Abstract
Background
Programmed death 1 (PD-1) and its ligand PD-L1 play a key dysfunction of T lymphocytes. The purpose of this study was to assess and compare the prognostic role of tumor- TILs and its relationship with PD-L1 expression in stage II and III colon cancer.
Methods
Immunohistochemisty was used to assess the densities of CD8+, CD4+, and FOXP3+ cells, and PD-L1 expression in intraepithelial tumor site from 58 stage II and III colon cancers. These were evaluated for association with histopathologic features and overall survival.
Results
PD-L1-positive tumors contained a higher number of CD8+ TILs with statistical significance (p = 0.001). CD4+ TILs showed positive correlation with PD-L1 expression (p = 0.034). There were no associations between PD-L1 expression and FOXP3+ TILs. Microsatellite instability (MSI)-high status (p = 0.001; Odd ration 18.0; 95% CI = 4.3–74.8) was the strongest prognostic factor along with mucinous/poor cell differentiation, CD8 and right tumor location was associated with PD-L1 expression (p = 0.024, 0.035 and 0.033, respectively).
Conclusion
This study demonstrated that PD-L1 expression was associated with MSI-high, increased CD8+ TILs, mucinous and poor cell differentiation, and right-sided tumor location.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world and exhibits heterogeneous characteristics in terms of genomic alterations, expression signature and drug responsiveness (Citation1). Currently, the most reliable prognostic factor in colon cancer is the TNM stage. Although the prognosis for individual patients is dependent upon the extent of the disease, the prognosis of patients with the same stage varies widely, especially in those with stage II and III tumors (Citation2). Given a significantly reduced recurrence rate from adjuvant chemotherapy, postoperative chemotherapy has become the standard treatment for stage-III colon cancer (Citation3). However, the role of adjuvant chemotherapy is still controversial in stage-II colon cancer and is currently not a standard of care for this group of patients (Citation4). The overall survival rate for stage-II colon cancer after surgery alone ranges from 70% to 80%, and the risk of relapse ranges from 25% to 30% (Citation4). Although recommendations are given for consideration of adjuvant therapy in stage-II patients with high‐risk features, the current guideline admittedly states that the current definition of “high‐risk” stage-II colon cancer remains inadequate (Citation2). The limitation with TNM staging suggests the need to scrutinize beyond clinicopathologic features for recurrence prediction to search for a better prognostic marker for survival and recurrence.
Recently, immune checkpoint inhibitors have dramatically improved long-term prognoses in few malignancies such as malignant melanoma, lung cancer, and renal cell cancer which had insufficient prognosis with conventional treatment (Citation5–7). Interaction of the programmed cell death 1 (PD-1) on T cells, and its ligand PD-L1, which is expressed on tumor cells, and inflammatory immune cells including B cells, dendritic cells, and macrophages plays a crucial role in immune check point inhibition (Citation8). It is well known that the binding of PD-L1 on tumor cells to PD-1 on T cells suppresses T-cell–mediated anti-tumor immune response, which is called “immune tolerance” (Citation8,Citation9), and the blockade of this pathway gives a better prognosis in some malignancies. Microsatellite instability high (MSI-H) tumors have been identified as the best candidates for immunotherapy treatment, and a number of studies have evaluated the efficacy of anti-PD-L1 and anti-CTLA-4 monoclonal antibodies in this setting. Two phase-II trials (KEYNOTE-016 and KEYNOTE-164) and one phase-II trial (CheckMate-142) conducted in previously treated MSI-H metastatic CRC patients provided objective response rates (ORR) ranging from 28% to 52%, progression-free survival (PFS) rates ranging from 34% to 59%, and overall survival (OS) rates of 72% to 76% (Citation10–12).
It was reported that biologic behaviors of colon cancers are associated with a unique immune response of the colon cancer microenvironment, and the outcome is governed predominantly by immune responses at the primary tumor site (Citation13). This suggests that precise evaluation of local immune response could be useful for predicting prognosis or even have prognostic value superior to and independent of the TNM classification (Citation13).
