Abstract
Objective
To evaluate the efficacy and safety of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors combined with endocrine therapy (ET) for hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER-2−) advanced breast cancer (ABC) patients.
Methods
We searched clinical trials of CDK4/6 inhibitors combined with ET and calculated the clinical outcomes.
Results
HR+/HER-2− ABC patients treated with CDK4/6 inhibitors combined with ET had significantly prolonged progression-free survival (PFS) and improved objective response rate (ORR) and clinical benefit rate (CBR).
Conclusions
CDK4/6 inhibitors combined with ET can bring more clinical benefits to ABC patients, and the safety profile is acceptable.
Background
Breast cancer is the most frequently diagnosed malignant cancer among women worldwide, and it accounts for 25.1% of all tumors (Citation1). It is also the leading cause of cancer-related death in women (Citation2). Approximately 5% of breast cancer patients have distant metastases at the time of diagnosis (Citation3). Approximately 30–40% of early-stage patients eventually develop advanced breast cancer (ABC) (Citation4). Every year, approximately half a million people die from ABC worldwide (Citation5). Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER-2−) breast cancer is the most common breast cancer subtype, and HR+/HER-2− patients account for approximately 60% of all ABC patients (Citation3). In the past, international guidelines unanimously recommended endocrine therapy (ET) as the initial treatment for HR + ABC patients (Citation6,Citation7), which has revolutionized the prognosis and overall treatment strategy for HR+/HER-2− ABC. However, the majority of patients progress during ET due to acquired resistance, and a proportion of patients may fail to respond to initial therapy due to primary resistance (Citation8). Therefore, resistance is a challenge of ET treatment in HR + ABC patients.
At present, the US Food and Drug Administration (FDA) has approved four cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors for medical use in the United States. Palbociclib, ribociclib, and abemaciclib were approved to be combined with endocrine drugs for clinical treatment of HR+/HER-2− ABC patients. Trilaciclib was approved for bone marrow protection in patients undergoing chemotherapy of small cell lung cancer. Palbociclib has been approved for first- and second-line treatment of HR+/HER-2− ABC patients. Ribociclib and abemaciclib have been approved for first- and back-line treatment of HR+/HER-2− ABC patients (Citation9,Citation10). CDK4/6 inhibitors combined with endocrine drugs can increase the treatment response rate and prolong disease control time in HR+/HER-2− ABC patients (Citation11). Moreover, CDK4/6 inhibitors are oral agents that are more tolerable by patients than traditional intravenous chemotherapeutic agents. CDK4/6 inhibitors combined with endocrine drugs can improve clinical outcomes; however, this approach may increase the incidence of adverse events (AEs) and the financial burden on patients. Therefore, this study systematically evaluated the efficacy and safety of CDK4/6 inhibitors combined with ET and endocrine monotherapy for HR+/HER-2− ABC patients to provide accurate evidence for clinical application.
Methods
Search strategy
Databases including PubMed, Embase, The Cochrane Library, and Web of Science were systematically searched by 2 researchers (Yongyan Li and Li Li) for published randomized controlled trials (RCTs) up to July 2020. RCTs involving CDK4/6 inhibitors combined with ET for the treatment of ABC patients were collected. In addition, referenced papers were included to expand the search scope. The search strategy consisted of a combination of variable keywords: breast neoplasm, breast cancer, breast tumor, abemaciclib, LY2835210, LY2385219, Verzenio, ribociclib, LEE011, palbociclib, Ibrance, PD 0332991, PD0332991, PD-0332991, endocrine therapy, letrozole, and fulvestrant. The proposed MeSH terms were as follows: #1 breast neoplasm OR breast cancer OR breast tumor; #2 palbociclib OR Ibrance OR PD 0332991 OR PD0332991 OR PD-0332991; #3 ribociclib OR LEE011; #4 abemaciclib OR abemaciclib OR LY2835210 OR LY2385219; #5 endocrine therapy OR letrozole OR fulvestrant; #6: #2 OR #3 OR #4; and #7: #1 AND #5 AND #6.
