Abstract
Potential future application of engineered gene drives (GDs), which bias their own inheritance and can spread genetic modifications in wild target populations, has sparked both enthusiasm and concern. Engineered GDs in insects could potentially be used to address long-standing challenges in control of disease vectors, agricultural pests and invasive species, or help to rescue endangered species, and thus provide important public benefits. However, there are concerns that the deliberate environmental release of GD modified insects may pose different or new harms to animal and human health and the wider environment, and raise novel challenges for risk assessment. Risk assessors, risk managers, developers, potential applicants and other stakeholders at many levels are currently discussing whether there is a need to develop new or additional risk assessment guidance for the environmental release of GD modified organisms, including insects. Developing new or additional guidance that is useful and practical is a challenge, especially at an international level, as risk assessors, risk managers and many other stakeholders have different, often contrasting, opinions and perspectives toward the environmental release of GD modified organisms, and on the adequacy of current risk assessment frameworks for such organisms. Here, we offer recommendations to overcome some of the challenges associated with the potential future development of new or additional risk assessment guidance for GD modified insects and provide considerations on areas where further risk assessment guidance may be required.
Introduction
For centuries, humans have sought to control insect disease vectors (such as disease-transmitting mosquitoes and ticks), agricultural insect pests and non-native invasive insect species through a variety of methods. These methods include: the use of biological or chemical insecticides, insect-resistant crops, biological control, and genetic control methods such as the sterile insect technique (SIT) and Wolbachia-mediated incompatible insect technique (IIT) [Citation1–3]. Controlling disease transmission by mosquitoes, for example, is a long-standing public health goal [Citation4,Citation5]. While effective on a local/regional scale, current control methods have not prevented the spread of mosquito-vectored diseases, not least due to the evolution of resistance to commonly used insecticides, difficulty in reaching all breeding/resting sites, increased human population densities, climate change and global trade [Citation6,Citation7]. This has prompted the development of new, yet complementary, genetic control approaches, such as Wolbachia-mediated pathogen interference (PI) and genetically modified insects (GMIs) carrying a dominant (female) lethal gene [(fs)RIDL] or containing engineered gene drives (GDs), to combat the spread of mosquito- and other vector-borne diseases worldwide [Citation5,Citation8]. Genetic control of insects involves the intended release of individuals that contain the heritable genetic modification of interest (for the purposes of control) that is introduced and disseminated into wild populations via mating [Citation1]. Similarly, challenges associated with increased insecticide resistance in agricultural insect pests and the invasion of non-native insect species are driving the exploration of novel genetic control approaches, including (fs)RIDL and engineered GDs [Citation3,Citation9–11].
GDs can be described as genetic elements that bias their own inheritance from generation to generation in order to gain a transmission advantage over the rest of the genome [Citation12]. During the sexual reproduction of diploid organisms, each of the two copies of a gene present in each parent has a 50% chance of being inherited according to Mendelian laws of inheritance. GDs can increase this probability and be transmitted to subsequent generations at a frequency greater than the expected 50%. This preferential inheritance may allow GDs to rapidly spread in interbreeding populations, increasing their prevalence and that of any genetically linked cargo/payload genes, even if the introduced genetic modification imposes some fitness costs on their host. The speed of this process is inversely correlated with the generation time of the organism (i.e. the shorter the generation time, the faster is the GD potential to spread) [Citation12].
First reported in 1920, naturally occurring GDs (such as transposable elements, homing endonuclease genes, segregation distorters, Medea, Wolbachia endosymbionts) have been observed in a variety of organisms [Citation13]. While the idea of harnessing naturally occurring GDs against disease vectors, agricultural pests and invasive species is not new [Citation14], it has proved difficult to engineer efficient GD systems using classical genetic approaches. Recent advances in molecular and synthetic biology (e.g. gene editing using the clustered regularly interspaced short palindromic repeats [CRISPR] and CRISPR-associated protein 9 [Cas9] system) enable the engineering of GDs with much greater ease in a wide range of organisms, with an initial focus on insects.
In this review, which builds on our experience as main contributors to a Scientific Opinion by the Panel of Genetically Modified Organisms (GMOs) of the European Food Safety Authority (EFSA) [Citation15], we (1) describe engineered GD strategies and approaches in insects, (2) address the potential benefits of GD technologies and risk concerns associated with the deliberate environmental release (termed hereafter release) of GMIs containing engineered GDs (termed hereafter GD modified insects [GDMIs]), (3) present some of the latest international/regional developments on the need to develop new or further (i.e. additional to the existing) risk assessment guidance for releases of GD modified organisms (GDMOs), (4), offer recommendations to overcome some of the challenges anticipated for the development of practical and useful guidance on engineered GDs, and (5) highlight some risk assessment issues for GDMIs that may require further guidance.
Engineered gene drive strategies and approaches in insects
Engineered GDs can be designed to either suppress target insect populations (population suppression) or modify them (population modification). Suppressive engineered GDs aim to reduce target populations. This can be achieved by imposing a substantial fitness cost through the inactivation of important genes involved in (sex-specific) survival (e.g. non-developing females) or reproduction of the target population (e.g. reduced offspring fertility, biased sex ratio to males), or via the introduction of genes that reduce the lifespan [Citation16]. Such strategies are expected to result in population reduction or even collapse, and theoretically may lead to (global) eradication of the target organism. Engineered GDs for population modification, primarily for disease vector control, are intended to modify the genetic makeup of target populations to be less able to transmit disease (impaired vector competence including disease refractory traits). Such strategies are based on the introduction of genes that kill the pathogen in the insect or produce molecules blocking pathogen development. They can also involve the inactivation of genes required for the target organism to transmit the pathogen [Citation16]. Cargo/payload genes must be genetically linked to the engineered GD to ensure they are inherited alongside the drive.
Depending on the engineered GD system (whose design and mode of action are diverse), theoretically, a genetic modification could spread spatially beyond target populations (non-localized) and persist indefinitely (self-sustaining), or be restricted in its spread (localized) or persistence (self-limiting). Self-sustaining systems can be designed to spread a genetic modification rapidly, widely and for an indeterminate time, perhaps for many generations or until the target population is either eliminated or modified, or until a viable resistant mutation becomes established. Self-sustaining systems that are spatially unrestricted could introgress into any interbreeding target species that has vertical gene flow with the target population where the GD modified individuals are released, within a relevant timeframe [Citation17]. Self-limiting engineered GDs, on the other hand, are those in which the genetic modification is expected to be temporally limited and disappears from the target population in the absence of additional releases.
Inherent to many engineered GD systems is the requirement for individuals to be released above a certain threshold density before they will drive the genetic modification through the target population [Citation1,Citation18–21]. This threshold refers to the proportion of GD modified individuals with respect to the total target population that will reliably initiate the spread of the genetic modification. The lower the threshold, the more likely that dispersal of low numbers of GD modified individuals could initiate the establishment of the genetic modification in (neighboring) target populations of the same species. High threshold GDs only spread if the density of the modified individuals reaches a high proportion within the target population. With relatively low levels of dispersal, a further spread would be inhibited, as the genetic modification would fail to reach the threshold density needed to drive into areas neighboring a release area, thus enabling local confinement.
