ABSTRACT
A Grouped ActiNide EXtraction (GANEX) process for the extraction of actinides from used nuclear fuel for transmutation purposes has been investigated. The studied solvent consists of phenyl trifluoromethyl sulfone (FS-13), CyMe4-BTBP, and TBP, a combination that has previously shown promising results. The time to reach extraction equilibrium for the system has been found to be less than 20 min. A 2:1 complex has been found between CyMe4-BTBP and americium(III) or curium(III), whereas plutonium(IV) and CyMe4-BTBP create a 1:1 complex. The extraction of fission product is low in the system.
Introduction
Nuclear power is an important electricity source and accounts for a large proportion of the electricity produced in the OECD countries (America, 18.3%; Europe, 22.3%; Pacific, 11%).[Citation1]One of the major problems related to nuclear power is the production of the radiotoxic used nuclear fuel. This fuel has to be isolated from the environment and stored in a final repository for around 100,000 years to reach the same radiotoxicity as natural uranium.[Citation2] Today, two main strategies are in use for handling the used nuclear fuel.[Citation3] One strategy is direct disposal, or the “once through” fuel cycle, where the fuel is used once in the reactor and then left for disposal in the final repository.[Citation4]The other option is “Reprocessing” to recover plutonium and uranium from the used nuclear fuel using the solvent extraction PUREX process.[Citation5,Citation6] A further development of this concept is to recover not only plutonium and uranium but also the rest of the long-lived actinides, such as americium, curium, and neptunium. Reusing the long-lived actinides as fuel instead of disposing them will not only increase the energy usage of the used fuel, but also decrease the heat load of the used fuel, making the final repository more volume efficient, [Citation7,Citation8] and will decrease the long-term radiotoxicity of the used fuel.[Citation3,Citation9,Citation10]
Several different separation processes for long-lived actinide recovery have been developed over the years. [Citation11–Citation13] In this study, the process known as the Grouped ActiNide EXtraction (GANEX) process has been investigated. The aim of the GANEX process is to simultaneously remove all the actinides from the dissolved used nuclear fuel.[Citation14] This renders a possible single process for separation of the actinides from both the lanthanides and the rest of the fission and corrosion/activation products.
The basic concept of a GANEX process is a grouped extraction where all the actinides together are separated from the fission products and corrosion/activation products. A typical GANEX process involves two successive steps. The first step is where the bulk uranium is removed, [Citation15] and the second step is where the transuranium elements (TRUs) are removed.[Citation16,Citation17] At Chalmers University of Technology, a GANEX process that uses the concept of combining two well-known extractants with a diluent has been developed. This enables the utilization of the specific properties of both extracting agents. The chosen ligands are 6,6ʹ-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[Citation1,Citation2,Citation4]triazin-3-yl)[2,2ʹ]bipyridine (CyMe4-BTBP),[Citation18,Citation19] designed to extract tri-butyl and pentavalent actinides without extracting trivalent lanthanides,[Citation20] and tri-butyl phosphate (TBP) (), which is mainly for extracting tetra- and hexavalent actinides.[Citation21] Combining these two extracting agents enables the necessity of redox control to be avoided, and it is possible to strip the actinides selectively or to reuse them directly in a homogeneous recycling.
Figure 1. Molecular structure: left, 6,6ʹ-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[Citation1,Citation2,Citation4]triazin-3-yl)[2,2ʹ]bipyridine (CyMe-BTBP); right, tri-butyl phosphate (TPB).
![Figure 1. Molecular structure: left, 6,6ʹ-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[Citation1,Citation2,Citation4]triazin-3-yl)[2,2ʹ]bipyridine (CyMe-BTBP); right, tri-butyl phosphate (TPB).](/cms/asset/23126356-3785-4f76-aaa5-15dc1adf13b1/lsei_a_1497043_f0001_b.gif)
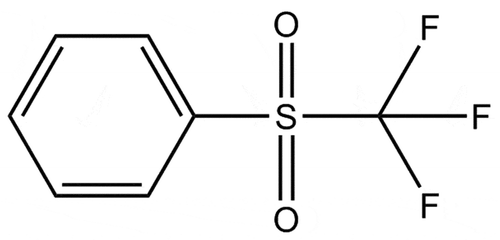
Over the years, many different chemicals have been investigated for use as diluents in the Chalmers GANEX system.[Citation22–Citation24], Lofstrom-Engdahl2014a. Owing to various issues, these chemicals have been shown not to be suitable, and there is hence a need to investigate alternative chemicals as substitutes. In this work, phenyl trifluoromethyl sulfone (FS-13) has been investigated as a GANEX diluent, as shown in . FS-13 was originally developed as a diluent in the UNiversal EXtraction (UNEX) process and has a good stability against nitric acid and high resistance against radiation. [Citation25,Citation26] Other properties indicating that FS-13 could be a good GANEX diluent are a low viscosity, a high density of 1.4 gmL
[Citation27] (making the organic phase heavier than the aqueous), the polarity of the molecule, and a good chemical stability.
Experimental methods
The experiments within this work have mainly been performed using the same composition of the organic phase, 10 mM CyMe4-BTBP (synthesized in house according to Foreman et al. [Citation18]), 30% TBP (Sigma-Aldrich, 97%), and 70%
FS-13 (CarboSynth,
99% or Marshallton,
99%). An aqueous phase consisting of 4 M HNO3 (Sigma Aldrich,
69% diluted with MilliQ-water,
18 M
) has been used in all cases.