Eligibility
The inclusion criteria were as follows: (1) prospective phase II or III RCTs; (2) the trial subjects were patients with histologically and/or cytologically confirmed HR+/HER-2− ABC; (3) the patients in experimental intervention groups were treated with CDK4/6 inhibitors (palbociclib, ribociclib, or abemaciclib) plus ET (letrozole, fulvestrant, tamoxifen (TAM), nonsteroidal aromatase inhibitors (NSAIs)); (4) the trial analyzed the efficacy and safety of CDK4/6 inhibitors combined with ET versus endocrine monotherapy and included the following measurements: progression-free survival (PFS), overall survival (OS), objective response rate (ORR), clinical benefit rate (CBR) and the rate of AEs; and (5) language restriction-English only. Where multiple articles analyzed the same trial, the most recent data were used.
Data extraction
Relevant information was extracted independently by two researchers (Yongyan Li and Li Li) according to a predefined form. Data extraction included (1) the basic characteristics of the included RCTs, namely, study ID, first author, publication year, published journal, and clinical stage, and (2) outcome indicators, namely, PFS, OS, ORR, CBR and the rate of grade 3–5 AEs. Data from relevant appendices were also extracted. All disagreements were resolved by discussion or negotiation with an arbitrator (Qin Li).
Statistical analysis
To evaluate the risk of bias in the RCTs, two researchers (Yongyan Li and Li Li) independently assessed the risk of bias in the included studies using the Cochrane Reviewer Handbook and cross-checked the results.
Forest plots were generated with Stata 16 (Stata Corporation, College Station, TX, USA). We calculated the pooled hazard ratio (HR) and 95% CI for PFS, the pooled odds ratio (OR), and 95% CI for the ORR, the CBR and the rate of grade 3–5 AEs. I-squared (I2) tests were used to evaluate the heterogeneity between trials. Either p < 0.1 or I2 > 50% indicated the existence of heterogeneity, and the randomized-effects model was used. Otherwise, the fixed-effects model was used. p < 0.05 was considered statistically significant.
Results
Main characteristics and the risk of bias in the included studies
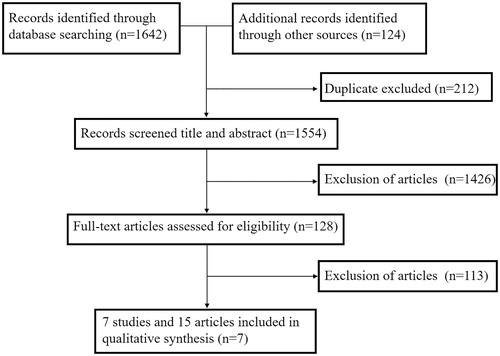
According to the search strategies, 1,766 records were retrieved from PubMed, Embase, The Cochrane Library, and Web of Science. After removing duplicates and irrelevant records, 7 RCT records containing 4,415 patients were ultimately included in the qualitative synthesis () (Citation12–26). Four RCTs enrolling 1,473 patients were available at the cutoff date for analysis of OS. The main characteristics of these included studies are listed in . Full details about the risk of bias in RCT studies are shown in .
Table 1. Main characteristics of included studies.
Table 2. Risk of bias: a summary table for each risk of bias item for each study.
Efficacy
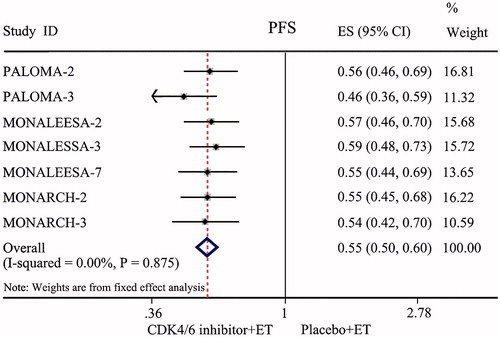
All seven RCTs enrolling 4,415 patients were available for the analysis of PFS at the end of the observation period. The fixed-effects model was used because there were no heterogeneities (I2 = 0.00%, p = 0.857) between these data. The pooled data showed that the groups treated with CDK4/6 inhibitors (palbociclib, ribociclib or abemaciclib) combined with ET had a prolonged PFS compared with the endocrine monotherapy groups (HR = 0.55, 95% CI: 0.50–0.60, p < 0.001; ).
