 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Polyamines (PAs) are important molecules that determine cell longevity or death. Studies have shown that nutritional supplementation with spermidine can reduce age-related pathology and increase life span in a number of organisms, including humans. In addition, applying PAs to plants prevents their senescence. This review aims to provide an integrated understanding of the regulation of PA metabolism and its effect(s) on cell homeostasis. PA metabolism is universal for plants and animals. Research has shown that increased levels of PA synthesizing enzymes are associated with cell proliferation, whereas activation of the PA catabolic pathway increases oxidative stress and leads to aging/senescence due to cellular damage. Intracellular PA levels are regulated at the transcriptional and translational levels of the PA metabolic genes. The cis-acting regulatory elements and transcription factors determine the tissue-, developmental stage-, and stress-specific expression of a gene. At the translational level, it is regulated by miRNAs targeting mRNAs for cleavage or translational suppression. The byproducts of PA metabolism, such as hypusine and acrolein, are important for cell survival or death. PAs and their metabolic enzymes play several other important roles in plant and animal physiology via their effects on chromatin condensation, histone acetylation, histone deacetylation, transmethylation, and protein-protein interactions. This review focuses on the role(s) of PAs as universal bioregulators in processes across kingdoms, with specific reference to regulation of cellular longevity and death.
Introduction
The Dutch microscopist Antonie van Leeuwenhoek described in a 1677 letter to Lord William Brouncker, the then President of the Royal Society of London, the discovery of spermatozoa in his own semen (Robertson et al., Citation2016). A year later, he discovered crystals in the dried sperm, later termed spermine phosphate. Spermine belongs to a group of polyamines (PAs) (Bachrach, Citation2010) called biogenic polyamines. These organic polycations are found in all eukaryotes and are essential for cell survival (Agostinelli et al., Citation2010a; Sánchez-Jiménez et al., Citation2019). Man’s existence is dependent on polyamines, but it also ends with a group of polyamines called cadaverine and putrescine, which are largely responsible for the foul odor of putrescent cadavers (Hussain et al., Citation2013).
The primary and secondary amino groups of polyamines are protonated at physiological pH, and interact electrostatically with negatively charged molecules such as DNA, RNA, proteins, and phospholipids (Bachrach, Citation2005). There is evidence that PAs regulate cellular activities at transcriptional, translational and post-translational levels. They differ from inorganic cations such as Mg2+ or Ca2+ in that their positive charges are spaced at defined distances by flexible methylene chains that can participate in hydrophobic interactions. PAs engage in stronger and more specific interactions with nucleic acids and acidic macromolecules than inorganic cations (Igarashi and Kashiwagi, Citation2000; Thomas and Thomas, Citation2001; Bachrach, Citation2005; Igarashi and Kashiwagi, Citation2010). Their net cellular concentrations are generally found to be at millimolar levels in eukaryotic cells (Park and Igarashi, Citation2013). Most intracellular PAs are compartmentalized and/or bound to negatively charged molecules. This allows for the free PAs to remain at much lower concentrations than the total cellular concentration. PA homeostasis is elaborately maintained by intricate multiple feedback mechanisms at the levels of biosynthesis, catabolism, uptake, and efflux (Thomas, 2001), suggesting the importance of homeostasis of PAs in plant and animal development and physiology. PAs also play a significant role in plant responses to abiotic stress, their levels rising in plants exposed to different stressors (Upadhyay et al., Citation2020; Citation2021a). Likewise, PA levels are also known to change in response to biotic stress in some situations that involve pathogen infection (Walters, Citation2003). Thus, their important function in stress tolerance offers an opportunity to increase plant tolerance under a variety of environmental challenges (Alcázar et al., Citation2006). PAs also have anti-inflammatory and anti-mutagenic properties, provide defense against various stressors, and can induce autophagy in animals (Matsumoto, Citation2015).
This review focuses on the role(s) of PAs as universal bioregulators in processes across kingdoms with specific reference to the regulation of cellular longevity and death.
Polyamine metabolism and biochemistry – plants vs animals
Polyamine biosynthesis pathways in plants and animals, in general, are conserved, with few differences (Tabor and Tabor, Citation1984). The PA biosynthesis pathway involves three amino acids: arginine, ornithine, and methionine (Bell and Malmberg, Citation1990; Michael et al., Citation1996; Bagni and Tassoni, Citation2001; Kusano et al., Citation2008) (). Arginine is converted to ornithine by hydrolyzing arginase (ARGAH, EC 3.5.3.1) and then to putrescine (Put) by ornithine decarboxylase (ODC, EC 4.1.1.17). Arginine can also be converted via arginine decarboxylase (ADC, EC 4.1.1.19) to agmatine. Agmatine is a substrate for two different pathways leading to Put. In one pathway, agmatine iminohydrolase (AIH, EC 3.5.3.12) catalyzes the formation of N-carbamoylputrescine (NCPut), and then NCPut is the substrate for the reaction catalyzed by N-carbamoylputrescine aminohydrolase (CPA, EC 3.5.1.53), the product of this two-step reaction is Put (Bell and Malmberg, Citation1990; Michael et al., Citation1996; Bagni and Tassoni, Citation2001; Kusano et al., Citation2008; Upadhyay et al., Citation2020). A second pathway agmatine is transformed directly to Put, and this reaction is catalyzed by arginase/agmatinase [ARGAH, EC 3.5.3.1(1)] (Wang et al., Citation2014; Lenis et al., Citation2017; Patel et al., Citation2017; Navakoudis and Kotzabasis, Citation2022). Interestingly, some plants, such as Arabidopsis thaliana, Spirodela polyrhiza, and the moss Physcomitrella patens, do not have genes encoding ODC, while some green algae do not have the ADC pathway (Fuell et al., Citation2010; Hanfrey et al., Citation2011; Upadhyay et al., Citation2021b). Nonetheless, both ADC and ODC are present in many plants, including barley. ODC expression is organ specific (Tanwar et al., Citation2022). Interestingly, HvODC1 and 2 occur only in barley roots, while the alternative ADC pathway is active in other organs. Another amino acid, methionine, is also involved in the formation of PAs. Methionine is converted by Sadenosyl-L-methionine synthetase (MAT, EC 2.5.1.6) to S-adenosyl-L-methionine (SAM) and then via Sadenosyl-L-methionine decarboxylase (SAMDC, EC 4.1.1.50) decarboxylated S-adenosyl-L-methionine (dcSAM). dcSAM serves as a donor of aminopropyl groups for spermidine synthesis from Put, and spermine (Spm) synthesis from spermidine (Spd). These reactions are catalyzed by spermidine synthase (SPDS, EC 2.5.1.16; SRM in humans) and spermine synthase (SPMS, EC 2.5.1.22; SMS in humans), respectively (Mattoo et al., Citation2015; Guo et al., Citation2020). In plants, the final reaction involves thermospermine, which is catalyzed by thermospermine synthase (TSPMS or ACL5, EC 2.5.1.B4) instead of SPMS. Thermospermine is not very abundant in plants, and it has yet to be described in animals (Minguet et al., Citation2008; Vera-Sirera et al., Citation2010). SPDS and SPMS gene families differ between animals and plants (Minguet et al., Citation2008; Vera-Sirera et al., Citation2010). Likely, SPMS evolved from SPDS independently in these two kingdoms.
Figure 1. Polyamine metabolic pathways in plants and animals. (A) Biosynthetic pathways are common between plants and animals, except for one reaction that leads exclusively to the formation of thermospermine in plants. (B) Animal catabolism pathways. (C) Plant catabolism pathways. Enzymes specific for plants are marked in green, enzymes exclusive for animals are marked in red, universal enzymes are marked in blue. Dotted arrow lines indicate spontaneous aldehyde decomposition into the toxic compound known as acrolein. Grey box indicates transcription factor synthesis pathway. Yellow boxes indicate conversion catabolism pathways. Orange boxes indicate terminal catabolism pathways. The question mark (?) indicates poorly understood pathways. Asterisk denotes unstable aldehydes that can spontaneously decompose into acrolein. AcPAO: acetylpolyamine oxidase; ADC: arginine decarboxylase; AIH: agmatine iminohydrolase; ARGAH: arginase/agmatinase; CPA: N-carbamoylputrescine aminohydrolase; CuAO: copper-containing amine oxidases; DHS: deoxyhypusine synthase; DOHH: deoxyhypusine hydroxylase; MAT: S-adenosyl-L-methionine synthetase; NATA: N-acetyltransferase activity; ODC: ornithine decarboxylase; PAO: polyamine oxidase; PAObc: polyamine back conversion oxidase; SAMDC: S-adenosyl-L-methionine decarboxylase; SPDS: spermidine synthase; SMO: spermine oxidase; SPMS: spermine synthase; SSAT spermidine/spermine N1-acetyltransferase; TSPMS: thermospermine synthase.
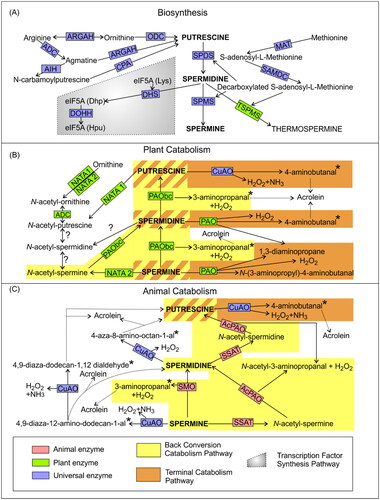
Spd is also an aminobutyl donor necessary for a two-stage reaction named hypusination, the product of which is a fully active translation elongation factor (Saini et al., Citation2009; Park et al., Citation2010). Deoxyhypusine synthase (DHS, EC 2.5.1.46) catalyzes the attachment of the aminobutyl moiety of Spd to lysine of the precursor protein eIF5A [eIF5A(Lys)]. The intermediate deoxyhypusine-eIF5A [eIF5A(Dhp)] is further converted to active hypusine [eIF5A(Hpu)] by deoxyhypusine hydroxylase (DOHH, EC 1.14.99.29) (Park, Citation2006; Park and Igarashi, Citation2013; Belda-Palazón et al., Citation2016), which anneals a hydroxyl group onto the appended aminobutyl chain with both regio- and stereo-specificity.
Put is also a substrate for the biosynthesis of tropane alkaloids such as nicotine, scopolamine, and cocaine of the Solanaceae plant family (Nathanson et al., Citation1993). Putrescine N-methyltransferase (PMT, EC 2.1.1.53) initiates the formation of these alkaloids (Takahashi and Tong, Citation2015). The aminobutyl group of Spd is also transferred to Put by the action of homospermidine synthase (HSS, EC 2.5.1.45; ) in plants. The product of this reaction is homospermidine – an essential precursor in the biosynthesis of pyrrolizidine alkaloids, which serve as defense compounds against predatory insects. The gene sequences encoding DHS and HSS have a high degree of identity (Ober and Hartmann, Citation1999; Ober et al., Citation2003).
Both biosynthesis and catabolism of PAs in plants and animals are similar. Universal for both groups are two distinct pathways of PA catabolism: i) the terminal catabolic pathway (TC) and ii) the back-conversion pathway (BC). The TC pathway leads to the complete decomposition of the PA, and the BC pathway leads to the breakdown of lower polyamines from higher polyamines (Spm → Spd → Put). Oxidases are the major enzymes of PA catabolism. In both animals and plants, two types of polyamine oxidases are present: (1) copper-containing amine oxidases (CuAO, EC 1.4.3.6) and (2) flavin-containing polyamine oxidases (PAO, EC 1.5.3.11) (Kusano et al., Citation2008; Tavladoraki et al., Citation2012; Navakoudis and Kotzabasis, Citation2022).
In plants, apart from CuAO, PAO is also involved in the TC pathway. PAO oxidizes Spd and Spm to form 4-aminobutanal and N-(3-aminopropyl)-4-aminobutanal, respectively, and also 1,3-diaminopropane (Dap) and H2O2. In the plant BC pathway, Spm and Spd are oxidized by PAObc to Spd and Put, respectively, and also to 3-aminopropanal and H2O2 in each reaction (Cona et al., Citation2006; Tavladoraki et al., Citation2006; Angelini et al., Citation2008). The 4-aminobutanal and 3-aminopropanal products of PA catabolism are unstable aldehydes that can spontaneously decompose into acrolein (Wood et al., Citation2007). Acetylated PAs include N-acetyl-putrescine, N-acetyl-spermidine and N-acetyl-spermine (Tassoni et al., Citation2000; Kamada-Nobusada et al., Citation2008; Lou et al., Citation2020). However, little is known about their downstream reaction mechanisms and the enzymes catalyzing them (Gerlin et al., Citation2021). Arabidopsis N-acetyltransferase activity (NATA) genes are the closest homologs of the genes encoding mammalian PA acetyltransferases (Toumi et al., Citation2019). NATA2 catalyzes the acetylation of spermine and the formation of N-acetyl-spermine, while acetylation of putrescine is catalyzed by NATA1. The enzyme responsible for spermidine acetylation is still unknown (Lou et al., Citation2020). Although most PAOs have very low catalytic activity with the acetylated PAs (Gerlin et al., Citation2021), Ahou et al. (Citation2014) reported that AtPAO5 can convert N-acetyl-spermine to Spd. This discovery may indicate that a parallel BC pathway involving acetylated PAs is present in plants as in animal systems. Also, NATA1 catalyzes the acetylation of ornithine and putrescine, resulting in the formation of Nδ-acetyl-ornithine and N-acetyl-putrescine, respectively. Another study showed that ADC is also responsible for converting Nδ-acetyl-ornithine into N-acetyl-putrescine in A. thaliana (Lou et al., Citation2020).
