Abstract
We examined if serum concentrations Interferon gamma-induced protein (IP-10) is a potential clinical biomarker for cancer-related-fatigue (CRF). Fatigue scores and IP-10 concentrations were measured from curatively treated fatigued cancer patients randomized to either cognitive behavioral therapy (CBT, n = 26) or waiting-list (WL, n = 13). No correlation was found between baseline IP-10 level and fatigue severity and no significant differences in IP-10 serum levels were observed between fatigued and matched non-fatigued patients (n = 22). Relative changes in IP-10 concentrations from baseline to six-month follow-up were not significantly different between the CBT and WL conditions. In this study, IP-10 showed low potential as clinical CRF biomarker.
Trial registration: This study is registered at ClinicalTrials.gov (NCT01096641).
Background
A substantial percentage of survivors of both hematological and solid malignancies experience severe fatigue, a condition known as cancer-related fatigue (CRF) (Citation1,Citation2). CRF is a severe and debilitating problem impairing quality of life (Citation1,Citation2). Cognitive behavioral therapy (CBT), specifically designed for CRF, is a proven effective treatment (Citation3). For clinical practice, it would be instrumental to have biomarkers at our disposal for the early identification of patients who are vulnerable to the development of CRF and to guide treatment of CRF.
Previous studies have suggested that components of the immune system are of relevance for the development of CRF. Activation of the immune system by the tumor or its treatment may lead to the release of cytokines, chemokines, and other immune related substances (Citation1). These substances play a central role in both the innate and adaptive immune response but also mediate symptoms such as fatigue (Citation4). Alterations in cytokine serum levels have been reported previously in fatigue-related disorders, such as depression and chronic fatigue syndrome (Citation5,Citation6). However, the exact role of chemokines in CRF has not frequently been studied as most studies only measure cytokines (Citation7,Citation8). Interferon gamma-induced protein (IP-10), also known as CXCL-10, plays a role in lymphocytic infiltration of the tumor site and is therefore associated with tumor progression and poor survival in patients with breast cancer (Citation9) and pancreatic cancer (Citation10,Citation11). Recently, indeed a relationship between chemokine IP-10 serum concentrations and CRF was found in patients with acute myeloid leukemia (Citation12). There was a significant correlation between changes in IP-10 serum concentrations and changes in fatigue severity before and after the first cycle of chemotherapy. However, another study found no correlation between fatigue and cytokines or chemokines (i.e., IP-10) adult survivors of childhood acute lymphoblastic leukemia and lymphoma (Citation13). Thus, it remains uncertain whether IP-10 can be used as a biomarker of CRF.
Three important limitations of the currently available evidence merit attention. Firstly, these previous studies did not incorporate a matched non-fatigued control group to compare the findings of the fatigued experimental group with. Secondly, only one of these studies showed longitudinal data of both IP-10 and CRF levels and found no relationship. Thirdly, there is no data available on CRF and the role of chemokines/IP-10 in solid malignancies yet. In the current study, our goal was to study the association between CRF and IP-10. Therefore, we used the highly sensitive Luminex array to analyze baseline and follow-up serum samples obtained from a previously conducted 6-month randomized controlled trial (RCT), in which CRF symptoms were assessed in fatigued survivors of solid and hematological malignancies before and after CBT or before and after a waiting list (WL) for CBT (Citation14). First, we made a cross-sectional comparison between the baseline IP-10 concentrations and fatigue levels of all fatigued patients (the pooled CBT and WL group) to those of a matched control group of non-fatigued cancer patients. Next, we compared the changes in IP-10 serum concentrations at baseline and follow-up between the CBT group and the WL group.
Methods
Study design
The original study is registered at ClinicalTrials.gov (NCT01096641) and patients were enrolled from March 2009 until April 2012. The eligibility criteria, procedure, and design were in line with the CONSORT statement (Supplementary data, CONSORT checklist) and have been described previously (Citation14). Fatigued cancer survivors were randomly assigned (3:1 ratio) to either the CBT intervention condition (n = 50) or the WL condition (n = 14). Random assignment was done by means of a sequence of labeled cards contained in sealed, numbered envelopes prepared by an independent statistical advisor. The envelopes were opened by the psychologist in presence of the patient. Patients randomized to the intervention group were immediately treated with CBT (Citation4). Treatment of patients randomized to the WL-group commenced after a waiting period of 6 months (Citation15).