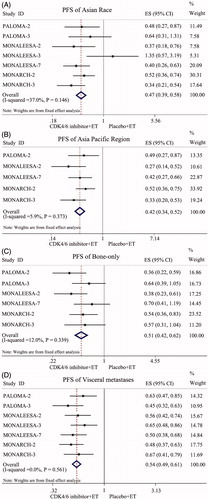
Subgroup analysis of PFS, according to stratification factors, confirmed a consistent conclusion across some subgroups that CDK4/6 inhibitors could decrease the incidence of disease progression. All 7 RCTs enrolling 4,415 patients were available for the analysis of PFS in Asian race subtypes. The pooled data showed that the CDK4/6 inhibitor combined with ET groups had a prolonged PFS compared with the endocrine monotherapy groups in Asian patients (HR = 0.47, 95% CI: 0.39–0.58, p < 0.001; ). Five RCTs enrolling 3,168 patients were available for the analysis of PFS in the Asia-Pacific region subtype. The pooled data showed that the combination treatment groups had a prolonged PFS compared with endocrine monotherapy groups in Asia-Pacific region patients (HR = 0.42, 95% CI: 0.34–0.52, p < 0.001; ). Six RCTs enrolling 3,894 patients were available for the analysis of PFS in the bone-only metastatic subtype. The pooled data showed that the groups treated with CDK4/6 inhibitors combined with ET had a prolonged PFS compared with the endocrine monotherapy groups of bone-only metastatic subtype patients (HR = 0.51, 95% CI: 0.42–0.62, p < 0.001; ). All 7 RCTs enrolling 4415 patients were available for the analysis of PFS in the visceral metastatic subtype. The pooled data showed that the combination treatment groups also had a prolonged PFS compared with the endocrine monotherapy groups in visceral metastasis subtype patients (HR = 0.54, 95% CI: 0.49–0.61, p < 0.001; ).
Figure 3. Forest plot of comparison: PFS of subgroup. (A) Asian Race Subtype; (B) Asia Pacific Region Subtype; (C) Bone-only metastases Subtype; (D) Visceral metastases Subtype.
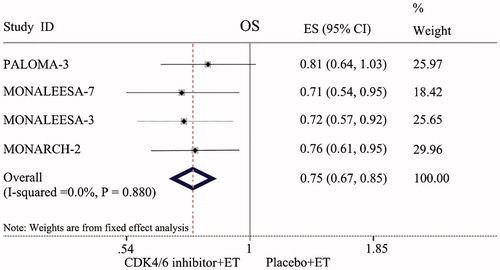
Our analysis showed that the groups treated with CDK4/6 inhibitors combined with ET had a prolonged OS compared with the endocrine monotherapy groups (HR = 0.75, 95% CI: 0.67–0.85, p < 0.001; ).
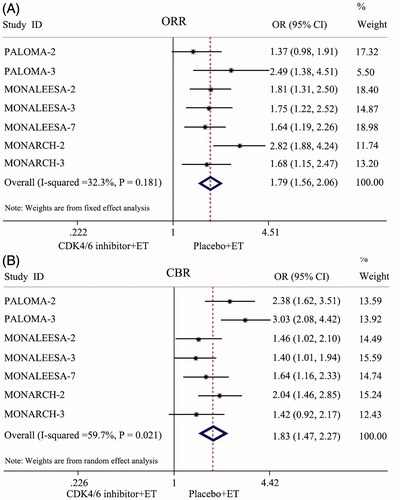
All seven RCTs enrolling 4,415 patients were available for the analysis of the ORR at the end of the observation period. Compared with patients treated with endocrine monotherapy, the ORR probability tended to be increased in patients who were treated with CDK4/6 inhibitors plus ET (OR = 1.79, 95% CI: 1.56–2.06, p < 0.001; ).
