?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Agitated thin-film drying (ATFD) has been proposed for efficient and mild drying of viscous liquid foods, pastes or pureed foods. We report a study on the influence of product and process parameters on ATFD. During ATFD of spinach leaf slurries, the wall temperature mainly affected the specific evaporation rate, while the absolute evaporation rate was proportional to the feed rate. The fact that blade rotation speed had limited effect on the drying rate suggested that the process is limited by heat transfer through the wall. ATFD is especially suited for slurries that show relatively limited sticky behavior during drying and liquid–solid phase transition with corresponding brittle viscoelastic behavior. This was demonstrated by drying juices from tomato and bell pepper, giving poor results, and by drying solutions from whey protein isolate (WPI) and sucrose, which could be successfully dried.
Introduction
Drying is a commonly used technology to preserve food products. Various types of dryers have been developed that are tailored to specific feed properties and product requirements.[Citation1] The most commonly applied drying technologies to liquid feeds can be categorized into two types, i.e. convective and conductive drying. Spray drying is one of the most commonly applied convective drying methods, where energy for evaporation is supplied by contacting a dispersion of the product with hot air.[Citation2] Drum drying, refractance window drying and agitated thin-film drying (ATFD) are examples of conductive drying methods, where energy for evaporation is supplied by steam condensation and/or hot water, which is then transferred through a wall to a thin product film that is present at the other side of the wall. ATFD is done by spreading the product on the inside of a hollow cylinder, and supplying heat from the outside. The product film is continuously agitated with a knife that rotates inside the cylinder. In general, conductive drying is especially suitable for high viscous (food) products. Drum drying is for example extensively applied for drying of starch, mashed potatoes, fruit purees, dry soup mixtures, casein, etc.[Citation3] Refractance window drying is reported especially suitable for drying of pureed fruits and vegetables, i.e. strawberry pulp, mango pulp, carrot puree, and pumpkin puree.[Citation4–6] Whereas drum drying and refractance window drying are more readily applied and effective for drying of viscous liquid foods, pastes or pureed foods, ATFD is not yet widely applied.[Citation1,Citation5,Citation7,Citation8]
The advantage of spray drying is that it is a mild and fast dehydration process due to the fast evaporation. Reported disadvantages of spray drying are its low energy efficiency, high capital costs and the relatively low-bulk density of the dried products.[Citation9] The lower energy efficiency of spray drying is related to the large energy loss via the warm and moist exhaust gas. In that respect, drum drying is more efficient as it consumes on average 40% less energy due to amongst others lower energy loss via the exhaust gas.[Citation2,Citation9] A major drawback of atmospheric drum drying is, however, that the product is exposed to the boiling temperature (100 °C), which can lead to undesired damage to heat sensitive foods.[Citation1,Citation7] Operation of drum drying processes under reduced pressure could be an attractive alternative, but the capital costs of vacuum drum dryers are relatively large. During refractance window drying, the product is dried as a thin film on a belt, which moves over a bath filled with hot water 90 °C and the film is dried under atmospheric conditions.[Citation6] Drawback is for example that very thin films need to be casted to facilitate the drying, which limits capacity and troubles scale-up. ATFD is therefore identified as a promising alternative conductive drying method.
