S.J., and Thomashow, M.F. 2009. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 21: 972–984.
- Dong, M.A., Farré, E.M., and Thomashow, M.F. 2011. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 108: 7241–7246.
- Duchêne, E., Butterlin, G., Dumas, V., and Merdinoglu, D. 2012. Towards the adaptation of grapevine varieties to climate change: QTLs and candidate genes for developmental stages. Theor. Appl. Genet. 126: 623–635.
- Elshire, R.J., Glaubitz, J.C., Sun, Q., Poland, J.A., Kawamoto, K., Buckler, E.S., and Mitchell, S.E. 2011. A robust, simple genotyping by sequencing (GBS) approach for high diversity species. PloS ONE. 6: e19379.
- Espinoza, C., Degenkolbe, T., Caldana, C., Zuther, E., Leisse, A., Willmitzer, L., Hincha, D.K., and Hannah, M.A. 2010. Interaction with diurnal and circadian regulation results in dynamic metabolic and transcriptional changes during cold acclimation in Arabidopsis. PloS ONE. 5: e14101.
- Fan, S., Bielenber, D.G., Zhebentyayeva, T.N., Reighard, G.L., Okie,W.R., Holland, D., and Abbott, A.G. 2009. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica). New Phytol. 185: 917–930.
- Fasoli, M., Santo, D.S., Zenoni, S., Tornielli, G.B., Farina, L., Zamboni, A., Porceddu, A., Venturini, L., Bicego, M., Murino, V., Ferrarini, A., Delledonne, M., and Pezzotti, M. 2012. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell. 24: 3489–3505.
- Fear, C.D., Lauer, F.I., Luby, J.J., and Stucker, R.L. 1985. Genetic components of variance for winter injury, fall growth cessation, and off-season flowering in blueberry progenies. J. Amer. Soc. Hort. Sci. 110: 262–266.
- Fejer, S.O. 1976. Combining ability and correlations of winter survival, electrical impedance and morphology in juvenile apple trees. Can. J. Plant Sci. 56: 303–309.
- Feng, X-M., Zhao, Q., Zhao, L-L., Qiao, Y., Xie, X-B., Li, H-F., Yao, Y-X., You, C-X., and Hao, Y-J. 2012. The cold-induced basic helix-loop-helix transcription factor gene MdClbHLH1 encodes an ICE-like protein in apple. BMC Plant Biol. 12: 22.
- Fennell, A. 2004. Freezing tolerance and injury in grapevines. J. Crop Improv. 10: 201–235.
- Fennell, A. and E. Hoover. 1991. Photoperiod influences growth, bud dormancy, and cold acclimation in Vitis labruscana and V. riparia. J. Amer. Soc. Hort. Sci. 116: 270–273.
- Garcia-Bañuelos, M.L., Gardea, A.A., Winzerling, J.J., and Vazquez-Moreno, L. 2009. Characterization of a midwinter-expressed dehydrin (DHN) gene from apple trees (Malus domestica). Plant Mol. Biol. Rep. 27: 476–487.
- Garris, A., Clark, L., Owens, C., McKay, S., Luby, J., Mathiason, K., and Fennell, A. 2009. Mapping of photoperiod-induced growth cessation in the wild grape Vitis riparia. J. Amer. Soc. Hort. Sci. 134: 261–272.
- George, M.F., Burke, M.J., Pellett, H.M., and Johnson, A.G. 1974. Low temperature exotherms and woody plant distribution. HortScience. 9: 519–522.
- Giorno, F., Guerriero, G., Baric, S., and Mariani, C. 2012. Heat shock trancriptional factors in Malus domestica: identification, classification and expression analysis. BMC Genomics 13: 639.
- Goes da Silva, F., Iandolino, S., Al-Kayal, F., Bohlmann, M.C., Cushman, M.A., Lim, H., Ergul, A., Figueroa, R., Kabuloglu, E.K., Osborne, C., Rowe, J., Tattersall, E., Leslie, A., Xu, J., Baek, J-M., Cramer, G.R., Cushman, J.C., and Cook, D.R. 2005. Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiol. 139: 574–597.
- Griffith, M., Lumb, C., Wiseman, S.B., Wisniewski, M.E., Johnson, R.W., and Marangoni, A.G. 2005. Antifreeze proteins modify the freezing process in planta. Plant Physiol. 138: 330–340.
