?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was undertaken to investigate the extraction of In3+ and Ga3+ by 7-(4-ethyl-1-methyloctyl)-8-hydroxy-quinoline (Kelex100), which has been found to be a slow extraction process. Homogeneous liquid-liquid extraction utilizing upper critical solution temperature of 4-methyl-1,3-dioxolan-2-one (propylene carbonate, abbreviated as PC) was used to eliminate the interface and speed up the extraction, since distribution between two phases was thought to be the rate-determining step. The effect of contact time on the percentage of metal extraction was examined at 80ºC using Kelex100 dissolved in toluene and PC solutions and the difference between two solvents was evaluated. The results clearly show that the use of homogeneous liquid-liquid extraction using PC with Kelex100 is faster than traditional two phase extraction.
Introduction
In the liquid-liquid extraction of metal ions, broadly speaking, the solvent is composed of at least two components, an extractant to interact with metal ions and a solvent. Although the extractant has significant influence on performance, it is also well known that some solvents also strongly affect metal extraction; these solvents are called functional solvents. Functional solvents are classified into two categories. The first is a solvent possessing metal extractability inherently and the second has an ability of a reversible phase transition from a homogeneous (water miscible) phase to heterogeneous (water immiscible) two-phase system. Typical examples of former solvent are diethyl ether or 4-methyl-2-pentanone used for the extraction of iron(III) chloride[Citation1] and 3-isopropoxy-N-isopropyl-propanamide, which extracted gold(III) ions directly without using any extractants.[Citation2] The second category contains solvents, where a phase transition from a two-phase system to a single system occurs. This extraction method is called as Homogeneous Liquid-Liquid Extraction (HLLE)[Citation3], a range of phase separation methods such as salting out[Citation4,Citation5], pH dependence[Citation6], solubility change with the temperature[Citation7,Citation8] etc. have been reported to strip the desired solute.
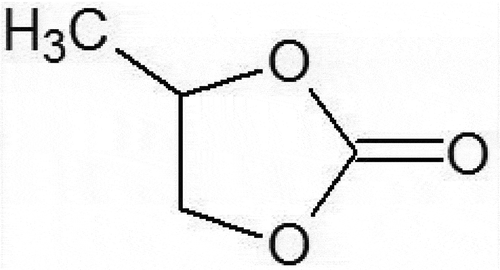
This study focused on the application of the use of the upper critical solution temperature (UCST) of 4-methyl-1,3-dioxolan-2-one (abbreviated as PC, see ) to extract the metal from an aqueous phase. Thus below UCST the aqueous phase containing metal ions is immiscible with the organic phase containing PC and extractant. When the temperature is increased beyond the UCST, the mixture forms a single phase providing enhancement in the extraction rate between the metal ions and the extractant. PC is less volatile, non-toxic, and a highly polar solvent and is used in cosmetics such as a nail polish remover and as an LSI washer in semiconductor manufacture. Although some studies on the application of PC in metal extraction have been reported[Citation9–Citation11], PC has not become popular as an extracting solvent yet. Other than the favorable properties as an extraction solvent mentioned above, PC has an UCST of 72ºC. However, the application of the UCST of PC to metal extraction has only been reported in the study on the extraction of iron(III) thenoyltrifluoroacetonate (TTA) reported by Murata et al.[Citation12] They reported qualitatively that the extraction rate of iron(III) (Fe3+) was promoted by elevating temperature and forming homogeneous phase in TTA-PC extraction system.
It was expected that the mass transfer of very hydrophobic extractants at an interface could be improved dramatically by making a homogeneous solution. For example, 7-(4-ethyl-1-methyloctyl)-8-hydroxy-quinoline (Kelex100) is well-known as a hydrophobic extractant. Kelex100 solubility in the aqueous phase is very small, complex formation proceeds mainly at the interface and therefore, the extraction rate is slow and dependent on the interfacial area compared to 8-quinolinol which is a basic structure of Kelex100. For example, it was reported that the extraction of gallium(III) (Ga3+) with Kelex100 from weakly acidic solution required 5 days to attain equilibrium.[Citation13] Therefore, the present study was undertaken to examine whether the use of PC in place of toluene in the extraction of trivalent metal ion such as aluminum(III) (Al3+), Fe3+, Ga3+ and indium(III) (In3+) with Kelex100 could significantly enhance the extraction rate.
