Abstract
Over the past 15 years, the seismic shifts caused by the convergence of biomolecular, chemical, physical, mathematical, and computational sciences alongside cutting-edge developments in information technology and engineering have erupted into a new field of scientific endeavor dubbed Synthetic Biology. Recent rapid advances in high-throughput DNA sequencing and DNA synthesis techniques are enabling the design and construction of new biological parts (genes), devices (gene networks) and modules (biosynthetic pathways), and the redesign of biological systems (cells and organisms) for useful purposes. In 2014, the budding yeast Saccharomyces cerevisiae became the first eukaryotic cell to be equipped with a fully functional synthetic chromosome. This was achieved following the synthesis of the first viral (poliovirus in 2002 and bacteriophage Phi-X174 in 2003) and bacterial (Mycoplasma genitalium in 2008 and Mycoplasma mycoides in 2010) genomes, and less than two decades after revealing the full genome sequence of a laboratory (S288c in 1996) and wine (AWRI1631 in 2008) yeast strain. A large international project – the Synthetic Yeast Genome (Sc2.0) Project – is now underway to synthesize all 16 chromosomes (∼12 Mb carrying ∼6000 genes) of the sequenced S288c laboratory strain by 2018. If successful, S. cerevisiae will become the first eukaryote to cross the horizon of in silico design of complex cells through de novo synthesis, reshuffling, and editing of genomes. In the meantime, yeasts are being used as cell factories for the semi-synthetic production of high-value compounds, such as the potent antimalarial artemisinin, and food ingredients, such as resveratrol, vanillin, stevia, nootkatone, and saffron. As a continuum of previously genetically engineered industrially important yeast strains, precision genome engineering is bound to also impact the study and development of wine yeast strains supercharged with synthetic DNA. The first taste of what the future holds is the de novo production of the raspberry ketone aroma compound, 4-[4-hydroxyphenyl]butan-2-one, in a wine yeast strain (AWRI1631), which was recently achieved via metabolic pathway engineering and synthetic enzyme fusion. A peek over the horizon is revealing that the future of “Wine Yeast 2.0” is already here. Therefore, this article seeks to help prepare the wine industry – an industry rich in history and tradition on the one hand, and innovation on the other – for the inevitable intersection of the ancient art practiced by winemakers and the inventive science of pioneering “synthetic genomicists”. It would be prudent to proactively engage all stakeholders – researchers, industry practitioners, policymakers, regulators, commentators, and consumers – in a meaningful dialog about the potential challenges and opportunities emanating from Synthetic Biology. To capitalize on the new vistas of synthetic yeast genomics, this paper presents wine yeast research in a fresh context, raises important questions and proposes new directions.
Synthesizing unscripted futures
Imagine, for a moment, it is 2050 and you are watching a television show called Who wants to be a Billionaire? A contestant – who happens to be a young winemaker – is one question away from the bonanza. The final question is a particularly intriguing and complex one: “What breakthrough-technology in biology emerged at the start of the twenty-first century that has had the most profound impact on the cost-effective production of things such as energy-rich molecules for renewable biofuels and sustainable industrial chemicals; compounds for the bioremediation of polluted environments; novel antibiotics, vaccines and personalized medicines; and adequate nutritious and safe food supplies to meet the demands of today’s global population of 9 billion people of which two-thirds are over the age of 60?” The contestant anxiously considers the four options as they come up. A: Molecular Biology; B: Systems Biology; C: Regenerative Biology; D: Synthetic Biology. As a professional winemaker with formal training in science, the contestant discounts options A and B because those two disciplines originated in the previous century. However, the stakes are high and to be absolutely sure, the contestant uses the last lifeline by asking the audience to vote. The audience responds unanimously with option D. The contestant instantly locks in D: Synthetic Biology. Under loud applause the host hands the contestant a $1-billion holographic e-coin voucher and the world has yet another billionaire. Pure fantasy or not?
Without the benefit of 20/20 hindsight we cannot accurately predict an unscripted future. We know all too well that even familiar things can play out in very unfamiliar ways. However, one certainty is that tomorrow is shaped by, amongst other things, the groundbreaking scientific achievements, and technological developments of yesterday and today. Our individual and collective fascinations and concerns regarding emerging sciences such as Synthetic Biology, and our present choices and actions, will sculpt the world inherited by future generations. The art of turning hindsight into foresight gives us the necessary insight to learn from the past, prepare for the future and to conduct our research in new emerging sciences in the present so that the past and the future are ever present when we frame our research questions, conceptualize our experiments and develop the necessary policy and regulatory frameworks.
Today’s fast-paced world craves Eureka! moments in science and “something-for-nothing” turning-point discoveries and big-picture technological advances that reveal new horizons at a stroke. Such headline-grabbing Eureka! breakthroughs are rare in the world of science, which is becoming increasingly specialized but, at the same time, also multi-disciplinary and interconnected. The gradual seismic forces build as researchers collaborate around the globe and across the boundaries of various disciplines to stack data point atop data point, and layer nano-insight on nano-insight – which is often too slow for society to feel the subtle tremors of an approaching tipping point during which shifting tectonic plates suddenly collide and irreversibly change the world. Such are the expected future-shaping impacts of Synthetic Biology on the world that today’s newborns will be living in by 2050.
During the first 15 years of this millennium, the magnitude of the early tremors from Synthetic Biology have become more pronounced as contributions from disciplines such as biomolecular, chemical, physical, mathematical and, computational sciences, information technologies, and engineering are closing the gaps from different directions and angles among themselves to give rise to this new trailblazing interdisciplinary science (). The forces of these merging disciplines are shifting our bioengineering capabilities from high-throughput “reading DNA” (i.e. DNA sequencing) to affordable “writing and editing DNA” (i.e. DNA synthesizing) paradigms. With the convergence of the latest developments in “wet” biomolecular sciences and “dry” information and engineering technologies, it is now possible to design and construct new biological parts (genes), devices (gene networks) and modules (biosynthetic pathways), and to redesign natural biological systems (cells and organisms) for useful purposes.[Citation1] It does not only mean that we can now do the same things differently; it also means that we can do totally different things. That is why a growing number of imaginative scientists, futurists, and commentators are coming to believe that Synthetic Biology will erupt into novel biological solutions to some of the most pressing challenges facing humanity and the planet this century.
Figure 1. The emerging science of Synthetic Biology originates from the recent convergence of several “wet” biomolecular, chemical and physical sciences, and “dry” mathematical and computational sciences, information technologies, and engineering. This is a twenty-first-century transdisciplinary science, which is barely 15 years old. This futurist and transformative technology holds the potential to improve quality of life and sustain the planet in very significant ways.
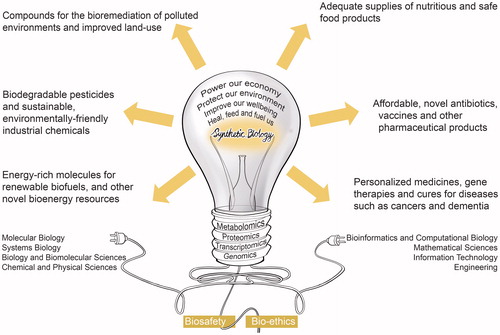
Unsurprisingly, the commercial world is responding swiftly. The global value of the Synthetic Biology market grew 10-fold between 2010 and 2016 to an estimated figure of more than $10 billion.[Citation2] Some of the world’s leading politicians and entrepreneurial business leaders are already referring to Synthetic Biology as one of the great trendsetting technologies that holds the promise to “heal us, feed us, fuel us” – a futurist technology that will improve our well-being, protect our environment, and power our economy.[Citation1] This promise, however, also poses great ethical challenges and risks, thereby placing great responsibility on researchers, policymakers, regulatory bodies, industry, and all citizen stakeholders to engage in a meaningful dialog about how to capitalize on the potential of Synthetic Biology to improve quality of life and sustain the planet while minimizing the risk of harm.[Citation3]
We can expect that the “hype-horror-hope” nature of a debate like this will intensify and polarize opinions in every sector and industry in the years to come. Thus, there is an urgency that well-informed opinion leaders in the agricultural, food and beverage industries also “lean in” to constructively contribute to this debate.
Given the pivotal role that yeast plays as a workhorse in the fermentation industry as well as an experimental research model organism in the advancement of Synthetic Biology, the wine industry, too, will be impacted by the outcomes of this debate. In fact, the use of Synthetic Biology techniques are already spilling over into the development of improved wine yeast strains.[Citation4]
To engage all stakeholders of the wine industry in a sensible debate over the complexities surrounding this controversial and disruptive new science, this paper briefly reviews the state-of-play in Synthetic Biology, illuminates the critically important role of the global Synthetic Yeast Genome (Sc2.0) Project (more detail available from www.syntheticyeast.org) in advancing the frontiers of synthetic genomics and proposes future directions and guiding principles for future wine yeast research.
Teetering on the edge of a new scientific frontier
The past and future of DNA science are ever present in Synthetic Biology
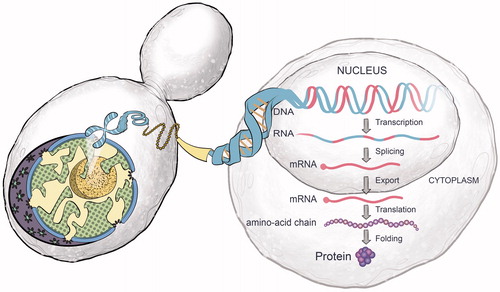
The discovery of the double-helical structure of DNA, more than 60 years ago, has captivated our imaginations and continues to shape our endeavors to understand and harness the programming of life’s genetic “software”. The complementary nature of perfectly matching deoxyribonucleic acid strands of the double helix and the simplicity of the digital code of DNA’s software – consisting of only four letters: adenine (A), cytosine (C), guanine (G), and thymine (T) – continue to fascinate the world (). From the beginning, scientists were intrigued by how, and under what circumstances, sequences of nucleotide triplets (codons) of genes are transcribed into messenger ribonucleic acid (mRNA) molecules, which in turn, are translated by the ribosomal machinery in cells into sequences of amino acids to form proteins ().
Figure 2. The structure of the deoxyribonucleic acid (DNA) double helix consists of two complementary strands, which are held together by two hydrogen bonds between adenine (A) and thymine (T) and three hydrogen bonds between guanine (G) and cytosine (C). The sequence of the A-T and G-C base pairs (bp) comprises the genetic code of genes which reside on chromosomes. Each gene is transcribed into a messenger ribonucleic acid (mRNA) – a polymer with a ribose and phosphate backbone and four different nitrogenous bases, adenine (A), guanine (G), cytosine (C) and uracil (U). Nucleotide triplets, called codons, specify which amino acid will be added next during protein synthesis.