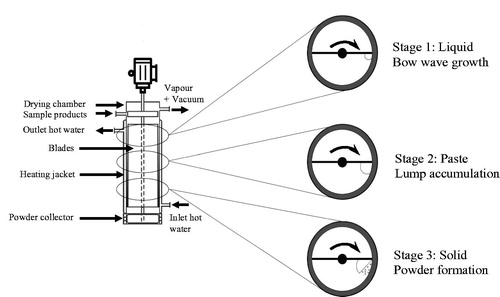
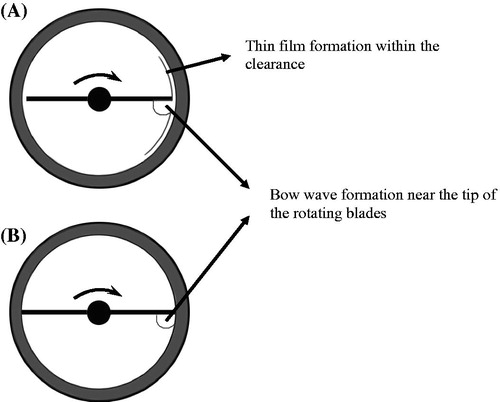
ATFD is a continuous drying process carried out in a scraped heat exchanger, which can be easily operated under reduced pressure and is thus suitable for heat sensitive products. The ATFD consists of two major elements, i.e. the drying chamber with a heating jacket and the rotor with fixed blades. Two different blade configurations can be distinguished, namely the small-gap and the scraped surface blades. For the small-gap blade configuration, a gap is present between the blade-tips and the wall. The rotating blades agitate the liquid feed which spreads as a falling thin film within the gap and a falling bow wave is created at the tip of the blades, as shown in .[Citation10,Citation11] For the scraped surface blade configuration, the blade-tips directly contact the wall with a negligible gap. This blade constitution forms a larger falling bow wave with minimal film formation, as shown in .[Citation12] The latter blade configuration is more efficient in preventing the formation of an undesired dry, insulating layer on the wall and thus promote efficient heat transfer, especially for materials with higher fouling tendency.[Citation13,Citation14]
Figure 1. Schematic representation of product distribution in the ATFD with different blade constitutions: (A) small-gap blades and (B) scraped surface blades.
provides a schematic drawing of the drying process within the ATFD with scraped surface blades. The product progressively passes through different consistencies, i.e. liquid, paste, and solid. When the feed enters the ATFD, the liquid feed forms two bow waves on the front edge of the blades. The blades rotate fast and contact the liquid feed to the heating wall. The high rotation speed generates a well-mixed liquid flow within the two bow waves. The liquid flows down spirally due to gravity and concentrates as water is removed by evaporation. Due to this concentration, the viscosity increases and thus the flow rate goes down. The concentrated product becomes increasingly viscous and transforms from a paste into a brittle material. Depending on the brittleness of the resulting product, which is dependent on its moisture content and material properties, the product will fracture into smaller powder particles due to the rotating action of the blades.
Several studies have investigated, flow distribution and heat and mass transfer during agitated thin-film evaporation (ATFE) processes, which are the first stages of ATFD. Komari et al.[Citation10] investigated the flow and mixing behavior of a fluid with a high viscosity in an agitated thin-film evaporator with a single small-gap blade. They found that more than 70% of the supplied feed end up in the bow waves and that mass transfer between the bow waves and the thin film was negligible. Komari et al.[Citation15] improved the apparatus by using multiple vertically aligned blades, and reported that the vertically aligned blades could strongly promote mass transfer between the bow waves and the thin film and thus increase the efficiency of the equipment. De Goede and De Jong[Citation16] studied the heat transfer properties of a scraped surface heat exchanger and developed a model by combining a regular model for turbulent pipe flow with the penetration theory. McKenna[Citation17] presented a model for the design of a wiped film evaporator, by considering both fluid and mass transport phenomena.
Only very few studies investigated ATFD as such. Pawar et al.[Citation1,Citation11] studied the heat and mass transfer during ATFD and proposed a stage-wise penetration theory model, by assuming ideal mixing between the bow wave and the thin film. Their model implies that the resistance for heat transfer would be in the film. The model was validated with ATFE instead of ATFD experiments, which is only one part of the overall ATFD process: during ATFD, the feed will transform from a viscous liquid into a paste and subsequently into a solid, and the mass and heat transfer during operation will be different.[Citation18] Hitherto, to the best of our knowledge no experimental studies have systematically investigated this product transformation during ATFD drying and its consequence on the drying behavior. Given the changes in product consistency, we expect that the product properties will have large influence on the drying behavior in an ATFD.