Experimental
Reagents
Kelex100 was supplied with purity of 96.3% by HighChem Company Limited and was used as it was. PC, toluene, and all other reagents were purchased from Kanto Chemical Co., Inc., as analytical-grade reagents.
Extraction procedure
Aqueous phases were prepared by mixing 0.1 mmol/L Al3+, Fe3+, Ga3+, In3+ nitrates with 0.01 mol/L NH4NO3, and 0.1 mol/L triammonium citrate and pH of the mixed solution was adjusted using 3 mol/L ammonia water and 3 mol/L HNO3. An organic phase was prepared by dissolving Kelex100 in PC or toluene to a concentration of 0.01 mol/L. Two phases were heated by immersing in a water bath at 80ºC for 30 min. Equal volumes of the organic and aqueous solutions were transferred into 50 mL extraction vial and shaken at 80ºC for 24 hours. After cooling to room temperature and phase separation by centrifugation, the metal ions in the organic phase were back-extracted into equal volume of 1 mol/L HCl. The concentration of metal ions in the aqueous phase and the receiving phase were measured using inductively coupled plasma optical emission spectrometry (Vista-Pro, Varian). The equilibrium pH values of the aqueous phases were also measured. The extraction at 25ºC was conducted in the same manner as described above except for maintaining the temperature of solution at 25ºC throughout the course of extraction. The extraction percentage (%E) was calculated by Equation (1).
where [M]aq,init and [M]org are the initial concentration of the metal ion in the aqueous phase and the final concentration of the metal ion in the organic phase, respectively.
Results and discussion
UCST of propylene carbonate
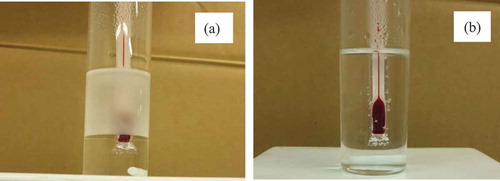
PC is fairly insoluble in water at room temperature, however, its solubility increases with increasing temperature. If both phases were heated to 60ºC, PC gradually dissolved in water and the water layer becomes opaque (). When the temperature was raised to more than 72ºC, PC and water dissolve in each other and become homogeneous (). PC and water separate into two phases again by lowering the temperature to less than 72ºC ().
Extraction at 25ºC
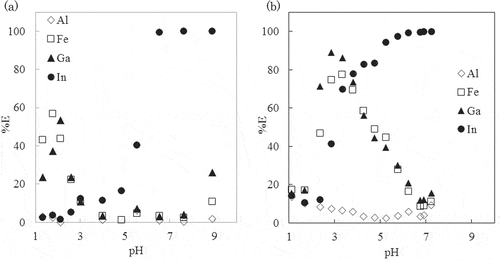
, show extraction curves obtained with toluene and the PC extraction system at 25ºC, respectively. The two extraction systems have shown some similarities in their extraction of these metals. For example, Al3+ is not extracted over the whole range of pH irrespective of solvent. As for Fe3+ and Ga3+, the maxima of extraction, %E, in PC system was 77% at pH 3.33 for Fe3+ and 89% at pH 2.86 for Ga3+. This was slightly higher than in the toluene system, that is, 57% at pH 1.78 for Fe3+ and 53% at pH 2.12 for Ga3+. The percentage extraction of In3+ increased with increasing pH and reached a maximum in both systems, in the pH range of 6.55–8.89 for toluene and in the pH range of 6.23–7.24 for PC. The clear advantage of PC as an extraction solvent over toluene was not recognized in the extraction at low temperature. Yamada et al. reported that 5 × 10–4 mol dm–3 Al3+, In3+, and Ga3+ were completely extracted within 3 h with 2 × 10–2 mol dm–3 8-quinolinol 1-octanol/octane mixed solution from neutral pH region in perchlorate solution.[Citation14] The lower extractability in the present extraction system compared with that in 8-quinolinol 1-octanol/octane extraction system can be ascribed to the use of a more hydrophobic extractant, Kelex100 and the addition of citrate salt as a masking reagent as to prevent hydrolysis of metal ions.