CuAO is an enzyme involved in the plant and animal TC pathway wherein oxidation of Put occurs. This PA catabolic reaction is present in both kingdoms. This reaction produces H2O2, NH3 and 4-aminobutanal, which can also be converted to Ɣ-aminobutyric acid (GABA), an amino acid important in signaling, in subsequent reactions (). Interestingly, the intracellular animal CuAOs and all plant CuAOs have higher catalytic efficiency for Put than for Spd and Spm. Animal serum CuAOs, such as bovine serum amine oxidase (BSAO, EC 1.4.3.6), preferentially oxidize Spd and Spm (Bagni and Tassoni, Citation2001; Agostinelli et al., Citation2010b).
In the animal BC pathway, the enzyme spermidine/spermine N1-acetyltransferase (SSAT, EC 2.3.1.57) catalyzes the transfer of the acetyl group from acetyl-CoA to Spd or Spm and the formation of N1-acetyl-spermidine or N1-acetyl-spermine, respectively (). N1-acetyl-spermidine and N1-acetyl-spermine are then oxidized by acetylpolyamine oxidase (AcPAO, EC 1.5.3.13) to form the reaction products Put and Spm, respectively, as well as N-acetyl-3-aminopropanal and H2O2 (Pegg, Citation2008; Park and Igarashi, Citation2013). Spm is also converted to Spd by an alternative route that uses spermine oxidase (SMO, EC 1.5.3.16). Other products of this reaction are H2O2 and 3-aminopropanal (Wang et al., Citation2001b; Vujcic et al., Citation2002; Cervelli et al., Citation2003; Tavladoraki et al., Citation2012). Another possible back conversion pathway which is characteristic only for animals involves CuAO. CuAO oxidizes Spd and Spm to form H2O2 and NH3, 4-aza-8-amino-acetate-1-al and 4,9-diaza-12-amino-dodecan-1-al, respectively. The latter can be oxidized by CuAO to 4,9-diaza-dodecan-1,12 dialdehyde. Aldehydes 4-aza-8-amino-octan-1-al, 4,9-diaza-12-amino-dodecan-1-al, and 4,9-diaza-dodecan-1,12 dialdehyde, if not metabolized by aldehyde dehydrogenases (ALDH, 1.2.1.5), can spontaneously convert to Put and Spd, respectively, and to acrolein (Agostinelli et al., Citation2006; Citation2010b). However, this alternative pathway of back conversion involving CuAO seems to be less physiologically important (Agostinelli et al., Citation2006; Citation2010b; Tavladoraki et al., Citation2012).
Plant and animal PAOs also differ in the mode of substrate binding at the catalytic site, which results in the oxidation of a different carbon atom, and in consequence leads to the formation of different products (Tavladoraki et al., Citation2012).
Although the pathways of PA metabolism in animals and plants differ partially in terms of individual reactions, they share the possibility of rapid interconversion, i.e., conversion through biosynthesis (Put → Spd → Spm) or reconversion (Spm → Spd → Put) (). Interconversion, along with transport, allows for effective control of PA levels in cell compartments (Seiler, Citation2004; Moschou et al., Citation2008; Tavladoraki et al., Citation2012; Handa et al., Citation2018; Navakoudis and Kotzabasis, Citation2022). In order to maintain homeostatic control over the complex biochemistry involved in polyamine metabolism, strict control of PA metabolic genes and enzymes in plants and mammals is necessary.
Table 1. Plant and animal terminal catabolism and back conversion catabolism pathways. AcPAO: acetylpolyamine oxidase; CuAO: copper-containing amine oxidase; NATA: N-acetyltransferase activity; PAO: polyamine oxidase; PAObc: polyamine back conversion oxidase; Put: putrescine; SMO: spermine oxidase; Spd: spermidine; Spm: spermine; SSAT spermidine/spermine N1-acetyltransferase.
Aspects of regulation of polyamine metabolism
The mechanisms that regulate PA biosynthesis are now known (reviewed in Gonzalez et al., Citation2021). Gene regulation is known to occur at various levels; however, transcriptional regulation seems crucial, involving two major regulatory components: cis-acting regulatory elements (CREs) and trans-acting transcription factors (TFs). Cis-regulatory elements determine the tissue, developmental stage, and stress-specific expression of a gene (Biłas et al., Citation2016). A transcribed ADC-box in the 5′-UTR of the tomato ADCs was hypothesized to produce secondary RNA structures and operate as a translational repressor based on mutational analyses (Wu et al., Citation2019). This motif has been proposed as an old cis-regulatory element that is conserved across plant seed species (Wu et al., Citation2019). Many in-silico studies have identified CREs in the PA metabolic genes in plants including Citrus sinensis, Solanum lycopersicum, Oryza sativa, Hordeum vulgare and Arabidopsis thaliana (Basu et al., Citation2014; Wang and Liu, Citation2015; Majumdar et al., Citation2017; Liu et al., Citation2018; Tanwar et al., Citation2022). The identified CREs are composed of conventional promoter elements (TATA-box, CAAT-box), hormone-responsive elements (ABRE, GARE motif, TATC-box and P-box; TCA, CGTCA, TGACG elements, etc.) and stress-responsive elements (LTR elements, TC-rich repeats, HSE, MBS, WUN-motifs, etc.) (Basu et al., Citation2014; Wang and Liu, Citation2015; Majumdar et al., Citation2017; Liu et al., Citation2018; Tanwar et al., Citation2022). In most of these studies, the CREs were identified in the promoter regions, however their functional investigation on the regulation of gene expression have not been well studied in terms of hormonal or stress response in plants. Very few studies demonstrated the effect of hormones application on the regulation of PA metabolic pathway genes associated with the presence of hormone responsive CREs in their promoter regions. In tomato, most of the examined PA synthesis genes have presented differential expression after being exposed to auxin, gibberellic acid (GA), abscisic acid (ABA), salicylic acid (SA), methyl jasmonate (MeJA), or ethephon (ETH) (Liu et al., Citation2018). Importantly, the promoters of these tomato PAs synthesis genes were found to contain nine hormone responsive cis-acting regions. On the other hand, SlADC2, SlODC1, SlSPDS1, and SlSPDS5 contain an ethylene-responsive cis-element (ERE) in their promoters, and their expression was found to be induced by ETH (Liu et al., Citation2018). Similarly with auxin GA, ABA, SA, or MeJA treatments, genes that were upregulated or downregulated also included the relevant responsive element in their promoters. Transcriptional regulation of PAs biosynthesis may involve various CREs such as ABRE, MYB, WRKY, LTRE, W-box and GATA. In human studies, Wilms tumor protein (WT1) was found to stimulate the promoter of the CuAO1 gene by interacting with a specific cis-regulatory element (Kirschner et al., Citation2014). These findings suggested that CuAO1 is a downstream target gene of WT1.
The interaction of PA metabolism genes with microRNAs (miRNAs) was studied via in-silico analysis, which led to the identification of hvu-miR5049b, hvu-miR6180, and hvu-miR6196 microRNAs associated with PA metabolic genes in barley (Tanwar et al., Citation2022). miRNA hvu-miR5049b was found to be upregulated under drought conditions (Hackenberg et al., Citation2015), while upregulation of hvu-miR6196 was observed under salt adaptation of the autopolyploid Hordeum bulbosum (Liu and Sun, Citation2017). Differential expression of hvu-miR6180 during fungal stress was reported in wheat (Inal et al., Citation2014), while Zma-miR167 positively modulates resistance to maize chlorotic mottle virus (MCMV) (Liu et al. (Citation2022). Zma-miR167 directly targets auxin response factor 3 (ZmARF3) and ZmARF30, which directly bind to the promoter of ZmPAO1, activating its expression to regulate H2O2 production as well as to confer resistance to MCMV (Liu et al., Citation2022). PA metabolism has been shown to be associated with microRNAs in mammalian pathophysiology. Higher expression of miR-34c-5p and miR-320c targets the polyamine gene SMOX in the prefrontal brain of suicidal individuals, while enhanced expression of miR-139-5p and miR-320c targets the PA catabolic gene SSAT1 (Lopez et al., Citation2014). In another study, in-silico analysis predicted binding between miRNA miR-1 and ODC in diabetic cardiomyopathy (DMCM) (Kambis et al., Citation2021). miR-1 overexpression and ODC activity are drastically decreased in the diabetic heart (Al‑Κafaji et al., 2021). These findings suggest a potential role of the dysregulated miRNA-polyamine axis in DMCM patients (Kambis et al., Citation2021).
Polyamine metabolic enzymes play several other important roles in cellular regulation and plant/animal physiology via protein-protein interactions (PPI). For example, the regulatory PPI network for the barley PA metabolic pathway genes has considerable interactive networks among several proteins including the aldehyde dehydrogenase (ALDH) family (Tanwar et al., Citation2022). ALDH, a family of enzymes involved in plant metabolism and aldehyde homeostasis, is important for eliminating toxic aldehydes. These include the response to stress conditions such as high temperature, high salinity, dehydration, oxidative stress, or heavy metals in plant tissues (Zhao et al., Citation2017). PAO overexpression in human degenerated disk samples showed that Spd supplementation balanced polyamine metabolism and delayed NP cell senescence in an IL-1-induced nucleus pulposus (NP) cell degeneration model (Che et al., Citation2022). In-silico PPI network analysis suggested that cyclin-dependent kinase inhibitor 2 A (Cdkn2A) regulates several oxidative stress genes and that PAO has a positive association with Cdkn2a. Thus, it was hypothesized that Cdkn2a is involved in the upstream activation/regulation of PAO.
Polyamine metabolism byproducts
Hypusine
Hypusine (Nϵ-4-amino-2-hydroxybutyl(lysine)) is a polyamine-derived natural amino acid, which, as mentioned above, is synthesized post-translationally via the aminobutyl moiety of Spd. Hypusine is solely found in the protein eIF5A () (Shiba et al., Citation1971; Park et al., Citation1981; Cooper et al., Citation1983). It is essential for eIF5A activity and cell viability in eukaryotes (Pällmann et al., Citation2015). The specific structure of Spd is necessary for the synthesis of hypusine, which entails an absolute requirement for the polyamine Spd in eukaryotes (Byers et al., Citation1994; Chattopadhyay et al., Citation2003; Citation2008). The name hypusine was proposed based on its structure, being built of two moieties, hydroxyputrescine and lysine (Shiba et al., Citation1971). Early on, researchers correlated the synthesis of the amino acid hypusine with cell growth and its critical role in cell proliferation (Cooper et al., Citation1982; Chen, Citation1983; Gerner et al., Citation1986). eIF5A containing this amino acid is essential for the survival of the cell, and modifications of hypusine are important for alleviating translation stalling in poly-proline sequences and other proline-containing motifs (Doerfel et al., Citation2013; Gutierrez et al., Citation2013; Schuller et al., Citation2017). This small protein is highly conserved, especially at the hypusination site, showing the importance of this polyamine-derived protein modification in the evolution of eukaryotes (Park, Citation2006).
In plants, eIF5A and its hypusination may have a role in adaptation to environmental conditions. Plant eIF5A proteins are also highly conserved and are involved in multiple biological processes (Pálfi et al., Citation2021). eIF5A is necessary for mRNA translation and translocation from the nucleus to the cytoplasm (Thompson et al., Citation2004). eIF5A also plays roles in supporting plant growth and in regulating responses to sub-lethal osmotic and nutrient stress (Ma et al., Citation2010). The activation of eIF5A via hypusination occurs in two enzymatic steps, catalyzed by DHS and DOHH (Figure 1A). The DHS in plants is highly over-expressed at the beginning of leaf senescence (Duguay et al., Citation2007) and during the response to osmotic and drought stresses (Wang et al., Citation2001a). The complete decrease in activity of DHS in transgenic plants was found to lead to delayed leaf and fruit senescence as well as inhibition of xylem formation (Wang et al., Citation2003; Citation2005; Liu et al., Citation2008). Hypusinated eIF5A is involved in plant senescence and promotes translation of mRNAs necessary for inducing programmed cell death (PCD) (Wang et al., Citation2001a). However, when DHS was moderately suppressed (ca. 50%), Arabidopsis plants showed increased growth (increased root biomass, bigger leaves, and enhanced seed yield) and tolerance of drought stress (Wang et al., Citation2003; Duguay et al., Citation2007). On the other hand, when DHS was suppressed in Petunia, anomalies in chloroplast development were evident (Liu et al., Citation2019). Gene alterations in Arabidopsis also showed that DHS mutants possess a lethal female gametophyte and thus it cannot be a homozygous diploid (Pagnussat et al., Citation2005). In this regard, studies on DHS inhibitors could be of interest, as these compounds could modify the hypusination process and thereby enhance plants’ tolerance of (a)biotic stressors (Pálfi et al., Citation2021). A recent study demonstrated that Spd, due to its participation in the hypusination process, plays a role in ribosomal quality checks. Disorders in the hypusination process of eIF5A can result either in ribosome stalling and the activation of no-go decay mechanisms or could induce nonstop ribosomal activities (Poidevin et al., Citation2019).