- Grimplet, J., Cramer, G.R., Dickerson, J., Van Hemert, J., Mathiason, K., and Fennell, A. 2009. VitisNet: “Omics” integration through grapevine molecular networks. PLoS ONE. 4: e8365.
- Grimplet, J., Dickerson, J., Adam-Blondon, A-F., and Cramer, G.R. 2011. Bioinformatic tools in grapevine genomics. In: Genetics, Genomics and Breeding of Grapes. pp. 317–331 Adam-Blondon, A-F., Martinez-Zapater, J.M., and Kole, C. eds. Enfield, NH: Science Publishers.
- Gusta, L.V. and Wisniewski, M.E. 2012. Understanding plant cold hardiness: an opinion. Physiol Plant. preprint. ISSN 0031-9317.
- Gusta, L.V., Wisniewski, M.E., and Trischuk, R.G. 2009. Patterns of freezing in plants: the influence of species, environment and experimental procedures. In: Plant Cold Hardiness: From Laboratory to the Field. pp.214–225. CABI, Cambridge, UK.
- Guy, C.L. 2003. Freezing tolerance of plants: current understanding and selected emerging concepts. Can. J. Bot. 81: 1216–1223.
- Harmer, S.L., Hogenesch, J.B., Straume, M., Chang, H-S., Han, B., Zhu, T., Wang, X., Kreps, J.A., and Kay, S.A. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 290: 2110–2113.
- Heide, O.M. and Prestrud, A.K. 2005. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 25: 109–114.
- Howell, G.S. and Shaulis, N. 1980. Factors influencing within-vine variation in the cold resistance of cane and primary bud tissues. Am. J. Enol. Vitic. 31: 158–161.
- Ibañez, C., Ramos, A., Acebo, P., Contreras, A., Casado, R., Alona, I., and Aragoncillo, C. 2008. Overall alteration of circadian clock gene expression in the chestnut cold response. PLoS ONE. 3: e3567.
- Jaillon, O., Aury, J.M., Noel, B., Policriti, A., Clepet, C., Casagrande, A., Choisne, N., et al. 2007. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 449: 463–467.
- Janska, A., Marsik, P., Zelenkova, S., and Ovesna, J. 2010. Cold stress and acclimation – what is important for metabolic adjustment. Plant Biol. 12: 395–405.
- Jian, L-C., Li, J-H., and Li, P. 2000. Seasonal alterations in amount of Ca2+ in apical bud cells of mulberry (Morus bombciz Koidz): an electron microscopy-cytochemica study. Tree Physiol. 20: 623–628.
- Jimenez, S., Li, Z., Reighard, G.L., and Bielenberg, D.G. 2010a. Gene expression of DAM5 and DAM6 is suppressed by chilling temperatures and inversely correlated with bud break rate. Plant Mol. Biol. 73: 157–167.
- Jimenez, S., Li, Z., Reighard, G.L., and Bielenberg, D.G. 2010b. Identfication of genes associated with growth cessation and bud dormancy entrance using a dormancy-incapable tree mutant. BMC Plant Biol. 10: 25.
- Jones, K.S., McKersie, B.D., and Paroschy, J. 2000. Prevention of ice propagation by permeability barriers in bud axes of Vitis vinifera. Can. J. Bot. 78: 3–9.
- Kader, S.A., and Proebsting, E.L. 1992. Freezing behavior of Prunus, subgenus Padus, flower buds. J. Amer. Soc. Hort. Sci. 117: 995–960.
- Khan, S.A., Chibon, P-Y., de Vos, R.C. H., Schipper, B.A., Walraven, E., Beekwilder, J., van Dijk, R., Finkers, R., Visser, R.G. F., van de Weg, E.W., Bovy, A., Cestaro, A., Velasco, R., Jacobsen, E., and Schouten, H.J. 2012. Genetic analysis of metabolites in apple fruits indicates an mQTL hotspot for phenolic compounds on linkage group 16. J. Exp. Bot. 63: 2895–2908.
- Kitashiba, H., Ishizaka, T., Isuzugawa, K., Nishimura, K., and Suzuki, T. 2004. Expression of a sweet cherry DREB1/CBF ortholog in Arabidopsis confers salt and freezing tolerance. J. Plant Physiol. 161: 1171–1176.
