Abstract
The study of reproductive mechanisms is of particular interest for a real understanding of seed plant evolution. Spermatophytes comprise angiosperms and four orders of gymnosperms (Cycadales, Ginkgoales, Coniferales, and Gnetales) whose main characteristic is the reproduction via seeds. Ginkgo and cycads form a sister clade to the other gymnosperms and occupy a key phylogenetic position in-between the extinct Paleozoic seed ferns and the other extant gymnosperms. This review focuses on the similarities and differences between the reproductive mechanisms of Ginkgo and Cycas, from the morphogenesis of the male and female organs to the pollination and fertilization events. Together with the morphological and cytological description, the latest available molecular data on the reproductive organ development of the two plant genera are discussed. This will, hopefully, pave the path for new studies aiming at filling the gaps in our understanding of the hormonal and genetic regulation of their reproductive mechanisms. The whole reproductive process is presented in detail, providing a comprehensive and organic picture together with complete illustrations and photographic material. Each phase of the reproductive process is dissected, pointing out the main similarities and differences found among the two genera. The comparison comprises the male and female reproductive organs development, with a focus on pollen ontogeny, shape, ultrastructure, and germination as well as ovule development and patterning, female gametophyte formation, and ovule integument differentiation. Particular attention is given to the pollination and fertilization events focusing on the role of reproductive fluids as well as zoogamy.
1. Introduction
The seed is the key feature characterizing spermatophytes and has allowed their diffusion and diversification around the whole world. Its origin traces back to the Devonian period, about 360 million years ago. The appearance of this structure was a central event during the evolution of land plants (Meade et al., Citation2021). Most of the studies on the reproduction of spermatophytes have been conducted on model organisms belonging to the group of angiosperms. However, of the five main lineages of seed plants, four of them belong to gymnosperms: Cycadales, Ginkgoales, Coniferales, and Gnetales. Hence, for a real understanding of spermatophyte reproduction, it is important to extend our knowledge investigating the reproductive mechanisms of plants belonging to gymnosperms. As reported by Ran et al. (Citation2018), cycads and Ginkgo form a sister clade to the other extant gymnosperm lineages, thus belonging to the oldest branches of seed plants. A sister relationship between cycads and Ginkgo is also supported by some morphological characteristics, especially in regard their reproductive biology, such as the haustorial pollen tube development and the flagellated male gametes.
Ginkgo biloba is the only surviving species in the clade Ginkgophytes. Leaves very similar in form and vegetation to the ones of the modern Ginkgo have been found as fossils probably deposited during the early Mesozoic Era, about 250 million years ago (Zhao et al., Citation2019). In particular, the study of fossils has demonstrated that Ginkgoaceae originated in the Permian, and reached their maximum diffusion in the Jurassic. Later, probably due to the changes in the climate conditions, Ginkgo species declined progressively in their distribution and survived as a relict in China. Since then, humans strongly contributed to its redistribution, being G. biloba widely cultivated as a park specimen or street tree (Zhao et al., Citation2019). Ginkgo is a large dioecious tree with characteristic fan-shaped dichotomously veined leaves that turn a beautiful golden-yellow in autumn.
Cycads belong to the order Cycadales, comprising three families (Cycadaceae, Stangeriaceae, and Zamiaceae), ten genera (Bowenia, Ceratozamia, Cycas, Dioon, Encephalartos, Lepidozamia, Macrozamia, Microcycas, Stangeria, and Zamia) and about 330 species (Condamine et al., Citation2015). Cycads date from the late Paleozoic Era, about 350–280 million years ago (Nagalingum et al., Citation2011). During the last decades, evidence has accumulated showing that cycads are facing a strong threat of extinction (Donaldson, Citation2003). The genus Cycas is the most widespread with species living in Australia, Pacific islands, southern Asia, and Madagascar.
This review will focus on the main similarities and differences among the reproductive mechanisms of Ginkgo and Cycas. Male cones, microsporophylls, pollen grains development and morphogenesis, the pollination mechanisms and pollen tube germination will be discussed in detail. Furthermore, the female megasporophylls, the ovule development and the fertilization mechanisms will be explored in depth. In the last part of the review, the more recent molecular data will be presented together with research proposals in the hope that this fascinating field of study will be extended by filling in the current gaps.
2. Male cones and microsporophylls
Both Ginkgo and Cycas species are dioecious.
In G. biloba, the male cones are organized in catkin-like clusters at the end of a brachyblast. Each mature male cone is pendulous and composed of many microsporophylls arranged loosely on a central axis with a spiral pattern ( and ) (Mundry and Stützel, Citation2004). The orientation and positioning of the male cones is peculiar: at the time of buds opening, they are arranged externally and perpendicularly to the leaves () and, at pollination time, this specific arrangement results even more evident, because they elongate becoming pendulous and more separated from the leaves that remain central (). This is suggested to be an evolutionary adaptation that ensures an efficient pollen dispersal, being Ginkgo anemophilous (Lu, Jin, et al., Citation2011). Each microsporophyll consists of a long stalk terminating into a hump or knob and bearing two pendant microsporangia ( and ). Interestingly, the knob of the microsporophylls contains from 1 to 3 air sacs (). The microsporangia are tubular in shape and surrounded by distinct wall layers, including the tapetum (). The wall layers enclose a central sporogenous tissue: from the internal microsporocytes (or microspore mother cells), the pollen grains will develop through a process comprising microsporogenesis and microgametogenesis (Lu, Wang, et al., Citation2011).
Figure 1. Male reproductive structures of Ginkgo (A–E) and Cycas (F–J). (A) Ginkgo biloba male brachyblast before pollination time; scale bar 1 cm. (B) Detail of G. biloba microsporophylls on a sigle cone; scale bar 2 mm (* indicates an air sac in the knob of the microsporophyll). (C) Ginkgo male cone cut transversely with microsporophylls spirally arranged around the central axis, adaxial view; scale bar 2 mm. (D) G. biloba microsporophyll with an apical sterile knob and two pendant microsporangia; scale bar 2 mm (* indicates an air sac in the knob of the microsporophyll). (E) G. biloba male cone soon after dehiscence of microsporangia; scale bar 2 mm. (F) Cycas revoluta male cone at around pollination time; scale bar 10 cm. (G) Detail of C. revoluta microsporophylls attached perpendicularly to the main cone axis; scale bar 2 cm. (H) Cycas circinalis male cone cut transversely with microsporophylls spirally arranged around the central axis, abaxial view; scale bar 4 cm. (I) C. circinalis microsporophyll with an apical sterile apophysis and the microsporangia arranged in sori; scale bar 1 cm. (J) C. circinalis sori soon after dehiscence of microsporangia, with pollen still inside most of them; scale bar 2 mm.
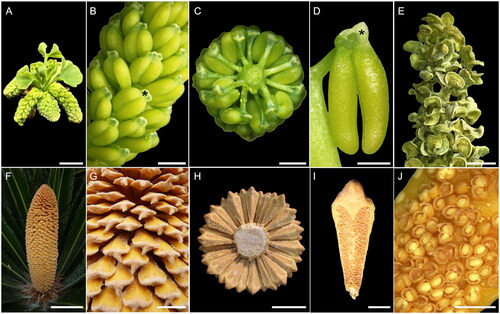
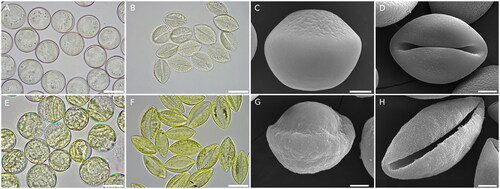
Figure 2. Pollen morphology and ultrastructure of Cycas and Ginkgo. (A) Cycas circinalis hydrated pollen, scale bar 20 µm. (B) C. circinalis dehydrated pollen, scale bar 20 µm. (C) C. circinalis hydrated pollen observed under the scanning electron microscope (SEM), scale bar 5 µm. (D) C. circinalis dehydrated pollen observed at SEM, scale bar 5 µm. (E) Ginkgo biloba hydrated pollen, scale bar 20 µm. (F) G. biloba dehydrated pollen, scale bar 20 µm. (G) G. biloba hydrated pollen observed at SEM, scale bar 5 µm. (H) G. biloba dehydrated pollen observed at SEM, scale bar 5 µm. For C and G, pollen was fresh collected, rehydrated in water, fixed, ethanol dehydrated, critical point dried and coated with gold.
Male strobili in Cycas differ from Ginkgo in that male plants produce each year a single male cone at their apex from the apical meristem ( and ). Later, an apical meristem forms at the base of the cone, pushing it on one side, thus allowing the resume of stem growth. Each strobilus has a conical shape and consists of a central axis around which microsporophylls are attached at right angle in a compact, spiral, and acropetal succession (). Although varying in size and form, in general each microsporophyll is flattened, woody and with a scale-like structure. It differentiates into a distal sterile region called an apophysis and a proximal fertile part ( and ). Each microsporophyll bears hundreds of microsporangia on its abaxial surface, this resembles the abaxial position of sporangia typical of many ferns. This resemblance to ferns is strengthened by the fact that the microsporangia are arranged in clusters of 3–6, similarly to the sori of ferns () (Norstog and Nicholls, Citation1998; Stevenson, Citation2013).