Current research efforts have focused on the development of engineered GDs that are specific, stable and avoid or delay the evolution of resistance against them. Resistance evolution can impede the continued spread of an engineered GD, inducing GD failure [Citation22–24]. Resistance can result from genetic variation in the population or can be induced by the nuclease activity itself, where repair by end-joining or imprecise/incomplete homology-directed repair can produce variant, non-cleavable alleles [Citation25]. In the case of CRISPR‐Cas9-mediated homing-based GDs, single nucleotide differences at the guide RNA (gRNA) target site can make it resistant to recognition by Cas9 and preclude further cutting/homing, because CRISPR‐Cas9 cleavage requires a near‐perfect match between the ∼ 20 base pair gRNA sequence and the genomic target site [Citation26]. Since population modification strategies operate over long periods of time, resistance due to spontaneous mutation in target sequences is more likely to be selected for in such systems [Citation25]. In suppressive GD systems, declining population numbers reduce the frequency of potential mutation events that could lead to resistance, despite the higher selection pressure for resistance mutations compared to population modification GDs. Various strategies are currently being explored to overcome resistance evolution: multiplexing gRNA targeting different target DNAs [Citation26,Citation27], targeting ultra-conserved and functionally constrained genes essential for survival or fertility [Citation28–30], optimising/regulating GD expression to reduce end-joining activity [Citation31], stacking multiple cargo/payload genes in the same host individual [Citation32], designing engineered GDs that target conserved or haploinsufficient genes that also carry a recoded cDNA restoring endogenous gene activities [Citation30,Citation33–37], minimizing fitness costs [Citation38], and combining multiple engineered GD approaches [Citation39]. While potential future GD failure (i.e. loss of efficacy) is often mentioned as a concern, it is not unique to engineered GDs or other genetic control systems [Citation23,Citation40].
Recent research efforts also aim to develop engineered GDs that are confinable (i.e. limited in their spread and persistence) and reversible (i.e. recallable from the environment in the event of unwanted consequences) [Citation24,Citation41–44]. Several approaches have been proposed to restrict the spread of engineered GDs within a specified target population or geographic region, or to reduce their persistence in target populations over the course of several generations [Citation44–46]. Theoretically, localized GDs are not expected to establish themselves in neighboring target populations when dispersal is low [Citation47]. Engineered localized GDs may thus constitute a form of biological or molecular confinement that could supplement physical and ecological confinement [Citation16]. Reversal GDs have been proposed as genetic remediation or neutralizing systems that could reverse the effects of a previously released GDMO in the event of unintended consequences. The development of reversal GDs is proceeding in insects such as mosquitoes and fruit flies, and their potential use is explored with population genetic models [Citation42,Citation48,Citation49]. Systems have also been designed to either turn on or turn off GD activity in the presence or absence of small organic molecules that can easily enter cells [Citation50,Citation51]. While reversal and inducible GD systems hold promise, developers themselves caution against unforeseen consequences [Citation49], and indicate that reversal GDs may not necessarily be the first choice for remediation efforts due to the associated uncertainty of introducing another GD approach [Citation52].
While current research is investigating the development of engineered GDs in insect populations and deploying them, it will take many years before they can be applied to practical disease vector/pest management. At present, some GDMIs are either in development or have been tested experimentally in the laboratory, often with multigenerational data and model simulations [Citation17,Citation19,Citation20,Citation27,Citation28,Citation33,Citation35,Citation39,Citation43,Citation45,Citation46,Citation53–67]. However, no “contemporary” GDMIs have been assessed in small-scale physically and/or ecologically confined field trials, or open release trials [Citation5,Citation8,Citation10,Citation15,Citation68].
Potential benefits and risk concerns
Potential future application of engineered GDs has sparked both enthusiasm and concern [Citation69]. GDMIs could potentially be used to address long-standing challenges in the control of disease vectors, agricultural pests and invasive species, or help to rescue endangered species. However, despite their potential to provide important public benefits, there are concerns that GDMI releases may pose different or new harms to animal and human health and the wider environment. Potential benefits and risk concerns are briefly described below.
Potential benefits
The use of engineered GDs in insects could have three main benefits: (1) reach parts of target populations that are missed by conventional methods, (2) very high target specificity, and (3) provide ongoing effects with relatively little or no further input. As is the case with any other genetic control method for insects (such as SIT, Wolbachia-mediated IIT and PI, and [fs]RIDL), GD technologies exploit the mate-seeking behavior of GD modified individuals, which self-disperse and actively seek mates [Citation1]. Due to the self-propagating nature of engineered GDs, theoretically, no enabling infrastructure is needed to ensure spread. Thus, GDMIs may be capable of extending into areas that are difficult to reach, and that are only served by limited infrastructure [Citation70].
Insect genetic control approaches, including GDMIs, are species-specific or at least limited to the related species complex (i.e. a group of closely related partially interbreeding, but distinct, species), and depending on the engineered GD system, they may even be population-specific (i.e. with limited ability to spread the genetic modification beyond the initial target population into which it is released). Thus, the wild-type organisms targeted by GDMIs may include: specific populations, single species, a species complex (covering all strains and sibling species where reasonable levels of hybridization or introgression can occur in the field), or a set of partially reproductively connected species. Depending on the extent of the set of target organisms, intended outcomes may differ across the spectrum of such a complex. Overall, species specificity could reduce undesired direct effects on non-target organisms (NTOs) compared to chemical insecticides used for indoor residual spraying, outdoor insecticide fogging, or chemical larvicides in breeding sites [Citation70].
Due to preferential inheritance, the acquisition of the genetic modification is expected to occur much faster than with most other genetic changes driven by natural selection [Citation12]. Consequently, engineered GDs may have a potentially higher efficiency at suppressing/modifying target populations than other genetic control methods based on classical Mendelian inheritance [Citation70]. Moreover, in the absence of mutation, heritable resistance or assortative mating, self-sustaining engineered GDs are intended to be relatively stable, potentially requiring only small and infrequent secondary releases over time to reach and maintain population suppression or modification [Citation61]. In some cases, environmental stressors could affect the continuation of an engineered GD in a target population, allowing a managed fade-out.
Except for Wolbachia-mediated PI, existing genetic methods for insect disease vector/pest control are self-limiting and mostly used to suppress target populations. Theoretically, some engineered GDs may enable: (1) rapid and non-localized spread of a genetic modification through target populations from low initial introductions, even if it imposes some fitness costs on the host, (2) persistence of a genetic modification in a target population until this population is locally eliminated, and (3) modifying the genetic makeup of target populations, while maintaining normal population densities. Owing to these features, GDMIs may potentially complement and expand the range of genetic methods for disease vector/pest control () [Citation5,Citation8,Citation10,Citation15].
