 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Spray drying of emulsions is a widespread encapsulation technique to produce a large variety of powdered formulations. The oil droplet size (ODS) in the powder is critical for product quality, influencing key product parameters such as the encapsulation efficiency (EE) of the oil. To expand the understanding of changes in ODS and structure formation during the drying step. For this purpose, model oil-in-water emulsions were spray-dried at varying air inlet and outlet temperatures. The powders were characterized regarding ODS and EE. Smaller ODS were observed for parameter combinations where higher drying rates are expected due to decreased coalescence. The results for the EE revealed no clear trend. An increase of air outlet temperature first led to a small decrease in EE followed by a distinct increase of EE. The first decrease may indicate a collapse of the particle morphology but more detailed investigations are necessary to explore these phenomena.
Introduction
Spray drying is a commonly used technique for the production of oily powder products such as infant formula, dairy powders and products with encapsulated oil soluble components such as flavors or vitamins.[Citation1] In this process, an oil-in-water emulsion is atomized into fine droplets, which are subsequently dried into particles by contact with hot air.[Citation2] Generally speaking, the oil droplet size (ODS) and amount of free surface oil are the two most important quality parameters determining product properties of the reconstituted emulsion such as color, creaminess and bioavailability of encapsulated active substances as well as powder product properties such as flowability and stability.[Citation3–5] Powder properties depend also on the matrix material and the particle size, which is mainly dominated by the spray droplet size after atomization.
In industrial processes, the ODS is commonly set to a specific value in the feed emulsion under the assumption, that it is stable during the spray drying process. However, previous studies have shown that the ODS can undergo changes during the atomization as well as the spray droplet drying process, resulting in differences between the ODS in the final powder product compared to the feed emulsion.[Citation6,Citation7] It is therefore mandatory to build a good understanding of the parameters influencing the oil droplet size distribution (ODSD) during the drying of a spray droplet.
The drying of a spray droplet is commonly divided into two stages.[Citation8,Citation9] In the beginning, the droplet is quickly heated until droplet temperature reaches the wet bulb temperature. The droplet temperature remains constant and the drying takes place at an almost constant rate. The droplet shrinks and the concentration of solutes and oil droplets increases. As the spray droplet shrinks and the oil concentration increases, the oil droplets are more likely to come into contact and the collision frequency increases.[Citation10] This leads to an increase in ODS, a phenomenon that has been previously described for spray drying of emulsions.[Citation11,Citation12]
Depending on the relationship of the velocity of shrinking and the diffusion rate of the solute, a more or less pronounced concentration gradient can develop with higher solute concentration at the droplet surface. After reaching a critical concentration of solute at the droplet surface, a skin forms and heat and mass transfer are slowed down. This typically marks the start of the second drying step, where surface moisture is completely evaporated and the drying rate is controlled by internal moisture migration.[Citation8] Once the moisture removal is complete, the dried particle heats up until it reaches the temperature of the drying air.[Citation13] This process can be described by heat and mass transfer equations as formulated by Nešić and Vodnik[Citation9] and Ranz.[Citation14] From these assumptions it is quite clear, that with an increasing heat transfer to the drying droplet, the drying rate increases. This can either be achieved by an increase in drying air temperature or an increase in air velocity. These correlations have been shown in single droplet experiments.[Citation15,Citation16] It was shown that the drying rate increases for higher air temperatures or air velocities and that the locking point is reached earlier in the process.
Different particle morphologies may develop during the last stage of droplet drying.[Citation17] If the drying rate is slow, there is sufficient time for equilibration of the concentration gradient within the particle and a solid particle is formed. For high drying rates, a hollow particle is expected to form, as the droplet heats up and vapor is formed inside. In extreme cases, this can even lead to the formation of blow holes. Depending on the rigidity of the surface the droplet can also go through a cycle of inflation and deflation periods, and may form different structures. A commonly used tool to predict if a droplet will form a solid or hollow powder particle is the Péclet number, which is defined as the ratio between the evaporation rate at the drying front to the diffusion coefficient of the solute in the water phase.[Citation18] Matrix materials, such as starch, have very small diffusion coefficients compared to the drying rates in a spray dryer, thus usually leading to very high Péclet number. Hollow particle structures are expected in this case.