In this study, we aimed to evaluate the relationship between PD-L1 expression in tumor cells and clinicopathological characteristics, including FOXP3+, CD8+ and CD4+ tumor-infiltrating lymphocytes (TILs) density, with survival.
Patients and methods
Patients and specimens
Between January 2011 and December 2014, 507 patients underwent curative resection of CRC at the Gil Medical Center, Gachon University. Of these patients, 342 patients were diagnosed as pathologic stage II and III colon cancer according to the American Joint Committee on Cancer staging system (Citation14). Twenty archival specimens per year were randomly selected according to their microsatellite status. The criteria for case inclusion were as follows: (Citation1) availability of formalin-fixed, paraffin-embedded tissue; (Citation2) a complete set of clinical and pathologic information such as age, gender, size of tumor, lymphatic invasion, vascular invasion, and perineural invasion were included; (Citation3) no prior history of any form of colitis during the past 1 year, (Citation4) no rectal cancer and (Citation5) no prior history of chemotherapy or radiation therapy. Among the 80 randomly selected archival specimens, 58 archival specimens fulfilled the inclusion criteria and were available for analysis.
Ethics statement
All the clinical specimens were approved by the clinical research ethics committee of the Gachon University, Gil Medical Center. Written informed consent was obtained from all patients according to the policies of the committee (IRB# GIRBA2216). Any information that could identify the patients was not included in this article. All methods were performed in accordance with the Helsinki guidelines and regulations.
Surveillance
Surveillance for recurrence following surgery is outlined as follows: physical examination, serum CEA (carcino-embryonic antigen), chest radiography, and spiral abdominal computed tomography were performed every 6 months for the first three years and annually thereafter. Other examinations, such as colonoscopy and abdominal ultrasound, were selected and performed every six to 12 months depending on the status of the patients. The median follow-up duration was 53.5 months (20.0–83.7 months).
Immunohistochemistry
After reviewing H&E slides of each tumor, a representative paraffin block that showed well-fixed, viable tumor was selected. Four consecutive 4 μm thick sections were cut with a microtome and transferred to poly-L-lysine-coated slides. The sections were dewaxed in xylene and rehydrated with 100% alcohol. Immunohistochemical staining was performed using Bond III automated IHC/ISH stainer (Leika Biosystems, Newcastle, UK). Antigen retrieval was done by heating in Epitope Retrieval Solution 1/2 (ER1/ER2) (Leika) for 20 minutes. Endogenous peroxidase activity was blocked by incubating the sections in 0.3% hydrogen peroxide for 10 minutes. The sections were incubated with primary antibodies [rabbit monoclonal antibody to PD-L1 (Abcam, ab205921, clone 28-8, 1:100 dilution), mouse monoclonal antibody to CD4 (Novocastra, ORG-8756, clone 1F6, 1:50 dilution), mouse monoclonal antibody to CD8 (Novocastra, NCL-L-CD8-4B11, clone 4B11), and mouse monoclonal antibody to FOXP3 (Abcam ab20034, clone 236 A/E7, 1:100 dilution)] for 15 minutes at 37 °C, followed by visualization with Bond Intesne Detection Kit (Leika). Each immunohistochemistry run contained a positive control (on-slide tonsil tissue) and a negative antibody control (buffer, no primary antibody).
PDL-1 expression
PD-L1 staining was defined as complete circumferential or partial linear plasma membrane staining at any intensity. Cytoplasmic staining was not considered positive. The percentage of viable tumor cells exhibiting positive-membrane staining in the entire specimen was measured and categorized as one of “less than 1%”, “1% to less than 5%”, “5% to less than 10%”, and “10% or greater” groups. Cases of 1% or greater of staining percentage were regarded as PD-L1-positive. The expression of PD-L1 in tumor cells were estimated using IHC method mentioned above.