- Kobayashi, M., Horiuchi, H., Fujita, K., Takuhara, Y., and Suziki, S. 2012. Characterization of grape C-repeat-binding factor 2 and B-box-type zinc finger protein in transgenic Arabidopsis plants under stress conditions. Mol. Biol. Rep. 39: 7933–7939.
- Lee, D-M. and Thomashow, M.F. 2012. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA. 109: 15054–15059.
- Leida, C., Conesa, A., LLácer. G., Badenes, M.L., and Ríos, G. 2011. Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytol. 193: 67–80.
- Leida, C., Terol, J., Marti, G, Agusti, M., LLácer. G., Badenes, M.L., and Ríos, G. 2010. Identification of genes associated with bud dormancy release in Prunus persica by suppression subtractive hybridization. Tree Physiol. 30: 655–666.
- Levitt, J. 1980. Responses of Plants to Environmental Stresses I. Chilling, Freezing, and Temperature Stresses. 2nd Ed. Academic Press, New York.
- Li, Z., Reighard, G.L., Abbott, A.G., and Bielenberg, D.G. 2009. Dormancy-associated MADS genes from the EVG locus of peach [Prunus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. J. Exp. Bot. 60: 3521–3530.
- Licausi, F., Giorgi, F.M., Zenoni, S., Osti, F., Pezzotti, M., and Perata, P. 2010. Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis viniferea. BMC Genomics. 11: 719.
- Liang, D., Xia, H., Wu, S., and Ma, F. 2012. Genome-wide identification and expression profiling of dehydrin gen family in Malus domestica. Mol. Biol. Rep. 39: 10759–10768.
- Lindlőf, A., 2010. Interplay between low-temperature pathways and light reduction. Plant Sig. Behavior. 5: 820–825.
- Mathers, H.M. 2004. Supercooling and cold hardiness in sour cherry germplasm: Flower buds. J. Amer. Soc. Hort. Sci. 129: 675–681.
- Mathiason, K., He, D., Grimplet, J., Venkateswari, J., Galbraith, D.W., Or, E., and Fennell, A. 2009. Transcript profiling in Vitis riparia during chilling requirement fulfillment reveals coordination of gene expression pattersn with optimized bud break. Funct. Integr. Genomics. 9: 81–96.
- Michelmore, R.W. 2012. CoGePedia. . http://genomevolution.org/CoGe/ Accessed 11/2012.
- Ming, R., Hou, S., Feng, Y., Yu, Q., Dionne-Laporte, A., Saw, J.H., Senin, P., et al. 2008. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature. 452: 991–997.
- Miura, K., Ohta, M., Nakazawa, M., Ono, M., and Hasegwa, P.M. 2011. ICE1 Ser403 is necessary for protein stabilization and regulation of cold signaling and tolerance. Plant J. 67: 269–279.
- Muthalif, M.M. and Rowland, L.J. 1994. Identification of dehydrin-like proteins responsive to chilling in floral buds of blueberry (Vaccinium, section Cyanococcus). Plant Physiol. 104: 1439–1447.
- Obata, T. and Fernie, A.R. 2012. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 69: 3225–3243.
- Olukolu, B.A., Trainin, T., Fan, S., Kole, C., Bielenberg, G., Reighard, G.L., Abbott, A.G., and Holland, D. 2009. Genetic linkage mapping for molecular dissection of chilling requirement and budbreak in apricot (Prunus armeniaca L.). Genome. 52: 819–828.
- Owens, C.L. 2005. Breeding temperate fruit crops for improved freezing tolerance. HortScience. 40: 1950–1953.
- Owens, C.L., Thomashow, M.F., Hancock, J.F., and Iezzoni, A.F. 2002. CBF1 orthologs in sour cherry and strawberry and the heterologous expression of CBF1 in strawberry. J. Amer. Soc. Hort. Sci. 127: 489–494.
- Palonen, P. and D. Buszard. 1997. Current state of cold hardiness research on fruit crops. Can. J. Plant Sci. 77: 399–420.
- Panta, G.R., Rowland, L.J., Arora, R., Ogden, E.L., and Lim, C-C. 2004. Inheritance of cold hardiness and dehydrin genes in diploid mapping populations of blueberry. J. Crop Improv. 10: 37–52.
