ABSTRACT
Mitochondria are responsible for providing energy currency to life processes in the molecular form of ATP and are therefore typically referred to as the power factories of cells. Plant mitochondria are also relevant to the common phenomenon of cytoplasmic male sterility, which is agronomically important in various crop species. Cytoplasmic male sterility (CMS) is a complex trait that may be influenced by patterns of mitochondrial genome evolution, and by intergenomic gene transfer among the organellar and nuclear compartments of plant cells. Here, we review patterns and processes that shape plant mitochondrial genomes, some relevant interactions between organelles, and the general features shared by the majority of cytoplasmic male-sterile genes in plants to further the goal of understanding CMS.
I. Mitochondria and cytoplasmic male sterility: An overview
Mitochondria are a semi-autonomous and primarily maternally inherited genetic organellar system responsible for producing cellular ATP by oxidative phosphorylation (Gray et al., Citation1999; Gray, Citation2012). Land plant mitochondrial genomes (mitogenomes) are notably different from other eukaryotic mitochondria due to their paradoxical mixture of extraordinarily fast and slow rates of evolution. Flowering plant mitogenomes exemplify the former, with substantial variation in genome size and structure even among close relatives (Francis and Fernand, Citation1977; Alverson et al., Citation2010; Tang et al., Citation2015; Chen et al., Citation2017). They have highly variable intergenetic regions containing diverse repeated sequences (Kitazaki and Kubo, Citation2010), frequent structural rearrangements (Galtier, Citation2011), massive genes loss, frequent endogenous and foreign DNA transfer (Bergthorsson et al., Citation2003; Kubo and Newton, Citation2008; Hao and Palmer, Citation2009; Bock, Citation2010; Liu et al., Citation2011), and a highly variable RNA editing process (Takenaka et al., Citation2008). Conversely, plant mitochondrial genes display exceptionally low rates of nucleotide substitution (Wolfe et al., Citation1987; Palmer et al., Citation2000; Mower et al., Citation2007; Galtier, Citation2011). This contrast between the pace of mitochondrial genomic and genic evolution has led to many questions regarding these disparate patterns of evolution. Among these is the question of how mitochondrial gene and genome evolution influence the interactions of the nuclear genome and the origin of cytoplasmic male sterility (CMS).
CMS is a maternally inherited trait conferred by the mitochondrial genome that results in a failure to produce functional pollen and/or male reproductive organs (Pruitt and Hanson, Citation1991; Budar and Pelletier, Citation2001; Chase, Citation2007; Kubo et al., Citation2011; Suzuki et al., Citation2013), except in the presence of restorer-of-fertility genes (Chase, Citation2007; Cho et al., Citation2012; Lee et al., Citation2014; Huang et al., Citation2015). CMS is also important in specifying gynodioecy in natural populations (Hanson and Bentolila, Citation2004; Miller and Bruns, Citation2016), a phylogenetically widespread reproductive strategy in flowering plants. CMS has been detected in more than 150 species (Carlsson et al., Citation2008), and gynodioecy may occur in as many as 7% of angiosperm species (Ornduff, Citation1986; Budar and Pelletier, Citation2001; McCauley and Bailey, Citation2009). Not only does CMS confer a prominent breeding system (second only to hermaphrodism [Budar and Pelletier, Citation2001]), but it also has led to significant gains in agriculture mediated by heterosis in crop plants and large-scale commercial production of F1 hybrid seed (Havey, Citation2004; Chen and Liu, Citation2014; Bohra et al., Citation2016). Understanding the mechanisms of CMS, therefore, has broad importance to plant biology and agronomy.
In this review, we describe the composition and characteristics of plant mitogenomes, and the functional interactions and propensity for endogenous gene transfer between plant nuclear and organellar genomes. We review recent advances in understanding several mechanisms that are known to underlie CMS in plants, with a focus on peculiarities specific to CMS-associated genes. Throughout, we emphasize the connection between processes of mitogenome evolution and the role of CMS in plant biology and crop improvement.
II. Characteristics of plant mitochondrial genomes: High variability and complexity conferred by repeated sequences
Plant mitogenomes are notable for both their extraordinary variation in genome size and their remarkable variability in structure and organization. By contrast, vertebrate animal mitogenomes are rather small (∼14–20 kb) and highly conserved in both size and structure (Boore, Citation1999; Lavrov et al., Citation2016), and plant mitochondrial genomes exhibit substantial variation in both these features (Kitazaki and Kubo, Citation2010; Galtier, Citation2011; Mower et al., Citation2012b), although exceptions exist in nonflowering plants (Liu et al., Citation2014; Guo et al., Citation2016). Plant mitochondrial genomes may vary enormously in size even within single plant families; in the Cucurbitaceae, for example, mitochondrial genomes vary over 7-fold in size, from 379 kb in Citrullus lanatus (Alverson et al., Citation2010) to 2,740 kb in Cucumis melo (Rodriguez-Moreno et al., Citation2011). Even more spectacularly, within the single genus Silene, mitogenome sizes vary over 40-fold in size, from 253 kb in Silene latifolia (Sloan et al., Citation2010) to more than 11 Mb in Silene conica (Sloan et al., Citation2010). Analyses of composition reveal that the size variability of these mitogenomes does not reflect differences in the number of functional genes (Clifton et al., Citation2004), but rather differences in the intergenic space (Cupp and Nielsen, Citation2014). The factors contributing to this variation are diverse and include variable accumulation of diverse repeated sequences; inclusion of unknown sequences (Palmer et al., Citation2000; Bergthorsson et al., Citation2004; Alverson et al., Citation2010; Rice et al., Citation2013); variation in intronic size (Laroche et al., Citation1997; Satoh et al., Citation2004); and differences in pseudogene fragment number and size (Knoop, Citation2013).
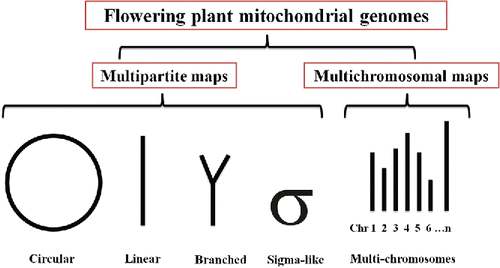
In addition to variability in size, the structure of mitogenomes is remarkably variable (Mower et al., Citation2012b). While mitogenomes typically are depicted as single circular rings (Kitazaki and Kubo, Citation2010), many other configurations for plant mitochondrial chromosomes have been reported (), including diverse linear and circular forms, highly branched and sigma-like morphologies, as well as multichromosomal structures that are capable of substoichiometric co-occurrence. The mitochondrial genomes of some CMS lines in maize (Allen et al., Citation2007) and rice, for example, have linear configurations (Notsu et al., Citation2002). Conversely, the mitochondrial genome of garden rocket (Eruca sativa Mill.) is multipartite, consisting of six master circles and four smaller subgenomic circles which likely result from repeat-induced genomic reorganization (Wang et al., Citation2014). A similar configuration has been observed in Brassica oleracea, which displays a tripartite structure consisting of a 220 kb master circle that is also split into two subgenomic circles (170 and 50 kb each) via homologous recombination of repeated sequences (Grewe et al., Citation2014). Observations in the hau CMS line of Brassica and its maintainer line in B. juncea suggest not only a multipartite structure, but also the substoichiometric coexistence of different mitotypes (Heng et al., Citation2014). Multichromosomal mitochondrial genomes have been identified in four independent angiosperm lineages (Alverson et al., Citation2011a; Sloan et al., Citation2012a; Rice et al., Citation2013; Sanchez-Puerta et al., Citation2017). These two configurations, i.e., multipartite and multichromosomal maps, are not intuitively different and indeed may be alternative representations of the same genome. The distinction typically relies on the number of chromosomes (Gualberto and Newton, Citation2017; Sanchez-Puerta et al., Citation2017). That is, multipartite maps typically contain fewer than three chromosomes that can be assembled into the forms noted in (Gualberto and Newton, Citation2017), whereas multichromosomal maps contain up to 50–100 linear or circular chromosomes (Alverson et al., Citation2011a; Sloan et al., Citation2012a; Rice et al., Citation2013; Sanchez-Puerta et al., Citation2017).
The accumulated evidence, therefore, is that plant mitochondrial genomes frequently have a multipartite organization (Kitazaki and Kubo, Citation2010) associated with complex recombination among repeated sequences (Oldenburg and Bendich, Citation1996; Backert and Borner, Citation2000). In general, mitogenomes are arranged in a main ring (i.e., a “master chromosome”) that contains a complete set of genes and a number of sub-rings (Alverson et al., Citation2011a,Citationb). Alternatively, they can be reconstructed configurations produced by frequent recombination among repeated sequences (Lonsdale et al., Citation1988; Kubo et al., Citation2000; Handa, Citation2003; Sugiyama et al., Citation2005; Marechal and Brisson, Citation2010).
Repeated sequences are common in plant mitochondrial genomes, with estimates of up to 38% of the mitochondrial genome occupied by repeats of variable size and copy number (Mower et al., Citation2012b). This number surely is an underestimate, as some of the large mitogenomes, e.g. in Silene or the Curcurbitaceae noted above, likely reflect the accumulation and decay of multiple diverse repeated elements. The origin of these repeats is often unclear, as are the mechanisms that underlie the propensity for mitogenomes to acquire repetitive sequences. From the >60 sequenced mitogenomes (Mower et al., Citation2012b; Gualberto et al., Citation2014; Liu et al., Citation2014), it is clear that plant mitogenomes vary widely in repeat content and composition, which, in conjunction with the phylogenetically widespread occurrence of large mitogenomes, lend support to the idea that multiple divergent repeated sequences are acquired independently during evolution (Andre et al., Citation1992). Consequently, as the presence of repeated sequences is associated with recombination in the mitogenome (Manchekar et al., Citation2006; Kitazaki and Kubo, Citation2010; Chen et al., Citation2017), the high structural variability (Ogihara et al., Citation2005), complexity, and multipartite organization of plant mtDNA (Abdelnoor et al., Citation2003) should come as little surprise.
The highly recombinogenic, repetitive nature of plant mitogenomes has been linked to various traits, including CMS (Galtier, Citation2011), and, intriguingly, the presence of CMS may be associated with the presence of large repeats. CMS is typically conferred via chimeric genes (Section VI.A.1), whose generation has been associated with the presence of large repeats. In Brassica juncea, for example, comparison between the hau CMS line and its maintainer line revealed that repeats in the CMS line were typically twice the size as those in the maintainer line (for repeats >100 bp). Furthermore, the presence of three large repeats downstream from the hau CMS-associated gene orf288 has been implicated in the formation of this chimera (Heng et al., Citation2014). Comparative analysis between the mitogenomes of CMS and male-fertile lines of pepper (Capsicum annuum L.) showed that CMS candidate genes orf507 and ψatp6-2 were proximal to edges of highly rearranged CMS-specific DNA regions, whose evolution may be the result of nearby intermediate or large-sized repeats (Jo et al., Citation2014). While recombination in short repeats is rare and has yet to be associated with CMS (Touzet and Meyer, Citation2014), these repeats may have played other roles in evolution (Andre et al., Citation1992), leading to other deficiencies such as respiratory impairments (Kitazaki and Kubo, Citation2010). A final comment is that many nuclear genes, including Msh1 (Abdelnoor et al., Citation2003; Shedge et al., Citation2007; Galtier, Citation2011), RecA2 (Shedge et al., Citation2007), RecA1/4 (Odahara et al., Citation2009) and OSB1 (Zaegel et al., Citation2006), appear to participate in the regulation of recombination events for different repeated sequences (Lillestol et al., Citation2009; Cupp and Nielsen, Citation2014; Gualberto and Newton, Citation2017), which may represent an additional cytonuclear process important in the origin of CMS.