Table 1. Overview of current and emerging genetic disease vector/pest control strategies in insects (reprinted and adapted from [Citation15]).
Risk concerns
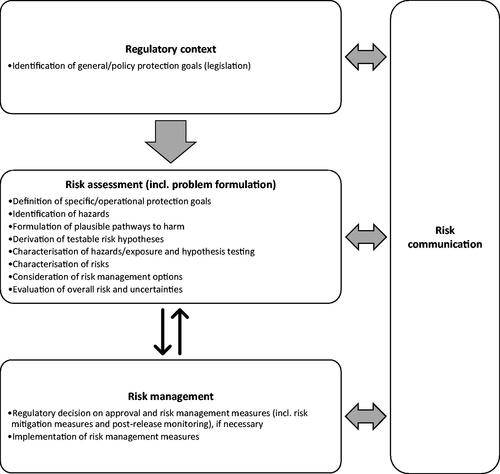
The fact that some GDMIs may potentially have higher efficiency at suppressing/modifying target populations than other genetic control methods, and operate at greater spatial and temporal scales, has raised concerns about harm to human health, animal health and the wider environment as a consequence of their application. Several publications have addressed various possible harms to existing broad protection goals (such as human and animal health, and the wider environment) associated with GDMO releases, including insects [Citation3,Citation9,Citation15,Citation16,Citation25,Citation70–80]. While some of the potential risk concerns are similar in many conventional or other genetic control systems, additional concerns have been raised about novel harms and pathways to established harms to humans, animals and the environment associated with GDMI releases. It is impossible here to capture fully all specific potential risk concerns, so this section aims to highlight general categories of concern, which are mentioned below. Potential risk concerns will vary dependent on the biology and ecology of the insect species under consideration, engineered GD design and strategy, nature of the introduced traits, intended GDMI uses, scale and frequency of the release, receiving environments (covering the receiving environments where GDMIs will be released and into which they may spread), and the interactions amongst these variables. Consequently, risk concerns will need to be identified on a case-by-case basis using a problem formulation approach, and addressed as part of the risk assessment process ().
In most jurisdictions, GMO releases are subject to risk assessment and regulatory approval. In the risk analysis process, the role of risk assessors is to assess any plausible risk that the specific proposed release (open release trials or deployment) of a GMO may pose to human and animal health and the environment, and provide risk management options to risk managers (). Risk is characterized by testing specific hypotheses about the probability that harm (i.e. an adverse effect on something of value defined by relevant protection goals) will occur and the severity of harm if it occurs based on reasonable scenarios outlining causal events. This process is framed by a problem formulation approach that articulates protection goals and decision-making criteria for assessing risks and devising tests of hypotheses that meet those criteria [Citation81–84]. Decisions to approve a permit for release, given potential risk management, is are taken by risk managers [Citation9].
Previously proposed risk concerns to human and animal health and the wider environment (covering biodiversity, food webs, ecosystems, ecosystem functions and ecosystem services) associated with potential GDMI releases, include:
The potential for toxicity and/or allergenicity due to the transmission of toxic or allergenic substances (related to the components of an engineered GD) either directly by biting or indirectly by exposure from such substances released into the environment (e.g. incidental exposure through inhalation or ingestion);
The potential for disease transmission (for disease vectors) due to:
○Increased abundance of disease vectors (through niche replacement whereby another insect pest [e.g. other mosquito species in aquatic habitats during larval stages] invades the ecological niche vacated by the suppression of the target organism);
○Increased competence for the transmission of existing or novel pathogens by the target population;
○Modified mating, host seeking or feeding behaviors, or geographic range (broader temperature tolerance) of a disease vector;
Modified pathogen virulence in the case of population modification;
Displacement of other insect species due to higher persistence and invasiveness of GD modified individuals compared to the wild-type (mainly for population modification strategies);
Harm to NTOs due to:
○Unintended spread of the genetic modification beyond the target population (i.e. spillover), to other closely related species through vertical gene flow (i.e. hybridization) and other species through horizontal gene transfer;
○Exposure to toxic substances (related to the components of an engineered GD) through consumption of GD modified individuals;
○Reduced quality of the GD modified individuals serving as a food source (e.g. prey, host) for the NTO (e.g. predator);
○Suppression and potential eradication of the target organism that serves as a food source for the NTO, or on which NTOs rely for the delivery of ecosystem functions and services;
Altered water quality due to the suppression of the target organism (e.g. mosquito larvae in aquatic habitats) which results in reduced larval consumption of algae causing levels of algae to increase as well as their associated toxins produced from algal bloom;
Resurgence of an intrinsically harmful target organism due to future failure of an engineered GD (e.g. genetic and phenotypic instability) or resistance to either the GD or its cargo/payload genes, possibly coupled with reduced immunity in human populations that have had a reduced disease challenge while the control was effective.
International/regional discussions on the need for new or additional risk assessment guidance
Potential future GMO releases may include GDMOs. While regulations are in place to guard against potential adverse effects of GMO releases in most jurisdictions, these regulations were developed for crop plants and animals that typically do not spread intentionally on their own or persist in the environment [Citation69], so the associated risk assessment guidelines may not necessarily be sufficiently adequate for GDMOs [Citation9]. Moreover, some features of engineered GDs may pose different or new harms and risk assessment challenges. Therefore, discussions on the need to develop new or additional risk assessment guidance for GDMO releases are ongoing among risk assessors and risk managers at the international/regional/national level. In these discussions and for the possible development of new or further risk assessment guidance, international organizations are likely to play a key role, because some potential GDMI releases are expected to occur across many nations [Citation85]. Guidance from international organizations help support consistent risk assessment frames in diverse jurisdictions. Here, we report some of the latest international/regional developments, focusing on the activities performed by an Ad Hoc Technical Expert Group (AHTEG) on risk assessment operating under the Cartagena Protocol on Biosafety (CPB), EFSA, the World Health Organization (WHO), the International Union for the Conservation of Nature (IUCN), and the African Union’s African High-Level Panel on Emerging Technologies (APET).