All solvent extraction experiments within this work have been conducted in similar ways, unless otherwise stated, using freshly prepared solvent batches and separate samples for each actinide. All experiments were performed in triplicate. The uncertainties are, in all cases, calculated as standard deviations from triplicate samples.
For phase contact, 3.5 mL glass vials with plastic lids have been used. All samples contained equal amounts of organic and aqueous phases, 300–1000 L. Trace amounts of the actinides and europium were added to the samples from stock solutions: U(VI)-235 (84.44% enrichment, 40 mM), Np((V, IV)-239 (extracted from an Am-243-loaded silica column), Pu(IV)-238 (0.28 MBq mL
), Am(III)-241 (0.42 MBq mL
), Cm(III)-244 (0.23 MBq mL
), and Eu(III)-152 (23 kBq mL
). Americium and europium were in most cases investigated together, i.e. added to the same samples. The other actinides were investigated separately. The fission product experiments were performed using inactive metal solutions of the corresponding elements, as listed in . Concentrations were chosen to represent fission products present in a PUREX feed. Phase contact was, in all cases, facilitated in a mechanical shaker (IKA, VIBRAX VXR 1,500 rpm) at 25°C for a duration of 1 h, unless otherwise stated.
Table 1. Inactive metals used to simulate fission product extraction.
Analysis
The samples containing both americium-241 and europium-152 were measured using a high-purity germanium detector (HPGe)(Canberra, Gamma Analyst GEM 23195, or Ortec, GEM 15180-S), using gamma energies of 59.5 keV and 121.8 keV, respectively. All samples containing plutonium-238, uranium-235, neptunium-239, americium-241, or curium-244, separately, were measured using a liquid scintillation counting (LSC) detector (Wallac, 1414 WinSpectral). Experiments to examine possible quenching of the samples have been performed. No quenching using FS-13 has been observed within the concentration range studied. All samples containing both americium-241 and curium-244 were measured using an alpha spectrometer (Ortec, Alpha Duo, Octête TM PC) using alpha energies of 5.5 MeV and 5.8 MeV, respectively. The inactive metals were measured on an inductively coupled plasma mass spectrometer (ICP-MS)(Perkin Elmer Elan 6100 DRC). Only the aqueous phase can be measured on the ICP-MS used, and the metal extraction was hence determined by measuring the aqueous phase prior to and after the phase contact. Each sample was measured until the measurement uncertainty was below 5%. No differences in the efficacy between the different detectors were observed.
Analytical HPLC was used to determine the remaining amount of CyMe4-BTBP in the samples after both radiolysis and aging. The measurement system used consisted of a Merck-Hitachi HPLC system (LaChrom 7000 series) equipped with a Pump L7100, a DAD 7450 detector, an Intelligent Injector L7250, and an interface. Chromatographic conditions were as follows: column, Merck Purospher STAR RP-C 8e Endcaped (5 m, 250 × 2 mm I.D.); mobile phase, Solvent A: 80 mM aqueous triethylamine acetate (TEAA, pH 8.2) and Solvent B: CH
CN; isocratic elution with mixing, A: 22%, B: 78%; flow rate, 0.3 mL/min; detection, DAD (220–350 nm); and selected wavelength, 235 nm. The samples with a volume of 25
L were syringed twice into two different vials, and the volume was adjusted to 1.0 mL by adding acetonitrile. The injection volumes were 3
L. The data represent mean values from six injections made from two vials of each sample. The calibration curve was constructed using the nonirradiated reference sample with a concentration of 10 mM CyMe4-BTBP (aged under the same conditions as the irradiated samples) and data from four to five injections of volumes between 1 and 10
L. The calibration curve has a linear fit over the whole range of R
= 0.9969.
Mass spectrometry (Thermo-Finnigan Fleet Ion Trap Instrument) with atmospheric pressure chemical ionization (APCI) in positive mode was used to identify the decomposition products in the samples after irradiation. All the samples and their corresponding reference solution were stored in a freezer at −33°C, both before and after analyses. Then, 25 L of each sample was diluted to a volume of 1.0 mL by adding acetonitrile. Infusion from the syringe into an ion source was used. Experimental conditions for the MS interface were as follows: flow rate from a syringe infusion pump, 10
L/min; sheath gas flow, 20 L/min; auxiliary gas flow, 9 L/min; source voltage, 4.17 kV; vaporizer temperature, 400°C; capillary temperature, 250°C; capillary voltage, 3 V; mass range from 50 to 1000 mass units; and [M + H]
peaks were observed.
During the Time-Resolved Laser Fluorescence Spectroscopy (TRLFS) measurements, Cm(III) (10 M) was extracted from 4 M HNO3 into organic phases composed of 10 mM CyMe4-BTBP in (a) 100%
FS-13 or (b) 70%
FS-13 and 30%
TBP. Phase contacting time was 1 h or 4 h. The separated organic phase was transferred into a quartz cuvette (1 cm path length) and measured (T = 25°C). TRLFS studies were performed using a Nd:YAG laser-pumped (Continuum Surelite Laser) dye laser system (NARROWscan D-R Dye Laser) with a repetition rate of 10 Hz. Cm(III) was excited using a wavelength of
= 396.6 nm. Following spectral decomposition by a spectrograph (Shamrock 303i, 1199 lines mm
), the spectra were recorded by an Intensified Charge-Coupled Devices (ICCD) camera (iStar Gen III, ANDOR) with an integrated delay controller. The fluorescence signal was detected after a delay time of 1
s to discriminate scattering light and short-lived fluorescence of organic compounds.
