Abstract
Inrecent years, the use of spray drying for the production of anhydrobiotics has gained the interest of functional food manufacturers, mainly due to cost efficiencies and enhanced product and process flexibility (e.g., enhanced shelf life). In the present work, spray-drying conditions (air inlet temperature and feed flow rate) were optimized for the microencapsulation of the thermo sensitive probiotic lactobacilli strains Lactobacillus acidophilus stabilized in a 60:20:20 (w/w) maltodextrin: whey protein concentrate: D-glucose carrier. A 23 full-factorial experimental design was constructed with air inlet temperature (120, 140, and 160°C) and feed flow rate (6, 7.5, and 9.0 mL/min) as the independent variables and total viable counts (TVC), water activity (a w ), and cyclone recovery (CR) defined as the dependent variables. The increase in air inlet temperature from 120 to 160°C induced a significant (p < 0.001) reduction in the TVC from 9.02 to 7.20 log cfu/g, which corresponds to a97.5% loss of the L. acidophilus viable counts. On the other hand, the increase in the feed flow rate from 6 to 7.5 mL/min significantly reduced (p < 0.001) the heat-induced viability loss. A further increase in the feeding rate did not further modify the achieved thermo protection, and a detrimental impact of cyclone recovery (reduction) and water activity (increase) of the powder was observed. Using pruned quadratic mathematical models, the optimum spray-drying conditions for the production of maximally viable microencapsulated L. acidophilus were 133.34°C and 7.14 mL/min. The physicochemical and structural characteristics of the powders produced were acceptable for application with regards to residual water content, particles mean size, and thermo physical properties to ensure appropriate storage stability under room temperature conditions, with a low inactivation rate of L. acidophilus. Microcapsules appeared partially collapsed by scanning electron microscope with a spherical shape with surface concavities.
INTRODUCTION
The term probiotics refers to live microorganisms, which when administered at sufficient amounts (usually at 106–107 cfu/g of product), confer health effects that are beneficial to the host.[ Citation 1 ] The incorporation of probiotics in real food systems is challenging for food manufacturers due to their sensitivity to the harsh processing and chemical conditions used in the food process industry. Conditions may induce cellular damage through osmotic and thermal stresses (e.g., thermal processing, high solutes concentration) and through increased redox potential.[ Citation2-4 ] In addition, if storage conditions are not ideal, physical state changes (glassy to rubbery) may take place, triggering biochemical and enzymatic reactions that detrimentally affect the bacterial survival.[ Citation2-4 ] Over the last few years, the probiotics industry has experienced a remarkable market share increase, as a broad range of food products containing probiotics have been launched, including classic yogurt-based carriers, ice creams, fruit beverages, non-dairy spreads, breakfast cereals, and health supplements for direct consumption.[ Citation 3 , Citation5-12 ]
Dehydration processes such freeze drying, spray drying, freeze–spray drying, vacuum, and fluidized bed drying are among the most common practices for the production of anhydrobiotics for incorporation into food systems while retaining cell viability after processing.[ Citation 2 ] Although freeze drying is considered one of the best practices for reducing thermal damage, spray drying is more advantageous in terms of cost, energy, and throughput.[ Citation 13 ] Despite the wide demand for low-cost probiotics, the use of spray drying as a standard production technique is challenging due its detrimental impact on the cellular integrity of probiotics during drying (if not correctly optimized) and resultant reduction in stability of encapsulated bacteria during product aging.[ Citation 2 , Citation 3 , Citation 13 ] It has been demonstrated that heat-induced cellular damage is primarily associated with changes in the physical state of the membranes; for example, crystalline to gel phase transition, modification of the cytoplasm fluidity, peroxidation of the lipid membrane bilayer, structural modification of macromolecular constructs (e.g., proteins or nucleic acids unfolding), and alteration of other biological processes in the cells.[ Citation 2 , Citation 14 ] In addition to the carrier–probiotic interaction, the impact of the spray-drying process is associated with process parameters (inlet and outlet air temperature, feed flow rate, residence time at the drying chamber, design parameters of the drying chamber, temperature of the drying medium, etc.), thermodynamic processes (heat and mass transfer rates during droplets dehydration), drying kinetics (impact of steady and falling drying rates), and the biology of the bacteria to be encapsulated (species and strain type, adaptation of the bacteria to heat or osmotic stress conditions, growth state of the culture media).[ Citation 3 , Citation15-27 ] Gardiner et al.[ Citation 3 ] reported a strain-dependent decline of viable counts of Lactobacillus paracasei NFBC and Lactobacillus salivarius UCC as function of increasing outlet temperature, with the latter strains exhibiting the highest sensitivity to NaCl (a marker of thermal damage). The same researchers reported that the viability of the bacteria was optimized when an air outlet temperature of 80–85°C was used. For most thermo sensitive strains such as L. acidophilus or Lactobacillus rhamnosus GGan outlet air temperature ranging from 70 to 80°C is recommend to minimize spray drying–induced cellular injuries.[ Citation 5 , Citation 17 ] The use of high feed flow rates can also reduce heat-induced cell damage, but this approach resulted in an increased water activity of the final product and changes to the morphological features of the spray-dried powder.[ Citation 22 , Citation 27 ]