III. Gene loss and collinearity
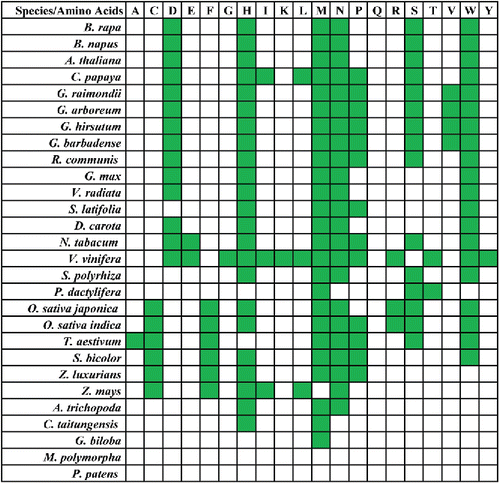
While most genes required for mitochondrial metabolism are encoded by the nuclear genome, plant mitochondria commonly contain a suite of approximately 67 essential genes (Kurland and Andersson, Citation2000) that typically are conserved in both type and number (Kitazaki and Kubo, Citation2010). These include protein-encoding, rRNA, and tRNA genes, in addition to pseudogenes and ORFs of unknown function, the latter having been implicated in CMS (Satoh et al., Citation2004; Luo et al., Citation2013). Conservation and loss of these genes in different plant groups have been extensively studied (Rice et al., Citation2013; Skippington et al., Citation2015; Skippington et al., Citation2017). Plant mitochondrial protein-encoding genes are commonly divided into five categories (participating in five complexes or subunits associated with mitochondrial respiratory metabolism; [Lei et al., Citation2013]), which typically are conserved among plants, albeit to varying degrees. Genes classified in complex II, for example, have been lost in most higher plants, particularly in monocots, whereas complexes I, III, IV, and V show much greater conservation across land plant species with occasionally loss of nad genes (Viscum scurruloideum) and cox2 (legumes) (Palmer et al., Citation2000; Fujii et al., Citation2007; Bentolila and Stefanov, Citation2012; Rice et al., Citation2013; Skippington et al., Citation2015; Skippington et al., Citation2017). Conversely, both the ribosomal subunit and tRNA-encoding genes have experienced a more dynamic pattern of evolution (), often displaying progressive loss, and collectively representing the majority of cases of genic variation among plant mitogenomes (Clifton et al., Citation2004; Lei et al., Citation2013). Most gene “losses” are not complete removals but rather reflect functional transfer to the nuclear genome, where they encode products that return to the mitochondria (Palmer et al., Citation2000; Adams and Palmer, Citation2003). Other true losses, such as “missing” tRNAs, have been compensated for by native analogs in the nuclear or chloroplast genomes. Namely, nuclear-derived tRNA are expressed and then imported into mitochondria. In contrast, plastid tRNA genes have been transferred into the mitochondrial genome where they are locally expressed (Marechal-Drouard et al., Citation1990; Schneider, Citation2011; Liu et al., Citation2013).
Figure 2. Gene content in sequenced mitogenomes from 38 plant species (2074A, 2074S, and E5903 were two sterile lines and one restoring line associated with cotton, respectively). The genes included encode proteins, rRNAs, and tRNAs. Protein-coding genes are shown in the following order (top to bottom): those associated with complex I-V, cytochrome C synthetase subunits, ribosomal protein large and small subunits, and intron maturase. Boxes in light green, dark green, and white represent intact genes, pseudogenes, and the absence of genes, respectively. The maximum likelihood phylogenetic tree was constructed based on 17 mitochondial genes related to the respiratory chain: nad1, nad2, nad3, nad4, nad4L, nad5, nad6, nad9, cob, cox1, cox2, cox3, atp1, atp4, atp6, atp8, and atp9.
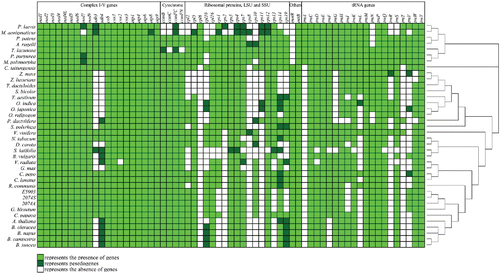
Notwithstanding the many cases of mitochondrial gene loss, gene content is far more conserved than gene order, which varies enormously among angiosperm plants. This variation can be observed even between accessions of the same species (Liu et al., Citation2013; Tuteja et al., Citation2013). This frequent recombination common to plant mitogenomes disrupts and reorganizes syntenic blocks, as has been demonstrated in the comparison among Gossypium species (Tang et al., Citation2015; Chen et al., Citation2017). Notably, some evidence suggests that at least in some cases, natural selection may have played a key role in shaping the evolution of syntenic gene clusters. Specifically, some syntenic clusters are co-transcribed, such as rrn5-rrn18, rps3-rpl16 and nad3-rps12 in rice and palm, among others (Nakazono et al., Citation1995; Fang et al., Citation2012). While the origin of these syntenic co-transcribed gene clusters is unclear, they appear to be distributed consistently throughout plant taxonomic groups (Liu et al., Citation2013).
IV. Unidirectional gene transfer and bidirectional functional interactions between chloroplasts and mitochondria
A characteristic of plant mitogenomes is their propensity for becoming a genetic “dumping ground” for sequences from both the nuclear and chloroplast (cp) genomes. Whereas this process is bidirectional between the mitochondrial and nuclear genomes (Knoop et al., Citation1996; Stupar et al., Citation2001; Timmis et al., Citation2004; Lough et al., Citation2008), sequence transfer from chloroplast to mitochondrial genomes typically is one-way (Hao and Palmer, Citation2009; Smith, Citation2011; Tsunewaki, Citation2011), with two notable exceptions of mitochondrion-to-plastid transfer in Daucus carota (Goremykin et al., Citation2009) and Asclepias syriaca (Smith, Citation2014). Genetic transfer from the chloroplast to the mitochondrial genome is well documented (Oldenburg and Bendich, Citation1996; Bock, Citation2010; Smith, Citation2011), with cpDNA-like sequences detected in many mitogenomes from a variety of species (Alverson et al., Citation2010; Kitazaki and Kubo, Citation2010; Rodriguez-Moreno et al., Citation2011). As an indication of the scale of this process, between 0.1 and 10.3% of mtDNA has an origin tracing back to the chloroplast genome (Kitazaki and Kubo, Citation2010; Sloan and Wu, Citation2014); again, this likely underestimates the upper end of the range (Alverson et al., Citation2010).
Conversely, transfers in the reverse direction, i.e., mitochondria to chloroplasts, are rare. Aside from a few notable exceptions (Daucus carota, Asclepias syriaca and sensu lato) of horizontal gene transfer into the chloroplast (Mackenzie and Chase, Citation1990; Goremykin et al., Citation2009; Lei et al., Citation2013; Knox, Citation2014; Smith, Citation2014), the chloroplast genome is highly conserved among plant species, and is unlikely to accept foreign sequences (Kitazaki and Kubo, Citation2010). Those infrequent cases of horizontal gene transfer into the chloroplast most commonly are associated with double-strand break repair and interestingly, are more likely to occur if a plastid-to-mitochondria transfer event had occurred previously (Mackenzie and Chase, Citation1990; Christensen, Citation2013).
Exogenous sequence uptake into plant mitochondrial is common and ongoing, occurring throughout land plants (Kitazaki and Kubo, Citation2010; Rodriguez-Moreno, Citation2011). There is, however, a notable difference in the propensity for chloroplast DNA (e.g. cpDNA-derived tRNA genes) uptake among mitochondria in land plants (), with a greater number and longer average length in seed plants. Additionally, while a variety of chloroplast sequences are found in land plant mitogenomes, most functionally transferred genes are tRNAs (). Almost half of the donated cpDNA-like sequences are related to tRNAs, although a few are related to genes involved in photosynthesis or other functions (Nakazono et al., Citation1996; Adams et al., Citation2002a; Wang et al., Citation2012; Liu et al., Citation2013). Many of these are maintained after transfer (Dietrich et al., Citation1996; Clifton et al., Citation2004; Sloan et al., Citation2010). However, some copies become degenerate upon integration and/or over time (Wang et al., Citation2007), which can lead to the formation of detrimental CMS-introducing ORFs. Perhaps more intriguing is the common conversion of regions of the atp1/atpA mitochondrial gene copy such that the chloroplast and mitochondrial copies are unusually conserved (Hao and Palmer, Citation2009). Moreover, some chloroplast-derived sequences have been shown to maintain syntenic relationships with specific mitochondrial sequences, even among species separated by millions of years of divergence (Liu et al., Citation2013).
Unlike the pattern observed with the chloroplast, nuclear-mitochondrial transfers are bidirectional (Francis and Fernand, Citation1977; Bock, Citation2010) and can contribute a significant amount of DNA to either genome. Nuclear-derived sequences have been found in the mitogenomes of multiple species (Unseld et al., Citation1997; Alverson et al., Citation2010; Rodriguez-Moreno et al., Citation2011), comprising nearly half of the maize mitogenome (Goremykin et al., Citation2012) and typically falling between 0.1 and 38.6% (Kubo and Newton, Citation2008; Rodriguez-Moreno et al., Citation2011; Goremykin et al., Citation2012). Reciprocally, there exist numerous examples of mitochondrial sequences becoming incorporated in the nuclear genome, often as nonfunctional NUMTs (i.e. NUclear MiTochondrial DNAs). These range in size from short transfers of only ∼20 kb nucleotides (Liu et al., Citation2014) to hundreds of kilobases of mtDNA in complex arrangements (Lin et al., Citation1999; Stupar et al., Citation2001; Bergthorsson et al., Citation2003; Yu et al., Citation2003; Lough et al., Citation2008). The origin and fate of transferred sequences have been facilitated to be clarified by the increased availability of genomic sequences (Adams et al., Citation2000, Citation2002b; Noutsos et al., Citation2005; Mower et al., Citation2012a; Michalovova et al., Citation2013).
In addition to nonfunctional transfers, the nuclear genome can also uptake genic sequences from the mitochondria (Adams et al., Citation2002a; Adams and Palmer, Citation2003; Mower et al., Citation2012a), subsequently acquiring both the regulatory factors (de Longevialle et al., Citation2007) and targeting information necessary for successful transfer of the protein products back to the mitochondria (Peeters and Small, Citation2001; Liu et al., Citation2009). This is perhaps not entirely surprising in that communication between the mitochondrial and nuclear genomes is necessary for successful mitochondrial function (Poyton and McEwen, Citation1996; Cannino et al., Citation2007; Jacoby et al., Citation2012; Arenas-M et al., Citation2014; Braun et al., Citation2014), as well as regulation of some nuclear genes (Yuan and Yang, Citation1999), such as those involved in pathogen response (Colombatti et al., Citation2014). Indeed, of the more than 2000 proteins contained within angiosperm mitochondria, typically fewer than 41 proteins are encoded by the mitogenome itself (Millar et al., Citation2005).
