?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Liquid–liquid extraction and liquid membrane transport behavior of tetravalent actinide ions viz. Th(IV), Np(IV), and Pu(IV) were investigated for the first time using a diglycolamide (DGA) based dendrimer with a tris(2-aminoethyl)amine (TREN) scaffold as the organic extractant. The generation of 1 dendrimer with six DGA pendent moieties (termed as TREN-G1-DenDGA) extracted Np(IV) more effectively than the other two ions, the trend being Np(IV) > Pu(IV) > Th(IV). The extraction studies of Np(IV) from 3 M HNO3 indicated a 1:1 (metal:ligand) species and the extraction efficiency increased with increasing nitric acid concentration (1–6 M). The transport efficiency of Np(IV) increased with the nitric acid concentration (1–6 M) as well as with the ligand concentration. A very low concentration of 5.75 × 10−4 M ligand, when used as the carrier, resulted in the transport of ca. 25% metal ion transport in 5 h, which increased to >85% with 4.4 × 10−3 M ligand. The transport efficiency of the metal ion across the SLM followed the trend Np(IV) > Th(IV) > Pu(IV). The membrane stability was not satisfactory as seen over a period of 5 days suggesting long-term use may require regular replenishment of the carrier solvent. The effective diffusion coefficient (Deff) of Np(IV)-TREN-G1-DenDGA were determined by the lag-time method and was found to be 5.1 × 10−8 cm2/s.
Introduction
Neptunium is an important element in the group of actinide elements and is produced as an activation product in nuclear reactors. 237Np is used as a target for the production of 238Pu, which has found application as a power source in the thermoelectric generator in the space program due to its high heat output.[Citation1] The quantity of 237Np (t1/2: 2.14 × 106 yr) in the spent fuel of Pressurized Heavy Water Reactor (PHWR) origin with a burn up of about 6700 MWD/Te (megawatt-day per tonne) is approximately 25 g per tone of the fuel discharged and the quantity may be higher with high burn up fuels.[Citation2] Hence, it is very important to separate this long-lived isotope from the spent fuel not only for its subsequent use to produce 238Pu but also to get rid of the long-term radiotoxicity and health hazards arising out of this isotope.[Citation3] Neptunium can be present in the spent fuel dissolver solution in different oxidation states (+4, +5, +6) and during the PUREX (Plutonium Uranium Redox Extraction) process it gets distributed among the organic and aqueous phases because of the different extractability of its different ionic species.[Citation4–6] The +4 and +6 oxidation states of neptunium are extractable by the PUREX organic phase, while the +5 state is weakly extractable. Generally, more than 40% of neptunium stays in the aqueous raffinate, while around 60% neptunium goes to the TBP (tri-n-butyl phosphate) phase during the first cycle of the PUREX process.[Citation7]The chemistry of neptunium in nitric acid medium is quite complicated due to its complex redox reactions.[Citation8–12] Therefore, it is very important to adjust the neptunium oxidation state in the nitric acid solution to the extractable (+4, +6) or weakly extractable (+5) oxidation state to route it to the organic phase or to the aqueous phase, respectively. Neptunium can exist in different oxidation states (+4, +5 and +6) in 3 M HNO3 due to the close proximation of the reduction potentials of different oxidation state couples of neptunium.[Citation13,Citation14] By using stronger reducing agent, such as the mixture of ferrous sulfamate and hydroxyl amine hydrochloride it can be possible to reduce +6 and +5 oxidation states of neptunium in dilute nitric acid to the +4 oxidation state[Citation2,Citation15] and therefore, can be extracted in the organic phase containing suitable extractant. Similarly, using a suitable complexing agent, the extracted Np(IV) can also be stripped to the aqueous solution.
The chemistry of the extraction of neptunium by TBP is well known. However, TBP forms degraded phosphorus-containing products due to radiolysiswhich interfere while stripping the metal ions from the organic phase and also facilitate the formation of insoluble phosphate compounds,and can limit the amount of waste loading into a glass matrix.[Citation16] On the other hand, diglycolamide-based extractants are green extractants (CHON principle) and form degraded products that are innocuous in nature.[Citation17] These extractants in suitable diluents (isodecanol-n-dodecane mixtures) have a very high extraction ability for the trivalent actinides/lanthanides and tetravalent actinides from acidic feed solutions.[Citation18–20] However, multiple DGA (diglycolamide) extractants where three or four DGA moieties are appended to a central nitrogen or carbon atom are superior extractants for the extraction of trivalent actinides/lanthanides and tetravalent actinides than simple DGA ligands such as TODGA (N,N,N,’N’-tetra-n-octyl diglycolamide, ).[Citation21–25] Generally 3 to 4 TODGA molecules form aggregate in non-polar diluents (n-dodecane) when contacted with dilute nitric acid. Therefore, use of multiple DGA extractant where 3 to 4 DGA moieties are there in the molecule can alleviate the aggregation formation and make the phenomena diluent independent. Moreover, multiple-DGA-based extractant can enhance the extraction efficiency of metal ion due to cooperative action.
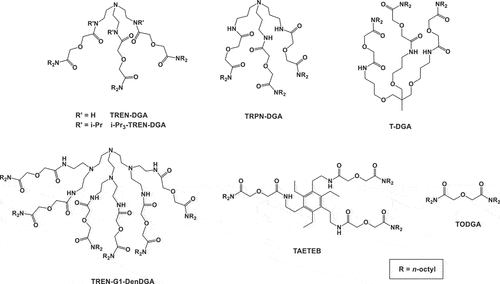
Figure 1. Structures of TREN-DGA, i-Pr3-TREN-DGA, TRPN-DGA, T-DGA, TAETEB, TODGA, and TREN-G1-DenDGA.
Due to the very high extraction efficiency for the tri- and tetravalent actinide ions and also their favorable extraction and stripping kinetics, these tailor-made ‘exotic’ multiple DGA ligands can be used in very small quantities (millimolar concentration) in molecular diluents (5% isodecanol-95% n-dodecane) as carrier extractants in supported liquid membranes (SLMs) for transport studies of metal ions. There are many advantages of using SLMs for metal ion transport, such as simultaneous extraction and stripping, small inventory of carrier ligands, no third-phase formation, no phase entrainment, etc.[Citation26–28]
Recently, we have reported the extraction and transport behavior of tetravalent actinides (Np and Pu) with two different alkyl derivatives of N-pivoted multiple DGA ligands (termed as TRPN-DGA and i-Pr3-TREN-DGA, ) using SLM, which showed very promising extraction and transport of Np(IV) and Pu(IV) in nitric acid solution.[Citation29] Another multiple DGA extractant, TAETEB () where 3 DGA units were attached to a benzene ring platform offers more flexibility for the DGA units to bind with the metal ion compared to the DGA units attached to a single C or N atom, thereby expecting higher extraction efficiency for the metal ions. The objective of the present study was to see the extraction and transport behaviour of the very important neptunium in the tetravalent oxidation state by TREN-G1-DenDGA () where 6 DGA moieties are present in the structure thereby expecting higher metal ion extraction not only due to the presence of more number of DGA units in the structure but also due to the enhanced hydrophobicity of the metal ion-ligand complex formed. Furthermore, the exotic ligands are better suited for a SLM study where the ligand inventory was very low. The diluent composition of 5% isodecanol-95% n-dodecane was chosen based on our previous studies.[Citation29,Citation30] For comparison purpose, we have also carried out some studies with Th(IV) and Pu(IV) with this ligand. Extraction and stripping kinetic and transport studies were performed with Np(IV) and to a lesser extent also with Th(IV) and Pu(IV) for comparison. To our knowledge, this is the first report on liquid–liquid extraction and supported liquid membrane transport studies involving tetravalent actinide ions with a TREN-based dendrimer.