Ad Hoc Technical Expert Group (AHTEG) on risk assessment
In April 2020, an AHTEG on risk assessment operating under the CPB to the Convention on Biological Diversity (CBD) recommended the development of “additional guidance materials” for the risk assessment of GDMOs [Citation86]. The CPB is an internationally binding agreement that provides a framework for the safe handling, transport and use of living modified organisms (LMOs) that contain a novel combination of genetic material and are obtained through the use of modern biotechnology. The CPB also provides a framework for the risk assessment of LMOs with the objective to “identify and evaluate the potential adverse effects of LMOs on the conservation and sustainable use of biological diversity in the likely potential receiving environment, also taking into account risks to human health”. AHTEG’s assessment considered a specific study on engineered GDs commissioned by the CBD Secretariat [Citation79] and the outcome of discussions of an open-ended online forum. Amongst other aspects, the AHTEG recognized that “existing risk assessment methodology may still be applicable for LMOs containing engineered gene drives”, but that “there are specific technical or methodological challenges that require further attention”. AHTEG’s report and recommendation will be considered by the Subsidiary Body on Scientific, Technical and Technological Advice at its 24th meeting (SBSTTA-24; tentatively scheduled in 2021). On the basis of the recommendation by SBSTTA-24, the Parties may adopt a possible decision on the development of “additional guidance materials” for LMOs containing engineered GDs at the 10th Biannual Conference of the Parties (COP) serving as the meeting of the Parties to the CPB (COP-MOP-10; tentatively scheduled the second half of 2021) [Citation87].
European Food Safety Authority (EFSA)
To support the European Union in its future work on GDMOs under the CPB/CBD, EFSA assessed, through a problem formulation exercise, whether: (1) GDMO releases could pose risks and potential novel hazards to human and animal health and the environment, (2) the scientific considerations given in its previously published guidance for the risk assessment of GMOs [Citation88,Citation89] are adequate and sufficient for GDMOs, and (3) there is need for updated guidance in relation to the previously published guidance. EFSA focused its activities on disease-transmitting insects, primarily mosquitoes, as they represent the most likely cases of GDMOs for release in the near future, but also considered agricultural insect pests and non-native invasive insects [Citation15]. Based on a review of the relevant information reported in the scientific literature and progress in developing GDMIs, and an assessment of the considerations given in [Citation88,Citation89], EFSA concluded that its guidelines for GMIs, that do not contain engineered GDs, provide an appropriate basis for the risk assessment of GDMIs, but they should be more specific to address the challenges that GDMI releases may pose. While the risk assessment of GDMIs can build on the existing framework for GMIs, it was concluded that there are specific areas within the molecular characterization, environmental risk assessment and post-release monitoring, where further guidance is needed [Citation15].
Comments gathered during a stakeholder workshop [Citation90] and online public consultation [Citation91] revealed different, often contrasting, opinions and perspectives toward GDMI releases for insect disease vector/pest control (reflecting divergent views on precaution), and on the adequacy of current risk assessment frameworks for such insects. Several interested parties/persons considered that the current framework for GMIs, that do not contain engineered GDs, is inadequate for the risk assessment of GDMIs. Since they are of the opinion that no reliable risk assessment can be conducted, they advocate invoking the precautionary principle to refrain from GDMI releases. Other interested parties/persons considered that the risk assessment of GDMIs can build on the existing framework for GMIs, while acknowledging that there are specific areas where further guidance may be required.
World Health Organization (WHO)
Currently, the WHO is in the process of updating its 2014 guidance framework for testing genetically modified (GM) mosquitoes for use against disease vectors [Citation92] in order to incorporate specific considerations for engineered GDs [Citation5,Citation85]. The guidance framework describes a phased testing pathway and best practices for evaluating GM mosquitoes intended as a public health tool. According to this framework, testing proceeds iteratively through multiple phases (from contained laboratories, indoor cages and insectaries, to physically and/or ecologically confined field trials [e.g. large cages, physical islands], to small-scale open release trials, to large-scale open release trials, to releases), with each phase involving a larger spatial and/or longer temporal scale and a higher degree of human or environmental exposure and realism [Citation16,Citation72]. Since some engineered GDs are predicted to have potential impacts during the multiple phases of testing recommended in this framework, there have been calls for additional guidance and oversight before any field testing begins [Citation16]. The updated guidance framework is expected to be published in the first half of 2021. It has been noted that an updated WHO guidance framework would also be a valuable resource for international and regional organizations that focus on agricultural insects and other arthropod pests [Citation85]. Looking into the future, the WHO Regulation and Prequalification Department is also working on how to successfully monitor GDMIs for disease vector control in terms of regulatory approval, quality, safety and performance.
International Union for the Conservation of Nature (IUCN)
Following a resolution at the 2016 IUCN World Congress (WCC-2016-Res-086), a Task Force on Synthetic Biology and Biodiversity Conservation and its accompanying Technical Subgroup has been established to support the development of an IUCN guidance on biodiversity conservation and synthetic biology. As a first step, IUCN commissioned the Task Force to assess the state of science and policy around synthetic biology techniques, including GD technologies. This assessment aimed to explore the implications of synthetic biology, including GDs, and its potential impact on conservation and sustainable use of biological diversity as well as the equitable sharing of benefits arising from genetic resources. The IUCN report took a case-by-case approach by examining case studies, and made a plea for the policy debate to be grounded in evidence [Citation93].
The IUCN assessment led to a draft set of principles on the intersection of biodiversity conservation and synthetic biology, which will be voted on as a motion at the next IUCN World Conservation Congress in 2021. Principles referred to include: case-by-case and evidence-based decision-making, evaluation of existing alternatives for potential applications of synthetic biology intended for conservation goals, steps to ensure that potential applications of synthetic biology are intended for purposes other than conservation do not threaten biodiversity and its sustainable use, staged assessment of risks and benefits, filling knowledge gaps, facilitating transnational knowledge transfer and capacity building, potential introduction of moratoria, intergenerational equity, dialogue between conservationists and synthetic biologists, and ethics. The outcomes of the synthetic biology and GD discussions at the World Conservation Congress will likely influence discussions and decisions at SBSTTA-24 and COP-MOP-10 [Citation87].
African High-Level Panel on Emerging Technologies (APET)
In an APET report [Citation94], the African Union recognized emerging GD technologies as having great potential to contribute significantly to Africa’s development, especially to combat mosquito-borne diseases such as malaria. The report emphasized that risk assessment will be essential for the development of GD technologies, and that there is need to develop the capacity and capabilities for making informed decisions about whether to adopt GDMIs or not. In this frame, a series of four consultative meetings, focusing on building capacity in problem formulation, were held in Africa considering GD technologies. These 4-day workshops, held in Ghana, Kenya, Botswana and Gabon, during 2016–2018, were organized by the New Partnership for Africa’s Development Agency [Citation77]. Among other things, the APET recommended governance measures, including a network of African researchers who register, self-regulate and peer review their work; national guidelines, frameworks and enabling legislation that considers both potentials and risks; and the development, coordination and harmonization of regulations and guidelines for regulating the development, approval and use of potential GD products [Citation94].