Results and discussion
Kinetics of actinides and europium (representing the lanthanides) extraction were investigated to evaluate the time it takes for the system to reach equilibrium compared to other systems using the same equipment, as shown in .
Figure 3. Distribution ratios over time for uranium, neptunium, plutonium, americium, curium, and europium in 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 (lines added to guide the eye).
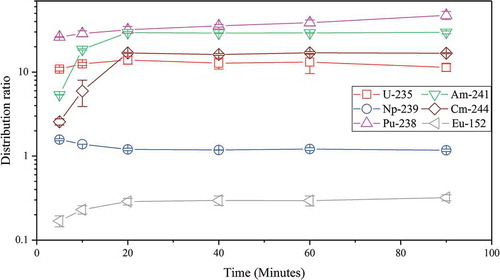
Uranium and plutonium extract quickly and reach equilibrium more or less instantly. Neptunium extraction however decreases between 5 and 20 min, which might be due to a shift in the dominating oxidation state of neptunium. Neptunium is easily oxidized and reduced by the acidic conditions in this experiment and is most likely present in mixed oxidation states. [Citation28,Citation29] Americium and curium extraction reach equilibrium after 20 min and show a significant increase in the distribution ratio increase between 5 and 20 min. Europium is not extracted to any greater extent and has similar kinetics to americium and curium. Hence, all actinides and europium reached equilibrium after 20 min, which is fast compared to other GANEX systems with CyMe4-BTBP as an extracting agent, using the same equipment. [Citation30]
The results in also provide information about the metal equilibrium distribution in the two phases, as listed in . The system has high distribution ratios for plutonium and americium, slightly lower ratios for uranium and curium, and a ratio well below one for europium. Comparing these distribution ratios with previously investigated GANEX systems using other diluents, it is found that the values are found to be in a similar order of magnitude. [Citation30–Citation32]Desirable distribution ratios should render a high metal extraction, while still maintaining a feasible actinide stripping. Neptunium has a rather low distribution ratio, and therefore further investigations are needed to successfully extract neptunium together with the other actinides in this system.
Table 2. Distribution ratios for selected actinides and europium, as well as separation factors for selected actinides over europium in 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
Separation factors were calculated for evaluation of the actinide extraction in comparison to europium, used as a reference for the lanthanides, as listed in . The only actinide with a low separation factor toward europium is neptunium. The separation factor is high enough, however, to enable separation. Americium/curium separation can also be desirable in certain suggested reprocessing options, such as the Amine Extraction (AmEX) process.[Citation33] The separation factor between americium and curium is, however, small (1.5 0.2), in agreement with the selectivity observed in an octanol diluent.[Citation19] This indicates that americium and curium may be hard to separate from each other.
It is important to understand the behavior of the ingoing components in the extraction system in order to predict their future behavior in a process. A previously published study has shown that the combination of TBP and CyMe4-BTBP is beneficial for both americium and curium extraction using FS-13 as the diluent[Citation34]. The distribution ratios of americium and curium reach 0.05 and 0.4, respectively, after 1 h of phase contacting in both the systems containing only one of the two extracting agents (70% FS-13 and 30%
TBP or 100%
FS-13 and 10 mM CyMe4-BTBP). In the system containing both extracting agents (70%
FS-13, 30%
TBP, and 10 mM CyMe4-BTBP) americium and curium, however, reach distribution ratios of 25 and 17, respectively, as shown in .
Figure 4. Distribution ratios of actinides and europium using A: 70% FS-13 and 30%
TBP; B: 100%
FS-13 and 10 mM CyMe
-BTBP; and C: 70%
FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as the organic phase and 4 M HNO3 as the aqueous phase. Data retrieved from Halleröd et al. [Citation34].
![Figure 4. Distribution ratios of actinides and europium using A: 70% FS-13 and 30% TBP; B: 100% FS-13 and 10 mM CyMe-BTBP; and C: 70% FS-13, 30% TBP, and 10 mM CyMe4-BTBP as the organic phase and 4 M HNO3 as the aqueous phase. Data retrieved from Halleröd et al. [Citation34].](/cms/asset/a751e3be-137b-4449-ba7f-f678b2e7b476/lsei_a_1497043_f0004_b.gif)
TRLFS experiments were performed to confirm the extraction of trivalent actinides as 1:2 complexes. Cm(III) was extracted from 4 M HNO3 into 10 mM CyMe4-BTBP in 100% FS-13 or 10 mM CyMe4-BTBP in 70%
FS-13, 30%
TBP. Organic-phase samples were investigated by TRLFS. Similar emission spectra were found in both cases (), showing an emission maximum at a wavelength of 620 nm. The spectra match the spectrum of the [Cm(CyMe4-BTBP)
(NO
)]
complex in octanol.[Citation35]
Figure 5. Graph A: normalized fluorescence spectrum of Cm(III)-CyMe4-BTBP complexes in the organic phase using 100% FS-13 or 70%
FS-13 and 30%
TBP and 4 M HNO3 as the aqueous phase. Graph B: fluorescence spectra of Cm(III)-CyMe4-BTBP-complexes in the organic phase using 100%
FS-13 and 10 mM CyMe4-BTBP and 4 M HNO3 as aqueous phase after 1 h and 4 h phase contacting.