Experimental
Materials
Chemicals
TREN-G1-DenDGA was synthesized as reported previously.[Citation30] The characterization of the ligands was done by 1H NMR spectroscopy and HR-MS and the purity was found to be >99%. The diluents, n-dodecane and isodecanol, were procured from Lancaster, UK, and SRL, Mumbai, respectively, and were used as obtained. N-(2-Hydroxyethyl)ethylenediamine-N,N’N’-triacetic acid (HEDTA) (Sigma-Aldrich, USA), oxalic acid (SRL, India), α-hydroxyisobutyric acid (α-HIBA) (Sigma-Aldrich, USA) and 2-thenoyltrifluoroacetone (TTA) (Fluka, Switzerland) were obtained and used as such. The required feed nitric acid solutions were prepared on diluting suprapur nitric acid (Merck) using milliQ water (Millipore, USA). The feed acid solutions were standardized using known strength NaOH, and the end point was detected by phenolphthalein (Fluka) as indicator. A ferrous sulfamate solution (0.3 M) was prepared by dissolving iron powder (BDH) in sulfamic acid (Aldrich) and the required quantity was used in the experiment. All the other chemicals used here were of AR grade.
Membranes
Porous PTFE (polytetrafluoroethylene) flat sheet membranes (dimensions: diameter 47 mm, thickness 80 µm, pore size 0.45 µm, and porosity 72%) were procured from Sartorius, Germany. The pore size was confirmed from Hg porosimetry measurements, while the thickness was checked by a Mututoya digital micrometer.
Radiotracer
239Np tracer was prepared on irradiating uranyl nitrate hexahydrate in the Dhruva reactor at BARC, Mumbai, at a thermal neutron flux of 1 × 1014 n cm−2 s−1. The radiotracer239Np is formed by (n, γ) reaction with 238U followed by β- emission. 239Np was separated from the host matrix using a reported method[Citation31] in which ca. 2 mL of 1 M HNO3 was used to dissolve the irradiated product, and a few drops of ferrous sulfamate (FS) (0.3 M) and a 50–100 mg of hydroxylamine hydrochloride (HA) solid were added into it to convert Np into its +4 state. The mixture was contacted with 2 mL of 0.5 M HTTA in xylene (Merck) for a few minutes. This two-phase mixture was centrifuged at 5000 rpm for 10 minutes, and the organic phase was collected in a vial. This separated organic phase was contacted with 2 mL of 8 M HNO3 to strip neptunium. Subsequently, the aqueous phase containing the neptunium was contacted two times with xylene to remove any traces of organic material present in the aqueous phase. The aqueous phase containing neptunium(IV) in 8 M HNO3 was used as the 239Np stock. Its radionuclidic purity was checked using gamma ray spectrometry and scintillation counting with an alpha-beta discriminator, and the purity was found to be >99%. Carrier-free 234Th tracer was freshly ‘milked’ in the laboratory from natural uranium, which was previously loaded in Aliquat-336/chloroform following a reported method.[Citation32] The purity of the 234Th was checked using gamma ray spectrometry. Laboratory stock Pu (mainly 239Pu) was purified by an anion exchange resin to remove 241Am traces, prior to its use, and the purity was checked by alpha as well as gamma ray spectrometry.[Citation33] The oxidation state of Pu(IV) and Np(IV) was adjusted to +4 using NaNO2 and an FS-HA mixture, respectively. The oxidation state of +4 was confirmed from the slope analysis of the linear fitting of the log–log plots of DPu or DNp with HTTA concentrations in xylene.[Citation34]
234Th and 239Np were assayed by gamma ray counting using a NaI(Tl) scintillation counter (Para Electronics) coupled to a multi-channel analyzer (ECIL, India). Pu was assayed by liquid scintillation counting (Hidex, Finland) using the Ultima gold scintillation cocktail (Perkin Elmer). The concentration of the metal ions, viz., Th4+, Np4+, and Pu4+ in the studies was <10−12 M, 10−13 M, and 10−6 M, respectively.
Methods
Liquid–Liquid extraction studies
Liquid–liquid extraction studies were carried out by equilibrating equal volumes (usually 1 mL) of the organic and aqueous phases together in 10 mL capacity stoppered Pyrex glass tubes in a thermostated water bath at 25.0 ± 0.1ºC with constant shaking for about 1 h. After the required time of shaking, the tubes were settled, centrifuged (at 3000 rpm for 3 minutes) and 100 µL aliquots of organic as well as aqueous phases were removed and assayed radiometrically. Generally, the organic phase contained 5.75 × 10−4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane. The presence of isodecanol was required to improve the solubility of the ligand.[Citation35] On the other hand, the aqueous phase was generally taken as low to moderate concentrations of nitric acid (1 M − 6 M) spiked with the required radiotracer. The D-ratio is defined as the ratio of the activity per unit volume in the organic phase to that in the aqueous phase. Since equal volumes of the organic and aqueous phases were used, the percentage stripping (%S) can be defined as follows:
The solvent extraction studies were done in duplicate which showed a reproducibility within ±2% and the mass balance was found to be within ±5% (which was determined from the difference of counts per 100 µL of the initial aqueous sample and the total counts in 100 µL of organic and aqueous phases after equilibration).
Supported liquid membrane transport studies
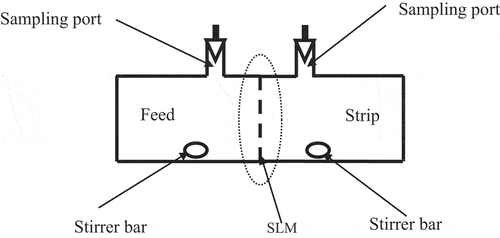
The supported liquid membrane studies were performed in a two-compartment transport cell () in which the PTFE membrane, containing the ligand solution in the membrane pores, was fixed in between the two compartments and was tightened using a Parafilm strip. The compartments were clamped together with a metallic clamp. However, prior to that, the PTFE membrane was soaked in the ligand solution for 60 minutes in a Petri dish to ensure uniform soaking of the membrane. Before fixing the membrane in the transport cell, the soaked membrane was gently wiped with a tissue paper to remove the extra ligand solution stuck to the membrane. One side of the transport cell was filled with the feed solution, while the other side was filled with the receiver solution. Both solutions were stirred continuously by a stirrer bar and a synchronous magnetic stirrer. The samples (usually 100 µL) were removed simultaneously from both the source and strip phases through their respective sampling port as shown in and assayed radiometrically. All the transport studies were carried out at room temperature (25 ± 1°C) under ambient conditions.
Different transport parameters, viz., diffusion coefficient (D0), effective diffusion coefficient (Deff), permeability coefficient (P), were determined from the transport data (vide infra). In addition, the percentage transport (%T) of the metal ion to the receiver phase was determined from EquationEquation (2)(2)
(2) to quantify the transport of the metal ion at a given time.
The % metal ion retained in the feed phase at a given time t is determined by EquationEquation (3)(3)
(3) :
where Cf,t and Cr,t represent the concentrations of the metal ions in the source and the receiver phases, respectively, at time ‘t’ and Cf,0 represents that in the feed phase at the start of the experiment. The permeability coefficient (P) is determined using the following equation[Citation36]:
where Q stands for the effective surface area, which is obtained by multiplying the geometrical surface area (A) and the porosity (ε) of the PTFE membrane used, and Vf is the feed volume, which is kept at 20 mL in the present study. The geometric surface area of the membrane exposed towards the solution was 4.94 cm2, while the porosity was 72%.