The effect of carrier wall material on probiotic survival through spray drying has been studied previously.[ Citation 5 , Citation 10 , Citation17-21 ] The total solids concentration of the carrier aliquot as well as the presence of ingredients that can induce a significant depression of the melting temperature of the micro particles has been reported to critically affect the structural integrity of the cytoplasmic membranes and control the osmotic pressure that leads to membranes rupture.[ Citation 2 ] For that reason, materials that ensure good encapsulating capacity and impart acceptable powder functional characteristics (porosity, free-flowing ability, anticaking, and good wetting and dispersing properties) are often chosen as the carrier wall material, in addition to other ingredients that exhibit thermo protective features, such as disaccharides (lactose, sucrose, or trehalose), dextrose, or polyols (mannitol, sorbitol), or act as probiotic growth stimulants (fructo- and galacto-oligosaccharides).[ Citation 2 , Citation 3 , Citation17-19 , Citation 26 ]
Recently, Ying et al.[ Citation 5 ] demonstrated that the use of complex carbohydrate–protein systems as potential carriers for anhydrobiotics can confer improved performance over storage. In the present study, the impact of the spray-drying conditions (inlet temperature and feeding rate) on the thermo sensitive L. acidophilus NCIMB 701748 strain microencapsulated in a ternary carrier system comprised of maltodextrin, whey protein concentrate, and D-glucose systems was investigated. The optimum spray-drying conditions required for the production of maximally viable dry probiotic formulations with acceptable physicochemical and structural characteristics were determined.
MATERIALS AND METHODS
Preparation of Probiotics
L. acidophilus NCIMB 701748 obtained from the NCIMB culture collection (NCIMB Ltd., Aberdeen, Scotland) was incubated at 37°C with 5% CO2 for 24 h. A small amount of the colonies was collected with a sterilized loop and suspended in the cryo-medium of Roti-Store systems (Roti-Store, Carl-Roth GmbH, Karlsruhe, Germany) and the plastic bead cultures were stored in a freezer (New Brunswick Scientific, U57085, Histon, Cambridge, UK) at −80°C.
Five beads of the deep-frozen L. acidophilus were placed in 500 mL of MRS broth (Oxoid Ltd., Basingstoke, UK) and incubated for 48 h at 37°C under anaerobic conditions in plastic jars containing Anaero Gen (Oxoid Ltd.). Bacterial cells were harvested by centrifugation (Sigma Laborzentifugen, SciQuip 2-16, Osterode am Harz, Germany) at 3,000 g for 5 min, and after discarding the supernatant liquid, the recovered pellets were washed once with phosphate buffer saline pH 7.0 (Dulbecco A PBS, Oxoid Ltd.). The cell suspensions were centrifuged at the same conditions and the supernatant PBS was carefully discarded.
Preparation of the Drying Media
Twelve grams of maltodextrin 15 DE (C Dry MD 01910, Cargill Ltd., Manchester, UK), 4 g of whey protein concentrate (Lacprodan DI-8090, Arla A/S, Viby, Denmark), and 4 g of D-glucose (Fisher Chemicals, Loughborough, UK) were blended together and balanced to 100 g with distilled water. The solutions were left to fully hydrate for 1 h at room temperature under magnetic stirring and subsequently they were heat-treated at 90°C for 10 min to destroy pathogens and allow complete protein denaturation. The carbohydrate–protein aliquots were rapidly cooled at room temperature using an ice bath and the L. acidophilus pellets were suspended. Non significant differences in the initial viable L. acidophilus counts of the carrier aliquots were observed (9.02 ± 0.02 log cfu/g).
Spray Drying and Storage of Probiotic Powders
The inoculated media with L. acidophilus were dried using a Buchi B-290 laboratory spray dryer (Buchi, Flawil, Switzerland) and the carrier aliquot was kept under low-speed agitation throughout the spray-drying process using a magnetic stirrer. A 23 factorial experimental design (Table 1) used to establish the optimum spray-drying conditions (inlet and feeding rate) in terms of maximal strain survival and cyclone recovery and minimum moisture levels. The spray dryer was operated at three different air inlet temperatures (120, 140, and 160°C) and feed flow rates (6, 7.5, and 9) mL min−1, smf the drying air flow rate (35 m3 h−1) and compressor air pressure (0.5 MPa) were kept constant throughout the drying process. The outlet temperature varied proportionally with air inlet temperature and feed flow rate conditions (Table ). The dry probiotic formulations were collected from the cyclone separator vessel, placed in sealed glass vials, and stored at room temperature in desiccators containing saturated lithium chloride (LiCl, Fisher Scientific) solutions to provide dry conditions (a w = 0.11).