V. Mechanisms of cytoplasmic male sterility (CMS)
CMS is a maternally conferred reproductive trait that relies on the expression of CMS-inducing mitochondrial sequences and the presence (or absence) of nuclear-based fertility restoring sequences (Chen and Liu, Citation2014). Factors implicated in the evolution of CMS include wide interspecific or intergeneric hybridization (Gobron et al., Citation2013), cell fusion (Yamagishi and Bhat, 2014), and intron cleavage (Jin et al., Citation2013). At the molecular level, the development of CMS can be broadly binned into the following three main categories: (1) mtDNA recombination and interactions between mitochondrial and nuclear genomes; (2) aberrant RNA editing; and (3) accumulation of toxic protein products.
A. Three routes to CMS
1. mtDNA recombination and cytonuclear interaction
Both the rampant recombination in plant mitogenomes and the propensity for intergenomic sequence transfer are involved in CMS determination at the genome level, with the former acting to induce CMS and the latter to resolve it. As described above, mitogenome recombinations generate novel chimeric sequences. Many examples of CMS stem from these consequences of recombination (Sloan et al., Citation2012b; Chen and Liu, Citation2014; Yamagishi and Bhat, 2014; Charlesworth, Citation2017; Tang et al., Citation2017), and include chimeric gene fusions (Rathburn and Hedgcoth, Citation1991; Tuteja et al., Citation2013; Wang et al., Citation2013b; Szklarczyk et al., Citation2014; Tang et al., Citation2017), partial/orphan ORFs (Shearman et al., Citation2014), and disruptions in gene orientation/promoter association (Horn et al., Citation2014). Often, these chimeric CMS genes exhibit co-transcription with upstream or downstream functional genes, such as T-urf13 in CMS-T maize (Wise et al., Citation1987; Kennell and Pring, Citation1989), orf352 in RT102-CMS rice (Okazaki et al., Citation2013), and orf355 and orf77 in CMS-S maize (Wen and Chase, Citation1999; Gallagher et al., Citation2002). CMS genes typically affect the mitochondrial electron transfer chain pathways, and are commonly classified into four categories according to the components they affect: [1] complex I (NADH dehydrogenase); [2] complex IV (cytochrome oxidase); [3] complex V (F0F1 ATP synthase); and [4] “other” (Horn et al., Citation2014; Wesołowski et al., Citation2015).
If the development of CMS-inducing genes in the mitochondria blocks successful reproduction, compensation for these defects is required for fertility. Indeed, restorer-of-fertility genes (RFs) have been identified in and/or suggested for many CMS systems (Forde et al., Citation1978; Bonhomme et al., Citation1991; Rathburn and Hedgcoth, Citation1991; Ducos et al., Citation2001; Liu et al., Citation2007; Luo et al., Citation2013; Wang et al., Citation2013a; Table 1 in Hu et al., Citation2014; Table 1 in Chen and Liu, Citation2014; Kim et al., Citation2015). These are encoded not by the mitochondria, but by sequences in the nuclear genome. That is, sterility is reversed through synergistic cytonuclear interactions and antero/retrograde signaling between the mitochondrial and nuclear genomes (Rhoads and Subbaiah, Citation2007; Chen and Liu, Citation2014; Kitazaki et al., Citation2015; Liu et al., Citation2016). The exact nature of many of these RF loci, however, remains unknown. In a comprehensive review regarding the mechanisms underlying CMS, Chen and Liu (Citation2014) tabulated a list of known CMS + RF systems from diverse species, including maize, sunflower, and radish (Chen and Liu, Citation2014) and their mode of action, if known. Remarkably, many of the properties of the RF loci remain unknown. However, the coding capacity for those that are known (or can be predicted) are quite diverse, from an aldehyde dehydrogenase in maize (the first isolated plant restorer gene, Cui et al., Citation1996; Liu et al., Citation2001) to a putative peptidase in sugar beet (Matsuhira et al., Citation2012) to a suite of PPR proteins in diverse species (Brown et al., Citation2003; Kazama and Toriyama, Citation2003; Koizuka et al., Citation2003; Akagi et al., Citation2004; Komori et al., Citation2004; Klein et al., 2005; Wang et al., Citation2006; Uyttewaal et al., Citation2008; Jordan et al., Citation2010; Hu et al., Citation2012; Wang et al., Citation2013c; Kitazaki et al., Citation2015; Liu et al., Citation2016).
The modes of action for CMS-related mitochondrial genes appear equally as diverse. In Brassica napus, CMS-related orf224/atp6 was found to downregulate pollen development by causing an energy deficiency (An et al., Citation2014). Whereas CMS in Chinese cabbage has been associated with retrograde signaling (i.e., signals from the plastid or mitochondrion that control nuclear gene expression) from the mitochondrion that interferes with nuclear gene expression in a suite of pathways, including auxin response, ATP synthesis, and more (Dong et al., Citation2013). Maladaptive retrograde signaling has also been implicated in mitochondrial interference with the rice nuclear gene COX11, resulting in the premature programmed cell death of tapetal cells and subsequent pollen abortion (Luo et al., Citation2013). Still, other species have the features and function of cytotoxic proteins (Dewey et al., Citation1987; Korth et al., Citation1991; Levings, Citation1993; Korth and Levings, Citation1993; Nakai et al., Citation1995; Duroc et al., Citation2005; Wang et al., Citation2006; Jing et al., Citation2012).
Broad characterization of the mechanisms that cause CMS has led to the following four accepted models: (1) the energy deficiency model; (2) the retrograde regulation module; (3) the aberrant programmed cell death model; and (4) the cytotoxicity model. However, as is the case with rice COX11 and other CMS systems, the mode of action may affect more than one category. An additional interesting dimension is that while the expression of CMS genes does not appear to be restricted to the male reproductive tissues in many species (Warmke and Lee, Citation1978; Abad et al., Citation1995; Yamamoto et al., Citation2008; Chen and Liu, Citation2014), accumulation of CMS-related proteins can be highly spatiotemporally specific to induce CMS (Abad et al., Citation1995; Wang et al., Citation2006; Luo et al., Citation2013).
2. Regulation of CMS transcripts via RNA editing
Post-transcriptional RNA editing of mitochondrial genes is both ubiquitous and important for regulation (Marchfelder and Binder, Citation2004; Takenaka et al., Citation2008; Wang et al., Citation2009; Grewe et al., Citation2014). Typically, RNA editing of mitochondrial transcripts in flowering plants occurs in coding regions of mitochondrial transcripts (Hanson et al., Citation1996) to convert specific cytosine residues to uracil (C → U) (Yi et al., Citation2004; Bentolila et al., Citation2008; Suzuki et al., Citation2013; Szklarczyk et al., Citation2014; Takenaka et al., Citation2014). Defects in proper RNA editing have been associated with truncated or repressed transcripts, ultimately resulting in plant or cell death (Amuthan et al., Citation2001). In the case of CMS, RF genes modulate specific RNA editing through cleavage and/or degradation of the CMS products, thereby restoring fertility (Chen and Liu, Citation2014; Yan et al., Citation2015). Regulation of CMS products by RF-mediated, RNA editing occurs diverse species, from sorghum (Tang et al., Citation1999) to Brassica (Menassa et al., Citation1999) to rice (Wang et al., Citation2006), and reduction in RNA editing of CMS genes has been associated with sterility (Suzuki et al., Citation2013). Maize CMS-associated orf77 shows significant reduction in editing in sterile lines (Gallagher et al., Citation2002), as does CMS-associated atp6 in Sorghum bicolor (Howad and Kempken, Citation1997) and atp9 in rice and soybean (Wei et al., Citation2008). The number of RNA editing sites can vary among species, e.g., an average 43 different editable sites among mitochondrial protein-coding regions of Arabidopsis thaliana (Giege and Brennicke, Citation1999), Brassica napus (Handa, Citation2003), and Oryza sativa (Notsu et al., Citation2002), as well as among tissues, ecotypes, or genes (Bentolila et al., Citation2008). This link between RNA editing and CMS is not ubiquitous for all plants (Horn et al., Citation2014). Finally, recent degradome sequencing implicates a possible role for microRNAs in the regulatory network of Brassica juncea and Raphanus sativus CMS (Yang et al., Citation2013; Zhang et al., Citation2016).
3. Specific protein products
In all known cases, the protein products of CMS genes are the likely agents of CMS, not the transcripts themselves that. Most CMS-associated proteins possess transmembrane configurations capable of disrupting the mitochondrial membrane structure and/or altering the permeability and potential of mitochondrial membrane (Li et al., Citation2014). These proteins can directly interfere with energy production (Jing et al., Citation2012; Wang et al., Citation2013c; Wu et al., Citation2013), induce the release of cytochrome C via accumulation of unusually large numbers of reactive oxygen species (ROS) (Yan et al., Citation2014), and stimulate premature programmed cell death in male reproductive tissues (Luo et al., Citation2013; Horn et al., Citation2014). Several CMS proteins have demonstrated toxicity, such as URF13 in CMS-T maize (Korth et al., Citation1991), ORFH79 in HL-CMS rice (Hu et al., Citation2013), Orf507 in CMS pepper (Li et al., Citation2013), and ROS homeostasis associated protein in cotton (Yang et al., Citation2014). Restoration of fertility can occur at the translational or post-translational level. That is, in many CMS systems, RF genes do not affect accumulation of the CMS transcript; rather, restored lines are characterized by a marked decrease in toxic CMS protein accumulation (Chen and Liu, Citation2014). Reduced protein accumulation (without similar transcript reduction) has been observed in maize (Dewey et al., Citation1991), common bean (Sarria et al., Citation1998), Brassica (Landgren et al., Citation1996), radish (Uyttewaal et al., Citation2008), and rice (Itabashi et al., Citation2011), among others. Many of these suggest that restoration of fertility occurs via reduction in the production of toxic proteins; however, in at least one maize CMS system, there is evidence that fertility is restored via the degradation of toxic products (versus proteins; Liu et al., Citation2001).
VI. Conclusions
Mitochondria are the primary source of cellular energy, providing the necessary means for growth, development, and reproduction. These highly conserved functions render the slow evolutionary pace of plant mitochdondrial genes understandable. However, the rapidity with which plant mitogenomes evolve is far more puzzling, given both the potential and observed consequences of this lability, including massive alterations in genome size, organization, and composition. The consequences of and adaptation to mitochondrial gene loss (transfer to the nucleus) and genomic recombination are particularly interesting in that both appear to require cooperation from the nucleus to ensure viability. The tolerance for recombination, and the resulting consequences, while perhaps surprising for such an essential cellular component, relies largely on a co-evolutionary dynamic among the nucleus and its extra-nuclear genomes.