In mammalian cells, inhibitors of the two main enzymes responsible for hypusine synthesis, DHS and DOHH, cause an arrest in cell growth (Hanauske-Abel et al., Citation1994; Park et al., Citation1994). Also, application of an S-adenosyl-L-methionine decarboxylase inhibitor, which causes the lack of Spd in cells, results in cytostasis due to a decrease in hypusinated eIF5A (Byers et al., Citation1994). Moreover, gene modification studies have shown that knock-out of the DHS gene produces a lethal phenotype in Saccharomyces cerevisiae, as does the lack of translation initiation factors 51 A and 51B (yeast homologs of eIF5A), indicating the importance of deoxyhypusine modification in the viability of eukaryotic cells (Schnier et al., Citation1991; Wöhl et al., Citation1993; Sasaki et al., Citation1996; Park et al., Citation1998). Such an effect was also observed in mice in which knock-out of the gene encoding deoxyhypusine synthase caused deformations of blastocysts and death of mice embryos in early embryonic development stages. These findings indicate the essential nature of DHS in cell growth and animal development (Nishimura et al., Citation2012). Similarly, genetic data provide evidence that defects in DHS can lead to neurodevelopmental disorders in humans. Mutations causing a lack of or reduced activity of DHS lead to clinical phenotypes, which supports the importance of eIF5A hypusination in human brain development (Zeesman et al., Citation2012; Park and Wolff, Citation2018). Interestingly, the latest research revealed evidence that impaired eIF5A hypusination may cause serious Mendelian disorders (e.g., microcephaly) in humans. These disorders can be partly restored by supplementation with Spd (Faundes et al., Citation2021).
Acrolein
Acrolein is a reactive carbonyl species (RCS) and is another important by-product of polyamine metabolism. This highly reactive unsaturated aldehyde is formed by β-elimination of the aldehydes formed from polyamine oxidation and readily reacts with lysine residues of proteins to form Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-lysine) (Uchida et al., Citation1998). Acrolein can also be created from lipid peroxidation and occurs in cigarette smoke and as an environmental contaminant (Moghe et al., Citation2015). It is also produced from Spm in higher levels compared to production from arachidonic acid (Pegg, Citation2013). The first studies on this compound were reported almost 60 years ago and revealed its potential role in cell toxicity (Tabor et al., Citation1964). Later, the assumption of the key role of acrolein formation in the toxicity of oxidized polyamines was supported by experiments that studied the rate of inhibition of bacterial macromolecular synthesis by Spm in the presence of serum amine oxidases (Kimes and Morris, Citation1971). It is now well known that acrolein is toxic compound leading to oxidative damage of proteins and DNA, which can contribute to cell death (Pegg, Citation2013; Stewart et al., Citation2018). As an electrophile, acrolein is highly reactive with nucleophiles present in cells such as those found in specific side chains of proteins and nucleic acids. For example, it easily binds to the imidazole group of histidine, the sulfhydryl group of cysteine and the amino group of lysine. These amino acids are important molecules that take part in many processes, from enzyme catalysis to redox signaling and reactive oxygen species (ROS) detection. The formation of a complex with acrolein may lead to significant alterations in protein function (Moghe et al., Citation2015).
In plants, acrolein levels increase during stress conditions (Mano et al., Citation2010; Yin et al., Citation2010; Yamauchi et al., Citation2012). Among known RCS in plants, acrolein is one of the most potent (Mano et al., Citation2017), leading to higher inhibition of photosynthesis, mainly by creating adducts with enzymes in chloroplast stroma (Mano et al., Citation2009). Acrolein was also found to increase in the early stages of cell death, suggesting that it can initiate PCD in cell cultures, such as in tobacco. Thus, it has been suggested that it targets proteins in vivo, specifically mitochondrial proteins, causing apoptosis-like cell death (Biswas and Mano, Citation2015). Later it was found that acrolein depletes the reduced glutathione pool, causes a decrease in ascorbate level, and causes an increase in ROS in tobacco cells. It also activates two caspase-like proteases which play a role in PCD activation (Biswas and Mano, Citation2016). Following these findings, Mano et al. (Citation2017) reported that glutathione S-transferase has acrolein-scavenging activity and is a key enzyme in maintaining the correct level of acrolein in plant tissues (Mano et al., Citation2017). Recently, it was found that acrolein takes part in plant ferroptosis-like cell death. For instance, when the caspase inhibitor Z-VAD-FMK was used in A. thaliana, the cells were more viable when treated with acrolein (Hajdinák et al., Citation2019).
In animals, an increased level of polyamine oxidation products is a cause of serious acute and chronic diseases (Pegg, Citation2013). Acrolein is a critical intermediate in brain damage, corresponding to the degree of cerebral injury (Zahedi et al., Citation2010). Therefore, acrolein level may be a useful marker of brain injury in patients suffering from stroke (Igarashi and Kashiwagi, Citation2011). An important finding is that an increased level of acrolein is observed in patients with moderate symptoms, who need magnetic resonance imaging analysis to confirm the presence of brain damage. Detecting silent brain infarctions can be accomplished by measuring the acrolein level together with C-reactive protein and interleukin-6 (Yoshida et al., Citation2009). Also, the combined measurement of acrolein level and β-amyloid appears to be a useful biochemical marker for detecting patients with Alzheimer’s disease (Waragai et al., Citation2012; Mizoi et al., Citation2014). The findings in humans indicating that acrolein is important in neurological injuries were also supported in mice (Saiki et al., Citation2009; Citation2011). Acrolein levels also increase in patients with chronic renal failure and in a variety of kidney diseases (Sakata et al., Citation2003) as well as in liver damage (Burcham et al., Citation2012) and diabetes (Daimon et al., Citation2003). Additionally, acrolein was found to affect muscle regeneration in mice, probably through inhibition of protein B kinase signaling (Chen et al., Citation2019).
The role of polyamines in regulation of gene expression
PAs are involved in gene expression regulation through wide-ranging mechanisms including direct binding to nucleic acids, gene transcription, translation initiation, and epigenetic processes (Aye et al., Citation2022). Cationic PAs have binding capacity to negatively-charged molecules such as DNA, RNA, and proteins and can alter the function of these biomolecules (Imre et al., Citation2022). Direct binding of PAs in parallel to the longitudinal axis of DNA promotes stabilization of the DNA double helix and its aggregation (Thomas et al., Citation2016; Nishio et al., Citation2018). Moreover, the binding of PAs to DNA may favor the formation of noncanonical DNA structures that have been found to stabilize nucleosomes and promote condensation of chromatin (Raspaud et al., Citation1999; Brooks, Citation2013; Visvanathan et al., Citation2013; Imre et al., Citation2022). PAs have been observed to facilitate the reversible oligomerization of nucleosomal arrays in vitro and to stabilize highly condensed states of chromosomal fibers. Therefore PAs are considered as relevant repressors of gene transcription in vivo (Pollard et al., Citation1999).
The repressor effect of PAs on gene transcription can be antagonized by histone acetylation, which is one of the most well-characterized histone post-translational modifications (PTM) and epigenetic marks (Kim et al., Citation2023). Histone acetylation is reversible and dynamically modulated by the action of histone acetyltransferases (HATs) and histone deacetylases (HDACs). These enzymes are crucial for regulating essential cellular processes, and their abundance and activity in cells are tightly regulated (Kumar et al., Citation2021). PAs have been shown to influence widespread changes in gene expression by affecting the availability of acetyl-CoA, which is necessary for histone acetylation. Specifically, PA depletion has been shown to affect glycolysis and mitochondrial metabolism, resulting in limitations in acetyl-CoA and a decrease of global histone acetylation (Aye et al., Citation2022). Moreover, PA deficiency is associated with reduction of acetyltransferase activity (Igarashi and Kashiwagi, Citation2019; Sakamoto et al., Citation2020). This effect is due to the PA-mediated enhancement of interactions between specific microRNA and the 5′-UTR of mRNAs of acetyltransferase enzymes such as GCN5 (general control nonderepressible 5) and HAT1 (histone acetyltransferase 1). Under standard conditions enhanced microRNA (mRNA) interactions lead to an increase in translation of involved mRNAs, resulting in elevated abundance of acetyltransferase proteins and a subsequent increased acetylation level of specific lysine residues on histone H3 and H4 (Sakamoto et al., Citation2020).
The above-described GCN5 and HAT1 acetyltransferases have been proposed to be a part of the ‘polyamine modulon’ – a term describing a set of genes whose expression is enhanced by PAs at the level of translation (Igarashi and Kashiwagi, Citation2006). Moreover, the presence of a transcription factors among genes belonging to the polyamine modulon suggests that the regulatory effect of PAs on gene expression is not limited to the mRNA translation process (Igarashi and Kashiwagi, Citation2018; Citation2019). Indeed, the stimulating effect of PAs on gene transcription has been reported. Namely, transcription of human polyamine-modulated factor-1 has been shown to be upregulated by PAs through interaction with a PA-response element in the promoter region (Wang et al., Citation1999). PA-modulated factor-1 enhances in turn the transcription of several other genes including SSAT encoding Spd/Spm N1-acetyltransferase (Stephenson and Seidel, Citation2006). However, the repressing effect of PAs on gene transcription has also been reported in several cases, including Smad proteins, which are transcription activators involved in cell proliferation (Ten Dijke et al., Citation2002; Liu et al., Citation2003) and the TGF-β/TGF-β (transforming growth factor-β) receptor in intestinal epithelial cells (Patel et al., Citation1998; Rao et al., Citation2000; Liu et al., Citation2003).
Alterations in PA levels also modulate nucleosome stability, which is a key regulatory factor in eukaryotic gene control. Thus, altered PA levels may cause changes in the epigenome by changing nucleosome stability because nucleosomes are strong barriers to enzymes involved in DNA methylation and demethylation against gaining access to their targets (Imre et al., Citation2022). PA metabolism is also closely associated with the availability of a major methyl group donor in biological transmethylation reactions, namely S-adenosyl-L-methionine (SAM). Apart from being a substrate for transmethylation reaction, SAM is also a substrate for the synthesis of PAs () as well as ethylene, biotin and nicotinamide (Van de Poel et al., Citation2013). SAM is a source of byproducts in PA synthesis reactions, including decarboxylated S-adenosyl-L-methionine (dcSAM) and S-adenosyl-L-homocysteine (SAH). SAM byproducts may also act as competitive inhibitors of various DNMT enzymes (Frostesjö et al., Citation1997; Fukui et al., Citation2019) affecting the DNA methylation level in living cells (Yamamoto et al., Citation2010). A reduced dcSAM level has been suggested to increase DNMT activity, thereby emphasizing the degree of DNA methylation and plausibly causing gene silencing (Tiburcio et al., Citation2014). However, an increased dcSAM concentration with a decreased PA level has been associated with aberrant methylation of the entire genome (Kano et al., Citation2013; Soda et al., Citation2013; Perez et al., Citation2018).
The action of PAs in the regulation of DNA methylation has been recently discussed in terms of aging-related pathologies and life span. Moreover, the control of DNA methylation may be an important target of interference with cancer development. The methylation state of individual genomic CpG sites remains constant in most normal cell populations, whereas measurements of the methylcytosine content of the DNA show great reduction in many tumors (Wahlfors et al., Citation1992; Schipper et al., Citation2007).
In addition to modifications of DNA via SAM metabolites, PAs have been proposed to regulate a wider range of transmethylation reactions. Here, the regulatory function is mostly related to the effective usage of SAM, as its availability was shown to be crucial for the methylation status of various cell molecules (Parrish et al., Citation2015; Shojaei Saadi et al., Citation2016). Diverting SAM pools into the polyamine biosynthesis pathway diverts one carbon metabolite from other important pathways. Several studies on simultaneous operation of three main metabolic pathways using SAM – ethylene biosynthesis, polyamine biosynthesis and transmethylation reactions – in plant and animal models have indicated the relevance of the balance between different SAM utilizing pathways in development and biology of living organisms (Locke et al., Citation2000; Tari and Csiszár, Citation2003; Van de Poel et al., Citation2013; Yu et al., Citation2016; Asgher et al., Citation2018; McKenna et al., Citation2018; Mahajan et al., Citation2020).
