Abstract
The brown algae (Phaeophyceae) are a group of multicellular heterokonts that are ubiquitous in today’s oceans. Large brown algae from multiple orders are the foundation to temperate coastal ecosystems globally, a role that extends into arctic and tropical regions, providing services indirectly through increased coastal productivity and habitat provisioning, and directly as a source of food and commercially important extracts. Recent multi-locus and genome-scale analyses have revolutionized our understanding of the brown algal phylogeny, providing a robust framework to test evolutionary hypotheses and interpret genomic variation across diverse brown algal lineages. Here, we review recent developments in our understanding of brown algal evolution based on modern advances in phylogenetics and functional genomics. We begin by summarizing modern phylogenetic hypotheses, illuminating the timescales over which the various brown algal orders diversified. We then discuss key insights on our understanding of brown algal life cycle variation and sexual reproduction systems derived from modern genomic techniques. We also review brown algal speciation mechanisms and the associated biogeographic patterns that have emerged globally. We conclude our review by discussing promising avenues for future research opened by genomic datasets, directions that are expected to reveal critical insights into brown algal evolution in past, present, and future oceans.
I. The nature and origin of brown algae
The brown algae (Phaeophyceae) comprise approximately 2000 described species, and are one of few eukaryotic lineages to have evolved complex multicellularity (Charrier et al., Citation2008; Knoll, Citation2011; Cock et al., Citation2014). Along with other multicellular groups such as metazoans, fungi and green plants, brown algae possess several key characteristics that have enabled them to thrive as macroscopic organisms (Charrier et al., Citation2008), including cell-to-cell adhesion and communication (Charrier et al., Citation2008; Cock et al., Citation2010; Citation2014; Deniaud-Bouët et al., Citation2014), tissue differentiation (Fritsch, Citation1935; Kloareg and Quatrano, Citation1988), internal transport of sugars (Fritsch, Citation1935; Schmitz and Srivastava, Citation1976) and the capacity for three dimensional growth (Fritsch, Citation1935; Starko and Martone, Citation2016a). These features have contributed to the emergence and diversification of the world’s largest marine autotrophs (e.g. Laminariales, Fucales) besides clonal plants (e.g. Arnaud-Haond et al., Citation2012), and have restructured the dynamics of coastal marine ecosystems around the world (Steinberg et al., Citation1995; Steneck et al., Citation2002; Pyenson and Vermeij, Citation2016; Starko et al., Citation2019; Vermeij et al., Citation2019). Brown algae also exhibit striking morphological variation across species, differing substantially in their level of complexity at the levels of cells, tissues and organs (Fritsch, Citation1935). A thorough understanding of brown algal evolution and systematics is essential for disentangling the processes underlying the evolution of complexity in this group and its implications for coastal ecosystems globally.
Brown algae play fundamental roles in the functioning of coastal marine ecosystems. Large brown algae, particularly those in the orders Laminariales, Tilopteridales, Fucales, and Desmarestiales, act as ecosystem engineers (Bruno and Bertness, Citation2001; Schiel and Foster, Citation2006; Mineur et al., Citation2015, Teagle et al., Citation2017) and are dominant members of intertidal and shallow subtidal ecosystems worldwide (Steneck et al., Citation2002; Schiel and Foster, Citation2006; Teagle et al., Citation2017). Large brown algae form complex underwater forests that dramatically increase the structural complexity of marine ecosystems (Steneck et al., Citation2002; Teagle et al., Citation2017) and alter environmental factors such as light (Gerard, Citation1984; Connell, Citation2003a; Gattuso et al., Citation2006), fluid dynamics (Hurd and Stevens, Citation1997; Stephens and Hepburn, Citation2014), sedimentation (Connell, Citation2003b; Filbee-Dexter et al., Citation2016) and food availability (Duggins et al., Citation1989; Estes et al., Citation2016). Large brown algae also provide habitat for a wide range of other taxa (Steneck et al., Citation2002; Graham, Citation2004; Teagle et al., Citation2017; Hind et al., Citation2019), including many commercially important animals (Bologna and Steneck, Citation1993; Smale et al., Citation2013; Markel et al., Citation2017), and serve as essential nursery grounds for many species (Holbrook et al., Citation1990; Kitada et al., Citation2019). Besides habitat provision, brown algae are a key source of productivity along the coast (Mann, Citation1973; Pfister et al., Citation2019) and can significantly increase secondary productivity in nearshore ecosystems through direct herbivory and increased detrital production (Duggins et al., Citation1989; Krumhansl and Scheibling, Citation2012). This energy input plays an important role in maintaining food security for many large mammals (Estes et al., Citation2016; Pyenson and Vermeij, Citation2016; Vermeij et al., Citation2019), including humans, and is believed to have facilitated the spread of human populations from Asia to North America prior to the Holocene, the so-called “kelp highway” hypothesis (Erlandson et al., Citation2015; Braje et al., Citation2017). As humanity further ventures into the Anthropocene, brown algae are becoming key players in ocean-based strategies for combating climate change given their role in sequestering carbon (Krause-Jensen and Duarte Citation2016; Krause-Jensen et al., Citation2018; Hoegh-Guldberg et al., Citation2019). A new frontier of “charismatic carbon” in the form of seaweed farming could regionally offset carbon emissions from agriculture and provide additional benefits by restoring coastal habitats and alleviating ocean acidity (Froehlich et al., Citation2019). Altogether, the ecosystem services provided by brown algal forests are conservatively estimated to value USD $500,000–1,000,000 per year per km of coastline (Filbee-Dexter and Wernberg, Citation2018). Given that forests of large brown algae dominate approximately 25% of the world’s coastlines (Wernberg et al., Citation2019), the global value of ecosystem services provided by brown algae is likely to be in the hundreds of billions of USD per year.
In addition to the indirect benefits that they provide to humans by maintaining ecosystem functioning in nearshore marine environments, brown algae hold direct economic value through food harvests and commercial extracts (Mautner, Citation1954; Vásquez et al., Citation2014; Bennett et al., Citation2016; Milledge et al., Citation2016). Brown algae have long been used as a food source by human communities with coastal access (Tseng, Citation1981; Druehl, Citation1988; McHugh, Citation2003). Today, brown algae are harvested from the wild and through aquaculture operations around the world (Fleurence et al., Citation2012; Charrier et al., Citation2017; Bennion et al., Citation2019). The global harvest of brown macroalgae from wild stocks is estimated at more than half a million tonnes per year and has been increasing in recent decades (Mac Monagail et al., Citation2017). The polysaccharide metabolism of brown algae is unique among photoautotrophs (including red and green algae) and many of these polysaccharides are desirable for their bioactive properties. For example, fucose-containing sulfated polysaccharides (FCSPs), found in the cell wall and extracellular matrices of brown algae (Deniaud-Bouet et al., 2014; Kloareg and Quatrano, Citation1988), can have anti-inflammatory, anti-viral, anti-biotic, anti-oxidant, anti-coagulant, and anti-adhesive properties (Li et al., Citation2008; Morya et al., Citation2012), and are widely used in medicine and cosmetics (Li et al., Citation2008; Fitton, Citation2011). Alginates, carbohydrate polymers made up of mannuronic and guluronic acids (Kloareg and Quatrano, Citation1988) act as gelling agents in food products, have medical and commercial applications as absorbents (Skaugrud et al., Citation1999; Lee and Mooney, Citation2012), and serve as the basis of macroalgal bio-fuel development (Wargacki et al., Citation2012). Brown algae also concentrate halogens, such as iodine, as a means of coping with various forms of stress (e.g. heat stress, ultraviolet radiation, herbivory, and oxidative stress; La Barre et al., Citation2010). As a result of micronutrient sequestration, brown algae are harvested and sold commercially as dietary supplements, both for human consumption (Fitton, Citation2003; Leblanc et al., Citation2006), and for animal feed (Øverland et al., Citation2019; Pereira et al., Citation2019).
In the broader context of eukaryotic evolution, brown algae originated within the heterokonts (i.e. stramenopiles), one of four major groups in the eukaryotic lineage containing Telonemea, Stramenopiles, Alveolata, and Rhizaria (TSAR lineage; Burki et al., Citation2019). The photosynthetic heterokonts share an ancestral endosymbiotic event of phagocytosis of a red alga, giving rise to their plastids (Keeling, Citation2013), and are part of the Ochrophyta, a lineage of mainly unicellular and mostly photosynthetic lineages including diatoms, chrysophytes, synurophytes, xanthophytes and many less well-known groups (). While the relationships among many classes of Ochrophyta remain unresolved, three main groups (SI, SII, SIII) are supported in most phylogenies (). The brown algae are situated within lineage SI, as part of a radiation of classes during the late Paleozoic (∼310 Ma; asterisk in ). However, phylogenetic analyses have struggled to resolve the SI lineage further due to insufficient power of the selected genetic markers (e.g. Yang et al., Citation2012, Wetherbee et al., Citation2019). Moreover, genome-scale studies have not included many of the lesser-known classes (e.g. Derelle et al., Citation2016; Kim et al., Citation2019; Thakur et al., Citation2019). Multicellularity in the Ochrophyta is not exclusive to the brown algae, with several classes in the clade SI-radiation having at least some simple multicellular representatives (e.g. Phaeothamniophyceae) or macroscopic siphonous species (e.g. Vaucheria and Botrydium in the Xanthophyceae). There is still considerable debate about the evolutionary processes that underlie transitions to multicellularity but several possible advantages have been proposed, including reduced predation due to increased size, increased production of reproductive cells for dispersal, and efficient allocation of distinct biological functions to different specialized cell types. The class Schizocladiophyceae includes only one species, Schizocladia ischiensis, which forms small filaments. Schizocladiophyceae is the sister lineage to brown algae, suggesting that simple multicellularity was already present in the Paleozoic ancestor of the brown algae (Brown and Sorhannus, Citation2010; CAP in ).
Figure 1. Summary diagram of phylogenetic relationships among the classes of photosynthetic heterokonts, derived from results presented in Kawai et al. (Citation2003), Yang et al. (Citation2012), Derelle et al. (Citation2016), Han et al. (Citation2019), Kim et al. (Citation2019), Thakur et al. (Citation2019) and Wetherbee et al. (Citation2019). The asterisk represents the ancestral node of the majority of the SI clade, dated to the late Paleozoic (∼310 Ma; Brown and Sorhannus, Citation2010). The species counts given in parentheses after each class are from AlgaeBase (Guiry and Guiry, Citation2020).
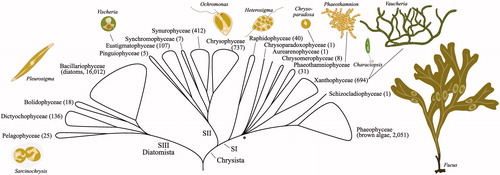
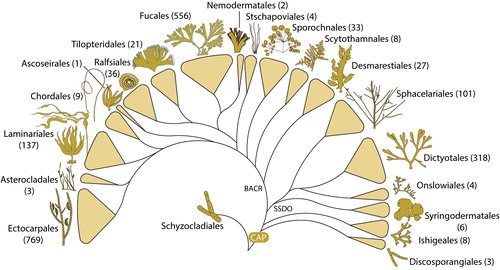
Figure 2. Phylogeny of the brown algal orders based on 12 markers (18S, 5.8S, 28S, atpB, psbA, psaB, psaA, rbcL, psbC, cox1, cox3, nad1). In brackets are the numbers of species within each order according to AlgaeBase (Guiry and Guiry, Citation2020). CAP: common ancestor of Phaeophyceae; SSDO: Sphacelariales, Syringodermatales, Dictyotales, Onslowiales clade; BACR: brown algal crown radiation.
Brown algal classification schemes have traditionally relied on a combination of thallus morphology, life history traits, types of spores and gametes, and cytoskeletal characteristics (reviewed by de Reviers et al., Citation2007). DNA sequencing dramatically altered our view of brown algal relationships and the evolution of traits. The earliest phylogenetic studies were limited by the coarse resolution of chosen markers (i.e. 18S; Tan and Druehl, Citation1993), but further work has dramatically enhanced our knowledge of brown algal systematics by including multiple markers, time calibrated phylogenies (Silberfeld et al., Citation2010; Martin and Zuccarello, Citation2012; Starko et al., Citation2019; Yip et al., Citation2020), and, more recently, genome-scale datasets for some brown algal groups (Jackson et al., Citation2017; Starko et al., Citation2019).
Given the socio-ecological importance of brown algae, their relevance to key evolutionary transitions and processes, and the considerable new insight being shed on brown algae today, we aim to review contemporary knowledge of brown algal evolution. In this review paper, we provide the latest interpretation of the phylogenetic relationships of brown algal lineages derived from molecular data, summarize new insights on the evolution of life history traits, and provide an up-to-date overview of biogeography and mechanisms promoting reproductive isolation. We conclude our review by offering perspectives on promising avenues for further understanding brown algal evolution.
II. Phylogenetic history of the brown algae
Molecular phylogenies overturned the traditional, 20th century view of brown algal classification, which had been based on a combination of life cycle structure, thallus architecture and gametic traits. For example, the widely held hypothesis that the morphologically more complex orders had diverged from filamentous Ectocarpales early in the diversification of the brown algae was confidently rejected by phylogenetic evidence. Instead, the Ectocarpales were close relatives of one the most morphologically complex groups of brown algae, the Laminariales. Ancestral state reconstructions based on molecular phylogenies indicate that parenchymatous growth has probably reverted to filamentous growth multiple times (Silberfeld et al., Citation2010). Likewise, life history traits and gametic differentiation display complex evolutionary patterns with transitions from isogamy through anisogamy to oogamy having occurred several times independently (Silberfeld et al., Citation2010), the genetic underpinnings of which have only recently been described (see life history traits, section III.B). Such pliability means molecular data has been instrumental in confidently defining brown algal relationships. In this section, we review the brown algal orders, characteristic features within the groups, and provide information on evolutionary events based on molecular evidence, where available.
Molecular data have clearly shown that the overall phylogenetic structure of the brown algae includes two orders that resulted from early divergence events (), a large clade composed of four orders (Sphacelariales, Syringodermatales, Dictyotales, Onslowiales, coined the SSDO clade), and a large and initially poorly resolved radiation comprising all remaining brown algal orders and referred to as the brown algal crown radiation (BACR). Increased gene-sampling has significantly improved the resolution of the BACR and has created a robust phylogenetic framework to interpret brown algal evolution, though the precise affinities among a small number of lineages remain unresolved.
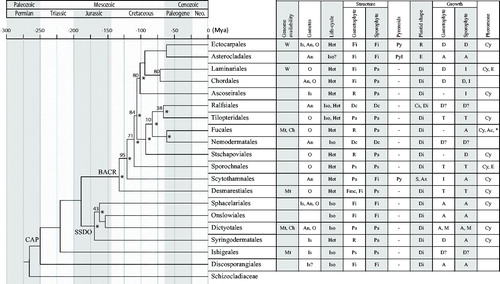
Calibrated phylogenies, although admittedly based on limited fossil evidence, have also provided insight into the timescales of brown algal evolution. Fossilization of brown algae is rare, especially given hard bodied lineages are known in only two extant genera (Newhousia and Padina, the latter of which deposits a thin layer of aragonite on the surface of the thallus only: Miyata et al., Citation1977; Kraft et al., Citation2004). Red and green macroalgal lineages were also present alongside the brown algae, leading to confusion and debate as to how to classify fossil specimens with simple and convergent features. For example, Upper Devonian species Drydenia foliata, Hungerfordia dichotoma, and Enfieldia mutilata (380–360 Ma; Fry and Banks, Citation1955), and the Late Pennsylvanian-Early Permian genus Perissothallus (300 Ma; Krings et al., Citation2007) are fossil specimens variously linked to extant brown, green, and red algal species. Only a few fossils have been assigned to brown algal lineages with enough confidence to time-calibrate phylogenetic trees (; Silberfeld et al., Citation2010). The oldest is a species preserved in Early Cretaceous (145–100 Ma) clay shales of the Gangapur Formation in India. The specimen displays a fan-shaped thallus and a zonation pattern consistent with the extant genus Padina (Rajanikanth, Citation1989). Miocene deposits (dated to 13–17 Ma) from the Monterey Formation in California offer three more fossilized brown algae: Julescraneia grandicornis, an extinct species intermediate in morphology between the extant laminarialean genera, Pelagophycus and Nereocystis, and the extinct genera Paleocystophora and Paleohalidrys, the root names of which are derived from extant genera of the Sargassaceae that display a characteristic sympodial branching pattern (Parker and Dawson, Citation1965). Though earlier brown algal fossils have been proposed (citations above), the fossil evidence used to calibrate phylogenetic trees suggests the brown algal orders diversified almost entirely within the Mesozoic Era (252–66 Ma), surviving the cataclysm that claimed the Dinosaurs.
Figure 3. Time calibrated maximum likelihood phylogeny of the brown algae, using the following fossil evidence: Paleocystophora (Fucales) and Julescraneia grandicornis (Laminariales) from the Monterey Formation Miocene deposits (13–17 Ma; Parker and Dawson, Citation1965), and Padina-like species (Dictyotales) from the Gangapur formation, Early Cretaceous (145.5–99.6 Ma; Rajanikanth, Citation1989). Nodes lacking bootstrap values are fully supported for both maximum likelihood and bayesian phylogenetic trees. Topological conflicts with bayesian trees are indicated by an *. W: Whole; Mt: Mitochondrion; Ch: Chloroplast; Nu: Nuclear; Is: Isogamous; An: Anisogamous; O: Oogamous; Het: Heteromorphic; Iso: Isomorphic; Fmc: Few microscopic cells; Fi: Filamentous; Ps: Pseudoparenchymatous (haplostichous); Pa: Parenchymatous (polystichous); R: reduced; Dc: Pseudoparenchymatous discoid; Py: Pyrenoids without invaginations; PyI: Pyrenoids with invaginations; A: Apical; D: Diffuse; I: Intercalary; M: Marginal; T: Trichothallic; R: Ribbon-shaped; E: Elongated; Di: Discoid; S: Stellate; Ax: Axial; Cs: Cap-shaped; Cy: Cyclic hydrocarbons; Ac: Acyclic hydrocarbons; Ep: Epoxyde; *C11 and C8-olefins. Empty cells: no data available; ?: doubtful identification, –: absence of trait; CAP: common ancestor of Phaeophyceae; SSDO: Sphacelariales, Syringodermatales, Dictyotales, Onslowiales clade; BACR: brown algal crown radiation.
A. Early divergence events
The orders Discosporangiales and Ishigeales branched from the other brown algal lineages early in the evolutionary history of the brown algae sometime at the beginning of the Mesozoic Era (∼250 Ma; ). These two orders only contain a total of 11 recorded species (Guiry and Guiry, Citation2020) but differ markedly from other brown algae. Discosporangiales exhibit uniseriate, branched filaments with apical meristematic cells, but lack the heterotrichous growth pattern (prostrate and upright thalli projections) common in many other brown algal orders. Discosporangium mesarthrocarpum also features unique disc-shaped reproductive organs. Traditionally, Discosporangium and Choristocarpus were treated as members of the Sphacelariales on the basis of their apical growth, but molecular phylogenies have indicated that these genera are from a distinct order (Draisma et al., Citation2001; Burrowes et al., Citation2003; Kawai et al., Citation2007), leading to the reinstatement of Discosporangiales as originally proposed by Schmidt (Citation1937) and the inclusion of Choristocarpaceae in the order (Kawai et al., Citation2007).
The Ishigeales include branched, upright or flattened parenchymatous thalli up to 10–20 cm high. The genus Ishige was traditionally classified in the defunct order Chordariales (now Chordariaceae within Ectocarpales), however, its higher rank taxonomy was controversial given the lack of important features such as pyrenoids in the chloroplast (Hori, Citation1971) and a heteromorphic life history (Hori, Citation1993). Phylogenetic analyses revealed its distinct position apart from most of the brown algae (Tan and Druehl, Citation1994; Peters and Ramírez, Citation2001), leading to creation of a new order Ishigeales (Cho et al., Citation2004).
B. Mid-Mesozoic diversification
Sometime during the Mid-Mesozoic (approximate timeframe for the Jurassic period, 200–145 Ma; ), the SSDO clade split from the lineage that gave rise to the remaining extant brown algal orders and diversified into what are now four orders: Sphacelariales, Syringodermatales, Dictyotales, and Onslowiales. Of these lineages, the most prominent is the Dictyotales, which currently encompasses a great deal of brown algal species diversity (318; ). The success of Dictyotales can be partially attributed to their affinity for tropical climates, a biome largely uninhabited by the other brown algal orders (except for the tropical fucoids, see tropical biogeography, subsection IV.B.3). Molecular work has revealed remarkable diversity concentrated in a few genera, including Dictyota (Bittner et al., Citation2008; Tronholm et al., Citation2010), Lobophora (Sun et al., Citation2012; Vieira et al., Citation2016; Camacho et al., Citation2019), and Padina (Ni-Ni-Win et al., Citation2008; Citation2010; Citation2012; Citation2018; Ni-Ni-Win, Arai, et al., Citation2011; Ni-Ni-Win, Draisma, et al., Citation2011). All Dictyotales are characterized by an isomorphic alternation of generations, with parenchymatous sporophyte and gametophyte thalli. Two tribes are recognized based on either having a single apical cell (Dictyoteae; De Clerck et al., Citation2006; Bittner et al., Citation2008) or having many localized or marginal apical cells forming dichotomously branched or fan-shaped thalli (Zonarieae). Dictyotales are also unique among brown algae in that they include calcified taxa (i.e. Padina and Newhousia; Kraft et al., Citation2004), a feature that may have facilitated their presence in the fossil record (Rajanikanth, Citation1989). Meiosis typically produces four nonmotile spores that produce the isomorphic gametophyte. Sexual reproduction is oogamous and the sperm have only an anterior flagellum, possessing, however, a second flagellar basal body (Manton, Citation1959), with the exception of several species of Zonaria which are reported to have two flagella (Phillips and Clayton, Citation1991; Citation1993; Phillips Citation1997).
Sphacelariales has been characterized by a thallus structure in which branched filaments grow from a conspicuous parenchymatous, terete thallus (Prud'homme van Reine, Citation1982; 1993). They display isomorphic life histories with various types of sexual reproduction including isogamy, anisogamy and oogamy (). The taxonomy of Sphacelariales was revised considerably by Draisma, Prud’homme van Reine, et al., (Citation2010), broadening the description of the order to include foliose and crustose taxa (Kawai et al., Citation2005; Kawai, Hanyuda, Draisma, et al., Citation2015; Silberfeld et al., Citation2014). Likely owing to the diminutive character of the Sphacelariales, considerable genetic diversity discordant with recorded morphospecies has been revealed in some locations (e.g. Northeast Pacific; Chan, Citation2018). Onslowiales is a small order comprising two genera which were traditionally classified in Sphacelariales. Members of the Syringodermatales have fan-shaped thalli that develop by lateral cohesion of filaments arising from a marginal meristem. The life history patterns are remarkably divergent within the order’s single genus Microzonia (Camacho et al., Citation2018), with gametophytes being either filamentous or reduced to only 4 or 2 cells (Henry and Müller, Citation1983; Henry, Citation1984; Kawai and Yamada, Citation1990).
C. The brown algal crown radiation
The remaining brown algal orders form a conspicuous clade that radiated throughout the Cretaceous period (145–66 Ma), the BACR (). The BACR contains the most ecologically and economically important orders, including the Fucales and Laminariales. Silberfeld et al., (Citation2010) suggested that the BACR resulted from recovery following an extinction event, potentially linked with volcanic activity and subsequent oxygen depletion in earth’s oceans 129–134 Ma (though dysoxia has not been confirmed during this event; Peate, Citation1997). Hypotheses explaining diversification of the BACR at the ordinal level are otherwise scant. Ancestral state reconstructions provided by Silberfeld et al., (Citation2010) suggest that the ancestor to the BACR had a heteromorphic life history, oogamous fertilization, intercalary growth of pseudoparenchymatous tissue, and chloroplasts containing several pyrenoids. Orders have variously reverted back to isomorphic life histories and terminal growth, and adoption of anisogamous or isogamous fertilization. Parenchymatous tissue and reductions to single plastids have also evolved independently multiple times. Such pliability in key features remains a mystery within the brown algae, and the genetic underpinnings of such dynamic evolutionary events are only beginning to be worked out (see life history traits, section III.B).
Desmarestiales and Scytothamnales represent the earliest branching orders within the BACR (approximately 125 Ma), and together account for only 35 described species (primarily within the genus Desmarestia). Members of Desmarestiales are globally distributed but are hypothesized to have evolved in the Southern Hemisphere, where they are a prominent member of Antarctic assemblages (Peters et al., Citation1997). Members of Scytothamnales are predominantly known from temperate to cold water regions of the Southern Hemisphere, with two globally distributed tropical species. Desmarestiales develop a pseudoparenchymatous thallus through apical meristems and are notable for the ability of some members to accumulate sulfuric acid within cells, which is interpreted as an anti-herbivory defense mechanism (Pelletreau and Muller-Parker, Citation2002). Scytothamnales features branched, filamentous or terete, parenchymatous species (Tanaka et al., Citation2007).
The remaining taxa of the BACR are classified into 12 orders, which vary dramatically in external morphology, cytology, and life history traits. Ascoseirales is a monotypic order featuring the Antarctic species Ascoseira mirabilis, which forms a large parenchymatous thallus with intercalary growth, holdfast and stipe, and is characterized by a diplontic life cycle, a feature that appears in only one other brown algal order, the Fucales. Reproductive structures in Ascoseira are borne in conceptacles that produce chains of large cells containing eight isogamous, flagellate gametes (Moe and Henry, Citation1982; Clayton, Citation1987), while unfused gametes may also develop into sporophytes (i.e. parthenogenesis/antherogenesis).
Fucales is a large order of more than 500 species and 9 families, members of which are major components of coastal ecosystems globally, including cold water regions of the Northern (Fucus, Ascophyllum, Pelvetiopsis, Silvetia, etc.) and Southern Hemisphere (Durvillaea, Cystophora, etc.), as well as in warm temperate to tropical coastal ecosystems (Cystoseira s.l., Sargassum, Turbinaria, etc.). Diversity is highly skewed toward Sargassaceae, which comprises ca. 30 genera and over 90% of described species. Fucaceae and Seirococcaceae comprise 5 genera each and ca. 28 species, whereas the remaining 6 families are monospecific or monogeneric and together comprise no more than 15 species. Erect thalli are parenchymatous, often with differentiation between holdfast, stipe and branches (terete or leafy), and pneumatocysts (air bladders) that provide buoyancy (). Growth results from the division of apical cells and cell division in associated meristematic regions except for Durvillaea and Notheia. The life cycles of Fucales are oogamous with oogonia and spermatangia borne on specialized branches known as receptacles. Gametophytic stages are highly reduced and retained in the conceptacles in the sporophytic thallus, so that plants superficially regenerate only from sporophytic thalli. Unfertilized eggs are incapable of developing parthenogenetically. The phylogeny and diversification of Fucales has been the subject of several studies (e.g. Serrão et al., Citation1999; Coyer et al., Citation2006; Fraser et al., Citation2010; Draisma, Ballesteros, et al., Citation2010; Cánovas et al., Citation2011; Bruno de Sousa et al., Citation2019; Yip et al., Citation2020).