Results and discussion
Extraction and stripping studies
Kinetics of extraction and stripping
To achieve rapid transport of the metal ions across a SLM, the extraction kinetics at the source–membrane interface and the stripping kinetics at the membrane–receiver interface should be reasonably fast. Therefore, separate liquid–liquid extraction and stripping kinetic studies of the metal ions were conducted prior to investigate their transport across an SLM. The extraction kinetics of Th(IV), Np(IV), and Pu(IV) was determined from a feed solution of 3 M HNO3 with 5.75 × 10−4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane, whereas the stripping kinetics was followed with the extracted metal ions in the organic phase by 1 M α-HIBA. The stripping agent, 1 M α-HIBA, was chosen due to its very high stripping efficiency for Np(IV) as evident from . The efficacy of different stripping agents for Np(IV) stripping follows the order 1 M α-HIBA > 0.1 M HEDTA +0.05 M HNO3 > 0.1 M HEDTA +0.5 M HNO3 > 0.5 M oxalic acid +0.5 M HNO3 (). The extraction kinetics data of different metal ions suggested that the equilibrium D-ratios were obtained within 30 minutes, whereas those of the stripping kinetics studies were obtained within 10 minutes (). These studies reveal that the extraction kinetics is comparatively slower than the stripping kinetics. An earlier investigation with the comparatively less bulky ligand TREN-DGA () suggested relatively faster extraction kinetics (within 10 minutes, equilibrium D-ratio was achieved) with both Np(IV) and Pu(IV).[Citation37] This indicates that the bulkiness in the ligand structure plays an important factor in the extraction kinetics. A similar slower kinetics of extraction and faster stripping were found in our earlier investigation with Eu(III) and Am(III) with this ligand.[Citation38]
Figure 3. Extraction and stripping kinetics of (a) Np(IV), (b) Pu(IV) and (c) Th(IV) ions. Extraction: [TREN-G1-DenDGA]: 5.75 × 10−4 M in 5% isodecanol-95% n-dodecane; Aqueous phase: 3 M HNO3 containing the metal ions. Stripping: Organic phase: Above extracted complex; Aqueous phase: 1 M α-HIBA.
![Figure 3. Extraction and stripping kinetics of (a) Np(IV), (b) Pu(IV) and (c) Th(IV) ions. Extraction: [TREN-G1-DenDGA]: 5.75 × 10−4 M in 5% isodecanol-95% n-dodecane; Aqueous phase: 3 M HNO3 containing the metal ions. Stripping: Organic phase: Above extracted complex; Aqueous phase: 1 M α-HIBA.](/cms/asset/ead293d3-5934-4431-8e6e-496cd13a2a24/lsei_a_2074501_f0003_oc.jpg)
Table 1. Stripping of Np(IV) extract in 5.75 × 10 −4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane with different stripping agents.
The D-ratios upon extraction follow the trend: DNp(IV) > DPu(IV) > DTh(IV). The higher D-ratio of Np(IV) than that of Pu(IV) is surprising and cannot be explained based on the ionic potential of the metal ion. One of the reasons it may be due to the higher overall aqueous nitrate complexation constant of Pu(IV) compared to Np(IV) that drives to form stronger metal–nitrate complexes in the aqueous phases with Pu(IV). Dendrimer being a bulky ligand, it is quite reasonable to assume that it cannot effectively displace nitrate ion to accommodate itself in the structure with Pu(IV) compared to that with Np(IV). A higher DNp(IV) than DPu(IV) was also reported by Sengupta et al. in the case of CMPO (carbamoylmethyl phosphine oxide).[Citation39] Due to the encouraging extraction and stripping results of Np(IV) obtained with TREN-G1-DenDGA and considering the importance of neptunium, most of the studies were carried out with Np(IV), while a few experiments were carried out with other tetravalent metal ions, viz., Th(IV) and Pu(IV), for comparison purposes.
Effect of nitric acid concentration
The effect of the nitric acid concentration on the D-ratio of Np(IV) was studied by varying it in the range of 0.5 M to 6 M keeping the ligand concentration as 5.75 × 10−4 M in 5% isodecanol-95% n-dodecane. shows that the DNp(IV)-value increases monotonously with increasing nitric acid concentration, suggesting a solvation mechanism for the extraction of the metal ion. This extraction by the ligand occurred after metal–ligand complexation at the aqueous–organic interface, followed by extraction of the complex into the organic phase according to the following equation:
Figure 4. DNp(IV) at varying concentrations of (a) nitric acid with [TREN-G1-DenDGA]: 5.75 × 10−4 M in 5% isodecanol-95% n-dodecane and (b) TREN-G1-DenDGA at 3 M HNO3; Temperature: 25ºC.
![Figure 4. DNp(IV) at varying concentrations of (a) nitric acid with [TREN-G1-DenDGA]: 5.75 × 10−4 M in 5% isodecanol-95% n-dodecane and (b) TREN-G1-DenDGA at 3 M HNO3; Temperature: 25ºC.](/cms/asset/50367d78-140d-4596-b602-f89d943473c9/lsei_a_2074501_f0004_b.gif)
where M is Np, L is TREN-G1-DenDGA and y is the number of L molecules involved in EquationEquation (5)(5)
(5) . The species with the subscripts ‘(aq)’ and ‘(org)’ represent those in the aqueous and the organic phases, respectively.
Comparison of the extraction behavior of Np(IV) with that of the lower homologue of the present ligand, viz., TREN-DGA, showed a similar trend.[Citation37] However, the DNp(IV)-ratio is higher with TREN-G1-DenDGA at a given nitric acid concentration. For example, DNp(IV) at 3 M HNO3 was found to be 3.8 with 1 × 10−3 M TREN-DGA, whereas it is 6.1 with 5.75 × 10−4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane (). This may be explained by the higher lipophilicity of TREN-G1-DenDGA than TREN-DGA due to the presence of more DGA moieties in the former. Consequently, it is obvious that the DNp(IV)-ratio with TODGA is much lower than that of TREN-G1-DenDGA at a corresponding ligand concentration (assuming ML type species extracted with TREN-G1-DenDGA) (vide infra) (). This suggests that due to the cooperative metal-ligand complexation by the multi-dentate TREN-G1-DenDGA, a stronger metal-ligand complex is formed than that with TODGA where generally three to four TODGA molecules are required for metal-ligand complex formation.[Citation40] Comparing different DGA ligands, the DNp(IV)-values follow the trend: i-Pr3-TREN-DGA > TAETEB > TRPN-DGA > T-DGA > TREN-G1-DenDGA > TREN-DGA >> TODGA (assuming three to four TODGA ligands are required to bind one metal ion) (). DNp(IV) is much higher with multiple DGA-based ligands compared to TODGA. However, the lower DNp(IV)-ratio with TREN-G1-DenDGA compared to other multiple DGA ligands, except for TREN-DGA, but especially with i-Pr3-TREN-DGA may be due to its bulkier structure, which may cause steric hindrance upon complexing with a Np(IV) ion. Therefore, in addition to cooperative complexation, other factors, such as lipophilicity, steric hindrance, +I effect of the alkyl group, etc., are important for the D-ratios of a metal ion.
Table 2. DNp(IV) values with different ligands in 5% isodecanol-95% n-dodecane at 3.0 M HNO3.