The aim of this study was therefore to create better understanding of drying behavior of foods during ATFD drying and how it is affected by process parameters and product properties. Juice from spinach leaves was selected as a model system to investigate the influence of drying parameters, i.e. heating temperatures, feeding rates and rotation speeds. The obtained results were compared to the existing ATFD model proposed by Pawar et al.[Citation1] Different food formulations, such as whey protein isolate (WPI) solution, sucrose solution, bell pepper juice and tomato juice, were dried using ATFD. Drying behavior of spinach juice was taken as a reference and compared to drying behavior of the other materials in the ATFD.
Materials and methods
Materials
Spinach suspensions for ATFD experiments were prepared starting from fresh spinach leaves, which were purchased from the local supermarket. The fresh spinach leaves were juiced by an Angelia AG-7500 Juicer (Angel Co. Ltd, Busan Republic of Korea). After juicing, the squeezed juice and fiber residues were collected separately. The obtained insoluble fiber residues were dried in a hot air oven (Binder, Tuttlingen, Germany) at 60 °C for 20 h. The dried fibers were milled by the Rotor Mill Pulverisette-14 (FRITSH International, Pittsboro, NC) at 6000 rpm with a 0.2 mm sieve. The drying and milling of fibers was necessary because the fiber particles had to be small enough to avoid blockage of the feeding pump. Subsequently, the milled fibers were added back into the squeezed juice to obtain a spinach suspension with approximately again 2% w/w fibers. In addition, around five drops of antifoam B aqueous silicone emulsion (Sigma-Aldrich®, Zwijndrecht, the Netherlands) were added into 1 L juice to prevent excessive foaming.
Lab-scale ATFD
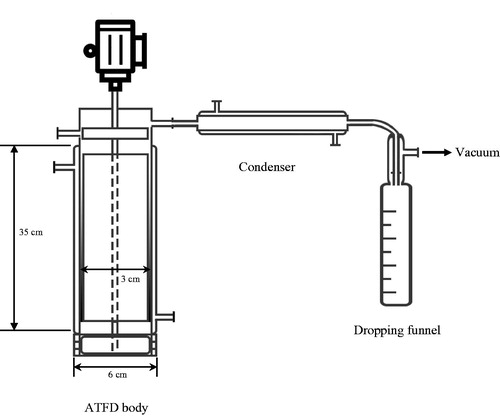
shows a schematic diagram of the experimental lab-scale apparatus. The ATFD chamber was made from transparent glass to facilitate observation of the heat exchange area. The ATFD chamber was equipped with a Liebig condenser and a dropping funnel (Laboratory Glass Specialists B.V, Ubbena, the Netherlands) to condense and quantify the vapor release. The entire system was operated under reduced pressure (50 mbar) using a vacuum pump (SC 950; KNF Neuberger GmbH, Freiburg, Germany). The dimensions of the lab-scale ATFD are shown in .
Table 1. Dimensions of the lab-scale agitated thin-film dryer.
Experiments were performed to determine the (specific) evaporation rates of the spinach suspensions at different drying conditions. The spinach suspension was preheated to 33 °C and then supplied to the system with a flow rate ranging from 0.3 to 0.5 kg/h, by a peristaltic pump (205S; Watson Marlow, Falmouth, UK) with rotation speeds from 30 to 50 rpm. The drying temperatures of the heating chamber ranged from 70 to 90 °C. The condenser was operated with cooling water of 2 °C. The amount of condensed water was measured and used to obtain the evaporation rate. The specific evaporation rate was calculated by dividing the evaporation rate by the used heat exchange area.
Analysis of the moisture content and water activity
Powders were collected at the bottom of the lab-scale ATFD for analysis of the moisture content and water activity. To determine the moisture content, around 1.5 g of the powder was dried in a hot air oven (Binder) at 105 °C for 16 h. The water activity was measured by an AquaLab 4TE dew point water activity meter (METER Food, Munich, Germany). Measurements were carried out in duplicate.