The sister orders Ectocarpales and Asterocladales emerged late within the BACR, close to the end of the Cretaceous period (66 Ma), with markedly different outcomes in species diversity. Asterocladales comprise a single genus Asterocladon, which is distributed from tropical to temperate coasts. Ectocarpales, on the other hand, is the most speciose brown algal order, with more than 750 species in more than 100 genera and 5 to 6 families, though the taxonomy at the genus and family rank is far from resolved. Traditionally, four orders were recognized based on a combination of two characters: thallus construction and chloroplast morphology (i.e. Ectocarpales s.s., Chordariales, Dictyosiphonales and Scytosiphonales). However, the presence of intermediate forms and subsequent phylogenetic work led to the collapse of these orders into Ectocarpales s.l. New families were also established, such as Adenocystaceae (Rousseau et al., Citation2000) and Petrospongiaceae (Racault et al., Citation2009), or reinstated (e.g. Acinetosporaceae, Peters and Ramírez, Citation2001). The phylogenetic resolution of the genetic markers used for these revisions was limited, however, and phenotypic characters for defining these families remain scarce. Ectocarpus was the first brown alga to have a fully annotated genome, and insights into brown algal evolution continue to develop from this model organism (Cock et al., Citation2010; detailed in life history traits, section III). Among the key discoveries were genes potentially associated with multicellular development, a high proportion of introns (40.4% of the genome), an integrated viral genome, red algal genes derived from the secondary endosymbiosis event that initiated the divergence of the Ochrophyta, and insights into various metabolic functions as compared to other photosynthetic lineages (Cock et al., Citation2012).
Laminariales was also late to emerge in the BACR, branching from its sister Chordales in the early Cenozoic. Members of the Laminariales are often referred to as “kelps,” although debate remains about whether this is a taxonomic or functional term, as some large brown algae from other orders are also commonly referred to as kelp (see Fraser, Citation2012, for a review of the debate). Today, the Laminariales includes the largest marine macroalgae, and often form large ‘kelp forests,’ which provide habitats for a wide range of other taxa. The largest among them, Macrocystis, can achieve lengths exceeding 50 m and is one of the fastest growing organisms on the planet. Although the Laminariales are most common in cold and temperate waters (Krumhansl et al., Citation2016), they also grow in tropical waters where they are confined to deeper (colder) habitats (Graham et al., Citation2007). Laminariales are the most structurally complex macroalgae and possess significant cellular and tissue differentiation, including phloem-like structures that transport sugar throughout their large thalli and distinctive differentiation between stipe, blade and holdfast that may facilitate their ability to achieve large sizes in biomechanically challenging environments (Johnson and Koehl, Citation1994; Drobnitch et al., Citation2015; Starko and Martone, Citation2016a; Liggan and Martone, Citation2018). Kelp growth is mediated by an intercalary meristem that is in the transition between stipe and lamina, allowing the development of perennial sporophytes. The unique position of this intercalary meristem has been hypothesized to facilitate long term coexistence between laminarialean algae and surface-feeding herbivores (i.e. Vermeij et al., Citation2019).
Laminariales possess two distinctive generations: a large parenchymatous sporophyte that alternates with a microscopic, filamentous gametophyte stage. Evolutionary and ecological knowledge of gametophytes remains poor relative to the sporophyte stage, though sequence data have been used to detect gametophytes in situ (Fox and Swanson, Citation2007; Robuchon et al., Citation2014; Bringloe et al., Citation2018). These studies indicate gametophyte distributions are, at times, disjunct with the accompanying sporophytes, suggesting gametophytes could persist in locations where environmental conditions do not favor growth and survival of the sporophyte. During reproduction events, female gametophytes are known to produce lamoxirene, a gamete-attracting pheromone that also functions to stimulate the synchronized release of sperm from antheridia (Maier, Citation1995).
A number of phylogenetic studies based on increasingly large datasets have gradually resolved relationships among species of the previously broader Laminariales, and revealed knowledge about their evolution (Lane et al., Citation2006; Kawai et al., Citation2008; Citation2013; Jackson et al., Citation2017; Starko et al., Citation2019). Phylogenomic analyses recently distinguished the Chordales (Starko et al., Citation2019), which were historically viewed as “simple” kelps with traits that were thought to be ancestral compared with the remaining (“complex”) kelp families. Ancestral state reconstruction subsequently revealed several characters unique to the Chordales (simple kelps), rather than ancestral to the Laminariales (complex kelps) as originally supposed. For instance, a hapteral (root-like), rather than a discoid, holdfast was likely the ancestral state to the Laminariales, and annual life histories have evolved multiple times throughout the order with a perennial life history featured in the ancestor. Complex traits such as a stiff stipe, tissue cavitation, and various forms of branching have also evolved independently across multiple lineages. Phylogenomic analyses have revealed that the crown age of the Laminariales (31.5 Ma) corresponded with the Eocene-Oligocene boundary. Laminariales are hypothesized to have expanded into niches that were opened by global cooling and mass extinction of marine life at this time (Ivany et al., Citation2000), leading to the accelerated diversification of this group. Their diversification predated the appearance of sea otters and various benthic feeding fauna, suggesting Laminariales provided an important food and habitat resource driving the evolution of marine faunal lineages in the North Pacific.
Chordales is a small order, sister to the Laminariales, with nine known species in three genera and three families distributed in temperate to cold-water regions of the Northern Hemisphere. Morphology and life history patterns are diverse among families, although all members show heteromorphic life histories with annual, large parenchymatous sporophytes and minute filamentous gametophytes. Akkesiphycus (Akkesiphycaceae) has fragile lanceolate sporophytes with diffuse growth, and sexually monomorphic dioecious gametophytes producing anisogamous gametes (Kawai, Citation1986; Kawai and Sasaki, Citation2000). Pseudochorda (Pseudochordaceae) has terete sporophytes without localized meristems and sexually monomorphic or dimorphic, filamentous, oogamous gametophytes (Kawai and Kurogi, Citation1985; Kawai and Nabata, Citation1990). Chorda (Chordaceae) has terete sporophytes with localized meristems and trumpet-shaped hyphae, and sexually dimorphic filamentous oogamous gametophytes (Kylin, Citation1933). Although traditionally only one species C. filum was recognized, molecular phylogenetic studies have revealed considerable species diversity, including four new species recently described from the Pacific and the Arctic regions (Kawai et al., Citation2019). In contrast to Laminariales, sex attractants have not been identified in Chordales.
The remaining BACR orders are Tilopteridales, Stschapoviales, Sporochnales, Ralfsiales, and Nemodermatales, and each further showcases the plasticity in morphology and life history traits of brown algae below the ordinal level. The thallus structures observed in the Tilopteridales are highly diverse across families. The family Tilopteridaceae includes three filamentous genera (South, Citation1975; Hooper et al., Citation1988) with nearly isomorphic life histories, whereas members of Cutleriaceae have heteromorphic life histories with terete or membranous gametophytes and crustose sporophytes (Fritsch, Citation1945); finally, Phyllariaceae is composed of genera forming large sporophytes that also form forests and microscopic gametophytes, both resembling Laminariales in external morphology. The order Stschapoviales is known only from cold water regions in the Northern Hemisphere, with the three monotypic genera Halosiphon (Halosiphonaceae), Platysiphon (Platysiphonaceae) and Stschapovia (Stschapoviaceae; Kawai and Sasaki, Citation2004; Kawai, Hanyuda, Draisma, et al., Citation2015; Kawai, Hanyuda, Yamagishi, et al., Citation2015). The thalli are terete with assimilatory filaments in whorls. Halosiphon shows a typical heteromorphic life history with large sporophytes and monoecious filamentous gametophytes. In contrast, Stschapovia and Platysiphon appear to show a modified life history without alternation between two different generations, as in Ascoseirales and Fucales. The order Sporochnales contains 33 species in 11 genera that are distributed in temperate to sub-tropical regions. Pseudoparenchymatous thalli are filamentous to terete, and macroscopic sporophytes alternate with minute, filamentous gametophytes producing eggs and sperm. Ralfsiales is composed of 36 species from 7 genera distributed from tropical to cold water regions. The order is primarily composed of species with crustose thalli, but some have terete erect thalli (Kawai, Citation1989), characterized by discoidal early development of the thallus, intercalary plurilocular gametangia with terminal cells, terminal unilocular zoidangia, and a crustose phase in the life history. Neoralfsiaceae (Lim et al., Citation2007) and Hapalospongidiaceae (León-Alvarez et al., Citation2017) are recent additions to the order. The order Nemodermatales consists of two crustose monotypic genera from temperate coasts, Nemoderma (Nemodermataceae) and Zeacarpa (Zeacarpaceae) (Phillips et al., Citation2008; Kawai et al., Citation2016).
III. Evolution of life history traits and sexual reproduction
The life cycle is a fundamental biological feature that influences the evolution of various traits including reproduction systems and modes of dispersion and must be taken into account to fully understand the biology of a species. Brown macroalgae exhibit a wide variety of life cycles, sexual systems, and reproductive modes (for a recent review see Liu et al., Citation2017). Their life cycles range from isomorphic haplodiplontic life cycles, in which both the gametophyte and sporophyte exhibit similar levels of multicellular development (e.g., Dictyota dichotoma), to diplontic life cycles, where only the diploid generation is multicellular (e.g., Fucus spp.). When gametophytes and sporophytes are morphologically different, the cycle is considered heteromorphic. In the brown algae, the diploid sporophyte is generally dominant (i.e., larger) compared to the haploid gametophyte, except in a few genera, such as Scytosiphon, where the haploid phase is a large, upright thallus and the diploid phase a prostrate crust (Heesch et al., Citation2019).
Phylogenies based on morphological and molecular characters suggest that the ancestral condition of brown algal sexual reproduction was haplodiplontic, with similar haploid and diploid phases (i.e., isomorphic; Fritsch, Citation1949; Henry, Citation1984; Clayton, Citation1988; Cho et al., Citation2004; Silberfeld et al., Citation2010; Heesch et al., Citation2019). Modifications of this isomorphic life cycle have occurred in several lineages, which involved either a reduction in size of the gametophyte generation (transition to a heteromorphic cycle, e.g., Syringodermatales, prior to the ancestor of the BACR) or loss of this haploid generation (transition to a diplontic life cycle, e.g., Ascoseirales, Fucales, genus Tilopteris in Tilopteridales; Silberfeld et al., Citation2010; Heesch et al., Citation2019). Transitions to diplontic cycles appear to have been irreversible, as there have been no transitions back to a haplodiplontic life cycle. In contrast, multiple transitions have occurred from heteromorphic to isomorphic life cycles (Silberfeld et al., Citation2010; Heesch et al., Citation2019). Analysis of the evolutionary processes driving these transitions remains a productive area of research for the brown algae. The annotated genome of Ectocarpus has also provided invaluable insights into the genes that regulate life history traits. In this section, we review emerging knowledge relating to life history traits in the brown algae, including the mechanisms underlying the maintenance of life cycle types, and the genes involved in alternating life history stages and sexual differentiation.
A. Evolutionary drivers of brown algal life cycles
Bell (Citation1982) remarked that “the casualness of the few attempts to provide a functional account of haploidy and diploidy constitutes a major scandal.” Since Bell’s comment, however, new theoretical models and experimental studies have emerged (Valero et al., Citation1992; Mable and Otto, Citation1998; Coelho et al., Citation2007). Masking of deleterious mutations and short-term benefits of diploidy compared with haploidy were the first explanations of dominance of the diploid phase in most plants and animals (Crow and Kimura, Citation1965). As most deleterious mutations are recessive (Manna et al., Citation2012), diploids (but not haploids) benefit from the short-term advantage of masking but they suffer from the long-term disadvantage of accumulating deleterious mutations in populations that ultimately reach the species genome (i.e., genetic load; Crow and Kimura, Citation1965). Later it was shown that the low level of genetic load in haploids, due to purging, can overcome this short-term advantage of diploids if there is strong linkage between the locus that determines life cycle structure (i.e., either a haplontic or diplontic life cycle) and the locus subject to deleterious mutations (Perrot et al., Citation1991; Otto and Marks, Citation1996).
In this context, transitions between haplontic and diplontic life cycles over evolutionary time have been interpreted as tradeoffs between short-term individual-level benefits due to masking (diploidy) and longer-term advantages of more efficient selection against deleterious mutations (haploidy). Specifically, haploidy could be favored if there is little mixing (i.e., crossing) with diploids (Otto and Marks, Citation1996). Recently, Heesch et al., (Citation2019) tested the prediction of Otto and Marks (Citation1996) that inbreeding or asexual reproduction favors haploid life cycles, extensively examining the correlation between the sexual system of a species (monoecious/dioecious) and the relative dominance (i.e., size) of the haploid and diploid phases of the life cycle for over 70 species of brown algae. This analysis supported the prediction that transitions toward dominance of the haploid phase would be more frequent when the sexual system was monoecious. Nevertheless, as having separate sexes is not always a good proxy for the mating system (Krueger-Hadfield et al., Citation2015), estimates of inbreeding coefficients within natural populations should be carried out to shed further light on the link between mating system and ploidy level (Heesch et al., Citation2019).
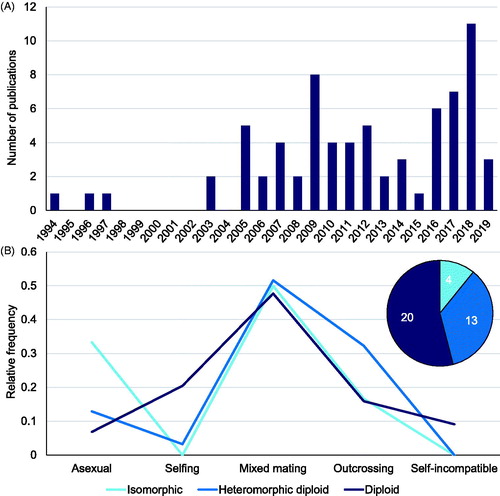
To further investigate the relationship between reproductive system and life cycle type in brown algae, we surveyed 177 peer-reviewed papers published between 1984 and 2019, 72 of which provided information about reproductive systems in 37 species of brown algae, of which 20 had a diplontic life cycle (e.g., Fucales), 13 were heteromorphic with a dominant diploid phase (12 Laminariales and 1 Tilopteridales) and only 4 exhibited an isomorphic life cycle (i.e., Dictyotales and Ectocarpales, but see Couceiro et al., Citation2015; see Supplemental Material for methods; ). This new literature survey indicated marked intraspecific reproductive system variability at the population level. Mating system variation within species has been widely reported in land plants, where it is due to environmental and genetic factors (Goodwillie et al., Citation2005). In particular, selfing (and asexual reproduction) may be favored in newly colonized sites or at range margins by providing reproductive assurance (Baker, Citation1955; Peck et al., Citation1998; Hargreaves et al., Citation2014). A textbook example of this phenomenon in the brown algae is Fucus vesiculosus, which shows contrasting reproductive systems in the Atlantic Ocean compared to the Baltic Sea, in that asexual reproduction becomes common at its ecological (Baltic Sea) margin (Tatarenkov et al., Citation2005) where sexual reproduction is impaired by salinity (Serrão et al., Citation1996). Other examples include the laminarialean kelp Laminaria digitata and the isomorphic Dictyota dichotoma, which both show increased asexual reproduction at the southern limit of their range distributions (Oppliger et al., Citation2014; Steen et al., Citation2019). The number of publications describing reproductive systems in brown algae has increased 20-fold since the Bell (Citation1997) and the Mable and Otto (Citation1998) studies (). The new literature survey only partially supported Otto and Marks' model (Citation1996). As expected, increased recombination (mixed mating systems and outcrossing) appeared to be linked to the dominant diploid phase (diplontic or species with a heteromorphic life cycle with diploid dominance); however, an increased degree of asexuality with haploid dominance was not consistently detected, as both asexual reproduction and mixed mating appeared to be prevalent in species with isomorphic life cycles (). These results may be attributed to the relatively low number of population genetics studies published in species characterized by a haploid dominant life cycle (see Supplemental Material). Moreover, no information for haplontic life cycles was available in the literature, likely owing to the rarity of these cycles among brown algae.
Figure 4. Literature survey of studies conducted prior to January 2020 that have addressed the relationship between mating system and life cycle type (dominant ploidy phase). (A) Number of publications concerning different reproductive systems over the last 25 years. (B) Relative frequencies of species showing different reproductive systems according to their life cycle. The pie chart represents the total number of studied species for each type of life cycle. Heteromorphic diploid refers to a diploid-dominant haplodiplontic life cycle. No heteromorphic haploid-dominant life cycle studies were found. Additional information can be found in the Supplemental Material.
Different models predict that differences in ecological niches, or differences in survival/fertility between haploid and diploid individuals, can play an important role in the evolution of life cycles, favoring the stable coexistence of haploid and diploid stages (Hughes and Otto, Citation1999; Rescan et al., Citation2016; Scott and Rescan, Citation2017). Differences in ecological niches between life cycle generations have been observed in many seaweed species, including brown algae with heteromorphic life cycles (Valero et al., Citation1992; Mable and Otto, Citation1998; Thornber and Gaines, Citation2004; Coelho et al., Citation2007). Few studies, however, have attempted to estimate differences in ecological niche or fitness between haploid and diploid phases in isomorphic haplodiplontic seaweeds (mainly in the red algae, Rhodophyta; Destombe et al., Citation1993; Pacheco-Ruiz et al., Citation2011). Demographic studies carried out on laminarialean kelp populations (heteromorphic haplodiplontic) have taken into account the fitness of individuals during the dominant diploid phase (sporophyte) in the field (Pereira et al., Citation2017), or during the haploid phase (gametophyte) under laboratory conditions (Pereira et al., Citation2011; Oppliger et al., Citation2012), but the fitness of haploids and diploids have not been compared directly, largely due to the challenges of studying the gametophyte in situ (Schiel and Foster, Citation2006) and cultivating large sporophytes in the lab.
Compounding the challenge of understanding fitness tradeoffs in brown algal life cycles, work on Ectocarpus spp. life cycles indicates there is potential for intraspecific variability of stage dominancy. Earlier work by Müller (Citation1964) produced Ectocarpus siliculosus gametophytes and sporophytes of similar size in culture, with sporophytes absent in field observations. However, the life cycle of this species can be rather heteromorphic in the field, as microscopic epilithic diploid sporophytes and macroscopic epiphytic gametophytes were reported growing on Scytosiphon in Naples (Couceiro et al., Citation2015). In contrast, the same species studied at another location, near Roscoff, had only diploid individuals, either epilithic or epiphytic, and reproducing clonally on various seaweeds (Couceiro et al., Citation2015). Thus, within-species variation for brown algal life cycles occurs in nature, as in red seaweeds (Destombe et al., Citation1989).
B. The genetic basis of life cycle alternation
With the recent emergence of the filamentous brown alga Ectocarpus as a model system for genetic and genomic analyses (Cock et al., Citation2011; Coelho et al., Citation2012; Brodie et al., Citation2017), it has become possible to investigate the genetic mechanisms underlying diverse aspects of brown algal biology, including the regulation of life cycle transitions. The recent advent of tools such as high-quality genome assembly (Cormier et al., Citation2017), high-density genetic maps (Heesch et al., Citation2010; Avia et al., Citation2017), extensive transcriptomic data, and cloning-by-sequencing methodologies (Godfroy et al., Citation2017) now make it possible to use the Ectocarpus model to identify genetic loci underlying phenotypic variation.
This forward genetic approach has been applied to the analysis of two Ectocarpus life cycle mutants, ouroboros (oro) and samsara (sam; Coelho et al., Citation2011; Arun et al., Citation2019). Ectocarpus has an isomorphic haplodiplontic life cycle, which involves alternation between two types of filamentous thallus corresponding to the sporophyte and gametophyte generations. Individuals that lack functional copies of either the ORO or the SAM gene are unable to deploy the sporophyte developmental program and, instead, develop as gametophytes. Genetic characterization of the ORO and SAM genes showed that they encode two different three amino acid loop extension homeodomain transcription factors (TALE HD TFs) (Arun et al., Citation2019). TALE HD TFs have been also implicated in life cycle regulation in the green lineage (Viridiplantae) in both green algal models and land plants. This similarity between life cycle regulators in the brown and green lineages suggests that they probably have common ancestry and are therefore derived from a regulatory system that already existed at the crown radiation of the eukaryotic supergroups (Arun et al., Citation2019). Given that mating type factors are thought to function primarily as detectors of syngamy, to initiate the diploid phase of the life cycle (Perrin, Citation2012), the hypothesis of a deep evolutionary origin of life cycle regulators is further supported by reports that distantly-related homeodomain or homeodomain-like proteins act as mating type factors in both fungi and social amebae (Nasmyth and Shore, Citation1987; Van Heeckeren et al., Citation1998; Hull et al., Citation2005; Hedgethorne et al., Citation2017). Consistent with the deep evolutionary history of this life cycle regulatory system, ORO and SAM orthologues were found in a broad range of brown algae (Arun et al., Citation2019). Other Ochrophyta lineages also possess TALE HD TFs but they are too divergent from the brown algal proteins to confidently identify them as ORO or SAM orthologues. Functional analysis of TALE HD TFs from other lineages will therefore be needed to further trace the detailed evolutionary history of the ORO and SAM genes within Ochrophyta.
Several hundred genes are differentially expressed between the sporophyte and gametophyte generations of the Ectocarpus life cycle (Coelho et al., Citation2011; Arun et al., Citation2019; Lipinska et al., Citation2019), indicating that ORO and SAM regulate a complex program of gene expression. These generation-biased genes are predicted to carry out diverse functions but, interestingly, there appears to be a correlation between enriched gene functions (gene ontology terms) and phenology. Analysis of the Ectocarpus life cycle in the field indicates that the sporophyte is probably the overwintering stage as it is present, often in microscopic form, for most of the year, whereas the gametophyte only appears in the spring for a limited period (Couceiro et al., Citation2015). These respective features of each generation are correlated with a general preponderance of metabolic genes upregulated in the sporophyte (i.e., survival and maintenance) compared to genes with roles in growth and cell division upregulated in the gametophyte (i.e., rapid seasonal growth; Coelho et al., Citation2011).
The developmental complexity of brown algae varies enormously. Moreover, because of the broad range of haplodiplontic life cycles in brown algae, the sporophyte and gametophyte generations can vary in terms of size, morphological complexity and ecological function. A recent analysis of generation-biased gene expression in four different brown algae with different life cycles and different levels of developmental complexity indicated that generation-biased gene sets turn over rapidly during evolution (Lipinska et al., Citation2019). Therefore, a picture is emerging of strongly conserved master regulators, such as ORO and SAM combined with highly variable sets of downstream differentially expressed genes. This conservation of master regulators is a theme that has also been observed in other developmental contexts, for example in animals, where conserved orthologues of the eyeless gene direct the construction of very different types of eye in organisms as diverse as mammals and fruit flies (Quiring et al., Citation1994).
Recently, forward genetics has also been used to identify genes playing key roles during the development of the sporophyte and/or gametophyte generations. One of the questions that these experiments aimed to address concerns the evolutionary origins of the sporophyte and gametophyte developmental programs, in particular the extent to which these programs have emerged independently for the two generations. The recent identification of two genes that play key roles in the development of the basal systems that attach Ectocarpus individuals to substrata has begun to address this question. The first of these genes, DISTAG (DIS), is required for the deployment of basal systems during both the sporophyte and gametophyte generations (Godfroy et al., Citation2017). This was a surprising observation because the basal systems of the two generations are very different (Peters et al., Citation2008). In the gametophyte, asymmetric division of the initial cell, a meiospore, leads to the production of a small rhizoid and an upright filament that will develop into the apical thallus. In the sporophyte, on the other hand, the initial cell divides symmetrically, and an extensive network of basal filaments is established before the upright filaments of the apical system are produced (). The basal systems of the two generations therefore differ in terms of their developmental programs, their size and the cell types involved (i.e., rhizoid cells in the gametophyte, round and elongated filament cells in the sporophyte) but nonetheless depend on a common genetic component for their development. The DIS gene encodes tubulin binding cofactor Cd1 (TBCCd1), which is thought to have a role associated with the cytoskeleton (Godfroy et al., Citation2017). TBCCd1 is an ancient protein that has been conserved across diverse eukaryotic supergroups and DIS orthologues have been found in all brown algae analyzed to date. However, the functions of these genes in the other brown algae remain to be investigated.
Figure 5. Phenotypes of the Ectocarpus immediate upright (imm) and distag (dis) mutants during the sporophyte generation. Schematic representations of development from a single initial cell with apical and basal tissues in red and blue, respectively. Wild type individuals produce an extensive network of basal filaments before producing upright (apical) filaments, whereas the imm mutant only produces a small rhizoid and the dis mutant completely lacks basal tissues.
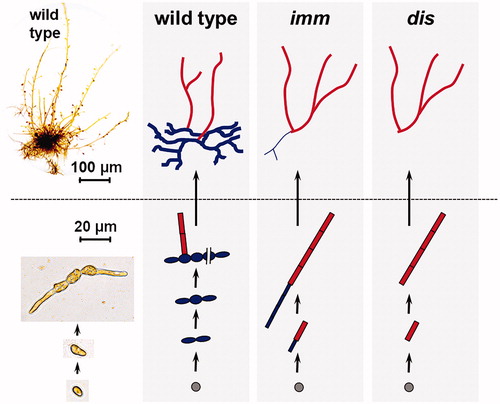
Mutation of the second gene, IMMEDIATE UPRIGHT (IMM), leads to the extensive basal system of the sporophyte being replaced by a small rhizoid, which resembles the rhizoid of the gametophyte (; Peters et al., Citation2008). There is no visible phenotype during the gametophyte generation. The sporophyte phenotype has been interpreted to indicate that the extensive basal system of this generation evolved from a simpler rhizoid-like system, an evolutionary event that specifically affected the sporophyte generation (Macaisne et al., Citation2017). Taken together, therefore, analysis of the dis and imm mutants has indicated that evolution of the sporophyte and gametophyte developmental programs has involved both sharing of genetic components (DIS) and the evolution of generation-specific programs (IMM). IMM encodes a protein of unknown function with a conserved, repeated cysteine-rich domain (Macaisne et al., Citation2017). IMM orthologues have been identified in several brown algal orders but the function of these proteins is unclear as these species do not exhibit delayed deployment of apical organs during the sporophyte generation. The conserved cysteine-rich domain of the IMM protein has been called the EsV-1-7 domain because it was first observed in a gene from the Ectocarpus virus EsV-1. Other Ochrophyta lineages possess no more than one EsV-1-7 domain gene but there appears to have been a spectacular expansion of this gene family in the brown algae, for example, with 91 gene family members being detected in Ectocarpus (Macaisne et al., Citation2017). Given the key developmental role of the IMM gene, it has been suggested that the expansion of this gene family may have played a role in the evolution of complex multicellularity in the brown algae. Interestingly, EsV-1-7 domain genes are patchily distributed across the eukaryotic tree and have been found in three diverse viral genomes, leading to the suggestion that viruses may have mediated horizontal transfer of these genes during eukaryotic evolution (Macaisne et al., Citation2017).