Effect of the ligand concentration
To understand the metal ion extraction from the feed–membrane interface to the membrane phase, the metal–ligand stoichiometry was determined from a ligand concentration variation experiment at 3 M HNO3. Therefore, a liquid–liquid extraction study was conducted in which the extraction of Np(IV) was carried out with different concentrations of TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane (). The data were fitted to the log–log plot of DNp(IV) versus ligand concentration (EquationEquation (6)(6)
(6) ). This equation was derived from EquationEquation (5)
(5)
(5) .
Figure 5. Transport profile of Np(IV) across flat sheet SLMs from 1 M, 3 M and 6 M HNO3 feed solutions using 5.75 × 10−4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane. Stripping solution in receiver is 1 M α-HIBA.
where L is TREN-G1-DGA.
The results of the solvent extraction experiments are given in .
Table 3. Slopes of log–log plot of DNp(IV) versus TREN-G1-DenDGA concentration; extraction carried out at 3 M HNO3.
The two-phase extraction equilibrium constant (Kex) and the distribution ratio (DM) are defined as follows (EquationEquations (7)(7)
(7) and (Equation8
(8)
(8) )):
where ‘a’ is the activity of the species and a=γ.c, where ‘γ’ is the activity coefficient of the respective species and ‘c’ is the concentration of the species in the solution. The details are given in the supporting information (SI)
From and it follows that TREN-G1-DenDGA forms an ML type complex with Np(IV), whereas its lower homologue TREN-DGA reportedly formed ML and ML2 type species. Moreover, the higher homologues of TREN-DGA, viz., TRPN-DGA and i-Pr3-TREN-DGA, also formed ML type species. This suggests that as the bulkiness or the flexibility in the ligand structure increases, the chances of formation of ML-type species increase. Previously, we have reported that TREN-G1-DenDGA preferred to coordinate to the trivalent Eu(III) in a ‘crab like’ fashion forming an ‘inclusion’ complex, in which three DGA moieties of the ligand coordinate to the central metal ion to satisfy the nine-coordination of the Eu(III) ion, while nitrate ions remained in the outer sphere.[Citation30,Citation31] Therefore, it is highly likely that in case of the Np(IV) ion, being smaller in size than Eu(III), the eight-coordination of Np(IV) may be fully satisfied by the DGA moieties of the TREN-G1-DenDGA ligand. The four nitrate ions remain in the outer sphere to neutralize the charge of the metal ion forming [Np(NO3)4.L neutral species, which get extracted into the organic medium.
Transport studies
Transport studies were carried out to investigate the effect of feed composition and ligand concentration in the membrane on the transport behavior and to compare the transport behavior of other tetravalent metal ions by comparing the transport flux and permeability coefficients. In addition, diffusion coefficients and stability of the membrane were also studied.
Effect of feed acid concentration
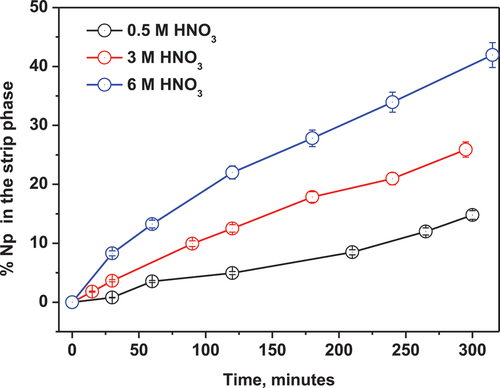
The feed nitric acid concentration of high-level waste (HLW) lies between 3 and 4 M. Therefore, a study on the effect of the nitric acid concentration on the separation of Np(IV) is very relevant and important to understand its role on the transport efficiency of the metal ion.
The nitric acid concentration in the feed phase was varied from 1 to 6 M keeping the ligand concentration in the membrane as 5.75 × 10−4 M in 5% isodecanol-95% n-dodecane and 1 M α-HIBA as the stripping agent, while monitoring the transport of Np(IV) in the receiver phase with time. It can be seen in that the transport efficiency of the metal ion in the receiver phase increases with time at a given nitric acid concentration in the feed phase. Similarly, the transport efficiency also increases with the nitric acid concentration at a given time. For example, at 1 M, 3 M, and 6 M HNO3, the % transport of Np(IV) in the strippant after 5 h was found to be 14.8%, 26.0%, and 41.2%, respectively. This can be easily explained by EquationEquation (6)(6)
(6) , which shows a positive dependence on the nitrate ion concentration in the feed (vide supra). With an increase in the nitric concentration, the equilibrium shifts to the forward direction and results in more extraction of the metal ion in the membrane phase. Consequently, there was an increase in the transport efficiency. However, if one compares the transport efficiency of Np(IV) by TREN-G1-DenDGA with TRPN-DGA and i-Pr3-TREN-DGA,[Citation26] there are two noticeable differences. Firstly, the percentage of cumulative transport of Np(IV) follows the order: i-Pr3-TREN-DGA > TRPN-DGA >> TREN-G1-DenDGA (). Secondly, whereas transport of Np(IV) increases with nitric acid concentration with TREN-G1-DenDGA, there is a decreasing trend at higher nitric acid concentration with TRPN-DGA and i-Pr3-TREN-DGA.[Citation29] The lower transport of Np(IV) with TREN-G1-DenDGA compared to i-Pr3-TREN-DGA and TRPN-DGA may be due to the lower DNp(IV) and the lower diffusion coefficient (Deff) of its metal-ligand complex (vide infra) (due to formation of a bulkier metal-ligand complex). The increasing trend of the transport of Np(IV) with nitric acid by TREN-G1-DenDGA indicates that the competitive reaction of acid uptake by the ligand (EquationEq. 9
(9)
(9) ) may not be important for the ligand to affect the transport efficiency compared to that with TRPN-DGA and i-Pr3-TREN-DGA.
Table 4. Transport of Np(IV) from different feed nitric acid concentrations after 5 h. Receiver phase: 1 M α-HIBA, [Ligand]: 5.75 × 10−4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.
where L is TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.
This is also evident from , showing that the DNp(IV)-ratios monotonously increase with nitric acid concentration, whereas for TRPN-DGA and i-Pr3-TREN-DGA, a plateau and a decreasing trend are observed at higher nitric acid concentration (>2 M HNO3), respectively.[Citation29] TREN-G1-DenDGA contains six DGA moieties in a molecule, whereas TRPN-DGA and i-Pr3-TREN-DGA only have three DGAs. So, even if 50% DGAs are forming adduct with HNO3 in case of TREN-G1-DenDGA, still three DGA moieties are free to complex with the metal ion. However, the same number of protons would form adduct with all DGA moieties of the other two ligands, and consequently, no sites will be left to coordinate to the metal ion. Therefore, even though the transport efficiency with this dendrimer DGA ligand is comparatively lower than that with TRPN-DGA and i-Pr3-TREN-DGA, these ligands may be very useful for metal ion transport at higher nitric acid concentrations.
Effect of ligand concentration
The above discussion showed that transport of Np(IV) is low (26%) at 3 M HNO3 with 5.75 × 10−4 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane. For an efficient separation of metal ions, useful for practical purpose, high throughput, selectivity, and stability of the membrane are required. Therefore, it is very important to increase the transport rate of Np(IV) through the membrane. As one of the ways, the transport rate can be increased by increasing the ligand concentration in the membrane, which eventually increases the extraction of metal ions in the membrane from the source–membrane interface according to EquationEquation (6)(6)
(6) . Therefore, the ligand concentration in the membrane was varied from 5.75 × 10−4 M to 4.42 × 10−3 M to investigate the effect of the ligand concentration on the transport of Np(IV) keeping the feed as 3 M HNO3 and the strip as 1 M α-HIBA. The results are given in and . It is clear from that with the increase of the ligand concentration, the transport efficiency increases. For example, at ligand concentrations of 5.75 × 10−4 M, 2.21 × 10−3 M and 4.42 × 10−3 M, the transport of Np(IV) is 26.0%, 64.6%, and 85.2%, respectively. The permeability coefficient (P) is also increasing with the ligand concentration. This can be explained from EquationEquation (10)
(10)
(10) .