TABLE 1 Codification of the independent variables (air inlet temperature and feed flow rate) used for the construction of the response surface design
TABLE 2 Effects of the spray-drying conditions (air inlet temperature and feed flow rate) on outlet temperature, water activity, total viable counts of L. acidophilus NCIMB 701748, and recovery rate of the powders in the cyclone separator
Design of the Experiments
A full-factorial design (n = 32) was used for this course of experiments with air inlet temperature (X 1) and feed flow rate (X 2) as factors and the total viable counts (TVC) after spray drying, the water activity of the spray-dried product (a w ), and the cyclone recovery percentage as the responses. Full-factorial design of experiments (DOE) have previously been successfully applied in a wide range of applications; for example, quality and process optimizations studies in food systems and consumer preference studies.[ Citation28-30 ] Although full-factorial DOE approaches are generally regarded as time and cost consuming due to the large number of experiments required, they do offer the best practice approach for process or product optimization where the factor interactions cannot be neglected.[ Citation 31 ]
Analysis of variance (ANOVA) was performed to estimate the significance (p < 0.05) of the main effects (linear and quadratic) and their interactions (linear, linear–quadratic, and quadratic–quadratic). The effects of the inlet air temperature and feed flow rate were modeled in a full quadratic mathematical model using response surface methodology as described in Eq. (Equation1):
Enumeration of the Bacteria
The L. acidophilus microcapsule powders as well as the drying media aliquots were suspended and diluted in phosphate buffer saline (Dulbecco A PBS, Oxoid Ltd.) under constant shaking for 10 min at room temperature to ensure complete dissolution of the powders. Serial dilutions in PBS were carried out and subsequently pour plated on molten MRS agar and the plates were incubated at 37°C for 72 h under anaerobic conditions. Enumeration of the bacteria was performed in triplicate following the standard plating methodology and the total counts of the viable bacteria were expressed as log colony forming units per gram (log cfu/g).
The viability of the bacteria after the spray-drying process was calculated according to the formula
Characterization of the Optimized Final Product
Moisture Content and Water Activity
The moisture content was calculated according to American Association of Cereal Chemists (St. Paul, MN, USA) method AA-15A (Approved Methods of Analysis, 11th ed.). Two grams of the powder were placed in aluminum pans and dried at 105°C for 24 h. Residual moisture content was calculated according to the formula
DSC Measurements
A standard power-compensated Perkin Elmer DSC-7 (Perkin Elmer Ltd., Beaconsfield, UK) was used for calculation of the glass transition temperature of the optimized formulation. A small portion (15–20 mg) of the powder was weighted in a high-pressure, stainless steel pan and heated from −30 to 150°C at a rate of 10°C/min. A double heating–cooling scanning step was performed, and thermal properties (onset, midpoint, and offset glass transition temperatures and specific heat capacity change, ΔC p ) were calculated using Mettler Toledo Star (Columbus, OH, USA) software from the second heating step thermographs.
Particle Mean Size Analysis
The particle mean size analysis was performed on a laser diffraction particle size analyzer equipped with the Tornado dry powder system (LS 13320, Beckman Coulter, USA). The Fraunhofer theory was used for the determination of the mean diameters of the microcapsules. The volume distributions of the samples were calculated and the results are presented as mean particle size diameter.
Color Measurement
One gram of powder was put in plastic cuvettes and color measurements were performed using a Hunter lab (Color Quest XE, HunterLab, Reston, VA, USA) colorimeter. The CIE Lab color scale was used to measure the L* (black to white), a* (red to green), and b* (yellow to blue) parameters. The total color difference, ΔE*, between a white standard tile (L* = 92.59, a* = −0.78, b* = 0.67) and each individual dry probiotic formulation were calculated according to the formula
Hygroscopicity
The hygroscopicity of the probiotic powders was determined according to the procedure described by Fritzen-Freire et al.[ Citation 19 ] More specifically, a 1 g sample of the powder was placed in a desiccator equilibrated at 75% relative humidity containing a saturated sodium chloride (NaCl) solution at room temperature. Samples were kept for 7 days and hygroscopicity was calculated gravimetrically according to the formula
Dissolution
The dissolution capacity of the powders was calculated according to the method of Fritzen-Freire et al.[ Citation 19 ] One gram of powder was added to 50 mL of distilled water and dispersed under magnetic stirring (Ika GmbH, Germany) at 892 rpm using a 2 mm × 7 mm stirring bar. The time required for complete dissolution of the powder was recorded.