Study population
The study population has been described previously (Citation14). In brief, patients between 18 and 65 years old could be included in the study after successful completion of curative treatment for a malignant, solid tumor or (non-)Hodgkin Lymphoma at least one year earlier. Patients were excluded if they had comorbid psychiatric conditions, used concomitant psychoactive medication (benzodiazepines, anticonvulsants, anti-depressants), or if they had any physical condition that could explain symptoms of fatigue. Fatigued patients were included if they had a checklist individual strength (CIS)-fatigue score ≥35. We included a matched control group of those patients whose CIS-fatigue score was <27 (Citation3,Citation16–18) indicating lack of fatigue. These non-fatigued patients were matched to the fatigued patients with respect to age, sex, and previous cancer treatment. The local Radboud University Medical Centre ethical committee approved the research protocol. All patients provided written informed consent.
Measurements
Fatigue
Fatigue severity was assessed using the validated fatigue subscale of the CIS-fatigue, which is an 8-items rating scale with scores ranging from 8 to 56 (Citation19,Citation20). A higher score indicates a higher level of fatigue.
Serum IP-10 concentrations
Blood samples were taken by vena puncture and serum aliquots were stored at −80 °C. IP-10 levels were measured with suspension bead assays (Bio-Rad, Richmond, CA) using a high sensitivity Luminex reader (BioRad, BioSource, Linco, Colchester, UK) according to the manufacturer’s recommendations. The serum concentration was expressed as pg/ml.
Statistical analyses
Preliminary analyses encompassed the comparison of baseline characteristics between the fatigued and non-fatigued patients and between the CBT and WL conditions (i.e., age, gender, time since cancer treatment, cancer diagnosis, and cancer treatment) with unpaired t-tests, Mann-Whitney U tests, and Chi-square tests. Next, we examined the comparability of baseline fatigue scores between the CBT and WL conditions with Mann-Whitney U tests. Thereafter, the relative decrease in fatigue scores over time between the CBT and the WL conditions was statistically tested with Mann-Whitney U tests.
In the cross-sectional part of the study, median baseline IP-10 concentration was compared between the fatigued (pooled CBT and WL group) versus the matched non-fatigued group and statistically tested with a Mann-Whitney U test. Also, baseline IP-10 concentrations were correlated with the corresponding CIS-fatigue scores using Spearman’s correlation.
In the longitudinal part of the study, again we first calculated serum IP-10 concentration relative change scores between baseline and 6-month follow-up of the fatigued cancer survivors. Thereafter, we statistically tested the relative decrease in IP-10 concentration of the CBT versus the WL group using a Mann-Whitney-U test. Also, the IP-10 concentration relative change scores were correlated with the relative change fatigue scores using Spearman’s correlation.
Statistical analyses were conducted with Graphpad Prism version 5 for Windows and SPSS 20. All tests were performed two-sided at an α = 0.05 level of significance.
Results
Baseline Characteristics
From March 2009 until April 2012, 64 fatigued cancer survivors were included in the RCT as described earlier (Citation14). For the present analysis, paired serum samples were available of 26 patients in the CBT condition (49% male) and 13 in the WL condition (38% male) (). Furthermore, 22 serum samples at baseline were available of non-fatigued patients (50% male) to be compared with the fatigued patients (49% male). Mean age ± standard deviation (SD) was similar between the fatigued (pooled CBT and WL groups) and matched non-fatigued controls (49.4 ± 9.9 and 48.3 ± 10.5 years, respectively, p = 0.67), as well as between the participants in the CBT condition and the WL condition (48.9 ± 9.2 and 50.5 ± 11.4 years, respectively, p = 0.62). In addition, the median time ± SD since completion of the cancer treatment was similar in the fatigued versus matched non-fatigued patients (51.6 ± 53.4 and 60.5 ± 43.6 months, respectively, p = 0.212) and in the CBT versus WL condition (54.0 ± 60.7 and 46.9 ± 36.6 months, respectively, p = 0.59). For further information on baseline characteristics ().