Polyamines and aging
PAs have been identified as “juvenility” factors. Thus, applying PAs to plants prevents their senescence, which is a long-known phenomenon (reviewed in Sobieszczuk-Nowicka, Citation2017). Transformations between different PAs were shown to contribute to dark-induced barley leaf senescence responses (Sobieszczuk-Nowicka et al., Citation2015a). Importantly, transcript levels and corresponding PA catabolic enzymes, DAO and PAO, increase during induced and developmental senescence, thus making them important components of senescence-related mechanisms (Ioannidis et al., Citation2014; Sobieszczuk-Nowicka et al., Citation2015a; Tanwar et al., Citation2022). Moreover, inhibition of PAO activity slows down the senescence-associated chlorophyll loss (Sobieszczuk-Nowicka et al., Citation2015a). Arabidopsis PA back conversion oxidase mutants have also been utilized for studying dark-induced senescence. In these mutants, conversion of Spm to Spd, and/or Spd to Put, does not occur and their senescence is delayed (Sequera-Mutiozabal et al., Citation2016). The delayed dark-induced senescence in these mutants was found to be associated with the accumulation of Spm (Sequera-Mutiozabal et al., Citation2016). These plants were found to have reduced production of ROS and, interestingly, increased levels of the signaling molecule nitric oxide. These data suggest that Spm is likely a “signaling” metabolite that protects against stress via metabolic conversions such as ascorbate/dehydro-ascorbate redox state transitions, changes in sugar and nitrogen metabolism, cross-talk with ethylene biosynthesis, and mitochondrial electron transport chain modulation (Sequera-Mutiozabal et al., Citation2016). Thus, metabolic interactions between PAs, particularly Spm, occur with cellular oxidative balance and transport/biosynthesis of amino acids, likely suggestive of a strategy to cope with damage during senescence. Plants also respond to environmental factors by secreting Spd to the apoplast, where its catabolism leads to H2O2 production (a hypersensitive response). Based on the H2O2 concentration, these cells initiate either a defense response or a cell death program (Yoda et al., Citation2003; Citation2006). Moreover, high Spd and Spm pools accumulate in the apoplast during induced senescence, which is associated with gradual accumulation of the apoplastic PA catabolism intermediate diaminopropane, and H2O2 (Sobieszczuk-Nowicka et al., Citation2015a). The basal Put levels in the apoplastic pool of PAs are an order of magnitude lower, increasing only slightly during senescence. However, Put is a dominant PA in the free PA fraction and accumulates to higher levels before decreasing. The decrease in free Put is concomitant with the formation of PCA-soluble Put conjugates that accumulate at high levels in the senescing leaf, indicating that the Put-conjugating enzymes are active in senescing cells (Sobieszczuk-Nowicka et al., Citation2015a). Senescence-dependent remobilization of nitrogen (N) and carbon (C) flow may contribute to PA conjugation, since the expression of respective protein-coding genes also increases (Sobieszczuk-Nowicka et al., Citation2015a). The fact that plant cells can sense PAs as organic-N and stimulate turnover of N molecules has been previously substantiated and discussed (Mattoo et al., Citation2010). Physiological and structural changes in chloroplasts during senescence are also associated with PA conjugation and modification of chloroplast proteins via chloroplast-localized transglutaminases (ChlTGases) (reviewed in Parrotta et al., Citation2022). The ChlTGase in barley was found to be activated during dark-induced leaf senescence, and associated with enhanced local TGase accumulation and activity together with increased expression of the barley HvPng1-like gene (Sobieszczuk-Nowicka et al., Citation2015b). These findings also suggest that PAs and plant senescence cross paths. Moreover, SAM, which functions as a precursor in the biosynthesis pathways of both PAs and the leaf senescence promoter ethylene, needs to be examined metabolically, as has recently been detailed in the tomato system (Lasanajak et al., Citation2014). SAM is a significant substrate in senescence-dependent 1-C metabolism. According to research by Luka et al. (Citation2009), the regulation of SAM levels in mammalian cells involves the post-translational inhibition of glycine N-methyltransferase by folate. An important area for research is how plant cells control SAM levels during plant senescence.
Polyamine levels decline with age (Green, Citation2019), and these molecules have been implicated in the aging process. Nutritional supplementation of Spd can reduce age-related pathology and can increase life span in animals (Eisenberg et al., Citation2009; Madeo et al., Citation2010; Pucciarelli et al., Citation2012; Eisenberg et al., Citation2016; Wirth et al., Citation2021). The many health-promoting effects of oral Spd treatment have made the production of Spd-rich dietary supplements important in food technology. Numerous supplements, named longevity elixirs, have been developed, such as soybeans, wheat germ and algae (Madeo et al., Citation2010; van den Oever and Mayer, Citation2022). There is evidence for nutritional supplementation of polyamines, especially Spd. Polyamines are inter-convertible into different forms that have been shown to extend life span in yeast, nematodes, and Drosophila, as well as preventing aging in human cells (Eisenberg et al., Citation2009) and providing cardioprotection and an extended life span in humans (Eisenberg et al., Citation2016; Kiechl et al., Citation2018). These ‘anti-aging’ effects induce autophagy by Spd, whereas genetic disruption of autophagy in yeast, worms, and flies counteracts such beneficial effects (Eisenberg et al., Citation2009). A mechanism that links the role of polyamines in eiF5A hypusination to autophagy and the function of B lymphocytes from aged individuals has also been presented (Zhang et al., Citation2019). Inhibition of hypusination using an Spd analog (GC7) was found to prevent these beneficial effects of polyamine supplementation in vitro.
Conclusions
Polyamines are important molecules that determine cell longevity and death. It is now known that nutritional supplementation with Spd can reduce age-related pathology and increase the life span in a number of animal organisms as well as in humans. In addition, applying PAs to plants prevents their senescence. Even though this is a mature field, more work is needed to better understand and define the broad reach of polyamine metabolism in regulating life processes. This review aimed to achieve an integrated understanding of the regulation of PA metabolism and its effect(s) on cell homeostasis (). Polyamine titer is regulated by transcriptional and translational levels of PA metabolism genes. CREs and TFs act at the transcriptional level and determine stress-specific expression of specific genes, while at the translational level they are regulated by miRNAs by targeting mRNAs for cleavage or translational suppression.
Figure 2. Polyamines and cell biology. (A) The upper part of the figure shows a schematic plant cell, and the lower part shows an animal cell. Polyamines regulate gene expression at the transcriptional level (DNA binding, histone deacetylation/acetylation) and at the translational level by affecting RNA structure and protein synthesis. Acrolein, which is a by-product of PA catabolism, is a toxic molecule that can cause inhibition of photosynthesis and increase in early stages of cell death, suggesting that it takes part in initiation of PCD. Acrolein targets proteins, specifically mitochondrial proteins, causing apoptosis-like cell death. Another by-product of PA catabolism is hypusine – a compound essential for eIF5A activity, and absolutely vital for survival of the cell. Polyamines in the plant cell accumulate in the vacuole, and in the animal cell in lysosomes. In the plant cell, inhibition of PA catabolic enzyme activities drastically slows down the senescence-associated chlorophyll loss. In the animal cell, polyamines also have anti-aging effects and immune functions. (B) Imbalance in the homeostasis of PA levels in the cell can lead to many disorders at both the cellular and organismal level. Increased biosynthesis of PAs and overaccumulation of PAs lead to cell transformation and hyper-proliferation. On the other hand, increased PA catabolism and consequently low levels of polyamines increase oxidative stress, which leads to senescence, aging, and cellular damage.
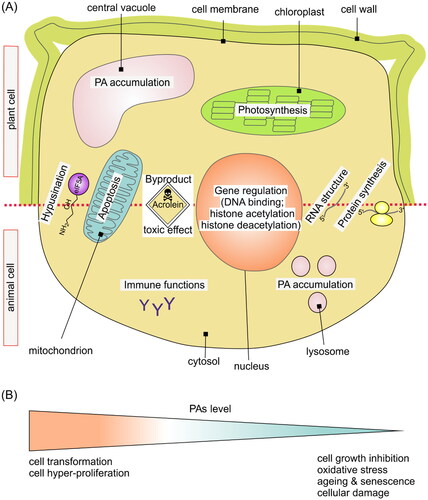
Moreover, the byproducts of PA metabolism such as hypusine and acrolein are important for cell survival and death. Hypusine is essential for eIF5A activity, which is vital for cell survival. Acrolein is a toxic compound leading to oxidative damage of proteins and DNA, which can contribute to cell death. Polyamines play other important roles in plant and animal physiology, via their interaction with DNA structure, effects on chromatin condensation, and histone acetyltransferases and deacetylases. Furthermore, PAs also control a variety of transmethylation reactions in addition to DNA modification. SAM availability has been found to be essential for the methylation status of numerous cellular molecules. The regulatory function in this instance is primarily related to the effective utilization of SAM.
These studies clearly suggest that PAs in plant and animal physiology have multifunctional roles including their function in facilitating macromolecular synthesis and biomolecular conformations. Their wide-ranging pleiotropic effects make them crucial for cell viability and proliferation along the entire phylogenetic scale. Thus, further exploration of the interplay between PA metabolic genes, CREs, miRNAs, and PPI, is of great interest to help decipher the cross-talk between PA metabolism and cellular processes (Alexander et al., Citation2017; Alexander et al., Citation2020).
It is also important to note that acetylated forms of PAs may have equally important roles, a perspective for future exploration. Acetylated PAs in plants and animals are at very low concentrations, since they are exported outside the cell (Pegg, Citation2013). However, in plants acetylated PAs suppress antibacterial defense (Lou et al., Citation2016; Torres et al., Citation2005), while in animals acetylated PAs are present in higher concentrations in cancers (Hiramatsu et al., Citation1997). Indeed, derivatives of the natural PAs have the potential to boost SSAT expression and increase the cytotoxicity of chemotherapies in tumor cells. PA- and SSAT-targeting drugs have the future potential to be turned into novel cancer therapies (Tse et al., Citation2022; Tavladoraki et al., Citation2012; Hiramatsu et al., Citation2005). Moreover, structural analogs of polyamines have also been shown to affect cancer cell metastasis and inhibit polyamine import (Muth et al., Citation2014; Dobrovolskaite et al., Citation2022). These simple linear biomolecules provide scaffolds for future drug design, as they impart significant water solubility and can target specific cellular uptake processes, e.g. PA uptake by ATP13A3 (Sekhar et al., Citation2022). More research on the role of acetylated polyamines is needed to evaluate their potential to support plant resistance to stress. Thus, all the PA-based metabolic pathways highlight the roles that PAs play in determining whether a cell will live or die. Defining the role of PAs in plants and animals at both the cellular and molecular levels should allow a better understanding of the mechanisms leading to cell longevity or death. This knowledge will contribute to improving the quality and longevity of human and animal life, as well as possibly intensifying the cultivation of economically important crops.