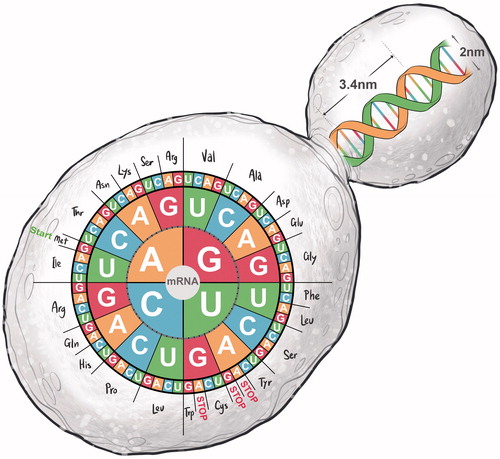
Figure 3. The process of protein biosynthesis starts with the transcription of a gene’s DNA to messenger RNA (mRNA) in the nucleus. Once the mRNA molecules are processed (e.g. the removal of non-coding introns separating the exons), they are exported through the pores of the nuclear membrane to the ribosomes where they are translated into proteins.
The late 1950s saw the uncovering of the mysteries of the universal genetic code, which was followed in the 1960s with the unraveling of the workings of regulatory genetic circuits and programmed gene expression. Building on these new insights, the 1970s were marked by the development of recombinant DNA and molecular cloning techniques. With the improvement of transformation procedures and the arrival of polymerase chain reaction (PCR) techniques in the 1980s, genetic engineering of microorganisms became fully entrenched in mainstream research. This was followed by the advent of automated DNA sequencing, high-throughput ‘omics technologies, bioinformatics, and improved computational tools, which, together, paved the way for the decoding of complete genomes in the 1990s and beyond.
These developments expanded the discipline of Biology – defined as “the study of the morphology, physiology, anatomy, behavior, origin, and distribution of living organisms” – and branched into Molecular Biology and Systems Biology during the latter half of the previous century (). With Molecular Biology, researchers sought to understand interactions between the various components of a cell, including interactions between the different types of DNA, RNA, and protein biosynthesis, and to learn how these interactions are regulated. Systems Biology extended this “reductionist” approach to a more “holistic” one by using computational and mathematical modeling of complex biological systems to “reverse-engineer” cellular networks. Building on the conceptual and technological advances in Molecular and Systems Biology, the new millennium welcomed the green sprouts of a new offshoot of Biology. Synthetic Biology combines molecular approaches with engineering principles to “forward-engineer” genetic systems by constructing collections of modular parts to design, build, and fine-tune gene regulatory networks (e.g. “quorum-sensing” circuitry for multicellular partnering) analogous to controllable electrical circuits.[Citation5] In short, Synthetic Biology is on a continuum with Molecular Biology and Systems Biology and entails the design and construction of novel biological systems, including complex “synthetic” organisms.
Figure 4. The evolution of Biology, Genetics, Molecular Biology and Systems Biology into Synthetic Biology, and the overlap between Metabolic Engineering and Synthetic Biology (as described by Nielsen and Keasling [Citation90]). The first approach (bottom metabolic pathway in the diagram) summarizes the standard approach where a naturally producing yeast is selected as a “cell factory” for production of the desirable product. Typically the flux toward the product is naturally low, but through the use of traditional, non-GM strain improvement or the use of directed genetic modifications – metabolic engineering – it is possible to increase the flux toward the product. In the second approach (middle metabolic pathway in the diagram), the yeast cell does not naturally produce the product of interest. By including a synthetically designed pathway into the yeast cell, the yeast acquires the ability to produce the product, often in small amounts initially. However, through pathway optimization the flux through this synthetic pathway can be increased to ensure a high flux toward the product. This approach applies concepts from both Metabolic Engineering and Synthetic Biology. In the third approach (top metabolic pathway in the diagram), a complete synthetic yeast cell could potentially be constructed such that it is dedicated to producing a desirable product.[Citation90]
![Figure 4. The evolution of Biology, Genetics, Molecular Biology and Systems Biology into Synthetic Biology, and the overlap between Metabolic Engineering and Synthetic Biology (as described by Nielsen and Keasling [Citation90]). The first approach (bottom metabolic pathway in the diagram) summarizes the standard approach where a naturally producing yeast is selected as a “cell factory” for production of the desirable product. Typically the flux toward the product is naturally low, but through the use of traditional, non-GM strain improvement or the use of directed genetic modifications – metabolic engineering – it is possible to increase the flux toward the product. In the second approach (middle metabolic pathway in the diagram), the yeast cell does not naturally produce the product of interest. By including a synthetically designed pathway into the yeast cell, the yeast acquires the ability to produce the product, often in small amounts initially. However, through pathway optimization the flux through this synthetic pathway can be increased to ensure a high flux toward the product. This approach applies concepts from both Metabolic Engineering and Synthetic Biology. In the third approach (top metabolic pathway in the diagram), a complete synthetic yeast cell could potentially be constructed such that it is dedicated to producing a desirable product.[Citation90]](/cms/asset/da3bbace-806f-4629-8e27-b28a0380806d/ibty_a_1214945_f0004_c.jpg)
Genetic engineering upgrades to genome engineering
With the arrival of next-generation “genetic engineering” tools at the beginning of this century, synthetic biologists started to acquire the equipment to rewrite, reshuffle and edit genomes. These twenty-first-century molecular tools reinvented “genetic engineering” and raised the bar to the level of “genome engineering”.
The synthesis of a DNA copy of the 7458-nucleotide poliovirus genome [Citation6] and the 5386-bp genome of the bacteriophage Phi-X174 [Citation7] gave the first glimpses of whole-genome synthesis. When the chemically synthesized genome of Phi-X174 was incorporated into Escherichia coli host cells, the transduced bacteria self-destructively produced new phage particles just as with natural Phi-X174 infections.
However, the size of viral genomes is miniscule when compared to the genomes of even the tiniest bacteria such as Mycoplasma. So, to synthesize an entire bacterial genome required a step-change; fortunately, this was made possible by rapid advances in lower-cost and improved DNA synthesis techniques.
The first bacterial genome – the 583-kb genome of Mycoplasma genitalium – was chemically synthesized in 2008.[Citation8] This was a watershed moment; however, in 2010, whole-genome engineering became much more of a reality with the creation of a cell of one bacterial species controlled by a chemically synthesized genome of another species.[Citation9] Synthesized DNA cassettes were assembled via in vivo recombination in yeast to recreate the 1.1 Mb Mycoplasma mycoides genome, which was then transplanted into Mycoplasma capricolum, resulting in viable bacteria that contained only the synthesized genome (). To make the “synthetic” version of the bacteria recognizably different and detectable, 14 non-essential M. mycoides genes were deleted and some novel “watermarking signature DNA” were added and inscribed into the synthetic genome. The “secret” watermark branded the synthetic bacteria with a unique serial number of the J. Craig Venter Institute (JCVI-syn1.0) and an encryption that contains the URL of a website and three quotations.
Figure 5. The synthesis of the world’s first synthetic bacterial genome. A synthetic version (1079 kb) of the 1.1 Mb genome of Mycoplasma mycoides was transplanted into Mycoplasma capricolum, resulting in viable bacteria.[Citation9,Citation10]
![Figure 5. The synthesis of the world’s first synthetic bacterial genome. A synthetic version (1079 kb) of the 1.1 Mb genome of Mycoplasma mycoides was transplanted into Mycoplasma capricolum, resulting in viable bacteria.[Citation9,Citation10]](/cms/asset/e36add6c-a160-4b47-bfaa-c26c93bff869/ibty_a_1214945_f0005_c.jpg)
This was a world-first and, in subsequent commentary, the JCVI team eloquently explained their groundbreaking achievement by likening a biological cell to a computer with the genome as the software that encodes the cell’s instructions.[Citation10] They referred to the cellular machinery as the hardware that interprets and runs the software. They further explained that advances in DNA technology have made it possible for scientists to act as biological “software engineers”, programming new biological “operating systems” into cells. Commentators in the popular press responded by reporting that “a Rubicon was crossed” with this “synthetic” bacterium containing “an artificial genome, which was designed on a computer and manufactured in a laboratory” – “a living creature with no ancestor”. These commentaries sparked an ongoing debate.
This evolving debate has not deterred the JCVI research team. This year, they succeeded in reducing the 1079-kb synthetic genome of M. mycoides JCVI-syn1.0 to a bare minimum of 531 kb, carrying 473 genes.[Citation11] By retaining essential and quasi-essential genes through three cycles of design, synthesis, and testing, they produced strain JCVI-syn3.0 with a genome smaller than that of any autonomously replicating cell found in nature. This minimalist JCVI-syn3.0 genome contains almost all genes involved in the synthesis and processing of macromolecules, as well as 149 genes with unknown biological functions. It is widely believed that the use of this JCVI-syn3.0 versatile platform will boost further investigations into the core functions of life and exploration of whole-genome design.[Citation11]
This remarkable scientific achievement is likely to raise the temperature of the broader debate regarding the ethical and regulatory concerns of society at large because, suddenly, it has become possible to conceive of a future world in which new bacteria and, eventually, new complex microorganisms, plants, and animals are designed and built from scratch to predetermined specifications.
While the debate about the pros and cons of “whole-genome engineering” is intensifying, the focus of the scientific world is turning toward further improvements of DNA synthesis and genome editing techniques, the genetic recoding of bacteria such as E. coli,[Citation12,Citation13] and a well-studied unicellular eukaryote with a remarkable track-record in bridging scientific successes achieved with viruses and bacteria to more complex multicellular organisms – the yeast, Saccharomyces cerevisiae.[Citation14]
Raising the “synthetic” bar with yeast
Yeast is a workhorse with sound academic and industrial credentials
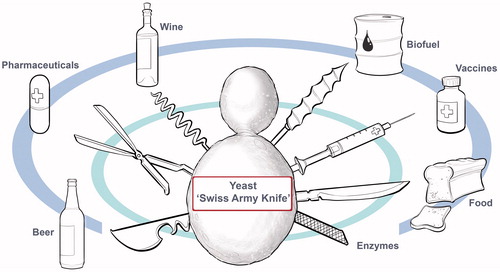
Metaphorically speaking, of the ∼150 described yeast genera and 1500 known yeast species,[Citation15] the “sugar fungus”, S. cerevisiae, can be described as the most revered, “graduating” with top marks in both majors of “fundamental research” and “practical applications”. S. cerevisiae is the most versatile yeast and excels equally well in laboratory and industrial settings.[Citation16] In the world of microbes, its unequaled versatility has earned S. cerevisiae the reputation as the “Swiss Army Knife” yeast ().