All seven RCTs enrolling 4,415 patients were available for the analysis of the CBR at the end of the observation period. Because the data heterogeneity of the CBR was apparent (I2 = 59.7%, p = 0.021), a random-effects model was used. The results of the meta-analysis showed that the CDK4/6 inhibitor and ET combination groups had a higher CBR than the endocrine monotherapy groups (OR = 1.83, 95% CI: 1.47–2.27, p < 0.001; ).
Adverse effects (AEs)
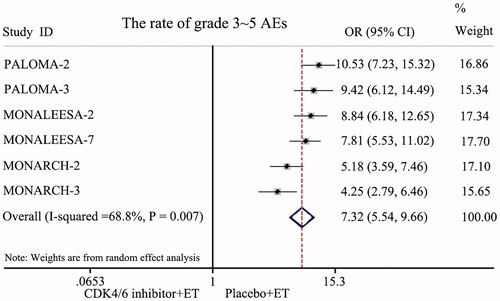
All seven RCTs reported safety data. The most common AEs of the combined treatment groups were neutropenia, leukopenia, fatigue, nausea, arthralgia, diarrhea, increased ALT, increased AST, etc. Six RCTs enrolling 3,671 patients reported the incidence of grade 3–5 AEs. A randomized-effects model was used because of the existence of heterogeneity (I2 = 68.8%, p = 0.007). The rate of grade 3–5 AEs in the CDK4/6 inhibitor and ET combination groups was significantly higher than in the endocrine monotherapy groups (HR = 7.32, 95% CI: 5.54–9.66, p < 0.001; ). However, the symptoms of the AEs could be controlled with simple treatments.
Discussion
In the past, ET was the standard treatment for postmenopausal HR+/HER-2− ABC patients. Aromatase inhibitors (AIs) are the most frequently used first-line options for postmenopausal women with HR + advanced or metastatic breast cancer. Tamoxifen (TAM) or AI plus ovarian suppression is the recommended therapy for premenopausal women (Citation27). However, drug resistance and disease progression occur in the vast majority of patients after a period of ET. The emergence of CDK4/6 inhibitors has changed the therapeutic strategy for HR+/HER-2− ABC patients. A number of large clinical trials have confirmed that compared with endocrine monotherapy, a CDK4/6 inhibitor combined with ET could significantly improve efficacy indexes such as PFS, OS, the ORR, and the CBR. Based on these clinical trials, the FDA has approved the use of a CDK4/6 inhibitor in combination with ET for patients with advanced or metastatic HR+/HER-2− breast cancer. Currently, worldwide, the combination of CDK4/6 inhibitors and ET is widely used as a first- and second-line treatment for patients with HR+/HER-2− advanced or metastatic breast cancer without the presence of visceral crisis (Citation28).
All seven RCTs included in this study were large double-blind, double-arm clinical trials, and intention-to-treat (ITT) analysis was used on the data of those lost to follow-up. The risk of selection bias was small, and the results were reliable. The results of this meta-analysis showed that compared with those treated with placebo combined with ET, HR+/HER-2− ABC patients treated with CDK4/6 inhibitors combined with ET had significantly prolonged PFS (HR = 0.55, 95% CI: 0.50–0.60, p < 0.001) and markedly improved ORR and CBR (OR = 1.79, 95% CI: 1.56–2.06, p < 0.001; OR = 1.83, 95% CI: 1.47–2.27, p < 0.001). This result indicated that CDK4/6 inhibitors plus ET could significantly improve the short-term prognosis in HR+/HER-2− ABC patients. Further analysis of OS in PALOMA-3, MONALEESA-3, MONALEESA-7, and MONARCH-2 also confirmed that compared with those treated with endocrine monotherapy, HR+/HER-2− ABC patients treated with CDK4/6 inhibitors combined with ET also had significantly prolonged OS (HR = 0.75, 95% CI: 0.67–0.85, p < 0.001). This revealed that the long-term efficacy was also enhanced. Subgroup analysis was conducted according to race, region, and metastatic sites. The results showed that CDK4/6 inhibitors combined with ET could significantly improve the PFS of HR+/HER-2− ABC patients in the Asian race, Asia-Pacific region, bone-only metastatic, and visceral metastatic subtypes. The study revealed that CDK4/6 inhibitors were also applicable to Asian race patients, Asian Pacific region patients, bone-only metastatic patients, and visceral metastatic patients. For patients with a heavy tumor burden, such as bone metastasis and visceral metastasis, it was found that CDK4/6 inhibitors plus ET can still achieve satisfactory therapeutic effects.