C. Evolution of brown algal sex chromosomes and sexual differentiation systems
Brown algae are characterized by a striking diversity of sexual systems, levels of sexual dimorphism and reproductive modes, and these traits are labile across the different groups (see above). The most prevalent system has separate sexes during the haploid stage of the life cycle (dioicy) but several transitions to co-sexuality (monoicy) have occurred during brown algal evolution (Luthringer et al., Citation2014; Heesch et al., Citation2019). Only one group, the Fucales, has evolved sex determination (dioecy) in the diploid stage of the life cycle, and, again, shifts have occurred between separate sexes and co-sexuality (monoecy) multiple times within this lineage (Cánovas et al., Citation2011). This broad diversity of sex-related traits makes the brown algae exceptional models to investigate the forces driving the evolution of sex determination. In particular, the range of analytical tools available for Ectocarpus sp. has allowed significant progress to be made in our understanding of the mechanisms underlying sex determination and differentiation in this model species. Ectocarpus sp. has a haploid, dioicous sexual system in which male and female sexes are determined after meiosis in the sporophyte, depending on whether the meio-spores inherit a U (female) or a V (male) chromosome (Coelho et al., Citation2018). The sex chromosomes of Ectocarpus sp. were the first eukaryotic UV system to be described in detail (Ahmed et al., Citation2014). The nonrecombining sex-determining regions (SDR) of the U and V chromosomes are ∼1 Mbp in length, occupying about a 10th of the sex chromosome (Cormier et al., Citation2017). The male haplotype of the SDR contains 17 protein-coding genes and three pseudogenes, whereas the female haplotype contains 15 protein-coding genes and seven pseudogenes. Genes at the male and female SDRs in Ectocarpus sp. are highly divergent at the sequence level, suggesting that these regions have been evolving independently for a long period of evolutionary time. Because of the lack of recombination, both SDRs are enriched in transposable elements and gene poor compared with autosomes, a characteristic that is shared with Y and W nonrecombining regions (Bachtrog et al., Citation2014).
The availability of classical genetic tools for Ectocarpus has been an asset to understand sex determination and sex chromosomal dominance in UV systems. Genetic crosses using the oro life cycle mutant (producing a functional diploid gametophyte; Coelho et al., Citation2011; Arun et al., Citation2019; see above) have shown that the male SDR on the V chromosome is dominant over the female SDR, implying the existence of a male master sex-determining gene(s) within the male SDR. One male-specific SDR gene, which is strongly upregulated during fertility and is predicted to encode a high mobility group (HMG) domain transcription factor, is a good candidate for this master regulator (Ahmed et al., Citation2014). HMG domain genes are involved in sex determination in animals and mating type determination in fungi (Idnurm et al., Citation2008; Graves and Peichel, Citation2010). Interestingly, orthologues of the Ectocarpus HMG domain gene are consistently male-linked in all brown algal species that have been investigated so far (Lipinska et al., Citation2017).
Comparative genomic analyses across nine brown algal species has identified a core set of genes that has been stably maintained within their SDRs, suggesting that these genes play a role in sex determination, and/or that these genes were present on the ancestral chromosome and have been trapped in this chromosomal region since the recombination suppression event that gave rise to the SDR. This set of genes includes the HMG domain gene. In addition to this conserved set of genes, substantial modifications occurred in each of the brown algal SDRs, involving gene loss, gene gain and relocation of genes from the SDR to autosomes (Lipinska et al., Citation2017). Gene loss and gene gain events have also played a role in the evolution of sex-determination systems in several metazoan lineages (Emerson et al., Citation2004; Potrzebowski et al., Citation2008), highlighting common features between haploid and diploid sexual systems. Gene gain in brown algal U and V sex chromosomes has occurred via transposition from other chromosomes and engulfment of neighboring genes located in the pseudo-autosomal region (PAR, Lipinska et al., Citation2017). The genes that have been acquired by the nonrecombining regions are expressed mainly during the haploid, gametophyte generation. This observation agrees with models predicting that haploid sex chromosomes should evolve by gaining genes favorable for the haploid phase of the life cycle (Bull, Citation1983).
The PAR of the Ectocarpus sex chromosome recombines at a similar rate to the autosomes (Luthringer et al., Citation2015). Therefore, the expectation was that the structural and evolutionary characteristics of this region would be similar to those of the autosomes (Otto et al., Citation2011). Surprisingly, however, this is not the case (Luthringer et al., Citation2015). Moreover, the PAR is enriched in genes that are preferentially or exclusively expressed during the sporophyte (diploid) generation of the life cycle, and many of these genes do not have homologs in other brown algal clades (Luthringer et al., Citation2015). A modelling-based approach evaluating the enrichment in sporophyte-biased gene expression for the PAR suggested differential pressures in males and females acting on alleles that are advantageous during the sporophyte generation of the life cycle. Recent data indicates that the PAR exhibits more neutral evolution compared with autosomal regions, and that genes in this region may be under balancing selection (Avia et al., Citation2018), in agreement with theoretical expectations that linked neutral diversity increases exponentially with the number of selected loci (Navarro and Barton, Citation2002). It is currently unknown if the PAR of Ectocarpus is representative of PARs in other UV systems, and investigations of sex chromosomes in other brown algae will be crucial to address this question.
Sex chromosomes play a major role in sex determination, but most of the phenotypic differences between males and females are caused by differential expression of genes that are present in both sexes, a phenomenon known as sex-biased gene expression (Parsch and Ellegren, Citation2013). Only about 10% of the transcriptome is sex-biased in Ectocarpus (Lipinska et al., Citation2015), which is not surprising given the phenotypic similarities between sexes in this species (Ahmed et al., Citation2014). Interestingly, other brown algae with more marked morphological differences between sexes had only a small fraction of sex-biased genes, ca. 7-12% in the kelp Saccharina latissima (Monteiro et al., Citation2019), and 9-14% in Fucus vesiculosus (Martins et al., Citation2013). It appears that overall, brown algae exhibit less conspicuous sex-biased transcriptomes compared with animal systems, where a large proportion of the genome may be differentially regulated in males and females. This is probably because phenotypic sexual dimorphism is less pronounced in the brown algae (Luthringer et al., Citation2014; Lipinska et al., Citation2015). Interaction between the sexes in most brown algae is indirect, through broadcast spawning of gametes that meet and fuse in the seawater, without any further intervention of the gametophyte. The success of reproduction is therefore ensured by strategies such as releasing gametes at the optimal phase of the tide or by providing gametes with efficient phototactic and pheromone systems (Maier, Citation1995; Pearson and Serrão, Citation2006) and not by developing a high level of sexual dimorphism at the gametophyte level.
It is a common observation that male-biased genes in XY sexual systems tend to evolve more rapidly than unbiased genes at the level of their protein-coding sequence (Ellegren and Parsch, Citation2007). Analysis of the evolutionary rates of sex-biased compared with unbiased genes in Ectocarpus indicated that both male- and female-biased genes had faster evolutionary rates than unbiased genes (Lipinska et al., Citation2015). These genes also showed evidence of stronger positive selection compared to autosomal genes, suggesting that their faster evolutionary rates are at least partly driven by adaptive evolution.
IV. Reproductive isolation and speciation
A. Speciation mechanisms
The “species problem” has been with us since before Darwin. While defining the meaning of “species” is a thorny and complex problem beyond the scope of this review, recent advances in our understanding of brown algae have shed light on the mechanisms by which lineages diverge. Brown algae vary widely in traits that influence their potential for speciation and diversification, such as life history strategies, dispersal mechanisms and potential, as well as apparent scope for ecological diversification. For instance, the potential for dispersal in brown algae can vary from a few centimeters (Hays, Citation2007; Barner et al., Citation2011) to hundreds of kilometers (Smith, Citation2002; Fraser et al., Citation2020). In the sister species Postelsia palmaeformis and Nereocystis luetkeana, the difference in potential for long distance dispersal is dramatic, in that P. palmaeformis is characterized by drooping, deeply grooved blades, promoting highly localized dispersal and selfing (Barner et al., Citation2011), while N. luetkeana produces dehiscent sori on blades near the surface- up to tens of meters from the substratum, presumably promoting greater dispersal distances (Dayton, Citation1985). This type of variation in dispersal potential is widely represented throughout the brown algae, with some species capable of forming enormous rafts that can cross oceans (Smith, Citation2002) while others generally disperse only locally or through a series of “stepping stones” (Billot et al., Citation2003). The substantial differences in traits among lineages are expected to manifest as variation in diversification rates, which is supported by diversification analyses (Cánovas et al., Citation2011; Starko et al., Citation2019) and the heterogeneity in the species richness of lineages across the brown algal phylogeny (; Silberfeld et al., Citation2010). In this subsection, we summarize the main mechanisms known to drive speciation and lineage diversification across the brown algae and critically evaluate the state of knowledge of these various mechanisms.
1. Barriers to reproduction
Natural populations of brown algae reproduce sexually by external fertilization (broadcast spawning; e.g., many Fucoids, Ectocarpales and Dictyotales) or functional brooding (i.e., retention of fertilized eggs, e.g., Sargassaceae). The frequent aggregation of closely related species in natural habitats suggests that barriers to hybridization should be very important. Nevertheless, pheromonal systems for sperm attraction are simple; often the same active molecule is shared across a large group of species (Müller et al., Citation1971; Citation1979; Citation1981; Müller and Jaenicke, Citation1973; Jaenicke et al., Citation1974; Müller and Gassmann, Citation1980). Moreover, the high cross-specific fertility in experimental crosses within and even between genera (Bolton et al., Citation1983; Kraan and Guiry, Citation2000; Coyer et al., Citation2002; Müller et al., Citation2019) have led these authors to assume that specificity in gamete recognition or gamete incompatibility might not be the key factors in producing or maintaining species boundaries. This provides an apparent paradox, where substantial species diversity appears to have arisen, and maintained in sympatry, with few intrinsic barriers to reproduction. There are, however, examples where barriers to crossing are observed between closely related species pairs (Tom Dieck, Citation1992; reviewed by Bartsch et al., Citation2008). Interestingly, hybrid inviability between sympatric sister species may contrast with viability at greater evolutionary distances and/or geographic isolation (Tom Dieck, Citation1992; Martins et al., Citation2019). For example, sister species Laminaria hyperborea and L. digitata are incompatible (Tom Dieck, Citation1992), while Laminaria digitata and L. pallida, which are more distantly related but differ in geographic range (Rothman et al., Citation2017; Martins et al., Citation2019) are partially compatible, suggesting that reproductive barriers may only be reinforced when species are sympatric. Reinforcement can even be population specific within species ranges, as exemplified by Fucus vesiculosus and F. spiralis. These species co-occur along most of their range with limited introgression, but are extensively introgressed where allopatric populations (separated by habitat) contact, suggesting lower reinforcement of the allopatric versus sympatric populations (Moalic et al., Citation2011). Similarly, hybrids appear to be more common in recent versus old contact zones in Atlantic F. distichus and F. serratus (Hoarau et al., Citation2015).
Ecological factors and life history traits can also affect gene flow between diverging populations and reinforce species or lineage boundaries. Alone or in concert, variations in reproductive phenology, niche occupancy, and mating system can be strong segregating factors. For example, both the sex ratio and the length of the vegetative growth stage during the gametophyte generation can be influenced by temperature (Oppliger et al., Citation2011; Citation2012). Such variation in contact zones could contribute to reduced cross fertility between species. Gamete or meio-spore dispersal distances are generally limited in brown algae, minimizing potential adverse effects of dilution on fertilization efficiency (Reed et al., Citation2004; Pearson and Serrão, Citation2006). High densities of individuals are also likely important because pheromone gradients for sperm attraction are effective only at mm scales (Lüning and Müller, Citation1978). Additionally, the prevalence of negative phototaxis and/or negative buoyancy of gametes, or a microscopic gametophyte phase suggests that fertilization has evolved to take place at or near the (2-dimensional) benthos rather than in the water column. There is good evidence from fucoids that the sophisticated sensing mechanisms used to synchronize gamete release to narrow temporal windows (reviewed by Pearson and Serrão, Citation2006) vary at hourly scales between sympatric congenerics (Monteiro et al., Citation2012, Citation2016). Such small variations in reproductive timing may provide strong reproductive isolation (e.g. in sympatric interfertile corals; Levitan et al., Citation2004). Controls over gamete release and dispersal may therefore largely restrict opportunities for natural hybridization, in addition to post-zygotic effects on fitness.
A major shift in speciation research has come with the recognition that reproductive barriers often remain semipermeable and gene flow may occur even while species differentiate (see Hausdorf, Citation2011, and refs therein). Although most interspecific hybrids are sterile or less fit than their parents, some may survive and reproduce, enabling the transfer of neutral and adaptive variants across species boundaries (introgressive hybridization), or even the formation of novel evolutionary lineages (homo- and allopolyploid speciation, and hybrid clones). Brown algae have been pivotal in linking hybridization and introgression with historical biogeography. Periodic range dissections and expansions associated with climatic oscillations (e.g., glacial-interglacial cycles) often result in secondary contact between vicariant lineages and sister species, where heterosis (Martins et al., Citation2019) and/or lack of reinforcement (Hoarau et al., Citation2015) can potentially result in increased genetic transfer. These contact zones are predicted to be more frequent at (but not restricted to) higher latitudes, for instance along trans-Arctic routes and the Aleutian Arch (where western and eastern expanding Pacific populations meet), but have seldom been objectively examined beyond a few case studies showing very limited intra-specific gene-flow in sibling species (Tellier, Tapia, et al., Citation2011; Neiva et al., Citation2018). Fine-scale studies of these contact zones are likely to clarify isolation mechanisms underlying brown algal speciation. Signatures of past and ongoing hybridization and introgression, typically genetic admixture (e.g. hybrid microsatellite genotypes), conflicts among organellar genomes (Tellier, Faugeron, et al., Citation2011), and conflicts between organellar and nuclear genomes, have been detected in natural populations for a wide range of brown algal taxa (Hodge et al., Citation2010; Neiva et al., Citation2010; Moalic et al., Citation2011; Zardi et al., Citation2011; Geoffroy et al., Citation2015; Kogame et al., Citation2015; Montecinos et al., Citation2017a), pinpointing their taxonomic ubiquity and importance in brown algal evolution.
Macromutations (e.g., polyploidy) can also cause barriers to reproduction and result in “instant speciation,” often with accompanying evolutionary opportunity (see Sousa et al., Citation2019). By comparison with other groups, like embryophytes, the incidence of polyploidy is poorly understood in brown algae. The recent confirmation of two late-Pleistocene allopolyploid lineages in the fucoid genus Pelvetiopsis are unique thus far, remarkably involving the same extant paternal ancestor (Neiva et al., Citation2017; Sousa et al., Citation2019). In Pelvetiopsis, the allopolyploid P. limitata is currently the most widespread and abundant species of the genus, whereas its paternal and maternal diploid ancestors are a narrow-endemic climatic relict (P. arborescens) and a presumably extinct species (Sousa et al., Citation2019). Climatic shifts, perhaps associated with hybrid vigor, have been invoked to explain its successful establishment beyond the contracting ancestors' ranges. Perenniality, self-compatible hermaphroditism, and other correlates of polyploidy in land plants are also found in some species-rich genera of brown algae, suggesting that it might be more widespread. Furthermore, across some of the most species-rich orders of brown algae (e.g., Laminariales, Ectocarpales, Fucales, Dictyotales) genomes sizes can vary several-fold (Phillips et al., Citation2011), leaving open the possibility that polyploid speciation has occurred deeper in the past. However, current genome data do not support hypotheses of ancient polyploidy, at least for Laminariales and Ectocarpales (Cock et al., Citation2010; Ye et al., Citation2015; Nishitsuji et al., Citation2016; Citation2019; Dittami et al., Citation2020; Shan et al., Citation2020). Thus, further analyses are required to understand the processes that have led to observed variation in genome size.
2. Allopatric speciation
There is substantial evidence that geographical isolation has played a major role in patterns of speciation and diversification across various brown algal clades. Vicariance events are common when tectonic or climatic shifts cause two or more populations to be isolated from each other. Long distance dispersal events may also allow populations to invade new parts of the globe, potentially leading to isolation from the parent population. This is presumably the dominant process in lineages such as Lessonia or Durvillaea, where species rarely overlap in range and patterns of speciation tend to represent lineage dispersal pathways (Fraser et al., Citation2010; Zuccarello and Martin, Citation2016). Similarly, recent phylogeographic analyses of both the Laminariales (Starko et al., Citation2019) and the genus Sargassum (Yip et al., Citation2020) indicate that region-specific diversification is likely a common process. The high number of regionally endemic large brown algae in each of the Southern Hemisphere temperate regions (S. America, S. Africa and Australia/New Zealand) suggests that trans-oceanic distances remain effective barriers to dispersal (Peters et al., Citation1997; Phillips, Citation2001; Bolton, Citation2010), contrasting with the comparatively contiguous landmasses in the Northern Hemisphere.
A history of repeated cycles of environmental or ecological change resulting in population subdivision is theoretically conducive to speciation (Gavrilets et al., Citation1998; Gavrilets, Citation2003). While glaciations periodically erase and re-distribute genetic lineages, they also promote evolutionary novelty, and have played an important role in promoting allopatric lineage divergence in the brown macroalgae during the Pleistocene (2.6 Ma–12 ka). Widespread amphiboreal taxa provide a paradigmatic example. Exchanges between Pacific and Atlantic basins (and to a lesser degree across the Atlantic) are only possible during brief interglacial periods when seasonally ice-free marine routes allow dispersal and colonization past the Bering Strait and across the Arctic. The subdivision of Saccharina latissima and Fucus distichus – two of the most widespread, polymorphic and ecologically plastic brown algae of the Northern Hemisphere – into vicariant Atlantic and Pacific phylogroups support this general background of episodic dispersal versus chronic isolation (Coyer et al., Citation2011; Neiva et al., Citation2018). In Saccharina latissima, NW Atlantic and Pacific lineages reveal remarkable genetic integrity in a large zone of high-Arctic secondary contact, suggestive of reproductive isolation and incipient speciation (Neiva et al., Citation2018). Similar patterns of vicariance between trans-Arctic populations are evident in many other brown algae but remain to be rigorously assessed using large population level datasets (exemplar genera include Chordaria, Desmarestia, Dictyosiphon, Petalonia, and Pylaiella; Saunders and McDevit, Citation2013; Bringloe and Saunders, Citation2019). The full extent to which glacial cycles act as a trans-Arctic “speciation pump” to amplify brown algae diversification also remains unassessed.
3. Sympatric, parapatric and peripatric speciation
Although allopatric speciation arising from geological and climatic processes is likely to have been an important process in establishing the modern diversity and distribution of brown algal species across modern oceans, there are many examples of brown algal speciation that did not depend on strict geographical isolation. Geographical clines may be associated with gradients in environmental conditions, and segregation of populations along these gradients can lead to lineage divergence or speciation, presumably through parapatric or peripatric speciation (sensu Funk and Omland, Citation2003). In the Lessonia nigrescens species complex (Laminariales), two cryptic species are recognized along the coast of Chile, with only a narrow overlap in ranges (Tellier et al., Citation2009) but complete reproductive isolation (Tellier, Tapia, et al., Citation2011). The heat tolerance of these cryptic species differs and matches their corresponding latitudinal distributions, suggesting that genetic and ecological divergence are linked (Oppliger et al., Citation2012; López-Cristoffanini et al., Citation2013). In this case, the phylogeny recovered a monophyletic clade for the northern species, nested within the southern species (Tellier et al., Citation2009), suggesting that speciation occurred at the northern range margin of the southern species following a combination of adaptation and founder effects (i.e., parapatric speciation; Funk and Omland, Citation2003). Similarly, the widespread species Fucus vesiculosus shows intraspecific lineage divergence both near the southern range edge (Cánovas et al., Citation2011) and in the marginal brackish Baltic Sea (Tatarenkov et al., Citation2007). In both cases, divergence is associated with habitat shifts and physiological changes consistent with local adaptation (Serrão et al., Citation1996; Pearson et al., Citation2000; Saada et al., Citation2016).
Ecological speciation is a mechanism of sympatric speciation linking ecology and evolution, whereby divergent selection within a species leads to speciation. The best studied examples of this process in brown algae are in the order Laminariales, where ecological explanations have been invoked to understand the diversity of species with overlapping ranges. Kelps (Laminariales) of the Northeast Pacific coast began diversifying recently (∼30 Ma, Starko et al., Citation2019) and predominantly in one oceanic basin (the North Pacific), suggesting ecological speciation played an important role early in their evolution. Between Mexico and Alaska, upwards of two dozen species of Laminariales are currently recognized (Guiry and Guiry, Citation2020), many of which have extensive and overlapping latitudinal ranges but play different ecological roles. Estes and Steinberg (Citation1988) proposed that ecological differences such as susceptibility to grazing may have been essential in driving species divergence and lineage diversification. Interestingly, ecological differences between species often lead to some degree of spatial segregation between species. For example, where the ranges of Laminaria digitata and Laminaria hyperborea overlap in the North Atlantic, these species tend to occupy differing tidal heights. Moreover, intraspecific lineage differentiation and, in some cases, true speciation events have been known to occur over local environmental gradients such as tidal height, depth, and wave exposure (e.g. Blanchette et al., Citation2002; Miller et al., Citation2000; Roberson and Coyer, Citation2004; Fraser, Hay, et al., Citation2009; Augyte et al., Citation2018). This pattern further holds true across the laminarialean phylogeny where niche partitioning across local gradients of wave exposure may have promoted diversification (Starko et al., Citation2020). For example, it is common for large brown algal sister species to have largely overlapping geographical ranges but use entirely different habitats, such as in Postelsia palmaeformis (high intertidal) and Nereocystis luetkeana (subtidal) or Saccharina latissima (sheltered coasts) and S. angustissima (wave exposed coasts) (Augyte et al., Citation2018; Starko et al., Citation2020). Moreover, several ecologically relevant characteristics, such as buoyancy, branching and wave resistance strategies have evolved repeatedly across the kelps (Starko & Martone Citation2016b, Starko et al., Citation2019), suggesting that species diversification was associated with ecological diversification in sympatry.
Further evidence of speciation in the absence of geographical isolation is evident in the Fucaceae. The multilocus phylogenetic approach of Cánovas et al., (Citation2011) showed that hermaphroditism is a derived trait and that the hermaphrodite lineage (F. spiralis and relatives) forms a paraphyletic sister clade with the ancestral extant southern populations of dioecious Fucus vesiculosus. Given the absolute habitat differentiation that prevails currently (saltmarsh versus open coast intertidal) in this southern range, dioecious and hermaphrodite divergence may have been parapatric/peripatric, with a switch in mating system from outcrossing to selfing-dominated (Perrin et al., Citation2007) likely contributing to reproductive isolation between the nascent lineages. Such association between ecological speciation and shifts in mating systems and/or life cycles have been observed also in kelps (see above) and Ectocarpus spp. (Couceiro et al., Citation2015). Even though these are still few examples, they highlight the possible role of the complex and variable life cycles of brown algae in promoting speciation without strong geographic isolation.
B. Historical biogeography
The underlying mechanisms for reproductive isolation are widely known in the brown algae, however, the trajectories leading to notable differences in assemblages across the globe follow in the wake of geological history and climate. Though the biogeography of brown algae could be subdivided into increasingly fine scale ecoregions (Lüning, Citation1990b, lists 23), here we review the evolution of assemblages with respect to three broad and historically recognized climate delineations: temperate (cold and warm), polar, and tropical assemblages (). We also conclude our review with a brief overview of the rare occasions in which the brown algae have ventured into freshwater.
Figure 6. Biogeographic subdivisions of marine biodiversity, modified from those described by Spalding et al. (Citation2007). Dark blue = Arctic; light blue = cold-temperate; pink = warm-temperate; red = tropical.
1. Temperate
Most brown algae live in temperate waters where some lineages act as the dominant foundation species in coastal ecosystems. As the oceans cooled following the Eocene optimum (∼32 Ma), several lineages of brown algae convergently evolved large thallus sizes and began to form upright, structurally complex habitats. The Laminariales, Fucales, Desmarestiales, and Tilopteridales all have members that form important marine habitats or ‘forests.’ The Laminariales diversified in the North Pacific as the oceans cooled in the late Cenozoic (Starko et al., Citation2019) and now dominate the temperate coastlines in this basin (Bolton, Citation2010). Members of the Laminariales have only recently spread to other oceanic basins (within the past 10 Ma; i.e., Atlantic, Southern Hemisphere), and are consequently much less diverse in these areas (Bolton, Citation2010; Starko et al., Citation2019). Rather, Fucales form important habitats in these areas. In the North Atlantic, the largely intertidal family Fucaceae dominate and reach their highest diversity, forming habitat along with Saccorhiza, one of two members of Tilopteridales (the other being related Phyllariopsis) to have achieved a large thallus. In the Southern Hemisphere, endemic fucoids such as Durvillaea, Phyllospora, and Scytothalia dominate temperate areas, coexisting with a small number of laminarialean species. Members of the Sargassaceae (Fucales), although largely tropical (see below), are also important constituents of temperate brown algal forests. While fucoids are among the dominant coastal habitat forming species throughout the globe, fucoid diversity in the Northern Hemisphere is largely restricted to the intertidal zone, potentially reflecting an effect of competition with (subtidal) laminarialean kelps.