- Pearce, R.S. 2001. Plant freezing and damage. Ann. of Bot. 87: 417–424.
- Pin, P.A. and Nilsson, O. 2012. The multifaceted roles of FLOWERING LOCUS T in plant development. Plant Cell Environ. 35: 1742–1755.
- Polashock, J.J., Arora, R., Peng, Y., Dhananjay, N., and Rowland, L.J. 2010. Functional identification of a blueberry CBF/DREB-like element associated with cold acclimation and freezing tolerance. J. Amer. Soc. Hort. Sci. 135: 40–48.
- Quamme, H.A. 1973. The mechanism of freezing injury in xylem of winter apple twigs. Plant Physiol. 51: 273–277.
- Quamme, H.A. 1976. Relationship of the low temperature exotherm to apple and pear production in North America. Can. J. Plant Sci. 56: 493–500.
- Quamme, H.A., Su, W.A., and Veto, L.J. 1995. Anatomical features facilitating supercooling of the flower within the dormant peach flower bud. J. Amer. Soc. Hort. Sci. 120: 814–822.
- Quamme, H., Wieser, C.J., and Stushnoff, C. 1973. The mechanism of freezing injury in xylem of winter apple twigs. Plant Physiol. 51: 273–277.
- Renaut, J., Hausman, J-F., Bassett, C., Artlip, T., Cauchie, H-M., Witters, E., and Wisniewski, M.E. 2008. Quatitative proteomic analysis of short photoperiod and low temperature responses in bark tissues of peach (Prunus persica L. Batsch). Tree Genet.Genomes. 4: 589–600.
- Rodriguez, J., Sherman, W.B., Scorza, R., Wisniewski, M., and Okie, W.R. 1994. Evergreen peach, its inheritance and dormant behavior. J. Amer. Soc. Hort. Sci. 119: 789–792.
- Rowland, L.J., Alkharouf, N.W., Darwish, O., Ogden, E.L., Polashock, J.J., Bassil, N.V., and Main, D. 2012. Generation and analysis of blueberry transcriptome sequences from leaves, developing fruit, and flowers from cold acclimation through deacclimation. BMC Plant Biol. 12: 46.
- Rowland, L.J., Dhanaraj, A.L., Naik, D., Alkharouf, N.W., Matthews, B., and Arora, R. 2008. Study of cold tolerance in blueberry using EST libraries cDNA microarrays, and subtractive hybridization. HortScience. 43: 1975–1981.
- Rowland, L.J., Panta, G.R., Mehra, S., and Parmentier-Line, C. 2004. Molecular genetic and physiological analysis of the cold-responsive dehydrins of blueberry. J. Crop Improv. 10: 53–76.
- Sarnighousen, E., Karlson, D.T., Zeng, Y., Goldsbrough, P.B., Raghothama, K.G., and Ashworth, E.N. 2004. Seasonal regulation of a novel YnSKn class of dehydrin-like cDNAs from cold acclimated red-osier dogwood (Cornus sericea L.) xylem. J. Crop Improv. 10: 17–35.
- Santamaria, M.E., Rodriguez, R., Canal, M.J., and Toorop, P.E. 2011. Transcriptome analysis of chestnut (Castanea sativa) tree buds suggests a putative role for epigenetic control for bud dormancy. Ann Bot. 108: 485–498.
- Sasaki, R., Yamne, H., Ooka, T., Jotatsu, H., Kitamura, Y., Akagi, T., and Tao, R. 2011. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japenese apricot. Plant Physiol. 157: 485–497.
- Schnabel, B.J. and Wample, R.L. 1987. Dormancy and cold hardiness in Vitis vinifera L. cv. White Riesling as influenced by photoperiod and temperature. Amer. J. Enol. Vitic. 38: 265–272.
- Shulaev, V., Sargent, D.J., Crowhurst, R.N., Mockler, T.C., Folkerts, O., Delcher, A.L., Jaiswal, P., et al. 2011. The genome of woodland strawberry (Fragaria vesca). Nature Genet. 43: 109–116.
- Siddiqua, M. and Nassuth, A. 2011. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant, Cell Environ. 34: 1345–1359.
- Smallwood, M. and Bowles, D.J. 2002. Plants in a cold climate. Phil. Trans. R. Soc. Lond. B 357: 831–847.