CMS is a prime example of recombination-induced mitochondrial dysfunction that requires nuclear input for resolution (Hanson and Bentolila, Citation2004; Pelletier and Budar, Citation2007). Novel and chimeric mitochondrial sequences are a frequent result of this recombination (Wise et al., Citation1987; Kennell and Pring, Citation1989; Wen and Chase, Citation1999; Gallagher et al., Citation2002; Okazaki et al., Citation2013; Yamagishi and Bhat, 2014; Tang et al., Citation2017), in which recombination sometimes leads to the creation of transcripts that interfere with normal male gametophyte development (Kitazaki and Kubo, Citation2010) via the generation of toxic and/or disruptive transmembrane proteins (Korth et al., Citation1991; Kim et al., Citation2007; Wan et al., Citation2007; Zhang et al., Citation2007; Yang et al., Citation2009; Gulyas et al., Citation2010; Jing et al., Citation2012; Flores-Renteria et al., Citation2013; Ji et al., Citation2013; Luo et al., Citation2013; Okazaki et al., Citation2013; Park et al., Citation2013; Hu et al., Citation2014). Surprisingly, such genes are not only abundant in many fertile plants, such as Arabidopsis thaliana (Marienfeld et al., Citation1997; Unseld et al., Citation1997), Beta vulgaris (Kubo et al., Citation2000), Oryza sativa (Notsu et al., Citation2002), Brassica napus (Handa, Citation2003), Zea mays (Clifton et al., Citation2004), Triticum aestivum (Ogihara et al., Citation2005), and Nicotiana tabacum (Sugiyama et al., Citation2005), but are also constitutively expressed. Research into this phenomenon has revealed much about the distribution and occurrence of CMS, the tissue-specificity of the phenotype (male gametophytic tissues only) (Jing et al., Citation2012; Hu et al., Citation2014), the modes of action behind CMS, and the cytonuclear cooperation that suppresses the phenotype (Carlsson et al., Citation2008). As more genomes (both nuclear and mitochondrial) and other “-omic” data become available (Du et al., Citation2016; Jacoby et al., Citation2016; Wang et al., Citation2016), the potential for increasing our understanding and manipulating this vitally important phenomenon will be enhanced.
Acknowledgments
The authors thank Daniel B. Sloan (Colorado State University), Yingguo Zhu and Shaoqing Li (Wuhan University), Xuequn Liu (South-central University for Nationalities), and Shu-Miaw Chao (Biodiversity Research Center of Academia Sinica, Taipei, China) for their many valuable comments and suggestions. They are also grateful to anonymous reviewers for their helpful comments.
Funding
This work was supported by the National Key R & D Program for Crop Breeding (2016YFD0101407).
References
- Abad, A. R., Mehrtens, B. J., and Mackenzie, S. A. 1995. Specific expression in reproductive tissues and fate of a mitochondrial sterility-associated protein in cytoplasmic male-sterile bean. Plant Cell 7: 271–285.
- Abdelnoor, R. V., Yule, R., Elo, A., Christensen, A. C., Meyer-Gauen, G., and Mackenzie, S. A. 2003. Substoichiometric shifting in the plant mitochondrial genome is influenced by a gene homologous to MutS. Proc. Natl. Acad. Sci. U.S.A. 100: 5968–5973.
- Adams, K. L., Daley, D. O., Qiu, Y. L., Whelan, J., and Palmer, J. D. 2000. Repeated, recent and diverse transfers of a mitochondrial gene to the nucleus in flowering plants. Nature 408: 354–357.
- Adams, K. L., Daley, D. O., Whelan, J., and Palmer, J. D. 2002a. Genes for two mitochondrial ribosomal proteins in flowering plants are derived from their chloroplast or cytosolic counterparts. Plant Cell 14: 931–943.
- Adams, K. L. and Palmer, J. D. 2003. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol. Phylogenet. Evol. 29: 380–395.
- Adams, K. L., Qiu, Y. L., Stoutemyer, M., and Palmer, J. D. 2002b. Punctuated evolution of mitochondrial gene content: High and variable rates of mitochondrial gene loss and transfer to the nucleus during angiosperm evolution. Proc. Natl. Acad. Sci. U.S.A. 99: 9905–9912.
- Akagi, H., Nakamura, A., Yokozeki-Misono, Y., Inagaki, A., Takahashi, H., Mori, K., and Fujimura, T. 2004. Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor. Appl. Genet. 108: 1449–1457.
- Allen, J. O., Fauron, C. M., Minx, P., Roark, L., Oddiraju, S., Lin, G. N., Meyer, L., Sun, H., Kim, K., Wang, C., Du, F., Xu, D., Gibson, M., Cifrese, J., Clifton, S. W., and Newton, K. J. 2007. Comparisons among two fertile and three male-sterile mitochondrial genomes of maize. Genetics 177: 1173–1192.
- Alverson, A. J., Rice, D. W., Dickinson, S., Barry, K., and Palmer, J. D. 2011a. Origins and recombination of the bacterial-sized multichromosomal mitochondrial genome of cucumber. Plant Cell 23: 2499–2513.
- Alverson, A. J., Wei, X., Rice, D. W., Stern, D. B., Barry, K., and Palmer, J. D. 2010. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae). Mol. Biol. Evol. 27: 1436–1448.
- Alverson, A. J., Zhuo, S., Rice, D. W., Sloan, D. B., and Palmer, J. D. 2011b. The mitochondrial genome of the legume Vigna radiata and the analysis of recombination across short mitochondrial repeats. PLoS ONE 6: e16404.
- Amuthan, G., Biswas, G., Zhang, S. Y., Klein-Szanto, A., Vijayasarathy, C., and Avadhani, N. G. 2001. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 20: 1910–1920.
- An, H., Yang, Z., Yi, B., Wen, J., Shen, J., Tu, J., Ma, C., and Fu, T. 2014. Comparative transcript profiling of the fertile and sterile flower buds of pol CMS in B. napus. BMC Genom. 15: 258.
- Andre, C., Levy, A., and Walbot, V. 1992. Small repeated sequences and the structure of plant mitochondrial genomes. Trends Genet. 8: 128–132.
- Arenas-M, A., Zehrmann, A., Moreno, S., Takenaka, M., and Jordana, X. 2014. The pentatricopeptide repeat protein MEF26 participates in RNA editing in mitochondrial cox3 and nad4 transcripts. Mitochondrion 19, Part B: 126–134.
- Backert, S. and Borner, T. 2000. Phage T4-like intermediates of DNA replication and recombination in the mitochondria of the higher plant Chenopodium album (L.). Curr. Genet. 37: 304–314.
- Bentolila, S., Elliott, L. E., and Hanson, M. R. 2008. Genetic architecture of mitochondrial editing in Arabidopsis thaliana. Genetics 178: 1693–1708.
- Bentolila, S. and Stefanov, S. 2012. A reevaluation of rice mitochondrial evolution based on the complete sequence of male-fertile and male-sterile mitochondrial genomes. Plant Physiol. 158: 996–1017.
- Bergthorsson, U., Adams, K. L., Thomason, B., and Palmer, J. D. 2003. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 424: 197–201.
- Bergthorsson, U., Richardson, A. O., Young, G. J., Goertzen, L. R., and Palmer, J. D. 2004. Massive horizontal transfer of mitochondrial genes from diverse land plant donors to the basal angiosperm Amborella. Proc. Natl. Acad. Sci. U.S.A. 101: 17747–17752.
- Bock, R. 2010. The give-and-take of DNA: horizontal gene transfer in plants. Trends Plant Sci. 15: 11–22.
- Bohra, A., Jha, U. C., Adhimoolam, P., Bisht, D., and Singh, N. P. 2016. Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Reports. 35: 967–993.
- Bonhomme, S., Budar, F., Ferault, M., and Pelletier, G. 1991. A 2.5 kb’ NcoI fragment of Ogura radish mitochondrial DNA is correlated with cytoplasmic male-sterility in Brassica cybrids. Curr. Genet. 19: 121–127.
- Boore, J. L. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27: 1767–1780.
- Braun, H. P., Binder, S., Brennicke, A., Eubel, H., Fernie, A. R., Finkemeier, I., Klodmann, J., König, A. C., Kühn, K., Meyer, E., Obata, T., Schwarzländer, M., Takenaka, M., and Zehrmann, A. 2014. The life of plant mitochondrial complex I. Mitochondrion 19, Part B: 295–313.
- Brown, G. G., Formanova, N., Jin, H., Wargachuk, R., Dendy, C., Patil, P., Laforest, M., Zhang, J. F., Cheung, W. Y., and Landry, B. S. 2003. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J. 35: 262–272.
- Budar, F. and Pelletier, G. 2001. Male sterility in plants: occurrence, determinism, significance and use. C. R. Acad. Sci. III 324: 543–550.
- Cannino, G., Di Liegro, C. M., and Rinaldi, A. M. 2007. Nuclear-mitochondrial interaction. Mitochondrion 7: 359–366.
- Carlsson, J., Leino, M., Sohlberg, J., Sundstrom, J. F., and Glimelius, K. 2008. Mitochondrial regulation of flower development. Mitochondrion 8: 74–86.
- Chacinska, A., Koehler, C. M., Milenkovic, D., Lithgow, T., and Pfanner, N. 2009. Importing mitochondrial proteins: machineries and mechanisms. Cell 138: 628–644.
- Charlesworth, D. 2017. Origins of rice cytoplasmic male sterility genes. Cell Res. 27: 3–4.
- Chase, C. D. 2007. Cytoplasmic male sterility: a window to the world of plant mitochondrial–nuclear interactions. Trends Genet. 23: 81–90.
- Chen, L. T. and Liu, Y. G. 2014. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 65: 579–606.
- Chen, Z., Nie, H., Grover, C. E., Wang, Y., Li, P., Wang, M., Pei, H., Zhao, Y., Li, S., Wendel, J. F., and Hua, J. 2017. Entire nucleotide sequences of Gossypium raimondii and G. arboreum mitochondrial genomes revealed A-genome species as cytoplasmic donor of the allotetraploid species. Plant Biol. 19(3): 484–493.
- Cho, Y., Lee, Y. P., Park, B. S., Han, T. H., and Kim, S. 2012. Construction of a high-resolution linkage map of Rfd1, a restorer-of-fertility locus for cytoplasmic male sterility conferred by DCGMS cytoplasm in radish (Raphanus sativus L.) using synteny between radish and Arabidopsis genomes. Theor. Appl. Genet. 125: 467–477.
- Christensen, A. C. 2013. Plant mitochondrial genome evolution can be explained by DNA repair mechanisms. Genome Biol. Evol. 5: 1079–1086.
- Clifton, S. W., Minx, P., Fauron, C. M., Gibson, M., Allen, J. O., Sun, H., Thompson, M., Barbazuk, W. B., Kanuganti, S., Tayloe, C., Meyer, L., Wilson, R. K., and Newton, K. J. 2004. Sequence and comparative analysis of the maize NB mitochondrial genome. Plant Physiol. 136: 3486–3503.
- Colombatti, F., Gonzalez, D. H., and Welchen, E. 2014. Plant mitochondria under pathogen attack: a sigh of relief or a last breath? Mitochondrion 19, Part B: 238–244.
- Cui, X., Wise, R. P., and Schnable, P. S. 1996. The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 272: 1334–1336.
- Cupp, J. D. and Nielsen, B. L. 2014. Minireview: DNA replication in plant mitochondria. Mitochondrion 19, Part B: 231–237.
- de Longevialle, A. F., Meyer, E. H., Andrés, C., Taylor, N. L., Lurin, C., Millar, A. H., and Small, I. D. 2007. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 intron 1 in Arabidopsis thaliana. Plant Cell 19: 3256–3265.
- Dewey, R. E., Timothy, D. H., and Levings, C. S. 1987. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc. Natl. Acad. Sci. U.S.A. 84: 5374–5378.
- Dewey, R. E., Timothy, D. H., and Levings, C. S. 1991. Chimeric mitochondrial genes expressed in the C male-sterile cytoplasm of maize. Curr. Genet. 20: 475–482.
