Abstract
Despite the incorporation of trastuzumab biosimilars (to treat HER2-positive breast cancer) in clinical practice guidelines, gaps remain such as patient and clinician education. We hosted a webinar comprised of a panel of biosimilars experts, oncologists, pharmacist, infusion nurse, and a patient advocate. The outcomes of the webinar include audience responses to pre- and post-webinar questionnaires, educational benefits, real-time opportunities to ask questions, and a recording. Education needs to be tailored to the needs of both, patients and clinicians.
In the last decade, the Federal Drug Administration (FDA) has approved five trastuzumab biosimilars for treating early-stage and metastatic HER2-positive breast cancer, as alternatives to trastuzumab biologic. The FDA defines biosimilars as highly similar (but not identical) to the reference product in terms of safety, efficacy, and quality (Citation1). A phase 3 and extension study conducted with 347 patients with HER2-positive breast cancer, found that 3-year event-free survival was higher for a trastuzumab biosimilar compared to reference medication, and cardiotoxicity was similar (Citation2). Studies highlight the importance of monitoring critical quality attributes to ensure consistent quality of trastuzumab biosimilars, given their potential to improve accessibility to trastuzumab therapy with no compromise to safety and efficacy (Citation3).
American Society of Clinical Oncology has been proactive in keeping information up-to-date and creating opportunities and resources for clinician-facing education on biosimilars (Citation4,Citation5). A Continuing Medical Education (CME)-certified educational initiative for cancer care clinicians, comprised of 3 live meetings (in conjunction with ASCO meetings) and an online course, was developed and implemented in 2017–2018. Metrics showed that the initiative improved clinical knowledge, awareness, and confidence regarding biosimilars. However, the authors suggest that more education is needed in this dynamic domain (Citation6).
In 2020, ASCO published updated guidelines for the selection of optimal adjuvant chemotherapy for breast cancer, highlighting that clinicians may offer any approved and available formulations of trastuzumab, including biosimilars (Citation7). A 2022 ASCO expert panel update on the use of biosimilars in oncology highlights the remaining gaps including variance in use and suggests there is room for improvement in the uptake of these lower-cost alternatives (Citation8). This is the case despite active efforts to address safety concerns by ensuring a rigorous regulatory approval process by the FDA along with thorough preclinical and clinical evaluation (Citation9).
Along with continued clinician-facing education, patient knowledge and comprehension regarding biosimilars have been an area that has received little attention. Survey research suggests that non-medical switching of patients to trastuzumab biosimilars has contributed to patient distress and highlighted knowledge gaps. Additionally, patients and oncologists diverge in their perspectives about switching (Citation10). These findings highlight the need for patient-facing education, as well as examination and improvement of patient-clinician communication on this topic.
Although there has been a shift to engage patients in conversations regarding solutions that pertain to them, historically, patient perspectives and lived experiences have been left out (Citation11). Triangulating perspectives from all stakeholders in a problem space (Citation12), especially for patients given they are directly impacted, is critical to appropriately address knowledge gaps, information needs, and mitigating avoidable distress. In other words, to inform solutions in care delivery that center on the patient, it is critical to include the experiences and perspectives of patients alongside the perspectives of clinicians (and other relevant roles).
As part of a project to develop an informational toolkit on trastuzumab biosimilars, we hosted a panel webinar to share consumable information, identify knowledge gaps, and elicit experiences from multiple perspectives. Our intended audience included patients and cancer care clinicians. The objective of the webinar was three-fold: to (1) provide up-to-date information on the science of trastuzumab biosimilars in a consumable manner; (2) represent informed perspectives, through targeted discussion, from multiple stakeholder roles, including a patient advocate, clinicians, and administrative decision makers on communication regarding trastuzumab biosimilars and treatment changes; (3) create a video informational resource, and (4) inform future work by identifying gaps in knowledge and outstanding questions.