Figure 6. Transport profile of Np(IV) across flat sheet SLMs from 3 M HNO3 feed solutions using different concentrations of TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane. Stripping solution in receiver is 1 M α-HIBA.
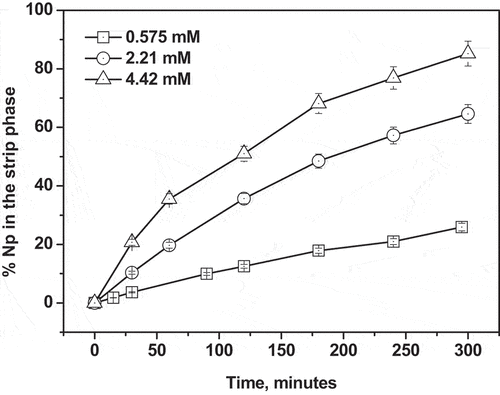
Table 5. Transport of Np(IV) with different ligand concentrations in the membrane. Feed: 3 M nitric acid; Receiver phase: 1 M α-HIBA; [Ligand]: TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane; Data after 5 h.
Here, DNp(IV) is the distribution ratio, Da and D0 are the aqueous and membrane diffusion coefficients of Np(IV), respectively, whereas da, d0, and τ are the aqueous diffusion layer thickness, membrane thickness, and tortuosity factor, respectively. Assuming that the rate-limiting step is the diffusion of the Np(IV)-TREN-G1-DenDGA complex, the first term in the denominator of EquationEquation (10)(10)
(10) can be ignored and, therefore, the equation simplifies to:
This equation shows that with the increase of the TREN-G1-DenDGA concentration, the permeability coefficient value (P) may increase or decrease depending on the relative values of DNp(IV) and D0, and hence, the permeability coefficient values are dependent on the ligand concentration. The increase of the ligand concentration can increase the D-ratios, whereas it may decrease the D0 values due to increase of the viscosity of the membrane phase. In the present case, the permeability coefficient values increase with the ligand concentration, indicating that DNp(IV) increases more than the decrease of D0 with the ligand concentration. The effect of D0 on the concentration may not be significant owing to the small concentration of the ligand used here, which may not change the viscosity of the medium significantly. A similar increase of the permeability coefficient value was also noticed in our previous study with the same ligands with Eu(III) and Am(III) transport.[Citation38]
Comparative transport behavior of Th(IV), Np(IV), and Pu(IV)
The encouraging results of the transport of Np(IV) with TREN-G1-DenDGA led us to compare the transport behavior of other tetravalent metal ions, viz., Th(IV) and Pu(IV), with this ligand under identical experimental conditions. For the transport study, 4.42 × 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane was taken in the membrane, whereas the feed and the strip phases were 3 M HNO3 and 1 M α-HIBA, respectively. The transport profiles of Th(IV), Np(IV), and Pu(IV) are given in , whereas the 5 h transport data and the permeability coefficient values are presented in . The transport values of Th(IV), Np(IV), and Pu(IV) are 72.4%, 85.2%, and 60.5%, respectively, while the corresponding permeability coefficient values (P) are 0.446 ± 0.010 cm s−1, 0.643 ± 0.015 cm s−1, and 0.316 ± 0.030 cm s−1. Compared to the tetravalent metal ions, the transport of the trivalent actinide ion, Am(III), was significantly lower.[Citation38] As the sizes of all these complexes are nearly the same, assuming 1:1 (M:L) complex formation, the diffusion of the species across the liquid membrane should take nearly the same time, and the difference in the transport rates are attributed to the difference in the extraction efficiency of the ligand towards the metal ions. As discussed below, ionic potential considerations may not be entirely true for the tetravalent ions, while this may be true for the trivalent ions.
Figure 7. Transport profile of different actinide ions across a flat sheet supported liquid membrane from 3 M HNO3 as feed phase and 1 M α-HIBA as stripping phase. [Ligand]: 4.42 x 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.
![Figure 7. Transport profile of different actinide ions across a flat sheet supported liquid membrane from 3 M HNO3 as feed phase and 1 M α-HIBA as stripping phase. [Ligand]: 4.42 x 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.](/cms/asset/c1c08f87-7e0c-4b88-99e5-d34c91d144d8/lsei_a_2074501_f0007_b.gif)
Figure 8. Lag-Time of Np(IV) transport across a flat sheet SLM from 3 M HNO3 as feed phase and 1 M α-HIBA as stripping phase. [Ligand]: 4.42 x 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.
![Figure 8. Lag-Time of Np(IV) transport across a flat sheet SLM from 3 M HNO3 as feed phase and 1 M α-HIBA as stripping phase. [Ligand]: 4.42 x 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.](/cms/asset/5d76c188-6af9-4993-a96d-40b3c5bd5805/lsei_a_2074501_f0008_b.gif)
Table 6. Transport of different actinides from 3 M HNO3 as feed acidity after 5 h. Receiver phase: 1 M α-HIBA; [Ligand]: 4.42 × 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.
The lowest transport rate of Pu(IV) compared to that of Np(IV) and Th(IV) is very unusual and does not follow the ionic potential trend of the metal ion, which is the highest with Pu(IV).[Citation41] The experimental data showed that the D-ratios follow the order: DNp(IV) > DPu(IV) > DTh(IV) (vide supra), which explains why the highest transport was observed with Np(IV) among the tetravalent ions. On the other hand, the higher transport of Th(IV) compared to that of Pu(IV) after >4 hours may be due to the slightly different stripping kinetics of the metal ions. A similar trend of higher transport of Np(IV) than that of Pu(IV) was reported by us with TRPN-DGA and i-Pr3-TREN-DGA, although the D-ratio of the latter ion was higher than that of the former ion.[Citation26]
Mechanism of transport
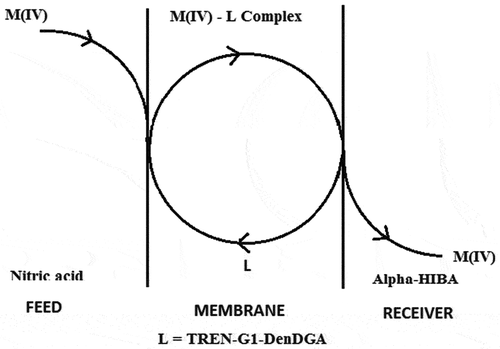
The mechanism of transport in a supported liquid membrane system is generally given in four parts[Citation33] viz. i) extraction of the metal ion at the feed–membrane interface, ii) diffusion of the M-L complex species along the thickness of the membrane, iii) decomplexation or stripping of the metal ion at the membrane–receiver interface and iv) diffusion of the carrier extractant from the receiver side of the SLM to its feed side.
As shown in , the extraction step takes longer time than the stripping step and the slow transport of the metal ions in the present case is a consequence of the slow extraction kinetics. Furthermore, the slow transport of the metal ions is also dependent on the diffusion of the metal–ligand complex, which arguably is rather bulky in view of the six DGA moieties attached to the TREN backbone. The mechanism of the transport is depicted in .