Recommendations for new or additional guidance development
Developers and potential applicants need useful and practical guidance to ensure that GD products meet the regulatory standards of safety for human and animal health and the environment, and receive public acceptance. The development of new or additional guidance for the risk assessment of GDMO releases is a challenge, especially at an international level [Citation95]. Due to different, often contrasting, opinions toward GDMOs, and on the adequacy of current risk assessment frameworks for such organisms [Citation91,Citation96], defining the scope of guidance and topics to prioritize may be contentious, and so may be the procedure to develop such guidance (i.e. which experts or expert groups to involve in terms of expertise, and how to appoint/nominate them) [Citation97]. Moreover, GD technologies are evolving very rapidly, and may yield diverse applications across various organisms and technical strategies for GD approaches with a wide spectrum of possible properties. Therefore, it will not be possible to evaluate them as one group [Citation9]. While guidance may offer potentially the most responsive approach to rapidly advancing technologies compared to regulations and statutes [Citation85], it can easily be outpaced by scientific advances, especially if its requirements are overly prescriptive and aim to encompass all possible GDMO applications. For guidance to be proportionate, practical and current, we recommend tailoring it to the most likely cases moving to practical applications for release (i.e. disease-transmitting mosquitoes whose control is a long-standing public health goal), requiring prioritization of its scope and regular updates as application priorities change [Citation95]. We also consider that guidance should differentiate between diverse GD systems and refrain from taking single cases to make broader, more general statements that may not be applicable for all GDMOs [Citation95]. Ideally, guidance should offer an overarching framework that is flexible and outlines general principles and methodology for risk assessment instead of being overly prescriptive [Citation95].
Another challenge is the limited direct experience conducting risk assessment of GDMI releases. Therefore, we suggest that the development of guidance involves an iterative process of design, revision and refinement, including a review of actual case studies by risk assessment experts and consultation with relevant stakeholders. Once in place, regular review must be continued to establish overall guidance utility and applicability, and assess where any refinements are necessary. This may ensure that guidance is realistic and proportionate, and remains consistent with the weight of scientific evidence and familiarity that will be gained with future GDMI releases [Citation95].
Risk assessment considerations for releases of gene drive modified insects
Novel technologies may present new hazards and/or pathways to harm, as well as sharing hazards similar to those from more established technologies for which there is experience in risk assessment. Several publications have anticipated that specific features of GDMOs (including GDMIs) may raise novel risk assessment challenges [Citation9,Citation72,Citation73,Citation78,Citation80,Citation86], while others did not identify new hazards of GDMIs compared to those of GMIs that do not contain engineered GDs [Citation3,Citation71,Citation77]. In the following sections, some considerations about the risk assessment of GDMI releases and associated challenges are given, building on those reported by the EFSA GMO Panel [Citation15] and James et al. [Citation16,Citation25].
Novel features of gene drive modified insects
Precedents suggest that: (1) the scale of population suppression, (2) the nature of target populations and receiving environments, and (3) the current lack of spatio-temporal controllability, may not be considered as novel aspects of GDMIs when compared with a control using naturally occurring GDs, GMIs that do not contain engineered GDs ([fs]RIDL) and non-GMIs (SIT, Wolbachia-mediated IIT and PI, and classical biological control [CBC]). This is because: (1) SIT and CBC have been used at a local and area-wide scale to suppress target populations (including area-wide elimination), often involving repeated releases over time to reach and maintain suppression, (2) current and emerging disease vector/pest control strategies can target non-domesticated or wild-type species in non-managed receiving environments, and (3) Wolbachia-mediated PI and CBC often lack spatio-temporal controllability [Citation3,Citation15].
In contrast: (1) the intended preferential inheritance of a transgenic construct, (2) the intended spatial and temporal scale of spread of a transgenic construct, and (3) population modification strategies should be considered as novel aspects of GDMIs, when compared with naturally occurring GDs and existing disease vector/pest control strategies involving insect release [Citation15].
The intended preferential inheritance of a transgenic construct does not occur in GMIs that do not contain engineered GDs. While preferential inheritance of genetic elements (which are not transgenic) is observed in many naturally occurring GDs and Wolbachia-mediated PI, currently, such systems are not tailored to spread a transgenic construct to achieve intended outcomes.
The potential to spread a transgenic construct widely and for an indeterminate time is different from disease vector/pest control strategies involving the release of GMIs and non-GMIs, as they are generally intended to be self-limiting (i.e. localized and transient), with the exception of Wolbachia-mediated PI.
Wolbachia-mediated PI is currently the only strategy applied for population modification. Theoretically, engineered GDs may enable modifying target populations in the field, and expand the means to achieve population modification (including the spectrum and nature of novel cargo/payload genes) compared to Wolbachia-mediated PI.
Whether the novel aspects of GDMIs present potential new hazards, lead to novel pathways to harm, and may pose additional or unique challenges for the risk assessment, needs to be assessed carefully on a case-by-case basis as part of a specific problem formulation ().
Problem formulation
The problem formulation, which serves as the starting point for conducting a risk assessment, can be applied in case-by-case evaluations of GDMI releases [Citation3,Citation71,Citation77,Citation90,Citation98]. Precedents suggest that the problem formulation approach provides a compelling framework to organize existing knowledge and identify relevant new knowledge and uncertainties on engineered GDs to support case-specific risk assessments and decision-making [Citation3,Citation71,Citation77,Citation98]. The approach involves (), (1) identifying protection goals and making them operational in risk assessment, (2) devising plausible pathways to harm that describe how a release could be harmful, (3) formulating risk hypotheses about the likelihood and severity of such events, (4) identifying the information needed to test risk hypotheses, and (5) developing plans to acquire new data for hypothesis testing if tests with existing information are insufficient for decision-making [Citation15,Citation81–84]. Enabling the testing of risk hypotheses makes the prospective pathway to harm approach very powerful for risk assessment because harm is defined explicitly, existing information is used effectively, new data are collected with a clear purpose, and risk is characterized against well-defined criteria of hypothesis corroboration or falsification.
Transparency on how problem formulation is conducted is important to all stakeholders. Sufficient detail about the methods, data, assumptions and uncertainties must be reported to promote transparency, facilitate an appropriate assessment of the quality of the problem formulation, ensure relevance, and enable reproducibility [Citation15]. In particular, when devising pathways to harm, all potential pathways should be systematically explored, and then those pathways would be prioritized based on their likelihood and potential consequences. Since it can be challenging to adequately devise multiple, complex pathways over a long period of time, a wide area, and/or heterogeneous receiving environments, it is important that all potential pathways are reported transparently. Moreover, a rationale justifying why potential pathways are not considered sufficiently plausible and/or consequential should be reported transparently for each potential pathway rejected [Citation15]. Active stakeholder engagement on problem formulation (including the setting of specific/operational protection goals) will improve the value of risk assessment, as it may help to ensure that risk assessment is meaningful and informative to the decisions that affect stakeholders.