- Stushnoff, C. 1972. Breeding and selection methods for cold hardiness in deciduous fruit crops. HortScience. 7: 10–13.
- Takuhara, Y., Kobayshi, M., and Suzuki, S. 2011. Low-temperature-induced transcription factors in grapevine enhance cold tolerance in transgenic Arabidopsis plants. J. Plant Physiol. 168: 967–975.
- Tanino, K.K., Kalcsits, L., Silim, S., Kendall, E., and Gray, G.R. 2010. Temperature-driven plasticity in growth cessation and dormancy development in deciduous woody plants: a working hypothesis suggesting how molecular and cellular function is affected by temperature during dormancy induction. Plant Mol. Biol. 73: 49–65.
- Tattersall, A.R., Grimplet, J., Deluc, L., Wheatley, M.D., Vincent, D., Osborne, C., Ergül, A., Lomen, E., Blank, R.R., Schlauch, K.A., Cushman, J.C., and Cramer, G.R. 2007. Transcript abundance profiles reveal larger and more complex responses of grapevine to chilling compared to osmotic and salinity stress. Funct. Integr. Genomics. 7: 317–333.
- Theocharis, A., Clément, C., and Barka, E.A. 2012. Physiological and molecular changes in plants grown at low temperatures. Planta. 235: 1091–1105.
- Tillett, R.L., Wheatley, M.D., Tattersall, E.A. R., Schlauch, K.A., Cramer, G.R., and Cushman, J.C. 2012. The Vitis vinifera C-repeat binding protein 4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Biotech. J. 10: 105–124.
- Thomashow, M.F. 2010. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 154: 571–577.
- Velasco, R., Zharkikh, A., Affourtit, J., Dhingra, A., Cestaro, A., Kalyanaraman, A., Fontana, P., Bhatnagar, S.K., et al. 2010. The genome of the domesticated apple (Malus x domestica Borkh.). Nature Genet. 42: 833–839.
- Velasco, R., Zharkikh, A., Troggio, M., Cartwright, D.A., Cestaro, A., Dmitry, P., Pruss, D., et al. 2007. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE.12: e1326.
- Victor, K.J., Fennell, A.Y., and Grimplet, J. 2010. Proteomic analysis of shoot tissue during photoperiod induced growth cessation in V. riparia Michx. Grapevines. Proteome Sci. 8: 44.
- Vogel, J.T., Zarka, D.G., Van Buskirk, H.A., Fowler, S.G., and Thomashow, M.F. 2005. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 41: 195–211.
- Walworth, A.E., Rowland, L.J., Polashock, J.J., Hancock, J.F., and Song, G. 2012. Overexpression of a blueberry-derived CBF enhances cold tolerance in a southern highbush blueberry cultivar. Mol. Breeding. 30: 1313–1323.
- Wang, R.-K., Li, L-L., Cao, Z-H., Zhoa, Q., Li, M., Zhang, L.-Y., and Hao,Y.-J. 2012. Molecular cloning and functional characterization of a novel apple MdCIPK6L gene reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Plant Mol. Biol. 79: 123–135.
- Wang, H.B., Wang, X.D., Cheng, C.G., Wang, B.L., Li, M., Gao, D.S., and Liu, F.Z. 2008. Natural inducing factors of peach bud dormancy and roles of Ca2+ in the dormancy induction. Ying Yong Sheng Tai Bao. 19: 2333–2338.
- Welling, A. and Palva, E.T. 2006. Molecular control of cold acclimation in trees. Physiol. Plant. 127: 167–181.
- Welling, A., Rinne, P., Vihera-Aarnio, A., Kontunen,-Soppela, S., Heino, P., and Palva, E.T. 2004. Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens Ehrh.). J. Exp. Bot. 55: 507–516.
- Wisniewski, M.E., Bassett, C.L., Norelli, J.L., Macarisin, D., Artlip, T.S., Gasic, K., and Korban, S. 2008. Expressed sequence tag analysis of the response of apple (Malus x domestica ‘Royal Gala’) to low temperature and water deficit. Physiol. Plant. 133: 298–317.
- Wisniewski, M.E., Bassett, C.L., Renaut, J., Farrell, R., Tworkoski, T., and Artlip, T., 2006. Differential regulation of two dehydrin genes from peach (Prunus persica) by photoperiod, low temperature and water deficit. Tree Physiol. 26: 575–584.