Diffusion coefficient
The diffusion coefficient is an important transport parameter, which gives an idea about the amount of metal ion transport per unit area of the membrane per unit time under the influence of a unit concentration gradient. This parameter for Np(IV) was determined by the lag-time method using the following formula:
where do is the membrane thickness (cm), ε is the membrane porosity and tlag is the lag-time. The membrane thickness is 0.007 cm; ε is reported to be 0.64. The lag-time is the time that is required for the metal ion complex to first appear in the receiver solution from the start of the experiment. The lag-time plot for Np(IV) is shown in and the lag-time is determined by the intersection point of the two straight lines. The lag-time data along with the diffusion coefficient values are given in . The diffusion coefficient (D0) can also be obtained using the Wilke-Chang equation[Citation42] which is defined as
Table 7. Diffusion coefficients of Np(IV) determined by the lag-time and Wilke-Chang equations. Feed: 3 M HNO3; Receiver: 1 M α-HIBA; [L]: 4.42 × 10 −3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane.
where M is the molecular weight of the complex, Np(NO3)4.L (L=TREN-G1-DenDGA), χ and η are the solvent association parameter and the viscosity of the solvent, respectively, Vm is the molar volume of the Np-L complex, and T is the temperature in degree Kelvin. The molar volume of TREN-G1-DenDGA was calculated as 5462.16 cm3 mol−1[Citation43] whereas the molecular weight of the Np(NO3)4.TREN-G1-DenDGA complex was 2925. The dynamic viscosity of n-dodecane was used as an approximation for the diluent system containing 5% isodecanol-95% n-dodecane being 1.34 mPa.s and χ is taken as unity for the solvent system. The diffusion coefficient values determined by the lag-time method were compared to those obtained by the Wilke-Chang equation (). The diffusion coefficients calculated with the Wilke-Chang equation are around two orders of magnitude higher than those obtained from the lag-time method. This could be due to the non-spherical complex diffusion in the actual complex vis-à-vis nearly spherical species diffusion assumed in the Wilke-Chang diffusion model. The diffusion coefficients obtained with the analogous ligands TRPN-DGA and i-Pr3-TREN-DGA are also included in the table for comparison purpose, which indicated higher diffusion coefficients of Np(IV) with these ligands, pointing to the effect of a bulkier size of the ligand on the diffusion coefficient.
Stability of the membrane
For a liquid membrane involving transport of metal ions, it is important to show a high trans-membrane flux stability in consecutive cycles of operations. Generally, it has been observed that SLMs exhibit poor stability and the transport efficiency for metal ion transport decreases from day 1 itself. Therefore, the stability of the present membrane was investigated by determining the transport profile of Np(IV) transport, with the present membrane system consisting of 4.42 × 10−3 M TREN-G1-DenDGA in 5% isodecanol-95% n-dodecane in the membrane, while feed phase and strip phase are 3 M HNO3 and 1 M α-HIBA, respectively. The transport profile of Np(IV) transport is measured up to 5 days and before the start of each day/cycle, the feed and strip-phase solutions were changed with fresh solutions of the same composition without disturbing the membrane. The transport profile on each day of transport (up to 5 h) is shown in , whereas the transport data at 5 h and the permeability coefficient values are given in . In this case, the transport efficiency also decreases already from day 1 itself, demonstrating the poor stability of the membrane. For example, the transport of Np(IV) at 5 h on days 1, 2, and 5 is 85.2%, 79.0%, and 48.9%, respectively. A stability study at comparatively lower ligand concentration (5.75 × 10−4 M) with Eu(III) as the metal ion under identical feed and strip compositions showed a comparatively poorer stability of the membrane,[Citation35] indicating that the ligand concentration may affect its stability. This indicates that the ligand is possibly leaching from the membrane into the adjacent aqueous phases while stirring the phases during the experiment.
Figure 9. Stability of the SLMs for Np(IV) transport studies. Feed: 3 M HNO3; Strip: 1 M α -HIBA; [Ligand]: 4.42 x 10−3 M TREN-G1-DGA in 5% isodecanol-95% n-dodecane.
![Figure 9. Stability of the SLMs for Np(IV) transport studies. Feed: 3 M HNO3; Strip: 1 M α -HIBA; [Ligand]: 4.42 x 10−3 M TREN-G1-DGA in 5% isodecanol-95% n-dodecane.](/cms/asset/cf1f4a05-4fb3-4380-b4f6-b336e4f1314d/lsei_a_2074501_f0009_oc.jpg)
Table 8. Stability of the SLMs for Np(IV) transport studies. Feed nitric acid concentration: 3 M; Receiver phase: 1 M α-HIBA; [Ligand]: 4.42 × 10−3 M TREN-G1-DGA in 5% isodecanol-95% n-dodecane. Data after 5 h.
When the ligand concentration in the membrane is quite low, the net ligand concentration in the membrane after leaching becomes very small and hence there is a large decrease in the permeability coefficient values upon increasing the number of cycles of operation. For example, the permeability coefficient values of Eu(III) under identical feed and strip compositions at 5.75 × 10−4 M TREN-G1-DenDGA in the membrane on days 1, 2 and 6 are 0.159 ± 0.002 cm s−1, 0.082 ± 0.001 cm s−1, and 0.014 ± 0.002 cm s−1, respectively. On the other hand, the permeability coefficient values obtained with the present system for Np(IV) transport on days 1, 2 and 5 are 0.643 ± 0.015 cm s−1, 0.554 ± 0.003 cm s−1, and 0.241 ± 0.016 cm s−1, respectively. Therefore, the percentage decrease of permeability coefficient values from day 1 to day 2 is 13.8% for Np(IV) transport at an initial ligand concentration in the membrane of 4.42 × 10−3 M, whereas it is 48.4% for Eu(III) transport at an initial ligand concentration in the membrane of 5.75 × 10−4 M. A similar poor stability of the membrane was also noticed with a TREN-DGA containing membrane.[Citation44]
Conclusions
The present investigation deals with extraction and transport studies of Np(IV) with an N-pivoted dendrimer-DGA ligand (TREN-G1-DenDGA) dissolved in 5% isodecanol-95% n-dodecane. In select cases, extraction and transport studies were also performed for Th(IV) and Pu(IV) for comparison purpose. Slow extraction (within 30 minutes equilibrium D-ratio is reached) and fast stripping (within 10 minutes equilibrium D-ratio is reached) kinetics of the metal ions was found. The solvent extraction data showed the formation of a 1:1 (metal:ligand) species with Np(IV) when extracted at 3 M HNO3 and an increasing trend of extraction of Np(IV) to the organic phase with nitric acid concentration (1–6 M). Similarly, an increasing trend of Np(IV) ion transport across a SLM to the receiver phase (1 M α-HIBA) was observed with the nitric acid concentration, which contrasts with our earlier investigations with TRPN-DGA and i-Pr3-TREN-DGA. This demonstrates the effectiveness of the TREN-G1-DenDGA ligand for metal ion transport at higher nitric acid concentrations. This may be due to the presence of a larger number of DGA-moieties in TREN-G1-DenDGA compared to that in TRPN-DGA and i-Pr3-TREN-DGA. This results in a lesser fraction of DGA.HNO3 adduct moieties with TREN-G1-DenDGA (or conversely more free DGAs available for binding a metal ion) and hence a better transport of metal ions compared to TRPN-DGA and i-Pr3-TREN-DGA at higher nitric acid concentrations. The permeability coefficient values of Np(IV) transport increased with increasing ligand concentration in the membrane. A permeability coefficient value of 0.643 ± 0.015 cm s−1 was obtained for Np(IV) transport of 85.2% after 5 h when the ligand concentration in the membrane was 4.42 × 10−3 M. The cumulative transport data at 5 h follow the order: Np(IV) > Th(IV) > Pu(IV), which deviates from their respective D-ratios being DNp(IV) > DPu(IV) > DTh(IV). Though the liquid membrane stability was not satisfactory, it can be alleviated by regular replenishment in the case of a longer run for large-scale processing. It has been clearly demonstrated that TREN-G1-DenDGA is superior to other related ligands, especially at higher acidities.