Figure 6. The budding yeast Saccharomyces cerevisiae is a multi-purpose single-celled fungus with GRAS (Generally Regarded As Safe) status. On the one hand, this “Swiss Army Knife” yeast is the best-studied eukaryotic experimental model organism in numerous research laboratories; and on the other hand, it is the fermentation industry’s “Black Belt” workhorse producing a broad range of fermented foods, beverages, biofuel, and pharmaceutical products.
In the research laboratory, this single-celled eukaryote is “well qualified” to answer basic biological questions about cellular, genetic, and metabolic processes asked by state-of-the-art science. It is one of the most intensively studied biological model systems (for a review see [Citation43] and references therein). The round to ovoid cells of S. cerevisiae are 5–10 μm in diameter and compartmentalized (including an encapsulated nucleus) like most other eukaryotic cells (). The three basic cell types of this budding yeast – a, α, and a/α cells – are easy and relatively inexpensive to grow in the laboratory and, under optimal nutritional and cultural conditions, they double their mass every 90 minutes (). Through an asexual mitotic budding process, heterothallic strains can multiply as stable a or α haploids while both heterothallic and homothallic strains can reproduce in the a/α diploid state or in a state of higher ploidy (). Most laboratory strains are haploid or diploid, whereas industrial wine strains are predominantly diploid or aneuploid, and occasionally polyploid. Two haploid cells of opposite mating types, a and α, can mate and produce a/α diploids, which, in turn, can undergo meiosis to generate four haploid ascospores, two of each mating type according to Mendelian segregation laws. Homothallic haploids can switch their mating-type from a to α and vice versa through the HO-controlled MATa, MATα, HML, and HMR loci on Chromosome 3, and conjugate with cells from the same single colony ().[Citation17]
Figure 7. The sub-cellular compartmentalization and architecture of the budding yeast Saccharomyces cerevisiae. The cell envelope, consisting of a cell wall, periplasm and plasma membrane, surrounds and encases the cell’s cytoplasm. The cytoskeleton maintains the structural organization of the organelles such as the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, and vacuoles. The nucleus of a haploid yeast cell contains a relatively compact genome of ∼12 Mb (non-redundant) to 14 Mb (total), separated into 16 linear chromosomes carrying ∼6000 genes of which ∼5000 are individually nonessential.
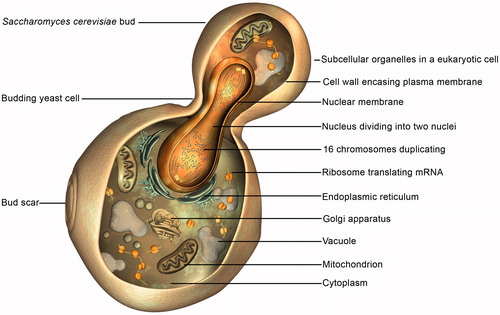
Figure 8. The cell cycle of a budding Saccharomyces cerevisiae cell, comprising four phases, – G1, S, G2 and M – runs for 90 minutes under optimal cultural conditions. Buds may arise at any point on the mother cell surface but never again at the same site.
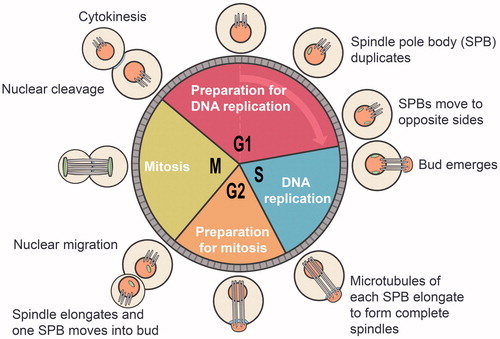
Figure 9. The life cycle of heterothallic and homothallic yeast strains. Haploid cells of the α mating-type produce a 13 amino acid long α factor; while the a mating type cells produce a peptide of 12 amino acids: the a factor. An a/α diploid cell formed by mating two haploid cells of opposite mating-types can neither produce nor respond to mating pheromones and will, under satisfactory nutritional and cultural conditions, grow and divide, maintaining the diploid state. Upon nutritional starvation, an a/α diploid cell undergoes meiosis, generating four haploid ascospores (two a and two α ascospores) encapsulated within an ascus. When released from the ascus, the ascospores germinate to commence new rounds of haploid existence. Heterothallic strains can be maintained as stable haploids for multiple generations; however, sex reversals, cell fusion, and diploid formation in homothallic strains prevent them from stable haploid states.
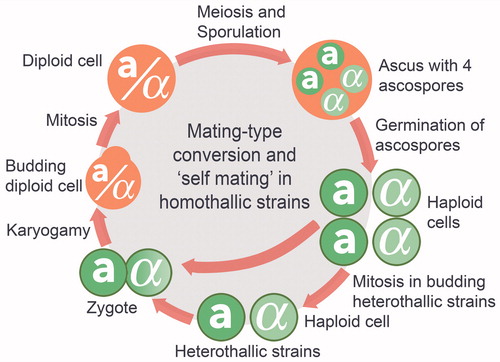
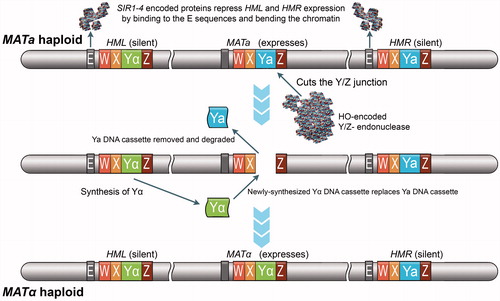
Figure 10. Chromosome 3 carries the two MAT genes (MATa and MATα) that determine the mating-type of Saccharomyces cerevisiae. The Ya and Yα regions are unique regions within these two MAT loci, each encoding two divergently transcribed mRNA molecules. In haploids, the two transcripts of the MATa locus code for the a1 and a2 polypeptides while the transcripts of MATα code for α1 and α2 polypeptides. MATa1 and MATa2, as well as all other a-specific genes, are constitutively expressed in the absence of the MATα2-encoded repressor, whereas all α-specific genes can only be transcribed in heterothallic haploids that express both MATα1 and MATα2. While expression of MATa1 and MATa2 is not required for a-mating, expression of MATa1 is required, along with the expression of MATα2, to repress haploid-specific gene expression in MATa/α diploids. Such heterothallic diploids can neither mate nor express other haploid-specific genes because of the action of the MATa1/MATα2-encoded co-repressor. Interestingly, homothallic haploid cells of S. cerevisiae can switch their mating-type from a to α and vice versa through the HO-controlled cassette model and conjugate with cells from the same single-spore colony. For example, an a cell can switch to an α cell by replacing the MATa allele with the MATα allele. The switching of the allele in the MAT locus is possible because homothallic haploids express the HO gene as well as an additional silenced copy of both the MATα and MATa alleles: the HML (Hidden MAT Left) locus carries a silenced copy of the MATα allele, and the HMR (Hidden MAT Right) locus carries a silenced copy of the MATa allele. The gene conversion event required for mating-type switching is initiated by the haploid-specific HO gene, which is tightly regulated and only activated in haploid cells during the G1 phase of the cell cycle. The HO-encoded DNA endonuclease cleaves a specific sequence at the MAT locus so that either the HML or HMR information can be duplicated and swapped into the active MAT locus. Thus, the silenced alleles of MATa and MATα present at HML and HMR serve as a reservoir of genetic information to repair the HO-induced DNA cleavage at the active MAT locus.
This accessible genetic system, which can be followed through asexual and sexual cycles, together with the ease of yeast transformation procedures, make S. cerevisiae amenable to almost any modification (breeding, mutagenesis, and cloning) that biomolecular science can throw at a eukaryote with GRAS (Generally Regarded As Safe) status. Due to its tractability, S. cerevisiae provided the “nuts and bolts” that shaped the foundations of Genetics, Molecular Biology, Systems Biology, and Synthetic Biology and it remains the mainstay eukaryotic chassis of choice.[Citation18]
In the context of industry application, the GRAS status of Saccharomyces yeast has been hard earned through its parallel development with civilization and human activity.[Citation19] Yeast fermentation was one of the primary forces to move the ancient world beyond hunting and gathering. That is why yeast became the first microorganism to be “domesticated”: for several millennia, this yeast has been used to raise dough, brew beer, and sparkle wine. Today, this industry workhorse drives multi-billion-dollar fermentations – churning out, on a daily basis, a wide spectrum of fermented foods and beverages, on the one hand, and biofuel and pharmaceutical products on the other.[Citation20]
The remarkable academic and industrial track-record of S. cerevisiae is highlighted by many world-firsts (). Not only was this yeast the first microorganism to be domesticated for the production of fermented food and beverages in ancient times, it was also the first microbe to be observed under the microscope and described as a living biochemical agent of transformation. In more recent times, S. cerevisiae was the host for the production of the first recombinant vaccine (against hepatitis B) and the first recombinant food enzyme (the milk coagulation enzyme, chymosin, for cheese-making).[Citation16] In 1996, the ∼12 Mb (non-redundant) to ∼14 Mb (total) genome of a haploid laboratory strain (S288c) of S. cerevisiae became the first eukaryotic genome to be fully sequenced, revealing that its 16 chromosomes encode ∼6000 genes of which ∼5000 are individually non-essential.[Citation21,Citation22] This publicly available genome sequence paved the way for building the world’s first systematic collection of bar-coded gene deletion mutants enabling high-throughput functional genomics experiments.[Citation23] This Yeast Deletion Library comprising yeasts with specific genes removed, together with the freely available Saccharomyces Genome Database (SGD; available from http://www.yeastgenome.org), resourced yeast biologists with unparalleled laboratory tools to shift scientific frontiers forward at a more rapid pace. In 2014, S. cerevisiae became the first eukaryotic cell to be equipped with a fully functional synthetic chromosome.[Citation24] A large international project – the Synthetic Yeast Genome (Sc2.0) Project – is now underway to synthesize all 16 linear chromosomes (ranging between 200 and 2000 kb in length) thereby positioning S. cerevisiae S288c to become the first eukaryote with a synthetic genome (see http://www.syntheticyeast.org).