Organization and planning
Our goal was to include multiple stakeholders (administrator, oncologist, pharmacist, infusion nurse, and patient advocate). One oncologist (Dr. Lustberg) and the patient advocate (Ms. Johnson) are members of the immediate project team. All additional manuscript authors took active roles in planning, promoting, and executing the webinar. We recruited additional panel participants through our professional networks, initially reaching out over email, followed by scheduling a phone conversation to answer questions. Dr. Lyman and Dr. Harvey have both administrative experience by serving on the American Society of Clinical Oncology (ASCO) Task Force on Biosimilars and clinical experience associated with trastuzumab biosimilars. An infusion nurse (Ms. Valero) has extensive clinical experience working with underserved patients receiving chemotherapy (including trastuzumab) infusions at the University of Illinois Hospital. Participants outside of the immediate project team received an honorarium upon the completion of the webinar. The resulting panel composition is represented in .
Table 1. Panel composition – names, affiliations, role on the panel, and biographies.
We identified the following learning objectives for the webinar: (1) improve understanding of trastuzumab biosimilars, including efficacy and safety; (2) gain an awareness of existing knowledge gaps from multiple perspectives; (3) appreciate the value of lived experiences of patient-clinician communication regarding cancer treatment changes. We also developed targeted questions to be directed to the panel and proposed which panelist might be in a position to respond to each. These questions were informed by knowledge gaps identified in datasets comprised of patient and oncologist surveys (Citation10,Citation13). We shared an agenda (including timing) along with the predetermined questions with panelists several days prior to the webinar. We allotted 60 minutes for discussion to engage panelists and to elicit audience questions.
Promotion
The project team developed a promotional flyer () for the webinar to be shared on social media. We made a conscious decision to differentiate the flyer from the standard template of the webinar host university, the University of Illinois at Chicago. The flyer design included a project logo and an overview. The University of Illinois Cancer Center (UICC) provided support by promoting the webinar formally through email and on their website (alongside other UICC events). The project team posted the flyer repeatedly and daily on social media (Twitter), using the hashtag #bcsm.
Execution
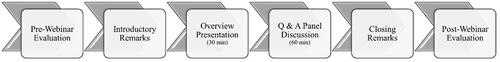
The webinar took place on Wednesday, October 20, 2021, 11 AM – 12:30 PM CST on Zoom. We had 15 audience members, which is 39% (15/38) of all registrants. The event began with an introduction by Dr. Papautsky (first author of this paper), followed by Dr. Lyman’s talk, and a discussion that included predetermined and audience questions. and represent the sequence of activities during the webinar and a screenshot of the panelists and one of Dr. Lyman’s presentation slides, respectively. Dr. Papautsky assured adherence to the timing in the agenda. She monitored chat and texted audience questions to Dr. Lustberg. In , we provided the questions (along with the source) and the corresponding responses by the panelists. At the end of the panel, all panelists provided final thoughts for steps forward:
Table 2. Discussion questions and responses.
“…sharing what questions patients should ask, patients sharing their experience with other patients.” (Ms. Johnson)
“For nursing, I think it’s important to keep educating ourselves on the information, and just keep being open to sharing that with patients…Say to patients: ’Just keep asking questions.’” (Ms. Valero)
“Use your pharmacist…get them out of their room, engage them, everyone - doctors, nurses, patients.” (Dr. Harvey)
“Knowledge is power, and knowledge requires communication. If the doctor’s not hearing from you about a problem, and then conveying that to appropriate channels, then we don’t learn and we don’t improve.” “There is no bad question and no complaint that shouldn’t be considered. No matter how much you think it isn’t a problem, share it, and, you know, discuss it.” (Dr. Lyman)
Evaluation
We developed pre- and post-webinar evaluation questions for the audience to characterize attendee background and their knowledge gaps and to identify which knowledge gaps remain, as a function of potentially missing content in the webinar or content that needs to be presented in a more comprehendible manner. We used quick response (QR) codes presented on a Microsoft Powerpoint slide to share a link to the pre- and post- webinar questions hosted on Poll Everywhere (polleverywhere.com), an online audience response system. Specifically, the QR codes were shared at the beginning and at the end of the webinar. Dr. Papautsky reminded the audience verbally and over chat to complete the questions.