For early-stage breast cancer (EBC) patients, positive lymph nodes, large tumor size, high histological grade, high Ki-67 expression, and heavy tumor load were important risk factors for postoperative recurrence and metastasis. These factors seriously affect the survival and quality of life of EBC patients after surgery. MonarchE (Citation29) indicated that in HR+/HER-2− EBC patients with positive nodes and high risk, abemaciclib plus ET still demonstrated superior invasive disease-free survival (IDFS) versus ET alone (92.2% and 88.7%). However, the PALLAS trial (Citation30) reached the opposite conclusion. Compared with endocrine monotherapy, palbociclib combined with ET did not improve IDFS in HR+/HER-2− EBC patients (88.2% vs 88.5%). This opposite conclusion may be due to differences in the baseline characteristics of the enrolled patients, differences in the type of CDK4/6 inhibitors, differences in the administered dose, and a shorter follow-up time. Long-term follow-up of the MonarchE and PALLAS studies is needed to evaluate whether the benefit of using CDK4/6 inhibitors is limited to first-line treatment in ABC patients.
It is worth noting that estrogen receptor and progesterone receptor expression are decisive factors in the efficacy of ET treatment in breast cancer. All the patients enrolled in our study were HR+/HER-2− ABC patients and our study revealed that HR+/HER-2− ABC patients could benefit from the combination of CDK4/6 inhibitors and ET. CDK4/6 inhibitors may also have an effect in HR − or HER-2+ ABC patients or triple-negative breast cancer (TNBC) patients. MonarcHER (Citation31) confirmed that in HR+/HER-2+ ABC patients, the combination of abemaciclib, fulvestrant, and trastuzumab could significantly improve PFS compared with standard chemotherapy plus trastuzumab. This indicates that CDK4/6 inhibitors may also have a role in the treatment of HER-2+ ABC patients. Some basic scientific studies have suggested that CDK4/6 inhibitors can block the proliferation of TNBC tumor cells. Studies have found that TNBC cell lines with high Rb gene expression are sensitive to palbociclib because this drug can arrest those cells in the G1 phase (Citation32). However, only 1 clinical trial has been completed assessing clinical benefits and toxicities of CDK4/6 inhibitors in TNBC patients (NCT01037790). This trial enrolled 4 Rb-positive TNBC patients treated with palbociclib monotherapy. Three patients experienced progressive disease, and 1 patient experienced stable disease, indicating insufficient efficacy. Encouragingly, a new study suggested that the combination of trilaciclib and chemotherapy could significantly reduce the damage of bone marrow cells and enhance immune system function of TNBC patients. It was meaningful to increase the cycles of chemotherapy and improve the quality of survival for TNBC patients (Citation33). There are ongoing clinical trials involving TNBC patients treated with CDK4/6 inhibitors as a monotherapy or combined with chemotherapy drugs or androgen inhibitors (NCT03805399, NCT02605486, NCT03519178). The effectiveness of CDK4/6 inhibitors alone or in combination with other drugs in HR − or TNBC patients is worth further study.