Much of the cryptic brown algal diversity observed today has a strong geographical signal, to a large degree imprinted by repeated cycles of population extinctions/colonizations and vicariance/contact events during the last stages of the Pleistocene (2.5 Ma ago to 12 ka). During glacial periods, including the Last Glacial Maximum (LGM, 26-19 ka), ice-sheet expansion into temperate latitudes extirpated seaweeds from much of their modern distributions. Such local extinctions under the direct effect of the ice expansion occurred along all circumpolar regions but were especially extensive along shallower and enclosed seas and coastlines. Because of the equatorward shifts of isotherms, seaweed glacial ranges reached lower latitudes and are assumed to have been latitudinally compressed. The rise of trans-oceanic land barriers (e.g., Beringia, currently at −50 m) associated with global sea level regressions (down to −120 m), and the replacement of high-latitude stepping stones with ice barriers in the Northern Hemisphere, also contributed to lengthy isolation of many regional assemblages, which is reflected in the genetics of contemporary populations (Neiva et al., Citation2018; Bringloe and Saunders, Citation2018; Citation2019; Bringloe et al., Citation2020). In the current interglacial period (Holocene, 12 ka- present), warmer climates, marine transgressions and seasonally ice-free marine routes allowed macroalgal assemblages (or subsets of populations within species) to vastly expand their ranges polewards (e.g. Fraser, Nikula, et al., Citation2009; Neiva, Pearson, et al., Citation2012), often associated with range contraction at lower latitudes (Neiva et al., Citation2014; Assis, Araújo, et al., Citation2018). This led to novel, sometimes transient, trans-oceanic colonizations and secondary contact in several brown algal species (Neiva et al., Citation2018; Bringloe et al., Citation2020).
In the NE Atlantic, where isotherms are more splayed latitudinally, agreement between genetic hotspots (characterized by high levels of genetic diversity, and even unique endemic lineages), and climatic refugia (areas with suitable climatic conditions during both glacial and interglacial extremes) has been documented across many brown algal taxa (Hoarau et al., Citation2007; Neiva, Pearson, et al., Citation2012; Neiva, et al., Citation2014; Assis et al., Citation2016; Assis, Serrão, et al., Citation2018), underscoring the importance of long-term regional persistence in driving regional diversity. These areas were identified beyond ice-sheet limits, along western Ireland, Brittany and northwest Iberia, in addition to the Canadian Maritimes in the NW Atlantic (Neiva et al., Citation2016; Assis, Araújo, et al., Citation2018). More recently, upwelling areas (Lourenço et al., Citation2016), deep off-shore reefs (Assis et al., Citation2016; Assis, Araújo, et al., Citation2018) and other thermally buffered regions (e.g. Neiva et al., Citation2017) have been recognized as important areas where relict populations (and gene-pools) persevere (Assis, Araújo, et al., Citation2018). In contrast, areas colonized only post-glacially (e.g. Scandinavia) tend to have homogenous genetic compositions. This pattern is expected at high latitudes due to consecutive genetic bottlenecks and gene surfing experienced along expanding fronts (Excoffier and Ray, Citation2008).
Similar links between climatic shifts, range dynamics and intra- or inter-specific diversity are evident in other temperate assemblages as well (Fraser, Spencer, et al., Citation2009; Hu et al., Citation2017; Zhang et al., Citation2019). In the SE Pacific (Chile), many seaweeds show complex genetic subdivisions at 42° S (Guillemin et al., Citation2016), matching the limits of coastal ice during the last glacial maximum (LGM; McCulloch et al., Citation2000) and around 30° S, possibly matching the northward limit of the Western Drift during the same period (Guillemin et al., Citation2016). Moreover, many species are endemic to the most southern region (i.e. the Magellan region; Santelices and Meneses, Citation2000) indicating that glacial refuges retained regional diversity. Similarly, in the Northeast Pacific, refugial islands and archipelagos have been found to possess endemic species or genotypes (e.g., Aleutian archipelago: Starko et al., Citation2018, St. George Island: Kawai et al., Citation2013; Haida Gwaii: Saunders and McDevit, Citation2014) suggesting that these areas represent relic assemblages.
2. Polar
While species diversity of the brown algae predominantly resides in temperate climates, the Arctic region nonetheless played a major role in the evolution of Northern Hemisphere lineages. The Arctic basin began as an embayment of the Pacific Ocean until this connection was severed approximately 66 Ma (Lawver et al., Citation1990; Marincovich et al., Citation1990). Aside from intermittent connections with the world’s oceans and seas, brown algae in the Arctic basin would have evolved in relative isolation until the North Atlantic opened 15 Ma ago (Lawver et al., Citation1990). Global temperatures early in the evolution of the Arctic (66 Ma; Jenkyns et al., Citation2004) were 30 °C warmer than today, and gradually cooled until the initiation of glacial cycles 2.6 Ma (Miller et al., Citation2010). The Arctic was likely an important area for “biotic innovations” in response to colder, seasonal waters that trickled southwards and settled into contemporary temperate assemblages (Hickey et al., Citation1983), particularly in the geologically younger Atlantic basin. As with temperate assemblages, recent cycles of glaciation had a major impact on contemporary distributions of brown algae in the Arctic.
Genetic surveys have nonetheless revealed surprising levels of diversity unique to the Arctic basin (Saunders and McDevit, Citation2013; Laughinghouse et al., Citation2015; Küpper et al., Citation2016; Bringloe et al., Citation2020), a finding difficult to reconcile with the historical view of Arctic populations as extensions of temperate species (Lee, Citation1973), a simple depiction perhaps further justified by the dull character of Arctic seaweeds. Genetic surveys and hindcasting of macroalgal distributions during the LGM, however, have recently revealed potential refugial locations much further north than previously recognized (Assis, Araújo, et al., Citation2018), including southern Greenland, which is proposed as the epicenter for the evolution of novel diversity in the East Canadian Arctic (Bringloe et al., Citation2020). Notable cases of brown algae with distinct intraspecific Arctic lineages include Alaria esculenta (Bringloe and Saunders, Citation2018; Bringloe et al., Citation2020), Saccharina latissima (a “cold temperate” lineage; Neiva et al., Citation2018), and Fucus distichus (Laughinghouse et al., Citation2015). Unknown species of Acinetosporaceae, Chordariaceae, Desmarestiaceae, and Scytosiphonaceae are reported from the Arctic (Küpper et al., Citation2016), among others reviewed by Bringloe et al., (Citation2020). Resolving the extent of perennial ice cover, the importance of marine encroachment by continental ice-sheets, and ultimately reaching a consensus regarding the locations of refugial populations during the last glacial maximum are areas of research that continue to evolve in the brown algae.
The Antarctic experienced a markedly different geological history compared to its northern counterpart, a history reflected in its brown algae. Antarctica has evolved largely free from the other continents since the early Cenozoic (66 Ma), with the Antarctic Circumpolar Current (ACC) further promoting this isolation over the past 30 Ma (Lüning, Citation1990a). As such, endemism has been more favorably viewed as a prominent feature of the Antarctic brown algae, with more than a third of its species considered endemic (Wiencke and Amsler, Citation2012, estimate 44% across the heterokonts). While Laminariales dominate as canopy forming species in the Arctic, the Antarctic is devoid of laminarialean kelps and instead dominated by Desmarestiales, which likely originated in the Southern Hemisphere (Peters et al., Citation1997). Antarctica also features the endemic habitat forming Ascoseira mirabilis, the only species of the order Ascoseirales. As with the Arctic, phylogenetic knowledge of Antarctic brown macroalgae is limited to a few studies (Hu et al., Citation2016), with only a handful of articles presenting genetic data (Mystikou et al., Citation2014; Küpper et al., Citation2019), and only Peters et al., (Citation1997) presenting phylogenetic interpretations (see above, and Yang et al., Citation2014 for an updated phylogeny of the Desmarestiales). Genetic structure in Macrocystis pyrifera is likely influenced by the ACC, which appears to carry haplotypes between distant subantarctic islands (Macaya and Zuccarello, Citation2010). However, this species does not occur anywhere on Antarctica and likely colonized the subantarctic region recently (Macaya and Zuccarello, Citation2010). The population structure of Durvillaea antarctica from Sub-Antarctic islands suggest sea ice was extensive during the LGM, pushing polar populations into refugia along the coastlines of New Zealand (Fraser, Hay, et al., Citation2009; Fraser, Hay, Nikula, et al., 2009 Fraser, Spencer, et al., Citation2009; Fraser et al., Citation2020).
Brown algae at higher latitudes face unique environmental challenges. Seasonal changes at high latitudes result in a condensed 24 h summer light regime followed by months of darkness, during which time ocean waters shift to freezing and ice conditions. Variances in nutrient availability and salinity are also important in the Arctic. Some brown macroalgae anticipate seasonal changes to time photosynthetic rate, growth, and reproduction, and in doing so maximize the efficiency of these processes (reviewed extensively by Wiencke et al., Citation2007, and more recently by Wiencke and Amsler, Citation2012). Perhaps the clearest example of a brown alga adapted for polar conditions, the Arctic kelp Laminaria solidungula is known to complete all of its growth under ice during months of darkness, using the summer months exclusively for carbon storage (Dunton and Schell, Citation1986). As a result, this kelp and similarly adapted seaweeds are recorded from extraordinary latitudes, as far as 82oN in Jörgen Brönlunds-Fjord where coastal waters remain frozen almost year-round (Lund, Citation1951).
3. Tropical
Brown algae are less prevalent in the tropics, with most tropical diversity stemming from Dictyotales (particularly Dictyota, Lobophora, and Padina; Silberfeld et al., Citation2014; Vieira et al., Citation2017) and Fucales (primarily Sargassum). Although most lineages of large habitat forming algae (e.g., Fucaceae, Laminariales, Durvilliaceae) are generally absent from tropical regions, the genus Sargassum is a notable exception with more than 350 currently recognized species (Yip et al., Citation2020). Despite the warm oligotrophic waters of the tropics, Sargassum manages to form analogous habitats to kelp forests in some areas (Coleman and Wernberg, Citation2017; Fulton et al., Citation2019), indicating that large brown algae are capable of evolving tolerance to low nitrogen and high temperatures. Sargassum diversified ∼4.3 Ma in the Indo-Pacific, where its species richness is the greatest, and spread globally into sub-tropical and temperate regions (Yip et al., Citation2020). Sargassum spp. compete with corals, and, although they can tolerate low levels of nitrogen, they tend to be more competitively successful against corals in areas of high nitrogen (Hughes et al., Citation1999) or low herbivory (Bellwood et al., Citation2006). Although other fucoids are present in the tropics (e.g., Turbinaria ornata), these tend to be smaller, lacking the scale of their large habitat forming counterparts.
The Dictyotales are species rich, widely distributed, and one of the few lineages common in the tropics. The Dictyotales arose through a divergence event relatively early in brown algal evolution (see brown algal phylogeny, subsection II.B) in the tropical southern Tethys of the Middle Jurassic, and today reaches peak species diversity in the tropical Indo-Pacific (Vieira et al., Citation2017; Steen et al., Citationunder revision). The high diversity of Dictyotales is mainly attributable to two diversification bursts, following the Cretaceous-Tertiary boundary, in Lobophora and Dictyota, respectively. Diversification rates in Dictyotales were markedly higher in the tropics. Lobophora radiated and remained in tropical to warm-temperate waters, while Dictyota expanded into colder temperate regions while preserving its presence in the tropics (Steen et al., Citationunder revision). Today, Dictyota, Lobophora and Padina are ecologically important benthic components in tropical and sub-tropical reef ecosystems (e.g. Briones-Fourzán and Lozano-Álvarez, Citation2001, Kaullysing et al., Citation2016; Vieira, Citation2020).
Aside from Dictyotales and Fucales, the paucity of brown algal representation across orders in the tropics suggests that there are important factors limiting their dispersal, persistence, and perhaps their diversification in warmer waters. Environmental conditions are likely not conducive to the success of many lineages of large brown algae. In particular, laminarialean kelps generally require cool temperatures and have high nitrogen demands, preventing them from establishing in tropical oligotrophic waters and mostly restricting them to temperate and arctic regions. Even the most heat tolerant genus Ecklonia barely occurs in the tropics, and is restricted to deep (i.e. cool) waters of tropical upwelling regions (Graham et al., Citation2007). Biotic interactions have also been invoked to explain the limited diversity and dominance of tropical brown algae. Unlike temperate regions where mass extinctions and global cooling may have provided ecological opportunities for brown algae to dominate (Estes and Steinberg, Citation1998; Cánovas et al., Citation2011; Vermeij et al., Citation2019; Starko et al., Citation2019), tropical climates have characterized much of earth’s history since the appearance of brown algae (). Corals and red algae have long dominated tropical regions, occupying niches and leading to less ecological opportunities for the brown algae. Recent work on tropicalization of temperate reefs has shown that large brown algae are quickly eliminated by the invasion of tropical herbivorous fishes (see Vergés et al., Citation2014; Citation2016; Citation2019), suggesting a mechanism that may have prevented the success of brown algae in the tropics historically. These factors likely do not occur in isolation. For example, Sargassum spp. continue to compete with corals, and the balance of this interaction is often mediated by herbivorous fish (Bellwood et al., Citation2006). This hypothesis is further supported by the fact that species of Dictyota, one of the few brown algal genera that is common in the tropics, are highly chemically defended and capable of maintaining high biomass even in the presence of heavy predation (Hay et al., Citation1987; Wiesemeier et al., Citation2007).
Substantial changes in the presence and abundance of certain tropical brown algal species have occurred in recent years owing to anthropogenic effects and climate-change-induced invasions. Notable examples of species introduced by transport vectors (e.g. boats, barges) include Colpomenia along Pacific shores of North America and both Atlantic and Pacific shores of South America (Lee et al., Citation2013), Dictyota flabellata and Sargassum muticum in Hawaii (Abbott and Huisman, Citation2003), and the general widespread invasion of S. muticum (Louime et al., Citation2017). Most concerning is the migration of S. fluitans and S. natans from the Sargasso Sea into various regions of the Atlantic, including Brazil (de Széchy et al., Citation2012), the Dominican Republic (Mendez-Tejeda and Rosado Jiménez, Citation2019), Ghana (Addico and deGraft-Johnson, Citation2016), and many other island nations of the Caribbean (Louime et al., Citation2017). Massive blooms have resulted in the “great Atlantic Sargassum belt,” a phenomenon that began in 2005. In 2018, the Sargassum belt stretched over 8850 km from West Africa to the Caribbean Sea, and generated over 20 million metric tons of biomass that smothered tropical coastlines (Wang et al., Citation2019). Climate change and increased micro- and macronutrients due to pollution are likely playing a role in the massive increase in biomass and movement of Sargassum around the Atlantic (Louime et al., Citation2017). These immense golden blooms have severe ecological, economical, and human health impacts, but also have the potential to inspire innovations in climate change mitigation (via carbon sequestration; Gouvêa et al., Citation2020), bio-fuel refinement, agriculture fertilizer, and eco-friendly charcoal alternatives (reviewed by Louime et al., Citation2017).
4. Freshwater
Despite the ubiquity of brown algae in marine environments globally, very few lineages have colonized freshwater ecosystems (Wynne and Bold, Citation1985; McCauley and Wehr, Citation2007; Sheath and Wehr, Citation2015; Wehr, Citation2015; Dittami et al., Citation2017). Although up to seven independent transitions from marine to freshwater habitats have occurred in brown algae (Dittami et al., Citation2017), none have resulted in widespread diversification, and freshwater lineages have remained restricted to filamentous or crustose forms (Wynne and Bold, Citation1985; McCauley and Wehr, Citation2007; Dittami et al., Citation2017).
Freshwater species are known from two orders: Ectocarpales and Sphacelariales. The first description of a freshwater brown alga was Pleurocladia lacustris (Braun, Citation1855), a widely distributed member of the Ectocarpales. Ectocarpus also invaded freshwater independently of Pleurocladia (West, Citation1996; McCauley and Wehr, Citation2007) with the freshwater species Ectocarpus subulatus found in Australia and Europe (Wehr et al., Citation2015). These species are not obligate freshwater taxa and are capable of surviving in saltwater (McCauley and Wehr, Citation2007; Dittami et al., Citation2012; Wehr et al., Citation2013). Differences in the gene expression profiles of freshwater and marine populations indicate that genomic changes have occurred to stabilize the transition to freshwater (Dittami et al., Citation2012; Citation2017; Citation2020; Meslet-Cladière et al., Citation2013). Most other freshwater brown algae are from the Sphacelariales, all of which are obligate freshwater inhabitants (Sheath and Wehr, Citation2015). Two sister species, Heribaudiella fluviatilis and Bodanella lauterborni likely share a freshwater ancestor and possibly represent the most ancient invasion of freshwater, leading to strong anti-coastal distributions (Wehr and Stein, Citation1985; McCauley and Wehr, Citation2007; Sheath and Wehr, Citation2015; Wehr, Citation2015). While these species can be easily distinguished based on morphology and variability in the rbcL plastid locus, no nucleotide polymorphisms exist between these species in the LSU rRNA gene, suggesting that speciation of these two entities nonetheless occurred recently in evolutionary time (McCauley and Wehr, Citation2007). Members of the genus Sphacelaria have also invaded freshwater, yielding the two freshwater obligate species Sphacelaria lacustris and S. fluviatilis (Dittami et al., Citation2017). Further work is needed to determine if this represents a single or multiple invasions of freshwater by the genus.
Porterinema fluviatile is another widespread freshwater brown alga whose phylogenetic placement remains unclear. While some authors have suggested that P. fluviatile may be a member of the Ralfsiales, the Sphacelariales, or the Scytosiphonaceae (Silberfeld et al., Citation2014), limited molecular data suggest that Porterinema is a distinct and poorly explored lineage (McCauley and Wehr, Citation2007). Similarly, another filamentous freshwater species was recently isolated from a freshwater aquarium in the United Kingdom and potentially represents a new taxonomic order (Belcher et al., Citation2009; Dittami et al., Citation2012). Cumulatively, these studies indicate that unexplored diversity remains in freshwater brown algae with the potential to influence our understanding of evolution in the brown algal phylogeny.
Although freshwater species are rare, many brown algae exhibit some level of tolerance to low salinity (Gordillo et al., Citation2002; Tatarenkov et al., Citation2005; Dittami et al., Citation2017), a feature most notable in the Fucales (Serrão et al., Citation1996; Tatarenkov et al., Citation2005). Populations of many species of Fucus can persist in salt marshes and estuaries (Kucera and Saunders, Citation2008; Neiva, Hansen, et al., Citation2012). Interestingly, these populations all assume a reduced and spindly morphology, which was previously recognized as Fucus cottonii (Kucera and Saunders, Citation2008; Neiva, Hansen, et al., Citation2012). Molecular evidence indicates that this distinct morphology and ability to inhabit low salinity environments has evolved multiple times in parallel across the genus, with members of Fucus distichus, F. vesiculosus and F. spiralis all possessing populations with this form (Kucera and Saunders, Citation2008; Neiva, Hansen, et al., Citation2012).
V. Conclusions and perspectives
Our knowledge of brown algal evolution has improved dramatically in recent decades, a process that has undoubtedly been accelerated by advances in sequencing capabilities. The affordability of high throughput sequence data will continue to propel the field of brown algal evolution toward large-scale genomic datasets. Complete genome sequences have been reported so far for six brown algae, the Ectocarpales Ectocarpus sp. (Cock et al., Citation2010), E. subulatus (Dittami et al., Citation2020), Cladosiphon okamuranus (Nishitsuji et al., Citation2016), Nemacystus decipiens (Nishitsuji et al., Citation2019) and the kelps Saccharina japonica (Ye et al., Citation2015) and Undaria pinnatifida (Shan et al., Citation2020). An important initiative for the future is the Phaeoexplorer project (led by Roscoff Biological Station and Genoscope), which aims to provide 67 annotated genome assemblies of 47 brown algal species, plus four related unicellular and multicellular Ochrophyta. In addition, at the level of individual species a considerable amount of transcriptomic data is being generated (e.g. Monteiro et al., Citation2012; Martins et al., Citation2013; Monteiro et al., Citation2019), together with genome-scale genotyping data based on RAD-seq or genome resequencing. Analysis of these new data is expected to provide further substantial advances in our understanding of brown algal evolution in several different contexts, including genome-wide investigations of spatial and temporal population structure (Guzinski et al., Citation2018; Kobayashi et al., Citation2018; Le Cam et al., Citation2020), genetic maps and Quantitative Trait Locus analyses (Avia et al., Citation2017), genome evolution (Avia et al., Citation2018), and phylogenomics (Fraser et al., Citation2016). We conclude our review by providing perspectives on promising avenues for studying brown algal evolution, opened up by the availability of genomic data.
A. Resolving the brown algal phylogeny
Phylogenetic placement of the brown algal orders is currently based on multiple genes, but relationships within some of the major clades are unresolved; for instance, the location of Sphacelariales within the SSDO clade (). Alignments of additional orthologous genes from genomic data will continue to clarify these relationships. It should also be noted that time calibration of the brown algal phylogeny is based on limited fossil records. Alignment of homologous regions across brown algal genomes and with genomes of other members of the Ochrophyta will offer alternative molecular clocks for revising and dating the evolutionary timelines presented here. Many relationships at lower taxonomic levels (family, genus, species) are also unresolved, particularly in less well-studied or more species-rich orders (e.g. Tilopteridales and Ectocarpales, respectively). Extending genome data for these orders will be an important step toward developing a comprehensive phylogeny of the brown algae.
Species delineations within the brown algae today are largely validated through the genetic species concept and rely heavily on organellar DNA barcode markers. While DNA barcoding has been an immensely successful tool for understanding species diversity within the brown algae, this method reflects the evolutionary history of the organelles themselves and not necessarily that of the host species, especially given the relatively recent evolution of brown algae in comparison to green and red algae. Consequently, organellar capture and introgression can obscure the true relationships between populations and species (see reproductive barriers, subsection IV.A.1). Genome-wide nuclear data can also be used to evaluate species boundaries, and this approach promises to offer significant advancement toward a more accurate brown algal species concept, which will either validate or rewrite the work that has been carried out using organellar sequence data.
B. Evolution of brown algal morphological, life cycle and reproductive traits
A recurring theme within the brown algal phylogeny is the incongruence between relationships inferred from molecular data and the morphological and life history traits exhibited by the various lineages. The development of a robust phylogenetic framework will provide a context in which to investigate the evolutionary history of these traits and, in particular, to relate trait variations and transitions to ecological and geographical events over evolutionary time. The availability of complete genome sequences for individual lineages will provide powerful tools to investigate these questions, though it will nonetheless be a major challenge to link genomic features (genotype) to morphological or life history traits (phenotype) in an evolutionary context. Recently developed genetic tools provide the means to address this problem by identifying genes associated with specific biological processes. For example, the ORO and SAM genes are conserved across the brown algae (Arun et al., Citation2019) and therefore represent a logical starting point when looking for genetic signatures associated with variations in the life cycle. Similarly, it will be important to identify the regulatory genes that underlie other brown algal traits and to analyze their function across the brown algae.
The evolution of life history traits also needs to be addressed using theoretical and empirical approaches based on population genetics in an evolutionary ecology framework. However, as mentioned above, the application of these approaches is currently heavily biased toward a small number of model brown algae, primarily from the Ectocarpales and Laminariales. The scope of these analytical approaches will therefore need to be broadened to include other model orders with markedly different ecological and life history strategies, such as Fucales and Dictyotales (e.g. tropical taxa), or orders that have converged on similar ecological roles (i.e. kelp in the broad sense, with members from Desmarestiales, Fucales, Laminariales, and Tilopteridales). Moreover, there is little to no information about species that have a dominant haploid phase (). Currently important questions include: What is the frequency of life cycle variants within and among populations? What are the drivers of such variation? Is life cycle variation plastic or genetically determined?
C. Epigenetics
To date, brown algal evolution has been investigated almost exclusively in a genetic framework, but epigenetic processes (i.e. processes that do not involve modification of the underlying genomic blueprint) also play important roles and therefore need to be taken into consideration. By responding to changes in the environment, epigenetic processes mediate acclimatization and therefore act as a buffer, allowing longer-term adaptive (genetic) responses. Moreover, the ability of a species to implement epigenetic responses will affect its long-term capacity to respond to environmental changes, an important characteristic in the context of climate change. Transcriptomic studies are already providing information about epigenetic processes in terms of the control of gene expression levels (Monteiro et al., Citation2012; Martins et al., Citation2013; Monteiro et al., Citation2019). A key challenge for the future will be to extend this type of analysis to a broader range of model species/orders and to additional environmental contexts and parameters (as mentioned above). It will also be important to better understand the processes underlying transcriptomic responses, in particular processes that occur at the chromatin level. Methods have been adapted to detect and quantify post-translational modifications of histones in brown algae (Bourdareau et al., Citation2020) and a recent study has reported a low level of DNA methylation in S. japonica (Fan et al., Citation2020). Note, however, that DNA methylation does not appear to be present in all brown algae, notably Ectocarpus (Cock et al., Citation2010). These emerging tools are expected to provide new insights into brown algal biology and the mechanisms underlying evolutionary innovations in this group of organisms.
D. Evolution of complex multicellularity
Brown algae are also important from an evolutionary point of view because they independently acquired complex multicellularity and have emerged as the third most complex group of multicellular organisms on the planet. Another major objective will be to improve our understanding of brown algal developmental biology, as comparisons of developmental processes in brown algae with those of animals and land plants is expected to provide important, general insights into the molecular events that underlie this key evolutionary transition. Based on recent advances, work in this area is expected to uncover both examples of deep conservation of some regulatory mechanisms (such as the involvement of TALE HD TFs in life cycle regulation for example; Arun et al., Citation2019) and lineage-specific novelties (such as the EsV-1-7 domain family, for example, which is absent from both plants and animals; Macaisne et al., Citation2017). At present, brown algal developmental biology is in its infancy so significant advances can be expected in the future.
E. Speciation mechanisms and biogeography
Our understanding of speciation mechanisms and contemporary distributions of brown algae across the globe continues to improve. A bridge between these two concepts, however, is lacking for the brown algae (as reflected in the structure of this review). What is the interplay between speciation and biogeography that ultimately governs brown algal distributions? The evolutionary events that lead to the diversification and global establishment of some orders have been broadly described (e.g. Oligocene cooling and subsequent dominance of Laminariales) but how were these events influenced by niche preferences and species traits? Strengthening the relationship between species and environment would help shift the study of brown algal biogeography from a descriptive to a hypothesis driven field.