Morphological Characterization
For visualization of the morphology of the microcapsules, a small amount of powder was carefully deposited onto carbon tabs (Agar Scientific, Stansted, UK) and coated with carbon (agar turbo carbon coater) to improve conductivity. Scanning electron microscope analysis (SEM) was performed on an FEI Quanta 3D 200 dual-beam focused ion beam scanning electron microscope (FIB-SEM). The images were acquired using secondary electron imaging at an accelerating voltage of 5–15 kV.
RESULTS AND DISCUSSION
Probiotics Survival throughout the Spray-Drying Process
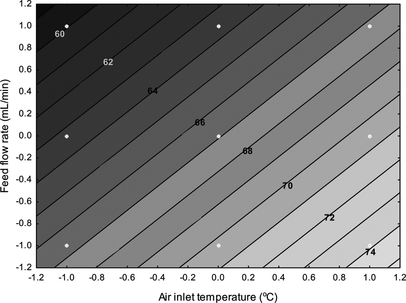
The impact of the tested spray-drying conditions (inlet air temperature and feed flow rate) on the viability of L. acidophilus throughout the process is displayed in Table and the resultant model is graphically illustrated in Fig. . As can be seen from Fig. , a reduction in the air inlet temperature was in almost all cases linked to an enhancement in L. acidophilus viability and at mid and high air inlet temperatures an elevation of the feed flow rate increased L. acidophilus viability, although this relationship was reversed at low air inlet temperatures. The most significant change was observed when the outlet temperature was reduced from 91.5 to 60°C; over this temperature range the survival rate of L. acidophilus increased from 2.5 to 84%. It is well established that the loss of probiotics viability during convective thermal processing is related to cellular injuries resulting from the combined effect of heat and mechanical stress. Examples include the denaturation of the informational macromolecules (DNA and RNA), damage to ribosomes, dehydration of cytoplasmic membranes, lipid peroxidation, and rupture and collapse of cell membrane due to water removal.[ Citation 2 , Citation 14 , Citation 16 , Citation 33 , Citation 34 ] Under excessive droplet heat transfer rate conditions (increase of the T d − T g driving force), the integrity of the cellular membranes can be lost due to crystalline to rubbery state transitions, which are responsible for the increase the membranes fluidity, leading eventually to the cells' fate.[ Citation 2 ] The changes observed as a result of alterations in the flow rate are a result of changes in the heat and mass transfer kinetics at the air–solid interface. Generally, the elevation of the feed flow rate causes a reduction in the droplets' surface temperature, which causes changes in both heat and water diffusivity,[ Citation 35 ] consequently reducing the physical damage to the cell membranes. As can be seen in the results of the ANOVA (Table ), both linear and quadratic coefficients are significant for air inlet temperature and the linear coefficients are significant for feed flow rates (p < 0.001).
FIG. 1 Effects of air inlet temperature and feed flow rate on the viable counts of L. acidophilus NCIMB 701748 after spray drying.
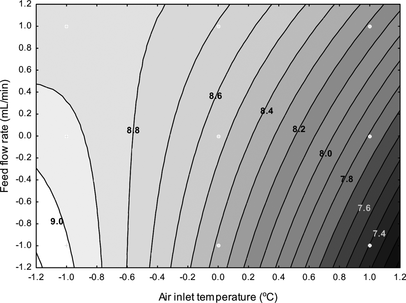
TABLE 3 Regression coefficients and their significance levels for pruned mathematical models used for the prediction of total viable counts of L. acidophilus NCIMB 701748, water activity, and powder recovery at the cyclone separator (ns = not significant)
Effects of Processing Conditions on Water Activity and Cyclone Recovery
The water activity of the spray-dried powder was significantly (p < 0.001) affected by air inlet temperature and feed flow rate as displayed in Fig. . In general, inlet air temperature was the more impactful factor when compared to feed flow rate for controlling water activity and residual water content (data not shown) of the finished powders; this can be seen in Fig. and is further detailed in Table . Products produced with the highest air temperature and lowest feed flow rate resulted in the driest formulations. Low a w values and residual moisture contents (<4–5% w/w) are prerequisites for the commercial production of spray-dried powders with good handling characteristics such as high flow ability, low stickiness and agglomeration, as well as maximum probiotics viability.[ Citation 35 ] The residual water content ranged from 1.7 to 5.4% w/w (data not shown), which complies with standard acceptable moisture levels for spray-dried powders.[ Citation 36 ] At conditions of low water activity, the matrix moves from the rubbery state toward the glassy state and, thus, water mobility is reduced. This inhibits cell metabolic activity of the bacterial cells, leading to extended shelf life.[ Citation 2 , Citation 5 , Citation 17 ]
FIG. 2 Effects of air inlet temperature and feed flow rate on the water activity of the spray-dried powders.