Authors’ contributions
E.S.-N. conceived the topic of the article; E.S.-N. and E.S. were responsible for the layout of the manuscript, and were involved in the writing of the “Introduction” section; E.S. prepared the table and was involved in the writing of the “Polyamine metabolism and biochemistry—plants vs animals” section; U.K.T. was involved in the writing of the “Aspects of Regulation of Polyamine Metabolism” section; E.P.-L. was involved in the writing of the “Polyamine metabolism byproducts”; M.G. drew the figures and was involved in the writing of the “Polyamine role in regulation of gene expression” section; E.S.-N and M.A.-J. were involved in the writing of the “Polyamines and aging" section; E.S.-N. and E.S. were involved in the writing of the “Conclusions” section; O.P. IV and A.K.M. provided suggestions for revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank Richard Ashcroft (bioscience editor) for the professional language editing of the manuscript. ES is an Adam Mickiewicz University Foundation scholar in the 2022/2023 academic year.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Agostinelli, E., Belli, F., Molinari, A., Condello, M., Palmigiani, P., Dalla Vedova, L., Marra, M., Seiler, N., and Arancia, G. 2006. Toxicity of enzymatic oxidation products of spermine to human melanoma cells (M14): sensitization by heat and MDL 72527. Biochim. Biophys. Acta. 1763:1040–1050. doi:10.1016/j.bbamcr.2006.07.014
- Agostinelli, E., Marques, M., Calheiros, R., Gil, F., Tempera, G., Viceconte, N., Battaglia, V., Grancara, S., and Toninello, A. 2010a. Polyamines: fundamental characters in chemistry and biology. Amino Acids. 38:393–403. doi:10.1007/s00726-009-0396-7
- Agostinelli, E., Tempera, G., Viceconte, N., Saccoccio, S., Battaglia, V., Grancara, S., Toninello, A., and Stevanato, R. 2010b. Potential anticancer application of polyamine oxidation products formed by amine oxidase: a new therapeutic approach. Amino Acids. 38:353–368. doi:10.1007/s00726-009-0431-8
- Ahou, A., Martignago, D., Alabdallah, O., Tavazza, R., Stano, P., Macone, A., Pivato, M., Masi, A., Rambla, J. L., Vera-Sirera, F., Angelini, R., Federico, R., and Tavladoraki, P. 2014. A plant spermine oxidase/dehydrogenase regulated by the proteasome and polyamines. J. Exp. Bot. 65:1585–1603. doi:10.1093/jxb/eru016
- Al-Kafaji, G., Al-Muhtaresh, H. A., and Salem, A. H. 2021. Expression and clinical significance of miR‑1 and miR‑133 in pre‑diabetes. Biomed. Rep. 14:33. doi:10.3892/br.2021.1409
- Alcázar, R., Marco, F., Cuevas, J. C., Patron, M., Ferrando, A., Carrasco, P., Tiburcio, A. F., and Altabella, T. 2006. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 28:1867–1876. doi:10.1007/s10529-006-9179-3
- Alexander, E. T., Mariner, K., Donnelly, J., Phanstiel, O., and Gilmour, S. K. 2020. Polyamine blocking therapy decreases survival of tumor-infiltrating immunosuppressive myeloid cells and enhances the antitumor efficacy of PD-1 blockade. Mol. Cancer Ther. 19:2012–2022. doi:10.1158/1535-7163.MCT-19-1116
- Alexander, E. T., Minton, A., Peters, M. C., Phanstiel, O., and Gilmour, S. K. 2017. A novel polyamine blockade therapy activates an anti-tumor immune response. Oncotarget 8:84140–84152. doi:10.18632/oncotarget.20493
- Angelini, R., Tisi, A., Rea, G., Chen, M. M., Botta, M., Federico, R., and Cona, A. 2008. Involvement of polyamine oxidase in wound healing. Plant Physiol. 146:162–177. doi:10.1104/pp.107.108902
- Asgher, M., Khan, M. I. R., Anjum, N. A., Verma, S., Vyas, D., Per, T. S., Masood, A., and Khan, N. A. 2018. Ethylene and polyamines in counteracting heavy metal phytotoxicity: a crosstalk perspective. J. Plant Growth Regul. 37:1050–1065. doi:10.1007/s00344-018-9823-x
- Aye, I. L., Gong, S., Avellino, G., Barbagallo, R., Gaccioli, F., Jenkins, B. J., Koulman, A., Murray, A. J., Stephen Charnock-Jones, D., and Smith, G. C. 2022. Placental sex-dependent spermine synthesis regulates trophoblast gene expression through acetyl-coA metabolism and histone acetylation. Commun. Biol. 5:586. doi:10.1038/s42003-022-03530-6
- Bachrach, U. 2005. Naturally occurring polyamines: interaction with macromolecules. Curr. Protein Pept. Sci. 6:559–566. doi:10.2174/138920305774933240
- Bachrach, U. 2010. The early history of polyamine research. Plant Physiol. Biochem. 48:490–495. doi:10.1016/j.plaphy.2010.02.003
- Bagni, N., and Tassoni, A. 2001. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids. 20:301–317. doi:10.1007/s007260170046
- Basu, S., Roychoudhury, A., and Sengupta, D. N. 2014. Deciphering the role of various cis-acting regulatory elements in controlling SamDC gene expression in rice. Plant Signal. Behav. 9:e28391. doi:10.4161/psb.28391
- Belda-Palazón, B., Almendáriz, C., Martí, E., Carbonell, J., and Ferrando, A. 2016. Relevance of the axis spermidine/eIF5A for plant growth and development. Front. Plant Sci. 7:245. doi:10.3389/fpls.2016.00245
- Bell, E., and Malmberg, R. L. 1990. Analysis of a cDNA encoding arginine decarboxylase from oat reveals similarity to the Escherichia coli arginine decarboxylase and evidence of protein processing. Mol. Gen. Genet. 224:431–436. doi:10.1007/BF00262438
- Biłas, R., Szafran, K., Hnatuszko-Konka, K., and Kononowicz, A. K. 2016. Cis-regulatory elements used to control gene expression in plants. Plant Cell. Tiss. Organ Cult. 127:269–287. doi:10.1007/s11240-016-1057-7
- Biswas, M. S., and Mano, J. 2015. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 168:885–898. doi:10.1104/pp.115.256834
- Biswas, M. S., and Mano, J. 2016. Reactive carbonyl species activate caspase-3-like protease to initiate programmed cell death in plants. Plant Cell Physiol. 57:1432–1442. doi:10.1093/pcp/pcw053
- Brooks, W. H. 2013. Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Front. Immunol. 4:91. doi:10.3389/fimmu.2013.00091
- Burcham, P. C., Raso, A., and Kaminskas, L. M. 2012. Chaperone heat shock protein 90 mobilization and hydralazine cytoprotection against acrolein-induced carbonyl stress. Mol. Pharmacol. 82:876–886. doi:10.1124/mol.112.078956
- Byers, T. L., Lakanen, J. R., Coward, J. K., and Pegg, A. E. 1994. The role of hypusine depletion in cytostasis induced by S-adenosyl-L-methionine decarboxylase inhibition: new evidence provided by 1-methylspermidine and 1,12-dimethylspermine. Biochem. J. 303 (Pt 2):363–368. doi:10.1042/bj3030363
- Cervelli, M., Polticelli, F., Federico, R., and Mariottini, P. 2003. Heterologous expression and characterization of mouse spermine oxidase. J. Biol. Chem. 278:5271–5276. doi:10.1074/jbc.M207888200
- Chattopadhyay, M. K., Park, M. H., and Tabor, H. 2008. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc. Natl. Acad. Sci. U S A. U S A 105:6554–6559. doi:10.1073/pnas.0710970105
- Chattopadhyay, M. K., Tabor, C. W., and Tabor, H. 2003. Spermidine but not spermine is essential for hypusine biosynthesis and growth in Saccharomyces cerevisiae: spermine is converted to spermidine in vivo by the FMS1-amine oxidase. Proc. Natl. Acad. Sci. USA. 100:13869–13874. doi:10.1073/pnas.1835918100
- Che, H., Ma, C., Li, H., Yu, F., Wei, Y., Chen, H., Wu, J., and Ren, Y. 2022. Rebalance of the polyamine metabolism suppresses oxidative stress and delays senescence in nucleus pulposus cells. Oxid. Med. Cell. Longev. 2022:8033353. doi:10.1155/2022/8033353
- Chen, H. J., Wang, C. C., Chan, D. C., Chiu, C. Y., Yang, R. S., and Liu, S. H. 2019. Adverse effects of acrolein, a ubiquitous environmental toxicant, on muscle regeneration and mass. J. Cachexia. Sarcopenia Muscle. 10:165–176. doi:10.1002/jcsm.12362
- Chen, K. Y. 1983. An 18000-dalton protein metabolically labeled by polyamines in various mammalian cell lines. Biochim. Biophys. Acta. 756:395–402. doi:10.1016/0304-4165(83)90350-1
- Cona, A., Rea, G., Angelini, R., Federico, R., and Tavladoraki, P. 2006. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11:80–88. doi:10.1016/j.tplants.2005.12.009
- Cooper, H. L., Park, M. H., and Folk, J. E. 1982. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell 29:791–797. doi:10.1016/0092-8674(82)90441-x
- Cooper, H. L., Park, M. H., Folk, J. E., Safer, B., and Braverman, R. 1983. Identification of the hypusine-containing protein hy + as translation initiation factor eIF-4D. Proc. Natl. Acad. Sci. U S A. U S A 80:1854–1857. doi:10.1073/pnas.80.7.1854
- Daimon, M., Sugiyama, K., Kameda, W., Saitoh, T., Oizumi, T., Hirata, A., Yamaguchi, H., Ohnuma, H., Igarashi, M., and Kato, T. 2003. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr. J. 50:61–67. doi:10.1507/endocrj.50.61
- Dobrovolskaite, A., Gardner, R. A., Delcros, J.-G., and Phanstiel, O. 2022. Development of polyamine lassos as polyamine transport inhibitors. ACS Med. Chem. Lett. 13:319–326. doi:10.1021/acsmedchemlett.1c00557
- Doerfel, L. K., Wohlgemuth, I., Kothe, C., Peske, F., Urlaub, H., and Rodnina, M. V. 2013. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 339:85–88. doi:10.1126/science.1229017
- Duguay, J., Jamal, S., Liu, Z., Wang, T. W., and Thompson, J. E. 2007. Leaf-specific suppression of deoxyhypusine synthase in Arabidopsis thaliana enhances growth without negative pleiotropic effects. J. Plant Physiol. 164:408–420. doi:10.1016/j.jplph.2006.02.001
- Eisenberg, T., Abdellatif, M., Schroeder, S., Primessnig, U., Stekovic, S., Pendl, T., Harger, A., Schipke, J., Zimmermann, A., Schmidt, A., Tong, M., Ruckenstuhl, C., Dammbrueck, C., Gross, A. S., Herbst, V., Magnes, C., Trausinger, G., Narath, S., Meinitzer, A., Hu, Z., Kirsch, A., Eller, K., Carmona-Gutierrez, D., Büttner, S., Pietrocola, F., Knittelfelder, O., Schrepfer, E., Rockenfeller, P., Simonini, C., Rahn, A., Horsch, M., Moreth, K., Beckers, J., Fuchs, H., Gailus-Durner, V., Neff, F., Janik, D., Rathkolb, B., Rozman, J., De Angelis, M. H., Moustafa, T., Haemmerle, G., Mayr, M., Willeit, P., Von Frieling-Salewsky, M., Pieske, B., Scorrano, L., Pieber, T., Pechlaner, R., Willeit, J., Sigrist, S. J., Linke, W. A., Mühlfeld, C., Sadoshima, J., Dengjel, J., Kiechl, S., Kroemer, G., Sedej, S., and Madeo, F. 2016. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22:1428–1438. doi:10.1038/nm.4222
- Eisenberg, T., Knauer, H., Schauer, A., Büttner, S., Ruckenstuhl, C., Carmona-Gutierrez, D., Ring, J., Schroeder, S., Magnes, C., Antonacci, L., Fussi, H., Deszcz, L., Hartl, R., Schraml, E., Criollo, A., Megalou, E., Weiskopf, D., Laun, P., Heeren, G., Breitenbach, M., Grubeck-Loebenstein, B., Herker, E., Fahrenkrog, B., Fröhlich, K.-U., Sinner, F., Tavernarakis, N., Minois, N., Kroemer, G., and Madeo, F. 2009. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11:1305–1314. doi:10.1038/ncb1975
- Faundes, V., Jennings, M. D., Crilly, S., Legraie, S., Withers, S. E., Cuvertino, S., Davies, S. J., Douglas, A. G. L., Fry, A. E., Harrison, V., Amiel, J., Lehalle, D., Newman, W. G., Newkirk, P., Ranells, J., Splitt, M., Cross, L. A., Saunders, C. J., Sullivan, B. R., Granadillo, J. L., Gordon, C. T., Kasher, P. R., Pavitt, G. D., and Banka, S. 2021. Impaired eIF5A function causes a Mendelian disorder that is partially rescued in model systems by spermidine. Nat. Commun. 12:833. doi:10.1038/s41467-021-21053-2
- Frostesjö, L., Holm, I., Grahn, B., Page, A. W., Bestor, T. H., and Heby, O. 1997. Interference with DNA methyltransferase activity and genome methylation during F9 teratocarcinoma stem cell differentiation induced by polyamine depletion. J. Biol. Chem. 272:4359–4366. doi:10.1074/jbc.272.7.4359
- Fuell, C., Elliott, K. A., Hanfrey, C. C., Franceschetti, M., and Michael, A. J. 2010. Polyamine biosynthetic diversity in plants and algae. Plant Physiol. Biochem. 48:513–520. doi:10.1016/j.plaphy.2010.02.008
- Fukui, T., Soda, K., Takao, K., and Rikiyama, T. 2019. Extracellular spermine activates DNA methyltransferase 3A and 3B. Int. J. Mol. Sci. 20:1254. doi:10.3390/ijms20051254
- Gerlin, L., Baroukh, C., and Genin, S. 2021. Polyamines: double agents in disease and plant immunity. Trends Plant Sci. 26:1061–1071. doi:10.1016/j.tplants.2021.05.007