A challenge for future biogeographic investigations of the brown algae will be to develop good model systems both for microevolutionary investigation of recent speciation events and macroevolutionary studies revealing large-scale patterns across space and time. In particular, with the development of genomics and high-throughput sequencing technologies, it is now possible to explore and characterize both the genetic basis of reproductive isolation and the factors (e.g. selection, genetic architecture) that favor speciation in natural populations at the level of the entire genome (Abbott et al., Citation2016, Ravinet et al., Citation2017). A good candidate to investigate micro-evolutionary aspects of speciation is Ectocarpus given that this genus is a complex of species constituting a continuum with respect to divergence times (Montecinos, Couceiro, et al., Citation2017), allopatric and sympatric populations with various levels of hybridization have been established (Montecinos, Guillemin, et al., Citation2017), and additional genomes are on the horizon (see above). Similarly, model systems at the ordinal level, together with well resolved phylogenies, are needed to drive advances in macro-evolutionary investigations, some of which exist or are emerging (viz. Dictyotales, Fucales, Laminariales; Bolton, Citation2010; Starko et al., Citation2019, Steen et al., Citationunder revision).
The impact of climate change on biogeographic distributions of brown algae will continue to be a hot topic in the near future. Many species are projected to retreat toward higher latitudes with the movement of isotherms (Assis, Araújo, et al., Citation2018; Martínez et al., Citation2018), and the loss of unique genetic diversity (Nicastro et al., Citation2013; Neiva et al., Citation2015; Assis, Araújo, et al., Citation2018) and functional diversity (Pereira et al., Citation2015; Mota et al., Citation2018) at retreating rear-edges is a concern. Moreover, as marine heatwaves continue to grow in prevalence, mid-range extinctions (Bennett et al., Citation2015) are also more likely to occur and genetic diversity is further threatened (Smale et al., Citation2019; Gurgel et al., Citation2020). Climate-driven range dynamics have been mainly investigated in south-north orientated coastlines (e.g. northeast Atlantic, the southeast Pacific), where continuous thermal gradients and absence of major dispersal barriers create few constraints for species latitudinal migration and habitat tracking. Predictive efforts should be extended to biogeographical settings where range shifts are physically or environmentally constrained, such as latitude-constrained continental limits (e.g. South Africa, but see Martínez et al., Citation2018 for an Australian study), semi-enclosed, longitudinally oriented seas (e.g. Mediterranean) and off-shore archipelagos, all of which tend to have rich assemblages with a large proportion of narrow-endemics facing limited dispersal options.
Marine heatwaves may act as a source of directional selection, potentially improving the thermal tolerance of populations (Gurgel et al., Citation2020) but risking maladaptation to other factors (Brady et al., Citation2019). Understanding interactions between neutral (e.g. founder effect) and adaptive processes (e.g. directional selection) during range expansions and pulse disturbance events would provide important context for current trends. Characterization of adaptive variation in nonmodel organisms is becoming possible with the development of genomic and analytic approaches (Lotterhos and Whitlock, Citation2015; Manel et al., Citation2016) and these types of analyses should help to distinguish between (and compare) neutral and adaptive variation in future studies. As climate change continues to progress, it will be both troubling and captivating to see how the trajectory of brown algal evolution is altered and what consequences this will have on coastal ecosystems across the globe.
F. Concluding remarks
Our understanding of brown algal evolution has changed rapidly in the wake of DNA sequence data. In particular, molecular analyses have revealed unexpected relationships in the phylogeny of the brown algae, highlighting numerous independent transitions between various life history strategies. Sequence data has also allowed us, for the first time, to estimate the diversification timeline of the brown algal orders, expanding insights derived from fossil evidence. The advent of a fully annotated brown algal genome has also offered surprises on life history and reproduction strategies, and has opened the path forward for studying underlying gene programs. In the field of speciation and biogeography, sequence data has revealed novel and cryptic species hiding in plain sight, at times changing the story of how the brown algal lineages diversified and came to dominate coastal ecosystems across the globe. As the field of evolutionary biology continues to develop, particularly in the applications of genomic datasets, we look forward to the exciting progress to come in our knowledge of brown algal evolution.
bpts_a_1787679_sm9001.docx
Download MS Word (23.8 KB)Acknowledgments
We are grateful to the reviewer who provided helpful edits and feedback. The authors would also like to thank the health professionals and other essential service providers around the world that have been working on the ‘frontlines’ during the SARS-CoV-2 pandemic, which began while this review was being written. We also recognize the Traditional Inhabitants of both ceded and unceded territory on which this research was conducted, and acknowledge that gains in contemporary knowledge invariably build on a history of race and gender discrimination.
Additional information
Funding
References
- Abbott, I. A., and Huisman, J. M. 2003. New species, observations, and a list of new records of brown algae (Phaeophyceae) from the Hawaiian Islands. Phycological Res. 51: 173–185.
- Abbott, R. J., Barton, N. H., and Good, J. M. 2016. Genomics of hybridization and its evolutionary consequences. Mol. Ecol. 25: 2325–2332.
- Addico, G. N. D., and deGraft-Johnson, K. A. A. 2016. Preliminary investigation into the chemical composition of the invasive brown seaweed Sargassum along the West Coast of Ghana. Afr. J. Biotechnol. 15: 2184–2191.
- Ahmed, S., Cock, J. M., Pessia, E., Luthringer, R., Cormier, A., Robuchon, M., Sterck, L., Peters, A. F., Dittami, S. M., Corre, E., Valero, M., Aury, J.-M., Roze, D., Van de Peer, Y., Bothwell, J., Marais, G. A. B., and Coelho, S. M. 2014. A haploid system of sex determination in the brown alga Ectocarpus sp. Curr. Biol. 24: 1945–1957.
- Arnaud-Haond, S., Duarte, C. M., Diaz-Almela, E., Marbà, N., Sintes, T., and Serrão, E. Á. 2012. Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS One 7: e30454.
- Arun, A., Coelho, S. M., Peters, A. F., Bourdareau, S., Pérès, L., Scornet, D., Strittmatter, M., Lipinska, A. P., Yao, H., Godfroy, O., Montecinos, G. J., Avia, K., Macaisne, N., Troadec, C., Bendahmane, A., and Cock, J. M. 2019. Convergent recruitment of TALE homeodomain life cycle regulators to direct sporophyte development in land plants and brown algae. ELife 8: e43101.
- Assis, J., Araújo, M. B., and Serrão, E. Á. 2018. Projected climate changes threaten ancient refugia of kelp forests in the North Atlantic. Glob. Chang. Biol. 24: e55–e66.
- Assis, J., Coelho, N. C., Lamy, T., Valero, M., Alberto, F., and Serrão, E. Á. 2016. Deep reefs are climatic refugia for genetic diversity of marine forests. J. Biogeogr. 43: 833–844.
- Assis, J., Serrão, E. Á., Coelho, N. C., Tempera, F., Valero, M., and Alberto, F. 2018. Past climate changes and strong oceanographic barriers structured low–latitude genetic relics for the golden kelp Laminaria ochroleuca. J. Biogeogr. 45: 2326–2336.
- Augyte, S., Lewis, L., Lin, S., Neefus, C. D., and Yarish, C. 2018. Speciation in the exposed intertidal zone: the case of Saccharina angustissima comb. nov. & stat. nov. (Laminariales, Phaeophyceae). Phycologia 57: 100–112.
- Avia, K., Coelho, S. M., Montecinos, G. J., Cormier, A., Lerck, F., Mauger, S., Faugeron, S., Valero, M., Cock, J. M., and Boudry, P. 2017. High-density genetic map and identification of QTLs for responses to temperature and salinity stresses in the model brown alga Ectocarpus. Sci. Rep. 7: 43241.
- Avia, K., Lipinska, A., Mignerot, L., Montecinos, A., Jamy, M., Ahmed, S., Valero, M., Peters, A., Cock, J., Roze, D., and Coelho, S. 2018. Genetic diversity in the UV sex chromosomes of the brown alga Ectocarpus. Genes 9: 286.
- Bachtrog, D., Mank, J. E., Peichel, C. L., Kirkpatrick, M., Otto, S. P., Ashman, T.-L., Hahn, M. W., Kitano, J., Mayrose, I., Ming, R., Perrin, N., Ross, L., Valenzuela, N., and Vamosi, J. C, Tree of Sex Consortium. 2014. Sex determination: why so many ways of doing it? PLOS Biol. 12: e1001899.
- Baker, H. G. 1955. Self-compatibility and establishment after “long-distance” dispersal. Evolution 9: 347–349.
- Barner, A. K., Pfister, C. A., and Wootton, J. T. 2011. The mixed mating system of the sea palm kelp Postelsia palmaeformis: few costs to selfing. Proc. Biol. Sci. 278: 1347–1355.
- Bartsch, I., Wiencke, C., Bischof, K., Buchholz, C. M., Buck, B. H., Eggert, A., Feuerpfeil, P., Hanelt, D., Jacobsen, S., Karez, R., Karsten, U., Molis, M., Roleda, M. Y., Schubert, H., Schumann, R., Valentin, K., Weinberger, F., and Wiese, J. 2008. The genus Laminaria sensu lato: Recent insights and developments. Euro. J. Phycol. 43: 1–86.
- Belcher, J. H., Carter, C. F., and Peters, A. F. 2009. An unknown filamentous brown alga (Phaeophyceae) from a domestic freshwater aquarium in Northampton UK. Phycologist 77: 13.
- Bell, G. 1982. The Masterpiece of Nature: The Evolution and Genetics on Sexuality. University of California Press, Berkeley, Los Angeles, USA.
- Bell, G. 1997. The evolution of the life cycle of brown seaweeds. Biol. J. Linn. Soc. 60: 21–38.
- Bellwood, D. R., Hughes, T. P., and Hoey, A. S. 2006. Sleeping functional group drives coral-reef recovery. Curr. Biol. 16: 2434–2439.
- Bennett, S., Wernberg, T., Connell, S. D., Hobday, A. J., Johnson, C. R., and Poloczanska, E. S. 2016. The ‘Great Southern Reef’: social, ecological and economic value of Australiás neglected kelp forests. Mar. Freshwater Res. 67: 47–56.
- Bennett, S., Wernberg, T., Joy, B. A., De Bettignies, T., and Campbell, A. H. 2015. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6: 1–7.
- Bennion, M., Fisher, J., Yesson, C., and Brodie, J. 2019. Remote sensing of kelp (Laminariales, Ochrophyta): monitoring tools and implications for wild harvesting. Rev. Fish. Sci. Aquac. 27: 127–141.
- Billot, C., Engel, C.R., Rousvoal, S., Kloare, G. B., and Valero, M. 2003. Current patterns, habitat discontinuities and population genetic structure: the case of the kelp Laminaria digitata in the English Channel. Mar. Ecol. Prog. Ser. 253: 111–121.
- Bittner, L., Payri, C. E., Couloux, A., Cruaud, C., de Reviers, B., and Rousseau, F. 2008. Molecular phylogeny of the Dictyotales and their position within the Phaeophyceae, based on nuclear, plastid and mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 49: 211–226.
- Blanchette, C. A., Miner, B. G., and Gaines, S. D. 2002. Geographic variability in form, size and survival of Egregia menziesii around Point Conception, California. Mar. Ecol. Prog. Ser. 239: 69–82.
- Bologna, P., and Steneck, R. 1993. Kelp beds as habitat for American lobster Homarus americanus. Mar. Ecol. Prog. Ser. 100: 127–134.
- Bolton, J.J. 2010. The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular genetics. Helgol. Mar. Res. 64: 263–279.
- Bolton, J. J., Germann, I., and Luning, K. 1983. Hybridization between Atlantic and Pacific representatives of the Simplices section of Laminaria (Phaeophyta). Phycologia 22: 133–140.
- Bourdareau, S., Tirichine, L., Lombard, B., Loew, D., Coelho, S. M., and Cock, J. M. 2020. Histone modifications during the life cycle of the brown alga Ectocarpus. BioRxiv. 980763.
- Brady, S. P., Bolnick, D. I., Angert, A. L., Gonzalez, A., Barrett, R. D. H., Crispo, E., Derry, A. M., Eckert, C. G., Fraser, D. J., Fussmann, G. F., Guichard, F., Lamy, T., McAdam, A. G., Newman, A. E. M., Paccard, A., Rolshausen, G., Simons, A. M., and Hendry, A. P. 2019. Causes of maladaptation. Evol. Appl. 12: 1229–1242.
- Braje, T. J., Dillehay, T. D., Erlandson, J. M., Klein, R. G., and Rick, T. C. 2017. Finding the first Americans. Science. 358: 592–594.
- Braun, A. 1855. Algarum unicellularium genera nova et minus cognita praemissis observationibus de algis unicellularibus in genere. Apud W. Engelmann, Leipzig, Germany.
- Bringloe, T. T., and Saunders, G. W. 2018. Mitochondrial DNA sequence data reveal the origins of postglacial marine macroalgal flora in the Northwest Atlantic. Mar. Ecol. Prog. Ser. 589: 45–58.
- Bringloe, T. T., and Saunders, G. W. 2019. DNA barcoding of the marine macroalgae of Nome, Alaska (Northern Bering Sea) reveals many trans-Arctic species. Polar Biol. 42: 851–864.
- Bringloe, T. T., Bartlett, C. A.B., Bergeron, E. S., Cripps, K. S.A., Daigle, N. J., Gallagher, P. O., Gallant, A. D., Giberson, R. O. J., Greenough, S. J., Lamb, J. M., Leonard, T. W., MacKay, J. A., McKenzie, A. D., Persaud, S. M., Sheng, T., Mills, A. M. E. S., Moore, T. E., and Saunders, G. W. 2018. Detecting Alaria esculenta and Laminaria digitata (Laminariales, Phaeophyceae) gametophytes in red algae, with consideration of distribution patterns in the intertidal zone. Phycologia 57: 1–8.
- Bringloe, T. T., Verbruggen, H., and Saunders, G. W. 2020. Population structure in Arctic marine forests is shaped by diverse recolonisation pathways and far northern glacial refugia. BioRxiv. 999466.
- Briones-Fourzán, P., and Lozano-Álvarez, E. 2001. The importance of Lobophora variegata (Phaeophyta: Dictyotales) as a habitat for small juveniles of Panulirus argus (Decapoda: Palinuridae) in a tropical reef lagoon. Bull. Mar. Sci. 68: 207–219.
- Brodie, J., Chan, C. X., De Clerck, O., Cock, J. M., Coelho, S. M., Gachon, C., Grossman, A. R., Mock, T., Raven, J. A., Smith, A. G., Yoon, H. S., and Bhattacharya, D. 2017. The algal revolution. Trends Plant Sci. 22: 726–738.
- Brown, J. W., and Sorhannus, U. L. F. 2010. A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): substantive underestimation of putative fossil ages. PLoS One 5: e12759.
- Bruno de Sousa, C., Cox, C. J., Brito, L., Pavão, M. M., Pereira, H., Ferreira, A., Ginja, C., Campino, L., Bermejo, R., Parente, M., and Varela, J. 2019. Improved phylogeny of brown algae Cystoseira (Fucales) from the Atlantic-Mediterranean region based on mitochondrial sequences. PLoS One 14: e0210143.
- Bruno, J., and Bertness, M. 2001. Habitat modification and facilitation in benthic marine communities. In Marine Community Ecology; Bertness, M., Gaines, S., and Hay, M., Eds. Sinauer Associates: Sunderland, UK, pp. 201–218.
- Bull, J. J. 1983. Evolution of Sex Determining Mechanisms. Benjamin/Cummings Publishing Company, California, USA.
- Burki, F., Roger, A. J., Brown, M. W., and Simpson, A. G. B. 2020. The new tree of Eukaryotes. Trends Ecol. Evol. (Amst.) 35: 43–55.
- Burrowes, R., Rousseau, F., Müller, D. G., and de Reviers, B. 2003. Taxonomic placement of Microzonia (Phaeophyceae) in Syringodermatales based on rbcL and 28S nrDNA sequences. Cryptog. Algol. 24: 63–73.
- Camacho, O., Fernández-García, C., Vieira, C., Gurgel, C. F. D., Norris, J. N., Freshwater, D. W., and Fredericq, S. 2019. The systematics of Lobophora (Dictyotales, Phaeophyceae) in the western Atlantic and eastern Pacific oceans: eight new species. J. Phycol. 55: 611–624.
- Camacho, O., Sauvage, T., and Fredericq, S. 2018. Taxonomic transfer of Syringoderma to Microzonia (Syringodermataceae, Syringodermatales), including a new record of Microzonia floridana comb. nov. for the Gulf of Mexico. Phycologia 57: 413–421.
- Cánovas, F. G., Mota, C. F., Serrão, E. Á., and Pearson, G. A. 2011. Driving south: a multi-gene phylogeny of the brown algal family Fucaceae reveals relationships and recent drivers of a marine radiation. BMC Evol. Biol. 11: 371.
- Chan, A. 2018. DNA barcode and phylogenetic analyses of Sphacelariales (Phaeophyceae) emphasizing Canada and contiguous coastal areas. Honours thesis, University of New Brunswick, Canada.
- Charrier, B., Abreu, M. H., Araujo, R., Bruhn, A., Coates, J. C., De Clerck, O., Katsaros, C., Robaina, R. R., and Wichard, T. 2017. Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol. 216: 967–975.
- Charrier, B., Coelho, S. M., Le Bail, A., Tonon, T., Michel, G., Potin, P., Kloareg, B., Boyen, C., Peters, A. F., and Cock, J. M. 2008. Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research. New Phytol. 177: 319–332.
- Cho, G. Y., Lee, S. H., and Boo, S. M. 2004. A new brown algal order, Ishigeales (Phaeophyceae), established on the basis of plastid protein-coding rbcL, psaA, psbA region comparisons. J. Phycol. 40: 921–936.
- Clayton, M. N. 1987. Isogamy and a fucalean type of life history in the Antarctic brown alga Ascoseira mirabilis (Ascoseirales, Phaeophyta). Bot. Mar. 30: 447–454.
- Clayton, M. N. 1988. Evolution and life histories of brown algae. Bot. Mar. 31: 379–387.
- Cock, J. M., Godfroy, O., Macaisne, N., Peters, A. F., and Coelho, S. M. 2014. Evolution and regulation of complex life cycles: a brown algal perspective. Curr. Opin. Plant Biol. 17: 1–6.
- Cock, J. M., Sterck, L., Rouzé, P., Scornet, D., Allen, A. E., Amoutzias, G., Anthouard, V., Artiguenave, F., Aury, J.-M., Badger, J. H., Beszteri, B., Billiau, K., Bonnet, E., Bothwell, J. H., Bowler, C., Boyen, C., Brownlee, C., Carrano, C. J., Charrier, B., Cho, G. Y., Coelho, S. M., Collén, J., Corre, E., Da Silva, C., Delage, L., Delaroque, N., Dittami, S. M., Doulbeau, S., Elias, M., Farnham, G., Gachon, C. M. M., Gschloessl, B., Heesch, S., Jabbari, K., Jubin, C., Kawai, H., Kimura, K., Kloareg, B., Küpper, F. C., Lang, D., Le Bail, A., Leblanc, C., Lerouge, P., Lohr, M., Lopez, P. J., Martens, C., Maumus, F., Michel, G., Miranda-Saavedra, D., Morales, J., Moreau, H., Motomura, T., Nagasato, C., Napoli, C. A., Nelson, D. R., Nyvall-Collén, P., Peters, A. F., Pommier, C., Potin, P., Poulain, J., Quesneville, H., Read, B., Rensing, S. A., Ritter, A., Rousvoal, S., Samanta, M., Samson, G., Schroeder, D. C., Ségurens, B., Strittmatter, M., Tonon, T., Tregear, J. W., Valentin, K., von Dassow, P., Yamagishi, T., Van de Peer, Y., and Wincker, P. 2010. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465: 617–621.
- Cock, J. M., Peters, A. F., and Coelho, S. M. 2011. Brown algae. Curr. Biol. 21: R573–5.
- Cock, J. M., Sterck, L., Ahmed, S., Allen, A. E., Amoutzias, G., Anthouard, V., Artiguenave, F., Arun, A., Aury, J.-M., Badger, J. H., Beszteri, B., Billiau, K., Bonnet, E., Bothwell, J. H., Bowler, C., Boyen, C., Brownlee, C., Carrano, C. J., Charrier, B., Cho, G. Y., Coelho, S. M., Collén, J., Le Corguillé, G., Corre, E., Dartevelle, L., Da Silva, C., Delage, L., Delaroque, N., Dittami, S. M., Doulbeau, S., Elias, M., Farnham, G., Gachon, C. M. M., Godfroy, O., Gschloessl, B., Heesch, S., Jabbari, K., Jubin, C., Kawai, H., Kimura, K., Kloareg, B., Küpper, F. C., Lang, D., Le Bail, A., Luthringer, R., Leblanc, C., Lerouge, P., Lohr, M., Lopez, P. J., Macaisne, N., Martens, C., Maumus, F., Michel, G., Miranda-Saavedra, D., Morales, J., Moreau, H., Motomura, T., Nagasato, C., Napoli, C. A., Nelson, D. R., Nyvall-Collén, P., Peters, A.F., Pommier, C., Potin, P., Poulain, J., Quesneville, H., Read, B., Rensing, S. A., Ritter, A., Rousvoal, S., Samanta, M., Samson, G., Schroeder, D. C., Scornet, D., Ségurens, B., Strittmatter, M., Tonon, T., Tregear, J.W., Valentin, K., von Dassow, P., Yamagishi, T., Rouzé, P., Van de Peer, Y., and Wincker, P. 2012. Chapter five – the Ectocarpus genome and brown algal genomics: the Ectocarpus genome consortium. Adv. Bot. Res. 64: 141–184.
- Coelho, S. M., Godfroy, O., Arun, A., Le Corguillé, G., Peters, A. F., and Cock, J. M. 2011. OUROBOROS is a master regulator of the gametophyte to sporophyte life cycle transition in the brown alga Ectocarpus. Proc. Natl. Acad. Sci. USA. 108: 11518–11523.
- Coelho, S. M., Gueno, J., Lipinska, A. P., Cock, J. M., and Umen, J. G. 2018. UV chromosomes and haploid sexual systems. Trends Plant Sci. 23: 794–807.
- Coelho, S. M., Peters, A. F., Charrier, B., Roze, D., Destombe, C., Valero, M., and Cock, J. M. 2007. Complex life cycles of multicellular eukaryotes: new approaches based on the use of model organisms. Gene 406: 152–170.
- Coelho, S. M., Scornet, D., Rousvoal, S., Peters, N., Dartevelle, L., Peters, A. F., and Cock, J. M. 2012. Ectocarpus: a model organism for the brown algae. Cold Spring Harb. Protoc. 2012: 193–198.
- Coleman, M. A., and Wernberg, T. 2017. Forgotten underwater forests: the key role of fucoids on Australian temperate reefs. Ecol. Evol. 7: 8406–8418.
- Connell, S. D. 2003a. The monopolization of understorey habitat by subtidal encrusting coralline algae: a test of the combined effects of canopy-mediated light and sedimentation. Mar. Biol. 142: 1065–1071.
- Connell, S. D. 2003b. Negative effects overpower the positive of kelp to exclude invertebrates from the understorey community. Oecologia 137: 97–103.
- Cormier, A., Avia, K., Sterck, L., Derrien, T., Wucher, V., Andres, G., Monsoor, M., Godfroy, O., Lipinska, A., Perrineau, M.-M., Van De Peer, Y., Hitte, C., Corre, E., Coelho, S. M., and Cock, J. M. 2017. Re-annotation, improved large-scale assembly and establishment of a catalogue of noncoding loci for the genome of the model brown alga Ectocarpus. New Phytol. 214: 219–232.
- Couceiro, L., Le Gac, M., Hunsperger, H. M., Mauger, S., Destombe, C., Cock, J. M., Ahmed, S., Coelho, S. M., Valero, M., and Peters, A. F. 2015. Evolution and maintenance of haploid-diploid life cycles in natural populations: the case of the marine brown alga Ectocarpus. Evolution 69: 1808–1822.
- Coyer, J. A., Hoarau, G., Oudot-Le Secq, M. P., Stam, W. T., and Olsen, J. L. 2006. A mtDNA-based phylogeny of the brown algal genus Fucus (Heterokontophyta; Phaeophyta). Mol. Phylogenet. Evol. 39: 209–222.
- Coyer, J. A., Hoarau, G., Van Schaik, J., Luijckx, P., and Olsen, J. L. 2011. Trans-Pacific and trans-Arctic pathways of the intertidal macroalga Fucus distichus L. reveal multiple glacial refugia and colonizations from the North Pacific to the North Atlantic. J. Biogeogr. 38: 756–771.
- Coyer, J. A., Peters, A. F., Hoarau, G., Stam, W. T., and Olsen, J. L. 2002. Inheritance patterns of ITS1, chloroplasts and mitochondria in artificial hybrids of the seaweeds Fucus serratus and F. evanescens (Phaeophyceae). Euro. J. Phycol. 37: 173–178.
- Crow, J. F., and Kimura, M. 1965. Evolution in sexual and asexual populations. Am. Nat. 99: 439–450.
- Dayton, P. K. 1985. Ecology of kelp communities. Annu. Rev. Ecol. Syst. 16: 215–245.
- De Clerck, O., Leliaert, F., Verbruggen, H., Lane, C. E., De Paula, J. C., Payo, D. A., and Coppejans, E. 2006. A revised classification of the Dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence analyses. J. Phycol. 42: 1271–1288.
- De Reviers, B., Rousseau, F., and Draisma, S. G. A. 2007. Classification of the Phaeophyceae from past to present and current challenges. In Unravelling the Algae, the past, Present, and Future of Algal Systematics; Brodie, J., and Lewis, J., Eds. CRC Press, Taylor & Francis Group, Boca Raton, FL, USA, pp. 267–284.
- De Széchy, M. T. M., Guedes, P. M., Baeta-Neves, M. H., and Oliveira, E. N. 2012. Verification of Sargassum natans (Linnaeus) Gaillon (Heterokontophyta: Phaeophyceae) from the Sargasso Sea off the coast of Brazil. Check List 8: 638–641.
- Deniaud-Bouët, E., Kervarec, N., Michel, G., Tonon, T., Kloareg, B., and Hervé, C. 2014. Chemical and enzymatic fractionation of cell walls from Fucales: insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 114: 1203–1216.
- Derelle, R., López-García, P., Timpano, H., and Moreira, D. 2016. A phylogenomic framework to study the diversity and evolution of Stramenopiles (=Heterokonts). Mol. Biol. Evol. 33: 2890–2898.
- Destombe, C., Godin, J., Nocher, M., Richerd, S., and Valero, M. 1993. Differences in response between haploid and diploid isomorphic phases of Gracilaria verrucosa (Rhodophyta, Gigartinales) exposed to artificial environmental conditions. Hydrobiologia 261: 131–137.
- Destombe, C., Valero, M., Vernet, P., and Couvet, D. 1989. What controls haploid-diploid ratio in the red alga Gracilaria verrucosa. J. Evolution. Biol. 2: 317–338.