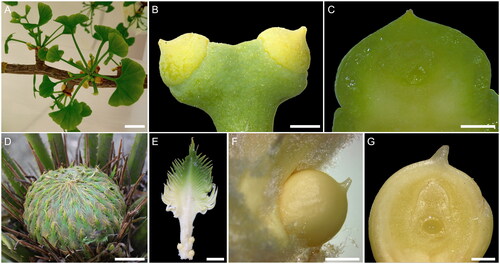
Figure 3. Female reproductive structures of Ginkgo (A–C) and Cycas (D–G). (A) Ginkgo biloba female brachyblast before pollination time; scale bar 1 cm. (B) Detail of a couple of G. biloba ovules present at the end of a stalk; scale bar 1 mm. (C) Fresh longitudinal medial section of a G. biloba ovule showing the coenocytic female gametophyte in the innermost part; scale bar 1 mm. (D) Cycas changjangensis female cone with megasporophylls loosely arranged, before pollination time; scale bar 4 cm. (E) C. changjangensis megasporophyll with a terminal pinnate leafy portion and a lower stalk bearing the ovules; scale bar 1 cm. (F) Detail of a single C. changjangensis ovule; scale bar 2 mm. (G) Fresh longitudinal medial section of a C. changjangensis ovule showing the coenocytic female gametophyte in the innermost part; scale bar 500 µm.
In both Ginkgo and Cycas, micro-sporangial dehiscence is longitudinal ().
Besides the differences in the number, distribution, and morphology of the male cones, as well as the organization of the microsporangia, Ginkgo and Cycas differ in the microsporangia wall. The wall of the mature microsporangium in almost all gymnosperms consists of an epidermis with thickenings in the cell walls, an exothecium (Stevenson, Citation2013). This is also the case of Cycas (), while in Ginkgo the microsporangia wall consists of an outermost tiny epidermis, not always visible in sections, and an endothecium of five to six layers () (Stevenson, Citation2013).
3. Pollen development, morphology, and ultrastructure
Pollen development in Ginkgo and Cycas is very similar (Moitra and Bhatnagar, Citation1982; Fernando et al., Citation2010; Breygina et al., Citation2021). In Ginkgo, the mature pollen released by the microsporangia is composed of four cells and forms through three mitotic divisions (). The free microspores that form inside the microsporangia are initially round in shape and surrounded by thin intine and thick exine layers. A centrally located nucleus contains one or two nucleoli and remains surrounded by a dense cytoplasm. The exine wall is not homogeneously deposited: an aperture area is present at the distal pole. This is a region in which the exine is thinner and the underlying intine is thicker. A large vacuole forms in the cytosol, enlarges, and pushes the nucleus to the side. The nucleus hence migrates toward the end underneath the aperture area. Here the first nuclear mitotic division takes place and the cytokinesis results in the formation of two asymmetric cells: one small flat cell called first prothallial cell and a larger antheridia initial cell. The antheridia initial cell undergoes immediately another round of mitosis, forming a second prothallial cell and an antheridia cell. The antheridia cell undergoes mitosis again with the formation of a tube cell and a generative cell in contact with the second prothallial cell () (Lee, Citation1955; Friedman and Gifford, Citation1997).
In the germinating pollen, one of the two prothallial cells degenerates, the generative cell divides into a stalk cell and a body cell (which will form the two sperm cells) and the tube cell forms the highly branched pollen tube () (Lee, Citation1955; Friedman and Gifford, Citation1997).
The ontogeny of the pollen grains in Cycas is very similar, with the only difference that only one prothallial cell – rather than two – is developed: in Cycas, mature pollen released by the microsporangia is composed by three cells and forms through two mitotic divisions (). Briefly, the microspore divides resulting in the formation of two unequal cells: a smaller prothallial cell and a larger antheridia cell. The prothallial cell does not divide any further, while the antheridia cell divides to form a generative cell near the prothallial cell and a large tube cell () (Foster and Gifford, Citation1974). The shedding of the microspores takes place at this three-celled stage. Further development of male gametophyte starts inside the ovule ().
In addition to the ontogeny, also the morphology and ultrastructure of the mature pollen grains in Ginkgo and Cycas is very similar () (Dehgan and Dehgan, Citation1988; Lu, Wang, et al., Citation2011; Citation2016). In both cases, the pollen shape is very different depending on the hydration state: the shape and ultrastructure change dramatically when considering mature hydrated pollen and dehydrated pollen. The hydrated pollen has a hemispherical shape with a large aperture area on the distal side in both Ginkgo and Cycas (). The prothallial cell(s) are located at the proximal end, and the large tube cell is located at the distal end, where the aperture area is found ( and ). When the pollen grain dehydrates, its shape changes completely: it closes around the aperture area, due to the different thickness of the wall there, and assumes a boat-like shape ().
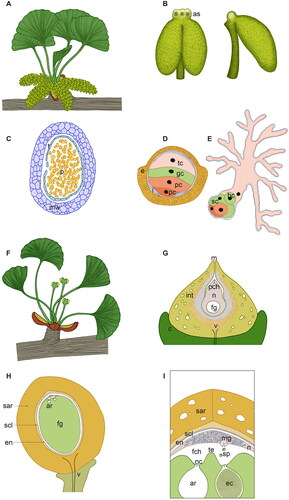
Figure 4. Scheme of the male (A–E) and female (F–I) reproductive structures of Ginkgo biloba at different magnification and developmental stages. (A) Male brachyblast of G. biloba with a magnification of 0.75×. The male cones develop on a dwarf male shoot in catkin-like clusters and are oriented perpendicularly to the leaves. Each male cone is pendulous and comprises many microsporophylls spirally and loosely arranged around a central axis. (B) Front and side view of a single microsporophyll of G. biloba, magnification of 12×. The microsporophyll consists in a long stalk terminating into a knob, which contains two or three air sacs (as), and bearing two pendant tubular microsporangia. (C) Transversal section of a Ginkgo microsporangium with pollen (p) inside, magnification of 22×. The microsporangia wall (mw) comprises an outermost epidermis, an endothecium with thickening in the cell wall and a tapetum (t), which will degenerate at maturity. (D) Ginkgo mature pollen grain, magnification 1250×. The mature pollen comprises a double wall: the external exine (e) of sporopollenin, which is thicker at the proximal side and thinner at the distal aperture area, and an inner pecto-cellulosic intine (i), which shows uneven thickenings too. Ginkgo pollen forms from the microspores via three mitoses and, at maturity, consists of four cells: a distal tube cell (tc), a generative cell (gc) and two proximal prothallial cells (pc). (E) Ginkgo germinated pollen. The same colors of D have been used to point out the fate of the different cells. The pollen tube is highly branched and has a primary haustorial role, it grows opportunistically in the available gaps in-between the nucellar cells. The generative cell divides giving origin to a stalk cell (sc) and a central body cell (bc) from which the two sperm cells will be produced. (F) G. biloba female brachyblast, magnification of 1×. Ovules form, generally in couples, at the end of the ovulate stalk and a collar (c) is present at their base. (G) Longitudinal section of a Ginkgo ovule soon after pollination, magnification of 17×. The ovule consists in a single integument (int), which at maturity will differentiate into three distinct layers as suggested by the different color shades, and a nucellus (n) inside which the female gametophyte (fg) develops. The nucellus is totally enclosed by the integument with the exception of the micropyle (m), from which the pollination drop will be emitted. Upon pollination, pollen enters into the ovule via the micropyle and arrives in the pollen chamber (pch) where it grows until fertilization. Differently from Cycas, the vasculature (v) of Ginkgo ovules is mainly restricted to the stalk and collar. (H) Ginkgo ovule cut in half longitudinally at fertilization time, magnification of 1.8×. Before fertilization occurs, the single ovule integument has already acquired the typical characteristics of the seed coat, differentiating an outer fleshy sarcotesta (sar), a middle lignified sclerotesta (scl) and an inner papery endotesta (en). The female gametophyte (fg) has cellularized and enlarged, at dispense of the nucellus (n). At the apical part of the female gametophyte, facing the micropyle, typically two archegonia (ar) are formed. (I) Fertilization in G. biloba, magnification of illustration H. Fertilization in Ginkgo occurs via zoogamy: the sperm cells (sp) are equipped with a large number of flagella (about 1000) arranged along approximately three coils and are released from the swollen unbranched basal end of the male gametophyte (mg). The flagellated sperm cells swim toward the archegonia (ar). Differently from Cycas, a tentpole (te) is visible in the middle of the fertilization chamber (fch) between the two archegonia. One sperm cell will penetrate inside the archegonium through the space in-between the neck cells (nc), and will fertilize the egg cell (eg). ar: archegonia; as: air sac; bc: body cell; c: collar; e: exine; ec: egg cell; en: endotesta; fch: fertilization chamber; fg: female gametophyte; gc: generative cell; i: intine; int: integument; m: micropyle; mg: male gametophyte; mw: microsporangial wall; n: nucellus; nc: neck cells; p: pollen; pc: prothallial cell; pch: pollen chamber; sar: sarcotesta; sc: stalk cell; scl: sclerotesta; sp: sperm cells; t: tapetum; tc: tube cell; te: tentpole; v: vasculature.