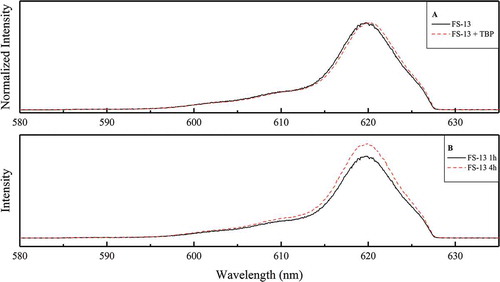
Thus, trivalent actinides seem to be extracted by CyMe4-BTBP in both systems despite the results shown in .[Citation34] Further time-resolved investigations of the system containing 100% FS-13 and 10 mM CyMe4-BTBP were performed with varied phase contact times (1 h and 4 h). The fluorescence emission spectra () show emission intensity increasing with time, indicating slow extraction kinetics.
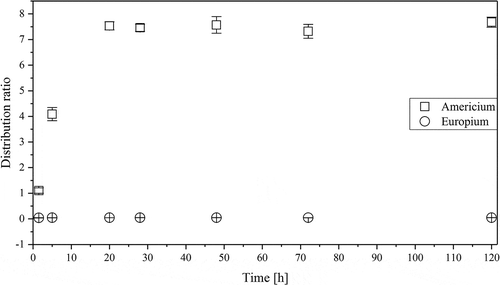
Owing to the TRLFS results in , a long-term extraction kinetics study of the system containing 100% FS-13 and 10 mM CyMe4-BTBP was performed, as shown in . The extraction of europium seems to be stable throughout the experiment, with a distribution ratio of 0.05, whereas the extraction of americium slowly increases over time. Americium extraction equilibrium is reached after approximately 20 h with a final distribution ratio of 7.5. This is slow compared to the system consisting of 70%
FS-13, 30%
TBP, and 10 mM CyMe4-BTBP: americium extraction equilibrium is reached after 20 min, with a distribution ratio of 29, as shown in . Hence, the presence of TBP strongly affects the minor actinide extraction kinetics by CyMe4-BTBP in FS-13 and also influences the corresponding equilibrium distribution ratio in a positive way. This indicates that TBP acts as a phase transfer catalyst. Similar behaviors have also been obtained when adding diMethyldiOctyl-hexaethoxy-malonamide (DMDOHEMA)[Citation19] or N, N, N', N'-tetraoctyldiglycolamide (TODGA).[Citation36,Citation37]
Figure 6. Long-term distribution ratios for americium and europium in 100% FS-13 and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
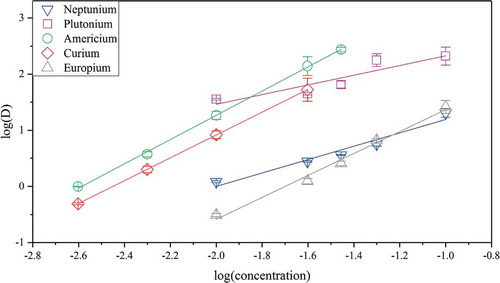
To be able to compare the complexation data retrieved for curium and CyMe4-BTBP by TRLFS with other metals, the complexation between CyMe4-BTBP and plutonium, americium, curium, and europium was investigated by extraction slope analysis using various concentrations of CyMe4-BTBP, as shown in . The results for the trivalent metals, americium, curium and europium, correspond well with the curium results obtained by TRLFS (2.16 0.04, 2.03
0.01, and 1.96
0.12, respectively), indicating a 2:1 complexation between CyMe4-BTBP and all three metals. The complexation between CyMe4-BTBP and plutonium (0.86
0.22), on the contrary, indicates a 1:1 relation. It should be noted, however, that plutonium (IV) in FS-13 is also extracted by TBP, as previously shown in . The influence of TBP in the system, however, has to be investigated further.
Figure 7. Distribution ratios as a function of CyMe4-BTBP concentration for the extraction of plutonium, americium, and europium in 70% FS-13, 30%
TBP, and CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
After concluding that the actinide extraction and lanthanide separation of the GANEX system using FS-13 as diluent is satisfactory, the stability properties of the system had to be investigated. The extraction of americium and europium in the system consisting of 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP has previously been found to be stable during both radiolysis and hydrolysis.[Citation38] In this work, however, HPLC measurements of the irradiated organic phase show that the CyMe4-BTBP concentration decreases with an increasing dose, as listed in . With increased dose, the HPLC chromatograms show that when the CyMe4-BTBP amount decreases, two other peaks increase correspondingly. These observed peaks (m/z = 551.33 and 567.25) have, by complementary MS measurements, showed the presence of two main degradation products, which could be assigned to two compositions corresponding to the hydroxyderivatives of BTBP, (HO)-CyMe4-BTBP and (HO)
-CyMe4-BTBP. The most likely position of the hydroxyl group(s) is on the pyridine ring of the BTBP. Although after 200 kGy none of the CyMe4-BTBP remains, the extraction ability of the system is, as previously mentioned, maintained. This indicates that the new molecules have similar extraction and complexation properties to CyMe4-BTBP.