- Dietrich, A., Small, I., Cosset, A., Weil, J. H., and Marechal-Drouard, L. 1996. Editing and import: strategies for providing plant mitochondria with a complete set of functional transfer RNAs. Biochimie 78: 518–529.
- Dong, X., Kim, W. K., Lim, Y. P., Kim, Y. K., and Hur, Y. 2013. Ogura-CMS in Chinese cabbage (Brassica rapa ssp. pekinensis) causes delayed expression of many nuclear genes. Plant Sci. 199–200: 7–17.
- Du, K., Liu, Q., Wu, X., Jiang, J., Wu, J., Fang, Y., Li, A., and Wang, Y. 2016. Morphological structure and transcriptome comparison of the cytoplasmic male sterility line in Brassica napus (SaNa-1A) derived from somatic hybridization and its maintainer line SaNa-1B. Front Plant Sci. 7: 1313.
- Ducos, E., Touzet, P., and Boutry, M. 2001. The male sterile G cytoplasm of wild beet displays modified mitochondrial respiratory complexes. Plant J. 26: 171–180.
- Duroc, Y., Gaillard, C., Hiard, S., Defrance, M., Pelletier, G., and Budar F. 2005. Biochemical and functional characterization of ORF138, a mitochondrial protein responsible for Ogura cytoplasmic male sterility in Brassiceae. Biochimie 87: 1089–1100.
- Fang, Y., Wu, H., Zhang, T., Yang, M., Yin, Y., Pan, L., Yu, X., Zhang, X., Hu, S., Al-Mssallem, I. S., and Yu, J. 2012. A complete sequence and transcriptomic analyses of date palm (Phoenix dactylifera L.) mitochondrial genome. PLoS ONE 7: e37164.
- Flores-Renteria, L., Orozco-Arroyo, G., Cruz-Garcia, F., Garcia-Campusano, F., Alfaro, I., and Vazquez-Santana, S. 2013. Programmed cell death promotes male sterility in the functional dioecious Opuntia stenopetala (Cactaceae). Ann. Bot. 112: 789–800.
- Forde, B. G., Oliver, R. J., and Leaver, C. J. 1978. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc. Natl. Acad. Sci. U.S.A. 75: 3841–3845.
- Francis, Q. and Fernand, V. 1977. Heterogeneous population of mitochondrial DNA molecules in higher plants. Nature 268: 365–368.
- Fujii, S., Komatsu, S., and Toriyama, K. 2007. Retrograde regulation of nuclear gene expression in CW-CMS of rice. Plant Mol. Biol. 63: 405–417.
- Gallagher, L. J., Betz, S. K., and Chase, C. D. 2002. Mitochondrial RNA editing truncates a chimeric open reading frame associated with S male-sterility in maize. Curr. Genet. 42: 179–184.
- Galtier, N. 2011. The intriguing evolutionary dynamics of plant mitochondrial DNA. BMC Biol. 9: 61.
- Giege, P. and Brennicke, A. 1999. RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc. Natl. Acad. Sci. U.S.A. 96: 15324–15329.
- Gobron, N., Waszczak, C., Simon, M., Hiard, S., Boivin, S., Charif, D., Ducamp, A., Wenes, E., and Budar, F. 2013. A cryptic cytoplasmic male sterility unveils a possible gynodioecious past for Arabidopsis thaliana. PLoS ONE 8: e62450.
- Goremykin, V. V., Lockhart, P. J., Viola, R., and Velasco, R. 2012. The mitochondrial genome of Malus domestica and the import-driven hypothesis of mitochondrial genome expansion in seed plants. Plant J. 71: 615–626.
- Goremykin, V. V., Salamini, F., Velasco, R., and Viola, R. 2009. Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Mol. Biol. Evol. 26: 99–110.
- Gray, M. W., Burger, G., and Lang, B. F. 1999. Mitochondrial evolution. Science 283: 1476–1481.
- Gray, M. W. 2012. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 4: a011403.
- Grewe, F., Edger, P. P., Keren, I., Sultan, L., Pires, J. C., Ostersetzer-Biran, O., and Mower, J. P. 2014. Comparative analysis of 11 Brassicales mitochondrial genomes and the mitochondrial transcriptome of Brassica oleracea. Mitochondrion 19, Part B: 135–143.
- Gualberto, J. M. and Newton, K. J. 2017. Plant mitochondrial genomes: dynamics and mechanisms of mutation. Annu. Rev. Plant Biol. 68: 17.1–17.28.
- Gualberto, J. M., Mileshina, D., Wallet, C., Niazi, A. K., Weber-Lotfi, F., and Dietrich, A. 2014. The plant mitochondrial genome: dynamics and maintenance. Biochimie 100: 107–120.
- Gulyas, G., Shin, Y., Kim, H., Lee, J.-S., and Hirata, Y. 2010. Altered transcript reveals an orf507 sterility-related gene in chili pepper (Capsicum annuum L.). Plant Mol. Biol. Rep. 28: 605–612.
- Guo, W., Grewe, F., Fan, W., Young, G. J., Knoop, V., Palmer, J. D., and Mower, J. P. 2016. Ginkgo and Welwitschia mitogenomes reveal extreme contrasts in gymnosperm mitochondrial evolution. Mol. Biol. Evol. 33: 1448–1460.
- Handa, H. 2003. The complete nucleotide sequence and RNA editing content of the mitochondrial genome of rapeseed (Brassica napus L.): comparative analysis of the mitochondrial genomes of rapeseed and Arabidopsis thaliana. Nucleic Acids Res. 31: 5907–5916.
- Hanson, M. R. and Bentolila, S. 2004. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16(Suppl): S154–S169.
- Hanson, M. R., Sutton, C., and Luis, B. 1996. Plant organelle gene expression: altered by RNA editing. Trends Plant Sci. 1: 57–64.
- Hao, W. and Palmer, J. D. 2009. Fine-scale mergers of chloroplast and mitochondrial genes create functional, transcompartmentally chimeric mitochondrial genes. Proc. Natl. Acad. Sci. U.S.A. 106: 16728–16733.
- Havey, M. 2004. The use of cytoplasmic male sterility for hybrid seed production. In: Molecular Biology and Biotechnology of Plant Organelles. pp. 623–634. Daniell, H. and Chase, C., Springer, Netherlands.
- Heng, S., Wei, C., Jing, B., Wan, Z., Wen, J., Yi, B., Ma, C., Tu, J., Fu, T., and Shen, J. 2014. Comparative analysis of mitochondrial genomes between the hau cytoplasmic male sterility (CMS) line and its iso-nuclear maintainer line in Brassica juncea to reveal the origin of the CMS-associated gene orf288. BMC Genomics 15: 322.
- Horn, R., Gupta, K. J., and Colombo, N. 2014. Mitochondrion role in molecular basis of cytoplasmic male sterility. Mitochondrion 19 Pt B: 198–205.
- Howad, W. and Kempken, F. 1997. Cell type-specific loss of atp6 RNA editing in cytoplasmic male sterile Sorghum bicolor. Proc. Natl. Acad. Sci. U.S.A. 94: 11090–11095.
- Hu, J., Huang, W., Huang, Q., Qin, X., Dan, Z., Yao, G., Zhu, R., and Zhu, Y. 2013. The mechanism of ORFH79 suppression with the artificial restorer fertility gene Mt-GRP162. New Phytol. 199: 52–58.
- Hu, J., Huang, W., Huang, Q., Qin, X., Yu, C., Wang, L., Li, S., Zhu, R., and Zhu, Y. 2014. Mitochondria and cytoplasmic male sterility in plants. Mitochondrion 19, Part B: 282–288.
- Hu, J., Wang, K., Huang, W. C., Liu, G., Gao, Y., Wang, J. M., Huang, Q., Ji, Y. X., Qin, X. J., Wan, L., Zhu, R. S., Li, S. Q., Yang, D. C., and Zhu, Y. G. 2012. The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant Cell 24: 109–122.
- Huang, W. C., Yu, C. C., Hu, J., Wang, L. L., Dan, Z. W., Zhou, W., He, C. L., Zeng, Y. F., Yao, G. X., Qi, J. Z., Zhang, Z. H., Zhu, R. S., Chen, X. F., and Zhu, Y. G. 2015. Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc. Natl. Acad. Sci. U.S.A. 112: 14984–14989.
- Itabashi, E., Iwata, N., Fujii, S., Kazama, T., and Toriyama, K. 2011. The fertility restorer gene, Rf2, for Lead Rice type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. Plant J. 65: 359–367.
- Jacoby, R. P., Li, L., Huang, S., Pong Lee, C., Millar, A. H., and Taylor, N. L. 2012. Mitochondrial composition, function and stress response in plants. J. Integr. Plant Biol. 54: 887–906.
- Jacoby, R. P., Millar, A. H., and Taylor, N. L. 2016. Opportunities for wheat proteomics to discover the biomarkers for respiration-dependent biomass production, stress tolerance and cytoplasmic male sterility. J Proteom. 143: 36–44.
- Ji, J., Huang, W., Yin, C., and Gong, Z. 2013. Mitochondrial cytochrome c oxidase and F1Fo-ATPase dysfunction in peppers (Capsicum annuum L.) with cytoplasmic male sterility and its association with orf507 and ψatp6-2 genes. Int. J. Mol. Sci. 14: 1050–1068.
- Jin, Z., Wu, L., Cao, J., Chen, Z., and Pei, Y. 2013. TinII intron, an enhancer to affect the function of the cytoplasmic male sterility related gene T in Brassica juncea. Sci. China Life Sci. 56: 1107–1112.
- Jing, B., Heng, S., Tong, D., Wan, Z., Fu, T., Tu, J., Ma, C., Yi, B., Wen, J., and Shen, J. 2012. A male sterility-associated cytotoxic protein ORF288 in Brassica juncea causes aborted pollen development. J. Exp. Bot. 63: 1285–1295.
- Jo, Y. D., Choi, Y., Kim, D. H., Kim, B. D., and Kang, B. C. 2014. Extensive structural variations between mitochondrial genomes of CMS and normal peppers (Capsicum annuum L.) revealed by complete nucleotide sequencing. BMC Genomics 15: 561.
- Jordan, D. R., Mace, E. S., Henzell, R. G., Klein, P. E., and Klein, R. R. 2010. Molecular mapping and candidate gene identification of the Rf2 gene for pollen fertility restoration in sorghum [Sorghum bicolor (L.) Moench]. Theor. Appl. Genet. 120: 1279–1287.
- Kazama, T. and Toriyama, K. 2003. A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Lett. 544: 99–102.
- Kennell, J. and Pring, D. 1989. Initiation and processing of atp6, T-urf13 and ORF221 transcripts from mitochondria of T cytoplasm maize. Mol. Gen. Genet. 216: 16–24.
- Kim, D. H., Kang, J. G., and Kim, B. D. 2007. Isolation and characterization of the cytoplasmic male sterility-associated orf456 gene of chili pepper (Capsicum annuum L.). Plant Mol. Biol. 63: 519–532.
- Kim, S., Kim, C. W., Park, M., and Choi, D. 2015. Identification of candidate genes associated with fertility restoration of cytoplasmic male-sterility in onion (Allium cepa L.) using a combination of bulked segregant analysis and RNA-seq. Theor. Appl. Genet. 128: 2289–2299.
- Kitazaki, K. and Kubo, T. 2010. Cost of having the largest mitochondrial genome: evolutionary mechanism of plant mitochondrial genome. J. Bot. 2010.