- Dittami, S. M., Corre, E., Brillet-Guéguen, L., Lipinska, A. P., Pontoizeau, N., Aite, M., and Avia, K. 2020. The genome of Ectocarpus subulatus - a highly stress-tolerant brown alga. Mar. Genom. 52: 100740.
- Dittami, S. M., Gravot, A., Goulitquer, S., Rousvoal, S., Peters, A. F., Bouchereau, A., Boyen, C., and Tonon, T. 2012. Towards deciphering dynamic changes and evolutionary mechanisms involved in the adaptation to low salinities in Ectocarpus (brown algae). Plant J. 71: 366–377.
- Dittami, S. M., Heesch, S., Olsen, J. L., and Collén, J. 2017. Transitions between marine and freshwater environments provide new clues about the origins of multicellular plants and algae. J. Phycol. 53: 731–745.
- Draisma, S. G. A., Ballesteros, E., Rousseau, F., and Thibaut, T. 2010. DNA sequence data demonstrate the polyphyly of the genus Cystoseira and other Sargassaceae genera (phaeophyceae). J. Phycol. 46: 1329–1345.
- Draisma, S. G. A., and Prud'homme Van Reine, W. F. 2001. Onslowiaceae fam. nov. (Phaeophyceae). J. Phycol. 37: 647–649.
- Draisma, S. G. A., Prud’homme van Reine, W., and Kawai, H. 2010. A revised classification of the Sphacelariales (Phaeophyceae) inferred from a psbC and rbcL based phylogeny. Europ. J. Phycol. 45: 308–326.
- Drobnitch, S. T., Jensen, K. H., Prentice, P., and Pittermann, J. 2015. Convergent evolution of vascular optimization in kelp (Laminariales). Proc. Biol. Sci. 282: 20151667.
- Druehl, L. D. 1988. Cultivated edible kelp. In Algae and Human Affairs; Lembi, C. A., and Waaland, J. R., Eds. Cambridge University Press, Cambridge, UK, pp. 119–134.
- Duggins, D. O., Simenstad, C. A., and Estes, J. A. 1989. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245: 170–173.
- Dunton, K. H., and Schell, D. M. 1986. Seasonal carbon budget and growth of Laminaria solidungula in the Alaskan High Arctic. Mar. Ecol. Prog. Ser. 31: 57–66.
- Ellegren, H., and Parsch, J. 2007. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 8: 689–698.
- Emerson, J. J., Kaessmann, H., Betrán, E., and Long, M. 2004. Extensive gene traffic on the mammalian X chromosome. Science 303: 537–540.
- Erlandson, J. M., Braje, T. J., Gill, K. M., and Graham, M. H. 2015. Ecology of the kelp highway: did marine resources facilitate human dispersal from Northeast Asia to the Americas? J. Isl. Coast. Archaeol. 10: 392–411.
- Estes, J. A., Burdin, A., and Doak, D. F. 2016. Sea otters, kelp forests, and the extinction of Steller’s sea cow. Proc. Natl. Acad. Sci. USA. 113: 880–885.
- Estes, J. A., and Steinberg, P. D. 1988. Predation, herbivory, and kelp evolution. Paleobiology 14: 19–36.
- Excoffier, L., and Ray, N. 2008. Surfing during population expansions promotes genetic revolutions and structuration. Trends Ecol. Evol. (Amst.). 23: 347–351.
- Fan, X., Han, W., Teng, L., Jiang, P., Zhang, X., Xu, D., Li, C., Pellegrini, M., Wu, C., Wang, Y., Kaczurowski, M. J. S., Lin, X., Tirichine, L., Mock, T., and Ye, N. 2020. Single-base methylome profiling of the giant kelp Saccharina japonica reveals significant differences in DNA methylation to microalgae and plants. New Phytol. 225: 234–249.
- Filbee-Dexter, K., Feehan, C. J., and Scheibling, R. E. 2016. Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Mar. Ecol. Prog. Ser. 543: 141–152.
- Filbee-Dexter, K., and Wernberg, T. 2018. Rise of turfs: a new battlefront for globally declining kelp forests. BioScience 68: 64–76.
- Fitton, J. H. 2003. Brown marine algae: a survey of therapeutic potentials. Altern. Complem. Ther. 9: 29–33.
- Fitton, J. H. 2011. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 9: 1731–1760.
- Fleurence, J., Morançais, M., Dumay, J., Decottignies, P., Turpin, V., Munier, M., Garcia-Bueno, N., and Jaouen, P. 2012. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Tech. 27: 57–61.
- Fox, C. H., and Swanson, A. K. 2007. Nested PCR detection of microscopic life-stages of laminarian macroalgae and comparison with adult forms along intertidal height gradients. Mar. Ecol. Prog. Ser. 332: 1–10.
- Fraser, C. I. 2012. Is bull-kelp kelp? The role of common names in science. New Zeal. J. Mar. Fresh. 46: 279–284.
- Fraser, C. I., Hay, C. H., Spencer, H. G., and Waters, J. M. 2009. Genetic and morphological analyses of the southern bull kelp Durvillaea antarctica (Phaeophyceae: Durvillaeales) in new zealand reveal cryptic species. J. Phycol. 45: 436–443.
- Fraser, C. I., McGaughran, A., Chuah, A., and Waters, J. M. 2016. The importance of replicating genomic analyses to verify phylogenetic signal for recently evolved lineages. Mol. Ecol. 25: 3683–3695.
- Fraser, C. I., Nikula, R., Spencer, H. G., and Waters, J. M. 2009. Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. Proc. Natl. Acad. Sci. USA. 106: 3249–3253.
- Fraser, C. I., Spencer, H. G., and Waters, J. M. 2009. Glacial oceanographic contrasts explain phylogeography of Australian bull kelp. Mol. Ecol. 18: 2287–2296.
- Fraser, C. I., Velásquez, M., Nelson, W. A., Macaya, E. C., and Hay, C. H. 2020. The biogeographic importance of buoyancy in macroalgae: a case study of the southern bull-kelp genus Durvillaea (Phaeophyceae), including descriptions of two new species. J. Phycol. 56: 23–36.
- Fraser, C. I., Winter, D. J., Spencer, H. G., and Waters, J. M. 2010. Multigene phylogeny of the southern bull-kelp genus Durvillaea (Phaeophyceae: Fucales). Mol. Phylogenet. Evol. 57: 1301–1311.
- Froehlich, H. E., Afflerbach, J. C., Frazier, M., and Halpern, B. S. 2019. Blue growth potential to mitigate climate change through seaweed offsetting. Curr. Biol. 29: 3087–3093.
- Fritsch, F. E. 1935. The Structure and Reproduction of the Algae. Cambridge University Press, Cambridge, UK.
- Fritsch, F. E. 1945. The Structure and Reproduction of the Algae. Vol. 2. Cambridge University Press, Cambridge, UK.
- Fritsch, F. E. 1949. The lines of algal advance. Biol. Rev. Camb. Philos. Soc. 24: 94–124.
- Fry, W. L., and Banks, H. P. 1955. Three new genera of algae from the Upper Devonian of New York. J. Paleontol. 29: 37–44.
- Fulton, C. J., Abesamis, R. A., Berkström, C., Depczynski, M., Graham, N. A. J., Holmes, T. H., Kulbicki, M., Noble, M. M., Radford, B. T., Tano, S., Tinkler, P., Wernberg, T., and Wilson, S. K. 2019. Form and function of tropical macroalgal reefs in the Anthropocene. Funct. Ecol. 33: 989–999.
- Funk, D. J., and Omland, K. E. 2003. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 34: 397–423.
- Gattuso, J.-P., Gentili, B., Duarte, C. M., Kleypas, J. A., Middelburg, J. J., and Antoine, D. 2006. Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences 3: 489–513.
- Gavrilets, S. 2003. Perspective: models of speciation: what have we learned in 40 years? Evolution 57: 2197–2215.
- Gavrilets, S., Li, H., and Vose, M. D. 1998. Rapid parapatric speciation on holey adaptive landscapes. Proc. Biol. Sci. 265: 1483–1489.
- Geoffroy, A., Mauger, S., De Jode, A., Le Gall, L., and Destombe, C. 2015. Molecular evidence for the coexistence of two sibling species in Pylaiella littoralis (Ectocarpales, Phaeophyceae) along the Brittany coast. J. Phycol. 51: 480–489.
- Gerard, V. A. 1984. The light environment in a giant kelp forest: influence of Macrocystis pyrifera on spatial and temporal variability. Mar. Biol. 84: 189–195.
- Godfroy, O., Uji, T., Nagasato, C., Lipinska, A. P., Scornet, D., Peters, A. F., Avia, K., Colin, S., Mignerot, L., Motomura, T., Cock, J. M., and Coelho, S. M. 2017. DISTAG/TBCCd1 is required for basal cell fate determination in Ectocarpus. Plant Cell 29: 3102–3122.
- Goodwillie, C., Kalisz, S., and Eckert, C. G. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 36: 47–79.
- Gordillo, F. J. L., Dring, M. J., and Savidge, G. 2002. Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity. Mar. Ecol. Prog. Ser. 234: 111–118.
- Gouvêa, L. P., Assis, J., Gurgel, C. F. D., Serrão, E. A., Silveira, T. C. L., Santos, R., Duarte, C. M., Peres, L. M. C., Carvalho, V. F., Batista, M., Bastos, E., Sissini, M. N., and Horta, P. A. 2020. Golden carbon of Sargassum forests revealed as an opportunity for climate change mitigation. Sci. Total Environ. 729: 138745.
- Graham, M. H. 2004. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems 7: 341–357.
- Graham, M. H., Kinlan, B. P., Druehl, L. D., Garske, L. E., and Banks, S. 2007. Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proc. Natl. Acad. Sci. USA. 104: 16576–16580.
- Graves, J. A. M., and Peichel, C. L. 2010. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 11: 205.
- Guillemin, M.L., Valero, M., Tellier, F., Macaya, E. C., Destombe, C., and Faugeron, S. 2016. Phylogeography of seaweeds in the South East Pacific: complex evolutionary processes along a latitudinal gradient. In Seaweed Phylogeography: adaptation and Evolution of Seaweeds under Environmental Change; Hu, Z.-M., and Fraser, C. I., Eds. Springer, Dordrecht, Netherlands, pp. 251–277.
- Guiry, M., and Guiry, G. 2020. AlgaeBase. http://www.algaebase.org (accessed Feb 2, 2020).
- Gurgel, C. F. D., Camacho, O., Minne, A. J., Wernberg, T., and Coleman, M. A. 2020. Marine heatwave drives cryptic loss of genetic diversity in underwater forests. Curr. Biol. 30: 1–8.
- Guzinski, J., Ballenghien, M., Daguin-Thiébaut, C., Lévêque, L., and Viard, F. 2018. Population genomics of the introduced and cultivated Pacific kelp Undaria pinnatifida: Marinas-not farms-drive regional connectivity and establishment in natural rocky reefs. Evol. Appl. 11: 1582–1597.
- Han, K. Y., Maciszewski, K., Graf, L., Yang, J. H., Andersen, R. A., Karnkowska, A., and Yoon, H. S. 2019. Dictyochophyceae plastid genomes reveal unusual variability in their organization. J. Phycol. 55: 1166–1180.
- Hargreaves, A.L., Eckert, C.G., and Bailey, J. 2014. Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Funct. Ecol. 28: 5–21.
- Hausdorf, B. 2011. Progress toward a general species concept. Evolution 65: 923–931.
- Hay, M. E., Duffy, J. E., Pfister, C. A., and Fenical, W. 1987. Chemical defense against different marine herbivores: are amphipods insect equivalents? Ecology. 68: 1567–1580.
- Hays, C. G. 2007. Adaptive phenotypic differentiation across the intertidal gradient in the alga Silvetia compressa. Ecology 88: 149–157.
- Hedgethorne, K., Eustermann, S., Yang, J.-C., Ogden, T. E. H., Neuhaus, D., and Bloomfield, G. 2017. Homeodomain-like DNA binding proteins control the haploid-to-diploid transition in Dictyostelium. Sci. Adv. 3: e1602937.
- Heesch, S., Cho, G. Y., Peters, A. F., Le Corguillé, G., Falentin, C., Boutet, G., Coëdel, S., Jubin, C., Samson, G., Corre, E., Coelho, S. M., and Cock, J. M. 2010. A sequence-tagged genetic map for the brown alga Ectocarpus siliculosus provides large-scale assembly of the genome sequence. New Phytol. 188: 42–51.
- Heesch, S., Serrano-Serrano, M., Luthringer, R., Peters, A. F., Destombe, C., Cock, J. M., and Valero, M. 2019. Evolution of life cycles and reproductive traits: insights from the brown algae. BioRxiv. 530477.
- Henry, E.C. 1984. Syringodermatales ord. nov. and Syringoderma floridana sp. nov. (Phaeophyceae). Phycologia 23: 419–426.
- Henry, E. C., and Müller, D. G. 1983. Studies on the life history of Syringoderma phinneyi sp. nov. (Phaeophyceae). Phycologia 22: 387–393.
- Hickey, L. J., West, R. M., Dawson, M. R., and Choi, D. K. 1983. Arctic terrestrial biota: paleomagnetic evidence of age disparity with mid-northern latitudes during the late Cretaceous and early Tertiary. Science 221: 1153–1156.
- Hind, K. R., Starko, S., Burt, J. M., Lemay, M. A., Salomon, A. K., and Martone, P. T. 2019. Trophic control of cryptic coralline algal diversity. Proc. Natl. Acad. Sci. USA. 116: 15080–15085.
- Hoarau, G., Coyer, J. A., Giesbers, M. C. W. G., Jueterbock, A., and Olsen, J. L. 2015. Pre-zygotic isolation in the macroalgal genus Fucus from four contact zones spanning 100–10 000 years: a tale of reinforcement? R. Soc. Open Sci. 2: 140538.
- Hoarau, G., Coyer, J. A., Veldsink, J. H., Stam, W. T., and Olsen, J. L. 2007. Glacial refugia and recolonization pathways in the brown seaweed Fucus serratus. Mol. Ecol. 16: 3606–3616.
- Hodge, F. J., Buchanan, J., and Zuccarello, G. C. 2010. Hybridization between the endemic brown algae Carpophyllum maschalocarpum and Carpophyllum angustifolium (Fucales): genetic and morphological evidence. Phycol. Res. 58: 239–247.
- Hoegh Guldberg, O., Chopin, T., Gaines, S., Haugan, P., Hemer, M., Howard, J., and Konar, M. 2019. The Ocean as a Solution to Climate Change: Five Opportunities for Action. World Resources Institute, Washington, DC, USA.
- Holbrook, S. J., Carr, M. H., Schmitt, R. J., and Coyer, J. A. 1990. Effect of giant kelp on local abundance of reef fishes: the importance of ontogenetic resource requirements. B. Mar. Sci. 47: 104–114.
- Hooper, R. G., Henry, E. C., and Kuhlenkamp, R. 1988. Phaeosiphoniella cryophila gen. et sp. nov., a third member of the Tilopteridales (Phaeophyceae). Phycologia 27: 395–404.
- Hori, T. 1971. Survey of pyrenoid distribution in brown algae. Bot. Mag., Tokyo 84: 231–242.
- Hori, T. 1993. Brown and red algae. Vol. 2. In An Illustrated Atlas of the Life History of Algae; T. Hori, Ed. Uchida Rokakuo, Tokyo, Japan, p. 345. (In Japanese).
- Hu, Z.-M., Duan, D. L., and Lopez-Bautista, J. 2016. Seaweed phylogeography from 1994–2014: an overview. In Seaweed Phylogeography: Adaptation and Evolution of Seaweeds Under Environmental Change; Hu, Z.-M., and Fraser, C. I., Eds. Springer, Dordrecht, Netherlands, pp 3–22.
- Hu, Z.-M., Li, J.-J., Sun, Z.-M., Gao, X., Yao, J.-T., Choi, H.-G., Endo, H., and Duan, D.-L. 2017. Hidden diversity and phylogeographic history provide conservation insights for the edible seaweed Sargassum fusiforme in the Northwest Pacific. Evol. Appl. 10: 366–378.
- Hughes, J. S., and Otto, S. P. 1999. Ecology and the evolution of biphasic life cycles. Am. Nat. 154: 306–320.
- Hughes, T., Szmant, A. M., Steneck, R., Carpenter, R., and Miller, S. 1999. Algal blooms on coral reefs: what are the causes? Limnol. Oceanogr. 44: 1583–1586.
- Hull, C. M., Boily, M.-J., and Heitman, J. 2005. Sex-specific homeodomain proteins Sxi1alpha and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot. Cell 4: 526–535.
- Hurd, C. L., and Stevens, C. L. 1997. Flow visualization around single- and multiple-bladed seaweeds with various morphologies. J. Phycol. 33: 360–367.
- Idnurm, A., Walton, F. J., Floyd, A., and Heitman, J. 2008. Identification of the sex genes in an early diverged fungus. Nature 451: 193–196.
- Ivany, L., Patterson, W. P., and Lohmann, K. C. 2000. Cooler winters as a possible cause of mass extinctions at the Eocene/Oligocene boundary. Nature 407: 887–890.
- Jackson, C., Salomaki, E. D., Lane, C. E., and Saunders, G. W. 2017. Kelp transcriptomes provide robust support for interfamilial relationships and revision of the little known Arthrothamnaceae (Laminariales). J. Phycol. 53: 1–6.
- Jaenicke, L., Muellar, D. G., and Moore, R. E. 1974. Multifidene and aucantene, C11 hydrocarbons in the male-attracting essential oil from the gynogametes of Cutleria multifida (Smith) Grev. (Phaeophyta). J. Am. Chem. Soc. 96: 3324–3325.
- Jenkyns, H. C., Forster, A., Schouten, S., and Sinninghe Damsté, J. S. 2004. High temperatures in the Late Cretaceous Arctic Ocean. Nature 432: 888–892.
- Johnson, A., and Koehl, M. 1994. Maintenance of dynamic strain similarity and environmental stress factor in different flow habitats: thallus allometry and material properties of a giant kelp. J. Exp. Biol. 195: 381–410.
- Kaullysing, D., Gopeechund, A., Mattan-Moorgawa, S., Taleb-Hossenkhan, N., Kulkarni, B., and Bhagooli, R. 2016. Increased density of the corallivore Drupella cornus on Acropora muricata colonies overgrown by Padina boryana. Proceedings of the 13th International Coral Reef Symposium, Honolulu, pp 288–304.
- Kawai, H. 1986. Life history and systematic position of Akkesiphycus lubricus (Phaeophyceae). J. Phycol. 22: 286–291.
- Kawai, H. 1989. Life history and systematic position of Heteroralfsia saxicola gen. et comb. nov. (Ralfsiaceae, Phaeophyceae). Phycologia 28: 243–251.
- Kawai, H., and Kurogi, M. 1985. On the life history of Pseudochorda nagaii (Pseudochordaceae fam. nov.) and its transfer from the Chordariales to the Laminariales (Phaeophyta). Phycologia 24: 289–296.
- Kawai, H., and Nabata, S. 1990. Life history and systematic position of Pseudochorda gracilis sp. nov. (Laminariales, Phaeophyceae). J. Phycol. 26: 721–727.
- Kawai, H., and Sasaki, H. 2000. Molecular phylogeny of the brown algal genera Akkesiphycus and Halosiphon (Laminariales), resulting in the circumscription of the new families Akkesiphycaceae and Halosiphonacacea. Phycologia 39: 416–428.
- Kawai, H., and Sasaki, H. 2004. Morphology, life history, and molecular phylogeny of Stschapovia flagellaris (Tilopteridales, Phaeophyceae) and the erection of the Stschapoviaceae. fam. nov. J. Phycol. 40: 1156–1169.
- Kawai, H., and Yamada, I. 1990. The specific identity and life history of Japanese Syringoderma (Syringodermatales, Phaeophyceae). Bot. Mag. Tokyo. 103: 325–334.
- Kawai, H., Hanyuda, T., Ridgway, L. M., and Holser, K. 2013. Ancestral reproductive structure in basal kelp Aureophycus aleuticus. Sci. Rep. 3: 2491.
- Kawai, H., Hanyuda, T., Bolton, J., and Anderson, R. 2016. Molecular phylogeny of Zeacarpa (Ralfsiales, Phaeophyceae) proposing a new family Zeacarpaceae and its transfer to Nemodermatales. J. Phycol. 52: 682–686.
- Kawai, H., Hanyuda, T., Draisma, S. G. A., and Müller, D. G. 2007. Molecular phylogeny of Discosporangium mesarthrocarpum (Phaeophyceae) with a reassessment of the Discosporangiales. J. Phycol. 43: 186–194.
- Kawai, H., Hanyuda, T., Draisma, S. G., Wilce, R. T., and Andersen, R. A. 2015. Molecular phylogeny of two unusual brown algae, Phaeostrophion irregulare and Platysiphon glacialis, proposal of the Stschapoviales ord. nov. and Platysiphonaceae fam. nov., and a re-examination of divergence times for brown algal orders. J. Phycol. 51: 918–928.
- Kawai, H., Hanyuda, T., Lindeberg, M., and Lindstrom, S. C. 2008. Morphology and molecular phylogeny of Aureophycus aleuticus gen. et sp. nov. (Laminariales, Phaeophyceae) from the Aleutian Islands. J. Phycol. 44: 1013–1021.
- Kawai, H., Hanyuda, T., Yamagishi, T., Kai, A., Lane, C. E., McDevit, D., Küpper, F. C., and Saunders, G. W. 2015. Reproductive morphology and DNA sequences of the brown alga Platysiphon verticillatus support the new combination Platysiphon glacialis. J. Phycol. 51: 910–917.
- Kawai, H., Maeba, S., Sasaki, H., Okuda, K., and Henry, E. C. 2003. Schizocladia ischiensis: a new filamentous marine chromophyte belonging to a new class, Schizocladiophyceae. Protist 154: 211–228.
- Kawai, H., Sasaki, H., Maeba, S., and Henry, E. C. 2005. Morphology and molecular phylogeny of Phaeostrophion irregulare (Phaeophyceae) with proposal for Phaeostrophiaceae fam. nov., and a review of Ishigeaceae. Phycologia 44: 169–182.
- Kawai, H., Suzuki, M., Saunders, G. W., and Hanyuda, T. 2019. Taxonomic study of the brown algal genus Chorda (Chordaceae, Laminariales) with description of the new species Chorda borealis from Alaska and northern Canada. Europ. J. Phycol. 54: 509–517.
- Keeling, P. J. 2013. The number, speed, and impact of plastid endosymbioses in Eukaryotic evolution. Annu. Rev. Plant Biol. 64: 583–607.
- Kim, J. I., Shin, H., Škaloud, P., Jung, J., Yoon, H. S., Archibald, J. M., and Shin, W. 2019. Comparative plastid genomics of Synurophyceae: inverted repeat dynamics and gene content variation. BMC Evol. Biol. 19: 20.
- Kitada, S., Nakajima, K., Hamasaki, K., Shishidou, H., Waples, R. S., and Kishino, H. 2019. Rigorous monitoring of a large-scale marine stock enhancement program demonstrates the need for comprehensive management of fisheries and nursery habitat. Sci. Rep. 9: 5290.
- Kloareg, B., and Quatrano, R. S. 1988. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. 26: 259–315.
- Knoll, A. H. 2011. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 39: 217–239.
- Kobayashi, H., Haino, Y., Iwasaki, T., Tezuka, A., Nagano, A. J., and Shimada, S. 2018. ddRAD-seq based phylogeographic study of Sargassum thunbergii (Phaeophyceae, Heterokonta) around Japanese coast. Mar. Environ. Res. 140: 104–113.
- Kogame, K., Rindi, F., Peters, A. F., and Guiry, M. D. 2015. Genetic diversity and mitochondrial introgression in Scytosiphon lomentaria (Ectocarpales, Phaeophyceae) in the north-eastern Atlantic Ocean. Phycologia 54: 367–374.
- Kraan, S., and Guiry, M. D. 2000. Sexual hybridization experiments and phylogenetic relationships as inferred from Rubisco spacer sequences in the genus Alaria (Phaeophyceae). J. Phycol. 36: 190–198.
- Kraft, G. T., Saunders, G. W., Abbott, I. A., and Haroun, H. J. 2004. A uniquely calcified brown alga from Hawaii: Newhousia imbricata gen. et sp. nov. (Dictyotales, Phaeophyceae). J. Phycol. 40: 383–394.
- Krause-Jensen, D., and Duarte, C. M. 2016. Substantial role of macroalgae in marine carbon sequestration. Nature Geosci. 9: 737–742.
- Krause-Jensen, D., Lavery, P., Serrano, O., Marbà, N., Masque, P., and Duarte, C. M. 2018. Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biol. Lett. 14: 20180236.
- Krings, M., Klavins, S. D., Barthel, M., Lausberg, S., Serbet, R., Taylor, T. N., and Taylor, E. L. 2007. Perissothallus, a new genus for Late Pennsylvanian-Early Permian noncalcareous algae conventionally assigned to Schizopteris (alphleboid foliage). Bot. J. Linn. Soc. 153: 477–488.
- Krueger-Hadfield, S. A., Roze, D., Correa, J. A., Destombe, C., and Valero, M. 2015. O father where art thou? Paternity analyses in a natural population of the haploid-diploid seaweed Chondrus crispus. Heredity 114: 185–194.
- Krumhansl, K. A., Okamoto, D. K., Rassweiler, A., Novak, M., Bolton, J. J., Cavanaugh, K. C., Connell, S. D., Johnson, C. R., Konar, B., Ling, S. D., Micheli, F., Norderhaug, K. M., Pérez-Matus, A., Sousa-Pinto, I., Reed, D. C., Salomon, A. K., Shears, N. T., Wernberg, T., Anderson, R. J., Barrett, N. S., Buschmann, A. H., Carr, M. H., Caselle, J. E., Derrien-Courtel, S., Edgar, G. J., Edwards, M., Estes, J. A., Goodwin, C., Kenner, M. C., Kushner, D. J., Moy, F. E., Nunn, J., Steneck, R. S., Vásquez, J., Watson, J., Witman, J. D., and Byrnes, J. E. K. 2016. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. USA. 113: 13785–13790.
- Krumhansl, K., and Scheibling, R. 2012. Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 467: 281–302.
- Kucera, H., and Saunders, G. W. 2008. Assigning morphological variants of Fucus (Fucales, Phaeophyceae) in Canadian waters to recognized species using DNA barcoding. Botany 86: 1065–1079.