Table 3. CyMe4-BTBP stability during irradiation using 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
During the hydrolysis studies, shown in , no decrease in the amount of CyMe4-BTBP could be observed. Although the measurements at weeks 4 and 5 could not be accurately measured due to small sample sizes, it is reasonable to believe that the CyMe4-BTBP concentration is constant during the entire investigated time interval. This is likely as no apparent decrease in the CyMe4-BTBP concentration can be observed between week 2 and week 6.
Table 4. CyMe4-BTBP stability over 7 weeks at room temperature (20–22°C) using 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
Temperature changes might occur during the separation and transmutation process, both due to heat released during the phase contacting and from irradiation. This could have an effect on the performance of the process, in terms of for example precipitation of the extracting agent and/or the complexed metal, and must therefore be investigated. Due to this, the metal extraction within a temperature interval between 20°C and 40°C has been investigated, as shown in . Within the temperature interval, it is not likely for the extraction reaction and speciation to change.[Citation39]A slight decrease with an increasing temperature is observed for the extraction of both americium and europium, indicating that the extraction reaction is exothermic. Similar slopes indicate selectively is maintained at higher temperatures.
Figure 8. The logarithm of the extraction equilibrium constant vs. 1/T. Using 77% FS-13, 23%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
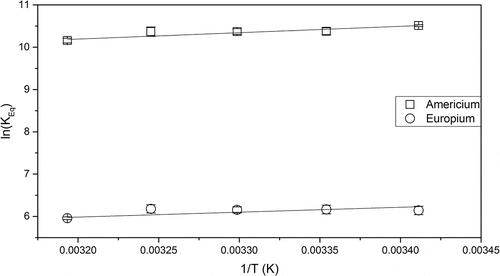
Presuming that enthalpy and entropy are constant for the investigated temperature interval, and
for the extraction of americium and europium can be calculated from the data points in . This is carried out by performing a linear regression where the van’t Hoff equation, Eq. 1, states that –
represents the slope and
represents the intercept of the linear fit.
where is the extraction equilibrium constant, T is the temperature, and R is the ideal gas constant.
The calculated enthalpy is negative, whereas the entropy is positive for both americium and europium (). Extraction is driven by both enthalpy and entropy. Similar results have been found for other GANEX systems studied using different diluents.[Citation40,Citation41]
Table 5. Enthalpy and entropy of complexation for the extraction of americium and europium using 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase.
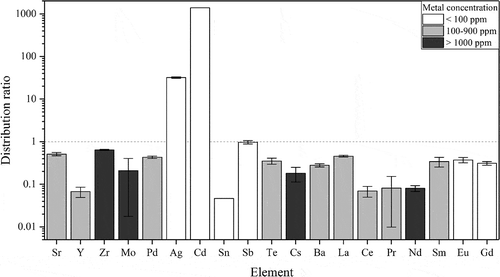
During a GANEX process, high extraction of the actinides alongside a low extraction of the fission products is desirable. In this study, inactive metals have been used as references for the actual fission products, and for a majority of the investigated fission products, a distribution ratio below 1 has been found (). Some of the fission product distribution ratios are, however, higher or close to 1 and might become a problem in a future process. The fission products with the highest distribution ratios are cadmium and silver, for which the abundance in the used fuel, however, is lower than 100 ppm. These unwanted metals can, despite this, be a problem in a future process if they cannot be scrubbed out. One metal with higher abundance (above 1000 ppm) in the used fuel is zirconium. Even if the distribution ratio remains below one, the high concentration can still cause problems. The distribution ratio of europium is high compared to other lanthanides present with a high abundance in the used fuel. Further studies into the decreasing extraction of fission products through possible suppression agents and stripping steps are therefore needed. Similar fission product behavior has been observed in another GANEX system with CyMe4-BTBP and TBP in cyclohexanone.[Citation22] Comparing the inactive europium () with the active europium (), distribution ratios of around 0.3 are found in both cases, indicating that the inactive metals can be used as substitutes for the active fission products.
Figure 9. Distribution ratios of selected fission products in 70% FS-13, 30%
TBP, and 10 mM CyMe4-BTBP as organic phase and 4 M HNO3 as aqueous phase. White bars correspond to a metal concentration below 100 ppm in the dissolved used fuel. Gray bars correspond to a metal concentration between 100 and 900 ppm in the dissolved used fuel. Black bars correspond to a metal concentration above 1000 ppm in the dissolved used fuel. The dashed line marks D = 1, which is the dividing line between extraction and strip.
Conclusions
A solvent extraction system containing 10 mM CyMe4-BTBP and 30% TBP in 70%
FS-13, intended for use within separation for transmutation, has been investigated with regard to actinide extraction kinetics, complex formation properties, radiolysis behavior, thermodynamic properties, and fission product extraction, with promising results.
All investigated actinides and europium reached full extraction after 20 min, which is comparably fast for a solvent containing 10 mM CyMe4-BTBP. As expected, americium and curium have similar extraction kinetics, indicating that they are extracted by the same ligand, CyMe4-BTBP. Uranium and plutonium, however, display faster extraction kinetics, indicating that they are not extracted by the same ligand as americium and curium but predominantly by TBP. Neptunium is most likely extracted by both ligands. High separation factors can be achieved between the actinides and europium in all cases except for neptunium. The low equilibrium distribution ratio for neptunium and the low separation factor between neptunium and europium might cause problems in a future process.