Ratio of soluble and insoluble compounds
The ratio of soluble and insoluble compounds was determined by adding ∼1 g of the powder into 30 mL of water and mixed for 60 min by the Multi Reax test tube shaker (Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). Subsequently, the suspensions were centrifuged by the Sorvall Legend XFR Centrifuge (Thermo Scientific Fisher, Waltham, MA) at 10,000g and 20 °C for 30 min. After centrifugation, the supernatant was removed and the pellet was dried in an oven at 105 °C for 16 h. The fraction of soluble powder (ηp) was calculated by:
(1)
(1)
where
is the moisture content of the ATFD-dried powders;
,
, and
are the mass of the ATFD-dried powders, the dried sediments and the centrifuge tube, respectively. The measurements were carried out in duplicate.
Morphology of the ATFD-dried powders
Scanning electron microscopy images of ATFD-dried powders were made using a Phenom G2 Pure SEM (Phenom-World BV, Eindhoven, the Netherlands). The dried powders were fixed on an aluminum pin-type mount [JEOL (Europe) BV. Nieuw-Vennep, the Netherlands] with carbon tabs. Pretreatment of the samples was not necessary.
Statistical analysis and mathematical modeling
A one-way analysis of variance (ANOVA) and a Tukey’s multiple regression analysis were applied to evaluate the obtained results at a significance level of 0.05. The ATFD process model from Pawar et al.[Citation1] was implemented and compared to experimental results. The statistical analysis and modeling work were carried out using MATLAB R2016b (MathWorks, Natick, MA).
Results and discussion
ATFD experiments with spinach leaf suspensions
The lab-scale ATFD rig was used to dry the spinach suspensions at different conditions, which are reported in . The effect of the drying temperature on the evaporation rate and the specific evaporation rate was determined. shows that with the increase of drying temperature, the overall evaporation rate does not change significantly (p > 0.05), but the specific evaporation rate increases significantly (p < 0.05), based on one-way ANOVA analysis. At a higher drying temperature, the surface area required for drying decreases, as shown in . Since the feed is dried completely, the total evaporation rate is fixed. When the drying temperature is increased, the specific evaporation will increase due to the larger driving force, which will result in a smaller total surface used for evaporation. Indeed, we see at higher drying temperatures that a larger part of the wall is not used.
Figure 4. (A) The evaporation rate and the specific evaporation rate in the ATFD for different temperatures (rotation speed =600 rpm, feed rate = 0.3 kg/h). The error bars represent the standard deviation of the experimental data (n = 3). The same letters represent no significant difference at the 95% confidence interval. The dotted lines are added to guide the eye. (B) Images showing the occupied surface area in the ATFD chamber at different drying temperatures.
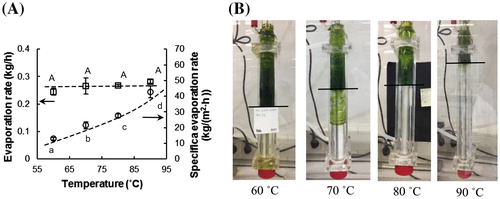
Table 2. The fraction of the used heating exchange area, the moisture content and water activity of the powder and fraction of soluble powder at different drying conditions.
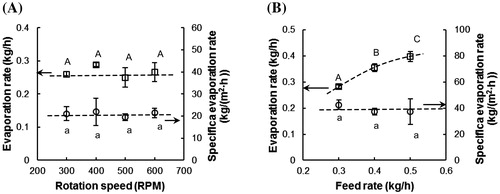
shows the influence of the rotation speed and the feed rate on the evaporation rate and the specific evaporation rate. As shown in , both the evaporation rate and the specific evaporation rate did not change at different rotation speeds (p > 0.05). The constant specific evaporation rate implies that mixing of the feed is not rate limiting for the drying process. Therefore, the heat transfer has to be limited by the heat transfer through the glass wall. This observation is opposite to the predictions by the model proposed by Pawar et al.,[Citation1] who proposed that the heat transfer coefficient of the ATFD may be derived from the heat penetration theory, and therefore should increase with the rotation speed. According to this model, the specific evaporation rate should increase as well. In fact, these authors also experimentally observed that the water evaporation rate was independent of the speed of the blades, when they concentrated a 20% w/w ammonium sulfate solution in an ATFE. Our conclusion is therefore that the blades do not directly influence the mass and heat transfer of the drying process, even though they are still essential for removing the solidified material from the wall. As shown in , we found that the effect of the feed rate is opposite to that of the temperature. The increased feed rate does not influence the specific evaporation rate (p > 0.05), but does influence the total evaporation rate (p < 0.05), since a larger feed rate leads to a larger surface covered by the drying feed, and therefore a larger total evaporation rate. The total evaporation rate depends on the feed rate, as long as the system is not at its maximum capacity.