Ginkgo and Cycas hydrated rounded pollen have a similar diameter. Comparing G. biloba and Cycas circinalis pollen, the latter results slightly smaller: Ginkgo pollen is about 25 µm in diameter while C. circinalis pollen is about 23 μm (). In the comparison between these two species, another feature that immediately stands out is the difference in color, with Ginkgo pollen exhibiting a brighter yellow appearance (). This probably depends on the different thicknesses of the exine wall, being sporopollenin responsible of the yellow pollen color: in Ginkgo the exine thickening at the proximal pole is greater than in C. circinalis. This peculiarity appears evident observing the hydrated pollen with both light and electron microscopes. Indeed, in Ginkgo the difference in thickness of the pollen wall at the distal and proximal poles is more pronounced (). Still, with regard to the exine appearance on the proximal side, a further difference concerns the surface roughness or smoothness. As it can already be seen from the images obtained under the light microscope, the Cycas pollen surface appears homogeneous (). This characteristic is even more evident from the electron microscope images in which the proximal surface appears essentially smooth (). On the contrary, in Ginkgo, the proximal pollen surface appears more rough, consistently with the greater wall thickness ().
In regards to the boat-like shaped dehydrated pollen, additional features emerge comparing the two genera. First, the ratio between the equatorial and the polar axes is lower in Cycas than in Ginkgo () (Lu, Jin, et al., Citation2011). This may be linked to the pollination mode: Cycas pollen results less aerodynamic suggesting, at least for some species, a biotic pollination rather than a wind pollination (Norstog, Citation1987; Stevenson et al., Citation1998; Ackerman, Citation2000). Indeed, insect pollination has been recently recorded in some cycads species (Kono and Tobe, Citation2007; Terry et al., Citation2012; Toon et al., Citation2020). This difference is also reflected in the different textures of the two mature pollen grains: the pollen of Cycas has a greater tendency to stick together.
Other more subtle differences are linked to the exine walls, which show peculiar ornamentations which have been described in detail thanks to electron microscopy studies: both have smooth exines with micro-reticulate decorations, but the patterns slightly differ from each other (Sahashi and Ueno, Citation1986; Lu, Jin, et al., Citation2011; PalDat, www.paldat.org). For example, the microreticulated ornamentation of C. circinalis appears more regular even if the lumina show a certain degree of variability in their size (). Differently, in Ginkgo the micro-reticulated ornamentation appears less regular with features resembling streaks or regulae with the occasional presence of holes ().
4. Female macrosporophylls and ovules
The organization of the female reproductive structures is quite different in Ginkgo and Cycas. The development of Ginkgo ovules has been precisely described in 13 stages (D’Apice et al., Citation2021). Ovuliferous organs arise in the axil of leaves or spur shoots (Jin et al., Citation2012). Ovules appear partially erect at the distal end of a stalk (). At the time of buds opening, the ovules are about 1–2 mm in length and yellowish. The stalk elongates rapidly pushing the ovules outward from the bud. A rim-like outgrowth termed collar (Douglas et al., Citation2007) is located at the base of each ovule ( and ). The nucellus is enclosed by a single integument that after pollination will differentiate into an outer fleshy layer (sarcotesta), a hard stony middle layer (sclerotesta) and a papery inner layer (endotesta) ().
Figure 5. Scheme of the male (A–E) and female (F–I) reproductive structures of Cycas revoluta at different magnification and developmental stages. (A) Male cone of C. revoluta with a magnification of 13×. A single male cone forms from the apical meristem and comprises a central axis with microsporophylls attached at right angle in a compact, spiral, acropetal succession. (B) Single microsporophyll of C. revoluta with a magnification of 1.15×. The microsporophyll is flattened and woody and comprises a sterile apophysis (ap) and a proximal fertile region with microsporangia arranged in sori (s) on the abaxial surface. (C) Transversal section of a Cycas microsporangium with pollen (p) inside, magnification of 45×. The microsporangia wall consists of an exothecium (ex) with thickening in the cell wall, more flattened microsporangial inner wall layers (mw) and a tapetum (t) which will degenerate at maturity. (D) Cycas mature pollen grain, magnification 1250×. The mature pollen comprises a double wall: the external exine (e) of sporopollenin, which is thicker at the proximal side and thinner at the distal aperture area, and an inner pecto-cellulosic intine (i), which shows uneven thickenings too. Cycas pollen forms from the microspores via two mitoses and, at maturity, consists of three cells: a distal tube cell (tc), a generative cell (gc) and a proximal prothallial cell (pc). (E) Cycas germinated pollen. The same colors of D have been used to point out the fate of the different cells. The pollen tube is typically unbranched or only slightly branched and has a primary haustorial role, it penetrates inside the nucellus via tip growth, enzymatically degrading the female tissue; later in development the basal portion swells. The generative cell divides giving origin to a stalk cell (sc) and a central body cell (bc) from which the two sperm cells will be produced. (F) C. revoluta megasporophyll, magnification of 1×. Each megasporophyll is a leaf-like structure, with an upper pinnate leafy portion and a lower stalk bearing 4–8 ovules. (G) Longitudinal section of a Cycas ovule soon after pollination, magnification of 17.5×. The ovule consists in a single integument (int), which at maturity will differentiate into three distinct layers as here suggested by the different color shades, and a nucellus (n) inside which the female gametophyte (fg) develops. The nucellus is totally enclosed by the integument with the exception of the micropyle (m) from which the pollination drop will be emitted. Upon pollination, pollen enters into the ovule via the micropyle and arrives in the pollen chamber (pch) where it grows until fertilization. Cycas ovules are characterized by a double vasculature: an unbranched outer vasculature (ov) and a ramified inner vasculature (iv), the exact growth pattern of the inner vasculature is not completely known hence here the iv branches are reported with a dashed line. (H) Cycas ovule cut in half longitudinally at fertilization time, magnification of 1×. Before fertilization occurs, the single ovule integument has already acquired the typical characteristics of the seed coat, differentiating an outer fleshy sarcotesta (sar), a middle lignified sclerotesta (scl) and an inner papery endotesta (en). The female gametophyte (fg) has cellularized and enlarged, at dispense of the nucellus (n). At the apical part of the female gametophyte, facing the micropyle, two-six archegonia (ar) are formed. (I) Fertilization in C. revoluta, magnification of illustration H. Fertilization in Cycas occurs via zoogamy: the sperm cells (sp) are equipped with a large number of flagella (about 40,000) arranged in 5–10 sinistral coils and are released from the swollen basal end of the male gametophyte (mg). The flagellated sperm cells swim in the fertilization chamber (fch) toward the archegonia (ar). One sperm cell will penetrate inside the archegonium through the space in-between the neck cells (nc) and will fertilize the egg cell (eg). ap: apophysis; ar: archegonia; bc: body cell; e: exine; ec: egg cell; en: endotesta; ex: exothecium; fch: fertilization chamber; fg: female gametophyte; gc: generative cell; i: intine; int: integument; iv: inner vasculature; m: micropyle; mg: male gametophyte; mw: microsporangial inner wall layers; n: nucellus; nc: neck cells; ov: outer vasculature; p: pollen; pc: prothallial cell; pch: pollen chamber; s: sori; sar: sarcotesta; sc: stalk cell; scl: sclerotesta; sp: sperm cells; t: tapetum; tc: tube cell.
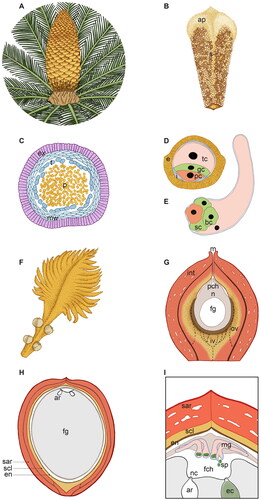
The organization of the female structure is remarkably different in Cycas, where ovules are beared by megasporophylls that form a loose and open cone on the top of the stem (). Each megasporophyll is a leaf-like structure, conspicuously pinnatifid, and reaches up to 20 cm or more in length, depending on the species. It has a flat body with an upper pinnate leafy portion and a lower stalk, bearing 4–8 ovules laterally arranged. Its surface is typically covered by many hairs ( and ). Ovules of cycads are erect and large in comparison to the ovules of other gymnosperms. As in Ginkgo, the nucellus is enclosed by a single integument which becomes histologically differentiated into an outer fleshy layer, a sclerified middle stony layer, and an inner thin papery layer (Zhang, Citation2019; Zumajo-Cardona et al., Citation2021) ().
A peculiar feature of cycads ovules is the development of two separate vascular systems: an outer vascular system and an inner vascular system. The outer vascular system consists of a series of unbranched veins that grow through the outer fleshy layer; while the inner vascular system consists in dichotomously branched strands that grow in the inner fleshy layer (). Interestingly, the vasculature of Ginkgo ovules is only weakly developed and restricted with vascular traces generally not found inside the ovule but mainly in the stalk up to the collar (Chamberlain, Citation1935; Zumajo-Cardona et al., Citation2021) ().
Important similarities can be found when considering female reproductive structures in Ginkgo and Cycas. First, the organization of the nucellus and the development of the female gametophyte, which will be considered in the next section; second, the differentiation of the ovule single integument into three anatomically different layers. The differentiation of the three layers starts before fertilization occurs and pollination seems to play a crucial role in triggering such developmental process () (D’Apice et al., Citation2021).
In Ginkgo the mature fleshy sarcotesta is yellowish, soft, and juicy. Mature seeds accumulate fatty acids in the sarcotesta that undergo oxidation during maturation (Nigris et al., Citation2021). This results in a characteristic bad smell resembling rotting organic material. Similarly, in Cycas the mature fleshy sarcotesta is composed by large, thin-walled undifferentiated cells, filled up with starch and tannins, and an epidermis (Zumajo-Cardona et al., Citation2021). Both Ginkgo and Cycas sarcotesta produce abundant mucilages, which increase the capacity to absorb or retain water. As for the middle layer, it is hard and stony, consisting of cells that are lignified at maturity. Cycas seeds have a thicker sclerotesta compared to the Ginkgo ones ( and ). Shortly after pollination, the cells of the ovule integument that will differentiate the sclerotesta are already histologically recognizable like small bricks compacted together, surrounded by the other integument cells (D’Apice et al., Citation2021). The inner endotesta is composed by parenchymatous thin-walled cells that will degenerate into a membranous tissue in the ripe seed ( and ) (Nigris et al., Citation2021; Zumajo-Cardona et al., Citation2021).