LSEI_2074501_Supplementary_Material
Download PDF (455.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/07366299.2022.2074501.
References
- Lange, R. G.; Carroll, W. P. Review of Recent Advances of Radioisotope Power Systems. Energy Convers. Manag. 2008, 49, 393–401. DOI: 10.1016/j.enconman.2007.10.028.
- Jagasia, P.; Dhami, P. S.; Rao, K. S.; Yadav, J. S.; Mohapatra, P. K.; Bhattacharyya, A.; Mahanty, B.; Kaushik, C. P.; Pujari, P. K. Extraction Chromatography Based Separation of Neptunium from Purex Dissolver and HLLW Stream. BARC technical report, BARC/2020/E/001.
- Thompson, R. C. Neptunium: The Neglected Actinide: A Review of the Biological and Environmental Literature. Radiat. Res. 1982, 90(1), 1–32. DOI: 10.2307/3575792.
- Srinivasan, N.; Ramaniah, M.; Patil, S.; Ramakrishna, V. Estimation of Neptunium in a Fuel Reprocessing Plant. J. Radioanal. Chem. 1971, 8(2), 223–229. DOI: 10.1007/BF02518186.
- Zhang, H.; Ye, G.-A.; Li, L.; Zheng, W.-F.; Cong, H.-F.; Xiao, S.-T.; Yang, H.; Lan, T. Simulation of the Neptunium Behavior During the First Solvent Extraction Cycle in the PUREX Process. J. Radioanal. Nucl. Chem. 2013, 295(2), 883–888. DOI: 10.1007/s10967-012-2297-6.
- Takanashi, M.; Homma, S.; Koga, J.; Matsumoto, S. Neptunium Concentration Profiles in the Purex Process. J. Alloys Comp. 1998, 271, 689–692. DOI: 10.1016/S0925-8388(98)00188-1.
- Edwards, S.; Andrieux, F.; Boxall, C.; Sarsfield, M. J.; Taylor, R. J.; Woodhead, D. Neptunium(iv)-Hydroxamate Complexes: Their Speciation, and Kinetics and Mechanism of Hydrolysis. Dalton Trans. 2019, 48(2), 673–687. DOI: 10.1039/C8DT02194E.
- Wang, Y.; Zhang, Z.; Abergel, R. J. Hydroxypyridinone-Based Stabilization of Np(iv) Enabling Efficient U/np/pu Separations in the Adapted PUREX Process. Sep. Purif. Technol. 2021, 259, 18178. DOI: 10.1016/j.seppur.2020.118178.
- Taylor, R. J.; Gregson, C. R.; Carrott, M. J.; Mason, C.; Sarsfield, M. J. Progress Towards the Full Recovery of Neptunium in an Advanced PUREX Process. Solv. Extr. Ion Exch. 2013, 31(4), 442–462. DOI: 10.1080/07366299.2013.800438.
- Precek, M.; Paulenova, A. Kinetics of Reduction of Hexavalent Neptunium by Nitrous Acid in Solutions of Nitric Acid. J. Radioanal. Nucl. Chem. 2010, 286(3), 771–776. DOI: 10.1007/s10967-010-0724-0.
- Nakahara, M., and Yoshikazu, K. Influence of Nitric Acid and Nitrous Acid on Oxidation and Extraction of Neptunium with Double Scrub Flow Sheet in Simplified Solvent Extraction Process. J. Chem. Eng. Japan. 2011,44, 1103280161.
- Morita, Y.; Kubota, M. Recovery of Neptunium, JAERI-M-84-043, 1984.
- Ohno, A.; Hennig, C.; Rossberg, A.; Funke, H.; Scheinost, A. C.; Bernhard, G.; Yaita, T. Electrochemical and Complexation Behavior of Neptunium in Aqueous Perchlorate and Nitrate Solutions. Inorg. Chem. 2008, 47, 8294–8305. DOI: 10.1021/ic8009095.
- Chatterjee, S.; Bryan, S. A.; Casella, A. J.; Peterson, J. M.; Levitskaia, T. G. Mechanisms of Neptunium Redox Reactions in Nitric Acid Solutions. Inorg. Chem. Front. 2017, 4(4), 581–594. DOI: 10.1039/C6QI00550K.
- Paulenova, A.; Vandegrift, I. G. F. Factors Controlling Redox Speciation of Plutonium and Neptunium in Extraction Separation Processes; Oregon State Univ: Corvallis, OR, 2013.
- Krishnamurthy, M. V.; Sipahimalani, A. T. Radiolytic Degradation of TBP-HNO3 System: Gas Chromatographic Determination of Radiation Chemical Yields of N-Butanol and Nitrobutane. J. Radioanal. Nucl. Chem. Lett. 1995, 199(3), 197–206. DOI: 10.1007/BF02162368.
- Zarzana, C. A.; Groenewold, G. S.; Mincher, B. J.; Mezyk, S. P.; Wilden, A.; Schmidt, H.; Modolo, G.; Wishart, J. F.; Cook, A. R. A Comparison of the γ -Radiolysis of TODGA and T(EH)DGA Using UHPLC-ESI-MS Analysis. Solvent. Extr. Ion Exch. 2015, 33(5), 431–447. DOI: 10.1080/07366299.2015.1012885.
- Sasaki, Y.; Sugo, Y.; Suzuki, S.; Tachimori, S. The NOVEL EXTRACTANTS, DIGLYCOLAMIDES, for the EXTRACTION of LANTHANIDES and ACTINIDES in HNO 3 – N -DODECANE SYSTEM. Solvent. Extr. Ion Exch. 2001, 19(1), 91–103. DOI: 10.1081/SEI-100001376.
- Ansari, S. A.; Pathak, P. N.; Manchanda, V. K.; Hussain, M.; Prasad, A.; Parmar, V. S. N,N,N′,N′-Tetraoctyl Diglycolamide (TODGA): A Promising Extractant for Actinide-Partitioning from High-Level Waste (HLW). Solvent. Extr. Ion Exch. 2005, 23(4), 463–479. DOI: 10.1081/SEI-200066296.
- Gujar, R. B.; Ansari, S. A.; Mohapatra, P. K.; Murali, M. S.; Manchanda, V. K. Comparative Evaluation of Two Substituted Diglycolamide Extractants for ‘Actinide Partitioning’. J. Radioanal. Nucl. Chem. 2010, 284(2), 377–385. DOI: 10.1007/s10967-010-0467-y.
- Mohapatra, P. K.; Iqbal, M.; Raut, D. R.; Verboom, W.; Huskens, J.; Godbole, S. V. Complexation of Novel Diglycolamide Functionalized Calix[4]arenes: Unusual Extraction Behaviour, Transport and Fluorescence Studies. Dalton Trans. 2012, 41(2), 360–363. DOI: 10.1039/C1DT11561H.