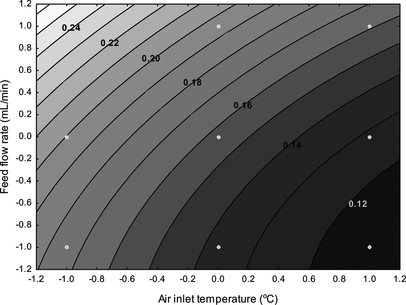
Both feed rate and inlet temperature significantly impacted cyclone recovery. In both cases this could be described by a linear relationship. In general, spray-drying yield was maximized when the spray dryer was operated at high air inlet temperatures and low feed flow rates (Fig. ). The amount of the dried product recovered via cyclone separation is influenced by many engineering and product parameters, such as drying air flow and local velocities; the spatial geometry of the separator; and the adhesiveness and cohesiveness of the particles while interacting with the drying chamber.[ Citation 36 , Citation 37 ] In our work, the air flow was kept constant, and thereby the parameters that affect the surface stickiness of the micro particles such as the hygroscopicity, glass transition temperature, moisture and thermal diffusivity of the carrier material, temperature of the droplets obtained in the drying chamber, etc., probably control the achieved powder recovery at the cyclone separator.[ Citation 36 ] According to the findings of Adhikari et al.,[ Citation 36 ] the presence of ingredients that act as plasticizers; for example, sugars increase the surface stickiness of the dried particles due to the increase in the glass transition–surface temperature gradient. Thus, as the T d − T g value increases the droplets move toward the rubbery state, sticking on the drying chamber surface and reducing the powder recovery rates. The effect is dependent on both heat and mass diffusion rates, because water acts as a plasticizer for macromolecules.[ Citation 38 ] In our case, the combination of high air inlet temperature and low feed flow rates led to less particle stickiness probably due to the lower residual contents achieved by the sufficient heat penetration and core-to-droplet surface water diffusion rate.
Optimization of Processing Conditions and Model Verification
It is not possible to simultaneously maximize the viability of L. acidophilus viability while minimizing water activity and maximizing cyclone yield. Therefore, compromises must be made and an optimal compromise must be sought. Optimal operating conditions that produced both viable L. acidophilus microencapsulates and a stable dry powder were calculated using a desirability function,[ Citation 23 ] which is further detailed in Eq. (Equation7):
TABLE 4 Optimum spray-drying conditions and validation of the pruned mathematical models constructed for the prediction of total viable counts of L. acidophilus NCIMB 701748, water activity, and powder recovery at the cyclone separator
Characterization of the Physicochemical and Structural Characteristics of Optimized Microcapsules
The physicochemical and structural properties of the microcapsules produced under the optimized spray dryer operating conditions are detailed in Table . Particle size analysis revealed a bimodal mean size distribution (Fig. ) that was characteristic of spray-dried powders with high bulk (tap) density; in general, bimodal distributions pack most efficiently as the smaller particles are included in the voids between the larger microcapsules.[ Citation 39 ] The volume-weighted mean diameter (d V,50) of the microcapsules was 10.96 µm, which is comparable to the values (10–20 µm) reported in the case of other spray-dried probiotic formulations.[ Citation 17 , Citation 40 ] The hygroscopic character of the powders (0.174 g/g of absorbed water) can be explained by the presence of D-glucose and lactose (both of which have hygroscopic properties), although the hygroscopicity values were much lower compared to other probiotic dry formulations.[ Citation 19 ] The glass transition temperature of the powder was 59°C, suggesting that the matrices can be stored under chilling or room temperature conditions while retaining their glassy state, irrespective of the plasticizing effect of D-glucose, lactose, and free water in the products.[ Citation 38 ] The spray-dried powders had a lower luminosity (L*) and higher yellow color intensity compared to other dairy-based spray-dried formulations,[ Citation 19 ] which was probably due to the presence of the milk proteins. Similar results have been also reported by other researchers for the color difference (ΔE*) for the white standard, which was 2.87; this color threshold is normally quoted for perceivable differences (ΔE* = 3).[ Citation 41 ]
FIG. 4 Particle mean size distribution of the optimized powders containing L. acidophilus NCIMB 701748.