5. Female gametophyte development
The female gametophyte development in Ginkgo and cycads is very similar (Chamberlain, Citation1906; Carothers Citation1907; Smith, Citation1910; Reynolds, Citation1924; Sedgwick, Citation1924; Soma, Citation1997). The female gametophyte arises from a single functional megaspore inside the nucellar tissue. The functional megaspore enlarges and undergoes a series of synchronized free nuclear divisions. Several thousand free nuclei are formed and become arranged in the peripheral cytoplasm between the megaspore wall and a large central vacuole. This is referred to as the coenocyte stage () (D’Apice et al., Citation2021). During this stage, the female gametophyte becomes surrounded by one or two layers of jacket cells, which provide nourishment to the enlarging megagametophyte. In parallel, the megaspore wall becomes thicker. The cellularization process follows a centripetal direction and involves an alveolation phase (Maheshwari and Singh, Citation1967). In particular, the process begins with the development of secondary spindles, so that every nucleus becomes connected to six adjacent nuclei. Anticlinal walls start to form centripetally and the gametophyte begins to appear like a honeycomb, where each cavity is called an “alveolus.” Each alveolus is open-ended with no wall near the central vacuole and the nucleus is typically found next to this open region. The vacuole reduces in size and the alveoli extend to the center of the gametophyte. Finally, each one becomes enclosed by the formation of an end wall and the nucleus moves back toward the periphery. Different alveoli become closed at variable distances from the center of the gametophyte, with most precociously closed alveoli found at the micropyle and chalazal ends. Through repeated formation of periclinal cell walls, each alveolus is divided into a row of cells. Later, the arrangement is lost due to divisions in irregular planes (Maheshwari and Singh, Citation1967).
Another important aspect concerns the formation of archegonia, usually 2–6 per ovule, from the archegonial initial cells that differentiate from the apical portion of the gametophyte (). A peculiar and curious aspect about the number and arrangement of the archegonia concerns the genus Microcycas: it has been observed that archegonia can form in groups or individually, not only in the apical portion of the gametophyte but also scattered all over the surface of the gametophyte and even inside it (Reynolds, Citation1924). The initial archegonial cell divides periclinally to form a large central cell and a much smaller initial neck cell. The initial neck cell forms a single tier of four neck cells by means of anticlinal divisions (Norstog, Citation1972; Wang et al., Citation2014). The larger central cell, instead, divides to form an egg cell and an ephemeral ventral canal cell. The ventral canal cell will rapidly degenerate, while the egg cell will enlarge and be surrounded by an archegonial jacket layer of cells. Interestingly, the egg cell in cycads can even reach a diameter of 500 µm and it is considered one of the largest in the plant kingdom (Maheshwari and Singh, Citation1967).
6. Pollination and secretory fluids
At the time of pollination, both Ginkgo and Cycas ovules produce a pollination drop. The pollination drop is a complex secretion that is important to receive the pollen grains and deliver them inside the ovule (Coulter et al., Citation2012; von Aderkas et al., Citation2018). The analysis of the composition of pollination drops from many different gymnosperms have brought to light their complexity: within these aqueous secretions, minerals, sugars, amino acids, antimicrobial compounds, and secretory proteins have been detected (Prior et al., Citation2019). This suggests a wider role of pollination drops in gymnosperm reproduction: beside the role in pollen capture, they probably play other important tasks related to pollen germination regulation, pollen-ovule communication, and defence from pathogens (Del Tredici, Citation2007; Nepi et al., Citation2009; Little et al., Citation2014; Nepi et al., Citation2017).
The pollination drop of Ginkgo has been shown to contain minerals (mainly Ca, B, Zn, K, S, Na, Mg, P, Si, and Al), sugars (mainly glucose, fructose and sucrose but also some other less appealing saccharides such as xylose, which are probably important for creating the right environment for pollen germination), alcohols, organic acids, fatty acids, amino acids, amides, nucleosides, phosphates, proteins and peptides, and microRNAs (Lu et al., Citation2020; Mao et al., Citation2022). The functions of the proteins of the Ginkgo pollination drop have been broadly determined by grouping them according to their GO annotations. The most enriched classes are: proteins associated with sugar modifications, proteins associated with defence, proteins associated with metabolism and proteins that facilitate the growth of the pollen tube. Among the proteins belonging to the last group, particular attention should be given to the arabinogalactan proteins (AGPs), which in angiosperms are important for the interaction between pollen and stigma or that between pollen and pollen, and calmoduline (CaM), which plays a crucial role in Ca2+ signaling (Cheng et al., Citation2018).
Cycas pollination drops are also complex and contain different minerals, sugars, alcohols, organic acids, amino acids, and proteins (Prior et al., Citation2019). The detected proteins are involved in cell wall/carbohydrate-modification, defence, and pollen recognition. The best-represented proteins in cycads pollination drops were those involved in carbohydrate modification processes. These enzymes are often involved in cell wall remodeling and are thought to act on pollen cell walls to facilitate pollen germination and pollen tube growth. Cell wall-modifying enzymes may also contribute to the formation of the pollen chamber, a cavity formed by the degeneration of the apical part of the nucellus in which the pollen grains will germinate. Pollination drop proteins may play a role in nutrient mobilization for the emerging pollen tube. Defence from pathogens is believed to be a key role for pollination drop proteins, given the exposed nature of pollination drops and their nutrients-rich composition. Finally, a collection of proteins found in cycads pollination drops fall into the category of AGPs (Prior et al., Citation2019). To date, no studies have been done about miRNAs in Cycas pollination drops, which might be an interesting area of investigation given the reported discoveries in Ginkgo (Lu et al., Citation2020; Mao et al., Citation2022).
It should be noticed that pollination drops are not the only interesting ovule secretory fluids in Ginkgo and cycads. After pollination and pollen germination in the pollination chamber, the pollen tube grows. This is a long process since in gymnosperms pollination and fertilization are separated by a time interval that can range from few weeks to several months (Stevenson, Citation2013). In the meantime, the ovule grows and the archegonial chambers are developed as previously described. In Ginkgo and Cycas fertilization occurs via zoogamy, with flagellated sperm cells swimming toward the archegonia for penetrating the egg cell ( and ). As it will be discussed later, this is an important feature that characterizes Cycas and Ginkgo and distinguishes them from the other seed plants, in which fertilization occurs via siphonogamy. In Cycas and Ginkgo the sperm cells are not delivered by the pollen tube, hence they should swim in an appropriate aqueous medium toward the egg cell. The composition of this medium is also probably important for the correct guidance of the flagellated sperms toward the egg (Mao et al., Citation2022; von Aderkas et al., Citation2022). However, the study of the composition of these archegonia secretions is quite challenging, since not only they are ephemeral (lasting few days) but are also found deep inside the ovule and difficult to collect. Recent studies in Cycas have begun to shed light on their composition, revealing that they significantly differ from pollination drops. These secretions are rich in proteins, while less in sugars and antimicrobial compounds, in line with their different roles (von Aderkas et al., Citation2022). Also, the types of proteins found in these secretions are different from the type of proteins found in pollination drops. Proteins contained in archegonia secretions show a broad range of functions and many of them are released by cells undergoing programmed cell death, a crucial event for the formation of the archegonial chamber (von Aderkas et al., Citation2022). Less is known about the composition of fertilization fluids in Ginkgo. Remarkably, probably in both Ginkgo and Cycas, such fluid composition is also tightly regulated by the controlled secretion of the archegonia wall cells, which are consistently characterized by a cytoplasm full of vesicles and with an extended endomembrane system (D’Apice et al., Citation2021; von Aderkas et al., Citation2022).
7. Pollen tube growth
A remarkable similarity between Cycas and Ginkgo reproductive mechanisms is the haustorial pollen tube development.
The pollen tube germination and growth in Ginkgo have been extensively studied by Friedman (Citation1987). Approximately one week after pollen’s arrival in the pollen chamber, the first signs of germination appear with the swelling of the tube cell and the appearance of a slightly bulbous protuberance. The growth of the pollen tube frequently initiates at approximately right angles to the original axial row of cells within the pollen grains. This angular tubular growth might be determined by physical constraints or nutrient gradients or might be random. The pollen tube elongates and penetrates into the available gaps among the nucellar cells. Shortly after, the growing tip of the pollen tube starts to ramify, giving rise to a considerable number of haustorial branches. The growth of the haustorial system is quite rapid with the branches proliferating in all directions (). Initially, the branches of the very extended haustorial system elongate and ramify remaining quite thin, and later the girth increases as well. The girth enlargement is uneven and depends on the available empty space, indeed it should be highlighted that the nucellar cells are not degraded, but that the male gametophyte growth is opportunistic, filling adjacent available spaces (Friedman, Citation1987). As Ginkgo is zoogamous, the role of this system is not the delivery of the sperm cells to the egg, but rather of anchoring the male gametophyte to the nucellar tissues to absorb nutrients ().