Ideally, the smallest possible oil droplets are encapsulated and the oil droplets are completely enclosed by matrix material.[Citation5] A large amount of unencapsulated surface oil usually leads to inferior powder properties, such as powder clumping due to liquid bridges formed between the particles by the oil.[Citation19] The amount of free surface oil is largely determined by the morphology of the dried powder and thus by the conditions of the drying process and used materials. High drying rates are known to lead to a collapse of the particle structure, i.e. due to the formation of cracks and blowholes.[Citation20] This may lead to an increased amount of oil leaking out of the powder matrix and covering the particles surface.
In the literature, the influence of the drying temperature on the free oil content has not been sufficiently clarified, with different studies yielding seemingly contradicting results. In general, higher temperatures, and thus higher drying rates, lead to shorter drying processes. This leads to an earlier skin formation and the locking point is reached earlier, too. Consequently, there is less time available for coalescence and redistribution of the oil droplets, leading to smaller oil droplets and an increased EE due to a larger proportion of the oil being enclosed within the starch matrix. This correlation has been shown in many studies.[Citation3,Citation21] However, several contrasting studies have shown that higher temperatures may result in vacuole formation and particle breakage, leading to increased oil leakage and lower EE.[Citation22]
A systematic investigation of the influence of the drying air temperature on surface oil content should help to better understand the relationship between drying conditions and the resulting particle structures. It is hypothesized, that the oil droplet size in the powder is smaller when drying at parameter combinations that are expected to lead to higher drying rates, as coalescence of the oil droplets should be reduced. It is furthermore hypothesized, that this shorter drying time also goes along with a higher EE due to an expected decrease in redistribution effects. To investigate these aspects, spray drying experiments at different air outlet temperature at constant air inlet temperature were conducted. This was achieved by increasing the heater power as well as the air mass flow. As both of these changes work toward an increase in heat transfer, it can be assumed that the drying rate increases. Furthermore, the impact of different air inlet temperatures at constant outlet temperature were investigated. The impact of this parameter variation on the drying rate is not obvious, due to the necessity of reducing the air mass flow and thereby the air velocity while increasing the heater power. The oil droplet size will be tracked and compared along the whole spray drying process to allow an isolated quantification of the impact of the drying step on the ODS. The influence of the drying rate on the product parameters will be discussed exemplary on the ODSD and the oil-EE, as further discussion would go beyond the scope of this paper.
Materials and methods
Model emulsion: preparation and characterization
All experiments were carried out with a model oil-in-water emulsion. MCT-oil (Witarix MCT-oil 60/40, IOI Oleochemical, Germany) was used as dispersed phase and a modified starch (C*Em-Cap, Cargill, Germany) solution as continuous phase. The used modified starch is an emulsifying starch, allowing to act for the purpose of matrix material and emulsifier simultaneously.
The emulsions were prepared in a two-step process similarly to the procedure by Taboada et al.[Citation23] In a first step, an ODS of 2 µm is adjusted by preparation of a concentrated emulsion using a colloid mill (IKA magic LAB, IKA Werke GmbH & Co. KG, Germany). The device was operated at 15,000 rpm for 2 min and a gap width of 0.159 mm. The composition of the concentrated emulsion was 40 wt.% water, 10 wt.% modified starch and 50 wt.% MCT oil. All reported mass fractions refer to the mass of the total emulsion. In the second step, the concentrated emulsion was diluted with a modified starch solution to reach a final composition of 15 wt.% MCT-oil and 20 wt.% modified starch in the feed emulsion for spray drying. This procedure was chosen to be able to produce large quantities of emulsion with a constant initial oil droplet size. The ODSD of the emulsions was measured via laser diffraction (HORIBA LA95, Retsch Technology GmbH, Germany).