- Küpper, F. C., Amsler, C. D., Morley, S., de Reviers, B., Reichardt, A., Peck, L. S., and Peters, A. F. 2019. Juvenile morphology of the large Antarctic canopy-forming brown alga Desmarestia menziesii. Polar Biol. 42: 2097–2103.
- Küpper, F. C., Peters, A. F., Shewring, D. M., Sayer, M. D. J., Mystikou, A., Brown, H., Azzopardi, E., Dargent, O., Strittmatter, M., Brennan, D., Asensi, A. O., van West, P., and Wilce, R. T. 2016. Arctic marine phytobenthos of northern Baffin Island. J. Phycol. 52: 532–549.
- Kylin, H. 1933. Über die Entwicklungsgeschichte der Phaeophyceen. Lunds Universitets Årsskrift N.F. Avd, 2, 29: 21–101.
- La Barre, S., Potin, P., Leblanc, C., and Delage, L. 2010. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs 8: 988–1010.
- Lane, C. E., Mayes, C., Druehl, L. D., and Saunders, G. W. 2006. A multi-gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic re-organization. J. Phycol. 42: 493–512.
- Laughinghouse, H. D., Müller, K. M., Adey, W. H., Lara, Y., Young, R., and Johnson, G. 2015. Evolution of the northern rockweed, Fucus distichus, in a regime of glacial cycling: implications for benthic algal phylogenetics. PLoS One 10: e0143795.
- Lawver, L., Müller, R., Srivastava, S., and Roest, W. 1990. The opening of the Arctic Ocean. In Geological History of the Polar Oceans: Arctic vs. Antarctic; Bleil U., and Thiede J., Eds. Kluwer Academic Publishers, Netherlands, pp 29–62.
- Le Cam, S., Daguin-Thiébaut, C., Bouchemousse, S., Engelen, A.H., Mieszkowska, N., and Viard, F. 2020. A genome-wide investigation of the worldwide invader Sargassum muticum shows high success albeit (almost) no genetic diversity . Evol. Appl. 13: 500–514.
- Leblanc, C., Colin, C., Cosse, A., Delage, L., La Barre, S., Morin, P., Fiévet, B., Voiseux, C., Ambroise, Y., Verhaeghe, E., Amouroux, D., Donard, O., Tessier, E., and Potin, P. 2006. Iodine transfers in the coastal marine environment: the key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie 88: 1773–1785.
- Lee, K. M., Boo, S. M., Kain, J. M., and Sherwood, A. R. 2013. Cryptic diversity and biogeography of the widespread brown alga Colpomenia sinuosa (Ectocarpales, Phaeophyceae). Bot. Mar. 56: 15–25.
- Lee, K. Y., and Mooney, D. J. 2012. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37: 106–126.
- Lee, R. K. S. 1973. General ecology of the Canadian Arctic benthic marine algae. Arctic 26: 32–43.
- Levitan, D. R., Fukami, H., Jara, J., Kline, D., McGovern, T. M., McGhee, K. E., Swanson, C. A., and Knowlton, N. 2004. Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58: 308–323.
- León-Alvarez, D., Reyes-Gómez, V. P., Wynne, M. J., Ponce-Márquez, M. E., and Quiróz-González. 2017. Morphological and molecular characterization of Hapalospongidion gelatinosum, Hapalospongidiaceae fam. nov. (Ralfsiales, Phaeophyceae) from Mexico. Bot. Mar. 60: 567–581.
- Li, B., Lu, F., Wei, X., and Zhao, R. 2008. Fucoidan: structure and bioactivity. Molecules 13: 1671–1695.
- Liggan, L. M., and Martone, P. T. 2018. Under pressure: biomechanical limitations of developing pneumatocysts in the bull kelp (Nereocystis luetkeana, Phaeophyceae). J. Phycol. 54: 608–615.
- Lim, P. E., Sakaguchi, M., Hanyuda, T., Kogame, K., Phang, S. -M., and Kawai, H. 2007. Molecular phylogeny of crustose brown algae (Ralfsiales, Phaeophyceae) inferred from rbcL sequences resulting in proposal for Neoralfsiaceae fam. nov. Phycologia 46: 456–466.
- Lipinska, A., Cormier, A., Luthringer, R., Peters, A. F., Corre, E., Gachon, C. M. M., Cock, J. M., and Coelho, S. M. 2015. Sexual dimorphism and the evolution of sex-biased gene expression in the brown alga Ectocarpus. Mol. Biol. Evol. 32: 1581–1597.
- Lipinska, A. P., Serrano-Serrano, M. L., Cormier, A., Peters, A. F., Kogame, K., Cock, J. M., and Coelho, S. M. 2019. Rapid turnover of life-cycle-related genes in the brown algae. Genome Biol. 20: 35.
- Lipinska, A., Toda, N., Heesch, S., Peters, A.F., Cock, J.M., and Coelho, S.M. 2017. Multiple gene movements into and out of haploid sex chromosomes. Genome Biol. 18: 104.
- Liu, X., Bogaert, K., Engelen, A. H., Leliaert, F., Roleda, M. Y., and De Clerck, O. 2017. Seaweed reproductive biology: environmental and genetic controls. Bot. Mar. 60: 89–108.
- López-Cristoffanini, C., Tellier, F., Otaíza, R., Correa, J. A., and Contreras-Porcia, L. 2013. Tolerance to air exposure: a feature driving the latitudinal distribution of two sibling kelp species. Bot. Mar. 56: 431–440.
- Lotterhos, K. E., and Whitlock, M. C. 2015. The relative power of genome scans to detect local adaptation depends on sampling design and statistical method. Mol. Ecol. 24: 1031–1046.
- Louime, C., Fortune, J., and Gervais, G. 2017. Sargassum invasion of coastal environments: a growing concern. Am. J. Environ. Sci. 13: 58–64.
- Lourenço, C. R., Zardi, G. I., McQuaid, C. D., Serrao, E. Á., Pearson, G. A., Jacinto, R., and Nicastro, K. R. 2016. Upwelling areas as climate change refugia for the distribution and genetic diversity of a marine macroalga. J. Biogeogr. 43: 1595–1607.
- Lund, S. 1951. Marine algae from Jörgen Brönlunds Fjord in eastern North Greenland. Meddr. Grønland. 128: 1–26.
- Lüning, K., and Müller, D. G. 1978. Chemical interaction in sexual reproduction of several Laminariales (Phaeophyceae): release and attraction of spermatozoids. Z. Pflanzenphysiol. 89: 333–341.
- Lüning, K. 1990a. Temperate and Polar seaweed vegetation of the southern Hemisphere. In Seaweeds: Their Environment, Biogeography, and Ecophysiology; Yarish, C., and Kirkman, H., Eds. John Wiley & Sons, New York, USA.
- Lüning, K. 1990b. Introduction to vertical and geographical distribution. In Seaweeds: Their Environment, Biogeography, and Ecophysiology; Yarish, C., and Kirkman, H., Eds. John Wiley & Sons, New York, USA.
- Luthringer, R., Cormier, A., Ahmed, S., Peters, A.F., Cock, J.M., and Coelho, S.M. 2014. Sexual dimorphism in the brown algae. Pers. Phycol. 1: 11–25.
- Luthringer, R., Lipinska, A. P., Roze, D., Cormier, A., Macaisne, N., Peters, A. F., Cock, J. M., and Coelho, S. M. 2015. The pseudoautosomal regions of the U/V sex chromosomes of the brown alga Ectocarpus exhibit unusual features. Mol. Biol. Evol. 32: 2973–2985.
- Mable, B. K., and Otto, S. P. 1998. The evolution of life cycles with haploid and diploid phases. Bioessays 20: 453–462.
- Mac Monagail, M., Cornish, L., Morrison, L., Araújo, R., and Critchley, A. T. 2017. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 52: 371–390.
- Macaisne, N., Liu, F., Scornet, D., Peters, A. F., Lipinska, A., Perrineau, M.-M., Henry, A., Strittmatter, M., Coelho, S. M., and Cock, J. M. 2017. The Ectocarpus IMMEDIATE UPRIGHT gene encodes a member of a novel family of cysteine-rich proteins with an unusual distribution across the eukaryotes. Development 144: 409–418.
- Macaya, E. C., and Zuccarello, G. C. 2010. Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Mar. Ecol. Prog. Ser. 420: 103–112.
- Maier, I. 1995. Brown algal pheromones. Vol. 11. In Progress in Phycological Research; Round, F. E. and Chapman, D. J., Eds. Biopress, Bristol, UK, pp 51–102.
- Manel, S., Perrier, C., Pratlong, M., Abi-Rached, L., Paganini, J., Pontarotti, P., and Aurelle, D. 2016. Genomic resources and their influence on the detection of the signal of positive selection in genome scans. Mol. Ecol. 25: 170–184.
- Mann, K. H. 1973. Seaweeds: their productivity and strategy for growth: The role of large marine algae in coastal productivity is far more important than has been suspected. Science 182: 975–981.
- Manna, F., Gallet, R., Martin, G., and Lenormand, T. 2012. The high-throughput yeast deletion fitness data and the theories of dominance. J. Evol. Biol. 25: 892–903.
- Manton, I. 1959. Observations on the internal structure of the spermatozoid of Dictyota. J. Exp. Bot. 10: 448–461.
- Marincovich, J. L., Brouwers, E. M., Hopkins, D. M., and McKenna, M. C. 1990. Late Mesozoic and Cenozoic paleogeographic and paleoclimatic history of the Arctic Ocean Basin, based on shallow-water marine faunas and terrestrial vertebrates. In The Arctic Ocean Region; Grantz, A., Johnson, L., and Sweeney, J., Eds. Geological Society of America, Boulder, Colorado, USA, pp 403–426.
- Markel, R. W., Lotterhos, K. E., and Robinson, C. L. K. 2017. Temporal variability in the environmental and geographic predictors of spatial-recruitment in nearshore rockfishes. Mar. Ecol. Prog. Ser. 574: 97–111.
- Martin, P., and Zuccarello, G. C. 2012. Molecular phylogeny and timing of radiation in Lessonia (Phaeophyceae, Laminariales). Phycol. Res. 60: 276–287.
- Martínez, B., Radford, B., Thomsen, M. S., Connell, S. D., Carreño, F., Bradshaw, C. J. A., Fordham, D. A., Russell, B. D., Gurgel, C. F. D., and Wernberg, T. 2018. Distribution models predict large contractions of habitat forming seaweeds in response to ocean warming. Divers. Distrib. 24: 1350–1366.
- Martins, M. J. F., Mota, C. F., and Pearson, G. A. 2013. Sex-biased gene expression in the brown alga Fucus vesiculosus. BMC Genomics 14: 294.
- Martins, N., Pearson, G. A., Gouveia, L., Tavares, A. I., Serrão, E. Á., and Bartsch, I. 2019. Hybrid vigour for thermal tolerance in hybrids between the allopatric kelps Laminaria digitata and L. pallida (Laminariales, Phaeophyceae) with contrasting thermal affinities. Eur. J. Phycol. 54: 548–561.
- Mautner, H.G. 1954. The chemistry of brown algae. Econ. Bot. 8: 174–192.
- McCauley, L. A. R., and Wehr, J. D. 2007. Taxonomic reappraisal of the freshwater brown algae Bodanella, Ectocarpus, Heribaudiella, and Pleurocladia (Phaeophyceae) on the basis of rbcL sequences and morphological characters. Phycologia 46: 429–439.
- McCulloch, R. D., Bentley, M. J., Purves, R. S., Hulton, N. R. J., Sugden, D. E., and Clapperton, C. M. 2000. Climatic inferences from glacial and palaeoecological evidence at the last glacial termination, southern South America. J. Quaternary Sci. 15: 409–417.
- McHugh, D. J. 2003. A guide to the seaweed industry. FAO Fisheries Technical Paper 441. Food and Agriculture Organization of the United Nations, Rome.
- Mendez-Tejeda, R., and Rosado Jiménez, G. A. 2019. Influence of climatic factors on Sargassum arrivals to the coasts of the Dominican Republic. J. Oceanogr. Mar. Sci. 10: 22–32.
- Meslet-Cladière, L., Delage, L., Leroux, C. J.-J., Goulitquer, S., Leblanc, C., Creis, E., Gall, E. A., Stiger-Pouvreau, V., Czjzek, M., and Potin, P. 2013. Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 25: 3089–3103.
- Milledge, J. J., Nielsen, B. V., and Bailey, D. 2016. High-value products from macroalgae: the potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 15: 67–88.
- Miller, G.H., Brigham-Grette, J., Alley, R.B., Anderson, L., Bauch, H.A., Douglas, M.S.V., Edwards, M.E., Elias, S.A., Finney, B.P., Fitzpatrick, J.J., Funder, S.V., Herbert, T.D., Hinzman, L.D., Kaufman, D.S., MacDonald, G.M., Polyak, L., Robock, A., Serreze, M.C., Smol, J.P., Spielhagen, R., White, J.W.C., Wolfe, A.P., and Wolff, E.W. 2010. Temperature and precipitation history of the Arctic. Quat. Sci. Rev. 29: 1679–1715.
- Miller, K. A., Olsen, J. L., and Stam, W. T. 2000. Genetic divergence correlates with morphological and ecological subdivision in the deep‐water elk kelp, Pelagophycus porra (Phaeophyceae). J. Phycol. 36: 862–870.
- Mineur, F., Arenas, F., Assis, J., Davies, A. J., Engelen, A. H., Fernandes, F., Malta, E-j., Thibaut, T., Van Nguyen, T., Vaz-Pinto, F., Vranken, S., Serrão, E. A., and De Clerck, O. 2015. European seaweeds under pressure: consequences for communities and ecosystem functioning. J. Sea Res. 98: 91–108.
- Miyata, M., Okazaki, M., and Furuya, K. 1977. Site and nature of calcium carbonate deposits in a calcareous brown alga Padina japonica (studies on the calcium carbonate deposition of algae). Bull. Jpn. Soc. Phycol. 25: 1–6.
- Moalic, Y., Arnaud-Haond, S., Perrin, C., Pearson, G. A., and Serrao, E. Á. 2011. Travelling in time with networks: revealing present day hybridization versus ancestral polymorphism between two species of brown algae, Fucus vesiculosus and F. spiralis. BMC Evol. Biol. 11: 33.
- Moe, R. L., and Henry, E. C. 1982. Reproduction and early development of Ascoseira mirabilis Skottsberg (Phaeophyta), with notes on Ascoseirales Petrov. Phycologia 21: 55–66.
- Montecinos, A. E., Couceiro, L., Peters, A. F., Desrut, A., Valero, M., and Guillemin, M. L. 2017. Species delimitation and phylogeographic analyses in the Ectocarpus subgroup siliculosi (Ectocarpales, Phaeophyceae). J. Phycol. 53: 17–31.
- Montecinos, A. E., Guillemin, M., Couceiro, L., Peters, A. F., Stoeckel, S., and Valero, M. 2017. Hybridization between two cryptic filamentous brown seaweeds along the shore: analysing pre- and postzygotic barriers in populations of individuals with varying ploidy levels. Mol. Ecol. 26: 3497–3512.
- Monteiro, C. A., Paulino, C., Jacinto, R., Serrão, E. Á., and Pearson, G. A. 2016. Temporal windows of reproductive opportunity reinforce species barriers in a marine broadcast spawning assemblage. Sci. Rep. 6: 29198.
- Monteiro, C. A., Serrão, E. Á., and Pearson, G. A. 2012. Prezygotic barriers to hybridization in marine broadcast spawners: reproductive timing and mating system variation. PLoS One 7: e35978.
- Monteiro, C., Heinrich, S., Bartsch, I., Valentin, K.U., Corre, E., Collén, J., Harms, L., Glöckner, G., and Bischof, K. 2019. Temperature dependent sex-biased gene expression in the gametophytes of the kelp Saccharina latissima. BioRxiv. 750455.
- Morya, V. K., Kim, J., and Kim, E.-K. 2012. Algal fucoidan: structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 93: 71–82.
- Mota, C. F., Engelen, A. H., Serrão, E. Á., Coelho, M. A. G., Marbà, N., Krause-Jensen, D., and Pearson, G. A. 2018. Differentiation in fitness-related traits in response to elevated temperatures between leading and trailing edge populations of marine macrophytes. PLoS One 13: e0203666.
- Müller, D. G. 1964. Life-cycle of Ectocarpus siliculosus from Naples, Italy. Nature 203: 1402–1402.
- Müller, D. G., and Gassmann, G. 1980. Sexual hormone specificity in Ectocarpus and Laminaria (Phaeophyceae). Naturwissenschaften 67: 462–463.
- Müller, D. G., Gassmann, G., Boland, W., Marner, F., and Jaenicke, L. 1981. Dictyota dichotoma (Phaeophyceae): identification of the sperm attractant. Science 212: 1040–1041.
- Müller, D. G., Gassmann, G., and Lüning, K. 1979. Isolation of a spermatozoid-releasing and -attracting substance from female gametophytes of Laminaria digitata. Nature 279: 430–431.
- Müller, D. G., and Jaenicke, L. 1973. Fucoserraten, the female sex attractant of Fucus serratus L. (Phaeophyta). FEBS Lett. 30: 137–139.
- Müller, D. G., Jaenicke, L., Donike, M., and Akintobi, T. 1971. Sex attractant in a brown alga: chemical structure. Science 171: 815–817.
- Müller, D. G., Murúa, P., and Westermeier, R. 2019. Reproductive strategies of Lessonia berteroana (Laminariales, Phaeophyceae) gametophytes from Chile: apogamy, parthenogenesis and cross-fertility with L. spicata. J. Appl. Phycol. 31: 1475–1481.
- Mystikou, A., Peters, A. F., Asensi, A. O., Fletcher, K. I., Brickle, P., van West, P., Convey, P., and Küpper, F. C. 2014. Seaweed biodiversity in the south-western Antarctic Peninsula: surveying macroalgal community composition in the Adelaide Island/Marguerite Bay region over a 35-year time span. Polar Biol. 37: 1607–1619.
- Nasmyth, K., and Shore, D. 1987. Transcriptional regulation in the yeast life cycle. Science 237: 1162–1170.
- Navarro, A., and Barton, N. H. 2002. The effects of multilocus balancing selection on neutral variability. Genetics 161: 849–863.
- Neiva, J., Assis, J., Coelho, N. C., Fernandes, F., Pearson, G. A., and Serrão, E. A. 2015. Genes left behind: climate change threatens cryptic genetic diversity in the canopy-forming seaweed Bifurcaria bifurcata. PLoS One 10: e0131530.
- Neiva, J., Assis, J., Fernandes, F., Pearson, G. A., and Serrão, E. Á. 2014. Species distribution models and mitochondrial DNA phylogeography suggest an extensive biogeographical shift in the high-intertidal seaweed Pelvetia canaliculata. J. Biogeogr. 41: 1137–1148.
- Neiva, J., Hansen, G. I., Pearson, G. A., Vliet, M. S. V. D., Maggs, C. A., and Serrão, E. Á. 2012. Fucus cottonii (Fucales, Phaeophyceae) is not a single genetic entity but a convergent salt-marsh morphotype with multiple independent origins. Eur. J. Phycol. 47: 461–468.
- Neiva, J., Paulino, C., Nielsen, M. M., Krause-Jensen, D., Saunders, G. W., Assis, J., Bárbara, I., Tamigneaux, É., Gouveia, L., Aires, T., Marbà, N., Bruhn, A., Pearson, G. A., and Serrão, E. A. 2018. Glacial vicariance drives phylogeographic diversification in the amphi-boreal kelp Saccharina latissima. Sci. Rep. 8: 1112.
- Neiva, J., Pearson, G. A., Valero, M., and Serrão, E. Á. 2010. Surfing the wave on a borrowed board: range expansion and spread of introgressed organellar genomes in the seaweed Fucus ceranoides L. Mol. Ecol. 19: 4812–4822.
- Neiva, J., Pearson, G. A., Valero, M., and Serrão, E. Á. 2012. Drifting fronds and drifting alleles: Range dynamics, local dispersal and habitat isolation shape the population structure of the estuarine seaweed Fucus ceranoides. J. Biogeogr. 39: 1167–1178.
- Neiva, J., Serrão, E. A., Anderson, L., Raimondi, P. T., Martins, N., Gouveia, L., Paulino, C., Coelho, N. C., Miller, K. A., Reed, D. C., Ladah, L. B., and Pearson, G. A. 2017. Cryptic diversity, geographical endemism and allopolyploidy in NE Pacific seaweeds. BMC Evol. Biol. 17: 30.
- Neiva, J., Serrão, E. Á., Assis, J., Pearson, G. A., Coyer, J. A., Olsen, J. L., Hoarau, G., and Valero, M. 2016. Climate oscillations, range shifts and phylogeographic patterns of North Atlantic Fucaceae. In Seaweed Phylogeography: adaptation and Evolution of Seaweeds under Environmental Change; Hu, Z.-M., and Fraser, C. I., Eds. Springer, Dordrecht, Netherlands, pp 279–308.
- Nicastro, K. R., Zardi, G., Teixeira, S., Neiva, J., Serrão, E. Á., and Pearson, G. A. 2013. Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 11: 6.
- Ni-Ni-Win, H. T., Arai, S., Uchimura, M., Abbott, I.A., and Kawai, H. 2008. New records of Padina species from the western coast of the Pacific Ocean. Phycol. Res 56: 288–300.
- Ni-Ni-Win, H. T., Arai, S., Uchimura, M., Prathep, A., Draisma, S. G. A., Soe, H., and Kawai, H. 2010. Four new species of Padina (Dictyotales, Phaeophyceae) from the western Pacific, Ocean, and reinstatement of Padina japonica. Phycologia 49: 136–153.
- Ni-Ni-Win, H. T., Arai, S., Uchimura, M., Prathep, A., Draisma, S. G. A., Phang, S. M., Abbott, I. A., Millar, A. J. K., and Kawai, H. 2011. A taxonomic study of the genus Padina (Dictyotales, Phaeophyceae) including the description of four new species from Japan, Hawaii, and the Andaman Sea. J. Phycol. 47: 1193–1209.
- Ni-Ni-Win, H. T.., Draisma, S.G.A., Furnari, G., Meinesz, A., and Kawai, H. 2011. Padina ditristromatica sp. nov. and Padina pavonicoides sp. nov. (Dictyotales, Phaeophyceae), two new species from the Mediterranean Sea based on morphological and molecular markers. Europ. J. Phycol. 46: 327–341.
- Ni-Ni-Win, H. T., Draisma, S. G. A., Prud'homme van Reine, W. F., Lim, P. E., Phang, S. M., and Kawai, H. 2012. Two new species of Padina (Dictyotales, Phaeophyceae), P. indiana and P. calcarea, from tropical Indo-Pacific regions based on morphological and molecular evidence. Phycologia 51: 576–585.
- Ni-Ni-Win, H. T., Kato, A., and Kawai, H. 2018. Two new species of Padina (Dictyotales, Phaeophyceae) from southern Japan, P. ogasawaraensis sp. nov. and P. reniformis sp. nov., based on morphology and molecular markers. Phycologia 57: 20–31.
- Nishitsuji, K., Arimoto, A., Higa, Y., Mekaru, M., Kawamitsu, M., Satoh, N., and Shoguchi, E. 2019. Draft genome of the brown alga, Nemacystus decipiens, Onna-1 strain: Fusion of genes involved in the sulfated fucan biosynthesis pathway. Sci. Rep. 9: 4607.
- Nishitsuji, K., Arimoto, A., Iwai, K., Sudo, Y., Hisata, K., Fujie, M., Arakaki, N., Kushiro, T., Konishi, T., Shinzato, C., Satoh, N., and Shoguchi, E. 2016. A draft genome of the brown alga, Cladosiphon okamuranus, S-strain: a platform for future studies of “mozuku” biology. DNA Res. 23: 561–570.
- Oppliger, L. V., Correa, J. A., Engelen, A. H., Tellier, F., Vieira, V., Faugeron, S., Valero, M., Gomez, G., and Destombe, C. 2012. Temperature effects on gametophyte life-history traits and geographic distribution of two cryptic kelp species. PLoS One 7: e39289.
- Oppliger, L. V., Correa, J. A., Faugeron, S., Beltrán, J., Tellier, F., Valero, M., and Destombe, C. 2011. Sex ratio variation in the Lessonia nigrescens complex (Laminariales, Phaeophyceae): effect of latitude, temperature, and marginality. J. Phycol. 47: 5–12.
- Oppliger, L. V., von Dassow, P., Bouchemousse, S., Robuchon, M., Valero, M., Correa, J. A., Mauger, S., and Destombe, C. 2014. Alteration of sexual reproduction and genetic diversity in the kelp species Laminaria digitata at the southern limit of its range. PLoS One 9: e102518.
- Otto, S. P., and Marks, J. C. 1996. Mating systems and the evolutionary transition between haploidy and diploidy. Biol. J. Linn. Soc. 57: 197–218.
- Otto, S. P., Pannell, J. R., Peichel, C. L., Ashman, T.-L., Charlesworth, D., Chippindale, A. K., Delph, L. F., Guerrero, R. F., Scarpino, S. V., and McAllister, B. F. 2011. About PAR: the distinct evolutionary dynamics of the pseudoautosomal region. Trends Genet. 27: 358–367.
- Øverland, M., Mydland, L. V., and Skrede, A. 2019. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 99: 13–24.
- Pacheco-Ruíz, I., Cabello-Pasini, A., Zertuche-Gonzalez, J. A., Murray, S., Espinoza-Avalos, J., and Dreyfus-Leon, M. J. 2011. Carpospore and tetraspore release and survival in Chondracanthus squarrulosus (Rhodophyta: Gigartinaceae) from the Gulf of California. Bot. Mar. 54: 127–134.
- Parker, B. C., and Dawson, E. Y. 1965. Non-calcareous marine algae from California Miocene deposits. Nova Hedwigia 10: 273–295.
- Parsch, J., and Ellegren, H. 2013. The evolutionary causes and consequences of sex-biased gene expression. Nat. Rev. Genet. 14: 83–87.
- Pearson, G. A., Kautsky, L., and Serrão, E. Á. 2000. Recent evolution in Baltic Fucus vesiculosus: reduced tolerance to emersion stresses compared to intertidal (North Sea) populations. Mar. Ecol. Prog. Ser. 202: 67–79.
- Pearson, G. A., and Serrão, E. Á. 2006. Revisiting synchronous gamete release by fucoid algae in the intertidal zone: fertilization success and beyond? Integr. Comp. Biol. 46: 587–597.
- Peate, D. W. 1997. The Paraná-Etendeka province. In Large Igneous Provinces: Continental, Oceanic, and Planetary Flood Volcanism. Geophysical Monographic Series, vol. 1; Mahoney, J. J., and Coffin, M. F., Eds. John Wiley & Sons, New York, USA, pp 217–245.