Cycas pollen tubes also have a primary haustorial role, but differently from those of Ginkgo, they are typically unbranched or only slightly branched (). Nevertheless, the penetration into the nucellus occurs via tip growth and tube elongation, and a swelling of the basal portion of the gametophyte is observed later in development. This is a feature common in Ginkgo and Cycas, and unique to them among the extant taxa of seed plants (Friedman, Citation1993). However, in cycads, the pollen tube growth within the nucellus is mediated by enzymatic and mechanical destruction of the nucellar tissues. Hence, differently from what happens in Ginkgo, the pollen tube growth results in a significant destruction of the female tissues, with the pollen tube penetrating and invading the nucellar cells (Friedman, Citation1993).
8. Zoogamy
One of the most striking similarities of Ginkgo and cycad reproduction that is unique to these two lineages is zoogamous fertilization (Paolillo, Citation1981; Hori and Miyamura, Citation1997).
In both cases, just before fertilization, the body cells undergo several important changes. The nucleus enlarges and assumes a spherical shape at the center of the cell. Two blepharoplasts (basal bodies) appear inside the cytoplasm and move at the two sides of the nucleus. When the body cell divides into two sperm cells, each male gamete will receive one of the two blepharoplasts that will assist the formation of the flagella. The motor apparatus has typically a spiral arrangement and consists of numerous flagella (Norstog et al., Citation2004).
In Ginkgo, the sperm cells have about 1,000 flagella arranged along approximately three coils (Norstog et al., Citation2004). In cycads the number of flagella can even be greater, and varies from about 2500 in Microcycas to approximately 50,000 in Zamia (about 40,000 in Cycas). They are arranged in 5–10 sinistral coils (Norstog et al., Citation2004). Cycads sperms are larger than Ginkgo sperms and are considered among the biggest among land plants: they are visible to the necked eye and can even reach 0.5 mm in diameter (Lee, Citation1955; Renzaglia and Garbary, Citation2001). Interestingly, while usually two sperm cells are produced, in Microcycas up to 22 spermatozoids may occur in a single microgametophyte (Norstog, Citation1990).
The newly formed spermatozoids appear hemispherical and are pressed against each other along their flat faces. The multi-flagellated spermatozoids are released, one after the other, from the pendulous swollen basal end of the gametophyte ( and ). Notably, the secretions of the ovule into the fertilization chamber play a key role in the induction of the release of the sperm cells (Takaso et al., Citation2013). This highlights again the importance of reproductive fluids in Ginkgo and cycad reproduction. When released, the sperm cells swim toward the archegonia combining a forward movement with a rotating one (Norstog et al., Citation2004).
9. Available molecular data on the development of the reproductive structures in Ginkgo and Cycas
Outside of the biochemical analysis conducted on the reproductive fluids, and especially the pollination drops, little is known about the molecular mechanisms that regulate Ginkgo and Cycas reproduction. This is certainly linked to the difficulties related to the study of nonmodel plants and the impossibility to obtain mutants and introduce desired mutations via genetic transformation as well as the lack of breeding programs. It is also important to consider that these plants take several years to reach sexual maturity; consequently, it is very difficult to evaluate the phenotype on the reproductive structures of any genetic defect or variant.
Nevertheless, the increasing availability of genome sequences (Guan et al., Citation2016; Liu et al., Citation2021; Citation2022), the technological developments of –omics techniques and the possibility to perform functional complementation analyses (Zhang et al., Citation2004) could help to fill these gaps.
The molecular studies done on Cycas and Ginkgo reproduction involve mainly the analysis of the genome sequences, searching for homologues to the genes that are known to play a crucial role in the ovule, pollen and embryo development in model plants, as well as some expression studies. For example, the analysis of the Cycas panzhihuaensis genome (Liu et al., Citation2022) identified members of the LAFL family, which includes master regulators of seed development such as LEAFY COTYLEDON1 (LEC1), ABSCISIC ACID INSENSITIVE3 (ABI3), FUSCA3 (FUS3), and LEAFY COTYLEDON2 (LEC2). In particular, the genomic analyses identified two LEC1 genes, one ABI3 gene and a group of FUS3 and LEC2 genes organized in tandem repeats. In addition, the Cycas genome contains also members of FUS3/LEC2-like families that are unique to gymnosperms (Liu et al., Citation2022). Similarly, members of the LAFL family are present also in the Ginkgo genome, including three LEC1 and one ABI3 (Liu et al., Citation2022).
An important molecular analysis of cycads reproductive biology concerns the study of the homologue of AGAMOUS gene CyAG. It is expressed in both female and male reproductive tissues including the ovule, the megasporophyll, the central axis of the cone, the microsporophyll, and the microsporangium (Zhang et al., Citation2004). Moreover, Zhang et al. (Citation2004) conducted a complementation analysis demonstrating that CyAG driven by the AG promoter can rescue the loss-of-function ag mutant of Arabidopsis. The functional characterization of CyAG suggested that the molecular mechanism of class C function controlling reproductive organs identity has been conserved during 300 million years of evolution. Studies on the homologue of AG have also been performed in Ginkgo. The AGAMOUS of Ginkgo, GBM5, is expressed not only in the female and male reproductive organs but also in young leaves (Jager et al., Citation2003), however the expression levels in the reproductive structures and especially in the ovules are much higher. In addition, GBM5 expression has been detected throughout development and ripening of the fleshy fruit-like structures of the seeds (Lovisetto et al., Citation2012). The conservation of the C function has been proven in experiments of heterologous gene expression, in which it has been shown that the ectopic expression of the Ginkgo GMB5 gene in tomato caused the homeotic transformation of the transgenic sepals into carpel-like structures (Lovisetto et al., Citation2015).
More molecular analyses have been conducted in Ginkgo compared to cycads, identifying also other key regulatory genes involved in ovule development (D’Apice et al., Citation2022) and investigating the importance of pollination in regulating the development of the female tissues (D’Apice et al., Citation2021).
In particular, omics analyses have been conducted on ovules before, during and after pollination, suggesting that the pollination event promotes further ovule development and triggers the activation of downstream pathways responsible for the switch from ovule integument into seed coat. Both metabolomics and transcriptomics results showed that the metabolic pathways involved in the seed coat formation are activated upon pollination (D’Apice et al., Citation2021). The observation that, similarly to Ginkgo, unpollinated ovules of cycads are aborted (Zumajo-Cardona et al., Citation2021) leads to hypothesize that a similar key role is played by pollination for cycads ovule development as well. However, no experimental data are available in support of this hypothesis.
Beside the aforementioned studies about AG orthologues, other MADS‐box genes and orthologues of key regulatory factors commonly associated with ovule development in angiosperms have been studied in Ginkgo including AGL6 and TM8‐like genes (Lovisetto et al., Citation2012) and WUSCHEL, EARLY FLOWERING 3, AINTEGUMENTA, and BELL1 (Wang et al., Citation2016). A clearer picture emerged thanks to in situ hybridization conducted by D’Apice et al. (Citation2022) comparing Ginkgo ovules at two different stages of development: a very early stage corresponding to ovule primordia within wintering buds and a later stage at pollination time. In this series of experiments, the expression of AG, AGL6, ANT, BEL1, Class III HD‐Zip, and YABBY genes has been analyzed extending the knowledge about the regulatory network underlying Ginkgo ovule development. In general, the investigated genes displayed expression patterns only partially comparable to those of model angiosperms, with certain genes been expressed in a pattern similar to Arabidopsis and others showing peculiarities unique to Ginkgo. Altogether, these data indicate that gymnosperms and angiosperms share partially similar gene regulatory pathways controlling ovule development.
In support of a conserved function of the MADS-box genes in the regulation of gymnosperms and angiosperms reproduction, data are available also for other species. Homologous MIKC-type MADS-box genes are present in both gymnosperms and angiosperms as well as ferns, suggesting they existed in the common ancestor of vascular plants, about 350-400 million years ago (Münster et al., Citation1997). The study of MADS-box genes and their implication in reproduction has been conducted in different gymnosperms including Gnetum (Shindo et al., Citation1999; Becker et al., Citation2003; Hou et al., Citation2020), Welwitschia (Moyroud et al., Citation2017), conifers (Sundström and Engström, Citation2002; Gramzow et al., Citation2014) and Taxus (Lovisetto et al., Citation2012).
Another interesting field of study concerns the investigation of the role of hormones, which in angiosperms have been shown to play a crucial role in the regulation of the development of reproductive organs, engaging in complex cross-talks with gene regulatory networks. Despite phytohormone physiology in reproductive structures remains a quite unexplored area of study for these two plants lineages, interesting studies have been conducted on other gymnosperms. For example, the amount and distribution of cytokinins, auxin, abscisic acid, and their metabolites were investigated during male and female cone initiation and early differentiation in long-shoot buds of Pinus contorta; this study revealed that the local hormonal status at specific developmental stages may play an important role in gender determination and cone yield (Kong et al., Citation2012). Additionally, the study of expression profiles of gibberellin metabolism genes in developing male and female cones of Pinus tabuliformis suggested that common ancestral mechanisms of control of GA regulate the development of male reproductive organs in P. tabuliformis and model angiosperms (Niu et al., Citation2014). On the same line, transcriptomic analysis of fertile and sterile ovules of P. tabuliformis evidenced a key role of auxin in the female gametophyte formation, the importance of cytokinins for ovule development and active ABA biosynthesis and signal transduction in sterile ovules (Yao et al., Citation2018). Moreover, analyses of the endogenous hormone dynamics in Dacrydium pectinatum showed that the levels of gibberellins, auxin, abscisic acid and cytokinins change during the process of female cone development (Lu et al. Citation2021; Wang et al., Citation2022). Similarly, endogenous levels of free and conjugated forms of auxin, cytokinins, and abscisic acid during seed development have also been detected in Douglas fir (Pseudotsuga menziesii) via high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA) (Chiwocha and von Aderkas, Citation2002). The role of phytohormones has also been studied in the white spruce Picea glauca analyzing ABA dynamics during seed development (Kong et al., Citation1997; Carrier et al., Citation1999) and in Picea abies and Pinus sylvestris analyzing auxin dynamics during seed maturation and germination (Sandberg & Ernstsen Citation1987; Sandberg et al., Citation1987). Finally, studies have been conducted on Gnetum parvifolium highlighting that the sexual differentiation process is accompanied by variations in levels of endogenous hormones (Lan et al., Citation2018).