Atomization of emulsions
Atomization experiments were carried out at an atomization test rig as described in Wittner et al.[Citation24] A three piston pump (Rannie LAB Typ 8.5, SPX FLOW Inc., USA) was used to supply the emulsion to the pressure swirl atomizer SKHN-MFP SprayDry (SprayingSystems Deutschland GmbH, Germany). The atomizers orifice diameter was set to 0.34 mm. Atomization experiments were conducted at atomization pressures of 10 MPa at room temperature. Volume flow rate at 10 MPa was between 0.3 and 0.4 L/min. ODSDs were measured offline by laser diffraction. For that purpose, a sample of the spray was collected during atomization. Due to the good repeatability of the measurements, atomization trials were performed with only one emulsion, with three samples being taken.
Spray drying of emulsion
Spray drying experiments have been conducted in a pilot-scale co-current spray dryer (Werco SD20, Hans G. Werner Industrietechnik GmbH, Germany). Atomization conditions were the same as in the atomization experiments. The spray dryer was operated with inlet air temperatures of 180–220 °C and outlet air temperatures of 65–85 °C. Air flow rate was set to around 500–700 m³/s depending on the targeted inlet and outlet temperature combinations. The collected powders were stored in air-tight containers or analysis. The ODS was measured with laser diffraction spectroscopy in the redispersed powder. Therefore, the powder was reconstituted in water with a concentration of 300 g/L. The oil droplet size was measured in triplicate.
Determination of free surface oil by solvent extraction
To determine the encapsulation efficiency (EE), the free surface oil was determined by solvent extraction. For that purpose, an experimental procedure according to Bae et al.[Citation25] was used. Spray-dried powder samples of 1.5 g were merged with 15 mL n-hexane (Carl Roth GmbH + Co. KG, Germany). The sample was shaken at 200 rpm in a vortex mixer. Subsequently, the n-hexane was removed by vacuum filtration through a filter paper (pore size 6 µm, Sigma-Aldrich, USA). The powder sample was then rinsed three times with 20 mL of n-Hexane by passing it through the powder to ensure that no surface oil remained. After evaporation of the n-hexane, the EE was calculated using the following equation:
(1)
(1)
where
is the total oil mass in the powder sample, and
is the surface oil that was extracted by the n-hexane extraction.
For the calculation of the EE, residual moisture content of the samples was taken into account. The residual moisture in the dried powder was determined by weight loss after oven-drying at 105 °C to constant mass. All measurements were performed in triplicate.
Results and discussion
Effect of air outlet temperature on the oil droplet size in the reconstituted powder
The resulting ODSD are depicted in for the whole spray drying process at different air outlet temperatures. The air outlet temperature was changed in a range of 65–85 °C, while the air inlet temperature was kept constant at 180 °C. This was achieved by increasing both the heater power as well as the air mass flow. The largest ODS is observed in the feed emulsion, with a Sauter mean diameter of 2 µm. The ODS decreases after atomization. This result is according to expectation, as it has been shown previously[Citation23,Citation26] that the atomization step can lead to oil droplet breakup and therefore changes in the ODS. The ODS after the drying step is larger than after atomization for all examined parameter combinations. This increase of the ODS during the drying step is consistent with the expectation described in the Introduction. As the drying spray droplet is shrinking, the oil droplets are more likely to collide, leading to coalescence of the oil droplets and the observed increase in ODS until the drying of the droplet is completed.
Figure 1. Volume cumulative distribution for the ODS before atomization, after atomization and in the redispersed powder at air outlet temperatures from 65 to 85 °C and a constant inlet air temperature of 180 °C.