- Gerner, E. W., Mamont, P. S., Bernhardt, A., and Siat, M. 1986. Post-translational modification of the protein-synthesis initiation factor eIF-4D by spermidine in rat hepatoma cells. Biochem. J. 239:379–386. doi:10.1042/bj2390379
- Gonzalez, M. E., Jasso‐Robles, F. I., Flores‐Hernández, E., Rodríguez‐Kessler, M., and Pieckenstain, F. L. 2021. Current status and perspectives on the role of polyamines in plant immunity. Ann. Appl. Biol. 178:244–255. doi:10.1111/aab.12670
- Green, D. R. 2019. Polyamines and aging: a CLEAR connection? Mol. Cell. 76:5–7. doi:10.1016/j.molcel.2019.09.003
- Guo, Y., Ye, Q., Deng, P., Cao, Y., He, D., Zhou, Z., Wang, C., Zaytseva, Y. Y., Schwartz, C. E., Lee, E. Y., Evers, B. M., Morris, A. J., Liu, S., and She, Q.-B. 2020. Spermine synthase and MYC cooperate to maintain colorectal cancer cell survival by repressing Bim expression. Nat. Commun. 11:3243. doi:10.1038/s41467-020-17067-x
- Gutierrez, E., Shin, B. S., Woolstenhulme, C. J., Kim, J. R., Saini, P., Buskirk, A. R., and Dever, T. E. 2013. eIF5A promotes translation of polyproline motifs. Mol. Cell. 51:35–45. doi:10.1016/j.molcel.2013.04.021
- Hackenberg, M., Gustafson, P., Langridge, P., and Shi, B. J. 2015. Differential expression of micro RNAs and other small RNAs in barley between water and drought conditions. Plant Biotechnol. J. 13:2–13. doi:10.1111/pbi.12220
- Hajdinák, P., Czobor, Á., and Szarka, A. 2019. The potential role of acrolein in plant ferroptosis-like cell death. PLOS One. 14:e0227278. doi:10.1371/journal.pone.0227278
- Hanauske-Abel, H., Park, M.-H., Hanauske, A.-R., Popowicz, A., Lalande, M., and Folk, J. 1994. Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim. Biophys. Acta. 1221:115–124. doi:10.1016/0167-4889(94)90003-5
- Handa, A. K., Fatima, T., and Mattoo, A. K. 2018. Polyamines: bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 6:10. doi:10.3389/fchem.2018.00010
- Hanfrey, C. C., Pearson, B. M., Hazeldine, S., Lee, J., Gaskin, D. J., Woster, P. M., Phillips, M. A., and Michael, A. J. 2011. Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota. J. Biol. Chem. 286:43301–43312. doi:10.1074/jbc.M111.307835
- Hiramatsu, K., Sugimoto, M., Kamei, S., Hoshino, M., Kinoshita, K., Iwasaki, K., and Kawakita, M. 1997. Diagnostic and prognostic usefulness of N 1, N 8-diacetylspermidine and N 1, N 12-diacetylspermine in urine as novel markers of malignancy. J. Cancer Res. Clin. Oncol. 123:539–545. doi:10.1007/s004320050102
- Hiramatsu, K., Takahashi, K., Yamaguchi, T., Matsumoto, H., Miyamoto, H., Tanaka, S., Tanaka, C., Tamamori, Y., Imajo, M., Kawaguchi, M., Toi, M., Mori, T., and Kawakita, M. 2005. N 1, N 12-diacetylspermine as a sensitive and specific novel marker for early-and late-stage colorectal and breast cancers. Clin. Cancer Res. 11:2986–2990. doi:10.1158/1078-0432.CCR-04-2275
- Hussain, A., Saraiva, L. R., Ferrero, D. M., Ahuja, G., Krishna, V. S., Liberles, S. D., and Korsching, S. I. 2013. High-affinity olfactory receptor for the death-associated odor cadaverine. Proc. Natl. Acad. Sci. U S A. U S A 110:19579–19584. doi:10.1073/pnas.1318596110
- Igarashi, K., and Kashiwagi, K. 2000. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 271:559–564. doi:10.1006/bbrc.2000.2601
- Igarashi, K., and Kashiwagi, K. 2006. Polyamine modulon in Escherichia coli: genes involved in the stimulation of cell growth by polyamines. J. Biochem. 139:11–16. doi:10.1093/jb/mvj020
- Igarashi, K., and Kashiwagi, K. 2010. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 42:39–51. doi:10.1016/j.biocel.2009.07.009
- Igarashi, K., and Kashiwagi, K. 2011. Protein-conjugated acrolein as a biochemical marker of brain infarction. Mol. Nutr. Food Res. 55:1332–1341. doi:10.1002/mnfr.201100068
- Igarashi, K., and Kashiwagi, K. 2018. Effects of polyamines on protein synthesis and growth of Escherichia coli. J. Biol. Chem. 293:18702–18709. doi:10.1074/jbc.TM118.003465
- Igarashi, K., and Kashiwagi, K. 2019. The functional role of polyamines in eukaryotic cells. Int. J. Biochem. Cell Biol. 107:104–115. doi:10.1016/j.biocel.2018.12.012
- Imre, L., Niaki, E. F., Bosire, R., Nanasi, P., Jr, Nagy, P., Bacso, Z., Hamidova, N., Pommier, Y., Jordan, A., and Szabo, G. 2022. Nucleosome destabilization by polyamines. Arch. Biochem. Biophys. 722:109184. doi:10.1016/j.abb.2022.109184
- Inal, B., Türktaş, M., Eren, H., Ilhan, E., Okay, S., Atak, M., Erayman, M., and Unver, T. 2014. Genome-wide fungal stress responsive miRNA expression in wheat. Planta 240:1287–1298. doi:10.1007/s00425-014-2153-8
- Ioannidis, N. E., Zschiesche, W., Barth, O., Kotakis, C., Navakoudis, E., Humbeck, K., and Kotzabasis, K. 2014. The genetic reprogramming of polyamine homeostasis during the functional assembly, maturation, and senescence-specific decline of the photosynthetic apparatus in Hordeum vulgare. J. Plant Growth Regul. 33:77–90. doi:10.1007/s00344-013-9387-8
- Kamada-Nobusada, T., Hayashi, M., Fukazawa, M., Sakakibara, H., and Nishimura, M. 2008. A putative peroxisomal polyamine oxidase, AtPAO4, is involved in polyamine catabolism in Arabidopsis thaliana. Plant Cell Physiol. 49:1272–1282. doi:10.1093/pcp/pcn114
- Kambis, T. N., Tofilau, H., Gawargi, F. I., Chandra, S., and Mishra, P. K. 2021. Regulating polyamine metabolism by miRNAs in diabetic cardiomyopathy. Curr. Diab. Rep. 21:52. doi:10.1007/s11892-021-01429-w
- Kano, Y., Soda, K., and Konishi, F. 2013. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area. PLoS One. 8:e56056. doi:10.1371/journal.pone.0056056
- Kiechl, S., Pechlaner, R., Willeit, P., Notdurfter, M., Paulweber, B., Willeit, K., Werner, P., Ruckenstuhl, C., Iglseder, B., Weger, S., Mairhofer, B., Gartner, M., Kedenko, L., Chmelikova, M., Stekovic, S., Stuppner, H., Oberhollenzer, F., Kroemer, G., Mayr, M., Eisenberg, T., Tilg, H., Madeo, F., and Willeit, J. 2018. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am. J. Clin. Nutr. 108:371–380. doi:10.1093/ajcn/nqy102
- Kim, T. H., Nosella, M. L., Bolik-Coulon, N., Harkness, R. W., Huang, S. K., and Kay, L. E. 2023. Correlating histone acetylation with nucleosome core particle dynamics and function. Proc. Natl. Acad. Sci. U S A. U S A 120:e2301063120. doi:10.1073/pnas.2301063120
- Kimes, B. W., and Morris, D. R. 1971. Inhibition of nucleic acid and protein synthesis in Escherichia coli by oxidized polyamines and acrolein. Biochim. Biophys. Acta. 228:235–244. doi:10.1016/0005-2787(71)90563-6
- Kirschner, K. M., Braun, J. F. W., Jacobi, C. L., Rudigier, L. J., Persson, A. B., and Scholz, H. 2014. Amine oxidase copper-containing 1 (AOC1) is a downstream target gene of the wilms tumor protein, WT1, during kidney development. J. Biol. Chem. 289:24452–24462. doi:10.1074/jbc.M114.564336
- Kumar, V., Thakur, J. K., and Prasad, M. 2021. Histone acetylation dynamics regulating plant development and stress responses. Cell. Mol. Life Sci. 78:4467–4486. doi:10.1007/s00018-021-03794-x
- Kusano, T., Berberich, T., Tateda, C., and Takahashi, Y. 2008. Polyamines: essential factors for growth and survival. Planta 228:367–381. doi:10.1007/s00425-008-0772-7
- Lasanajak, Y., Minocha, R., Minocha, S. C., Goyal, R., Fatima, T., Handa, A. K., and Mattoo, A. K. 2014. Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosylmethionine decarboxylase gene. Amino Acids. 46:729–742. doi:10.1007/s00726-013-1624-8
- Lenis, Y. Y., Elmetwally, M. A., Maldonado-Estrada, J. G., and Bazer, F. W. 2017. Physiological importance of polyamines. Zygote 25:244–255. doi:10.1017/S0967199417000120
- Liu, B., and Sun, G. 2017. Micro RNA s contribute to enhanced salt adaptation of the autopolyploid Hordeum bulbosum compared with its diploid ancestor. Plant J. 91:57–69. doi:10.1111/tpj.13546
- Liu, J., Chang, X., Ding, B., Zhong, S., Peng, L., Wei, Q., Meng, J., and Yu, Y. 2019. PhDHS Is Involved in Chloroplast Development in Petunia. Front. Plant Sci. 10:284. doi:10.3389/fpls.2019.00284
- Liu, L., Santora, R., Rao, J. N., Guo, X., Zou, T., Zhang, H. M., Turner, D. J., and Wang, J.-Y. 2003. Activation of TGF-β-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1056–G1067. doi:10.1152/ajpgi.00151.2003
- Liu, T., Huang, B., Chen, L., Xian, Z., Song, S., Chen, R., and Hao, Y. 2018. Genome-wide identification, phylogenetic analysis, and expression profiling of polyamine synthesis gene family members in tomato. Gene 661:1–10. doi:10.1016/j.gene.2018.03.084
- Liu, X., Liu, S., Chen, X., Prasanna, B. M., Ni, Z., Li, X., He, Y., Fan, Z., and Zhou, T. 2022. Maize miR167-ARF3/30-polyamine oxidase 1 module-regulated H2O2 production confers resistance to maize chlorotic mottle virus. Plant Physiol. 189:1065–1082. doi:10.1093/plphys/kiac099
- Liu, Z., Duguay, J., Ma, F., Wang, T. W., Tshin, R., Hopkins, M. T., Mcnamara, L., and Thompson, J. E. 2008. Modulation of eIF5A1 expression alters xylem abundance in Arabidopsis thaliana. J. Exp. Bot. 59:939–950. doi:10.1093/jxb/ern017
- Locke, J. M., Bryce, J. H., and Morris, P. C. 2000. Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J. Exp. Bot. 51:1843–1849. doi:10.1093/jexbot/51.352.1843
- Lopez, J. P., Fiori, L. M., Gross, J. A., Labonte, B., Yerko, V., Mechawar, N., and Turecki, G. 2014. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int. J. Neuropsychopharmacol. 17:23–32. doi:10.1017/S1461145713000941
- Lou, Y. R., Ahmed, S., Yan, J., Adio, A. M., Powell, H. M., Morris, P. F., and Jander, G. 2020. Arabidopsis ADC1 functions as an Nδ‐acetylornithine decarboxylase. J. Integr. Plant Biol. 62:601–613. doi:10.1111/jipb.12821
- Lou, Y. R., Bor, M., Yan, J., Preuss, A. S., and Jander, G. 2016. Arabidopsis NATA1 acetylates putrescine and decreases defense-related hydrogen peroxide accumulation. Plant Physiol. 171:1443–1455. doi:10.1104/pp.16.00446
- Luka, Z., Mudd, S. H., and Wagner, C. 2009. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J. Biol. Chem. 284:22507–22511. doi:10.1074/jbc.R109.019273
- Ma, F., Liu, Z., Wang, T. W., Hopkins, M. T., Peterson, C. A., and Thompson, J. E. 2010. Arabidopsis eIF5A3 influences growth and the response to osmotic and nutrient stress. Plant. Cell Environ. 33:1682–1696. doi:10.1111/j.1365-3040.2010.02173.x
- Madeo, F., Eisenberg, T., Büttner, S., Ruckenstuhl, C., and Kroemer, G. 2010. Spermidine: a novel autophagy inducer and longevity elixir. Autophagy 6:160–162. doi:10.4161/auto.6.1.10600
- Mahajan, U. V., Varma, V. R., Griswold, M. E., Blackshear, C. T., An, Y., Oommen, A. M., Varma, S., Troncoso, J. C., Pletnikova, O., O'Brien, R., Hohman, T. J., Legido-Quigley, C., and Thambisetty, M. 2020. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: a targeted metabolomic and transcriptomic study. PLOS Med. 17:e1003012. doi:10.1371/journal.pmed.1003012
- Majumdar, R., Shao, L., Turlapati, S. A., and Minocha, S. C. 2017. Polyamines in the life of Arabidopsis: profiling the expression of S-adenosylmethionine decarboxylase (SAMDC) gene family during its life cycle. BMC Plant Biol. 17:264. doi:10.1186/s12870-017-1208-y
- Mano, J., Ishibashi, A., Muneuchi, H., Morita, C., Sakai, H., Biswas, M. S., Koeduka, T., and Kitajima, S. 2017. Acrolein-detoxifying isozymes of glutathione transferase in plants. Planta 245:255–264. doi:10.1007/s00425-016-2604-5
- Mano, J., Miyatake, F., Hiraoka, E., and Tamoi, M. 2009. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 230:639–648. doi:10.1007/s00425-009-0964-9
- Mano, J. I., Tokushige, K., Mizoguchi, H., Fujii, H., and Khorobrykh, S. 2010. Accumulation of lipid peroxide-derived, toxic α, β-unsaturated aldehydes (E)-2-pentenal, acrolein and (E)-2-hexenal in leaves under photoinhibitory illumination. Plant Biotechnology 27:193–197. doi:10.5511/plantbiotechnology.27.193
- Matsumoto, M. 2015. Polyamines and Longevity in Mammals. In: Kusano, T., Suzuki, H. (eds) Polyamines. Springer: Tokyo.
- Mattoo, A., Fatima, T., Upadhyay, R., and Handa, A. 2015. Polyamines in plants: biosynthesis from arginine, and metabolic, physiological and stress-response roles. Amino Acids in Higher Plants, D’Mello, JPF, Ed., CABI: Wallingford, UK, p. 177–194.