Only preliminary phytohormones surveys are available in Cycas. These found a high production of salicylic acid and jasmonic acid in unpollinated ovules versus pollinated ovules and pointed to an involvement of gibberellins in the integument development and of abscisic acid during embryo development, seed coat formation, seed maturation and dormancy (Liu et al., Citation2022). These findings suggest even more that such area of research would be particularly fruitful and stimulating for extending our knowledge on seed plant reproduction.
10. Conclusions and final remarks
This review focused on the similarities and differences between the reproductive mechanisms of Ginkgo and Cycas, from the morphogenesis of the male and female organs to the pollination and fertilization events. The whole reproductive process was presented in detail, providing a comprehensive and organized picture together with complete illustrations and photographic material.
Among the similarities, this review examined in depth: pollen ontogeny, shape and ultrastructure, pollen germination and haustorial pollen tube development; ovule development with formation of a coenocytic female gametophyte and differentiation of the ovule integument, archegonia formation and zoogamy; reproductive fluids production and their role in pollination and fertilization. The comparison between Cycas and Ginkgo reproductive mechanisms has also brought to light some peculiar differences. Some of them are linked to the strongly diverse plant habits and/or the pollination mode. We pointed out: the different external morphology and organization of the male and female reproductive organs, the peculiar microsporangia wall structure, the different ovule vasculature, the distinct levels of pollen tube branching and mechanisms of penetration in the nucellus.
Beside the presentation of the morphological and developmental processes, a section has been dedicated to the latest available molecular data. Even if our knowledge concerning the molecular regulation of the reproductive development in Ginkgo and Cycas is far from being complete, -omics analyses as well as in situ hybridization studies suggest that similar gene regulatory pathways may control ovule development in these two gymnosperm lineages.
Despite the difficulties in working with these plants, further efforts are needed in order to extend our molecular knowledge of their reproductive biology, given their key phylogenetic position and remarkable evolutionary history. For example, in spite of the available morphological analyses of the male cones and male gametophyte development, as well as some cytological observations of pollen germination and pollen tube growth, almost no molecular data have been collected to clarify the genetic regulation of these processes.
Our review might stimulate future research on both plant genera, especially in regards the hormonal and genetic regulation of the development of the reproductive structures, as well as the role of pollination in ovule development and the cross-talks between the female and male gametophytes.
Acknowledgements
We would like to thank Luca Cacciavillani and Roberto Tacchetto (Botanical Garden of Padova, University of Padova, IT) for the valuable help in sampling Ginkgo and Cycas material. Many thanks to Prof. Manuel Casares Porcel, Director of the Botanical Garden of Granada (ES), for providing us with photos of Cycas revoluta (). We thank the illustrator Irene Fioretti for the artwork.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ackerman, J. D. 2000. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. In: Pollen and Pollination; Dafni A., Hesse M., and Pacini, E., Eds. Springer: Vienna, pp 167–185.
- Becker, A., Saedler, H., and Theissen, G. 2003. Distinct MADS-box gene expression patterns in the reproductive cones of the gymnosperm Gnetum gnemon. Dev. Genes Evol. 213: 567–572. doi:10.1007/s00427-003-0358-0
- Breygina, M., Klimenko, E., and Schekaleva, O. 2021. Pollen germination and pollen tube growth in gymnosperms. Plants. 10: 1301. doi:10.3390/plants10071301
- Carothers, I. E. 1907. Development of ovule and female gametophyte in Ginkgo biloba. Bot. Gaz. 43: 116–130.
- Carrier, D. J., Kendall, E. J., Bock, C. A., Cunningham, J. E., and Dunstan, D. I. 1999. Water content, lipid deposition, and (+)-abscisic acid content in developing white spruce seeds. J. Exp. Bot. 50:1359–1364. doi:10.1093/jxb/50.337.1359
- Chamberlain, C. J. 1935. Gymnosperms, structure and evolution. Chicago, Illinois: University of Chicago Press.
- Chamberlain, C. Y. 1906. The ovule and female gametophyte of Dioon. Bot. Gaz. 42: 321–358. doi:10.1086/329037
- Cheng, F., Zhao, B., Jiang, B., Lu, Y., Li, W., Jin, B., and Wang, L. 2018. Constituent analysis and proteomic evaluation of ovular secretions in Ginkgo biloba: not just a pollination medium. Plant Signal. Behav. 13: 1–8.
- Chiwocha, S., and von Aderkas, P. 2002. Endogenous levels of free and conjugated forms of auxin, cytokinins and abscisic acid during seed development in Douglas fir. Plant Growth Regul. 36: 191–200. doi:10.1023/A:1016522422983
- Condamine, F. L., Nagalingum, N. S., Marshall, C. R., and Morlon, H. 2015. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evol. Biol. 15: 65.
- Coulter, A., Poulis, B. A. D., and von Aderkas, P. 2012. Pollination drops as dynamic apoplastic secretions. Flora: Morphol. Distrib. Funct. Ecol. Plants. 207: 482–490. doi:10.1016/j.flora.2012.06.004
- D’Apice, G., Moschin, S., Araniti, F., Nigris, S., Di Marzo, M., Muto, A., Banfi, C., Bruno, L., Colombo, L., and Baldan, B. 2021. The role of pollination in controlling Ginkgo biloba ovule development. New Phytol. 232: 2353–2368. doi:10.1111/nph.17753
- D’Apice, G., Moschin, S., Nigris, S., Ciarle, R., Muto, A., Bruno, L., and Baldan, B. 2022. Identification of key regulatory genes involved in the sporophyte and gametophyte development in Ginkgo biloba ovules revealed by in situ expression analyses. Am. J. Bot. 109: 887–898. doi:10.1002/ajb2.1862
- Dehgan, B. and Dehgan, N. B. 1988. Comparative pollen morphology and taxonomic affinities in Cycadales. Am. J. Bot. 75: 1501–1516. doi:10.1002/j.1537-2197.1988.tb11224.x
- Del Tredici, P. 2007. The phenology of sexual reproduction in Ginkgo biloba: ecological and evolutionary implications. Bot. Rev. 73: 267–278.
- Donaldson, J .S. (ed.). 2003. Cycads. Status Survey and Conservation Action Plan. IUCN/SSC Cycad Specialist Group. IUCN, Gland (CH) and Cambridge (UK).
- Douglas, A. W., Stevenson, D. W., and Little, D. P. 2007. Ovule development in Ginkgo biloba L., with emphasis on the collar and nucellus. Int. J. Plant Sci. 168: 1207–1236. doi:10.1086/521693
- Fernando, D. D., Quinn, C. R., Brenner, E. D., and Owens, J. N. 2010. Male gametophyte development and evolution in extant gymnosperms. Int. J. Plant Dev. Biol. 4: 47–63.
- Foster, A. S. and Gifford, E. M. 1974. Comparative Morphology of Vascular Plants, 2nd ed. W. H. Freeman and Company: San Francisco. ISBN 978-0716707127.
- Friedman, W. E. 1987. Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo). Am. J. Bot. 74: 1797–1815. doi:10.1002/j.1537-2197.1987.tb08783.x
- Friedman, W. E. 1993. The evolutionary history of the seed plant male gametophyte. Trends Ecol. Evol. 8: 15–21. doi:10.1016/0169-5347(93)90125-9
- Friedman, W. E. and Gifford, E. M. 1997. Development of the male gametophyte of Ginkgo biloba: a window into the reproductive biology of early seed plants. In: Ginkgo Biloba: A Global Treasure; Hori, T., Ridge, R. W., Tulecke, W., Del Tredici, P., Trémouillaux-Guiller, J., and Tobe, H., Eds. Springer: Tokyo, pp 29–49.
- Gramzow, L., Weilandt, L., and Theißen, G. 2014. MADS goes genomic in conifers: towards determining the ancestral set of MADS-box genes in seed plants. Ann. Bot. 114: 1407–1429. doi:10.1093/aob/mcu066
- Guan, R., Zhao, Y., Zhang, H., Fan, G., Liu, X., Zhou, W., Shi, C., Wang, J., Liu, W., Liang, X., Fu, Y., Ma, K., Zhao, L., Zhang, F., Lu, Z., Lee, S. M., Xu, X., Wang, J., Yang, H., Fu, C., Ge, S., and Chen, W. 2016. Draft genome of the living fossil Ginkgo biloba. Gigascience. 5: 49. doi:10.1186/s13742-016-0154-1
- Hori, T., and Miyamura, S. 1997. Contribution to the knowledge of fertilization of gymnosperms with flagellated sperm cells: Ginkgo biloba and Cycas revoluta. In: Ginkgo Biloba a Global Treasure; Hori, T., Ridge, R. W., Tulecke, W., Del Tredici, P., Trémouillaux-Guiller, J., and Tobe, H., Eds. Springer: Tokyo, pp 67–84.