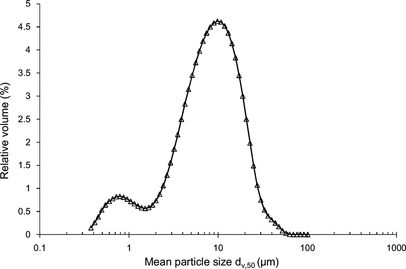
TABLE 5 Physicochemical characterization and storage stability of the optimized microcapsules containing L. acidophilus NCIMB 701748
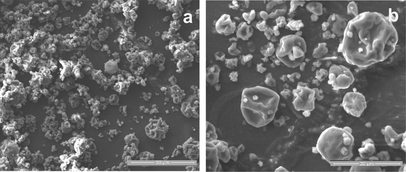
The morphology of the microcapsules is illustrated in the SEM micrographs shown in Fig. . The particulate structure of the product generated under the optimum spray-drying conditions had a partially collapsed structure, which is characteristic of many spray-dried powders analyzed under vacuum, that can be described as a deflated, flat, ball-like, spherical particles. The heat transfer rate and the water diffusion rate from the surface to the core of the droplets as well as the presence of whey protein ingredients critically affected the microstructure of spray-dried matrices, with intermediate heat and mass transfer rates (intermediate air inlet temperature and feed flow rate) to induce the highest collapse of the particles.[ Citation 42 ]
FIG. 5 SEM micrographs of the microcapsules containing L. acidophilus NCIMB 701748 produced under the optimized spray-drying conditions: (a) scale bar 50 µm and (b) scale bar 20 µm.
The inactivation rates of the powders stored for 30 days at chilled temperatures (4°C) and ambient room storage conditions (25°C) in sealed, airtight vials were calculated by fitting the TVC data to a first-order reaction model, which has successfully has been used in previous studies.[ Citation 5 , Citation 17 , Citation 20 , Citation 41 ] The total viable counts of L. acidophilus were 8.55 and 7.48 log cfu/g after 30 days of storage at 4 and 25°C, respectively, indicating a good storage stability of the probiotic powders. A fourfold increase in the inactivation rate of L. acidophilus was observed at room temperature (k 25°C = 0.041) compared to the lower temperature (k 4°C = 0.011). Storage temperature impacted the viability of an hydrobiotics through two main mechanisms: firstly, the increase in temperature increased the rate of metabolic activity in the cells (and other chemical or enzymatic reactions that may also occur; e.g., lipid oxidation) and, secondly, will modify the molecular mobility of water, as the environmental temperature approaches T g (the T − T g gradient), the matrix will move closer to the rubbery state and water molecular mobility will increase. It should be noted that in the optimized system generated herein, the inactivation rates of L. acidophilus at room temperature were considerably lower than those of other spray-dried thermo sensitive lactobacilli strains such as L. rhamnosus (0.08–0.19 days−1), Lactobacillus plantarum (0.055–0.062), L. acidophilus (0.0504), and Lactobacillus lactis (0.0574).[ Citation 17 , Citation 20 , Citation 43 ]
CONCLUSIONS
In the present work, we showed that optimization of the spray-drying process is essential for the production of dry spray powders containing viable thermo sensitive probiotics. The use of intermediate air inlet temperatures and feed flow rates are required to provide sufficient survivability of L. acidophilus through spray drying while retaining good powder recovery rates at the cyclone separator and low residual water activities. In addition, the microcapsules produced at the optimized spray-dried conditions were characterized as having acceptable physicochemical properties (total color, glass transition temperature, hygroscopicity) and low inactivation rates during storage at room and chilling temperature conditions. Although the results are promising and show that through compromises in techno-functional properties, yield, and shelf life, high-quality anhydrobiotics powders can be produced, it is anticipated that the shelf life of powders and the survival rates of L. acidophilus can be further improved by continual development of the carrier system and thermal profile of the drying chamber (through chamber geometry optimization and dynamic regulation of exhaust air).
ACKNOWLEDGMENTS
The authors gratefully acknowledge Val Street, Dr. Chris Parmenter, and Dr. Phil Richards for technical assistance and scientific advice with DSC measurements, SEM image analysis, and microbiological testing, respectively. Cargill Ltd. (Manchester, UK) and Arla A/S (Viby, Denmark) are also acknowledged for providing the maltodextrin and whey protein concentrate used in the present work.
Notes
a Starting cell concentration was 9.02 ± 0.02 log cfu/g.
X 1 = Inlet air temperature (°C); X 2 = feed flow rate (mL/min).
© Solmaz Behboudi-Jobbehdar, Christos Soukoulis, Lina Yonekura, and Ian Fisk
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. The moral rights of the named authors have been asserted.
REFERENCES
- Food and Agriculture Organization/World Health Organization. Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation. FAO Food and Nutrition Paper No. 85; FAO: Rome, Italy, 2006.
- Fu , N. ; Chen , X.D. Towards a maximal cell survival in convective thermal drying processes . Food Research International 2011 , 44 , 1127 – 1149 .
- Gardiner , G.E. ; O'Sullivan , E. ; Kelly , J. ; Auty , M.A.E. ; Fitzgerald , G.F. ; Collins , J.K. ; Ross , R.P. ; Stanton , C. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L salivarius strains during heat treatment and spray drying . Applied and Environmental Microbiology 2000 , 66 , 2605 – 2615 .
- Dave , R.I. ; Shah , N.P. Ingredient supplementation effects on viability of probiotic bacteria in yogurt . Journal of Dairy Science 1998 , 81 , 2804 – 2816 .