Yeast 2.0 is breaking through the “synthetic” sound barrier
Designing and building the world’s first synthetic yeast genome is set to deliver a significant “step change” in “synthetic genomics” as a sub-field of Synthetic Biology. A consortium of a dozen laboratories from five countries – the USA, UK, Australia, China, and Singapore – has embarked on a collaborative project aimed at the synthesis of the entire genome of a haploid laboratory strain (S288c) of S. cerevisiae by 2018 (). The goals of the Sc2.0 project include answering a broad spectrum of “profound questions about fundamental properties of chromosomes, genome organization, gene content, function of RNA splicing, the extent to which small RNAs play a role in yeast biology, the distinction between prokaryotes and eukaryotes, and questions relating to genome structure and evolution” while recognizing that “the eventual ‘synthetic yeast’ being designed and refined could ultimately play an important practical role” (see www.syntheticyeast.org).
Figure 12. The international Synthetic Yeast Genome (Sc2.0) Project aims to chemically synthesize all 16 linear chromosomes of the haploid S288c laboratory strain of Saccharomyces cerevisiae and use them to replace the entire native genome. In doing so, the goals are to answer some profound biological questions that will inform future fundamental research into the functioning of eukaryotic cells, as well as to benefit yeast strain development programs.
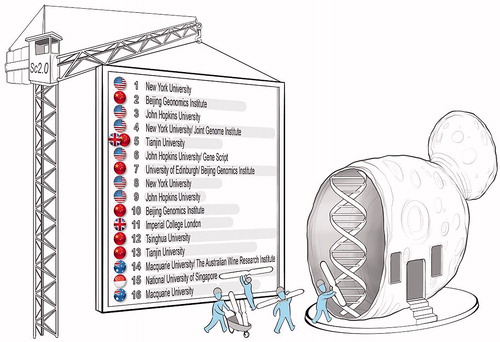
The first step toward building the ultimate yeast genome, as envisioned with the Sc2.0 project, was taken in 2011 with the construction of synthetic chromosome arms.[Citation25] This was followed up in 2014 with the synthesis of the first of the 16 chromosomes, namely Chromosome 3.[Citation24] Interestingly, this is the chromosome that carries the MATa, MATα, HML, and HMR loci, which determine the mating-type of S. cerevisiae cells (). Chromosome 3 is also the third smallest chromosome and it was the first S. cerevisiae chromosome that was fully sequenced almost two and a half decades ago.
The publicly available 316,617-bp DNA sequence of the native Chromosome 3 was used to redesign this chromosome on a computer according to a set of Sc2.0 principles relating to genome stability, genetic flexibility, and cell fitness (). These in silico “software edits” included the deletion of dispensable DNA sequences; the incorporation of uniquely recognizable sequences that could be used to differentiate between the native and synthetic DNA; the replacement of particular stop codons with other codons that terminate gene transcription equally well; and the flanking of non-essential genes with DNA sequences designed to catalyze their deletion on a specific signal.[Citation24] More specifically, subtelomeric regions, introns, transfer RNA (tRNA) genes, transposons, and silent mating-type loci (HML and HMR) were deleted; TAG stop codons were recoded to TAA stop codons, and loxPsym sites were added to enable genome scrambling when needed.
Figure 13. The synthesis of a functional 272,871-bp designer version (syn3) of Saccharomyces cerevisiae’s 316,617-bp native Chromosome 3. Changes to syn3 include the deletions of subtelomeric regions, introns, transfer RNA (tRNA) genes, transposons, and silent mating-type loci (HML and HMR), TAG/TAA stop-codons replacements, as well as the insertion of loxPsym sites to enable genome scrambling.[Citation24]
![Figure 13. The synthesis of a functional 272,871-bp designer version (syn3) of Saccharomyces cerevisiae’s 316,617-bp native Chromosome 3. Changes to syn3 include the deletions of subtelomeric regions, introns, transfer RNA (tRNA) genes, transposons, and silent mating-type loci (HML and HMR), TAG/TAA stop-codons replacements, as well as the insertion of loxPsym sites to enable genome scrambling.[Citation24]](/cms/asset/30f1c9aa-7746-4452-962c-a97d3a6dc55a/ibty_a_1214945_f0013_c.jpg)
The computer-designed sequence was used to construct a synthetic version of Chromosome 3.[Citation24] Overlapping stretches of 60–79 nucleotides were chemically synthesized in parallel and then assembled – by using standard PCR techniques and in vivo homologous recombination processes – into overlapping “minichunks” of approximately 3 kb in length. Together with the use of alternating selectable markers in each experiment, an average of 12 of these overlapping 3-kb synthetic DNA fragments were utilized to systematically replace the corresponding native sequences in 11 successive rounds of integrative transformation. The end-result was the successful replacement of the 316,617-bp native Chromosome 3 with a 272,871-bp synthetic version (labeled syn3). Despite the more than 50,000 alterations made in syn3 and the fact that the synthetic chromosome was 14% shorter than the native chromosome, the transformed yeast was not less fit than the original S288c S. cerevisiae strain.[Citation10] This result provides indicative answers to several fundamental biological questions. For example, from this work it is clear that the deletion of superfluous DNA among genes (introns), transposons (Ty elements) and tRNA genes, and the modification of unnecessary genetic code in syn3 did not negatively affect cell fitness.[Citation10] Also, with 5000 loxP sites, further tinkering with syn3 is now possible.
Building on the foundations of this pioneering work, and assisted by improved technologies that enable the commercial synthesis of much larger pieces of DNA at ever-decreasing costs, it is expected that the synthesis of at least another five of the 16 S. cerevisiae chromosomes will be reported during the course of this year. Thus, with approximately two-thirds of the genome synthesized (hosted in multiple cells) at this point in time, the Sc2.0 project is on track to produce the world’s first synthetic yeast genome by 2018.
Advances such as these are of interest to the entire field of Synthetic Biology because each technological innovation and new insight gained in understanding biological fundamentals creates new and stronger platforms from which we can launch into the design of entirely novel genes, chromosomes, genomes, and metabolic pathways for the biosynthesis of useful products. The first steps have already been taken to endow yeast, including wine yeast strains, with brand-new properties.
Equipping yeast with novel “synthetic” properties
Bioengineered yeast put synthetic DNA to work in industry
A laboratory strain of S. cerevisiae was first transformed in 1978.[Citation26] Since then, numerous genes were cloned into various strains, including industrial strains. Industrial yeasts have been genetically engineered for a wide variety of purposes over the past three decades. In the biotechnology-based industries, genes encoding enzymes, interferon, insulin, growth hormones, and vaccines were expressed in yeast. Patents were filed and several bioproducts generated by these genetically modified (GM) yeast strains were commercialized with varying degrees of success.[Citation27] The fiercest resistance from the general public was aimed against food products containing ingredients produced by genetically modified organisms (GMOs), such as the yeast-derived milk-clotting enzyme, chymosin. However, much less resistance was encountered with the commercialization of the yeast-derived recombinant vaccine against hepatitis B.
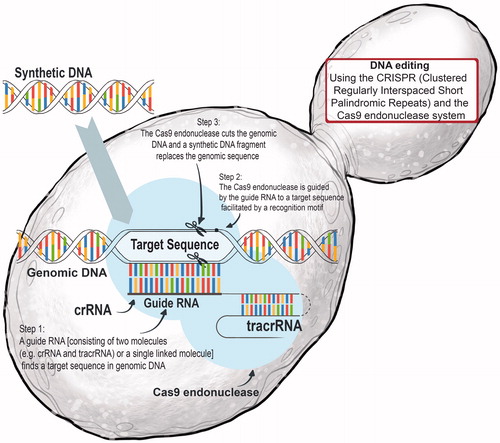
With the advent of Synthetic Biology, GM technologies have become much more powerful, precise, and sophisticated.[Citation28–30] In addition to the production of yeast-derived ethanol in the fermented beverage and biofuel industries, this mainstay eukaryotic chassis with GRAS status is increasingly being harnessed as “cell factories” for the production of high-value–low-volume products that could, for example, improve health, wellness, and nutrition.[Citation31] Commercial firms use a combination of genetic and genome engineering (including CRISPR editing technologies; ) approaches to (re)design, (re)construct, and analyze specific Saccharomyces strains by employing three major strategies: rational engineering; inverse metabolic engineering; and evolutionary strategies.[Citation32] Currently, the most common approach is to re-engineer the metabolism of existing cells and to retrofit existing strains. However, there are efforts underway to eventually create synthetic “minimal” yeast cells that could be used to generate valuable bioproducts.
Figure 14. The CRISPR-Cas9 system can be used as a “red pen” to correct and edit genetic blueprints of living cells in a precise and targeted way. CRISPR is an abbreviation for “clustered, regularly interspaced short palindromic repeats” found in select bacteria and archaea. This powerful DNA-editing tool is based on the CRISPR-associated protein-9 nuclease (Cas9) from bacteria such as Streptococcus pyogenes where it functions as an adaptive immunity, enabling the bacterial cells to respond to and eliminate invading genetic material. For example, invading DNA from viruses or plasmids is cut into small fragments and incorporated into a CRISPR locus amidst a series of short repeats (around 20 bps). These loci are transcribed, and transcripts are then processed to generate small RNA molecules (crRNA – CRISPR RNA) which are used to guide effector endonucleases that target invading DNA based on sequence complementarity. In a yeast cell, a CRISPR RNA will bind to a specific DNA segment which has a complementary sequence. The Cas9 nuclease will recognize the structure generated when a CRISPR RNA binds to a stretch of DNA and responds like a pair of molecular scissors, cutting through the DNA precisely at that point.