Quantitative analysis of TILs
The number of TILs were counted manually using a DP71 Digital camera (Olympus, Japan), installed on an Olympus light microscope (Olympus BX51, Japan) and attached to a personal computer. Three sets of independent 200x microscopic fields (0.366 mm2/field) containing viable tumor and modest to dense lymphocytic infiltration were selected for each patient sample and for each of immunoantibody (CD4, CD8 and FOXP3). Each set was made to include identical microscopic field. After taking a microphotography, the number of the immunostained lymphocytes were counted and three sets of the ratios of the TIL numbers of CD4, CD8 and FOXP3 (CD4-positive lymphocytes/CD8-positive lymphocytes, CD4-positive lymphocytes/FOXP3-positive lymphocytes, CD8-positive lymphocytes/FOXP3-positive lymphocytes) were calculated. The evaluation of TILs was performed by one pathologist without knowledge of the clinicopathologic data. For data analysis, mean values were used.
Statistical analysis
Comparisons between groups were performed using the Pearson’s chi-square test. For all immunohistochemical markers, the cutoff for definition of subgroup was the mean value. The mean cut off values for TILs are shown in Supplementary Table 1. Survival curves were estimated using the Kaplan–Meier product-limit method, and the significances of differences between survival curves were determined using the log-rank test. For multivariate analysis the Cox regression model was used to identify independent prognostic factors for overall survival and recurrence. Differences with p value less than 0.05 were considered statistically significant. The overall survival was calculated from the date of colon resection and the date of death or the last follow-up, and disease-free survival was measured from the date of colon resection to the date of tumor recurrence or the last follow-up.
Results
Correlation of immunohistochemical variables with clinicopathologic features
All cases were classified according to their mean numbers into either low- and high-density groups for each marker: CD8+, CD4+, and FOXP3+. In a total of 58 colon cancer patients, the median age was 64 years (range, 35–84) and 58.6% were male. PD-L1 expression was significantly associated with right colon (p < 0.0001), tumor size (p = 0.044), mucinous differentiation (p = 0.009) and MSI-high cancers (p < 0.0001) (). Gender, lymphovascular invasion, KRAS mutation and EGFR expression were not statistically associated with PD-L1 expression. The representative TILs for MSI-high and microsatellite stable (MSS) cancer is shown in and .
Figure 1. Representative examples of immunohistochemical findings of intraepithelial infiltrating lymphocytes. Microsatellite instability high PD-L1 expression (A) CD4+ T cells (B), CD8+ T cells (C), FOXP3+ T cells (D) (magnification × 400).
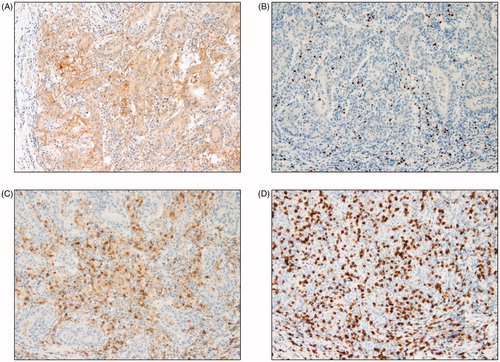
Figure 2. Representative examples of immunohistochemical findings of intraepithelial infiltrating lymphocytes. Microsatellite stable PD-L1 expression (A) CD4+ T cells (B), CD8+ T cells (C), FOXP3+ T cells (D) (magnification × 400).
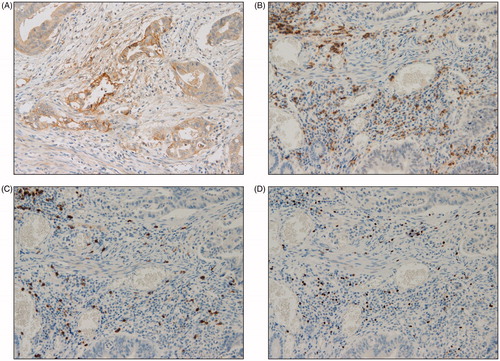
Table 1. Clinicopathologic features.
Correlation between PD-L1 expression and TILs
The correlations between PD-L1 expression in tumor cells and TILs are shown in . PD-L1 expression was evaluated in 58 tumors. PD-L1-positive tumors contained a higher number of CD8 positive TILs with statistical significance (p = 0.010). CD4 TILs showed positive correlation with PD-L1 expression (p = 0.034) A significant association between PD-L1 expression and FOXP3+ TILs was also shown (p value = 0.029) (). The ratios of CD4+/CD8+ TILs, CD4+/FOXP3+ TILs, and CD8+/FOXP3+ TILs were not significantly different between PD-L1-positive and -negative tumors (Supplementary Table S1 and Supplementary Figure S1).