Figure 5. The evaporation rate and the specific evaporation rate in the ATFD for different (A) rotation speeds (temperature = 70 °C, feed rate = 0.3 kg/h) and (B) feed rates (temperature = 90 °C, rotation speed = 600 rpm). The error bars represent the standard deviation of the experimental data (n = 3). The same letters represent no significant difference at the 95% confidence interval. The dotted lines are added to guide the eye.
shows the moisture content and water activity (aw) of the dried spinach powder and the fraction of soluble powder at different drying conditions. The moisture content of the powder ranges from 0.049 to 0.114 kg/kg total and the water activity ranges from 0.26 to 0.60. Although foods with aw<0.6 may be considered as microbiologically stable, they may still be susceptible to undesired chemical reactions and stickiness, and further drying in for example a fluidized bed would be recommended for vegetable powders to further decrease the water activity to aw<0.2.[Citation19] In most cases, the fraction of soluble powder was higher than 0.31. This was higher than that of spinach fibers after squeezing (0.21), indicating that the powders collected from ATFD were not only fibers. Therefore, ATFD did not selectively scrape insoluble particles from sticky lumps. It should be mentioned that during experiment no. 2, no powder was collected at the bottom of the setup. Instead, all the material stuck to the blades as lumps so that the moisture content, water activity and fraction of soluble powder in this experiment could not be measured. Probably this was due to the low rotation speed, which did not provide enough mechanical stress to fracture the material. Therefore, even though the rotation speed does not affect the heat transfer (), it is crucial for powder formation.
Comparison between experimental data and model predictions
As mentioned in the introduction a model was proposed by Pawar et al.[Citation1] for the ATFD process. compares the experimental and predicted fraction of the used heat exchange area. The predicted values are much lower than the experimental results. The underestimation by the model can be related to several inappropriate model assumptions.
Figure 6. Fraction of used heating area for different drying temperature in the ATFD compared to predicted values (rotation speed = 600 rpm; feed rate = 0.3 kg/h). The symbols represent the experimental data. The solid line represents the predictions from the model developed by Pawar et al. [Citation1]. The error bars show the standard deviation of the experimental data (n = 3).
![Figure 6. Fraction of used heating area for different drying temperature in the ATFD compared to predicted values (rotation speed = 600 rpm; feed rate = 0.3 kg/h). The symbols represent the experimental data. The solid line represents the predictions from the model developed by Pawar et al. [Citation1]. The error bars show the standard deviation of the experimental data (n = 3).](/cms/asset/4cdebdbb-486d-41ae-980a-495fb4323b86/ldrt_a_1458037_f0006_b.jpg)
Our finding of the independence of the specific evaporation rate of the blade rotation speed () points towards heat transfer limited by the wall (and possibly the heating fluid outside), but not by the feed slurry itself. One should note that Pawar et al.[Citation1] found the same independence in their experiments, which in fact contradicts their own model.
Their model assumes that the blades continuously remove the boundary layer and mix the feed suspension ideally. This assumption is not valid for highly concentrated products (Stage 2 in ), with high viscosity and thus a low rotational Reynolds number (ReR < 100).[Citation1] For a solid-like product (Stage 3 in ), mixing will stop completely. This leads to overestimation of the heat transfer coefficient in the concentrated and final paste/solid phases.
It is assumed that the rotating blade completely removes material from the heat exchange surface and thus heat transfer resistance due to fouling is neglected. In practice always some deposited material is observed on the wall probably leading to increased and significant heat transfer resistance over time, adding to the dominance of the heat transfer limitation of the wall (point 1).