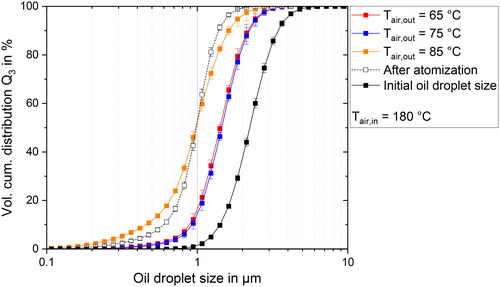
The smallest oil droplets are observed for a temperature of 85 °C, while larger ODSDs were observed for 65 and 75 °C. For the two lower temperatures, the ODSDs are overlapping. As we can expect higher drying rates for higher inlet temperatures, this goes along with the hypothesis from the Introduction that the oil droplets stay smaller during the drying step with increasing drying rates. As a reminder, at the investigated parameter combinations, air velocity and air mass flow increases, while the air inlet temperature, volume flow rate of the feed and the spray droplet size stays the same. The increased air velocity is expected to lead to shorter residence times in the spray dryer. Taking a look at the results for the residual moisture content in it can be seen, that the residual moisture content decreases for higher air outlet temperatures, despite shorter expected residence times. Based on this observation it can be assumed that the drying rate increases with increasing air outlet temperature.
Table 1. Residual moisture content for different air outlet temperature combinations at a constant air inlet temperature of 180 °C.
Effect of air inlet temperature on the oil droplet size in the reconstituted powder
The resulting ODSD for the whole spray drying process at different air inlet temperatures are depicted in . The air inlet temperature was changed in a range of 180–220 °C, while the outlet air temperature was kept constant at 75 °C. The largest ODS is again observed in the feed emulsion, while the atomization leads to a decrease in the ODS. The oil droplets after the drying step are again larger than after atomization for all examined parameter combinations. The smallest oil droplets are observed for the highest temperature of 220 °C, and the largest for 180 °C, with the results for 200 °C almost overlapping with the results 180 °C.
Figure 2. Volume cumulative distribution for the ODS before atomization, after atomization and in the redispersed powder at air inlet temperatures from 180 to 220 °C and a constant outlet air temperature of 75 °C.
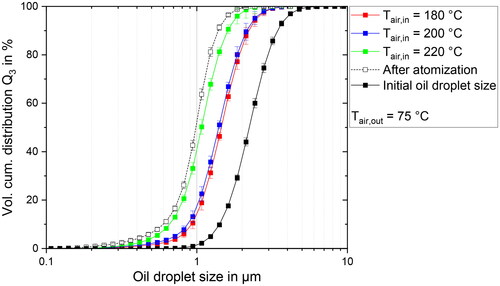
While the impact of a variation in air inlet temperature on the drying rate is not clear from theoretical considerations alone, it can be seen that the results for the ODS indicate less coalescence of the oil droplets for higher air inlet temperatures similarly to the results of a variation in air outlet temperature. Based on this observation alone, it can be assumed that the drying rate effectively increases for parameter combinations that lead to higher inlet temperatures as well. The residual moisture content is in a range of 2.37 to 2.73 wt.% for all investigated inlet temperatures, which does not give any indication on the drying rates.
Effect of drying rate on the encapsulation efficiency
The results for the EE of the oil in the spray dried powders are presented in . Parameter combinations for inlet temperatures of 180 and 200 °C were investigated for all outlet temperatures. Due to limitations associated with the high air inlet temperature of 220 °C, only the value for 75 °C is shown.
Figure 3. Encapsulation efficiency for different inlet air temperatures (180–220 °C) and outlet air temperatures (65–85 °C).
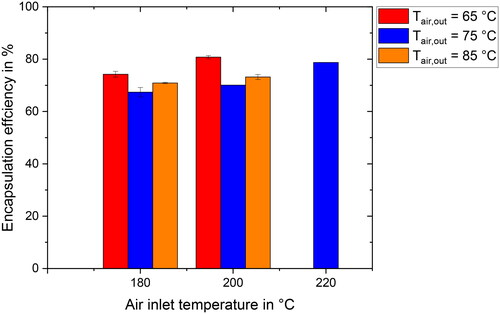
Encapsulation efficiencies ranging from approximately 65–80% were observed for all parameter combinations. Taking a closer look at the results for different air inlet temperatures at a constant outlet temperature of 75 °C, an increase in EE from 67.4 to 78.7% can be seen with increasing air inlet temperature. This supports the hypothesis presented in the Introduction, indicating that higher drying rates due to elevated air inlet temperatures lead to higher encapsulation efficiencies.