The list of products generated by bioengineered S. cerevisiae cell factories is long, ranging from aliphatic alcohols, β-amyrin, β-carotene, casbene, cinnamoyl anthranilates, cubebol, propanediol, and ribitol to acetic, adipic, artemisinic, and eicosapentaenoic acids. As an example, a company such as Evolva (see www.evolva.com) that specializes in the use of yeast cell factories for the production of high-value–low-volume ingredients has recently brought several bioproducts onto the market. These bioproducts include one of the most widely used flavoring agents, vanillin (a secondary metabolite and main constituent of natural vanilla originally obtained from cured seed pods of the vanilla orchid, Vanilla planifolia); the citrus-flavored sesquiterpene, valencene (a natural aroma component of citrus fruits such as Citrus sinensis oranges and commercially used as a flavor ingredient in food and drink, personal care, and household products); the citrus-flavored sesquiterpenoid nootkatone (naturally found in Citrus paradisi grapefruit with food-fragrance and insect-repellent properties); and the stilbenoid, resveratrol (a polyphenolic phytoalexin found in red Vitis vinifera grapes and believed to have several health benefits). Evolva expects to commercialize another two products within the next year: saffron (the world’s most expensive spice naturally derived from the flower Saffron crocus) and stevia (an increasingly sought-after zero-calorie sweetener usually extracted from the plant Stevia rebaudiana), and their work on sandalwood, agarwood, and ergot alkaloids is well advanced. At least two of these compounds, vanillin (3-methoxy-4-hydroxybenzaldehyde) and resveratrol (trans-3,5,4′-trihydroxystilbene), are also of interest to the wine industry.
Vanillin: Oak-aged red wines are known to contain traces of vanillin. De novo biosynthesis of vanillin in S. cerevisiae was achieved by incorporating an engineered pathway, which included 3-dehydroshikimate dehydratase from the dung mold Podospora pauciseta; an aromatic acid reductase from a bacterium belonging to the Nocardia genus; phosphopantetheinyl transferases from E. coli and/or Corynebacterium glutamicum; and overexpression of an O-methyltransferase gene from Homo sapiens ().[Citation33–38] To prevent the reduction of vanillin to vanillyl alcohol, this heterologous pathway was engineered into a mutant S. cerevisiae strain containing a deletion in the ADH6 alcohol dehydrogenase gene. To decrease product toxicity, the Arabidopsis thaliana UGT72E2 glycosyltransferase gene was added. The yield of free vanillin was further improved in this vanillin-glucoside producing strain, by using an in silico metabolic engineering strategy involving deletions of the native yeast PDC1 and GDH1 genes. Significantly, increased levels of free vanillin were achieved by employing a “global metabolic strategy” and “model-guided network engineering” prior to local pathway manipulation.
Figure 15. A bioengineered strain of Saccharomyces cerevisiae constructed for the de novo synthesis of the most widely used flavoring agent, vanillin.[Citation33–38]
![Figure 15. A bioengineered strain of Saccharomyces cerevisiae constructed for the de novo synthesis of the most widely used flavoring agent, vanillin.[Citation33–38]](/cms/asset/45c795e7-3706-4e5a-8fe9-a4a048ea2cf6/ibty_a_1214945_f0015_c.jpg)
Resveratrol: Red wines fermented with grape skins contain varying levels of resveratrol depending on the cultivar and growth conditions of the grapes and the length of skin contact during vinification.[Citation39] Resveratrol is a natural plant stress metabolite produced at increased levels when grapevines are exposed to fungal infection, wounding, or ultra-violet radiation in vineyards. In addition to its defense properties in grapevines, resveratrol in red wine is widely associated with human health benefits relating to anti-aging, anti-diabetic, anti-inflammatory, anti-thrombotic, and anti-tumor properties in pre-clinical trials. However, data from these inconclusive pre-clinical tests remain to be supported with clinical trials with large cohorts. Nevertheless, earlier work on the development of resveratrol-producing S. cerevisiae strains fed with expensive p-coumaric acid or aromatic amino acids [Citation39] has recently been superseded by the construction of a metabolically engineered strain capable of de novo production of resveratrol from glucose or ethanol via tyrosine as an intermediate ().[Citation40] The first step was to introduce the resveratrol biosynthetic pathway, comprising tyrosine ammonia-lyase from the bacterium Herpetosiphon aurantiacus, 4-coumaryl-CoA ligase from A. thaliana and resveratrol synthase from V. vinifera. The yield of resveratrol was significantly improved by the overexpression of feedback-insensitive alleles of the ARO4-encoded 3-deoxy-d-arabino-heptulosonate-7-phosphate and ARO7-encoded chorismate mutase, and the ACC1-encoded acetyl-CoA carboxylase boosting the supply of the precursor malonyl-CoA. Commercially relevant production levels in fed-batch fermentation were achieved by multiple integration of the resveratrol pathway genes into the genome of a robust S. cerevisiae strain.
Figure 16. A bioengineered strain of Saccharomyces cerevisiae constructed for the de novo synthesis of resveratrol from glucose or ethanol via tyrosine as an intermediate.[Citation40]
![Figure 16. A bioengineered strain of Saccharomyces cerevisiae constructed for the de novo synthesis of resveratrol from glucose or ethanol via tyrosine as an intermediate.[Citation40]](/cms/asset/195dae1f-8059-4844-8d65-1700c262e2cf/ibty_a_1214945_f0016_c.jpg)
However, the synthetically engineered yeast cell factory with the highest commercial profile is the high-yielding S. cerevisiae strain capable of producing artemisinic acid, a precursor of the potent anti-malarial compound, artemisinin (). Artemisinin is a sesquiterpene endoperoxide naturally produced by the sweet wormwood plant Artemisia annua. The unstable supply of plant-derived artemisinin caused regular shortages and price fluctuations for the manufacturers of “artemisinin-based combination therapies” (ACTs) for “uncomplicated” malaria caused by the parasite Plasmodium falciparum. Commercialization of a more reliable source of semi-synthetic artemisinin was achieved by engineering the complete biosynthetic pathway to artemisinic acid into S. cerevisiae.[Citation41]
Figure 17. A synthetically engineered strain of Saccharomyces cerevisiae designed and developed for the production of artemisinic acid, a precursor of the potent anti-malarial compound, artemisinin.[Citation41]
![Figure 17. A synthetically engineered strain of Saccharomyces cerevisiae designed and developed for the production of artemisinic acid, a precursor of the potent anti-malarial compound, artemisinin.[Citation41]](/cms/asset/3e983f99-35bd-4361-bb38-268bf278630c/ibty_a_1214945_f0017_c.jpg)
In this bioengineered strain, acetyl-CoA was converted to mevalonate through GAL-regulated overexpression of ERG10, ERG13, and tHMG1 (encoding a truncated HMG-CoA reductase). By placing ERG8, ERG12, and ERG19 under the GAL induction system, mevalonate was converted into isopentenyl diphosphate at increased levels.[Citation41] IDI1 overexpression was also controlled by the GAL system to efficiently convert isopentenyl diphosphate to dimethylallyl diphosphate, which, in turn, was converted to farnesyl diphosphate by GAL-induced overexpression of ERG20. To down-regulate a competing reaction of joining two farnesyl diphosphate moieties to form unwanted squalene, the ERG9 gene was linked to the copper-regulated CTR3 promoter. By adding copper sulfate to the medium, farnesyl diphosphate was converted via the amorpha-4,11-diene synthase (ADS) route to amorphadiene instead of squalene. Amorphadiene was oxidized to artemisinic alcohol by GAL-regulated overexpression of CYP7AV1, CPR1, and CYB5. GAL-induced overexpression of ADH1 led to the oxidization of artemisinic alcohol to artemisinic aldehyde, which was then oxidized to artemisinic acid by the overexpression of ALDH1 under the control of the GAL system. A practical, efficient, and scalable chemical process was developed to convert artemisinic acid to artemisinin by using a chemical source of singlet oxygen.[Citation41] These bioengineered yeast strains and chemical processes developed by renewable products company Amyris formed the basis for the commercial production of semi-synthetic artemisinin launched by pharmaceutical company Sanofi in 2014. It is hoped that this will help stabilize the supply of artemisinin for derivatization into active pharmaceutical ingredients (e.g. artesunate) for incorporation into anti-malarial ACTs.
Inspired by these early successes in using yeast as cell factories, several other research breakthroughs are in the making. For example, by imitating the modularity of diterpene biosynthesis in plants, more than 40 “new-to-nature” combinations of Class I and II diterpene synthases have recently been constructed. Four industrially relevant “targets” were achieved in scalable biotechnological production of therapeutically important diterpenoids involving the use of bioengineered strains of S. cerevisiae as cell factories.[Citation42]
Bioengineered wine yeast uncorks a future with synthetic DNA
History tells us that wine yeast strain development is well positioned to, once again, benefit from technological advances made with the genetic and genome engineering of non-wine strains of S. cerevisiae. To date, using genetic engineering [Citation20,Citation43,Citation44] and non-GM techniques,[Citation45] such as classical breeding (hybridization), adaptive laboratory evolution, and mutagenesis, several non-GM and GM wine strains have been developed with increased robustness, fermentation performance, health-related properties, and/or sensory attributes. Examples include strains that produce wines with lower alcohol levels;[Citation46–55] mutants that limit the production of unwanted hydrogen-sulfide off-flavors and volatile acidy;[Citation56–58] and strains that produce desirable esters,[Citation59,Citation60] terpenes,[Citation61] and thiols ().[Citation62–70] Efforts are also underway to express the α-guaiene-2-oxidase from grapevine in S. cerevisiae, thereby equipping wine yeast to transform grape-derived α-guaiene to the sought-after spicy aroma compound of Shiraz wine, rotundone.[Citation71–73] Several of these improved wine strains were patented but, so far, only two GM strains have been cleared by regulators in the USA and Canada for commercialization.
Figure 18. A range of genetically engineered wine strains from Saccharomyces cerevisiae (i) to decrease the concentration of alcohol, thereby reducing “hotness” in wine; (ii) to increase the safety of wine products by restricting the production of urea, thereby limiting the production of ethyl carbamate; (iii) to reduce the formation of hydrogen sulfide, thereby eliminating H2S-associated off-flavors; (iv) to increase the release and conversion of nonvolatile grape-derived thiols into volatile aromas, thereby increasing fruitiness in wine; (v) to enable yeast to assimilate malate and convert malic acid into lactic acid without the involvement of malolactic bacteria, thereby simplifying the winemaking process; and (vi) to optimize the ester content, thereby producing more-complex and well-balanced wines.