Figure 1. CONSORT flowchart of included patients. Flowchart of patients randomized to either the cognitive behavior therapy condition or the waiting-list condition. Only the participants who completed both baseline and follow-up IP-10 assessment were compared to non-fatigued patients in terms of baseline cytokine concentrations. Abbreviations: CBT: cognitive behavior therapy; WL: waiting list.
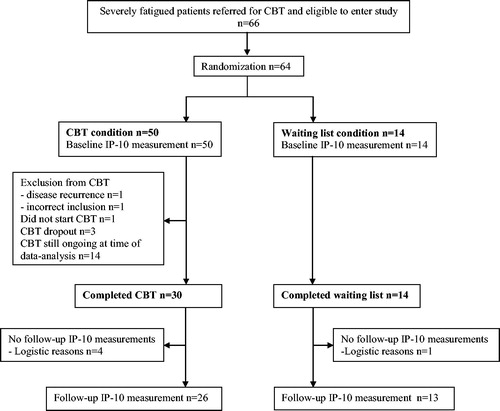
Table 1. Baseline characteristics of fatigued and non-fatigued patients (cross-sectional baseline comparison) and of fatigued patients in the therapy and waiting list condition (longitudinal comparison).
Fatigue
At baseline, the median score of fatigue severity was similar between the CBT group and the WL group (44 and 46, respectively, p = 0.31). After CBT, a significant decrease in the median fatigue score was observed in the intervention group (dropping from 44 to 22, p < 0.0001).The median fatigue score in the WL group also decreased significantly (from 46 to 40, p = 0.01). However, when we compared the relative decrease of fatigue scores over time in the CBT and the WL group, a significantly larger decrease was detected for the CBT group compared to the WL group (relative decrease = 49% and 10%, respectively, p < 0.001). In terms of clinical relevance, the number of participants reporting severe CRF (CIS-fatigue score ≥35), decreased from 26 to 3 in the CBT condition (p < 0.001), compared to a decrease of only 13 to 11 in the WL condition (p = 0.16).
Cross-sectional baseline comparison
First, we compared the median IP-10 concentrations in fatigued (CBT and WL condition pooled) versus matched non-fatigued individuals at baseline. No significant difference in median IP-10 serum concentrations (in pg/ml) was observed between fatigued (median = 738.0, n = 39) and non-fatigued patients (median = 902.4, n = 22), p = 0.74 (; ). Second, based onthe pooled baseline measurements of the CBT and WL condition, no significant correlation was found between IP-10 concentrations and fatigue severity (Spearman’s rho = 0.01, p = 0.94).
Figure 2. Longitudinal comparison of IP-10 concentration between CBT and WL condition. X-axis: Measurement at baseline and follow-up in the CBT and WL condition. Y-axis: IP-10 concentration in pg/mL. Abbreviations: CBT: cognitive behavior therapy; WL: waiting list.
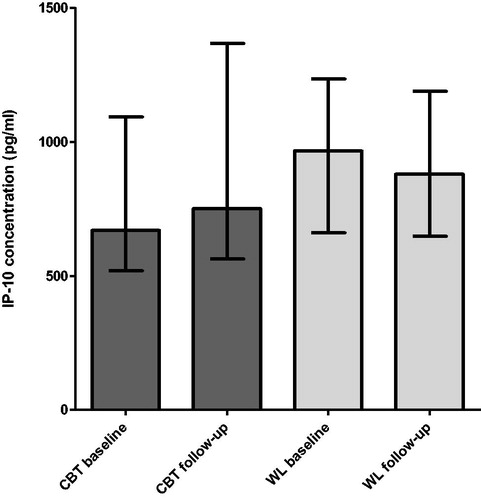
Table 2. Cross-sectional and longitudinal comparison of fatigue severity and IP-10 concentration.