- Ying , D.Y. ; Sun , J. ; Sanguansri , L. ; Weerakkody , R. ; Augustin , M.A. Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose . Journal of Food Engineering 2012 , 109 , 597 – 602 .
- Saarela , M. ; Virkajärvi , I. ; Nohynek , L. ; Vaari , A. ; Mättö , J. Fibres as carriers for Lactobacillus rhamnosus during freeze-drying and storage in apple juice and chocolate-coated breakfast cereals . International Journal of Food Microbiology 2006 , 112 ( 2 ), 171 – 178 .
- Ferraz , J.L. ; Cruz , A.G. ; Cadena , R.S. ; Freitas , M.Q. ; Pinto , U.M. ; Carvalho , C.C. ; Faria , J.A.F. ; Bolini , H.M.A. Sensory acceptance and survival of probiotic bacteria in ice cream produced with different overrun levels . Journal of Food Science 2012 , 77 ( 1 ), S24 – S28 .
- Cruz , A.G. ; Antunes , A.E.C. ; Sousa , A.L.O.P. ; Faria , J.A.F. ; Saad , S.M.I. Ice cream as probiotic food carrier . Food Research International 2009 , 42 ( 9 ), 1233 – 1239 .
- Ong , L. ; Shah , N.P. Probiotic cheddar cheese: Influence of ripening temperatures on proteolysis and sensory characteristics of cheddar cheeses . Journal of Food Science 2009 , 74 ( 5 ), S182 – S191 .
- Klu , Y.A.K. ; Williams , J.H. ; Phillips , R.D. ; Chen , J. Survival of Lactobacillus rhamnosus GG as influenced by storage conditions and product matrixes . Journal of Food Science 2012 , 77 ( 12 ), M659 – 663 .
- Lourens-Hattingh , A. ; Viljoen , B.C. Yogurt as probiotic carrier food . International Dairy Journal 2001 , 11 ( 1–2 ), 1 – 17 .
- Rodrigues , D. ; Sousa , S. ; Gomes , A.M. ; Pintado , M.M. ; Silva , J.P. ; Costa , P. ; Amaral , M.H. ; Rocha-Santos , T. ; Freitas , A.C. Stability of Lactobacillus paracasei as free cells or encapsulated in alginate-based microcapsules in low pH fruit juices . Food and Bioprocess Technology 2012 , 5 ( 7 ), 2748 – 2757 .
- Burgain , J. ; Gaiani , C. ; Linder , M. ; Scher , J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications . Journal of Food Engineering 2011 , 104 , 467 – 483 .
- Concoran , B.M. ; Stanton , C. ; Fitzgerald , G. ; Ross , R.P. Life under stress: The probiotic stress response and how it may be manipulated . Current Pharmaceutical Design 2008 , 14 , 1382 – 1399 .
- Corcoran , B.M. ; Ross , R.P. ; Fitzgerald , G.F. ; Stanton , C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances . Journal of Applied Microbiology 2004 , 96 , 1024 – 1039 .
- Teixeira , P. ; Castro , H. ; Mohacsi-Farkas , C. ; Kirby , R. Identification of sites of injury in Lactobacillus bulgaricus during heat stress . Journal of Applied Microbiology 1997 , 83 ( 2 ), 219 – 226 .
- Ananta , E. ; Volkert , M. ; Knorr , D. Cellular injuries and storage stability of spray dried Lactobacillus rhamnosus GG . International Dairy Journal 2005 , 15 , 399 – 409 .
- Sunny-Roberts , E.O. ; Knorr , D. The protective effect of monosodium glutamate on survival of Lactobacillus rhamnosus GG and Lactobacillus rhamnosus E-97800 (E800) strains during spray-drying and storage in trehalose-containing powders . International Dairy Journal 2009 , 19 , 209 – 214 .
- Fritzen-Freire , C.B. ; Prudencio , E.S. ; Amboni , R.D.M.C. ; Pinto , S.S. ; Negrao-Murakami , A.N. ; Murakami , F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics . Food Research International 2012 , 45 , 306 – 312 .
- Lapsiri , W. ; Bhandari , B. ; Wanchaitanawong , P. Viability of Lactobacillus plantarum TISTR 2075 in different protectants during spray drying and storage . Drying Technology 2012 , 30 , 1407 – 1412 .
- Bustos , P. ; Bórquez , R. Influence of osmotic stress and encapsulating materials on the stability of autochthonous Lactobacillus plantarum after spray drying . Drying Technology 2013 , 31 , 57 – 66 .
- Ahi , M. ; Hatamipour , M.S. ; Goodarzi , A. Optimization of leaving activity of baker's yeast during the spray-drying process . Drying Technology 2010 , 28 ( 4 ), 490 – 494 .