Pre- and Post-Webinar evaluation results
Approximately 15 participants attended the webinar. Not all participants responded to the pre- and post-webinar questions. Of 9 respondents, 5 were patients, and the rest were caregivers, pharmacist, oncologists, and nurses. Eight respondents generated 17 adjectives associated with trastuzumab biosimilars, with the most frequently (twice each) used being: breast cancer, effective, and less expensive. See for a word cloud comprised of the elicited adjectives. Six of 9 respondents rated their knowledge as either poor or fair and wanted to learn about side effects, efficacy, and whether biosimilars were equal to biologics. We received the below responses to the question What do you hope to learn from this webinar and why?. Please note that the responses are presented as they were provided including potential misspelling and grammatical issues.
“My insurance company wants me to switch to a biosimilar after 5 years on herceptin. I am NED on first line treatment stage 4 triple positive breast cancer and I worry about making any changes. Are they truely equal???”
“The differences in this medication VS Herceptin, and what feedback has been given about side effects”
“Are they an option for me after being on Herceptin for 4 years?”
“Patient perspectives on use”
“Efficacy-data. Better-communication”
“How and when biosimilars are being used? Is this a choice made by patients, providers, healthcare organizations, or insurance companies?”
“I learn that the change was safe and that I need to watch for side effects and talk to my oncologist if I have any side effects. It also empowers me to talk to my team and encourage them to have a more open conversation about biosimilars and not leading with an insurance change but that they are confidence in the switch.”
Only 3 individuals responded to the post-webinar questions. All reported that the webinar was easy to understand, with 1 highlighting that the differences between biologics and biosimilars are “over my head” and one wants to hear more about regulation and cost. All rated content and discussion as either excellent or good. The following statements reflect a remaining gap: “Still not 100% certain who has the authority/responsibility to choose a biosimilar over a biologic…” and a concern “I did find it disheartening that co-pays would not be helped with a biosimilar.” One respondent shared the following statement: “Great program! Very informative – engaging and interesting panelists.”
Takeaways
The webinar resulted in a lively discussion with no shortage of questions and responses, as well as received positive feedback from the audience and participants. In addition, through high visibility promotion by the project team, panelists, and their networks, along with UICC, achieved a large reach of exposure to the presence of a conversation on this topic. The promotional flyer posted on Twitter by Dr. Papautsky on September 28, 2021 (approximately 1 month prior to the event) achieved 12,005 impressions, 195 engagements, and 21 detail expands (according to Tweet Analytics). Based on the panel discussion, we have identified the following takeaways to inform the next steps in research and clinical practice associated with trastuzumab biosimilars (and perhaps beyond).
People have questions. Questions are not limited to patients, but clinicians as well. Panelists reported the need for continued conversation regarding biosimilars and the need to engage patients in that conversation.
This healthcare topic (and all topics) should engage all stakeholders, including patients and caregivers to account for lived experiences. Without engaging patients, challenges and issues may remain invisible and therefore, unaddressed.
Trastuzumab biosimilars have many advantages, but a number of knowledge gaps and misunderstandings exist. These gaps need dedicated attention through multi-stakeholder conversations, educational initiatives, and research (to elicit and charaterize barriers). Such efforts can form the basis for informing effective promotion and uptake of trastuzumab biosimilars, including mitigating avoidable patient distress.
There is a need for human-centered approaches for effective trastuzumab biosimilars resource design and dissemination that accounts for underserved populations.
Implications for managed care pharmacies
We identified several implications including the importance of: (1) ensuring comprehensive drug utilization reviews that account for patient experience of switching and side effects to inform prescriber recommendations; (2) promoting pharmacists as resources to patients (and clinicians) when faced with novel drug choices, (3) facilitating efficient and effective prior authorization to switch back to reference drug as needed, and (4) highlighting the need for culturally-sensitive patient-pharmacist consultations that are informative and effective in addressing patient questions.
Conclusion
Finally, the advantages of conducting an informational webinar are multi-fold. In addition to the real-time educational benefits and opportunities to ask questions of experts live, the recorded webinar serves as a resource that can be disseminated in service of future educational opportunities in complement other resources. The webinar is temporarily hosted on youtube.com on a UIC College of Applied Health Sciences (an affiliation of Dr. Papautsky) unlisted channel with 180 subscribers. As part of a poster on trastuzumab biosimilar we presented at the 2021 San Antonio Breast Cancer Symposium (Citation10), we included a QR code to the webinar that yielded 7 visits. We plan to include the webinar recording as part of the informational toolkit on trastuzumab biosimilars.