Longitudinal comparison: CBT versus WL
We did not find significant differences between the median relative change in IP-10 concentration from baseline to follow-up between the CBT condition (median relative increase = 13%, n = 26) and WL condition (median relative decrease = 5%, n = 13) (; ). Also, no significant correlations were observed between the intra-patient baseline and follow-up change scores for IP-10 concentrations and fatigue levels (Spearman’s rho = 0.025, p = 0.88).
Discussion
In this study, we could not identify an association between fatigue levels and IP-10 serum concentrations, neither by comparing fatigued with non-fatigued patients nor by assessing intra-patient variation over time. The results of this 6-month RCT thus question the usability of serum IP-10 as a reliable clinical biomarker for CRF.
For an immunomodulator, such as IP-10, to serve as a biomarker in clinical practice, the immunomodulator should first of all be easily and reliably measurable. Secondly, immunomodulator serum concentrations should be different in fatigued versus non-fatigued individuals and thirdly, immunomodulator levels should fluctuate in concert with fatigue severity changes over time. The current study design addressed all these aspects and was most appropriate to investigate the clinical utility of IP-10 as biomarker for CRF as (a) it was easily measurable in a blood sample from the participant and IP-10 concentrations were determined using one of the most sensitive assays, (b) a matched non-fatigued control group was included, and (c) changes in IP-10 and fatigue levels were compared over time, all in a controlled setting.
A limitation of this study was the heterogeneity of the included cancer-survivors in terms of previous diagnosis and type of treatment. Due to the small number of observations, we were not able to conduct sub-analyses to investigate whether patients with hematological and solid types of cancer or with different types of treatments showed higher serum IP-10 concentrations compared to others. However, studies show that both previous diagnosis and treatment characteristics are actually unrelated to CRF (Citation21–24). In this study, the majority of the patients were previously diagnosed with solid tumor, whereas only 13% had hematological malignancies. Some evidence indicates that patients who only received surgery without concurrent treatment are less at risk for CRF (Citation17), compared to patients who received surgery with adjuvant treatments, such as chemotherapy (Citation25,Citation26). In our total sample, only 10% of the patients received surgery only, whereas the other 90% did receive more aggressive treatments and these patients were equally distributed across all groups. Moreover, we intended to study IP-10 for CRF associated with any type of cancer, so sub-analyses would have been for exploratory purposes only.
Another limitation is the small sample size of the original study as compared to the previously conducted studies on IP-10 had larger sample-sizes (Citation12,Citation13). However, in our study, the correlation between IP-10 and fatigue levels was close to zero, suggesting that even with more power, no association could have been detected. Moreover, the sample size of our study was in line with those of previously conducted studies on other immunomudolators than IP-10 and conducting small studies is the first step to identify clinically utilizable biomarkers that can be explored in larger studies (Citation7,Citation8).
In conclusion, no association between IP-10 serum-levels and CRF was found in this RCT and therefore, IP-10 remains inadequate as a CRF biomarker for (solid) cancer survivors at the protein level in serum.
Declaration of interest
The authors declared that they have no conflict of interest.
Acknowledgments
We thank all patients who participated in this study. In special, we thank Jeanette Pots from the Department of Tumor Immunology, Radboud University Medical Centre, Nijmegen and Dr. Rene Lutter from the Department of Experimental Immunology, Academic Medical Center, Amsterdam for their assistance and prof. dr. Jan Willem Leer, Department of Radiology, Radboud University Nijmegen Medical Centre, Nijmegen for referral of patients for this study.
Additional information
Funding
Notes on contributors
Emil ter Veer
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Hetty Prinsen
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Mirjam A. G. Sprangers
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Koos A.H. Zwinderman
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Gijs Bleijenberg
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Tineke C.T.M. van der Pouw Kraan
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
I. Jolanda M. de Vries
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Eddy A. Wierenga
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
Hanneke W.M. van Laarhoven
The original study was conducted by HP, GB, KZ, JdV and HvL. For the current sub-analysis, EtV, HP, HvL, JdV and EW were responsible for the data acquisition. EtV conducted the data analysis. EtV, HvL, EW, MS and TvdPK were involved in interpretation of the results and drafting the manuscript. All authors gave final approval of the current manuscript.