- Kitazaki, K., Arakawa, T., Matsunaga, M., Yui-Kurino, R., Matsuhira, H., Mikami, T., and Kubo, T. 2015. Post-translational mechanisms are associated with fertility restoration of cytoplasmic male sterility in sugar beet (Beta vulgaris). Plant J. 83: 290–299.
- Klein, R. R., Klein, P. E., Mullet, J. E., Minx, P., Rooney, W. L., and Schertz, K. F. 2006. Fertility restorer locus Rf1 of sorghum (Sorghum bicolor L.) encodes a pentatricopeptide repeat protein not present in the colinear region of rice chromosome 12. Theor. Appl. Genet. 111: 994–1012.
- Knoop, V. 2013. Plant mitochondrial genome peculiarities evolving in the earliest vascular plant lineages. J. Syst. Evol. 51: 1–12.
- Knoop, V., Unseld, M., Marienfeld, J., Brandt, P., Sunkel, S., Ullrich, H., and Brennicke, A. 1996. copia-, gypsy and LINE-like retrotransposon fragments in the mitochondrial genome of Arabidopsis thaliana. Genetics 142: 579–585.
- Knox, E. B. 2014. The dynamic history of plastid genomes in the Campanulaceae sensu lato is unique among angiosperms. Proc. Natl. Acad. Sci. U.S.A. 111: 11097–11102.
- Koizuka, N., Imai, R., Fujimoto, H., Hayakawa, T., Kimura, Y., Kohno-Murase, J., Sakai, T., Kawasaki, S., and Imamura, J. 2003. Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J. 34: 407–415.
- Komori, T., Ohta, S., Murai, N., Takakura, Y., Kuraya, Y., Suzuki, S., Hiei, Y., Imaseki, H., and Nitta, N. 2004. Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J. 37: 315–325.
- Korth, K. L., Kaspi, C. I., Siedow, J. N., and Levings, C. S. 1991. URF13, a maize mitochondrial pore-forming protein, is oligomeric and has a mixed orientation in Escherichia coli plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 88: 10865–10869.
- Korth, K. L. and Levings, C. S. 1993. Baculovirus expression of the maize mitochondrial protein URF13 confers insecticidal activity in cell cultures and larvae. Proc. Natl. Acad. Sci. U.S.A. 90: 3388–3392.
- Kubo, T., Kitazaki, K., Matsunaga, M., Kagami, H., and Mikami, T. 2011. Male sterility-inducing mitochondrial genomes: how do they differ? Crit. Rev. Plant Sci. 30: 378–400.
- Kubo, T. and Newton, K. J. 2008. Angiosperm mitochondrial genomes and mutations. Mitochondrion 8: 5–14.
- Kubo, T., Nishizawa, S., Sugawara, A., Itchoda, N., Estiati, A., and Mikami, T. 2000. The complete nucleotide sequence of the mitochondrial genome of sugar beet (Beta vulgaris L.) reveals a novel gene for tRNA(Cys)(GCA). Nucleic Acids Res. 28: 2571–2576.
- Kurland, C. G. and Andersson, S. G. 2000. Origin and evolution of the mitochondrial proteome. Microbiol. Mol. Biol. Rev. 64: 786–820.
- Landgren, M., Zetterstrand, M., Sundberg, E., and Glimelius, K. 1996. Alloplasmic male-sterile Brassica lines containing B. tournefortii mitochondria express an ORF3 of the atp6 gene and a 32 kDa protein. Plant Mol. Biol. 32: 879–890.
- Laroche, J., Li, P., Maggia, L., and Bousquet, J. 1997. Molecular evolution of angiosperm mitochondrial introns and exons. Proc. Natl. Acad. Sci. U.S.A. 94: 5722–5727.
- Lavrov, D. V., Adamski, M., Chevaldonne, P., and Adamska, M. 2016. Extensive mitochondrial mRNA editing and unusual mitochondrial genome organization in Calcaronean sponges. Curr. Biol. 26: 86–92.
- Lee, Y. P., Cho, Y., and Kim, S. 2014. A high-resolution linkage map of the Rfd1, a restorer-of-fertility locus for cytoplasmic male sterility in radish (Raphanus sativus L.) produced by a combination of bulked segregant analysis and RNA-Seq. Theor. Appl. Genet. 127: 2243–2252.
- Lei, B., Li, S., Liu, G., Chen, Z., Su, A., Li, P., Li, Z., and Hua, J. 2013. Evolution of mitochondrial gene content: loss of genes, tRNAs and introns between Gossypium harknessii and other plants. Plant Syst. Evol. 299: 1889–1897.
- Levings, C. S. 1993. Thoughts on cytoplasmic male sterility in cms-T maize. Plant Cell 5: 1285.
- Li, J., Pandeya, D., Jo, Y. D., Liu, W. Y., and Kang, B. C. 2013. Reduced activity of ATP synthase in mitochondria causes cytoplasmic male sterility in chili pepper. Planta 237: 1097–1109.
- Li, Y., Liu, T., Duan, W., Song, X., Shi, G., Zhang, J., Deng, X., Zhang, S., and Hou, X. 2014. Instability in mitochondrial membranes in Polima cytoplasmic male sterility of Brassica rapa ssp. Chin. Funct. Integr. Genom. 14: 441–451.
- Lillestol, R. K., Shah, S. A., Brugger, K., Redder, P., Phan, H., Christiansen, J., and Garrett, R. A. 2009. CRISPR families of the crenarchaeal genus Sulfolobus: bidirectional transcription and dynamic properties. Mol. Microbiol. 72: 259–272.
- Lin, X. Y., Kaul, S. S., Rounsley, S., Shea, T. P., Benito, M. I., Town, C. D., Fujii, C. Y., Mason, T., Bowman, C. L., Barnstead, M., Feldblyum, T. V., Buell, C. R., Ketchum, K. A., Lee, J., Ronning, C. M., Koo, H. L., Moffat, K. S., Cronin, L. A., Shen, M., Pai, G., Van Aken, S., Umayam, L., Tallon, L. J., Gill, J. E., Adams, M. D., Carrera, A. J., Creasy, T. H., Goodman, H. M., Somerville, C. R., Copenhaver, G. P., Preuss, D., Nierman, W. C., White, O., Eisen, J. A., Salzberg, S. L., Fraser, C. M., and Venter, J. C. 1999. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402: 761–768.
- Liu, F., Cui, X. Q., Horner, H. T., Weiner, H., and Schnable, P. S. 2001. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant Cell 13: 1063–1078.
- Liu, G., Cao, D., Li, S., Su, A., Geng, J., Grover, C. E., Hu, S., and Hua, J. 2013. The complete mitochondrial genome of Gossypium hirsutum and evolutionary analysis of higher plant mitochondrial genomes. PLoS ONE 8: e69476.
- Liu, S. L., Zhuang, Y., Zhang, P., and Adams, K. L. 2009. Comparative analysis of structural diversity and sequence evolution in plant mitochondrial genes transferred to the nucleus. Mol. Biol. Evol. 26: 875–891.
- Liu, Y., Medina, R., and Goffinet, B. 2014. 350 My of mitochondrial genome stasis in mosses, an early land plant lineage. Mol. Biol. Evol. 31: 2586–2591.
- Liu, Y., Xue, J. Y., Wang, B., Li, L., and Qiu, Y. L. 2011. The mitochondrial genomes of the early land plants Treubia lacunosa and Anomodon rugelii: dynamic and conservative evolution. PLoS ONE 6: e25836.
- Liu, Z., Yang, Z., Wang, X., Li, K., An, H., Liu, J., Yang, G., Fu, T., Yi, B., and Hong, D. 2016. A mitochondria-targeted PPR protein restores pol cytoplasmic male sterility by reducing orf224 transcript levels in oilseed rape. Mol Plant 9: 1082–1084.
- Liu, Z. L., Xu, H., Guo, J. X., and Liu, Y. G. 2007. Structural and expressional variations of the mitochondrial genome conferring the wild abortive type of cytoplasmic male sterility in rice. J. Integr. Plant Biol. 49: 908–914.
- Lonsdale, D. M., Brears, T., Hodge, T. P., Melville, S. E., and Rottmann, W. H. 1988. The plant mitochondrial genome: homologous recombination as a mechanism for generating heterogeneity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 319: 149–163.
- Lough, A. N., Roark, L. M., Kato, A., Ream, T. S., Lamb, J. C., Birchler, J. A., and Newton, K. J. 2008. Mitochondrial DNA transfer to the nucleus generates extensive insertion site variation in maize. Genetics 178: 47–55.
- Luo, D., Xu, H., Liu, Z., Guo, J., Li, H., Chen, L., Fang, C., Zhang, Q., Bai, M., Yao, N., Wu, H., Wu, H., Ji, C., Zheng, H., Chen, Y., Ye, S., Li, X., Zhao, X., Li, R., and Liu, Y. G. 2013. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 45: 573–577.
- Mackenzie, S. A. and Chase, C. D. 1990. Fertility restoration is associated with loss of a portion of the mitochondrial genome in cytoplasmic male-sterile common bean. Plant Cell 2: 905–912.
- Manchekar, M., Scissum-Gunn, K., Song, D., Khazi, F., McLean, S. L., and Nielsen, B. L. 2006. DNA recombination activity in soybean mitochondria. J. Mol. Biol. 356: 288–299.
- Marchfelder, A. and Binder, S. 2004. Plastid and plant mitochondrial RNA processing and RNA stability. In: Molecular Biology and Biotechnology of Plant Organelles. pp. 261–294. Daniell, H. and Chase, C., Springer, Netherlands.
- Marechal-Drouard, L., Guillemaut, P., Cosset, A., Arbogast, M., Weber, F., Weil, J. H., and Dietrich, A. 1990. Transfer RNAs of potato (Solanum tuberosum) mitochondria have different genetic origins. Nucleic Acids Res. 18: 3689–3696.
- Marechal, A. and Brisson, N. 2010. Recombination and the maintenance of plant organelle genome stability. New Phytol. 186: 299–317.
- Marienfeld, J. R., Unseld, M., Brandt, P., and Brennicke, A. 1997. Mosaic open reading frames in the Arabidopsis thaliana mitochondrial genome. Biol. Chem. 378: 859–862.
- Matsuhira, H., Kagami, H., Kurata, M., Kitazaki, K., Matsunaga, M., Hamaguchi, Y., Hagihara, E., Ueda, M., Harada, M., Muramatsu, A., Yui-Kurino, R., Taguchi, K., Tamagake, H., Mikami, T., and Kubo, T. 2012. Unusual and typical features of a novel restorer-of-fertility gene of sugar beet (Beta vulgaris L.). Genetics 192: 1347–1358.
- McCauley, D. E. and Bailey, M. F. 2009. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Ann. Bot. 104: 611–620.
- Menassa, R. L., Homme, Y., and Brown, G. G. 1999. Post-transcriptional and developmental regulation of a CMS-associated mitochondrial gene region by a nuclear restorer gene. Plant J. 17: 491–499.
- Michalovova, M., Vyskot, B., and Kejnovsky, E. 2013. Analysis of plastid and mitochondrial DNA insertions in the nucleus (NUPTs and NUMTs) of six plant species: size, relative age and chromosomal localization. Heredity 111: 314–320.
- Millar, A. H., Heazlewood, J. L., Kristensen, B. K., Braun, H., and Møller, I. M. 2005. The plant mitochondrial proteome. Trends in Plant Sci. 10: 36–43.