Relevant experience
Currently, there is limited direct experience in conducting the risk assessment of GDMI releases. Nonetheless, principles and methodologies for risk assessment and risk management, experience from the risk assessment of GMIs that do not contain engineered GDs, and knowledge from other disease vector and pest control strategies, are relevant to performing GDMI risk assessments [Citation3,Citation15,Citation52]. For example, there is substantial experience with releasing insects for genetic and biological disease vector/pest control, including their risk assessment and post-release monitoring (where applicable), from which lessons can be learnt. This experience can be useful to identify some potential hazards, exposures and risks for GDMIs, and develop relevant, effective and efficient risk assessment guidance. Thus, it is appropriate to draw on the experience from current insect disease vector/pest control strategies, seek relevant precedents from more or less similar situations, and use this experience to inform the risk assessment of GDMIs. However, caution is required as specific control systems are likely to differ in various aspects [Citation15].
Risk assessment paradigm
GMO risk assessment guidance typically follows the comparative risk assessment paradigm, which is applied on a case-by-case basis, using an iterative and stepwise/staged/tiered testing method, and considers different lines of evidence, including modeling. However, this approach may leave some uncertainty before open field testing or release of some GDMIs, as it can be challenging to gather data from confined experimental systems (such as laboratories, indoor cages, small-scale physically and/or ecologically confined field trials) that would be fully applicable to field conditions [Citation15]. Mathematical modeling may help to fill this gap in data and extrapolate from data gathered from confined experimental systems to field conditions. Moreover, greater use of quantitative models is anticipated to address the long temporal scale and wide spatial scale of specific GDMI applications, and post-release monitoring. While there remains fundamental problems with models because extrapolation beyond the range of practical datasets involves considerable uncertainty, there are modeling processes that allow uncertainties to be more thoroughly understood (e.g. formal sensitivity analyses of predictions to parameter variation, consideration of outcomes at the bounds of acceptability in relation to specific/operational protection goals).
Field testing of self-sustaining and low threshold engineered gene drives
Gathering relevant data for assessing environmental risks of self-sustaining and low threshold engineered GDs in open release trials may be challenging due to their intended spatially and temporally unrestricted nature and the current inability for recall. Since self-sustaining engineered GDs are designed for widespread and long-standing control, spatially and/or temporally restricting their spread/persistence would not necessarily be in line with the intended outcome of their release. Therefore, besides gathering data under confined conditions, developers and potential applicants may wish to consider the utility of prior field testing of a related self-limiting strain (note however, that there should be a strong resemblance between the two systems, to the extent possible) as an intermediate step to gather evidence for model development and refinement, and reduce uncertainties in risk assessment [Citation15,Citation16,Citation52].
Molecular characterization
The molecular characterization of GMIs, that do not contain engineered GDs, is an important element for risk assessment [Citation88,Citation89], and will need to be expanded to assess the persistence and invasiveness potential of GDMIs, and the potential for resistance to evolve. This would include, among other aspects, a precise description of the mode of action and characteristics of the engineered GD itself, and a thorough description of the intended genetic target(s) in the insect host and target population(s). In addition, molecular tools will be needed to monitor intended and potential unintended effects post-release. These tools should permit evaluation of the efficiency, stability and inheritance of the engineered GD system, and follow the engineered GD spread and persistence in target and potential non-target populations.
Genetic and phenotypic stability of the transgenic construct
Target populations are expected to be genetically heterogeneous, so interactions between the transgenic construct and genetic background may be complex and difficult to predict. For engineered GDs that are intended to spread over wide areas and persist in target populations, the diversity of interactions with target populations and their diverse receiving environments is likely to be greater than for GMIs that do not contain engineered GDs. Thus, the concepts of inheritance and genetic and phenotypic stability of the transgenic construct may need further consideration compared to GMIs to address the preferential inheritance, and the broad array of possible GDMI applications whose mode of action and period of environmental exposure will differ. For example, assessment and monitoring of stability may be needed over multiple generations of GD modified individuals before release, and in the field after release as part of post-release monitoring, in order to demonstrate product consistency and the durability of efficacy after release [Citation15,Citation16,Citation25]. As part of the risk assessment, James et al. [Citation16,Citation25] recommended gathering data on the efficacy of engineered GDs from GD modified individuals with a genetic background as similar as possible to that of local target individuals found at the site(s) of the proposed release. If the release would occur at multiple sites, but those sites are connected geographically or otherwise not reproductively isolated, a single colony derived from locally collected individuals is deemed sufficient. If release sites are distant, the authors recommend the use of models to determine whether additional local colonies might be needed [Citation16,Citation25].
Unintended off-target effects and their consequences
For GD strategies based on site-directed nucleases, intended and unintended on-target and unintended off-target sequence modifications caused by the nuclease activity [Citation99] can affect the fitness of GD modified individuals and their offspring [Citation25]. Such effects may be more important for population modification strategies where the genetic modification must remain present and active at high frequency in target populations over long periods of time, whereas in population suppression systems the number of potential off-target events would decline over time as the population decreases. Unintended off-target effects are likely to be very heterogeneous at the individual level, and the frequency that such effects occur is assumed to be specific for the gRNA used in the GD construct. In silico analyses can help to identify and predict potential off-target effects in target populations prior to release as part of the risk assessment, but caution is required when interpreting such data, as they are subject to limitations (i.e. natural population heterogeneity). Potential adverse effects associated with mutations induced by the off-target activity should be evaluated in relation to spontaneous mutations. Off-target activity can be monitored post-release via molecular analyses, and addressed through management actions triggered by the identification and monitoring of fitness and other phenotypic changes over time in different genetic backgrounds.
Relevant comparators
For a given GDMI release, there will often not be a single comparator, but a range of relevant comparators (such as the non-GMI of the same species with a genetic background as close as possible and relevant to that of the GDMI, the target organism, or other disease vector/pest control systems) to inform a risk assessment and contextualize risks. Different comparators may be relevant for different component properties of a GDMI. Thus, more emphasis may need to be given to the purpose of risk assessment studies and comparisons when selecting relevant comparators. Given that some GDMI systems will operate at an ecosystem level, the definition of the comparator may need to be broadened from endpoints that solely consider genetic and phenotypic changes to those that can be indicative of potentially harmful ecosystem impacts. At the population and system level, multiple comparators may be needed to allow robust comparisons across a range of factors that are not sufficiently covered by any single comparator [Citation15].
Spread and persistence of engineered gene drives
Understanding how engineered GDs may spread and persist in target populations in the field, and their potential intended and unintended outcomes, is crucial for risk assessment. These processes are influenced by various ecological factors such as: the genetic diversity of target populations, density-dependent population dynamics, dispersal to neighboring populations, intraspecific competition, spatial heterogeneity, mating behavior and sexual selection, and heterogeneity of receiving environments. Prediction of the spread and persistence of engineered GDs in target populations in the field, which could be at a larger scale and a longer temporal scale for some self-sustaining engineered GDs, will require more robust mathematical models (in contrast to simple deterministic models) and data analysis appropriate to these temporal and spatial scales [Citation25]. Improved modeling capabilities and more empirical evidence are critical to support more realistic risk assessments [Citation100], because they would enhance the ability to understand the sensitivity of spread and persistence of engineered GDs to important ecological factors and their associated uncertainties, and predict how GDs might spread through target populations in the field [Citation9,Citation21,Citation101].