- Koc , K. ; Yilmazer , M.S. ; Balkir , P. ; Ertekin , F.K. Spray drying of yogurt: Optimization of process conditions for improving viability and other quality attributes . Drying Technology 2010 , 28 ( 4 ), 495 – 507 .
- Telang , A.M. ; Thorat , B.N. Optimization of process parameters for spray drying of fermented milk soy milk . Drying Technology 2010 , 28 ( 12 ), 1445 – 1456 .
- Chávez , B.E. ; Ledeboer , A.M. Drying of probiotics: Optimization of formulation and process to enhance storage survival . Drying Technology 2007 , 25 ( 7–8 ), 1193 – 1201 .
- Riveros , B. ; Ferrer , J. ; Borquez , R. Spray drying of a vaginal probiotic strain of Lactobacillus acidophilus . Drying Technology 2009 , 27 ( 1 ), 123 – 132 .
- Schutyser , A.I.M. ; Perdana , J. ; Boom , R.M. Single droplet drying for optimal spray drying of enzymes and probiotics . Trends in Food Science and Technology 2012 , 27 ( 2 ), 73 – 82 .
- Mestry , A.P. ; Mujumdar , A.S. ; Thorat , B.N. Optimization of spray drying of an innovative functional food: Fermented mixed juice of carrot and watermelon . Drying Technology 2011 , 29 ( 10 ), 1121 – 1131 .
- Goula , A.M. ; Adamopoulos , K.G. A new technique for spray-dried encapsulation of lycopene . Drying Technology 2012 , 30 ( 6 ), 641 – 652 .
- Myers , R.H. ; Montgomery , D.C. ; Anderson-Cook , C.M. Response Surface Methodology, , 3rd Ed. ; Wiley : Hoboken , NJ , 2009 .
- Cavaliere , C. ; Foglia , P. ; Marini , F. ; Samperi , R. ; Antonacci , D. ; Laganà , A. The interactive effects of irrigation, nitrogen fertilisation rate, delayed harvest and storage on thepolyphenol content in red grape (Vitis vinifera) berries: A factorial experimental design . Food Chemistry 2010 , 122 , 1176 – 1184 .
- Soukoulis , C. ; Tzia , C. Response surface mapping of the sensory characteristics and acceptability of chocolate ice cream containing alternate sweetening agents . Journal of Sensory Studies 2010 , 25 ( 1 ), 50 – 75 .
- Garre , E. ; Raginel , F. ; Palacios , A. ; Julien , A. ; Matallana , E. Oxidative stress responses and lipid peroxidation damage are induced during dehydration in the production of dry active wine yeasts . International Journal of Food Microbiology 2011 , 136 , 295 – 303 .
- Santivarangkna , C. ; Kulozik , U. ; Foerst , P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes . Journal of Applied Microbiology 2008 , 105 , 1 – 13 .
- Barbosa-Canovas , G.V. ; Ortega-Rivas , E. ; Juliano , P. ; Yan , H. Food Powders: Physical Properties, Processing and Functionality ; Kluwer Academic : New York , 2005.
- Adhikari , B. ; Howes , T. ; Lecomte , D. ; Bhandari , B.R. A glass transition temperature approach for the prediction of the surface stickiness of a drying droplet during spraying drying . Powder Technology 2005 , 149 , 168 – 179 .
- Barbosa-Canovas , G.V. ; Vega-Mercado , H. Dehydration of Foods ; Chapman & Hall : New York , 1996 .
- Roos , Y.H. Phase Transitions in Foods ; Academic Press : New York , 1995 .
- Tonon , R.V. ; Brabet , C. ; Hubinger , M.D. Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying . Journal of Food Engineering 2008 , 88 ( 3 ), 411 – 418 .
- Ying , D.Y. ; Phoon , M.C. ; Sanguansri , L. ; Weerakkody , R. ; Burgar , I. ; Augustin , M.A. Microencapsulated Lactobacillus rhamnosus GG powders: Relationship of powder physical properties to probiotic survival during storage . Journal of Food Science 2010 , 75 , E588 – E595 .
- Martinez-Cervera , S. ; Salvador , A. ; Muguerza , B. ; Moulay , L. ; Fizman , S.M. Cocoa fibre and its application as a fat replacer in chocolate muffins . LWT-Food Science and Technology 2011 , 44 , 729 – 736 .
- Reineccius , G.A. Multiple-core encapsulation—The spray drying of food ingredients . In Microencapsulation of Food Ingredients ; Vilstrup , P. , Ed.; Leatherhead Publishing : Surrey , UK , 2001 ; 151 – 185 .
- Dianawati , D. ; Mishra , V. ; Shah , N.P. Stability of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp . cremoris during storage at room temperature at low aw. Food Research International 2012 .
- © Solmaz Behboudi-Jobbehdar, Christos Soukoulis, Lina Yonekura, and Ian Fisk
- This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. The moral rights of the named authors have been asserted.