A previous study investigating various combinations of FS-13 and the two extracting agents has shown that the combination of TBP and CyMe4-BTBP extracts the minor trivalent actinides, whereas neither of the extracting agents seems to extract the minor trivalent actinides on their own. TRLFS studies investigating the complexation between CyMe4-BTBP and curium showed the formation of a 2:1 complex, both in 100% FS-13 and in 70%
FS-13, 30%
TBP, indicating that the trivalent actinides are indeed extracted by CyMe4-BTBP. TRLFS also indicated that this complex formation was very slow. Extended-duration kinetic extraction studies of CyMe4-BTBP and americium in 100% FS-13 confirmed these results, with extraction equilibrium reached after 20 h. The equilibrium distribution ratio is however lower than with TBP present in the extraction system. Supplementary slope analysis also shows that americium and europium form a 2:1 complex with CyMe4-BTBP in FS-13, whereas plutonium forms a 1:1 complex.
Previous radiolysis stability studies have shown that the extraction of americium and europium by CyMe4-BTBP and TBP in FS-13 is unaffected up to at least 500 kGy. Analysis of the irradiated solutions now shows that after 200 kGy, despite maintained distribution ratios, all of the CyMe4-BTBP present reacted and formed (HO)-CyMe4-BTBP and (HO)-CyMe4-BTBP.
Studies investigating the temperature dependence of americium and europium extraction show a slight decrease in the distribution ratios for both elements with increasing temperature, indicating an exothermic extraction reaction. Enthalpy and entropy calculations for the extraction of americium and europium show that the enthalpy is negative in both cases, whereas the entropy is positive. This increase of entropy during the extraction acts together with the enthalpy as a thermodynamic driving force for the reaction.
The extraction of selected fission products in the system is low, with a distribution ratio below 1 for most metals. A few elements, such as silver, cadmium, zirconium, and molybdenum, could however cause problems in a future process and require further investigations.
Acknowledgments
Udo Müllich at Karlsruhe Institute of Technology, Germany, is acknowledged for synthesizing and supplying CyMe4-BTBP.
Additional information
Funding
References
- OECD-NEA, Organisation for Economic Co-operation and Development – Nuclear En-ergy Agency; Nuclear Energy Data. http://www.oecd-nea.org/ndd/pubs/2017/7365-ned-2017.pdf, 2017; 2017-12-27.
- Madic, C.; Testard, F.; Hudson, M.; Liljenzin, J.-O.; Christiansen, B.; Ferrando, M.; Facchini, A.; Geist, A.; Modolo, G.; Gonzalez-Espartero, A.; De Mendoza, J. PART-NEW New Solvent Extraction Processes for Minor Actinides, Final Report, Report CEA-R-6066; 2004.
- IAEA. IAEA-TECDOC-1587, Spent Fuel Reprocessing Options; IAEA: Vienna, 2008.
- Choppin, G.; Liljenzin, J.-O.; Rydberg, J.; Ekberg, C. 2013. Radio Chemistry and Nuclear Chemistry, 4th; Woburn, MA: Elsevier, Chapter 21.
- Mtingwa, S. K.; Feasibility of Transmutation of Radioactive Elements. An international spent nuclear fuel storage facility-Exploring a Russian site as a prototype: Proceedings of an international workshop. 2005; pp 30–49.
- Madic, C.; Hudson, M.; Liljenzin, J.; Glatz, J.; Nannicini, R.; Facchini, A.; Kolarik, Z.; Odoj, R. New Partitioning Techniques for Minor Actinides. Final Report, NEWPART EUR 19149. Nuclear Science and Technology 2000,
- Bond, W.; Leuze, R. 1976. Transplutonium; Oak Ridge, Tennesse: Oak Ridge National Lab, 1975.
- Salvatores, M.; Slessarev, I.; Ritter, G.; Fougeras, P.; Tchistiakov, A.; Youinou, G.; Zaetta, A. Long-Lived Radioactive Waste Transmutation and the Role of Accelerator Driven (Hybrid) Systems. Nucl. Instrum. Methods Phys. Res., Sect. A 1998, 414, 5–20. DOI: 10.1016/S0168-9002(98)00522-1.
- Park, H.; Choi, J. 2005. An International Spent Nuclear Fuel Storage facility-Exploring a Russian Site as a Prototype: Proceedings of an International Workshop, The National Academies Press, Washington, DC. Chapter 10.
- Aoki, S.;. Research and Development in Japan on Long-Lived Nuclide Partitioning and Transmutation Technology. Prog. Nucl. Energy 2002, 40, 343–348. DOI: 10.1016/S0149-1970(02)00027-6.
- Weaver, B.; Kappelmann, F. TALSPEAK: A New Method of Separating Americium and Curiumfrom the Lanthanides by Extraction from an Aqueous Solution of an Aminopoly- Acetic Acid Complex with A Monoacidic Organophosphate or Phosphonate; Oak Ridge, Tennesse: Oak Ridge National Lab, 1964.
- Madic, C.; Hudson, M. J.; Liljenzin, J.; Glatz, J.; Nannicini, R.; Facchini, A.; Kolarik, Z.; Odoj, R. Recent Achievements in the Development of Partitioning Processes of Minor Actinides from Nuclear Wastes Obtained in the Frame of the NEWPART European Programme (1996–1999). Prog. Nucl. Energy 2002, 40, 523–526. DOI: 10.1016/S0149-1970(02)00046-X.