Extraction at 80ºC
shows extraction curves obtained with toluene extraction system at 80ºC. In the toluene extraction system, the extent of metal extraction was not affected by the increase in temperature. The extraction behaviors of metal ions is similar to those obtained at 25ºC (). That is, only In3+ was extracted effectively (95 ± 4.1%) in the pH range of 7.16–8.98, and other metals were not extracted sufficiently. While, in the PC extraction system, extraction behavior of metal ions at 80ºC () was quite different from those at 25ºC (). Surprisingly, Fe3+, In3+ and Ga3+ were all extracted with %E more than 98% over the pH range of 3.56–6.46; only Al3+ was not extracted. If chelate complex formation is exothermic, metal extraction is suppressed along with the increase of temperature and vice versa. Thus decreasing the distribution ratios along with increasing temperature has been reported for several extraction systems, such as extraction of silver(I) with dithizone chloroform(CHCl3) solution, silver(I) and cadmium(II) with 8-quinolinol in CHCl3[Citation15], and lanthanides with N,N-Diisobutyl-2-[octyl-(phenyl)- phosphoryl]-acetamide (CMPO).[Citation16] The values of enthalpy in Kelex100 extraction from NaOH solution, of which the extraction process was described by Equation (2), were determined to be −43 kJ mol−1 and −104 kJ mol−1 for Al3+ and Ga3+, respectively, and the distribution ratio for both metal ion diminished with increase of temperature.[Citation17]
where HL is Kelex100.
Effect of contact time
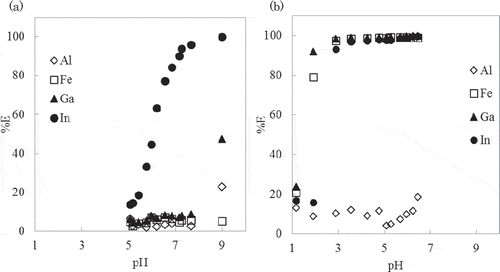
The effect of heating on the extraction was examined by changing the contact time at 80ºC. shows the relationship between %E and the contact time in toluene and shows the relationship between %E and the contact time in PC. As can be seen from ,, the rate of metal extraction was very slow at 25ºC in both toluene and PC extraction systems. So the change of equilibrium during the course of cooling from 80ºC to 25ºC can be considered to be negligibly small. Therefore, the contact time was defined as the shaking time at 80ºC and did not include the cooling time.
Figure 5. Effect of contact time on the extraction percentage of metal ions with toluene solution at 80°C.
pH: 8.06 ± 0.03.
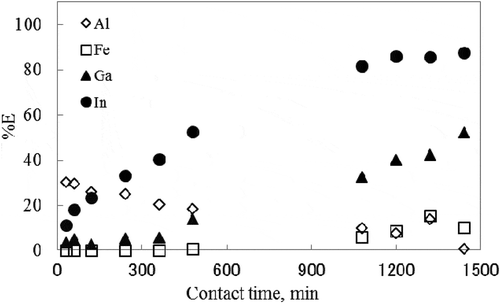
Figure 6. Effect of contact time on the extraction of metal ions with PC solution at 80°C.
pH: 6.08 ± 0.15.
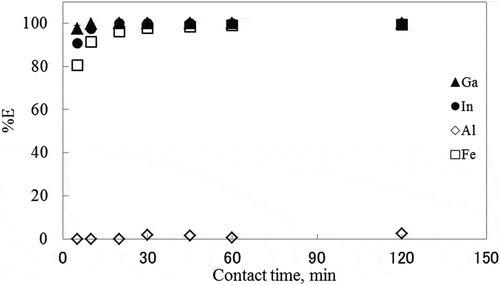
In the toluene extraction system, the extraction of In3+ took 1200 min to attain equilibrium, while other metals did not attain the extraction equilibrium even after 1440 min of contact. In the PC extraction system, %E of Fe3+, In3+, and Ga3+ exceeded 80%, 90%, and 97% in the first 5 min, respectively.