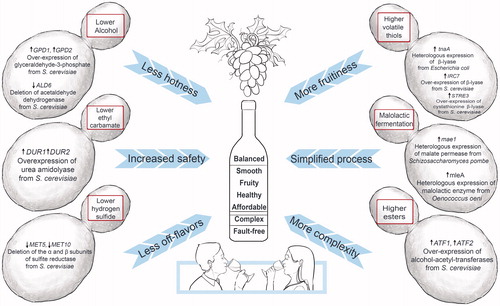
In 2005, a transgenic malolactic strain, ML01, became the first GM wine yeast to be released to market. This strain contains the mae1 malate transporter gene from the fission yeast Schizosaccharomyces pombe and the mleA malolactic gene from the wine bacterium Oenococcus oeni.[Citation74,Citation75] Placed under the control of the S. cerevisiae PGK1 promoter and terminator signals, both these genes were chromosomally integrated. A second commercially available strain, ECMo01, contains an extra copy of the S. cerevisiae DUR1,2 urea-amidolyase gene, which is expressed under the PGK1 regulatory sequences. Both GM strains offer potential benefits to winemakers and wine consumers alike. In the case of ML01, no malolactic fermentation driven by bacteria is required, thereby simplifying the winemaking process and avoiding the potential health risks associated with biogenic amine production by some bacteria. The constitutive overexpression of the DUR1,2 urea-amidolyase gene in ECMo01 drives the conversion of urea into ammonia and carbon dioxide, thereby removing the substrate for ethyl carbamate production, a suspected carcinogen.[Citation76] Despite these potential benefits, the ongoing anti-GMO campaigns prevented widespread uptake of these two GM wine yeasts in commercial wine production.[Citation27,Citation77,Citation78]
The 10-year delay caused by the anti-GM sentiments in the marketplace, however, did not deter scientific progress in yeast laboratories around the world. On the contrary, the pace of unraveling the genetic make-up of wine yeasts to inform further strain development accelerated over the past few years. For example, since the publication of the first complete genome sequence of a wine yeast strain (AWRI1631) in 2008,[Citation79] the genomes of at least six other widely used commercial wine yeast strains (AWRI1796, EC1118, QA23, VIN7, VIN13, and VL3) were sequenced and compared with the genomes of laboratory S. cerevisiae strains (S288c and Sigma1278b) as well as genomes of commercial Saccharomyces strains used in the baking, brewing, biofuel, ragi, and saké ().[Citation28,Citation29,Citation80–86] Valuable insights were gained from these studies – we are now much closer to fully understand what makes a wine yeast tick.[Citation87]
Figure 19. A cladogram depicting the genomic relationship among Saccharomyces cerevisiae strains originating from different geographical and industrial environments. Interestingly, there is a 0.6% difference in the nucleotide sequence of the S288c laboratory strain and that of the wine strain (AWRI1631) whose genome was first sequenced in 2008. By comparison, the difference between the nucleotide sequences of a human and a chimpanzee is 1% (adapted from Pretorius et al. [Citation78]).
![Figure 19. A cladogram depicting the genomic relationship among Saccharomyces cerevisiae strains originating from different geographical and industrial environments. Interestingly, there is a 0.6% difference in the nucleotide sequence of the S288c laboratory strain and that of the wine strain (AWRI1631) whose genome was first sequenced in 2008. By comparison, the difference between the nucleotide sequences of a human and a chimpanzee is 1% (adapted from Pretorius et al. [Citation78]).](/cms/asset/75891160-cd8d-4d1c-9f65-175065c47fc9/ibty_a_1214945_f0019_c.jpg)
As compared to the S. cerevisiae S288c reference strain, an additional stretch of ∼200 kb spread across distinct genomic loci (encoding single and multigene clusters) was found to be present in other S. cerevisiae strains, including wine strains.[Citation79] Strain-specific loci seem to reside in the subtelomeric regions – S. cerevisiae’s epicenter of genetic diversity. To date, at least three main loci were identified as loci that distinguish specific classes of industrial strains.[Citation87] First, the RTM1 locus (a member of a subtelomeric three-gene cluster) is mainly present in ale and distilling strains, and, to some extent, in strains that carry a set of genes specific to wine yeasts. This wine-specific set of genes constitutes a second industry-defining locus. This wine-specific locus comprises a cluster of five genes. Interestingly, this gene cluster displays strain differences in copy number, genomic location, and gene order most likely due to mobilization into, and throughout, wine-strain genomes as a circular intermediate via a yet to be understood process. The third industry-specific locus entails the evolutionary differences in biotin prototrophy amongst certain S. cerevisiae strains. More specifically, while the majority of strains, including those used in winemaking and brewing, are biotin auxotrophs, strains used for the production of saké acquired the capacity to synthesize biotin de novo over time, presumably because of evolutionary pressures in the low-biotin fermentations of saké mash.[Citation87]
In addition to these three industry-specific loci, several strain-specific genes were identified.[Citation87] Three wine-relevant examples of strain-specific genes are the FSY1 gene encoding a H+/fructose symporter, two paralogues, MPR1 and MPR2, conferring resistance to l-azetidine-2-carboxylic acid, and the β-lyase encoding gene, IRC7. An effective H+/fructose symporter is thought to provide a selective advantage to strains expressing FSY1 in highly concentrated mixtures of glucose and fructose during the fermentation of grape must. Wine strains that are able to consume fructose effectively are less likely to produce sluggish or stuck fermentations during vintages in which heat waves distort the usual 1:1 ratio of glucose and fructose in grape juice. Strains that carry the MPR-family paralogues are generally more stress tolerant by decreasing the toxic effects of reactive oxygen. It is believed that the fermentation performance of MPR-carrying strains is more robust. IRC7-expressing strains release more volatile thiols during fermentation, thereby producing wine with more fruity characters.[Citation64]
Valuable genomic insights were also gained into the Saccharomyces sensu stricto clade, comprising the S. cerevisiae complex (consisting of clearly defined “pure” lineages based strictly around geographic or industrial limits, and mosaic strains that appear to be the result of outcrossing between multiple pure lineages), and seven distinct Saccharomyces species (S. arboricolus, S. cariocanus, S. eubayanus, S. kudriavzevii, S. mikatae, S. paradoxus, and S. uvarum).[Citation87] As an example, genomic comparisons revealed that the thiol-releasing wine yeast, VIN7, has an allotriploid hybrid genome with S. cerevisiae and S. kudriavzevii origins.[Citation81] The understanding of the genetic basis of this “natural” hybrid’s unique capacity to produce wines with a guava-like aroma informed strain hybridization programs aimed at the improvement of fruitiness in specific styles of white wine. Once such strain-specific “flavor” genes are uncovered, they will undoubtedly also become the target for “synthetic software engineers”, constructing complex aroma-enhancing metabolic pathways in specific yeast strains.
In this regard, the first “synthetically engineered” wine yeast strain has just popped a cork revealing a whiff of raspberries in an experimental Chardonnay wine. In a world-first, a wine strain of S. cerevisiae was equipped with a biosynthetic pathway – comprising four separate enzymatic activities – to produce the highly desirable raspberry ketone, 4-[4-hydroxyphenyl]butan-2-one ().[Citation4] This phenylpropanoid is the primary aroma compound found in raspberries and it is also present in other fruits, vegetables and berries, such as blackberries, grapes, and rhubarb.
Figure 20. Development of the world’s first synthetically engineered wine strain of Saccharomyces cerevisiae for the de novo biosynthesis of the highly desirable raspberry ketone aroma compound, 4-[4-hydroxyphenyl]butan-2-one.[Citation4]
![Figure 20. Development of the world’s first synthetically engineered wine strain of Saccharomyces cerevisiae for the de novo biosynthesis of the highly desirable raspberry ketone aroma compound, 4-[4-hydroxyphenyl]butan-2-one.[Citation4]](/cms/asset/53fe32ad-d433-4a38-bc01-6b89b23b9275/ibty_a_1214945_f0020_c.jpg)
Plant-derived raspberry ketone is uneconomical for commercialization due to its low yield and chemically manufactured derivatives of this valuable flavoring agent fetch much lower prices than the naturally derived form.[Citation88] In earlier work, it was demonstrated that it is possible to produce raspberry ketone from p-coumaric acid in heterologous E. coli and S. cerevisiae strains.[Citation89] However, the high cost of p-coumaric acid as a substrate and the trace amounts of this phenylpropanoid obtained in the previous iteration of a bioengineered yeast strain, did not offer a profitable option for commercial production of raspberry ketone. The recent de novo production of raspberry ketone in a haploid wine strain (AWRI1631) of S. cerevisiae was achieved via metabolic pathway engineering and synthetic enzyme fusion.[Citation4]
The phenylpropanoid pathway starts with the conversion of phenylalanine to p-coumaric acid via cinnamate or directly from tyrosine to p-coumaric acid. Conversion of p-coumaric acid to raspberry ketone requires three additional enzymatic steps including a condensation reaction between coumaroyl-CoA and malonyl-CoA. To construct a biosynthetic pathway for the de novo production of raspberry ketone in a wine strain of S. cerevisiae, the following codon-optimized genes were synthesized and integrated into the yeast’s HO locus: the phenylalanine ammonia lyase from an oleaginous yeast, Rhodosporidium toruloides; the cinnamate-4-hydroxylase from the well-characterized model plant, A. thaliana; and the coumarate CoA ligase 2 gene from parsley, Petroselinum crispum, fused by a rigid linker to the benzalacetone synthase from rhubarb, Rheum palmatum.[Citation4] This “synthetic” wine yeast is capable of synthesizing raspberry ketone at concentrations almost two orders of magnitude above its predicted sensory threshold in Chardonnay grape juice under standard wine fermentation conditions, while retaining the ability to ferment the must to dryness. This first small step marks a giant leap forward for wine yeast into an unwritten future with synthetic DNA.
Treading the “synthetic” tightrope with care
Science is a constant balancing act, especially when it comes to walking the thin tightrope that spans the chasm between scientific freedom and social responsibility in the unknown territory of synthetic genomics. Even the best-balancing acrobats in research would find it challenging, at times, to make treading the high-tensioned wire of synthetic bioengineering or low-hanging slackline of “bio-error”/“bio-terror” look effortless – they have to carefully consider the complex gravitational forces of perception and control every step of the way across the gorge caused by this emerging science. The pioneering “extreme tightrope-daredevils” in Synthetic Biology cannot afford to lose their footing even once.
It is for this reason that the Sc2.0 partners (consisting of scientists from different backgrounds, diverse settings, and many nations) have developed and formally adopted a common set of principles to guide this massive, collaborative Synthetic Biology project (). To participate in the Sc2.0 project, every partner organization has signed a legally binding agreement that each individual researcher working across the “nodes” will strictly abide by the rules outlined in a published statement on ethics and governance. This Sc2.0 ethics and governance statement addresses issues that go to the heart of societal benefits, intellectual property, safety, and governance of this international Synthetic Yeast Genome Project.[Citation3]
Figure 21. Balancing scientific freedom and social responsibility with a project-level agreement and ethics and governance statement to guide the international Synthetic Yeast Genome (Sc2.0) Project (adapted from Silva et al. [Citation3]).