- Mattoo, A. K., Minocha, S. C., Minocha, R., and Handa, A. K. 2010. Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids. 38:405–413. doi:10.1007/s00726-009-0399-4
- McKenna, J., Kapfhamer, D., Kinchen, J. M., Wasek, B., Dunworth, M., Murray-Stewart, T., Bottiglieri, T., Casero, R. A., and Gambello, M. J. 2018. Metabolomic studies identify changes in transmethylation and polyamine metabolism in a brain-specific mouse model of tuberous sclerosis complex. Hum. Mol. Genet. 27:2113–2124. doi:10.1093/hmg/ddy118
- Michael, A. J., Furze, J. M., Rhodes, M. J., and Burtin, D. 1996. Molecular cloning and functional identification of a plant ornithine decarboxylase cDNA. Biochem. J. 314 (Pt 1):, 241–248. doi:10.1042/bj3140241
- Minguet, E. G., Vera-Sirera, F., Marina, A., Carbonell, J., and Blázquez, M. A. 2008. Evolutionary diversification in polyamine biosynthesis. Mol. Biol. Evol. 25:2119–2128. doi:10.1093/molbev/msn161
- Mizoi, M., Yoshida, M., Saiki, R., Waragai, M., Uemura, K., Akatsu, H., Kashiwagi, K., and Igarashi, K. 2014. Distinction between mild cognitive impairment and Alzheimer’s disease by CSF amyloid β40 and β42, and protein-conjugated acrolein. Clin. Chim. Acta. 430:150–155. doi:10.1016/j.cca.2014.01.007
- Moghe, A., Ghare, S., Lamoreau, B., Mohammad, M., Barve, S., Mcclain, C., and Joshi-Barve, S. 2015. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol. Sci. 143:242–255. doi:10.1093/toxsci/kfu233
- Moschou, P. N., Sanmartin, M., Andriopoulou, A. H., Rojo, E., Sanchez-Serrano, J. J., and Roubelakis-Angelakis, K. A. 2008. Bridging the gap between plant and mammalian polyamine catabolism: a novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 147:1845–1857. doi:10.1104/pp.108.123802
- Muth, A., Pandey, V., Kaur, N., Wason, M., Baker, C., Han, X., Johnson, T. R., Altomare, D. A., and Phanstiel, O. 2014. Synthesis and biological evaluation of antimetastatic agents predicated upon dihydromotuporamine C and its carbocyclic derivatives. J. Med. Chem. 57:4023–4034. doi:10.1021/jm401906v
- Nathanson, J. A., Hunnicutt, E. J., Kantham, L., and Scavone, C. 1993. Cocaine as a naturally occurring insecticide. Proc. Natl. Acad. Sci. U S A. U S A 90:9645–9648. doi:10.1073/pnas.90.20.9645
- Navakoudis, E., and Kotzabasis, K. 2022. Polyamines: α bioenergetic smart switch for plant protection and development. J. Plant Physiol. 270:153618. doi:10.1016/j.jplph.2022.153618
- Nishimura, K., Lee, S. B., Park, J. H., and Park, M. H. 2012. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 42:703–710. doi:10.1007/s00726-011-0986-z
- Nishio, T., Yoshikawa, Y., Fukuda, W., Umezawa, N., Higuchi, T., Fujiwara, S., Imanaka, T., and Yoshikawa, K. 2018. Branched‐chain polyamine found in hyperthermophiles induces unique temperature‐dependent structural changes in genome‐size DNA. Chemphyschem 19:2299–2304. doi:10.1002/cphc.201800396
- Ober, D., Gibas, L., Witte, L., and Hartmann, T. 2003. Evidence for general occurrence of homospermidine in plants and its supposed origin as by-product of deoxyhypusine synthase. Phytochemistry 62:339–344. doi:10.1016/s0031-9422(02)00553-8
- Ober, D., and Hartmann, T. 1999. Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. U S A. U S A 96:14777–14782. doi:10.1073/pnas.96.26.14777
- Pagnussat, G. C., Yu, H. J., Ngo, Q. A., Rajani, S., Mayalagu, S., Johnson, C. S., Capron, A., Xie, L. F., Ye, D., and Sundaresan, V. 2005. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132:603–614. doi:10.1242/dev.01595
- Pálfi, P., Bakacsy, L., Kovács, H., and Szepesi, Á. 2021. Hypusination, a Metabolic Posttranslational Modification of eIF5A in Plants during Development and Environmental Stress Responses. Plants 10:1261. doi:10.3390/plants10071261
- Pällmann, N., Braig, M., Sievert, H., Preukschas, M., Hermans-Borgmeyer, I., Schweizer, M., Nagel, C. H., Neumann, M., Wild, P., Haralambieva, E., Hagel, C., Bokemeyer, C., Hauber, J., and Balabanov, S. 2015. Biological relevance and therapeutic potential of the hypusine modification system. J. Biol. Chem. 290:18343–18360. doi:10.1074/jbc.M115.664490
- Park, M., Nishimura, K., Zanelli, C. F., and Valentini, S. R. 2010. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 38:491–500. doi:10.1007/s00726-009-0408-7
- Park, M. H. 2006. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 139:161–169. doi:10.1093/jb/mvj034
- Park, M. H., Cooper, H. L., and Folk, J. E. 1981. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. U S A. U S A 78:2869–2873. doi:10.1073/pnas.78.5.2869
- Park, M. H., and Igarashi, K. 2013. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol. Ther. 21:1–9. doi:10.4062/biomolther.2012.097
- Park, M. H., Joe, Y. A., and Kang, K. R. 1998. Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 273:1677–1683. doi:10.1074/jbc.273.3.1677
- Park, M. H., and Wolff, E. C. 2018. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J. Biol. Chem. 293:18710–18718. doi:10.1074/jbc.TM118.003341
- Park, M. H., Wolff, E. C., Lee, Y. B., and Folk, J. 1994. Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J. Biol. Chem. 269:27827–27832.
- Parrish, R. R., Buckingham, S. C., Mascia, K. L., Johnson, J. J., Matyjasik, M. M., Lockhart, R. M., and Lubin, F. D. 2015. Methionine increases BDNF DNA methylation and improves memory in epilepsy. Ann. Clin. Transl. Neurol. 2:401–416. doi:10.1002/acn3.183
- Parrotta, L., Tanwar, U. K., Aloisi, I., Sobieszczuk-Nowicka, E., Arasimowicz-Jelonek, M., and Del Duca, S. 2022. Plant transglutaminases: new insights in biochemistry, genetics, and physiology. Cells 11:1529. doi:10.3390/cells11091529
- Patel, A. R., Li, J., Bass, B. L., and Wang, J.-Y. 1998. Expression of the transforming growth factor-β gene during growth inhibition following polyamine depletion. Am. J. Physiol. 275:C590–C598. doi:10.1152/ajpcell.1998.275.2.C590
- Patel, J., Ariyaratne, M., Ahmed, S., Ge, L., Phuntumart, V., Kalinoski, A., and Morris, P. F. 2017. Dual functioning of plant arginases provides a third route for putrescine synthesis. Plant Sci. 262:62–73. doi:10.1016/j.plantsci.2017.05.011
- Pegg, A. E. 2008. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 294:E995–1010. doi:10.1152/ajpendo.90217.2008
- Pegg, A. E. 2013. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 26:1782–1800. doi:10.1021/tx400316s
- Perez, R. F., Tejedor, J. R., Bayon, G. F., Fernández, A. F., and Fraga, M. F. 2018. Distinct chromatin signatures of DNA hypomethylation in aging and cancer. Aging Cell. 17:e12744. doi:10.1111/acel.12744
- Poidevin, L., Unal, D., Belda-Palazón, B., and Ferrando, A. 2019. Polyamines as Quality Control Metabolites Operating at the Post-Transcriptional Level. Plants (Basel) 8:109. doi:10.3390/plants8040109
- Pollard, K. J., Samuels, M. L., Crowley, K. A., Hansen, J. C., and Peterson, C. L. 1999. Functional interaction between GCN5 and polyamines: a new role for core histone acetylation. Embo J. 18:5622–5633. doi:10.1093/emboj/18.20.5622
- Pucciarelli, S., Moreschini, B., Micozzi, D., De Fronzo, G. S., Carpi, F. M., Polzonetti, V., Vincenzetti, S., Mignini, F., and Napolioni, V. 2012. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 15:590–595. doi:10.1089/rej.2012.1349
- Rao, J. N., Li, L., Bass, B. L., and Wang, J.-Y. 2000. Expression of the TGF-β receptor gene and sensitivity to growth inhibition following polyamine depletion. Am. J. Physiol. Cell Physiol. 279:C1034–C1044. doi:10.1152/ajpcell.2000.279.4.C1034
- Raspaud, E., Chaperon, I., Leforestier, A., and Livolant, F. 1999. Spermine-induced aggregation of DNA, nucleosome, and chromatin. Biophys. J. 77:1547–1555. doi:10.1016/S0006-3495(99)77002-5
- Robertson, L., Backer, J., Biemans, C., Van Doorn, J., Krab, K., and Reijnders, W. 2016. Antoni Van Leeuwenhoek: Master of the Minuscule, Brill, Leiden: Boston.
- Saiki, R., Nishimura, K., Ishii, I., Omura, T., Okuyama, S., Kashiwagi, K., and Igarashi, K. 2009. Intense correlation between brain infarction and protein-conjugated acrolein. Stroke 40:3356–3361. doi:10.1161/STROKEAHA.109.553248
- Saiki, R., Park, H., Ishii, I., Yoshida, M., Nishimura, K., Toida, T., Tatsukawa, H., Kojima, S., Ikeguchi, Y., Pegg, A. E., Kashiwagi, K., and Igarashi, K. 2011. Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem. Biophys. Res. Commun. 404:1044–1049. doi:10.1016/j.bbrc.2010.12.107
- Saini, P., Eyler, D. E., Green, R., and Dever, T. E. 2009. Hypusine-containing protein eIF5A promotes translation elongation. Nature 459:118–121. doi:10.1038/nature08034
- Sakamoto, A., Terui, Y., Uemura, T., Igarashi, K., and Kashiwagi, K. 2020. Polyamines regulate gene expression by stimulating translation of histone acetyltransferase mRNAs. J. Biol. Chem. 295:8736–8745. doi:10.1074/jbc.RA120.013833
- Sakata, K., Kashiwagi, K., Sharmin, S., Ueda, S., Irie, Y., Murotani, N., and Igarashi, K. 2003. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 305:143–149. doi:10.1016/s0006-291x(03)00716-2
- Sánchez-Jiménez, F., Medina, M. Á., Villalobos-Rueda, L., and Urdiales, J. L. 2019. Polyamines in mammalian pathophysiology. Cell. Mol. Life Sci. 76:3987–4008. doi:10.1007/s00018-019-03196-0
- Sasaki, K., Abid, R., and Miyazaki, M. 1996. Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett. 384:151–154. doi:10.1016/0014-5793(96)00310-9
- Schipper, R. G., van den Heuvel, L.P., Verhofstad. A.A., De Abreu, R.A. 2007. Polyamines and DNA methylation in childhood leukaemia. Biochem. Soc. Trans. 35:331–335. doi:10.1042/BST0350331
- Schnier, J., Schwelberger, H., Smit-Mcbride, Z., Kang, H. A., and Hershey, J. 1991. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 11:3105–3114. doi:10.1128/mcb.11.6.3105-3114.1991
- Schuller, A. P., Wu, C. C., Dever, T. E., Buskirk, A. R., and Green, R. 2017. eIF5A functions globally in translation elongation and termination. Mol. Cell. 66:194–205.e5. doi:10.1016/j.molcel.2017.03.003
- Seiler, N. 2004. Catabolism of polyamines. Amino Acids. 26:217–233. doi:10.1007/s00726-004-0070-z
- Sekhar, V., Andl, T., and Phanstiel, O. 2022. ATP13A3 facilitates polyamine transport in human pancreatic cancer cells. Sci. Rep. 12:4045. doi:10.1038/s41598-022-07712-4
- Sequera-Mutiozabal, M. I., Erban, A., Kopka, J., Atanasov, K. E., Bastida, J., Fotopoulos, V., Alcázar, R., and Tiburcio, A. F. 2016. Global metabolic profiling of Arabidopsis polyamine oxidase 4 (AtPAO4) loss-of-function mutants exhibiting delayed dark-induced senescence. Front. Plant Sci. 7:173. doi:10.3389/fpls.2016.00173
- Shiba, T., Mizote, H., Kaneko, T., Nakajima, T., Yasuo, K., and Sano, I. 1971. Hypusine, a new amino acid occurring in bovine brain: isolation and structural determination. Biochim. Biophys. Acta. 244:523–531. doi:10.1016/0304-4165(71)90069-9
- Shojaei Saadi, H. A., Gagné, D., Fournier, É., Baldoceda Baldeon, L. M., Sirard, M.-A., and Robert, C. 2016. Responses of bovine early embryos to S-adenosyl methionine supplementation in culture. Epigenomics 8:1039–1060. doi:10.2217/epi-2016-0022
- Sobieszczuk-Nowicka, E. 2017. Polyamine catabolism adds fuel to leaf senescence. Amino Acids. 49:49–56. doi:10.1007/s00726-016-2377-y
- Sobieszczuk-Nowicka, E., Kubala, S., Zmienko, A., Małecka, A., and Legocka, J. 2015a. From accumulation to degradation: reprogramming polyamine metabolism facilitates dark-induced senescence in barley leaf cells. Front. Plant Sci. 6:1198. doi:10.3389/fpls.2015.01198
- Sobieszczuk-Nowicka, E., Zmienko, A., Samelak-Czajka, A., Łuczak, M., Pietrowska-Borek, M., Iorio, R., Del Duca, S., Figlerowicz, M., and Legocka, J. 2015b. Dark-induced senescence of barley leaves involves activation of plastid transglutaminases. Amino Acids. 47:825–838. doi:10.1007/s00726-014-1912-y
- Soda, K., Kano, Y., Chiba, F., Koizumi, K., and Miyaki, Y. 2013. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1, 2-dimethylhydrazine-induced tumorigenesis. PLOS One. 8:e64357. doi:10.1371/journal.pone.0064357
- Stephenson, A., and Seidel, E. 2006. Analysis of the interactions of Nrf-2, PMF-1, and CSN-7 with the 5′-flanking sequence of the mouse 4E-BP1 gene. Life Sci. 79:1221–1227. doi:10.1016/j.lfs.2006.03.042
- Stewart, T. M., Dunston, T. T., Woster, P. M., and Casero, R. A. 2018. Polyamine catabolism and oxidative damage. J. Biol. Chem. 293:18736–18745. doi:10.1074/jbc.TM118.003337
- Tabor, C. W., and Tabor, H. 1984. Polyamines. Annu. Rev. Biochem. 53:749–790. doi:10.1146/annurev.bi.53.070184.003533
- Tabor, C. W., Tabor, H., and Bachrach, U. 1964. Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma amine oxidase. J. Biol. Chem. 239:2194–2203.