Table 2. Correlation between tumor-infiltrating lymphocyte and PD-L1 expression.
Independent predictors of PD-L1 expression
A multivariate model was developed to test the independent prognostic factors and TIL densities, as proposed by Salama et al. (Citation15). The variables included this features were the densities of each T-cell marker with cell differentiation, MSI status, and tumor location. This analysis revealed that MSI-high status (p = 0.001; Odd ration 18.0; 95% CI = 4.3–34.8) was the strongest prognostic factor. It is interesting to note that mucinous histology, CD8 expression, and right tumor location were associated with PD-L1 expression (p = 0.024, 0.035, and 0.033 respectively). Expression of CD4 (p = 0.248) and FOXP3 (p = 1.555) was not significantly associated with PD-L1 expression ().
Table 3. Multivariate analysis on predictors of PD-L1 expression.
Overall survival
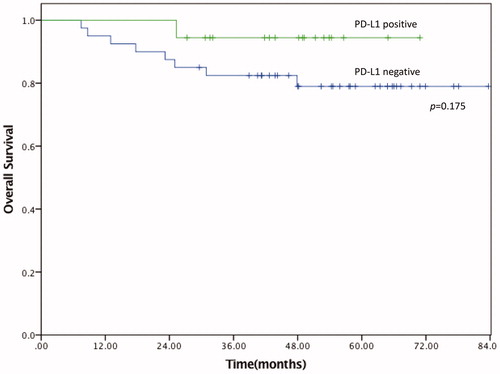
The overall survival 82.3% at 5- years for all patients. The forward conditional Cox regression model was used to delineate significant prognostic factors for survival. MSI-high status showed tendency toward better survival but did not showed significance (p = 0.059). Lymph node positivity was the only independent prognostic factor for overall survival. No significant advantage for overall survival was found for CD4 high or CD8 high groups (high-density groups). PD-L1 expression was not associated with increased overall survival (p = 0.175, , ) or disease free survival (p = 0.834, Supplementary Fig. S2).
Table 4. Multivariate analysis on predictors of survival.
Discussion
PD-L1 expression by tumor cells has been shown to provide a mechanism of immune evasion through downregulation of the active T-cell-mediated immune response (Citation16,Citation17). In this study, we demonstrate that PD-L1 expression is scarcely seen in sporadic colon cancer; however, it is associated with signatures MSI, mucinous/poor cell differentiation, right-sided colon, and frequent TILs. PD-L1 was not associated with overall survival in curatively resected stage II and III colon cancer.
To date, two different mechanisms of PD-L1 upregulation on tumor cells have been reported: innate immune resistance and adaptive immune resistance (Citation17,Citation18). The former refers to the upregulation of PD-L1 expression as a result of constitutive oncogenic signaling within tumor cells, as seen in ALK-rearranged and EGFR-mutant lung cancer (Citation17,Citation19,Citation20). By contrast, adaptive immune resistance refers to the induction of PD-L1 expression on tumor cells in response to local inflammatory signals produced by active anti-tumor immune response (cytotoxic T cell and/or Th1 pathway activation) (Citation17,Citation21). This in turn induces PD-1 expression on T cells (Citation22). When engaged by PD-L1 or other ligands, PD-1 inhibits kinases that are involved in T-cell activation through the phosphatase SHP250, which leads to the apoptosis of T cells, although additional signaling pathways are likely also induced (Citation17,Citation18,Citation23). Our findings suggest that upregulation of PD-L1 in colon cancer is probably due to an adaptive immune response with regards to frequent TILs expressing CD8, CD4, and FOXP3.