The model assumes that evaporation occurs only at the surface of the thin film, while the hottest location in the feed slurry is at the surface. Most probably, the evaporation already takes place close to the wall. Indeed we see the generation of bubbles indicating boiling inside the slurry. If this is the case, then the mass transfer from wall to bow wave does not contribute directly to the evaporation process; even though it is still important to remove the dry matter from the wall.
All of the above discussed assumptions contribute to the observed differences between predicted and experimental values and we therefore conclude that the penetration theory based model may be suitable for ATFE, but not for ATFD processes.
Application of ATFD to other food products
In addition to spinach juice, other food materials, i.e. WPI solution, sucrose solution, bell pepper juice and tomato juice, were dried in the lab-scale ATFD, to investigate the role of the properties of the feed materials in the ATFD process. Both WPI and sucrose solution were dried successfully in the setup, while bell pepper and tomato juices could not. These juices were only concentrated into a paste-like rubbery lump, which became very sticky and could not be scraped off by the blades anymore. The sticky rubbery lump accumulated in the setup impaired the operation of the dryer. The high concentrations of glucose and fructose, approximately 10 times higher than in spinach leaves, probably explains the observed drying behavior.[Citation20] These low molecular weight sugars do not crystallize easily and have a low glass transition temperature. Thus, these components contribute very much to sticky behavior during drying, leading to formation of a paste-like rubbery lump instead of a hard brittle solid.[Citation21–23] This was further confirmed by drying spinach juice with 2% w/w of glucose and 2% w/w fructose added. This sugar-rich spinach suspension could not be successfully dried, just as the bell pepper and tomato juices.
In contrast to glucose and fructose, sucrose solutions could be dried successfully, probably because of the fast crystallization of sucrose. indeed gives evidence of crystallized sucrose in the SEM micrograph of ATFD-dried sucrose particles. The sucrose consists of agglomerates of amorphous sugar and small regular hexagon crystals, which are indicated in the micrograph. It is indicative of that the fast crystallization may facilitate the solidification and fragmentation of the lump during drying. Also WPI could be dried with the lab-scale ATFD, as is shown in . The WPI powder particles are irregularly shaped with smooth break surfaces and some cracks, reflecting a brittle material fragmented by blade rotations. WPI consists of globular whey proteins that behave as ‘hard spheres’.[Citation24,Citation25] During drying, when the volume fraction of the proteins increases to a critical level, the hard spheres jam, which results in a dramatic change from a liquid-like to a solid-like state with a corresponding large viscosity change.[Citation26] The jammed WPI system is assumed to exhibit viscoelastic behavior similar to that of non-deformable hard sphere colloids.[Citation24,Citation25] The dried WPI resists to further deformation,[Citation24] being a hard brittle material, but can be broken by mechanical action via the blade rotation. Finally, shows that the ATFD-dried spinach powder consists of irregular-agglomerated particles with fibers.
Figure 7. Regular and scanning electron microscope images of ATFD-dried: (A) sucrose, (B) whey protein isolate (WPI), and (C) spinach and the ATFD chamber with the respective products.

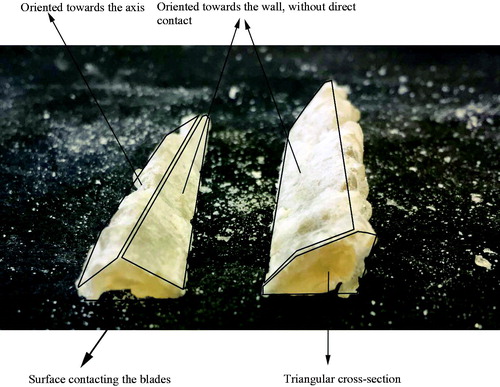
A solid lump could be removed from the equipment after drying of WPI, as shown in . The lump was tough (by manual tactile inspection), with a triangular cross section and probably did not contact the wall directly, but slid over a thin film on the surface. The powder formation may have resulted from attrition from the lump due to the mechanical action. This powder formation processes was also visualized in the schematic drawing of the ATFD process in . Because the WPI lumps are mechanically tough, the degree of attrition was not sufficient in the lab-scale ATFD and after a certain time impaired the operation of the dryer. In large scale operations, the tip speed of the blades can be much higher and combined with a stronger impeller motor, the disintegration of such a tough bow wave is faster and more thorough. Very fast blade rotation and a strong impeller motor could however also lead to local heating and damage to the product components.