- Todd, T.; Law, J.; Herbst, R.; Lumetta, G.; Moyer, B. 2000. Treatment of Radioactive Wastes Using Liquid-Liquid Extraction Technologies-Fears, Facts, and Issues. Proc. Waste Manag., 00, Tucson, AZ, Mar. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.606.2307&rep=rep1&type=pdf
- Miguirditchian, M.; Chareyre, L.; Hérès, X.; Hill, C.; Baron, P.; Masson, M. GANEX: Adaptation of the DIAMEX-SANEX Process for the Group Actinide Separation; Bagnols-sur-Ceze, France, 2007.
- Miguirditchian, M.; Roussel, H.; Chareyre, L.; Baron, P.; Espinoux, D.; Calor, J.; Viallesoubranne, C.; Lorrain, B.; Masson, M. HA Demonstration in the Atalante Facility of the Ganex 2. Cycle for the Grouped TRU Extraction. Proceedings GLOBAL 2009. 2009.
- Miguirditchian, M.; Sorel, C.; Camès, B.; Bisel, I.; Baron, P.; Espinoux, D.; Calor, J.; Viallesoubranne, C.; Lorrain, B.; Masson, M. HA Demonstration in the Atalante Facility of the Ganex 1. Cycle for the Selective Extraction of Uranium from HLW. Proceedings GLOBAL 2009. 2009.
- Carrott, M.; Geist, A.; Hères, X.; Lange, S.; Malmbeck, R.; Miguirditchian, M.; Mod-Olo, G.; Wilden, A.; Taylor, R. Distribution of Plutonium, Americium and Interfering Fission Products between Nitric Acid and a Mixed Organic Phase of TODGA and DMDO-HEMA in Kerosene, and Implications for the Design of the “EURO-GANEX” Process. Hydrometallurgy 2015, 152, 139–148. DOI: 10.1016/j.hydromet.2014.12.019.
- Foreman, M.; Hudson, M.; Drew, M.; Hill, C.; Madic, C. Complexes Formed Be-Tween the Quadridentate, Heterocyclic Molecules 6,6ʹ-Bis-(5,6-Dialkyl-1,2,4-Triazin-3-Yl)-2,2ʹ-Bipyridine (BTBP) and lanthanides(III): Implications for the Partitioning of ac-tinides(III) and lanthanides(III). Dalton Trans. 2006, 13, 1645–1653. DOI: 10.1039/B511321K.
- Geist, A.; Hill, C.; Modolo, G.; Foreman, M. R. S. J.; Weigl, M.; Gompper, K.; Hudson, M. J. 6,61-Bis(5,5,8,8-Tetramethyl-5,6,7,8-Tetrahydro-Benzo[1,2,4]Triazin-3-Yl) [2,21]Bipyridine, an Effective Extracting Agent for the Separation of Americium(III)and Curium(III) from the Lanthanides. Solvent Extr. Ion Exch. 2006, 24, 463–483. DOI: 10.1080/07366290600761936.
- Retegan, T.; Ekberg, C.; Dubois, I.; Fermvik, A.; Skarnemark, G.; Wass, T. J. Ex- Traction of Actinides with Different 6, 6ʹ-Bis (5, 6-Dialkyl-[1, 2, 4]-Triazin-3-Yl)-[2, 2ʹ]-Bipyridines (Btbps). Solvent Extr. Ion Exch. 2007, 25, 417–431. DOI: 10.1080/07366290701416000.
- Anderson, H.; Newton, M.; Asprey, L.; Richmond, C. Solvent Extraction Process for Plutonium, United States Patent Office, Patent no 2,924,506; 1960.
- Aneheim, E.; Ekberg, C.; Fermvik, A.; Foreman, M. R. S. J.; Retegan, T.; Skarnemark, G. A TBP/BTBP-based GANEX Separation Process. Part 1: Feasibility. Solvent Extr. Ion Exch. 2010, 28, 437–458. DOI: 10.1080/07366299.2010.480930.
- Aneheim, E.; Ekberg, C.; Fermvik, A.; Foreman, M. R. S. J.; Grűner, B.; Hajkova, Z.; Kvičalová, M. A TBP/BTBP-based GANEX Separation Process - Part 2: Ageing, Hydrolytic, and Radiolytic Stability. Solvent Extr. Ion Exch. 2011, 29, 157–175. DOI: 10.1080/07366299.2011.539462.
- Löfström-Engdahl, E.; On the Diluent and Solvent Effects in Liquid-Liquid Extraction Systems based on Bis-triazine-bipyridine (BTBP) ligands. Ph.D. thesis, Chalmers Uni- versity of Technology, 2014.
- Rzhekhina, E.; Karkozov, V.; Alyapyshev, M. Y.; Babain, V.; Smirnov, I.; Todd, P.; Law, J.; Herbst, R. Reprocessing of Spent Solvent of the UNEX Process. Radiochemistry 2007, 49, 493–498. DOI: 10.1134/S1066362207050086.