Recently, a trial found a significantly higher response rate and survival in colon cancer with MSI as compared with MSS (Citation24). The significance of MSI in identifying molecular profile-driven therapies in mCRC was recently elaborated in a phase-II trial enrolling 41 patients with treatment-refractory metastatic cancers stratified into three cohorts: MSI-high colon cancer (cohort A), MSS colon cancer (cohort B), and MSI-high non-CRC (cohort C) (Citation24). Treatment with the anti–PD-1 immune checkpoint inhibitor pembrolizumab (10 mg/kg intravenously every 14 days) showed significantly improved immune-related response rates and survival for cohorts A and C compared with cohort B. This study validated the concept that MSI high tumor and high tumor mutational burden can predict response to pembrolizumab in metastatic CRC (Citation24,Citation25). Predictive markers of anti-EGFR mAbs resistance has been extensively studied. Cetuximab or panitumumab are effective in 10–20% of unselected metastatic colorectal patients. KRAS mutations account for approximately 30–50% of patients who are not responsive. The oncogenic activation of BRAF affects the response to anti-EGFR mAbs in approximately 10% of mCRC. Other biomarkers, such as NRAS, PTEN and PI3K, may allow further selection of the appropriate patient group (Citation26).
Somatic mutations found in tumors can be recognized by the patient’s own immune system, and mismatch repair–deficient CRCs have ∼100 times as many somatic mutations as mismatch repair–proficient CRCs (Citation27). Two possible hypothesis explaining such event; (1) higher the mutational burden may result in the formation of more “neo-antigens” in microsatellite-unstable tumors, leading to a higher anti-tumor immune response (Citation17); (2) Mismatch repair–deficient cancers contain prominent lymphocyte infiltrates, a finding consistent with an immune response (Citation28). Upregulation of PD-L1 expression in tumor cells within this environment may lead to cascade of the active immune responses and more aggressive tumor biology (Citation17,Citation29). Although this study did not show an overall survival difference between the PD-L1-positive and PD-L1-negative groups, there was tendency toward worse survival outcome in PD-L1-negative group. This may be due to the small sample size. The correlation between mismatch-repair deficiency and PD-L1 expression in colon cancer supports the idea that immunogenic mismatch-repair deficiency cancers may acquire PD-L1 expression on the cancer cells to escape from the cytotoxic T-cell/Th1 immune response. Thus, leading to poor adaptive immune response and survival.
It is worth noting multivariate analysis revealed that increased CD8-positive TILs, and mucinous and poorly differentiated cell morphology, MSI, and right-sided tumor location were significant independent predictors of PD-L1 expression (). These results may insinuate the possibility that unique subgroups of colon cancer may arise within the DNA mismatch repair pathway of carcinogenesis, including a PD-L1-positive immune activated subtype, associated with increased TILs and adaptive immune resistance, and a PD-L1-negative immune-tolerant subtype (Citation17).
The prognostic role of nodal status was the only independent predictor of overall survival. PD-L1 expression was not correlated with significantly worse survival; however, there was a tendency toward prolonged survival and this finding should be interpreted with caution as our cohort had limited sample size (). In a recent report, PD-L1 expression was not correlated with prolonged survival in left-sided colon cancer and rectal cancer. However, right-sided colon cancer was associated with prolonged survival (Citation30). Although we did not statistical significance in overall survival due to small sample size, we are agreement with previous study that PD-L1 expression is associated with better survival (Citation30,Citation31).
We demonstrated that PD-L1 expression is associated with less aggressive cancer biology in colon cancer, which may result in better overall survival. Due to the small sample size our study did not reach statistical significance regarding overall survival. However, we demonstrated tendency toward better outcome (p = 0.175; ). This is in line with previous finding in colon cancer (Citation32,Citation33). The prognostic value of PD-L1 expression in colon cancer is highly debated. While some studies demonstrated with better outcome (Citation32), others have shown a worse outcome (Citation9,Citation31). Although our study is in agreement with Drosser et al. (Citation32), survival data should be interpreted with caution. In order to have a uniform study samples, we only included colon cancer and excluded rectal cancer. We believe rectal cancer is a difference disease with different tumor biology, treatment plan and prognosis. The discrepancy regarding overall survival with Masugi et al. (Citation9) may be also due to the time of patient recruitment. This study population was enrolled between 2011 and 2014. During this time, a considerable improvement in outcome was possible with addition of oxaliplatin. This contrast with Masugi et al. (Citation9) where more than 50% of patient population was diagnosed before 1999 and 5-FU was the main chemotherapeutic regimen at the time.