Non-target organisms
As is the case for any GMIs that do not contain engineered GDs, GDMIs can adversely affect NTOs either directly (e.g. through toxicity or allergenicity) or indirectly (e.g. disruption of food webs, ecosystem services) dependent on the GD construct and associated cargo/payload genes [Citation3,Citation15,Citation89]. While, in general, no novel harms of GDMIs on NTOs are foreseen, effects might be more severe (e.g. large scale, population wide) dependent on the engineered GD [Citation3]. Therefore, knowledge on the functional role of the target organism and potential cross-compatible species in the various ecosystems that may be encountered is crucial. Since most disease vectors, agricultural pests and invasive species (within their new receiving environments) are not keystone species, it is generally assumed that targeting them would not typically lead to new adverse effects on NTOs [Citation71,Citation74,Citation77], especially for suppressive GDs. The problem formulation approach would enable the risk assessment to focus on NTOs and receiving environments that are relevant for the specific GDMI, and would avoid disproportionate open-ended data collection exercises [Citation15].
Receiving environments
Receiving environments refer to the broader ecological and biological characteristics of an environment intended for GMO release, and are considered in risk assessments. For example, risk assessment requires information on the diversity and heterogeneity of potential receiving environments, and details on potential ecological interactions with natural receiving environments. The range of potential receiving environments is likely to be greater for specific GDMIs compared to GMIs that do not contain engineered GDs, given their broader spatial and longer temporal extent, and their potential to spread into further accessible environments following release into a known environment. As such, there may be a need to extend the approaches for risk assessment and recommend more prominent use of additional tools such as mathematical modeling to consider risks across the full range of potential receiving environments.
Risk management (risk mitigation and post-release monitoring)
Post-release monitoring would be required for any GDMI release. Depending on the engineered GD system, monitoring would need to be extensive in both time and space, and address potential outcomes from pathways to harm across any components of the receiving environments where plausible risks were identified. Given the potential for transboundary movement, such monitoring may need to be implemented consistently across different local regions and international jurisdictions, dependent on the nature of the engineered GD. Risk mitigation measures and a remediation plan for unacceptable levels of harm should be in place prior to release [Citation16]. Mitigation and management plans to ensure risks are at acceptable levels should undergo systematic evaluation in a manner similar to risk assessment.
Concluding remarks
Engineered GDs could potentially be used to address long-standing challenges in the control of insect disease vectors, agricultural pests and invasive species, or help to rescue endangered species, and thus provide important public benefits. However, there are concerns that GDMI releases may pose different or new harms to animal and human health and the wider environment, and raise novel risk assessment challenges. These risk concerns and associated risk assessment challenges have prompted some non-governmental organizations, parliamentarians, scientists and scientific bodies to call for either strict application of the precautionary principle or a (global) moratorium on GD research and GDMO releases [Citation102,Citation103]. Calls have also been made for a better understanding of the potential ecological and evolutionary impacts associated with GDMO releases, the phased testing of engineered GDs and their responsible and sustainable deployment, effective engagement of relevant stakeholders, and the implementation of regional approaches facilitating international governance of GDMOs that may spread across jurisdictional boundaries [Citation9,Citation16,Citation25,Citation104–109]. For example, in its recent position statement on the evaluation of GM mosquitoes for the control of vector-borne diseases, the WHO emphasized the need to evaluate the potential of new genetic control technologies (including the use of engineered GDs) to contribute to reducing the global burden of vector-borne diseases, while following a stepwise approach in the evaluation process [Citation8]. The WHO also acknowledged the critical role of community engagement in designing and implementing appropriate, sustainable public health responses [Citation5]. Similarly, the Entomological Society of America (ESA) recommended: (1) continued research to increase an understanding of the biological aspects that GD technologies may affect (population genetics, ecology, genomics, etc.), and assess their potential uses, risks and benefits in field scenarios, and (2) community engagement [Citation10]. Core commitments for field trials with GDMOs [Citation68] and a code of ethics for GD research [Citation110] have recently been issued by the research/GD community. They highlight, among other aspects, the need for engagement and public transparency, GD product efficacy and safety, regulatory evaluation (including risk-benefit assessments), and post-release monitoring and mitigation.
The potential need to develop new or additional risk assessment guidance for GDMO releases, including insects, is currently discussed at many levels by risk assessors, risk managers, developers, potential applicants and many other stakeholders. Some international/regional entities have suggested that the risk assessment of GDMIs can build on existing risk assessment frameworks for GMIs that do not contain engineered GDs, and be informed by the experience gained releasing insects for genetic and biological disease vector/pest control. Moreover, the problem formulation approach has been acknowledged to provide a compelling framework to organize existing knowledge and identify relevant new knowledge and uncertainties on engineered GDs to support case-specific risk assessments and decision-making [Citation15,Citation95,Citation98]. It is also recognized that there are specific areas where further guidance may be needed for the risk assessment of GDMIs [Citation15,Citation86]. These areas include: molecular characterization, genetic and phenotypic stability of the transgenic construct, assessment of unintended on-target and off-target activity of site-directed nucleases and their consequences, consideration of receiving environments, engineered GD spread and persistence in the field, knowledge on the functional role of the target organism and the potential cross-compatible species in the various ecosystems that may be encountered, definition of relevant comparators, model design, quality assurance, model validation, model interpretation, and post-release monitoring.
To ensure that the risk assessment guidance for GDMOs is proportionate, practical and current, it may need to be tailored to the most likely cases moving to practical applications for release. It should also offer an overarching framework that is flexible and outlines general principles and methodology for risk assessment instead of being overly prescriptive. The process of guidance development must ensure an iterative process of design, revision and refinement, including the review of actual case studies by risk assessment experts and consultation with relevant stakeholders [Citation95].
Disclaimer
The views expressed in this publication are those of the authors and should not be interpreted as representing the official position of EFSA. EFSA assumes no responsibility or liability for any errors or inaccuracies that may appear. Part of this publication builds on and reuses elements of an EFSA GMO Panel Scientific Opinion [Citation15], to which the authors contributed actively.
Author contributions
YD wrote the manuscript and conceived it. All authors read, reviewed, edited and approved the manuscript.
Acknowledgments
We thank the anonymous reviewers for their thoughtful feedback on the manuscript and suggestions for revision.
Disclosure statement
JDM has received funding from Bill and Melinda Gates Foundation, the WHO and the International Atomic Energy Agency (IAEA) related to the risk analysis of GDMIs, Wolbachia and radiation-based SIT.
References
- Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014;59:205–224.
- Flores HA, O’Neill SL. Controlling vector-borne diseases by releasing modified mosquitoes. Nat Rev Microbiol. 2018;16:508–518.
- Romeis J, Collatz J, Glandorf DCM, et al. The value of existing frameworks for the environmental risk assessment of agricultural pest control using gene drives. Environ Sci Pol. 2020;108:19–36.