When exploring the results for different air outlet temperatures at a constant inlet temperature, it can be observed that the EE decreases for an outlet temperature increase from 65 to 75 °C for the investigated inlet temperatures of 180 and 200 °C. This contradicts the expected higher EEs for higher drying rates. As was previously explained, an increase in air outlet temperature at constant inlet temperature increases the drying rate. Furthermore, it is anticipated that the outlet temperature increase leads to a higher final product temperature, as the temperature of the dry particles is determined by the surrounding air temperature. The combination of these two effects may lead to a collapse of the particle morphology and thus a decrease in EE. Similar results have been reported in previous studies.[Citation27,Citation28]
Further increasing the outlet temperature from 75 to 85 °C shows the trend of increasing EE with higher drying rates that was hypothesized in the Introduction. Oil droplets are expected to be immobilized within a shorter time span as the locking point is reached earlier in the process. It has to be noted, that the ODS in the final powder particles are smaller for an air outlet temperature of 85 °C compared to the other outlet temperatures. It has been shown previously[Citation29] that oil droplet size may impact EE in spray dried particles. In these publications, a reduction in ODS is generally linked to an increase in EE. With this in mind, the increase in EE from 75 to 85 °C could be caused due to both the higher drying rate as well as the differences in ODS. In future work, initial ODS in the feed emulsion should be set to a value at which no additional oil droplet breakup during atomization occurs, minimizing differences in ODS in the final powder product.
Nevertheless, it is an unsolved question if particle collapse still plays a role at these conditions. As the powder samples show a particle size distribution, it would be expected that the particle structure collapse occurs across a temperature range.
To better understand the influence of the drying rate on EE, single droplet drying experiments at different drying rates could be conducted. These experimental setups allow the monitoring of the particle morphology during the whole drying process. This is especially of interest, as a collapse of the particle morphology is expected to be pivotal for the decrease of EE for an air outlet temperature increase from 65 to 75 °C and may therefore present a great tool to deepen the understanding of the correlating mechanisms.
Conclusions
In the present study, different parameter combinations of air outlet and inlet temperatures were investigated during spray drying of a model emulsion containing modified starch and MCT-oil. In general, smaller oil droplets in the powder were observed for higher air outlet temperatures, as a higher drying rate leads to a shorter available time span for the coalescence of oil droplets. A similar result is observed for a change in air inlet temperature, indicating increased drying rates for the chosen parameter combinations and therefore the same influence on ODS.
It was found that increasing air inlet temperatures leads to a higher EE, as the time for a redistribution of the oil to the particles surface is shorter. A specific influence of the air outlet temperature on the EE was observed. At first, the EE decreased with increasing outlet temperature, an observation that may be attributed to a collapse of the particle morphology due to higher drying rates. A further increase in outlet temperature leads again to higher EE, although the impact of the drying rate and ODS on EE could not be properly separated. To mitigate the impact of the ODS, future work should aim to set the initial ODS to a sufficiently small value that oil droplet breakup during atomization is minimized. This would pave the way for further analysis of the impact of the drying rate on particle morphology development and its effect on EE. Additionally, single droplet drying experiments could prove beneficial, as they offer the possibility of a continuous monitoring of the particle morphology during drying.
Acknowledgements
Funding of the research project (AiF 21662 N) within the programme for promoting the Industrial Collective Research (IGF) of the German Federal Ministry for Economic Affairs and Climate Action (BMWK), supported via the Society for Process Engineering (GVT) based on a resolution of the German Parliament.
Disclosure statement
The authors report there are no competing interests to declare.
References
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. DOI: 10.1016/j.foodres.2007.07.004.
- Barbosa-Cánovas, G. V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum Publishers: Amsterdam, The Netherlands, 2005.