- Romanovskiy, V.; Smirnov, I.; Babain, V.; Todd, T.; Herbst, R.; Law, J.; Brewer, K. The Universal Solvent Extraction (UNEX) Process. I. Development of the UNEX Process Solvent for the Separation of Cesium, Strontium, and the Actinides from Acidic Radioactive Waste. Solvent Extr. Ion Exch. 2001, 19, 1–21. DOI: 10.1081/SEI-100001370.
- Law, J.; Herbst, R.; Todd, T.; Romanovskiy, V.; Babain, V.; Esimantovskiy, V.; Smirnov, I.; Zaitsev, B. The Universal Solvent Extraction (UNEX) Process. II. Flow- Sheet Development and Demonstration of the UNEX Process for the Separation of Ce- Sium, Strontium, and Actinides from Actual Acidic Radioactive Waste. Solvent Extr. Ion Exch. 2001, 19, 23–36. DOI: 10.1081/SEI-100001371.
- Huizenga, J. R.; Magnusson, L. B. Oxidation-Reduction Reactions of Neptunium (IV) and-(V)1. J. Am. Chem. Soc. 1951, 73, 3202–3206. DOI: 10.1021/ja01151a061.
- Taylor, R.; Gregson, C.; Carrott, M.; Mason, C.; Sarsfield, M. Progress Towards the Full Recovery of Neptunium in an Advanced PUREX Process. Solvent Extr. Ion Exch. 2013, 31, 442–462. DOI: 10.1080/07366299.2013.800438.
- Löfström-Engdahl, E.; Aneheim, E.; Ekberg, C.; Foreman, M.; Skarnemark, G. A. Comparison of Americium Extractions as a Function of Time Using Two Bis-Triazine- Bipyridine Ligands in Long-Chained Alcohol Diluents. Sep. Sci. Technol. 2014, 49, 2060–2065. DOI: 10.1080/01496395.2014.911325.
- Halleröd, J.; Ekberg, C.; Löfström-Engdahl, E.; Aneheim, E. Development of the Chalmers Grouped Actinide Extraction Process. Nukleonika 2015, 60, 829–835. DOI: 10.1515/nuka-2015-0115.
- Aneheim, E.; Ekberg, C.; Foreman, M. R.; Löfström-Engdahl, E.; Mabile, N. Studies of a Solvent for GANEX Applications Containing CyMe4-BTBP and DEHBA in Cyclo-Hexanone. Sep. Sci. Technol. 2012, 47, 663–669. DOI: 10.1080/01496395.2011.627908.
- Rainey, R.;. Development of the Amex Process for Americium Recovery; Oak Ridge, Tennesse: Oak Ridge National Lab. 1954.
- Halleröd, J.; Ekberg, C.; Aneheim, E. Phenyl Trifluoromethyl Sulfone as Diluent in a Grouped Actinide Extraction Process: Extraction Properties of the Solvent Components TBP and CyMe4-BTBP. J. Radioanal. Nucl. Chem. 2016, 307, 1711–1715. DOI: 10.1007/s10967-015-4416-7.
- Bremer, A.; Whittaker, D. M.; Sharrad, C. A.; Geist, A.; Panak, P. J. Complexation of Cm(III) and Eu(III) with CyMe4-BTPhen and CyMe4-BTBP Studied by Time Resolved Laser Fluorescence Spectroscopy. Dalton Trans. 2014, 43, 2684–2694. DOI: 10.1039/C3DT52204K.
- Modolo, G.; Wilden, A.; Daniels, H.; Geist, A.; Magnusson, D.; Malmbeck, R. Devel- Opment and Demonstration of a New SANEX Partitioning Process for Selective Actinide III)/lanthanide (III) Separation Using a Mixture of CyMe4BTBP and TODGA. Radiochim. Acta 2013, 101, 155–162. DOI: 10.1524/ract.2013.2016.
- Geist, A.; Magnusson, D.; Müllich, U. A Kinetic Study on the Extraction of Americium (III) into CyMe4-BTBP. Twelfth Information Exchange Meeting on Actinide and Fis- sion Product Partitioning and Transmutation (12-IEMPT), Prague, Czech Republic. 2012; pp 24–27.
- Halleröd, J.; Ekberg, C.; Foreman, M.; Löfström-Engdahl, E.; Aneheim, E. Stability of Phenyl Trifluoromethyl Sulfone as Diluent in a Grouped Actinide Extraction Process. J. Radioanal. Nucl. Chem. 2015, 304, 287–291. DOI: 10.1007/s10967-014-3657-1.
- Puzikov, E. A.; Zilberman, B. Y.; Fedorov, Y. S.; Blazheva, I. V.; Kudinov, A. S.; Ryabkov, D. V.; Shmidt, O. V. Influence of Temperature on the Extraction of Actinides from Nitric Acid Solutions into 30% TBP: Description within the Framework of a New Model. Radiochemistry 2015, 57, 136–142. DOI: 10.1134/S1066362215020058.
- Löfström-Engdahl, E.; Aneheim, E.; Ekberg, C.; Foreman, M.; Halleröd, J.; Skarne-Mark, G. Extraction Thermodynamics of Am (III) and Eu (III) Using CyMe4-BTBP in Various Organic Diluents. J. Chem. Thermodyn. 2014, 76, 64–69. DOI: 10.1016/j.jct.2014.03.004.
- Aneheim, E.; Development of a Solvent Extraction Process for Group Actinide Recovery from Used Nuclear Fuel. Ph.D. thesis, Chalmers University of Technology, 2012.