- Miller, I. and Bruns, E. 2016. The effect of disease on the evolution of females and the genetic basis of sex in populations with cytoplasmic male sterility. Proc. Biol. Sci. 283: 20153035.
- Mower, J. P., Touzet, P., Gummow, J. S., Delph, L. F., and Palmer, J. D. 2007. Extensive variation in synonymous substitution rates in mitochondrial genes of seed plants. BMC Evol. Biol. 7: 135.
- Mower, J. P., Jain, K., and Hepburn, N. J. 2012a. The role of horizontal transfer in shaping the plant mitochondrial genome. Adv. Bot. Res. 63: 41–69.
- Mower, J. P., Sloan, D., and Alverson, A. 2012b. Plant mitochondrial genome diversity: the genomics revolution. In: Plant Genome Diversity Volume 1. pp. 123–144. Wendel, J. F., Greilhuber, J., Dolezel, J., Leitch, I. J., Eds., Springer Vienna.
- Nakai, S., Noda, D., Kondo, M., and Terachi, T. 1995. High-level expression of a mitochondrial orf522 gene from the male-sterile sunflower is lethal to E. coli. Breed. Sci. 45: 233–236.
- Nakazono, M., Itadani, H., Wakasugi, T., Tsutsumi, N., Sugiura, M., and Hirai, A. 1995. The rps3-rpl16-nad3-rps12 gene cluster in rice mitochondrial DNA is transcribed from alternative promoters. Curr. Genet. 27: 184–189.
- Nakazono, M., Nishiwaki, S., Tsutsumi, N., and Hirai, A. 1996. A chloroplast-derived sequence is utilized as a source of promoter sequences for the gene for subunit 9 of NADH dehydrogenase (nad9) in rice mitochondria. Mol. Gen. Genet. 252: 371–378.
- Notsu, Y., Masood, S., Nishikawa, T., Kubo, N., Akiduki, G., Nakazono, M., Hirai, A., and Kadowaki, K. 2002. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol. Genet. Genom. 268: 434–445.
- Noutsos, C., Richly, E., and Leister, D. 2005. Generation and evolutionary fate of insertions of organelle DNA in the nuclear genomes of flowering plants. Genome Res. 15: 616–628.
- Odahara, M., Kuroiwa, H., Kuroiwa, T., and Sekine, Y. 2009. Suppression of repeat-mediated gross mitochondrial genome rearrangements by RecA in the moss Physcomitrella patens. Plant Cell 21: 1182–1194.
- Ogihara, Y., Yamazaki, Y., Murai, K., Kanno, A., Terachi, T., Shiina, T., Miyashita, N., Nasuda, S., Nakamura, C., Mori, N., Takumi, S., Murata, M., Futo, S., and Tsunewaki, K. 2005. Structural dynamics of cereal mitochondrial genomes as revealed by complete nucleotide sequencing of the wheat mitochondrial genome. Nucleic Acids Res. 33: 6235–6250.
- Okazaki, M., Kazama, T., Murata, H., Motomura, K., and Toriyama, K. 2013. Whole mitochondrial genome sequencing and transcriptional analysis to uncover an RT102-type cytoplasmic male sterility-associated candidate gene derived from Oryza rufipogon. Plant Cell Physiol. 54: 1560–1568.
- Oldenburg, D. J. and Bendich, A. J. 1996. Size and structure of replicating mitochondrial DNA in cultured tobacco cells. Plant Cell 8: 447–461.
- Ornduff, R. 1986. Angiosperm reproduction: plant breeding systems. Science 234: 620–621.
- Palmer, J. D., Adams, K. L., Cho, Y., Parkinson, C. L., Qiu, Y. L., and Song, K. 2000. Dynamic evolution of plant mitochondrial genomes: mobile genes and introns and highly variable mutation rates. Proc. Natl. Acad. Sci. U.S.A. 97: 6960–6966.
- Park, J. Y., Lee, Y. P., Lee, J., Choi, B. S., Kim, S., and Yang, T. J. 2013. Complete mitochondrial genome sequence and identification of a candidate gene responsible for cytoplasmic male sterility in radish (Raphanus sativus L.) containing DCGMS cytoplasm. Theor. Appl. Genet. 126: 1763–1774.
- Peeters, N. and Small, I. 2001. Dual targeting to mitochondria and chloroplasts. Biochim. Biophys. Acta 1541: 54–63.
- Pelletier, G. and Budar, F. 2007. The molecular biology of cytoplasmically inherited male sterility and prospects for its engineering. Curr. Opin. Biotechnol. 18: 121–125.
- Poyton, R. O. and McEwen, J. E. 1996. Crosstalk between nuclear and mitochondrial genomes. Annu. Rev. Biochem. 65: 563–607.
- Pruitt, K. D. and Hanson, M. R. 1991. Transcription of the Petunia mitochondrial CMS-associated Pcf locus in male sterile and fertility-restored lines. Mol. Gen. Genet. 227: 348–355.
- Rathburn, H. B. and Hedgcoth, C. 1991. A chimeric open reading frame in the 5 flanking region of coxI mitochondrial DNA from cytoplasmic male-sterile wheat. Plant Mol. Biol. 16: 909–912.
- Rhoads, D. M. and Subbaiah, C. C. 2007. Mitochondrial retrograde regulation in plants. Mitochondrion 7: 177–194.
- Rice, D. W., Alverson, A. J., Richardson, A. O., Young, G. J., Sanchez-Puerta, M. V., Munzinger, J., Barry, K., Boore, J. L., Zhang, Y., dePamphilis, C. W., Knox, E. B., and Palmer, J. D. 2013. Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science 342: 1468–1473.
- Rodriguez-Moreno, L., Gonzalez, V. M., Benjak, A., Marti, M. C., Puigdomenech, P., Aranda, M. A., and Garcia-Mas, J. 2011. Determination of the melon chloroplast and mitochondrial genome sequences reveals that the largest reported mitochondrial genome in plants contains a significant amount of DNA having a nuclear origin. BMC Genomics 12: 424.
- Sanchez-Puerta, M. V., Garcia, L. E., Wohlfeiler, J., and Ceriotti, L. F. 2017. Unparalleled replacement of native mitochondrial genes by foreign homologs in a holoparasitic plant. New Phytol. 214: 376–387.
- Sarria, R., Lyznik, A., Vallejos, C. E., and Mackenzie, S. A. 1998. A cytoplasmic male sterility-associated mitochondrial peptide in common bean is post-translationally regulated. Plant Cell 10: 1217–1228.
- Satoh, M., Kubo, T., Nishizawa, S., Estiati, A., Itchoda, N., and Mikami, T. 2004. The cytoplasmic male-sterile type and normal type mitochondrial genomes of sugar beet share the same complement of genes of known function but differ in the content of expressed ORFs. Mol. Genet. Genom. 272: 247–256.
- Schneider, A. 2011. Mitochondrial tRNA import and its consequences for mitochondrial translation. Annu. Rev. Biochem. 80: 1033–1053.
- Shearman, J. R., Sangsrakru, D., Ruang-Areerate, P., Sonthirod, C., Uthaipaisanwong, P., Yoocha, T., Poopear, S., Theerawattanasuk, K., Tragoonrung, S., and Tangphatsornruang, S. 2014. Assembly and analysis of a male sterile rubber tree mitochondrial genome reveals DNA rearrangement events and a novel transcript. BMC Plant Biol. 14: 45.
- Shedge, V., Arrieta-Montiel, M., Christensen, A. C., and Mackenzie, S. A. 2007. Plant mitochondrial recombination surveillance requires unusual RecA and MutS homologs. Plant Cell 19: 1251–1264.
- Siqueira, S. F., Dias, S. M., Lejeune, B., and de Souza, A. P. 2001. Marchantia polymorpha mitochondrial orf identifies transcribed sequence in angiosperm mitochondrial genome. Biochim. Biophys. Acta 1520: 203–211.
- Skippington, E., Barkman, T. J., Rice, D. W., and Palmer, J. D. 2015. Miniaturized mitogenome of the parasitic plant Viscum scurruloideum is extremely divergent and dynamic and has lost all nad genes. Proc. Natl. Acad. Sci. U.S.A. 112: E3515–E3524.
- Skippington, E., Barkman, T. J., Rice, D. W., and Palmer, J. D. 2017. Comparative mitogenomics indicates respiratory competence in parasitic Viscum despite loss of complex I and extreme sequence divergence, and reveals horizontal gene transfer and remarkable variation in genome size. BMC Plant Biol. 17: 49.
- Sloan, D. B., Alverson, A. J., Chuckalovcak, J. P., Wu, M., McCauley, D. E., Palmer, J. D., and Taylor, D. R. 2012a. Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol. 10: e1001241.
- Sloan, D. B., Alverson, A. J., Storchova, H., Palmer, J. D., and Taylor, D. R. 2010. Extensive loss of translational genes in the structurally dynamic mitochondrial genome of the angiosperm Silene latifolia. BMC Evol. Biol. 10: 274.
- Sloan, D. B. and Wu, Z. 2014. History of plastid DNA insertions reveals weak deletion and at mutation biases in angiosperm mitochondrial genomes. Genome Biol. Evol. 6: 3210–3221.
- Sloan, D. B., Muller, K., McCauley, D. E., Taylor, D. R., and Storchova, H. 2012b. Intraspecific variation in mitochondrial genome sequence, structure, and gene content in Silene vulgaris, an angiosperm with pervasive cytoplasmic male sterility. New Phytol. 196: 1228–1239.
- Smith, D. R. 2011. Extending the limited transfer window hypothesis to inter-organelle DNA migration. Genome Biol. Evol. 3: 743–748.
- Smith, D. R. 2014. Mitochondrion-to-plastid DNA transfer: it happens. New Phytol. 202: 736–738.
- Stupar, R. M., Lilly, J. W., Town, C. D., Cheng, Z., Kaul, S., Buell, C. R., and Jiang, J. 2001. Complex mtDNA constitutes an approximate 620-kb insertion on Arabidopsis thaliana chromosome 2: implication of potential sequencing errors caused by large-unit repeats. Proc. Natl. Acad. Sci. U.S.A. 98: 5099–5103.
- Sugiyama, Y., Watase, Y., Nagase, M., Makita, N., Yagura, S., Hirai, A., and Sugiura, M. 2005. The complete nucleotide sequence and multipartite organization of the tobacco mitochondrial genome: comparative analysis of mitochondrial genomes in higher plants. Mol. Genet. Genom. 272: 603–615.
- Suzuki, H., Yu, J., Ness, S., O'Connell, M., and Zhang, J. 2013. RNA editing events in mitochondrial genes by ultra-deep sequencing methods: a comparison of cytoplasmic male sterile, fertile and restored genotypes in cotton. Mol. Genet. Genomics 288: 445–457.
- Szklarczyk, M., Szyman´ski, M., Wójcik-Jagła, M., Simon, P. W., Weihe, A., and Börner, T. 2014. Mitochondrial atp9 genes from petaloid male-sterile and male-fertile carrots differ in their status of heteroplasmy, recombination involvement, post-transcriptional processing as well as accumulation of RNA and protein product. Theor. Appl. Genet. 127: 1689–1701.
- Takenaka, M., Verbitskiy, D., van der Merwe, J. A., Zehrmann, A., and Brennicke, A. 2008. The process of RNA editing in plant mitochondria. Mitochondrion 8: 35–46.