- Drusch, S.; Berg, S. Extractable Oil in Microcapsules Prepared by Spray-Drying: Localisation, Determination and Impact on Oxidative Stability. Food Chem. 2008, 109, 17–24. DOI: 10.1016/j.foodchem.2007.12.016.
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of Powder Particle Structure on the Oxidation Stability and Color of Encapsulated Crystalline and Emulsified Carotenoids in Carrot Concentrate Powders. J. Food Eng. 2019, 263, 398–408. DOI: 10.1016/j.jfoodeng.2019.07.025.
- Vega, C.; Roos, Y. H. Invited Review: Spray-Dried Dairy and Dairy-Like Emulsions—Compositional Considerations. J. Dairy Sci. 2006, 89, 383–401. DOI: 10.3168/jds.S0022-0302(06)72103-8.
- Serfert, Y.; Schröder, J.; Mescher, A.; Laackmann, J.; Rätzke, K.; Shaikh, M. Q.; Gaukel, V.; Moritz, H.-U.; Schuchmann, H. P.; Walzel, P.; et al. Spray Drying Behaviour and Functionality of Emulsions with β-Lactoglobulin/Pectin Interfacial Complexes. Food Hydrocolloids 2013, 31, 438–445. DOI: 10.1016/j.foodhyd.2012.11.037.
- Taboada, M. L.; Heiden‐Hecht, T.; Brückner‐Gühmann, M.; Karbstein, H. P.; Drusch, S.; Gaukel, V. Spray Drying of Emulsions: Influence of the Emulsifier System on Changes in Oil Droplet Size during the Drying Step. J. Food Process. Preserv. 2021, 45, e15753. DOI: 10.1111/jfpp.15753.
- Abdullahi, H.; Burcham, C. L.; Vetter, T. A Mechanistic Model to Predict Droplet Drying History and Particle Shell Formation in Multicomponent Systems. Chem. Eng. Sci. 2020, 224, 115713. DOI: 10.1016/j.ces.2020.115713.
- Nešić, S.; Vodnik, J. Kinetics of Droplet Evaporation. Chem. Eng. Sci. 1991, 46, 527–537. DOI: 10.1016/0009-2509(91)80013-O.
- Chesters, A. K. The Modelling of Coalescence Processes in Fluid-Liquid Dispersions: A Review of Current Understanding. Chem. Eng. Res. Des. 1991, 69, 259–270.
- Dollo, G.; Le Corre, P.; Guérin, A.; Chevanne, F.; Burgot, J. L.; Leverge, R. Spray-Dried Redispersible Oil-in-Water Emulsion to Improve Oral Bioavailability of Poorly Soluble Drugs. Eur. J. Pharm. Sci. 2003, 19, 273–280. DOI: 10.1016/S0928-0987(03)00134-9.
- Taboada, M. L.; Chutani, D.; Karbstein, H. P.; Gaukel, V. Breakup and Coalescence of Oil Droplets in Protein-Stabilized Emulsions During the Atomization and the Drying Step of a Spray Drying Process. Food Bioprocess Technol. 2021, 14, 854–865. DOI: 10.1007/s11947-021-02606-1.
- Gopireddy, S. R.; Gutheil, E. Numerical Simulation of Evaporation and Drying of a Bi-Component Droplet. Int. J. Heat Mass Transf. 2013, 66, 404–411. DOI: 10.1016/j.ijheatmasstransfer.2013.07.010.
- Ranz, W. E. Evaporation from Drops-I and-II. Chem. Eng. Progr. 1952, 48, 141–146, 173–180.
- Shamaei, S.; Kharaghani, A.; Seiiedlou, S. S.; Aghbashlo, M.; Sondej, F.; Tsotsas, E. Drying Behavior and Locking Point of Single Droplets Containing Functional Oil. Adv. Powder Technol. 2016, 27, 1750–1760. DOI: 10.1016/j.apt.2016.06.006.
- Walton, D. E. The Evaporation of Water Droplets. A Single Droplet Drying Experiment. Dry. Technol. 2004, 22, 431–456. DOI: 10.1081/DRT-120029992.