- Hou, C., Li, L., Liu, Z., Su, Y., and Wan, T. 2020. Diversity and expression patterns of MADS-Box genes in Gnetum luofuense - Implications for functional diversity and evolution. Trop. Plant Biol. 13: 36–49. doi:10.1007/s12042-019-09247-x
- Jager, M., Hassanin, A., Manuel, M., Le Guyader, H., and Deutsch, J. 2003. MADS-box genes in Ginkgo biloba and the evolution of the AGAMOUS family. Mol. Biol. Evol. 20: 842–854. doi:10.1093/molbev/msg089
- Jin, B., Jiang, X., Wang, D., Zhang, L., Wan, Y., and Wang, L. 2012. The behavior of pollination drop secretion in Ginkgo biloba L. Plant Signal. Behav. 7: 1168–1176. doi:10.4161/psb.21122
- Jin, B., Wang, D., Lu, Y., Jiang, X. X., Zhang, M., Zhang, L., and Wang, L. 2012. Female short shoot and ovule development in Ginkgo biloba L. with emphasis on structures associated with wind pollination. ISRN Bot. 2012: e230685–9. doi:10.5402/2012/230685 ]
- Kong, L., Attree, S., and Fowke, L.C. 1997. Changes of endogenous hormone levels in developing seeds, zygotic embryos and megagametophytes in Picea glauca. Physiol. Plant. 101: 23–30. doi:10.1034/j.1399-3054.1997.1010104.x
- Kong, L., von Aderkas, P., Zaharia, I., Abrams, S. R., Lee, T., and Woods, J. 2012. Analysis of phytohormone profiles during male and female cone initiation and early differentiation in longshoot buds of lodgepole pine. J. Plant Growth Regul. 31: 478–489. doi:10.1007/s00344-011-9257-1
- Kono, M., and Tobe, H. 2007. Is Cycas revoluta (Cycadaceae) wind- or insect-pollinated? Am. J. Bot. 94: 847–855. doi:10.3732/ajb.94.5.847
- Lan, Q., Liu, J.F., Shi, S.Q., Deng, N., Jiang, Z.P., and Chang, E.M. 2018. Anatomy, microstructure, and endogenous hormone changes in Gnetum parvifolium during anthesis. J. Sytematics Evol. 56: 14–24. doi:10.1111/jse.12263
- Lee, C. L. 1955. Fertilization in Ginkgo biloba. Bot. Gaz. 117: 79–100. doi:10.1086/335894
- Little, S. A., Prior, N. A., Pirone, C., and von Aderkas, P. 2014. Pollen–ovule interactions in gymnosperms. In: Reproductive Biology of Plants; Ramawat, K. G., Mérillon, J. M., and Shivanna, K. R., Eds. CRC Press: Boca Raton, pp. 91–117.
- Liu, H., Wang, X., Wang, G., Cui, P., Wu, S., Ai, C., Hu, N., Li, A., He, B., Shao, X., Wu, Z., Feng, H., Chang, Y., Mu, D., Hou, J., Dai, X., Yin, T., Ruan, J., and Cao, F. 2021. The nearly complete genome of Ginkgo biloba illuminates gymnosperm evolution. Nat. Plants. 7: 748–756. doi:10.1038/s41477-021-00933-x
- Liu, Y., Wang, S., Li, L., Yang, T., Dong, S., Wei, T., Wu, S., Liu, Y., Gong, Y., Feng, X., Ma, J., Chang, G., Huang, J., Yang, Y., Wang, H., Liu, M., Xu, Y., Liang, H., Yu, J., Cai, Y., Zhang, Z., Fan, Y., Mu, W., Sahu, S. K., Liu, S., Lang, X., Yang, L., Li, N., Habib, S., Yang, Y., Lindstrom, A. J., Liang, P., Goffinet, B., Zaman, S., Wegrzyn, J. L., Li, D., Liu, J., Cui, J., Sonnenschein, E. C., Wang, X., Ruan, J., Xue, J. Y., Shao, Z. Q., Song, C., Fan, G., Li, Z., Zhang, L., Liu, J., Liu, Z. J., Jiao, Y., Wang, X. Q., Wu, H., Wang, E., Lisby, M., Yang, H., Wang, J., Liu, X., Xu, X., Li, N., Soltis, P. S., Van de Peer, Y., Soltis, D. E., Gong, X., Liu, H., and Zhang, S. 2022. The Cycas genome and the early evolution of seed plants. Nat. Plants 8: 389–401. doi:10.1038/s41477-022-01129-7
- Lovisetto, A., Baldan, B., Pavanello, A., and Casadoro, G. 2015. Characterization of an AGAMOUS gene expressed throughout development of the fleshy fruit-like structure produced by Ginkgo biloba around its seeds. BMC Evol. Biol. 15: 139.
- Lovisetto, A., Guzzo, F., Tadiello, A., Toffali, K., Favretto, A., and Casadoro, G. 2012. Molecular analyses of MADS-box genes trace back to gymnosperms the invention of fleshy fruits. Mol. Biol. Evol. 29: 409–419. doi:10.1093/molbev/msr244
- Lu, W., Wang, E., Zhou, W., Li, Y., Li, Z., Song, X., Wang, J., Ren, M., Yang, D., Huo, S., Zhao, Y., and Liang, H. 2021. Morpho-histology, endogenous hormone dynamics, and transcriptome profiling in Dacrydium pectinatum during male cone development. Forests 12: 1598. doi:10.3390/f12111598
- Lu, Y., Jin, B., Wang, L., Wang, Y., Wang, D., Jiang, X., and Chen, P. 2011. Adaptation of male reproductive structures to wind pollination in gymnosperms: cones and pollen grains. Can. J. Plant Sci. 91: 897–906. doi:10.4141/cjps2011-020
- Lu, Y., Wang, L., Wang, D., Wang, Y., Zhang, M., Jin, B., and Chen, P. 2011. Male cone morphogenesis, pollen development and pollen dispersal mechanism in Ginkgo biloba L. Can. J. Plant Sci. 91: 971–981. doi:10.4141/cjps2011-036
- Lu, Y., Zhang, L., Cheng, F., Zhao, J., Cui, J., Li, W., Wang, L., and Jin, B. 2016. The morphology, ultrastructure, element distribution and motion behaviour in pollen of Ginkgo biloba L. Trees 30:2189–2201. doi:10.1007/s00468-016-1444-z
- Lu, Z., Jiang, B., Zhao, B., Mao, X., Lu, J., Jin, B., and Wang, L. 2020. Liquid profiling in plants: identification and analysis of extracellular metabolites and miRNAs in pollination drops of Ginkgo biloba. Tree Physiol. 40: 1420–1436. doi:10.1093/treephys/tpaa073
- Maheshwari, P., and Singh, H. 1967. The female gametophyte of gymnosperms. Biol. Rev. 42: 88–129. doi:10.1111/j.1469-185X.1967.tb01341.x
- Mao, D., Tang, H., Xiao, N., and Wang, L. 2022. Uncovering the secrets of secretory fluids during the reproductive process in Ginkgo biloba. Crit. Rev. Plant Sci. 41: 161–175. doi:10.1080/07352689.2022.2066805
- Meade, L. E., Plackett, A. R. G., and Hilton, J. 2021. Reconstructing development of the earliest seed integuments raises a new hypothesis for the evolution of ancestral seed-bearing structures. New Phytol. 229: 1782–1794. doi:10.1111/nph.16792
- Moitra, A. and Bhatnagar, S. P. 1982. Ultrastructure, cytochemical, and histochemical studies on pollen and male gamete development in gymnosperms. Gamete Res. 5:71–112. doi:10.1002/mrd.1120050108
- Moyroud, E., Monniaux, M., Thévenon, E., Dumas, R., Scutt, C. P., Frohlich, M. W., and Parcy, F. 2017. A link between LEAFY and B‐gene homologues in Welwitschia mirabilis sheds light on ancestral mechanisms prefiguring floral development. New Phytol. 216: 469–481. doi:10.1111/nph.14483
- Mundry, M., and Stützel, T. 2004. Morphogenesis of leaves and cones of male short-shoots of Ginkgo biloba L. Flora. Morphol. Distrib. Funct. Ecol. Plants. 199: 437–452. doi:10.1078/0367-2530-00171
- Münster, T., Pahnke, J., Di Rosa, A., Kim, J.T., Martin, W., Saedler, H., and Theissen, G. 1997. Floral homeotic genes were recruited from homologous MADS-box genes preexisting in the common ancestor of ferns and seed plants. Proc. Natl. Acad. Sci. USA. 94: 2415–2420. doi:10.1073/pnas.94.6.2415
- Nagalingum, N. S., Marshall, C. R., Quental, T. B., Rai, H. S., Little, D. P., and Mathews, S. 2011. Recent synchronous radiation of a living fossil. Science. 334: 796–799. doi:10.1126/science.1209926
- Nepi, M., Little, S., Guarnieri, M., Nocentini, D., Prior, N. A., Gill, J., Tomlinson, P. B., Ickert-Bond, S. R., Pirone, C., Pacini, E., and von Aderkas, P. 2017. Phylogenetic and functional signals in gymnosperm ovular secretions. Ann. Bot. 120: 923–936. doi:10.1093/aob/mcx103
- Nepi, M., von Aderkas, P., Wagner, R., Mugnaini, S., Coulter, A., and Pacini, E. 2009. Nectar and pollination drops: how different are they? Ann. Bot. 104: 205–219. doi:10.1093/aob/mcp124
- Nigris, S., D’Apice, G., Moschin, S., Ciarle, R., and Baldan, B. 2021. Fleshy structures associated with ovule protection and seed dispersal in gymnosperms: a systematic and evolutionary overview. Crit. Rev. Plant Sci. 40: 285–302. doi:10.1080/07352689.2021.1938397
- Niu, S., Yuan, L., Zhang, Y., Chen, X., and Li, W. 2014. Isolation and expression profiles of gibberellin metabolism genes in developing male and female cones of Pinus tabuliformis. Funct. Integr. Genomics 14: 697–705. doi:10.1007/s10142-014-0387-y
- Norstog, K. 1972. Role of archegonial neck cells of Zamia and other cycads. Phytomorphology. 22: 125–130.