The safety analysis showed that compared with placebo combined with ET, there was an increased rate of grade 3–5 AEs when CDK4/6 inhibitors were combined with ET (HR = 7.32, 95% CI: 5.54–9.66, p < 0.001). The most common AEs occurring with CDK4/6 inhibitor treatment were hematological AEs, such as neutropenia and leukopenia, most of which occurred in palbociclib and ribociclib trials. Myelosuppression caused by chemotherapy drugs always results from DNA damage and apoptosis, while myelosuppression caused by CDK4/6 inhibitors is due to cell cycle arrest (Citation34). Therefore, neutropenia and leukopenia caused by CDK4/6 inhibitors are reversible after drug treatment is interrupted or decreased. The common gastrointestinal system AEs occurring with CDK4/6 inhibitor treatment were diarrhea and nausea, most of which occurred in abemaciclib trials. Diarrhea and nausea could be well controlled by symptomatic treatments. It is important to note that diarrhea increases the risk of infection. If diarrhea is accompanied by neutropenia, it may have serious consequences for patients. Therefore, it is important that clinicians conduct rigorous detection and timely symptomatic treatment. Although CDK4/6 inhibitors can increase the rate of grade 3 ∼ 5 AEs, the associated risks are controllable. Thus, these inhibitors may still be considered a preeminent option for ABC patients.
There are several meta-analyses on the topic we have covered here. One meta-analysis (Citation35), published in 2017, summarized the efficacy and safety of 5 clinical trials, PALOMA-1, PALOMA-2, PALOMA-3, MONARCH-2, and MONALEESA-2. Another meta-analysis (Citation36), published in 2018, summarized the efficacy and safety of 8 clinical trials, PALOMA-1, PALOMA-2, PALOMA-3, MONARCH-2, MONARCH-3, MONALEESA-2, MONALEESA-3 and MONALEESA-7. Both articles included PALOMA-1, a phase II clinical trial. All the trials included in our research were phase III clinical trials and had a similar number of participants. Drug dose selection was carried out on the basis of phase II trials and had lower heterogeneity and more comparability among trials. In addition, several clinical trials conducted final or extended analyses in 2019 or 2020 and updated the PFS and grade 3 ∼ 5 AE data. Therefore, the results analyzed in our article are more in line with the actual results. Furthermore, we analyzed the OS of PALOMA-3, MONALEESA-3, MONALEESA-7, and MONARCH-2, demonstrating that the combination of CDK4/6 inhibitors and ET could significantly improve both short-term and long-term prognosis versus that with placebo combined with ET.
Although CDK4/6 inhibitors have achieved success in clinical treatment, acquired resistance should not be ignored (Citation37). The specific mechanism of resistance is incompletely understood. The absence of Rb function (Citation38), CDK6 amplification (Citation39), overactivation of the cyclin E-CDK2 axis, amplification of the CCNE1 gene (Citation40), and absence of FAT1 function (Citation41) have been considered to be potential mechanisms of CDK4/6 inhibitor resistance.
At present, the use of CDK4/6 inhibitors combined with ET for HR+/HER-2− ABC patients is a research “hot spot” and the therapy is gradually being applied in the clinic. Our study revealed that compared with endocrine monotherapy or placebo combined with ET, combining CDK4/6 inhibitors with ET significantly improved the therapeutic effect. Although the rate of high-grade AEs increased with CDK4/6 inhibitor treatment, the AEs could be well controlled by symptomatic treatments such as anti-diarrhea treatment, antiemetic treatment, and diet regulation. Investigating mechanisms of CDK4/6 inhibitor resistance, identifying biomarkers related to efficacy, optimizing drug delivery strategies, and matching the best combination regimens are future research directions.
Author contributions
Qin Li contributed to the design of the study and was responsible for the integrity of the data and the accuracy of the data analysis. Li Li, Yongyan Li, and Haifang Yang collected the clinical and pathological data. Yongfu Li and Qi Du were responsible for the statistical analysis. Yongyan Li wrote the manuscript and prepared the figures and tables. All authors contributed to the review and approved the paper. All authors agreed to be accountable for the content of this paper.
Declaration of interest
The authors have no conflicts of interest to disclose.
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43–46. doi:10.7314/apjcp.2016.17.s3.43.
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313.
- Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):11–19. doi:10.1093/annonc/mds232.
- Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005-2015). Breast. 2018;39:131–138. doi:10.1016/j.breast.2018.03.002.
- Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep. 2017;7:45411. doi:10.1038/srep45411.
- Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–1657. doi:10.1093/annonc/mdy192.
- Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34(25):3069–3103. doi:10.1200/JCO.2016.67.1487.
- Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: current evidence and future directions. World J Clin Oncol. 2014;5(5):990–1001. doi:10.5306/wjco.v5.i5.990.
- Rinnerthaler G, Gampenrieder SP, Greil R. ASCO 2018 highlights: metastatic breast cancer. Mag Euro Med Oncol. 2018;11(4):276–279. doi:10.1007/s12254-018-0450-9.
- Heptinstall AB, Adiyasa I, Cano C, Hardcastle IR. Recent advances in CDK inhibitors for cancer therapy. Future Med Chem. 2018;10(11):1369–1388. doi:10.4155/fmc-2017-0246.
- Murphy CG. The role of CDK4/6 inhibitors in breast cancer. Curr Treat Options Oncol. 2019;20(6):52. doi:10.1007/s11864-019-0651-4.
- Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303.
- Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi:10.1007/s10549-018-05125-4.
- Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi:10.1056/NEJMoa1505270.
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-0.
- Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi:10.1056/NEJMoa1810527.
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi:10.1056/NEJMoa1609709.
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi:10.1093/annonc/mdy155.
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi:10.1200/JCO.2018.78.9909.
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi:10.1056/NEJMoa1911149.
- Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi:10.1016/S1470-2045(18)30292-4.
- Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi:10.1056/NEJMoa1903765.
- Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi:10.1200/JCO.2017.73.7585.
- Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116. doi:10.1001/jamaoncol.2019.4782.
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi:10.1200/JCO.2017.75.6155.
- Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi:10.1038/s41523-018-0097-z.
- Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline update on ovarian suppression. J Clin Oncol. 2016;34(14):1689–1701. doi:10.1200/JCO.2015.65.9573.
- Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–1649. doi:10.1016/j.annonc.2020.09.010.
- Johnston S, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM. et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–3998. doi:10.1200/JCO.20.02514.
- Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–222. doi:10.1016/S1470-2045(20)30642-2.
- Tolaney SM, Wardley AM, Zambelli S, Hilton JF, Troso-Sandoval TA, Ricci F, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(6):763–775. doi:10.1016/S1470-2045(20)30112-1.
- Liu CY, Lau KY, Hsu CC, Chen JL, Lee CH, Huang TT, et al. Combination of palbociclib with enzalutamide shows in vitro activity in RB proficient and androgen receptor positive triple negative breast cancer cells. PLoS One. 2017;12(12):e0189007. doi:10.1371/journal.pone.0189007.
- Tan AR, Wright GS, Thummala AR, Danso MA, Popovic L, Pluard TJ, et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019;20(11):1587–1601. doi:10.1016/S1470-2045(19)30616-3.
- Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;22(8):2000–2008. doi:10.1158/1078-0432.CCR-15-1421.
- Messina C, Messina M, Zanardi E. Risks and benefits from CDK inhibitors for advanced HR + Her 2- breast cancer. Ann Oncol. 2017;28(12):3099–3100. doi:10.1093/annonc/mdx530.
- Messina C, Cattrini C, Buzzatti G, Cerbone L, Zanardi E, Messina M, et al. CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat. 2018;172(1):9–21. doi:10.1007/s10549-018-4901-0.
- Choo JR, Lee SC. CDK4-6 inhibitors in breast cancer: current status and future development. Expert Opin Drug Metab Toxicol. 2018;14(11):1123–1138. doi:10.1080/17425255.2018.1541347.
- Dean JL, McClendon AK, Hickey TE, Butler LM, Tilley WD, Witkiewicz AK, et al. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle. 2012;11(14):2756–2761. doi:10.4161/cc.21195.
- Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29(28):4018–4032. doi:10.1038/onc.2010.154.
- Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(8):2301–2313. doi:10.1158/0008-5472.CAN-15-0728.
- Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34(6):893–905. doi:10.1016/j.ccell.2018.11.006.