To compare the kinetics of extraction between toluene and PC more specifically, the extraction rate constant was calculated. The extraction mechanism of Al3+ in the sodium hydroxide solution using Kelex100 was reported as Equation (2) and the extraction rate was determined to be first order with respect to the Al3+ concentration in the aqueous phase.[Citation18] Inoue et al., reported that Ga3+ was extracted with Kelex100 from weakly acidic solution according to Equation (3) and the extraction reaction was first order.[Citation13]
Since aluminum, gallium, and indium belong to group 13 in periodic table, it was assumed that metal ions were extracted with a first-order reaction in the present extraction system. The extraction rate of the first-order reaction is expressed in Equation (4).
where [M]aq and [M]ini are metal ion concentration in the aqueous phase after shaking for a definite time and that before shaking with organic phase, respectively, and kex1 denotes the rate constant for metal ion extraction.
The percent extraction of metal ion in and were converted into ln([M]aq/[M]ini) and plotted against contact time. The approximate curves were obtained with these plots, and then the linear function and correlation coefficient (r2) were obtained. The slope in this linear function can be regarded as the apparent rate constant, kex1ʹ, of pseudo first order reaction and are summarized in . As can be seen , all the correlation coefficients for In3+ and Ga3+ were fairly good (r2 > 0.95) in both solvents, and consequently, the assumption of a pseudo first order reaction seems to be appropriate. However, correlation coefficients for Fe3+ shows poor fit with the correlation coefficient of 0.666 in this PC extraction system and 0.822 in toluene. Thus, it was assumed that Fe3+ was extracted via a second order reaction. Since the integrated rate equation for the second order reaction can be expressed by Equation (5), the inverse of the metal ion concentration in the aqueous phase after contact for a definite time was plotted against contact time,
Table 1. Rate constant for metal extraction.
where kex2 denotes the rate constant for metal ion extraction. The value of r2 obtained in PC extraction system were greater than 0.95, the slopes of these linear functions were regarded as the apparent rate constant, kex2ʹ, these values were used to evaluate the extraction rate of Fe3+. By simply comparing these rate constants, it can be concluded that the extraction rate of Fe3+, Ga3+, and In3+ in PC extraction system were around 15000, 740, and 380 times faster than those in toluene extraction system. Thus, the rate of metal extraction was accelerated dramatically by using PC at 80ºC in the present Kelex100 extraction system. Taking into account the above-mentioned results, this improvement in the extraction rate can be ascribed to the disappearance of the interface between water and PC due to UCST with PC.
Conclusion
This study clearly demonstrates that the HLLE method by utilizing upper critical solution temperature of PC accelerated the extraction rate of In3+ and Ga3+ in Kelex100 extraction system 740 and 380 times compared with those in toluene extraction system, respectively. In the Kelex100 toluene extraction system, the extractability of the metal ion was not improved significantly by increasing temperature from 25ºC to 80ºC, and therefore, the acceleration of extraction rate in PC extraction system can be ascribed to the disappearance of the interface due to the formation of homogeneous solution. It was shown that the present HLLE method by utilizing upper critical solution temperature of PC has potential to improve the extraction rate in the slow extraction systems using extremely hydrophobic extractant such as Kelex100.
References
- Rothe, J. W. The Extraction of Iron(III) from Hydrochloric Acidution by Ethylether. Chem.News. 1892, 66, 182.
- Tanaka, R.; Matsuo, S.; Noro, J.; Arakawa, R.; Komatsu, Y.; Fujinaga, K. Separation of Gold(III) from Transition Metals by the 3-isopropoxy-n-isopropylpropaneamide Extraction System. J. Ion Exchange. 2010, 21, 195–198.
- Anthemidis, A. N.; Kallirroy-Ioanna, G. I. Recent Developments in Homogeneous and Dispersive Liquid–Liquid Extraction for Inorganic Elements Determination. A Review. Talanta. 2009, 80, 413–421. DOI: 10.1016/j.talanta.2009.09.005.
- Matkovich, C. E.; Christian, G. D. Salting-out of Acetone from Water. Basis of a New Solvent Extraction System. Anal. Chem. 1973, 45, 1915–1921. DOI: 10.1021/ac60333a023.