- Takahashi, T., and Tong, W. 2015. Regulation and Diversity of Polyamine Biosynthesis in Plants. In: Kusano, T., Suzuki, H. (eds.), Polyamines. Springer: Tokyo.
- Tanwar, U. K., Stolarska, E., Paluch-Lubawa, E., Mattoo, A. K., Arasimowicz-Jelonek, M., and Sobieszczuk-Nowicka, E. 2022. Unraveling the genetics of polyamine metabolism in barley for senescence-related crop improvement. Int. J. Biol. Macromol. 221:585–603. doi:10.1016/j.ijbiomac.2022.09.006
- Tari, I., and Csiszár, J. 2003. Effects of NO2− or NO3− supply on polyamine accumulation and ethylene production of wheat roots at acidic and neutral pH: implications for root growth. Plant Growth Regulation 40:121–128. doi:10.1023/A:1024235211395
- Tassoni, A., Van Buuren, M., Franceschetti, M., Fornalè, S., and Bagni, N. 2000. Polyamine content and metabolism in Arabidopsis thaliana and effect of spermidine on plant development. Plant Physiol. Biochem. 38:383–393. doi:10.1016/S0981-9428(00)00757-9
- Tavladoraki, P., Cona, A., Federico, R., Tempera, G., Viceconte, N., Saccoccio, S., Battaglia, V., Toninello, A., and Agostinelli, E. 2012. Polyamine catabolism: target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids. 42:411–426. doi:10.1007/s00726-011-1012-1
- Tavladoraki, P., Rossi, M. N., Saccuti, G., Perez-Amador, M. A., Polticelli, F., Angelini, R., and Federico, R. 2006. Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol. 141:1519–1532. doi:10.1104/pp.106.080911
- Ten Dijke, P., Goumans, M. J., Itoh, F., and Itoh, S. 2002. Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 191:1–16. doi:10.1002/jcp.10066
- Thomas, T., and Thomas, T. J. 2001. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 58:244–258. doi:10.1007/PL00000852
- Thomas, T., Tajmir-Riahi, H., and Thomas, T. 2016. Polyamine–DNA interactions and development of gene delivery vehicles. Amino Acids. 48:2423–2431. doi:10.1007/s00726-016-2246-8
- Thompson, J. E., Hopkins, M. T., Taylor, C., and Wang, T. W. 2004. Regulation of senescence by eukaryotic translation initiation factor 5A: implications for plant growth and development. Trends Plant Sci. 9:174–179. doi:10.1016/j.tplants.2004.02.008
- Tiburcio, A. F., Altabella, T., Bitrián, M., and Alcázar, R. 2014. The roles of polyamines during the lifespan of plants: from development to stress. Planta 240:1–18. doi:10.1007/s00425-014-2055-9
- Torres, M. A., Jones, J. D., and Dangl, J. L. 2005. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37:1130–1134. doi:10.1038/ng1639
- Toumi, I., Pagoulatou, M. G., Margaritopoulou, T., Milioni, D., and Roubelakis-Angelakis, K. A. 2019. Genetically modified heat shock protein90s and polyamine oxidases in Arabidopsis reveal their interaction under heat stress affecting polyamine acetylation, oxidation and homeostasis of reactive oxygen species. Plants 8:323. doi:10.3390/plants8090323
- Tse, R.T.-H., Ding, X., Wong, C.Y.-P., Cheng, C.K.-L., Chiu, P.K.-F., Ng, C.-F. 2022.The Association between Spermidine/Spermine N1-Acetyltransferase (SSAT) and Human Malignancies. Int. J. Mol. Sci. 23:5926. doi:10.3390/ijms23115926
- Uchida, K., Kanematsu, M., Morimitsu, Y., Osawa, T., Noguchi, N., and Niki, E. 1998. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 273:16058–16066. doi:10.1074/jbc.273.26.16058
- Upadhyay, R. K., Fatima, T., Handa, A. K., and Mattoo, A. K. 2020. Polyamines and their biosynthesis/catabolism genes are differentially modulated in response to heat versus cold stress in tomato leaves (Solanum lycopersicum L.). Cells 9:1749. doi:10.3390/cells9081749
- Upadhyay, R. K., Fatima, T., Handa, A. K., and Mattoo, A. K. 2021a. Differential association of free, conjugated, and bound forms of polyamines and transcript abundance of their biosynthetic and catabolic genes during drought/salinity stress in tomato (Solanum lycopersicum L.) Leaves. Front. Plant Sci. 12:743568. doi:10.3389/fpls.2021.743568
- Upadhyay, R. K., Shao, J., and Mattoo, A. K. 2021b. Genomic analysis of the polyamine biosynthesis pathway in duckweed Spirodela polyrhiza L.: presence of the arginine decarboxylase pathway, absence of the ornithine decarboxylase pathway, and response to abiotic stresses. Planta 254:108. doi:10.1007/s00425-021-03755-5
- Van De Poel, B., Bulens, I., Oppermann, Y., Hertog, M. L., Nicolai, B. M., Sauter, M., and Geeraerd, A. H. 2013. S‐adenosyl‐l‐methionine usage during climacteric ripening of tomato in relation to ethylene and polyamine biosynthesis and transmethylation capacity. Physiol. Plant. 148:176–188. doi:10.1111/j.1399-3054.2012.01703.x
- Van Den Oever, S. P., and Mayer, H. K. 2022. Can oligomeric proanthocyanidins interfere with UHPLC analysis of spermidine in nutritional supplements? J. Food Compos. Anal. 109:104466. doi:10.1016/j.jfca.2022.104466
- Vera-Sirera, F., Minguet, E. G., Singh, S. K., Ljung, K., Tuominen, H., Blázquez, M. A., and Carbonell, J. 2010. Role of polyamines in plant vascular development. Plant Physiol. Biochem. 48:534–539. doi:10.1016/j.plaphy.2010.01.011
- Visvanathan, A., Ahmed, K., Even-Faitelson, L., Lleres, D., Bazett-Jones, D. P., and Lamond, A. I. 2013. Modulation of higher order chromatin conformation in mammalian cell nuclei can be mediated by polyamines and divalent cations. PLOS One. 8:e67689. doi:10.1371/journal.pone.0067689
- Vujcic, S., Diegelman, P., Bacchi, C. J., Kramer, D. L., and Porter, C. W. 2002. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem. J. 367:665–675. doi:10.1042/BJ20020720
- Walters, D. R. 2003. Polyamines and plant disease. Phytochem. 64:97–107. doi:10.1016/s0031-9422(03)00329-7
- Wahlfors, J., Hiltunen, H., Heinonen, K., Hamalainen, E., Alhonen, L. & Janne, J. 1992. Genomic hypomethylation in human chronic lymphocytic leukemia. Blood, 80:2074–80. doi:10.1182/blood.V80.8.2074.2074
- Wang, T. W., Lu, L., Wang, D., and Thompson, J. E. 2001a. Isolation and characterization of senescence-induced cDNAs encoding deoxyhypusine synthase and eucaryotic translation initiation factor 5A from tomato. J. Biol. Chem. 276:17541–17549. doi:10.1074/jbc.M008544200
- Wang, T. W., Lu, L., Zhang, C. G., Taylor, C., and Thompson, J. E. 2003. Pleiotropic effects of suppressing deoxyhypusine synthase expression in Arabidopsis thaliana. Plant Mol. Biol. 52:1223–1235. doi:10.1023/b:plan.0000004332.80792.4d
- Wang, T. W., Zhang, C. G., Wu, W., Nowack, L. M., Madey, E., and Thompson, J. E. 2005. Antisense suppression of deoxyhypusine synthase in tomato delays fruit softening and alters growth and development. Plant Physiol. 138:1372–1382. doi:10.1104/pp.105.060194
- Wang, W., and Liu, J.-H. 2015. Genome-wide identification and expression analysis of the polyamine oxidase gene family in sweet orange (Citrus sinensis). Gene 555:421–429. doi:10.1016/j.gene.2014.11.042
- Wang, X., Ying, W., Dunlap, K. A., Lin, G., Satterfield, M. C., Burghardt, R. C., Wu, G., and Bazer, F. W. 2014. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol. Reprod. 90:84. doi:10.1095/biolreprod.113.114637
- Wang, Y., Devereux, W., Stewart, T. M., and Casero, R. A. 1999. Cloning and characterization of human polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermineN 1-Acetyltransferase gene. J. Biol. Chem. 274:22095–22101. doi:10.1074/jbc.274.31.22095
- Wang, Y., Devereux, W., Woster, P. M., Stewart, T. M., Hacker, A., and Casero, R. A. Jr, 2001b. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Research 61:5370–5373.
- Waragai, M., Yoshida, M., Mizoi, M., Saiki, R., Kashiwagi, K., Takagi, K., Arai, H., Tashiro, J., Hashimoto, M., Iwai, N., Uemura, K., and Igarashi, K. 2012. Increased protein-conjugated acrolein and amyloid-β40/42 ratio in plasma of patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers. Dis. 32:33–41. doi:10.3233/JAD-2012-120253
- Wirth, A., Wolf, B., Huang, C.-K., Glage, S., Hofer, S. J., Bankstahl, M., Bär, C., Thum, T., Kahl, K. G., Sigrist, S. J., Madeo, F., Bankstahl, J. P., and Ponimaskin, E. 2021. Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. Geroscience. 43:673–690. doi:10.1007/s11357-020-00310-0
- Wöhl, T., Klier, H., Ammer, H., Lottspeich, F., and Magdolen, V. 1993. The HYP2 gene of Saccharomyces cerevisiae is essential for aerobic growth: characterization of different isoforms of the hypusine-containing protein Hyp2p and analysis of gene disruption mutants. Mol. Gen. Genet. 241:305–311. doi:10.1007/BF00284682
- Wood, P. L., Khan, M. A., and Moskal, J. R. 2007. The concept of “aldehyde load” in neurodegenerative mechanisms: cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res. 1145:150–156. doi:10.1016/j.brainres.2006.10.004
- Wu, D., von Roepenack-Lahaye, E., Buntru, M., de Lange, O., Schandry, N., Pérez-Quintero, A. L., Weinberg, Z., Lowe-Power, T. M., Szurek, B., Michael, A. J., Allen, C., Schillberg, S., and Lahaye, T. 2019. A plant pathogen type III effector protein subverts translational regulation to boost host polyamine levels. Cell Host Microbe. 26:638–649. e5. doi:10.1016/j.chom.2019.09.014
- Yamamoto, D., Shima, K., Matsuo, K., Nishioka, T., Chen, C. Y., Hu, G.-F., Sasaki, A., and Tsuji, T. 2010. Ornithine decarboxylase antizyme induces hypomethylation of genome DNA and histone H3 lysine 9 dimethylation (H3K9me2) in human oral cancer cell line. PloS One. 5:e12554. doi:10.1371/journal.pone.0012554
- Yamauchi, Y., Hasegawa, A., Mizutani, M., and Sugimoto, Y. 2012. Chloroplastic NADPH-dependent alkenal/one oxidoreductase contributes to the detoxification of reactive carbonyls produced under oxidative stress. FEBS Lett. 586:1208–1213. doi:10.1016/j.febslet.2012.03.013
- Yin, L., Mano, J., Wang, S., Tsuji, W., and Tanaka, K. 2010. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 152:1406–1417. doi:10.1104/pp.109.151449
- Yoda, H., Hiroi, Y., and Sano, H. 2006. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 142:193–206. doi:10.1104/pp.106.080515
- Yoda, H., Yamaguchi, Y., and Sano, H. 2003. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol. 132:1973–1981. doi:10.1104/pp.103.024737
- Yoshida, M., Tomitori, H., Machi, Y., Katagiri, D., Ueda, S., Horiguchi, K., Kobayashi, E., Saeki, N., Nishimura, K., Ishii, I., Kashiwagi, K., and Igarashi, K. 2009. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis 203:557–562. doi:10.1016/j.atherosclerosis.2008.07.022
- Yu, Y., Jin, C., Sun, C., Wang, J., Ye, Y., Zhou, W., Lu, L., and Lin, X. 2016. Inhibition of ethylene production by putrescine alleviates aluminium-induced root inhibition in wheat plants. Sci. Rep. 6:18888. doi:10.1038/srep18888
- Zahedi, K., Huttinger, F., Morrison, R., Murray-Stewart, T., Casero, R. A., and Strauss, K. I. 2010. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma. 27:515–525. doi:10.1089/neu.2009.1097
- Zeesman, S., Kjaergaard, S., Hove, H. D., Kirchhoff, M., Stevens, J. M., and Nowaczyk, M. J. 2012. Microdeletion in distal 17p13.1: a recognizable phenotype with microcephaly, distinctive facial features, and intellectual disability. Am. J. Med. Genet. A. 158a:1832–1836. doi:10.1002/ajmg.a.35508
- Zhang, H., Alsaleh, G., Feltham, J., Sun, Y., Napolitano, G., Riffelmacher, T., Charles, P., Frau, L., Hublitz, P., Yu, Z., Mohammed, S., Ballabio, A., Balabanov, S., Mellor, J., and Simon, A. K. 2019. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell. 76:110–125. e9. doi:10.1016/j.molcel.2019.08.005
- Zhao, J., Missihoun, T. D., and Bartels, D. 2017. The role of Arabidopsis aldehyde dehydrogenase genes in response to high temperature and stress combinations. J. Exp. Bot. 68:4295–4308. doi:10.1093/jxb/erx194