- Walton, D. E.; Mumford, C. J. Spray Dried Products—Characterization of Particle Morphology. Chem. Eng. Res. Des. 1999, 77, 21–38. DOI: 10.1205/026387699525846.
- Both, E. M.; Karlina, A. M.; Boom, R. M.; Schutyser, M. Morphology Development During Sessile Single Droplet Drying of Mixed Maltodextrin and Whey Protein Solutions. Food Hydrocolloids 2018, 75, 202–210. DOI: 10.1016/j.foodhyd.2017.08.022.
- Taneja, A.; Ye, A.; Jones, J. R.; Archer, R.; Singh, H. Behaviour of Oil Droplets During Spray Drying of Milk-Protein-Stabilised Oil-in-Water Emulsions. Int. Dairy J. 2013, 28, 15–23. DOI: 10.1016/j.idairyj.2012.08.004.
- Adhikari, B.; Howes, T.; Bhandari, B. R.; Truong, V. Experimental Studies and Kinetics of Single Drop Drying and Their Relevance in Drying of Sugar‐Rich Foods: A Review. Int. J. Food Prop. 2000, 3, 323–351. DOI: 10.1080/10942910009524639.
- Frascareli, E. C.; Silva, V. M.; Tonon, R. V.; Hubinger, M. D. Effect of Process Conditions on the Microencapsulation of Coffee Oil by Spray Drying. Food Bioprod. Process. 2012, 90, 413–424. DOI: 10.1016/j.fbp.2011.12.002.
- Vignolles, M.-L.; Jeantet, R.; Lopez, C.; Schuck, P. Free Fat, Surface Fat and Dairy Powders: Interactions Between Process and Product: A Review. Lait 2007, 87, 187–236. DOI: 10.1051/lait:2007010.
- Taboada, M. L.; Schäfer, A.-C.; Karbstein, H. P.; Gaukel, V. Oil Droplet Breakup During Pressure Swirl Atomization of Food Emulsions: Influence of Atomization Pressure and Initial Oil Droplet Size. J. Food Process Eng. 2021, 44, e13598. DOI: 10.1111/jfpe.13598.
- Wittner M. O., Ballesteros M. A., Link F. J., Karbstein H. P., Gaukel V.. Air–Core–Liquid-Ring (ACLR) Atomization Part II: Influence of Process Parameters on the Stability of Internal Liquid Film Thickness and Resulting Spray Droplet Sizes. Processes 2019, 7, 616. DOI: 10.3390/pr7090616.
- Bae, E. K.; Lee, S. J. Microencapsulation of Avocado Oil by Spray Drying Using Whey Protein and Maltodextrin. J. Microencapsul. 2008, 25, 549–560. DOI: 10.1080/02652040802075682.
- Bolszo, C. D.; Narvaez, A. A.; McDonell, V.; Dunn-Rankin, D.; Sirignano, W. A. Pressure—Swirl Atomization of Water-in-Oil Emulsions. Atomiz Spr. 2010, 20, 1077–1099. DOI: 10.1615/AtomizSpr.v20.i12.50.
- Bhandari, B. R.; Dumoulin, E. D.; Richard, H.; Noleau, I.; Lebert, A. M. Flavor Encapsulation by Spray Drying: Application to Citral and Linalyl Acetate. J. Food Sci. 1992, 57, 217–221. DOI: 10.1111/j.1365-2621.1992.tb05459.x.
- Hansen, P. S. Production of Agglomerated Fat-Filled Milk Powder. Int. J. Dairy Technol. 1980, 33, 19–23. DOI: 10.1111/j.1471-0307.1980.tb01459.x.
- Danviriyakul, S.; McClements, D. J.; Decker, E.; Nawar, W. W.; Chinachoti, P. Physical Stability of Spray-Dried Milk Fat Emulsion as Affected by Emulsifiers and Processing Conditions. J. Food Sci. 2002, 67, 2183–2189. DOI: 10.1111/j.1365-2621.2002.tb09524.x.