Figure 8. Pictures of solidified bow-waves formed by accumulation of material during drying of a WPI solution in the ATFD.
We conclude that the product properties, and especially the combination of phase transition and viscoelastic properties, determine whether ATFD is a suitable dying process.
Conclusions
Suspensions prepared from spinach leaves were dried in a lab-scale agitated thin-film dryer (ATFD). The spinach powders that were obtained had good flowability. The drying behavior was characterized and especially wall temperature was identified as important for the specific evaporation rate, giving a parameter to set the dryer capacity. The blade rotation speed does not affect the drying rate, but mechanically disintegrates the material into small powder particles during drying. The independence of the specific drying rate of the blade rotation speed shows that in our system the drying process is limited by heat transfer through the wall, which falsifies the existing model for ATFD, based on the penetration theory.
Successful drying is strongly dependent on the product properties. A liquid–solid phase transition at high concentrations and corresponding brittle viscoelastic behavior make a material potentially suitable for ATFD. This was demonstrated by drying of slurries of WPI, sucrose, and bell pepper and tomato juices. Larger scale ATFD enables higher blade velocities and stronger shearing, which may make the process somewhat less sensitive to the product properties. Disadvantage of high blade speed velocities may however be undesired elevation of the local temperature due to friction.
Acknowledgements
The authors thank Chaoyang Wang, Remco Boer, and Rens van Roekel for their support by performing experimental work.
Additional information
Funding
References
- Pawar, S. B.; Patil, R.; Mujumdar, A. S.; Thorat, B. N. Mathematical Modeling of Agitated Thin-Film Dryer. Dry. Technol. 2011, 29, 719–728. doi:10.1080/07373937.2010.526732
- Devahastin, S.; Mujumdar, A. S. Indirect Dryers. In Handbook of Industrial Drying, 4th ed.; CRC Press: Boca Raton, 2006; pp 137–149.
- Rodriguez, G.; Vasseur, J.; Courtois, F. Design and Control of Drum Dryers for the Food Industry. Part 1. Set-up of a Moisture Sensor and an Inductive Heater. J. Food Eng. 1996, 28, 271–282. doi:10.1016/0260-8774(95)00053-4
- Zotarelli, M. F.; Carciofi, B. A. M.; Laurindo, J. B. Effect of Process Variables on the Drying Rate of Mango Pulp by Refractance Window. Food Res. Int. 2015, 69, 410–417. doi:10.1016/j.foodres.2015.01.013
- Abonyi, B. I.; Feng, H.; Tang, J.; Edwards, C. G.; Chew, B. P.; Mattinson, D. S.; Fellman, J. K. Quality Retention in Strawberry and Carrot Purees Dried with Refractance™ Window System. J. Food Sci. 2002, 67, 1051–1056. doi:10.1111/j.1365-2621.2002.tb09452.x
- Nindo, C. I.; Feng, H.; Shen, G. Q.; Tang, J.; Kang, D. H. Energy Utilization and Microbial Reduction in a New Film Drying System. J. Food Process. Preserv. 2003, 27, 117–136. doi:10.1111/j.1745-4549.2003.tb00506.x
- Daud, W. R. W. Drum Dryers. In Handbook of Industrial Drying. 4th ed. CRC Press: Boca Raton, 2006.