- Norstog, K. 1987. Cycads and the origin of insect pollination. Am. Sci. 75: 270–279.
- Norstog, K. J. 1990. Spermatozoids of Microcycas calocoma: ultrastructure. Bot. Gaz. 151:275–284. doi:10.1086/337827
- Norstog, K. J., Gifford, E. M., and Stevenson, D.W. 2004. Comparative development of the spermatozoids of cycads and Ginkgo biloba. Bot. Rev. 70: 5–15. doi:10.1663/0006-8101(2004)070[0005:CDOTSO2.0.CO;2]
- Norstog, K., and Nicholls, T. 1998. The Biology of the Cycads. Cornell University Press: Ithaca, NY.
- PalDat. 2023. A palynological database, society for the promotion of palynological research in Austria (AutPal). https://www.paldat.org. (accessed Jun 2).
- Paolillo, D. J. 1981. The swimming sperms of land plants. BioScience. 31: 367–373. doi:10.2307/1308401
- Prior, N., Little, S. A., Boyes, I., Griffith, P., Husby, C., Pirone-Davies, C., Stevenson, D. W., Tomlinson, P. B., and von Aderkas, P. 2019. Complex reproductive secretions occur in all extant gymnosperm lineages: a proteomic survey of gymnosperm pollination drops. Plant Reprod. 32: 153–166. doi:10.1007/s00497-018-0348-z
- Ran, J. H., Shen, T. T., Wang, M. M., and Wang, X. Q. 2018. Phylogenomics resolves the deep phylogeny of seed plants and indicates partial convergent or homoplastic evolution between Gnetales and angiosperms. Proc. R Soc. B. 285: 20181012. doi:10.1098/rspb.2018.1012
- Renzaglia, K. S. and Garbary, D. J. 2001. Motile gametes of land plants: diversity, development, and evolution. Crit. Rev. Plant Sci 20: 107–213. doi:10.1080/20013591099209
- Reynolds, L. G. 1924. Female gametophyte of Microcycas. Bot. Gaz. 77:391–403. doi:10.1086/333340
- Sahashi, N. and Ueno, J. 1986. Pollen morphology of Ginkgo biloba and Cycas revoluta. Can. J. Bot. 64: 3075–3078. doi:10.1139/b86-406
- Sandberg, G. and Ernstsen, A. 1987. Dynamics of indole-3-acetic acid during germination of Picea abies seeds. Tree Physiol. 3: 185–192. doi:10.1093/treephys/3.2.185
- Sandberg, G., Ernstsen, A., and Hamnede, M. 1987. Dynamics of indole-3 acetic acid and indole-3 ethanol during maturation and germination of Pinus sylvestris seeds. Physiol. Plant. 71: 411–418. doi:10.1111/j.1399-3054.1987.tb02876.x
- Sedgwick, P. J. 1924. Life history of Encephalartos. Bot. Gaz. 77: 300–310. doi:10.1086/333317
- Shindo, S., Ito, M., Ueda, K., Kato, M., and Hasebe, M. 1999. Characterization of MADS genes in the gymnosperm Gnetum parvifolium and its implication on the evolution of reproductive organs in seed plants. Evol. Dev. 1: 180–190. doi:10.1046/j.1525-142x.1999.99024.x
- Smith, F. G. 1910. Development of the ovulate strobilus and young ovule of Zamia floridana. Bot. Gaz. 50: 128–141. doi:10.1086/330306
- Soma, S. 1997. Development of the female gametophyte and the embryogeny of Ginkgo biloba. In Ginkgo Biloba a Global Treasure; Hori, T., Ridge, R. W., Tulecke, W., Del Tredici, P., Trémouillaux-Guiller, J., and Tobe, H., Eds. Springer: Tokyo, pp 51–65.
- Stevenson, D. W. 2013. Chapter 5: Gymnosperm. In Annual Plant Reviews Volume 45: The Evolution of Plant Form; Ambrose, B., and Purrugganan, M., Eds. Hoboken, New Jersey, USA: Blackwell Publishing Ltd. pp 141–149.
- Stevenson, D. W., Norstog, K., and Fawcett, P. 1998. Pollination biology of cycads. In Reproductive Biology in Systematics, Conservation, and Economic Botany; Owens, S. and Rudall, P., Eds. Royal Botanic Gardens: Kew, UK. pp 277–294.
- Sundström, J. and Engström, P. 2002. Conifer reproductive development involves B-type MADS-box genes with distinct and different activities in male organ primordia. Plant J. 31: 161–169.
- Takaso, T., Kimoto, Y., Owens, J. N., Kono, M., and Mimura, T. 2013. Secretions from the female gametophyte and their role in spermatozoid induction in Cycas revoluta. Plant Reprod. 26: 17–23. doi:10.1007/s00497-012-0204-5
- Terry, I., Tang, W., Taylor, A., Singh, R., Vovides, A., and Cibrián Jaramillo, A. 2012. An overview of cycad pollination studies. In: Memoirs of the New York Botanical Garden, vol. 106. Springer: New York, NY, pp 352–394. doi:10.21135/893275150.024
- Toon, A., Terry, L. I., Tang, W., Walter, G. H., and Cook, L. G. 2020. Insect pollination of cycads. Austral Ecol. 45: 1033–1058.
- Von Aderkas, P., Little, S., Nepi, M., Guarnieri, M., Antony, M., and Takaso, T. 2022. Composition of sexual fluids in Cycas revoluta ovules during pollination and fertilization. Bot. Rev. 88: 453–484. doi:10.1007/s12229-021-09271-1
- Von Aderkas, P., Prior, N. A., and Little, S. A. 2018. The evolution of sexual fluids in gymnosperms from pollination drops to nectar. Front. Plant Sci. 9: 1844. doi:10.3389/fpls.2018.01844
- Wang, D., Lu, Y., Zhang, M., Lu, Z., Luo, K., Cheng, F., and Wang, L. 2014. Structure and function of the neck cell during fertilization in Ginkgo biloba L. Trees. 28: 995–1005. doi:10.1007/s00468-014-1013-2
- Wang, E., Lu, W., Liang, H., Zhang, X., Huo, S., Song, X., Wang, J., and Zhao, Y. 2022. Morpho-histology, endogenous hormone dynamics, and transcriptome profiling in Dacrydium pectinatum during female cone development. Front. Plant Sci. 13: 954788. doi:10.3389/fpls.2022.954788
- Wang, L., Lu, Z., Li, W., Xu, J., Luo, K., Lu, W., Zhang, L., and Jin, B. 2016. Global comparative analysis of expressed genes in ovules and leaves of Ginkgo biloba L. Tree Genet. Genomes. 12: 29. doi:10.1007/s11295-016-0989-8
- Yao, Y., Han, R., Gong, Z., Zheng, C., and Zhao, Y. 2018. RNA-seq analysis reveals gene expression profiling of female fertile and sterile ovules of Pinus tabulaeformis Carr. during free nuclear mitosis of the female gametophyte. Int. J. Mol. Sci. 19: 2246. doi:10.3390/ijms19082246
- Zhang, P., Tan, H. T., Pwee, K. H., and Kumar, P. P. 2004. Conservation of class C function of floral organ development during 300 million years of evolution from gymnosperms to angiosperms. Plant J. 37: 566–577. doi:10.1046/j.1365-313x.2003.01983.x
- Zhang, X. 2019. Ovule development in Cycads: observation on anatomy and nucellus morphology in Zamia and Cycas. bioRxiv 735837. doi:10.1101/735837
- Zhao, Y. P., Fan, G., Yin, P. P., Sun, S., Li, N., Hong, X., Hu, G., Zhang, H., Zhang, F. M., Han, J. D., Hao, Y. J., Xu, Q., Yang, X., Xia, W., Chen, W., Lin, H. Y., Zhang, R., Chen, J., Zheng, X. M., Lee, S.M., Lee, J., Uehara, K., Wang, J., Yang, H., Fu, C. X., Liu, X., Xu, X., and Ge, S. 2019. Resequencing 545 ginkgo genomes across the world reveals the evolutionary history of the living fossil. Nat. Commun. 10: 4201. doi:10.1038/s41467-019-12133-5
- Zumajo-Cardona, C., Frangos, S., and Stevenson, D. W. 2021. Seed anatomy and development in cycads and Ginkgo, keys for understanding the evolution of seeds. Flora. 285: 151951. doi:10.1016/j.flora.2021.151951