- Wisniewski, M.E., Close, T., Artlip, T.S., and Arora, R. 1996. Seasonal patterns of dehydrins and 70 kDa heat-shock proteins in bark tissues of eight species of woody plants. Physiol Plant. 96: 496–505.
- Wisniewski, M.E., Fuller, M., Palta, J., Carter, J., and Arora, R. 2004. Ice nucleation, propagation and deep supercooling in woody plants. J. Crop Improv. 10: 5–16.
- Wisniewski, M.E., Gusta, L.V., Fuller, M.P., and Karlson, D. 2009. Ice nucleation, propagation and deep supercooling: the lost tribes of freezing studies. In: Plant Cold Hardiness: From Laboratory to the Field. pp. 214–225. CABI, Cambridge, UK.
- Wisniewski, M.E., Norelli, J., Bassett, C., Artlip, T., and Macarisin, D. 2011. Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus x domestica) results in short-day induced dormancy and increased cold hardiness. Planta. 233: 971–983.
- Wisniewski, M.E., Webb, R., Balsamo, R., Close, T.J., Yu, X-M., and Griffith, M. 1999. Purification, immunolocation, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica). Physiol. Plant. 105: 600–608.
- Wu, J., Wang, Z., Shi, Z., Zhang, S., Ming, R., Zhu, S., Khan, M.A., et al. 2013. The genome of pear (Pyrus bretschneideri Rehd.). Genome Res. 23: 396–408.
- Xiao, H., Siddiqua, M., Braybrook, S., and Nassuth, A. 2006. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant, Cell Environ. 29: 1410–1421.
- Xiao, H., Tattersall, E.A. R., Siddiqua, M., Cramer, G.R., and Nassuth, A. 2008. CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant. Cell Environ. 31: 1–10.
- Xu, Q., Chen, L.L., Ruan, X., Chen, D., Zhu, A., Chen, C., Bertrand, D., et al. 2012. The draft genome of sweet orange (Citrus sinensis). Nature Genet. . Preprint.
- Yamane, H., Kashiwa, Y., Kakehi, E., Yonemori, K., Mori, H., Hayashi, K., Iwamoto, K., Tao, R., and Kataoka, I. 2006. Differential expression of dehydrin in flower buds of two Japanese apricot cultivars requiring different chilling requirements for bud break. Tree Physiol. 26: 1559–1563.
- Yamane, H., Ooka, T., Jatatsu, H., Hosaka, Y., Sasaki, R., and Tao, R. 2011. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS box genes, by low temperature and dormancy-breaking reagent treatment. J. Exp. Bot. 62: 3481–3488.
- Yang, T., Cudhuri, S., Yang, L., Du, L., and Pooviah, W.B. 2010. A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J. Biol. Chem. 285: 7119–7126.
- Yang, Y., He, M., Zhu, Z., Li, S., Xu, Y., Zhang, C., Singer, S.D., and Wang,Y. 2012. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 21: 140.
- Yang, W., Liu, X.D., Chi, X.J., Wu, C.A., Li, Y.Z., Song, L.L., Liu, X.M., Wang, Y.F., Wang, F.W., Zhang, C.M. Liu, Y.M. Xong, J.M., and Li, H.Y. 2011. Dwarf apple MbDREB1 enhances plant tolerance to low temperature, drought, and salt stress via both ABA-dependent and ABA-independent pathways. Planta. 233: 319–229.
- Zhao, T., Liang, D., Wang, P., Liu, J., and Ma, F. 2012. Genome-wide analysis and expression profiling of the DREB transcription factor gene family in Malus under abiotic stress. Mol. Genet. Genomics. 287: 423–436.
- Zhu, J., Dong, C.-H., and Zhu, J.-K., 2007. Interplay between cold-responsive gene regulation, metabolism, and RNA processing during plant cold acclimation. Curr. Op. Plant Biol. 10: 290–295.
- Zhuang, E.-B., Shi, T., Gao, Z.-H., Zhang, Z., and Zhang, J.-Y. 2012. Differential expression of proteins associated with seasonal bud dormancy at four critical stages in Japanese apricot. Plant Biol. 15: 233–242.