In conclusion, we demonstrate that PD-L1 expression is characteristic of MSI-high, with increased CD8-positive TILs, mucinous and poor cell differentiation, and right-sided tumor location, which being the independent predictors of high PD-L1 status.
Supplemental Material
Download PDF (280.4 KB)Disclosure statement
All of the listed authors claim no conflict of interest and financial disclosure.
Data availability statement
All data are available for disclosure.
Additional information
Funding
References
- Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47(2):127–41. doi:10.4143/crt.2015.060.
- Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al.; National Comprehensive Cancer Network. Localized colon cancer, version 3.2013 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(5):519–28. doi:10.6004/jnccn.2013.0069.
- Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9.
- Benson AB, 3rd;, Hamilton SR. Path toward prognostication and prediction: an evolving matrix. J Clin Oncol. 2011;29(35):4599–601. doi:10.1200/JCO.2011.37.8646.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate, I. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. doi:10.1056/NEJMoa1510665.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi:10.1056/NEJMoa1507643.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi:10.1056/NEJMoa1003466.
- Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813–20. doi:10.1158/1078-0432.CCR-15-1678.
- Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66(8):1463–73. doi:10.1136/gutjnl-2016-311421.
- Le DT, Kavan P, Kim TW, Burge ME, Van Cutsem E, Hara H, et al. Pembrolizumab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J Clin Oncol. 2018;36(15_suppl):3514.
- Diaz L, Marabelle A, Kim TW, Geva R, Van Cutsem E, André T, Ascierto, PA. et al. Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Ann Oncol. 2017;28(5 suppl):v128–v129.
- Thierry Andre SL, M, Wong H-J, Lenz F, Gelsomino M, Aglietta Michael Morse E Van Cutsem, et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): first report of the full cohort from CheckMate-142. J Clin Oncol. 2018;36(4_suppl):553–553.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Pages, F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139.
- Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002.
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–192. doi:10.1200/JCO.2008.18.7229.
- Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29(11):1433–1442. doi:10.1038/modpathol.2016.139.
- Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29(9):1104–1112. doi:10.1038/modpathol.2016.95.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi:10.1158/2159-8290.CD-13-0310.
- Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–4021. doi:10.1158/1078-0432.CCR-15-0016.
- Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48–61. doi:10.1016/j.cell.2014.12.033.
- Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–919. doi:10.1158/2159-8290.CD-15-0563.
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi:10.1158/1078-0432.CCR-13-3271.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596.
- Lin EI, Tseng LH, Gocke CD, Reil S, Le DT, Azad NS, Eshleman JR. Mutational profiling of colorectal cancers with microsatellite instability. Oncotarget. 2015;6(39):42334–42344. doi:10.18632/oncotarget.5997.
- Russo A, Rizzo S, Bronte G, Silvestris N, Colucci G, Gebbia N, et al. The long and winding road to useful predictive factors for anti-EGFR therapy in metastatic colorectal carcinoma: the KRAS/BRAF pathway. Oncology. 2009; 77( Suppl. 1):57–68. doi:10.1159/000258497.
- Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337.
- Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–2422. doi:10.1002/1097-0142(20010615)91:12<2417::AID-CNCR1276>3.0.CO;2-U.
- Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50(5):307–312. doi:10.1002/gcc.20854.
- Berntsson J, Eberhard J, Nodin B, Leandersson K, Larsson AH, Jirstrom K. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: relationship with sidedness and prognosis. Oncoimmunology. 2018;7(8):e1465165. doi:10.1080/2162402X.2018.1465165.
- Wyss J, Dislich B, Koelzer VH, Galvan JA, Dawson H, Hadrich M, Inderbitzin D, et al. Stromal PD-1/PD-L1 expression predicts outcome in colon cancer patients. Clin Colorectal Cancer. 2019;18(1):e20-e38. doi:10.1016/j.clcc.2018.09.007. Epub 2018 Sep 21.
- Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49(9):2233–2242. doi:10.1016/j.ejca.2013.02.015.
- Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. doi:10.1186/s12943-016-0539-x.