- Peck, J. R., Yearsley, J. M., and Waxman, D. 1998. Explaining the geographic distributions of sexual and asexual populations. Nature 391: 889–892.
- Pelletreau, K. N., and Muller-Parker, G. 2002. Sulfuric acid in the phaeophyte alga Desmarestia munda deters feeding by the sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 141: 1–9.
- Pereira, T. R., Engelen, A. H., Pearson, G. A., Serrão, E. Á., Destombe, C., and Valero, M. 2011. Temperature effects on the microscopic haploid stage development of Laminaria ochroleuca and Sacchoriza polyschides, kelps with contrasting life histories. Cah. Biol. Mar. 52: 395–403.
- Pereira, T. R., Engelen, A. H., Pearson, G. A., Valero, M., and Serrão, E. Á. 2017. Population dynamics of temperate kelp forests near their low-latitude limit. Aquat. Bot. 139: 8–18.
- Pereira, T.R., Engelen, A.H., Pearson, G.A., Valero, M., and Serrão, E. 2015. Response of kelps from different latitudes to consecutive heat shock. J. Exp. Mar. Biol. Ecol. 463: 57–62.
- Pereira, V., Marques, A., Gaivão, I., Rego, A., Abreu, H., Pereira, R., Santos, M. A., Guilherme, S., and Pacheco, M. 2019. Marine macroalgae as a dietary source of genoprotection in gilthead seabream (Sparus aurata) against endogenous and exogenous challenges. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 219: 12–24.
- Perrin, C., Daguin, C.V., De Vliet, M., Enge, l C. R., Pearson, G. A., and Serrão, E. Á. 2007. Implications of mating system for genetic diversity of sister algal species: Fucus spiralis and Fucus vesiculosus (Heterokontophyta, Phaeophyceae). Eur. J. Phycol. 42: 219–230.
- Perrin, N. 2012. What uses are mating types? The “developmental switch” model. Evolution 66: 947–956.
- Perrot, V., Richerd, S., and Valero, M. 1991. Transition from haploidy to diploidy. Nature 351: 315–317.
- Peters, A. F., and Ramírez, M. E. 2001. Molecular phylogeny of small brown algae, with special reference to the systematic position of Caepidium antarcticum (Adenocystaceae, Ectocarpales). Cryptogam. Algol. 22: 187–200.
- Peters, A. F., Scornet, D., Ratin, M., Charrier, B., Monnier, A., Merrien, Y., Corre, E., Coelho, S. M., and Cock, J. M. 2008. Life-cycle-generation-specific developmental processes are modified in the immediate upright mutant of the brown alga Ectocarpus siliculosus. Development 135: 1503–1512.
- Peters, A. F., Oppen, M. J. H., Wiencke, C., Stam, W. T., and Olsen, J. L. 1997. Phylogeny and historical ecology of the Desmarestiaceae (Phaeophyceae) support a southern hemisphere origin. J. Phycol. 33: 294–309.
- Pfister, C. A., Altabet, M. A., and Weigel, B. L. 2019. Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology 100: e02798.
- Phillips, J. A. 1997. Genus and species concepts in Zonaria and Homoeostrichus (Dictyotales, Phaeophyceae), including the description of Exallosorus gen. nov. Eur. J. Phycol. 32: 303–311.
- Phillips, J. A. 2001. Marine macroalgal biodiversity hotspots: why is there high species richness and endemism in southern Australian marine benthic flora? Biodivers. Conserv. 10: 1555–1577.
- Phillips, J. A., and Clayton, M. N. 1991. Biflagellate spermatozoids in the Dictyotales: the structure of gametes and gametangia in Zonaria angustata (Dictyotales, Phaeophyta). Phycologia 30: 205–214.
- Phillips, J. A., and Clayton, M. N. 1993. Comparative flagellar morphology of spermatozoids of the Dictyotales (Phaeophyceae). Eur. J. Phycol. 28: 123–127.
- Phillips, N., Burrowes, R., Rousseau, F., de Reviers, B., and Saunders, G. W. 2008. Resolving evolutionary relationships among the brown algae using chloroplast and nuclear genes. J. Phycol. 44: 394–405.
- Phillips, N., Kapraun, D. F., Gómez Garreta, A., Ribera Siguan, M. A., Rull, J. L., Salvador Soler, N., Lewis, R., and Kawai, H. 2011. Estimates of nuclear DNA content in 98 species of brown algae (Phaeophyta). AoB Plants 2011: plr001.
- Potrzebowski, L., Vinckenbosch, N., Marques, A. C., Chalmel, F., Jégou, B., and Kaessmann, H. 2008. Chromosomal gene movements reflect the recent origin and biology of therian sex chromosomes. PLoS Biol. 6: e80.
- Prud’homme van Reine, W. F. 1982. A Taxonomic Revision of the European Sphacelariaceae (Sphacelarial.es, Phaeophyceae). Brill/Leiden University Press, Leiden, Netherlands.
- Prud’homme van Reine, W. F. 1993. Sphacelariales (Phaeophyceae) of the world, a new synthesis. Kor. J. Phycol. 8: 145–160.
- Pyenson, N. D., and Vermeij, G. J. 2016. The rise of ocean giants: maximum body size in Cenozoic marine mammals as an indicator for productivity in the Pacific and Atlantic Oceans. Biol. Lett. 12: 20160186.
- Quiring, R., Walldorf, U., Kloter, U., and Gehring, W. J. 1994. Homology of the eyeless Gene of Drosophila to the Small eye Gene in Mice and Aniridia in humans. Science 265: 785–789.
- Racault, M.-F L. P., Fletcher, R. L., de Reviers, B., Cho, G. Y., Boo, S. M., Parente, M. I., and Rousseau, F. 2009. Molecular phylogeny of the brown algal genus Petrospongium Nägeli ex Kütz. (Phaeophyceae) with evidence for Petrospongiaceae fam. Nov. Cryptogam. Algolog. 30: 111–123.
- Rajanikanth, A. 1989. A fossil marine brown alga from the Gangapur Formation, Pranhita-Godavari Graben. Curr. Sci. 58: 78–80.
- Ravinet, M., Faria, R., Butlin, R. K., Galindo, J., Bierne, N., Rafajlović, M., Noor, M. A. F., Mehlig, B., and Westram, A. M. 2017. Interpreting the genomic landscape of speciation: a road map for finding barriers to gene flow. J. Evol. Biol. 30: 1450–1477.
- Reed, D. C., Schroeter, S. C., and Raimondi, P. T. 2004. Spore supply and habitat availability as sources of recruitment limitation in the giant kelp Macrocystis pyrifera (Phaeophyceae). J. Phycol. 40: 275–284.
- Rescan, M., Lenormand, T., and Roze, D. 2016. Interactions between genetic and ecological effects on the evolution of life cycles. Am. Nat. 187: 19–34.
- Roberson, L. M., and Coyer, J. A. 2004. Variation in blade morphology of the kelp Eisenia arborea: incipient speciation due to local water motion? Mar. Ecol. Prog. Ser. 282: 115–128.
- Robuchon, M., Couceiro, L., Peters, A. F., Destombe, C., and Valero, M. 2014. Examining the bank of microscopic stages in kelps using culturing and barcoding. Eur. J. Phycol. 49: 128–133.
- Rothman, M. D., Mattio, L., Anderson, R. J., and Bolton, J. J. 2017. A phylogeographic investigation of the kelp genus Laminaria (Laminariales, Phaeophyceae), with emphasis on the south Atlantic Ocean. J. Phycol. 53: 778–789.
- Rousseau, F., de Reviers, B., Leclerc, M.-C., Asensi, A., and Delépine, R. 2000. Adenocystaceae fam. nov. (Phaeophyceae) based on morphological and molecular evidence. Europ. J. Phycol. 35: 35–43.
- Saada, G., Nicastro, K. R., Jacinto, R., McQuaid, C. D., Serrão, E. Á., Pearson, G.A., and Zardi, G. I. 2016. Taking the heat: distinct vulnerability to thermal stress of central and threatened peripheral lineages of a marine macroalga. Diversity Distrib. 22: 1060–1068.
- Santelices, B., and Meneses, I. 2000. A reassessment of the phytogeographic characterization of temperate Pacific South America. Rev. Chil. Hist. Nat. 73: 605–614.
- Saunders, G. W., and McDevit, D. C. 2013. DNA barcoding unmasks overlooked diversity improving knowledge on the composition and origins of the Churchill algal flora. BMC Ecol. 13: 9.
- Saunders, G. W., and McDevit, D. C. 2014. A DNA barcode survey of Haida Gwaii kelp (Laminariales, Phaeophyceae) reveals novel ecological and distributional observations and Saccharina druehlii sp. nov. Botany 92: 821–826.
- Schiel, D. R., and Foster, M. S. 2006. The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Annu. Rev. Ecol. Evol. Syst. 37: 343–372.
- Schmidt, O. C. 1937. Choristocarpaceen und Discosporangiaceen. Hedwigia 77: 1–4.
- Schmitz, K., and Srivastava, L. M. 1976. The fine structure of sieve elements of Nereocystis Lütkeana. Am. J. Bot. 63: 679–693.
- Scott, M. F., and Rescan, M. 2017. Evolution of haploid-diploid life cycles when haploid and diploid fitnesses are not equal. Evolution 71: 215–226.
- Serrão, E. Á., Alice, L. A., and Brawley, S. H. 1999. Evolution of the Fucaceae (Phaeophyceae) inferred from nrDNA-ITS. J. Phycol. 35: 382–394.
- Serrão, E. Á., Kautsky, L., and Brawley, S. H. 1996. Distributional success of the marine seaweed Fucus vesiculosus L. in the brackish Baltic Sea correlates with osmotic capabilities of Baltic gametes. Oecologia 107: 1–12.
- Shan, T., Yuan, J., Su, L., Li, J., Leng, X., Zhang, Y., Gao, H., and Pang, S. 2020. First genome of the brown alga Undaria pinnatifida: chromosome-level assembly using PacBio and Hi-C technologies. Front. Genet. 11: 140–146.
- Sheath, R. G., and Wehr, J. D. 2015. Introduction to the freshwater algae. In Freshwater Algae of North America: Ecology and Classification; Wehr, J., Sheath, R., and Kociolek, J. P., Eds. Elsevier, Massachusetts, USA, pp 1–11.
- Silberfeld, T., Bittner, L., Fernández-García, C., Cruaud, C., Rousseau, F., Reviers, B., Leliaert, F., Payri, C. E., and De Clerck, O. 2013. Species diversity, phylogeny and large scale biogeographic patterns of the genus Padina (Phaeophyceae, Dictyotales). J. Phycol. 49: 130–142.
- Silberfeld, T., Leigh, J., Verbruggen, H., Cruaud, C., de Reviers, B., and Rousseau, F. 2010. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the “brown algal crown radiation. Mol. Phylogenet. Evol. 56: 659–674.
- Silberfeld, T., Rousseau, F., and de Reviers, B. 2014. An updated classification of brown algae (Ochrophyta, Phaeophyceae). Cryptogamie Algol. 35: 117–156.
- Skaugrud, Ø., Hagen, A., Borgersen, B., and Dornish, M. 1999. Biomedical and pharmaceutical applications of alginate and chitosan. Biotechnol. Genet. Eng. Rev. 16: 23–40.
- Smale, D. A., Burrows, M. T., Moore, P., O'Connor, N., and Hawkins, S. J. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a Northeast Atlantic perspective. Ecol. Evol. 3: 4016–4038.
- Smale, D. A., Wernberg, T., Oliver, E. C. J., Thomsen, M., Harvey, B. P., Straub, S. C., Burrows, M. T., Alexander, L. V., Benthuysen, J. A., Donat, M. G., Feng, M., Hobday, A. J., Holbrook, N. J., Perkins-Kirkpatrick, S. E., Scannell, H. A., Sen Gupta, A., Payne, B. L., and Moore, P. J. 2019. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 9: 306–312.
- Smith, S. D. 2002. Kelp rafts in the Southern Ocean. Global Ecol. Biogeogr. 11: 67–69.
- Sousa, F., Neiva, J., Martins, N., Jacinto, R., Anderson, L., Raimondi, P. T., Serrão, E. A., and Pearson, G. A. 2019. Increased evolutionary rates and conserved transcriptional response following allopolyploidization in brown algae. Evolution 73: 59–72.
- South, G. R. 1975. Contributions to the flora of marine algae of eastern Canada III. Order Tilopteridales. Le Natural. Canad. 102: 693–702.
- Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M., Halpern, B. S., Jorge, M. A., Lombana, A., Lourie, S. A., Martin, K. D., McManus, E., Molnar, J., Recchia, C. A., and Robertson, J. 2007. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57: 573–583.
- Starko, S., Boo, G. H., Martone, P. T., and Lindstrom, S. C. 2018. A molecular investigation of Saccharina sessilis from the Aleutian Islands reveals a species complex, necessitating the new combination Saccharina subsessilis. Algae 33: 157–166.
- Starko, S., Demes, K., Neufeld, C. J., and Martone, P. T. 2020. Convergent evolution of niche structure in Northeast Pacific kelp forests. Funct. Ecol.
- Starko, S., and Martone, P. T. 2016a. An empirical test of 'universal' biomass scaling relationships in kelps: evidence of convergence with seed plants. New Phytol. 212: 719–729.
- Starko, S., and Martone, P. T. 2016b. Evidence of an evolutionary-developmental trade-off between drag avoidance and tolerance strategies in wave-swept intertidal kelps (Laminariales, Phaeophyceae). ). J. Phycol. 52: 54–63.
- Starko, S., Soto Gomez, M., Darby, H., Demes, K. W., Kawai, H., Yotsukura, N., Lindstrom, S. C., Keeling, P. J., Graham, S. W., and Martone, P. T. 2019. A comprehensive kelp phylogeny sheds light on the evolution of an ecosystem. Mol. Phylogenet. Evol. 136: 138–150.
- Steen, F., Verlaque, M., Dhondt, S., Vieira, C., and De Clerck, O. 2019. Population structure and geographically structured reproductive strategies of the haplodiplontic seaweed Dictyota dichotoma. BioRxiv. 595587.
- Steen, F., Vieira, C., D’hondt, S., Tyberghein, L., Fernandez-García, C., Wysor, B., Tronholm, A., Mattio, L., Payri, C., Leliaert, F., Verbruggen, H., and Clerck, O. D. Under revision. Global diversification and biogeography of a group of brown seaweeds driven by different evolutionary processes across clades.
- Steen, F., Vieira, C., Leliaert, F., Payri, E. C., and De Clerck, O. 2015. Biogeographic affinities of Dictyotales from Madagascar: a phylogenetic approach. Cryptogamie Algol. 36: 129–141.
- Steinberg, P. D., Estes, J. A., and Winter, F. C. 1995. Evolutionary consequences of food chain length in kelp forest communities. Proc. Natl. Acad. Sci. USA. 92: 8145–8148.
- Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., and Tegner, M. J. 2002. Kelp forest ecosystems: biodiversity, stability, resilience and future. Envir. Conserv. 29: 436–459.
- Stephens, T., and Hepburn, C. 2014. Mass-transfer gradients across kelp beds influence Macrocystis pyrifera growth over small spatial scales. Mar. Ecol. Prog. Ser. 515: 97–109.
- Sun, Z., Hanyuda, T., Lim, P.-E., Tanaka, J., Gurgel, C. F. D., and Kawai, H. 2012. Taxonomic revision of the genus Lobophora (Dictyotales, Phaeophyceae) based on morphological evidence and analyses rbcL and cox3 gene sequences. Phycologia 51: 500–512.
- Tan, I. H., and Druehl, L. D. 1993. Phylogeny of the Northeast Pacific brown algal (Phaeophycean) orders as inferred from 18S rDNA gene sequences. In 14th International Seaweed Symposium; Chapman, A. R. O., Brown, M. T., and Lahaye, M., Eds. Springer, Dordrecht, Netherlands, pp 699–704.
- Tan, I. H., and Druehl, L. D. 1994. A molecular analysis of Analipus and Ralfsia (Phaeophyceae) suggests the order Ectocarpales is polyphyletic. J. Phycol. 30: 721–729.
- Tanaka, A., Nagasato, C., Uwai, S., Motomura, T., and Kawai, H. 2007. Re-examination of ultrastructures of the stellate chloroplast organization in brown algae: Structure and development of pyrenoids. Phyco. Res. 55: 203–213.
- Tatarenkov, A., Bergström, L., Jönsson, R. B., Serrão, E. Á., Kautsky, L., and Johannesson, K. 2005. Intriguing asexual life in marginal populations of the brown seaweed Fucus vesiculosus. Mol. Ecol. 14: 647–651.
- Tatarenkov, A., Jönsson, R. B., Kautsky, L., and Johannesson, K. 2007. Genetic structure in populations of Fucus vesiculosus (Phaeophyceae) over spatial scales from 10 m to 800 km. J. Phycol. 43: 675–685.
- Teagle, H., Hawkins, S. J., Moore, P. J., and Smale, D. A. 2017. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 492: 81–98.
- Tellier, F., Faugeron, S., and Valero, M. 2011. Possible role of a mitochondrial genome rearrangement in maintaining the spatial segregation of two cryptic species of the Lessonia nigrescens species complex. Cah. Biol. Mar. 52: 371–383.
- Tellier, F., Meynard, A., Correa, J., Faugeron, S., and Valero, M. 2009. Phylogeographic analyses of the 30 degrees S south-east Pacific biogeographic transition zone establish the occurrence of a sharp genetic discontinuity in the kelp Lessonia nigrescens: vicariance or parapatry? Mol. Phylogenet. Evol. 53: 679–693.
- Tellier, F., Tapia, J., Faugeron, S., Destombe, C., and Valero, M. 2011. The Lessonia nigrescens species complex (Laminariales, Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J. Phycol. 47: 894–903.
- Thakur, R., Shiratori, T., and Ishida, K. 2019. Taxon-rich multigene phylogenetic analyses resolve the phylogenetic relationship among deep-branching Stramenopiles. Protist 170: 125682.
- Thornber, C. S., and Gaines, S. D. 2004. Population demographics in species with biphasic life cycles. Ecology 85: 1661–1674.
- Tom Dieck, I. 1992. North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia 31: 147–163.
- Tronholm, A., Steen, F., Tyberghein, L., Leliaert, F., Verbruggen, H., Antonia Ribera Siguan, M., and De Clerck, O. 2010. Species delimitation, taxonomy, and biogeography of Dictyota in Europe (Dictyotales, Phaeophyceae). J. Phycol. 46: 1301–1321.
- Tseng, C. K. 1981. Commercial cultivation. Bot. Monogr. 17: 680–725.
- Valero, M., Richerd, S., Perrot, V., and Destombe, C. 1992. Evolution of alternation of haploid and diploid phases in life cycles. Trends Ecol. Evol. 7: 25–29.
- Van Heeckeren, W. J., Dorris, D. R., and Struhl, K. 1998. The mating-type proteins of fission yeast induce meiosis by directly activating mei3 transcription. Mol. Cell. Biol. 18: 7317–7326.
- Vásquez, J. A., Zuñiga, S., Tala, F., Piaget, N., Rodríguez, D. C., and Vega, J. M. A. 2014. Economic valuation of kelp forests in northern Chile: values of goods and services of the ecosystem. J. Appl. Phycol. 26: 1081–1088.
- Vergés, A., Doropoulos, C., Malcolm, H. A., Skye, M., Garcia-Pizá, M., Marzinelli, E. M., Campbell, A. H., Ballesteros, E., Hoey, A. S., Vila-Concejo, A., Bozec, Y.-M., and Steinberg, P. D. 2016. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA. 113: 13791–13796.
- Vergés, A., McCosker, E., Mayer‐Pinto, M., Coleman, M. A., Wernberg, T., Ainsworth, T., and Steinberg, P. D. 2019. Tropicalisation of temperate reefs: implications for ecosystem functions and management actions. Funct. Ecol. 33: 1000–1013.
- Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G. B., Campbell, A. H., Ballesteros, E., Heck, K. L., Booth, D. J., Coleman, M. A., Feary, D. A., Figueira, W., Langlois, T., Marzinelli, E. M., Mizerek, T., Mumby, P. J., Nakamura, Y., Roughan, M., van Sebille, E., Gupta, A. S., Smale, D. A., Tomas, F., Wernberg, T., and Wilson, S. K. 2014. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. Biol. Sci. 281: 20140846.
- Vermeij, G. J., Banker, R., Capece, L. R., Hernandez, E. S., Salley, S. O., Vriesman, V. P., and Wortham, B. E. 2019. The coastal North Pacific: origins and history of a dominant marine biota. J. Biogeogr. 46: 1–18.
- Vieira, C. 2020. Lobophora–coral interactions and phase shifts: summary of current knowledge and future directions. Aquat. Ecol. 54: 1–20.
- Vieira, C., Camacho, O., Sun, Z., Fredericq, S., Leliaert, F., Payri, C., and De Clerck, O. 2017. Historical biogeography of the highly diverse brown seaweed Lobophora (Dictyotales, Phaeophyceae). Mol. Phylogenet. Evol. 110: 81–92.
- Vieira, C., Camacho, O., Wynne, M. J., Mattio, L., Anderson, R. J., Bolton, J. J., Sansón, M., D'hondt, S., Leliaert, F., Fredericq, S., Payri, C., and De Clerck, O. 2016. Shedding new light on old algae: matching names and sequences in the brown algal genus Lobophora (Dictyotales, Phaeophyceae). Taxon 65: 689–707.
- Wang, M., Hu, C., Barnes, B.B., Mitchum, G., Lapointe, B., and Montoya, J. P. 2019. The great Atlantic Sargassum belt. Science 365: 83–87.
- Wargacki, A. J., Leonard, E., Win, M. N., Regitsky, D. D., Santos, C. N. S., Kim, P. B., Cooper, S. R., Raisner, R. M., Herman, A., Sivitz, A. B., Lakshmanaswamy, A., Kashiyama, Y., Baker, D., and Yoshikuni, Y. 2012. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335: 308–313.
- Wehr, J. D. 2015. Brown algae. In Freshwater Algae of North America: Ecology and Classification; Wehr, J., Sheath, R., and Kociolek, J. P., Eds. Elsevier, Massachusetts, USA, pp 851–871.
- Wehr, J. D., and Stein, J. R. 1985. Studies on the biogeography and ecology of the freshwater phaeophycean alga Heribaudiella Fluviatilis. J. Phycol. 21: 81–93.
- Wehr, J. D., Sheath, R. G., and Kociolek, J. P., Eds. 2015. Freshwater Algae of North America: Ecology and Classification. Elsevier, Massachusetts, USA.
- Wehr, J. D., Stancheva, R., Truhn, K., and Sheath, R. G. 2013. Discovery of the rare freshwater brown alga Pleurocladia lacustris (Ectocarpales, Phaeophyceae) in California streams. West. N. Am. Nat. 73: 148–157.
- Wernberg, T., Krumhansl, K., Filbee-Dexter, K., and Pedersen, M. F. 2019. Status and trends for the world’s kelp forests. In: World Seas: An Environmental Evaluation. p. 57–78. Sheppard, C., Ed. Elsevier, Massachusetts, USA.
- West, J. 1996. Ectocarpus siliculosus (Dillwyn) Lyngbye from the Hopkins River Falls, Victoria - the first record of a freshwater brown alga in Australia. Muelleria 9: 29–33.
- Wetherbee, R., Jackson, C. J., Repetti, S. I., Clementson, L. A., Costa, J. F., van de Meene, A., Crawford, S., and Verbruggen, H. 2019. The golden paradox – a new heterokont lineage with chloroplasts surrounded by two membranes. J. Phycol. 55: 257–278.
- Wiencke, C., and Amsler, C. D. 2012. Seaweeds and their communities in polar regions. Vol. 219. In Seaweed Biology. Ecological Studies (Analysis and Synthesis); Wiencke C., and Bischof K., Eds. Springer, Berlin, Germany, pp 265–291.
- Wiencke, C., Clayton, M. N., Gómez, I., Iken, K., Lüder, U. H., Amsler, C. D., Karsten, U., Hanelt, D., Bischof, K., and Dunton, K. 2007. Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev. Environ. Sci. Biotechnol. 6: 95–126.
- Wiesemeier, T., Hay, M., and Pohnert, G. 2007. The potential role of wound-activated volatile release in the chemical defence of the brown alga Dictyota dichotoma: blend recognition by marine herbivores. Aquat. Sci. 69: 403–412.
- Wynne, M. J., and Bold, H. C. 1985. Introduction to the Algae: Structure and Reproduction. Prentice-Hall, New Jersey, USA.
- Yang, E. C., Boo, G. H., Kim, H. J., Cho, S. M., Boo, S. M., Andersen, R. A., and Yoon, H. S. 2012. Supermatrix data highlight the phylogenetic relationships of photosynthetic Stramenopiles. Protist 163: 217–231.
- Yang, E. C., Peters, A. F., Kawai, H., Stern, R., Hanyuda, T., Bárbara, I., Müller, D. G., Strittmatter, M., van Reine, W. F. P., and Küpper, F. C. 2014. Ligulate Desmarestia (Desmarestiales, Phaeophyceae) revisited: D. japonica sp. nov. and D. dudresnayi differ from D. ligulata. J. Phycol. 50: 149–166.
- Ye, N., Zhang, X., Miao, M., Fan, X., Zheng, Y., Xu, D., Wang, J., Zhou, L., Wang, D., Gao, Y., Wang, Y., Shi, W., Ji, P., Li, D., Guan, Z., Shao, C., Zhuang, Z., Gao, Z., Qi, J., and Zhao, F. 2015. Saccharina genomes provide novel insight into kelp biology. Nat. Commun. 6: 6986.
- Yip, Z. T., Quek, R. Z. B., and Huang, D. 2020. Historical biogeography of the widespread macroalga Sargassum (Fucales, Phaeophyceae). J. Phycol. 56: 300–309.
- Zardi, G. I., Nicastro, K. R., Canovas, F., Costa, J. F., Serrao, E. Á., and Pearson, G. A. 2011. Adaptive traits are maintained on steep selective gradients despite gene flow and hybridization in the intertidal zone. PLoS One 6: e19402.
- Zhang, J., Yao, J., Hu, Z.-M., Jueterbock, A., Yotsukura, N., Krupnova, T. N., Nagasato, C., and Duan, D. 2019. Phylogeographic diversification and postglacial range dynamics shed light on the conservation of the kelp Saccharina japonica. Evol. Appl. 12: 791–803.
- Zuccarello, G. C., and Martin, P. 2016. Phylogeography of the Lessonia variegata species complex (Phaeophyceae, Laminariales) in New Zealand. Algae 31: 91–103.