![Figure 21. Balancing scientific freedom and social responsibility with a project-level agreement and ethics and governance statement to guide the international Synthetic Yeast Genome (Sc2.0) Project (adapted from Silva et al. [Citation3]).](/cms/asset/4d24c2b7-d6d0-4842-a064-f039120dcf09/ibty_a_1214945_f0021_c.jpg)
All members of the Sc2.0 consortium undertook to conduct and promote their work for the benefit of humankind and not to bring harm. The Sc2.0 team members agreed to engage with the public and to be transparent and open about their work. Intellectual property rights will not be taken on the ultimate strain containing the 16 synthetic chromosomes, nor on the intermediary clones and strains generated as part of the Sc2.0 project. Data and materials generated by this project will be made available to other researchers. All providers of synthetic Sc2.0 DNA sequences will be in compliance with the Screening Framework Guidance for Providers of Synthetic Double-Stranded DNA of the US Department of Health and Human Services. Non-member individuals requesting Sc2.0 data and/or materials will be assessed prior to shipment of any such materials to help reduce the chance that they are distributing materials to those with nefarious intent. The Sc2.0 team pledged that their laboratories, practices, and methods will have at their core an ethos of safety for both laboratory personnel and the communities outside their organizations. All Sc2.0 workers receive thorough training in biosafety, dual-use concerns, and other ethics issues as appropriate. It goes without saying that all Sc2.0 work is in compliance with national and local laws. The governing Executive Committee of the Sc2.0 project undertook to address any issues that might arise with regard to safety or compliance with the Sc2.0 agreement, and to revisit the Sc2.0 agreement as the project progresses and the technologies it uses develop to ensure that any risk by this work is appropriately managed.
This large international Synthetic Yeast Genome Project is, therefore, regulated by existing biosafety legislation and regulations pertaining to recombinant DNA research, and self-governed by the above Sc2.0 agreement and governance structure. To maintain a healthy balance between freedom and responsibility in synthetic genomics research, the Sc2.0 team is of the view that such project-level agreements are an important, valuable, and flexible model of self-regulation for similar global, large-scale Synthetic Biology projects to maximize the benefits and minimize potential harms.[Citation3]
Beyond the horizon
The world that we live in today is vastly different from the world of a century ago. We have become used to living in an ever-changing world of digital democracy and ubiquitous surveillance in smart megacities; a dangerously divided and income-polarized world of cyber and drone warfare; an overpopulated and polluted world with finite natural resources; and an interconnected world that is increasingly dependent on satellite-guided precision agriculture, “on-demand” big data analyses, cloud-computing, bionics, artificial intelligence, and automated robotics where “virtual reality” has indeed become reality. One wonders what the world will look like in 2050 or even 2100? When one pauses and imagines a future world, surreal visions – colored by science fiction movies – of brain-to-machine interfaces in post-human societies, space tourism, moon-mining, and even alien intelligence might slide through one’s mind. Perhaps more realistically from today’s perspective and in the context of this article’s topic, one could envisage a secure and “green” world powered by renewable biofuels; an adequate supply of affordable, nutritious, safe foods and clean water; and effective accessible medicines and treatments for all. Will synthetic chloroplasts and artificial leaves enable us to eventually grow green, self-powered buildings; will synthetic yeast cell factories provide the next generation biofuels, food enzymes, antibiotics, vaccines, and personalized medicines; and will synthetic genomics accelerate the discovery of beneficial gene therapies and cures for cancers and dementia?
Nobody can answer these questions with certainty; however, it is certain that Synthetic Biology will impact a future world in a very significant way. The unimaginable is about to be made possible by synthetic genome engineering. We are, yet again, standing on the brink of a new scientific frontier of unknown opportunities and unimaginable perils, a frontier of unfulfilled hopes and unfulfilled fears. Close collaboration among researchers across the boundaries of multiple disciplines, regulators and thought-leaders in society will have to carefully survey the wonders and terrors that await us and the legacy we will be leaving for future generations.
Past generations of pioneers, discoverers, inventors, and innovators demonstrated that the best way to navigate the uncharted and stormy oceans of a new science is not to take an overly pessimistic view complaining about the wind direction nor to be overly optimistic expecting the wind to change; the best way forward is to be realistic and adjust the sails. As realists, we need to embrace the winds of change and adjust our sails so that we can positively influence our destiny over the next fifty years and beyond.
As synthetic genomics is breathing new life into wine yeast research and stretching the realms of possibility, the winds of change originating from Synthetic Biology will undoubtedly also sweep through the vineyards, wineries, boardrooms, and consumer markets of the global wine industry. However, this will not be the first time that winemaking will have to weather the stormy winds of change generated by new discoveries, technologies, and inventions. It is well understood that the art and science of winemaking are natural companions and that the opposing forces of tradition and innovation have often tested this 7000-year-old companionship to its core. History shows us that when art and science combine harmoniously, great wines emerge; however, when the “natural” tension between tradition and scientific innovation is ill-informed and mismanaged, opinions diverge into centrifugal forces that prevent a constructive dialog and, ultimately, progress.
This article charges all stakeholders and thought-leaders in the global wine sector, to inform themselves about the opportunities and challenges afforded by Synthetic Biology. The time has come to engage in a constructive dialogue about the potential impact of synthetic genomics and the arrival of wine yeast 2.0 strains on their practices and products.
Acknowledgements
The author acknowledges the ongoing support from all members of the international Synthetic Yeast Genome Project (Sc2.0) consortium, and the research contributions of his team at Macquarie University: Thomas Williams, Heinrich Kroukamp, Hugh Goold, Natalie Curach, and Ian Paulsen and collaborators at The Australian Wine Research Institute, Anthony Borneman, Dariusz Kutyna, Danna Lee, and Daniel Johnson. I am grateful to Rae Blair, Tori Hocking, and Erin Semon for helping to proofread the manuscript, and to Bill Hope, Frantz Kantor and The Drawing Book Studios for creating the artwork used in the diagrams.
Disclosure statement
The author declares that he does not have any competing interests. The Synthetic Biology initiative at Macquarie University is financially supported by an internal grant from the University, and external grants from Bioplatforms Australia, the New South Wales (NSW) Chief Scientist and Engineer, and the NSW Government’s Department of Primary Industries.
References
- Willetts D. Eight great technologies. London: Policy Exchange; 2013.
- Kelley NJ, Whelan DJ, Kerr E, et al. Engineering biology to address global problems: Synthetic Biology markets, needs, and applications. Industrial Biotechnol. 2014;10:140–149.
- Silva A, Yang H, Boeke JD, et al. Freedom and responsibility in synthetic genomics: The Synthetic Yeast Project. Genetics. 2015;200:1021–1028.
- Lee D, Lloyd N, Pretorius IS, et al. Heterologous production of raspberry ketone in the wine yeast Saccharomyces cerevisiae via pathway engineering and synthetic enzyme fusion. Microb Cell Fact. 2016;15:49–55.
- Cameron DE, Bashor CJ, Collins JJ. A brief history of synthetic biology. Nat Rev Microbiol. 2014;12:381–390.
- Cello J, Paul AV, Wimmer E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science. 2002;297:1016–1018.
- Smith HO, Hutchison CA, Pfannkoch C, et al. Generating a synthetic genome by whole genome assembly: Phi-X174 bacteriophage from synthetic oligonucleotides. Proc Natl Acad Sci USA. 2003;100:15440–15445.
- Gibson DG, Benders GA, Andrews-Pfannkoch C, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008;319:1215–1220.
- Gibson DG, Glass JI, Lartigue C, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56.
- Gibson DG, Venter JG. Synthetic biology: construction of a yeast chromosome. Nature. 2014;509:168–169.
- Hutchison CA, Chuang RY, Noskov VN, et al. Design and synthesis of a minimal bacterial genome. Science. 2016;351:6253–6255.
- Isaacs FJ, Carr PA, Wang HH, et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333:348–353.
- Lajoie MJ, Rovner AJ, Goodman DB, et al. Genomically recoded organisms expand biological functions. Science. 2013;342:357–360.
- Pennisi E. Building the ultimate yeast genome. Science. 2014;343:1426–1429.
- Jolly NP, Varela C, Pretorius IS. Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14:215–237.
- Pretorius IS D, Toit MD, Van Rensburg P. Designer yeasts for the fermentation industry of the 21st Century. Food Technol Biotechnol. 2003;41:3–10.
- Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988;52:536–553.
- Jermy A. Milestones in synthetic (micro)biology. Nat Microbiol. 2014;12:309.
- Chambers PJ, Pretorius IS. Fermenting knowledge: the history of winemaking, science and yeast research. EMBO Rep. 2010;11:1–7.
- Pretorius IS, Curtin CD, Chambers PJ. The winemaker’s bug: from ancient wisdom to opening new vistas with frontier yeast science. Bioeng Bugs. 2012;3:147–156.
- Goffeau A, Barrell BG, Bussey H. Life with 6000 genes. Science. 1996;274:546–567.
- Oliver SG. From DNA sequence to biological function. Nature. 1996;379:597–600.
- Winzeler EA, Shoemaker DD, Astromoff A. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906.
- Annaluru N, Muller H, Mitchell LA, et al. Total synthesis of a functional designer eukaryotic chromosome. Science. 2014;344:55–58.
- Dymond JS, Richardson SM, Coombes CE, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature. 2011;477:471–476.
- Hinnen A, Hicks JB, Fink GR. Transformation of yeast. Proc Natl Acad Sci USA. 1978;75:1929–1933.
- Pretorius IS, Høj PB. Grape and wine biotechnology: challenges, opportunities and potential benefits. Austral J Grape Wine Res. 2005;11:83–108.
- Borneman AR, Pretorius IS, Chambers PJ. Comparative genomics: a revolutionary tool for wine yeast strain development. Curr Opin Biotechnol. 2013;24:192–199.
- Borneman AR, Schmidt SA, Pretorius IS. At the cutting-edge of grape and wine biotechnology. Trends Genet. 2013;29:263–271.
- Williams CT, Pretorius IS, Paulsen PT. Synthetic evolution of metabolic productivity using biosensors. Trends Biotechnol. 2016;34:371–381.