- Ochoa-Martínez, C. I.; Quintero, P. T.; Ayala, A. A.; Ortiz, M. J. Drying Characteristics of Mango Slices Using the Refractance Window™ Technique. J. Food Eng. 2012, 109, 69–75. doi:10.1016/j.jfoodeng.2011.09.032
- Nindo, C.; Tang, J. Refractance Window Dehydration Technology: A Novel Contact Drying Method. Dry. Technol. 2007, 25, 37–48. doi:10.1080/07373930601152673
- Komori, S.; Takata, K.; Murakami, Y. Flow Structure and Mixing Mechanism in an Agitated Thin-Film Evaporator. J. Chem. Eng. Japan. 1988, 21, 639–644. doi:10.1252/jcej.21.639
- Pawar, S. B.; Mujumdar, A.; Thorat, B. Flow Pattern and Heat Transfer in Agitated Thin Film Dryer. Chem. Eng. Process. 2011, 50, 687–693. doi:10.1016/j.cep.2011.04.005
- Keller, E. Oscillatory Rotor Blade for Treatment of Fluent Material in Thin Layers. U.S. Patent US3199575, Aug 10, 1965.
- Wilkerson, K. L. Ice-Making Machine. U.S. Patent US4538428, Sept 03, 1985.
- Stamatiou, E.; Meewisse, J.; Kawaji, M. Ice Slurry Generation Involving Moving Parts. Int. J. Refriger. 2005, 28, 60–72. doi:10.1016/j.ijrefrig.2004.07.016
- Komori, S.; Takata, K.; Murakami, Y. Flow and Mixing Characteristics in an Agitated Thin-Film Evaporator with Vertically Aligned Blades. J. Chem. Eng. Japan. 1989, 22, 346–351. doi:10.1252/jcej.22.346
- De Goede, R.; De Jong, E. Heat Transfer Properties of a Scraped-Surface Heat Exchanger in the Turbulent Flow Regime. Chem. Eng. Sci. 1993, 48, 1393–1404. doi:10.1016/0009-2509(93)80046-S
- McKenna, T. F. Design Model of a Wiped Film Evaporator: Applications to the Devolatilisation of Polymer Melts. Chem. Eng. Sci. 1995, 50, 453–467. doi:10.1016/0009-2509(94)00257-R
- Zeboudj, S.; Belhaneche-Bensemra, N.; Belabbes, R.; Bourseau, P. Modelling of Flow in a Wiped Film Evaporator. Chem. Eng. Sci. 2006, 61, 1293–1299. doi:10.1016/j.ces.2005.08.010
- Quek, S. Y.; Chok, N. K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chem. Eng. Process. 2007, 46, 386–392. doi:10.1016/j.cep.2006.06.020
- US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28 (Slightly revised). Version Current: May 2016. http://www.ars.usda.gov/ba/bhnrc/ndl
- Jaya, S.; Das, H. Glass Transition and Sticky Point Temperatures and Stability/Mobility Diagram of Fruit Powders. Food Bioprocess Technol. 2009, 2, 89–95. doi:10.1007/s11947-007-0047-5
- Muzaffar, K.; Nayik, G. A.; Kumar, P. Stickiness Problem Associated with Spray Drying of Sugar and Acid Rich Foods: A Mini Review. J. Nutr. Food Sci. 2015, S12, 003. doi:10.4172/2155-9600.S12-003
- O’Callaghan, D.; Hogan, S. The Physical Nature of Stickiness in the Spray Drying of Dairy Products—a Review. Dairy Sci. Technol. 2013, 93, 331–346. doi:10.1007/s13594-013-0114-9
- Sadek, C.; Pauchard, L.; Schuck, P.; Fallourd, Y.; Pradeau, N.; Le Floch-Fouéré, C.; Jeantet, R. Mechanical Properties of Milk Protein Skin Layers after Drying: Understanding the Mechanisms of Particle Formation from Whey Protein Isolate and Native Phosphocaseinate. Food Hydrocolloids. 2015, 48, 8–16. doi:10.1016/j.foodhyd.2015.01.014
- Nicolai, T.; Durand, D. Protein Aggregation and Gel Formation Studied with Scattering Methods and Computer Simulations. Curr. Opin. Colloid. Interface Sci. 2007, 12, 23–28. doi:10.1016/j.cocis.2007.03.002
- Hogan, S. A.; O’Loughlin, I. B.; Kelly, P. M. Soft Matter Characterisation of Whey Protein Powder Systems. Int. Dairy J. 2016, 52, 1–9. doi:10.1016/j.idairyj.2015.07.005