- Takenaka, M., Verbitskiy, D., Zehrmann, A., Härtel, B., Bayer-Császár, E., Glass, F., and Brennicke, A. 2014. RNA editing in plant mitochondria-connecting RNA target sequences and acting proteins. Mitochondrion 19, Part B: 191–197.
- Tang, H. V., Chen, W., and Pring, D. R. 1999. Mitochondrial orf107 transcription, editing, and nucleolytic cleavage conferred by the gene Rf3 are expressed in sorghum pollen. Sex. Plant Reprod. 12: 53–59.
- Tang, H., Zheng, X., Li, C., Xie, X., Chen, Y., Chen, L., Zhao, X., Zheng, H., Zhou, J., Ye, S., Guo, J., and Liu, Y. G. 2017. Multi-step formation, evolution, and functionalization of new cytoplasmic male sterility genes in the plant mitochondrial genomes. Cell Res. 27: 130–146.
- Tang, M. Y., Chen, Z. W., Grover, C. E., Wang, Y. M., Li, S. S., Liu, G. Z., Ma, Z. Y., Wendel, J. F., and Hua, J. P. 2015. Rapid evolutionary divergence of Gossypium barbadense and G. hirsutum mitochondrial genomes. BMC Genom. 16: 770.
- Timmis, J. N., Ayliffe, M. A., Huang, C. Y., and Martin, W. 2004. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 5: 123–135.
- Touzet, P. H. and Meyer, E. 2014. Cytoplasmic male sterility and mitochondrial metabolism in plants. Mitochondrion 19: 166–171.
- Tsunewaki, K. 2011. Interorganellar DNA transfer in wheat: dynamics and phylogenetic origin. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 87: 529–549.
- Tuteja, R., Saxena, R. K., Davila, J., Shah, T., Chen, W., Xiao, Y. L., Fan, G., Saxena, K. B., Alverson, A. J., Spillane, C., Town, C., and Varshney, R. K. 2013. Cytoplasmic male sterility-associated chimeric open reading frames identified by mitochondrial genome sequencing of four Cajanus genotypes. DNA Res. 20: 485–495.
- Unseld, M., Marienfeld, J. R., Brandt, P., and Brennicke, A. 1997. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 15: 57–61.
- Uyttewaal, M., Arnal, N., Quadrado, M., Martin-Canadell, A., Vrielynck, N., Hiard, S., Gherbi, H., Bendahmane, A., Budar, F., and Mireau, H. 2008. Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 20: 3331–3345.
- Wan, C., Li, S., Wen, L., Kong, J., Wang, K., and Zhu, Y. 2007. Damage of oxidative stress on mitochondria during microspores development in Honglian CMS line of rice. Plant Cell Rep. 26: 373–382.
- Wang, D., Rousseau-Gueutin, M., and Timmis, J. N. 2012. Plastid sequences contribute to some plant mitochondrial genes. Mol. Biol. Evol. 29: 1707–1711.
- Wang, D., Wu, Y. W., Shih, A. C., Wu, C. S., Wang, Y. N., and Chaw, S. M. 2007. Transfer of chloroplast genomic DNA to mitochondrial genome occurred at least 300 MYA. Mol. Biol. Evol. 24: 2040–2048.
- Wang, J., Cao, M. J., Pan, G. T., Lu, Y. L., and Rong, T. Z. 2009. RNA editing of mitochondrial functional genes atp6 and cox2 in maize (Zea mays L.). Mitochondrion 9: 364–369.
- Wang, J. W., Wang, X. L., Xu, H., Tang, H. W., Zhang, G. S., and Liu, Y. G. 2013a. Structural and expressional variation analyses of mitochondrial genomes reveal candidate transcripts for the S–V cytoplasmic male sterility in wheat (Triticum aestivum L.). J. Genet. Genomics 40: 437–439.
- Wang, J. Y., Zeng, L. H., Wang, S. Q., Wang, J., Lu, Y. L., Zhang, S. Z., Lan, H., and Cao, M. J. 2013b. Study on the expression of specific chimeric-orfs of C type cytoplasmic male sterile in maize. Isr J. Plant Sci. 61: 25–36.
- Wang, K., Gao, F., Ji, Y., Liu, Y., Dan, Z., Yang, P., Zhu, Y., and Li, S. 2013c. ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. New Phytol. 198: 408–418.
- Wang, S., Wang, C., Zhang, X. X., Chen, X., Liu, J. J., Jia, X. F., and Jia, S. Q. 2016. Transcriptome de novo assembly and analysis of differentially expressed genes related to cytoplasmic male sterility in cabbage. Plant Physiol. Biochem. 105: 224–232.
- Wang, Y., Chu, P., Yang, Q., Chang, S., Chen, J., Hu, M., and Guan, R. 2014. Complete mitochondrial genome of Eruca sativa Mill. (Garden Rocket). PLoS ONE 9: e105748.
- Wang, Z. H., Zou, Y. J., Li, X. Y., Zhang, Q. Y., Chen, L., Wu, H., Su, D. H., Chen, Y. L., Guo, J. X., Luo, D., Long, Y. M., Zhong, Y., and Liu, Y. G. 2006. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18: 676–687.
- Warmke, H. E. and Lee, S. L. 1978. Pollen abortion in T cytoplasmic male-sterile corn (Zea mays): a suggested mechanism. Science 200: 561–563.
- Wei, L., Yan, Z. X., and Ding, Y. 2008. Mitochondrial RNA editing of F0-ATPase subunit 9 gene (atp9) transcripts of Yunnan purple rice cytoplasmic male sterile line and its maintainer line. Acta Physiol. Planta. 30: 657–662.
- Wen, L. and Chase, C. D. 1999. Pleiotropic effects of a nuclear restorer-of-fertility locus on mitochondrial transcripts in male-fertile and S male-sterile maize. Curr. Genet. 35: 521–526.
- Wesołowski, W., Szklarczyk, M., Szalonek, M., and Słowińska, J. 2015. Analysis of the mitochondrial proteome in cytoplasmic male-sterile and male-fertile beets. J. Proteom. 119: 61–74.
- Wise, R. P., Fliss, A. E., Pring, D. R., and Gengenbach, B. G. 1987. urf13-T of T cytoplasm maize mitochondria encodes a 13 kD polypeptide. Plant Mol. Biol. 9: 121–126.
- Wolfe, K. H., Li, W. H., and Sharp, P. M. 1987. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl. Acad. Sci. U.S.A. 84: 9054–9058.
- Wu, Z., Cheng, J., Qin, C., Hu, Z., Yin, C., and Hu, K. 2013. Differential proteomic analysis of anthers between cytoplasmic male sterile and maintainer lines in Capsicum annuum L. Int. J. Mol. Sci. 14: 22982–22996.
- Yamamoto, M. P., Shinada, H., Onodera, Y., Komaki, C., Mikami, T., and Kubo, T. 2008. A male sterility-associated mitochondrial protein in wild beets causes pollen disruption in transgenic plants. Plant J. 54: 1027–1036.
- Yan, J., Tian, H., Wang, S., Shao, J., Zheng, Y., Zhang, H., Guo, L., and Ding, Y. 2014. Pollen developmental defects in ZD-CMS rice line explored by cytological, molecular and proteomic approaches. J. Proteom. 108: 110–123.
- Yan, J., Zhang, H., Zheng, Y., and Ding, Y. 2015. Comparative expression profiling of miRNAs between the cytoplasmic male sterile line MeixiangA and its maintainer line MeixiangB during rice anther development. Planta 241: 109–123.
- Yang, J., Liu, X., Xu, B., Zhao, N., Yang, X., and Zhang, M. 2013. Identification of miRNAs and their targets using high-throughput sequencing and degradome analysis in cytoplasmic male-sterile and its maintainer fertile lines of Brassica juncea. BMC Genom. 14: 9.
- Yang, J. H., Huai, Y., and Zhang, M. F. 2009. Mitochondrial atpA gene is altered in a new orf220-type cytoplasmic male-sterile line of stem mustard (Brassica juncea). Mol. Biol. Rep. 36: 273–280.
- Yang, P., Han, J., and Huang, J. 2014. Transcriptome sequencing and de novo analysis of cytoplasmic male sterility and maintenance in JA-CMS cotton. PLoS ONE 9: e112320.
- Yi, P., Huang, J. Y., Liu, Y., and Zhu, Y. G. 2004. Research progress of RNA editing in higher plant mitochondria. Acta Agron. Sin. 30: 735–738.
- Yu, Y. S., Rambo, T., Currie, J., Saski, C., Kim, H. R., Collura, K., Thompson, S., Simmons, J., Yang, T. J., Nah, G., Patel, A. J., Thurmond, S., Henry, D., Oates, R., Palmer, M., Pries, G., Gibson, J., Anderson, H., Paradkar, M., Crane, L., Dale, J., Carver, M. B., Wood, T., Frisch, D., Engler, F., Soderlund, C., Palmer, L. E., Tetylman, L., Nascimento, L., de la Bastide, M., Spiegel, L., Ware, D., O'Shaughnessy, A., Dike, S., Dedhia, N., Preston, R., Huang, E., Ferraro, K., Kuit, K., Miller, B., Zutavern, T., Katzenberger, F., Muller, S., Balija, V., Martienssen, R. A., Stein, L., Minx, P., Johnson, D., Cordum, H., Mardis, E., Cheng, Z. K., Jiang, J. M., Wilson, R., McCombie, W. R., Wing, R. A., Yuan, Q. P., Shu, O. Y., Liu, J., Jones, K. M., Gansberger, K., Moffat, K., Hill, J., Tsitrin, T., Overton, L., Bera, J., Kim, M., Jin, S. H., Tallon, L., Ciecko, A., Pai, G., Van Aken, S., Utterback, T., Reidmuller, S., Bormann, J., Feldblyum, T., Hsiao, J., Zismann, V., Blunt, S., de Vazeilles, A., Shaffer, T., Koo, H., Suh, B., Yang, Q., Haas, B., Peterson, J., Pertea, M., Volfovsky, N., Wortman, J., White, O., Salzberg, S. L., Fraser, C. M., Buell, C. R., Messing, J., Song, R. T., Fuks, G., Llaca, V., Kovchak, S., Young, S., Bowers, J. E., Paterson, A. H., Johns, M. A., Mao, L., Pan, H. Q., Dean, R. A., and Cons, R. C. S. 2003. In-depth view of structure, activity, and evolution of rice chromosome 10. Science 300: 1566–1569.
- Yuan, Z. Q. and Yang, J. S. 1999. Interaction between mitochondria and nucleus. Hereditas 21: 54–86.
- Zaegel, V., Guermann, B., Le Ret, M., Andres, C., Meyer, D., Erhardt, M., Canaday, J., Gualberto, J. M., and Imbault, P. 2006. The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis. Plant Cell 18: 3548–3563.
- Zhang, H., Li, S., Yi, P., Wan, C., Chen, Z., and Zhu, Y. 2007. A Honglian CMS line of rice displays aberrant F0 of F0F1-ATPase. Plant Cell Rep. 26: 1065–1071.
- Zhang, W., Xie, Y., Xu, L., Wang, Y., Zhu, X. W., Wang, R. H., Zhang, Y., Muleke, E. M., and Liu, L. W. 2016. Identification of microRNAs and their target genes explores miRNA-mediated regulatory network of cytoplasmic male sterility occurrence during anther development in radish (Raphanus sativus L.). Front Plant Sci. 7: 1054.