Declaration of interest
Dr. Lyman reports grants from Amgen, personal fees from Sandoz, personal fees from G1 Therapeutics, personal fees from BeyondSpring, personal fees from ER Squibb, personal fees from Merck, personal fees from Jazz Pharma, personal fees from TEVA, personal fees from Samsung, personal fees from Fresenius Kabi, personal fees from Partners Healthcare, outside the submitted work. The remaining authors report no conflicts of interest.
Acknowledgements
We are thankful to Sheridan Chaney, Director of Marketing and Communications for the University of Illinois Cancer Center for facilitating dissemination and promotion. We are grateful to numerous colleagues, patient advocates, community members, and friends who helped to promote this event.
Additional information
Funding
References
- FDA. Biosimilars. 2022. [cited 2022 Apr 13]. Available from: https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars.
- Pivot X, Pegram M, Cortes J, Lüftner D, Lyman GH, Curigliano G, et al. Three-year follow-up from a phase 3 study of SB3 (a trastuzumab biosimilar) versus reference trastuzumab in the neoadjuvant setting for human epidermal growth factor receptor 2–positive breast cancer. Eur J Cancer. 2019;120:1–9. doi:https://doi.org/10.1016/j.ejca.2019.07.015.
- Lüftner D, Lyman GH, Gonçalves J, Pivot X, Seo M. Biologic drug quality assurance to optimize HER2+ breast cancer treatment: insights from development of the trastuzumab biosimilar SB3. Target Oncol. 2020;15(4):467–75. doi:https://doi.org/10.1007/s11523-020-00742-w.
- Lyman GH. How biosimilars will impact costs and care in oncology. Clin Adv Hematol Oncol. 2019;17(10):544–7.
- Lyman GH, Balaban E, Diaz M, Ferris A, Tsao A, Voest E, et al. American Society of Clinical Oncology statement: biosimilars in oncology. J Clin Oncol. 2018;36(12):1260–5. doi:https://doi.org/10.1200/JCO.2017.77.4893.
- Williamson C, Berger L, Sullivan TP, Crawford J, Lyman GH. Addressing oncologists’ gaps in the use of biosimilar products. Am J Manag Care. 2019;25(6 Spec No):SP188–SP191.
- Denduluri N, Somerfield MR, Chavez-MacGregor M, Comander AH, Dayao Z, Eisen A, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO guideline update. JCO. 2021;39(6):685–93. doi:https://doi.org/10.1200/JCO.20.02510.
- Nahleh Z, Lyman GH, Schilsky RL, Peterson DE, Tagawa ST, Chavez-MacGregor M, et al. Use of biosimilar medications in oncology. JCO Oncol Pract. 2022;18(3):177–86. doi:https://doi.org/10.1200/OP.21.00771.
- Lyman GH, Kuderer N. Rumors of the death of biosimilars have been greatly exaggerated. Cancer Invest. 2019;37(8):325–26.
- Papautsky EL, Carlson M, Lustberg M. The patient-centered paradox: a US-based survey of physician- and patient-reported experiences with biosimilar trastuzumab. San Antonio, TX: San Antonio Breast Cancer Symposium; 2021.
- Papautsky EL, Patterson ES. Patients are knowledge workers in the clinical information space. Appl Clin Inform. 2021;12(1):133–40. doi:https://doi.org/10.1055/s-0041-1723022.
- Papautsky EL, Crandall B, Grome A, Greenberg JM. A case study of source triangulation: using artifacts as knowledge elicitation tools in healthcare space design. J Cogn Eng Decis Mak. 2015;9(4):347–58. doi:https://doi.org/10.1177/1555343415613720.
- Papautsky EL, Carlson M, Johnson SM, Montague H, Attai DJ, Lustberg MB. Characterizing experiences of non-medical switching to trastuzumab biosimilars using data from internet-based surveys with US-based oncologists and breast cancer patients. Breast Cancer Res Treat. 2022;1–9.