- Wu, L.; Fang, Y.; Jia, Y.; Yang, Y.; Liao, J.; Liu, N.; Yang, X.; Feng, W.; Ming, J.; Yuan, L. Pillar[5]arene-Based Diglycolamides for Highly Efficient Separation of Americium(III) and Europium(III). Dalton Trans. 2014, 43(10), 3835–3838. DOI: 10.1039/C3DT53336K.
- Wehbie, M.; Arrachart, G.; Karame, I.; Ghannam, L.; Pellet-Rostaing, S. Triazole Diglycolamide Cavitand for Lanthanide Extraction. Sep. Purif. Technol. 2016, 169, 17–24. DOI: 10.1016/j.seppur.2016.06.003.
- Wehbie, M.; Arrachart, G.; Arrambide Cruz, C.; Karame, I.; Ghannam, L.; Pellet-Rostaing, S. Organization of Diglycolamides on Resorcinarene Cavitand and Its Effect on the Selective Extraction and Separation of Hrees. Sep. Purif. Technol. 2017, 187, 311–318. DOI: 10.1016/j.seppur.2017.06.062.
- Mohapatra, P. K.; Iqbal, M.; Raut, D. R.; Verboom, W.; Huskens, J.; Manchanda, V. K. Evaluation of a Novel Tripodal Diglycolamide for Actinide Extraction: Solvent Extraction and SLM Transport Studies. J. Membr. Sci. 2011, 375(1–2), 141–149. DOI: 10.1016/j.memsci.2011.03.042.
- Noble, R., and Stern, S. Membrane Separations Technology: Principles and Applications; Amsterdam, The Netherlands: Elseviers, 1995.
- Kocherginsky, N. M.; Yang, Q.; Seelam, L. Recent Advances in Supported Liquid Membrane Technology. Sep. Purif. Technol. 2007, 53(2), 171–177. DOI: 10.1016/j.seppur.2006.06.022.
- De Gyves, J.; De San Miguel, E. R. Metal Ion Separations by Supported Liquid Membranes. Ind. Eng. Chem. Res. 1999, 38(6), 2182–2202. DOI: 10.1021/ie980374p.
- Mahanty, B.; Verma, P. K.; Mohapatra, P. K.; Leoncini, A.; Huskens, J.; Verboom, W. Pertraction of Np(iv) and Pu(iv) Across a Flat Sheet Supported Liquid Membrane Containing Two N-Pivoted Tripodal Diglycolamides. Sep. Purif. Technol. 2020, 238, 116418. DOI: 10.1016/j.seppur.2019.116418.
- Bhattacharyya, A.; Leoncini, A.; Mohapatra, P. K.; Verma, P. K.; Kanekar, A. S.; Yadav, A. K.; Jha, S.; Bhattacharyya, D.; Egberink, R. J. M.; Huskens, J., et al. A Diglycolamide-Functionalized TREN-Based Dendrimer with a ‘Crab-Like’ Grip for the Complexation of Actinides and Lanthanides. Dalton Trans. 2018, 47(42), 15164–15172. DOI: 10.1039/C8DT03051K.
- Mohapatra, P. K.; Ruikar, P. B.; Manchanda, V. K. Separation of Neptunium and Plutonium from Acidic Medium Using 3-Phenyl-4-Benzoyl-5-Isoxazolone. Radiochim. Acta. 2002, 90(6), 323–327. DOI: 10.1524/ract.2002.90.6.323.
- Thakur, P.; Veeraraghavan, R.; Mohapatra, P. K.; Manchanda, V. K.; Dash, K. C. Extraction of Ternary Complexes of Thorium(iv) with 3-Phenyl-4-Benzoyl-5-Oxazolone and Neutral Donors from Nitric Acid Medium. Talanta. 1996, 43(8), 1305–1312. DOI: 10.1016/0039-9140(96)01883-8.
- Kaya, A.; Kudo, H.; Shirahashi, J.; Suzuki, S. The Purification of Plutonium by Anion Exchange in Nitric Acid. J. Nucl. Sci. Technol. 1967, 4(6), 289–292. DOI: 10.1080/18811248.1967.9732746.
- Sengupta, A.; Mohapatra, P. K.; Iqbal, M.; Huskens, J.; Verboom, W. A Diglycolamide-Functionalized Task Specific Ionic Liquid (TSIL) for Actinide Extraction: Solvent Extraction, Thermodynamics and Radiolytic Stability Studies. Sep. Purif. Technol. 2013, 111, 264–270. DOI: 10.1016/j.seppur.2013.07.005.
- Sharma, J. N.; Kumar, A.; Kumar, V.; Pahan, S.; Janardanan, C.; Tessi, V.; Wattal, P. K. Process Development for Separation of Cesium from Acidic Nuclear Waste Solution Using 1,3-Dioctyloxycalix[4]arene-Crown-6 + Isodecyl Alcohol/n-Dodecane Solvent. Sep. Purif. Technol. 2014, 135, 176–182. DOI: 10.1016/j.seppur.2014.08.016.
- Danesi, P. R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19(11–12), 857–894. DOI: 10.1080/01496398408068598.
- Gujar, R. B.; Mohapatra, P. K.; Verboom, W. Extraction of Np4+ and Pu4+ from Nitric Acid Feeds Using Three Types of Tripodal Diglycolamide Ligands. Sep. Purif. Technol. 2020, 247, 116986. DOI: 10.1016/j.seppur.2020.116986.
- Mahanty, B.; Verma, P. K.; Mohapatra, P. K.; Leoncini, A.; Huskens, J.; Verboom, W. Pertraction of Americium(iii)/europium(iii) Ions Across a Flat Sheet Supported Liquid Membrane Containing a TREN Based Generation 1 Diglycolamide Dendrimer Ligand, Submitted for publication.
- Sengupta, A.; Mural, M. S.; Thulasidas, S. K.; Mohapatra, P. K. Solvent System Containing CMPO as the Extractant in a Diluent Mixture Containing N-Dodecane and Isodecanol for Actinide Partitioning Runs. Hydrometallurgy. 2014, 147-148, 228–233. DOI: 10.1016/j.hydromet.2014.05.014.
- Jensen, M. P.; Yaita, T.; Chiarizia, R. Reverse-Micelle Formation in the Partitioning of Trivalent F-Element Cations by Biphasic Systems Containing a Tetraalkyldiglycolamide. Langmuir. 2007, 23(9), 4765–4774. DOI: 10.1021/la0631926.
- Clark, D. L.; Hecker, S. S.; Jarvinen, G. D., and Neu, M. P. In The Chemistry of the Actinide and Transactinide Elements, eds; Morss, L. R., Edelstein, N. M., Fuger, J., and Katz, J. J., Eds.; Springer: The Netherlands, 2006; Vol. 2, 1157 .
- Wilke, C. R.; Chang, P. Correlation of Diffusion Coefficients in Dilute Solutions. AIChE J. 1955, 1(2), 264–270. DOI: 10.1002/aic.690010222.
- Schotte, W. Prediction of the Molar Volume at the Normal Boiling Point. Chem. Eng. J. 1992, 48(3), 167–172. DOI: 10.1016/0300-9467(92)80032-6.
- Mahanty, B.; Ansari, S. A.; Mohapatra, P. K.; Leoncini, A.; Huskens, J.; Verboom, W. Liquid-Liquid Extraction and Facilitated Transport of F-Elements Using an N-Pivot Tripodal Ligand. J. Hazard. Mater. 2018, 347, 478–485. DOI: 10.1016/j.jhazmat.2017.12.068.