References
- Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol. 2002;13(4):589–598.
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611.
- Gielissen MF, Verhagen S, Witjes F, Bleijenberg G. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol. 2006;24(30):4882–4887.
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW. et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 (Suppl 1):S112–S118.
- Gaab J, Rohleder N, Heitz V, Zhou JH, Shen WH, Johnson RW, Engert V, Schad T, Schürmeyer TH, et al. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30(2):188–198.
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158(8):1252–1257.
- Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23(8):2461–2478. doi:10.1007/s00520-015-2763-0.
- Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun. 2012;26(6):830–848.
- Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen, LT, O'Malley FP, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res. 2013;19(2):336–346. doi:10.1158/1078-0432.CCR 11-3314.
- Lunardi S, Lim SY, Muschel RJ, Brunner TB. IP-10/CXCL10 attracts regulatory T cells: implication for pancreatic cancer. OncoImmunology. 2015;4:e1027473. doi:10.1080/2162402X.2015.1027473
- Lunardi S, Jamieson NB, Lim SY, Griffiths KL, Carvalho-Gaspar M, Al-Assar O et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget. 2014;5:11064–11080. doi:10.18632/oncotarget.2519.
- Fung FY, Li M, Breunis H, Timilshina N, Minden MD, Alibhai SM. Correlation between cytokine levels and changes in fatigue and quality of life in patients with acute myeloid leukemia. Leuk Res. 2013;37:274–279. doi:10.1016/j.leukres.2012.11.013
- Hamre H, Zeller B, Kanellopoulos A Ruud E, Fosså SD, Loge JH, et al. Serum cytokines and chronic fatigue in adults surviving after childhood leukemia and lymphoma. Brain Behav Immun. 2013;30:80–87. doi:10.1016/j.bbi. 2013.01.006.
- Prinsen H, Bleijenberg G, Zwarts MJ, Hopman MT, Heerschap A, van Laarhoven HW. Physiological and neurophysiological determinants of postcancer fatigue: design of a randomized controlled trial. BMC Cancer. 2012;12:256.
- Prinsen H, Heerschap A, Bleijenberg G, Zwarts MJ, Leer JW, van Asten JJ. et al. Magnetic resonance spectroscopic imaging and volumetric measurements of the brain in patients with postcancer fatigue: a randomized controlled trial. PLoS One. 2013;8(9):e74638.
- Gielissen MF, Verhagen CA, Bleijenberg G. Cognitive behaviour therapy for fatigued cancer survivors: long-term follow-up. Br J Cancer. 2007;97(5):612–618.
- Servaes P, Verhagen S, Schreuder HW, Veth RP, Bleijenberg G. Fatigue after treatment for malignant and benign bone and soft tissue tumors. J Pain Symptom Manage. 2003;26(6):1113–22.
- Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer 2002;38(1):27–43.
- Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res. 2004;56(2):157–170.
- Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38(5):383–392.
- Bartsch HH, Weis J, Moser MT. Cancer-related fatigue in patients attending oncological rehabilitation programs: prevalence, patterns and predictors. Onkologie. 2003;26(1): 51–57.
- Dimeo F, Schmittel A, Fietz T, Schwartz S, Köhler P, Böning D. et al. Physical performance, depression, immune status and fatigue in patients with hematological malignancies after treatment. Ann Oncol. 2004;15(8):1237–1242.
- Gielissen MF, Schattenberg AV, Verhagen CA, Rinkes MJ, Bremmers ME, Bleijenberg G. Experience of severe fatigue in long-term survivors of stem cell transplantation. Bone Marrow Transplant. 2007;39(10):595–603.
- Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M. et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19(1):5–14.
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753.
- Woo B, Dibble SL, Piper BF, Keating SB, Weiss MC. Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum. 1998;25(5):915–920.