- Hori, T.; Fujinaga, T. Analytical Use of Solvent Extraction with acetonitrile/water/chloroform and 1-propanol/water/cyclohexane Mixtures. Talanta. 1985, 32, 735–743. DOI: 10.1016/0039-9140(85)80176-4.
- Igarashi, S.; Yotsuyanagi, T. Homogeneous Liquid-liquid Extraction by pH Dependent Phase Separation with a Fluorocarbon Ionic Surfactant and Its Application to the Preconcentration of Porphyrin Compounds. Mikrochim. Acta. 1992, 106, 37–44. DOI: 10.1007/BF01242697.
- Murata, K.; Ikeda, S. Homogeneous Liquid-liquid Extraction Method. Bunsekikagaku. 1969, 18, 1137. (In Japanese).
- Hoogerstraete, T. V.; Onghena, B.; Binnemans, K. Homogeneous Liquid−Liquid Extraction of Metal Ions with a Functionalized Ionic Liquid. J. Phys. Chem. Lett. 2013, 4, 1659−1663.
- Stephens, B. G.; Suddeth, H. A. Extraction of the 1,10-Phenanthroline, 4,7-diphenyl-1,10- Phenanthroline, and 2,4,6-tripyridyl-sym-triazine Complexes of Iron(II) into Propylene Carbonate. Application to the Determination of Iron in Sea Water and Aluminum Alloy. Anal. Chem. 1967, 39(12), 1478–1480. DOI: 10.1021/ac60256a034.
- Stephens, B. G.; Loftin, J. C.; Looney, W. C.; Williams, K. A. Propylene Carbonate Extraction of tris(pentan-2,4-dione)-Iron(III) from Aqueous Solution: Application to the Spectrophotometric Determination of Iron. Analyst. 1971, 96(140), 230–234. DOI: 10.1039/an9719600230.
- Stephens, B. G.; Felkel, H. L., Jr.; Spinelli, W. M. Spectrophotometric Determination of Copper and bis(2,4,6-tri(2- pyridyl)-1,3,5-triazine)iron(II) into Propylene Carbonate. Anal. Chem. 1974, 46(6), 692–696. DOI: 10.1021/ac60342a013.
- Murata, K.; Yokoyama, Y.; Ikeda, S. Homogeneous Liquid-liquid Extraction Method. Extraction of iron(III) Thenoyltrifluoroacetonate by Propylene Carbonate. Anal. Chem. 1972, 44, 805–810. DOI: 10.1021/ac60312a009.
- Inoue, K.; Nakayama, D. Solvent Extraction of Gallium with Kelex100 from Weakly Acidic Aqueous Solutions. J. Jpn. Inst. Metals. 1988, 52, 567–571. (In Japanese). DOI: 10.2320/jinstmet1952.52.6_567.
- Yamada, H.; Hayashi, H.; Yasui, T. Utility of 1-octanol/octane Mixed Solvents for the Solvent Extraction of Aluminum(III), Gallium(III), and Indium(III) with 8-quinolinol. Anal. Sci. 2006, 22, 371–376. DOI: 10.2116/analsci.22.371.
- Hellwege, H. E.; Schweitzer, G. K. Temperature Changes in Solvent Extractions of Cadmium Oxinate. Anal. Chim. Acta. 1963, 28, 236–241. DOI: 10.1016/S0003-2670(00)87225-6.
- Koma, Y.; Watanabe, M.; Nemoto, S.; Tanaka, Y. Trivalent F-element Intra-group Separation by Solvent Extraction with CMPO-complexant System. J. Nucl. Sci. Technol. 1998, 35, 130–136. DOI: 10.1080/18811248.1998.9733833.
- Sato, T. Solvent Extraction of Gallium (III) and Aluminum (III) from Alkaline Solutions by 7-alkylated 8-hydroxyquinoline. Keikinzoku. 1986, 36, 137–142. (In Japanese).
- Sato, T. Liquid-liquid Extraction of Aluminum (III) from Sodium Hydroxide Solutions by Alkylated Hydroxyquinoline. Keikinnzoku. 1990, 40, 429–434.