- David F, Siewers V. Advances in yeast genome engineering. FEMS Yeast Res. 2015;15:1–14. doi:10.1111/1567-1364.12200.
- Kim IL, Roldão A, Siewers V, et al. A systems-level approach for metabolic engineering of yeast cell factories. FEMS Yeast Res. 2012;12:228–248.
- Hansen EH, Møller BL, Kock GR, et al. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker’s yeast (Saccharomyces cerevisiae). Appl Environ Microbiol. 2009;75:2765–2774.
- Brochado AR, Matos C, Møller BL, et al. Improved vanillin production in baker’s yeast through in silico design. Microbial Cell Fact. 2010;9:1–15.
- Brochado AR, Patil KR. Overexpression of O-methyltransferase leads to improved vanillin production in baker’s yeast only when complemented with model-guided network engineering. Biotechnol Bioeng. 2013;110:656–659.
- Cankar K, Van Houwelingen A, Bosch D, et al. A chicory cytochrome P450 mono-oxygenase CYP71AV8 for the oxidation of (+)-valencene. FEBS Lett. 2011;585:178–182.
- Gallage NJ, Møller BL. Vanillin bioconversion and bioengineering of the most popular plant flavour and its de novo biosynthesis in the vanilla orchid. Mol Plant. 2015;8:40–57.
- Strucko T, Magnesko O, Mortensen UH. Benchmarking two commonly used Saccharomyces cerevisiae strains for heterologous vanillin-β-glucoside production. Metabolic Eng Comm. 2015;2:99–108.
- Becker VW, Armstrong GO, Van der Merwe MJ, et al. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003;4:79–85.
- Li M, Kildegaard KR, Chen Y, et al. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab Eng. 2015;32:1–11.
- Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532.
- Andersen-Ranberg J, Kongstad KT, Nielsen MT, et al. Expanding the landscape of diterpene structural diversity through stereochemically controlled combinatorial biosynthesis. Angewandte Chem. 2016;128:2182–2186.
- Pretorius IS. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast. 2000;16:675–729.
- Verstrepen KJ, Chambers PJ, Pretorius IS. The development of superior yeast strains for the food and beverage industries: challenges, opportunities and potential benefits. In: Querol A, Fleet GH, editors. The yeast handbook: yeasts in food and beverages. Heidelberg: Springer; 2006. p. 399–444.
- Chambers PJ, Bellon JR, Schmidt SA, et al. (2009). Non-genetic engineering approaches to isolating and generating novel yeasts for industrial applications. In: Kunze G, Satyanarayana T, editors. Yeast biotechnology: diversity and applications. Berlin: Springer; 2009. p. 433–457.
- Cambon B, Monteil V, Remize F, et al. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl Environ Microbiol. 2006;72:4688–4694.
- De Barros Lopes MA, Rehman AU, Gockowiak H, et al. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2). Austral J Grape Wine Res. 2000;6:208–215.
- Eglinton JM, Heinrich AJ, Pollnitz AP, et al. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast. 2002;19:295–301.
- Kutyna DR, Varela C, Henschke PA, et al. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci Technol. 2010;21:293–302.
- Kutyna DR, Varela C, Stanley GA, et al. Adaptive evolution of Saccharomyces cerevisiae to generate strains with enhanced glycerol production. Appl Microbiol Biotechnol. 2012;93:1175–1184.
- Michnick S, Roustan J-L, Remize F, et al. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast. 1997;13:783–793.
- Remize F, Roustan J, Sablayrolles J, et al. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol. 1999;65:143–149.
- Tilloy V, Ortiz-Julien A, Dequin S. Reduction of ethanol yield and improvement of glycerol formation by adaptive evolution of the wine yeast Saccharomyces cerevisiae under hyperosmotic conditions. Appl Environ Microbiol. 2014;80:2623–2632.
- Tilloy V, Cadiere A, Ehsani M, et al. Reducing alcohol levels in wines through rational and evolutionary engineering of Saccharomyces cerevisiae. Int J Food Microbiol. 2015;213:49–58.
- Varela C, Kutyna DR, Solomon M, et al. Evaluation of gene modification strategies to develop low-alcohol wine yeasts. Appl Environ Microbiol. 2012;17:6068–6077.
- Cordente AG, Heinrich AJ, Pretorius IS, et al. Isolation of sulfite reductase variants of a commercial wine yeast with significantly reduced hydrogen sulfide production. FEMS Yeast Res. 2009;9:446–459.
- Cordente AG, Curtin CD, Varela C, et al. Flavour-active wine yeasts. Appl Microbiol Biotechnol. 2012;96:601–618.
- Luo Z, Walkey CJ, Madilao LL, et al. Functional improvement of Saccharomyces cerevisiae to reduce volatile acidity in wine. FEMS Yeast Res. 2013;13:485–494.
- Lilly M, Bauer FF, Lambrechts MG, et al. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast. 2006;23:641–659.
- Lilly M, Styger G, Bauer FF, et al. The effect of increased yeast branched-chain amino acid transaminase activity and the production of higher alcohols on the flavor profiles of wine and distillates. FEMS Yeast Res. 2006;6:726–743.
- Carrau FM, Medina K, Boido E, et al. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett. 2005;243:107–115.
- Cordente AG, Cordero-Bueso G, Pretorius IS, et al. Novel wine yeast with mutations in YAP1 that produce less acetic acid during fermentation. FEMS Yeast Res. 2013;13:62–73.
- Holt S, Cordente AG, Williams SJ, et al. Engineering Saccharomyces cerevisiae to release 3-mercaptohexan-1-ol during fermentation through overexpression of an S. cerevisiae gene, STR3, for improvement of wine aroma. Appl Environ Microbiol. 2011;77:3626–3632.
- Roncoroni M, Santiago M, Hooks DO, et al. The yeast IRC7 gene encodes a β-lyase responsible for production of the varietal thiol 4-mercapto-4-methylpentan-2-one in wine. Food Microbiol. 2011;28:926–935.
- Swiegers JH, Bartowsky EJ, Henschke PA, et al. Yeast and bacterial modulation of wine aroma and flavour. Austral J Grape Wine Res. 2005;11:139–173.
- Swiegers JH, Capone DL, Pardon KH, et al. Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast. 2007;24:561–574.
- Swiegers JH, Pretorius IS. Modulation of volatile sulfur compounds by wine yeast. Appl Microbiol Biotechnol. 2007;74:954–960.
- Swiegers JH, Saerens SMG, Pretorius IS. Novel yeast strains as tools to adjust the flavour of fermented beverages to market specifications. In: Frenkel DH, Belanger F, editors. Biotechnology in flavour production. Oxford: Blackwell Publishing; 2008. p. 1–55.
- Swiegers JH, Kievit RL, Siebert T, et al. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 2009;26:204–211.
- Thibon C, Marullo P, Claisse O, et al. Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. FEMS Yeast Res. 2008;8:1076–1086.
- Wood C, Siebert TE, Parker M, et al. From wine to pepper: rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J Agric Food Chem. 2008;56:3738–3744.
- Takahashi S, Yeo Y, Greenhagen BT, et al. Metabolic engineering of sesquiterpene metabolism in yeast. Biotechnol Bioeng. 2007;97:170–181.
- Takase H, Sasaki K, Shinmori H, et al. Cytochrome P450 CYP71BE5 in grapevine (Vitis vinifera) catalyzes the formation of the spicy aroma compound (−)-rotundone. J Exp Bot. 2016;67:787–798.
- Volschenk H, Viljoen-Bloom M, Van Staden J, et al. Genetic engineering of an industrial strain of Saccharomyces cerevisiae for l-malic acid degradation via an efficient malo-ethanolic pathway. S Afr J Enol Vitic. 2004;25:63–73.
- Husnik JI, Volschenk H, Bauer F, et al. Metabolic engineering of malolactic wine yeast. Metabolic Eng. 2006;8:315–323.
- Coulon J, Husnik JI, Inglis DL, et al. Metabolic engineering of Saccharomyces cerevisiae to minimize the production of ethyl carbamate in wine. Am J Enol Vitic. 2006;57:113–124.
- Pretorius IS, Bauer FF. Meeting the consumer challenge through genetically customized wine-yeast strains. Trends Biotechnol. 2002;20:426–432.
- Pretorius IS, Curtin CD, Chambers PJ. Designing wine yeast for the future. In: Holzapfel W, editor. Advances in fermented foods and beverages: improving quality, technologies and health benefits. Cambridge: Woodhead Publishing; 2015. p. 195–226.
- Borneman AR, Forgan AH, Pretorius IS, et al. Comparative genome analysis of a Saccharomyces cerevisiae wine strain. FEMS Yeast Res. 2008;8:1185–1195.
- Borneman AR, Desany BA, Riches D, et al. Whole genome comparison reveals novel genetic elements that characterize the genome of industrial strains of Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1001287.
- Borneman AR, Desany BA, Riches D, et al. The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res. 2012;12:88–96.
- Dunn B, Richter C, Kvitek DJ, et al. Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res. 2012;22:908–924.
- Galeote V, Novo M, Salema-Oom M, et al. FSY1, a horizontally transferred gene in the Saccharomyces cerevisiae EC1118 wine yeast strain, encodes a high-affinity fructose/H + symporter. Microbiology. 2010;56:3754–3761.
- Galeote V, Bigey F, Beyne E, et al. Amplification of a Zygosaccharomyces bailii DNA segment in wine yeast genomes by extrachromosomal circular DNA formation. PLoS One. 2011;6:e17872.
- Novo M, Bigey F, Beyne E, et al. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc Natl Acad Sci USA. 2009;106:16333–16338.
- Wei W, McCusker JH, Hyman RW, et al. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc Natl Acad Sci USA. 2007;104:12825–12830.
- Liti G, Carter DM, Moses AM, et al. Population genomics of domestic and wild yeasts. Nature. 2009;458:337–341.
- Borneman AR, Pretorius IS. Genomic insights into the Saccharomyces sensu stricto complex. Genetics. 2015;199:281–291.
- Zabetakis I, Holden MA. Strawberry flavour: analysis and biosynthesis. J Sci Food Agric. 1997;74:421–434.
- Beekwilder J, van der Meer IM, Sibbesen O. Microbial production of natural raspberry ketone. Biotechnol J. 2007;2:1270–1279.
- Nielsen J, Keasling JD. Synergies between synthetic biology and metabolic engineering. Nat Biotechnol. 2011;29:693–695.