- Feachem RGA, Chen I, Akbari O, et al. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet. 2019;394:1056–1112.
- World Health Organization (WHO). Ethics and vector-borne diseases: WHO guidance. 2020. Available from: https://www.who.int/publications/i/item/ethics-and-vector-borne-diseases.
- Ritchie SA, Staunton KM. Reflections from an old Queenslander: can rear and release strategies be the next great era of vector control? Proc R Soc B. 2019;286(1905):20190973.
- World Health Organization (WHO). World malaria report 2019. Geneva: World Health Organization. 2019. Available from: https://www.who.int/publications-detail/world-malaria-report-2019.
- World Health Organization (WHO). Position statement: evaluation of genetically modified mosquitoes for the control of vector-borne diseases. 2020. Available from: https://www.who.int/publications/i/item/9789240013155.
- National Academies of Sciences Engineering and Medicine (NASEM). Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. Washington (DC): The National Academies Press; 2016. Available from: https://doi.org/https://doi.org/10.17226/23405
- Entomological Society of America. ESA position statement on the importance of continued innovation in gene drive technology. Ann Entomol Soc Am. 2020. 113:486–487.
- Lester PJ, Bulgarella M, Baty JW, et al. The potential for a CRISPR gene drive to eradicate or suppress globally invasive social wasps. Sci Rep. 2020;10:12398.
- Alphey LS, Crisanti A, Randazzo F, et al. Standardizing the definition of gene drive. Proc Natl Acad Sci USA. 2020;117:30864–30867.
- Burt A, Trivers RL. Genes in conflict. Boston (MA): Belknap Press of Harvard University Press; 2006.
- Burt A, Crisanti A. Gene drive: evolved and synthetic. ACS Chem Biol. 2018;13:343–346.
- EFSA Panel on Genetically Modified Organisms. Scientific Opinion on the adequacy and sufficiency evaluation of existing EFSA guidelines for the molecular characterisation, environmental risk assessment and post‐market environmental monitoring of genetically modified insects containing engineered gene drives. EFSA J. 2020;18:6297.
- James S, Collins FH, Welkhoff PA, et al. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in Sub-Saharan Africa: recommendations of a scientific working group. Am J Trop Med Hyg. 2018;98:1–49.
- Noble C, Adlam B, Church GM, et al. Current CRISPR gene drive systems are likely to be highly invasive in wild populations. eLife. 2018;7:e33423.
- Curtis C. Possible use of translocations to fix desirable genes in insect pest populations. Nat. 1968;218(5139):368–369.
- Leftwich PT, Edgington MP, Harvey-Samuel T, et al. Recent advances in threshold-dependent gene drives for mosquitoes. Biochem Soc Trans. 2018;46:1203–1212.
- Backus GA, Delborne JA. Threshold-dependent gene drives in the wild: spread, controllability, and ecological uncertainty. BioSci. 2019;69(11):900–907.
- Dhole S, Lloyd AL, Gould F. Gene drive dynamics in natural populations: the importance of density-dependence, space and sex. Annu Rev Ecol Evol Syst. 2020;51(1):505–531.
- KaramiNejadRanjbar M, Eckermann KN, Ahmed HMM, et al. Consequences of resistance evolution in a Cas9-based sex conversion-suppression gene drive for insect pest management. Proc Natl Acad Sci Usa. 2018;115(24):6189–6194.
- Price TAR, Windbichler N, Unckless RL, et al. Resistance to natural and synthetic gene drive systems. J Evol Biol. 2020;33:1345–1360.
- Raban RR, Marshall JM, Akbari OS. Progress towards engineering gene drives for population control. J Exp Biol. 2020;223:jeb208181.
- James SL, Marshall JM, Christophides GK, et al. Toward the definition of efficacy and safety criteria for advancing gene drive-modified mosquitoes to field testing. Vector Borne Zoonotic Dis. 2020;20:237–251.
- Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823.
- Oberhofer G, Ivy T, Hay BA. Behavior of homing endonuclease gene drives targeting genes required for viability or female fertility with multiplexed guide RNAs. Proc Natl Acad Sci USA. 2018;115:E9343–E9352.
- Kyrou K, Hammond AM, Galizi R, et al. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol. 2018;36:1062–1066.
- Schmidt H, Collier TC, Hanemaaijer MJ, et al. Abundance of conserved CRISPR-Cas9 target sites within the highly polymorphic genomes of Anopheles and Aedes mosquitoes. Nat Commun. 2020;11:1425.
- Champer J, Yang E, Lee YL, et al. A CRISPR homing gene drive targeting a haplolethal gene removes resistance alleles and successfully spreads through a cage population. Proc Natl Acad Sci USA. 2020;117:24377–24383.
- Hammond A, Karlsson X, Morianou I, et al. Regulating the expression of gene drives is key to increasing their invasive potential and the mitigation of resistance. PLoS Genet. 2021;17:e1009321.
- Gantz VM, Jasinskiene N, Tatarenkova O, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci USA. 2015;112:E6736–E6743.
- Oberhofer G, Ivy T, Hay BA. Cleave and Rescue, a novel selfish genetic element and general strategy for gene drive. Proc Natl Acad Sci USA. 2019;116:6250–6259.
- Adolfi A, Gantz VM, Jasinskiene N, et al. Efficient population modification gene-drive rescue system in the malaria mosquito Anopheles stephensi. Nat Commun. 2020;11:5553.
- Champer J, Lee YL, Yang E, et al. A toxin-antidote CRISPR gene drive system for regional population modification. Nat Commun. 2020;11:1082.
- Kandul NP, Liu J, Bennett JB, et al. A confinable home and rescue gene drive for population modification. eLife. 2021;10:e65939.
- Terradas G, Buchman AB, Bennett JB, et al. Inherently confinable split-drive systems in Drosophila. Nat Commun. 2021;12:1480.
- Beaghton AK, Hammond A, Nolan T, et al. Gene drive for population genetic control: non-functional resistance and parental effects. Proc Royal Soc B. 2019;286:20191586.
- Simoni A, Hammond AM, Beaghton AK, et al. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat Biotechnol. 2020;38(9):1054–1060.
- Marshall JM, Raban RR, Kandul NP, et al. Winning the tug-of-war between effector gene design and pathogen evolution in vector population replacement strategies. Front Genet. 2019;10:1072.
- Marshall JM, Akbari OS. Can CRISPR-based gene drive be confined in the wild? A question for molecular and population biology. ACS Chem Biol. 2018;13:424–430.
- Friedman RM, Marshall JM, Akbari OS. Gene drives: new and improved. Issues Sci Technol. 2020;36:72–78.
- Sánchez HMC, Bennett JB, Wu SL, et al. Modeling confinement and reversibility of threshold-dependent gene drive systems in spatially-explicit Aedes aegypti populations. BMC Biol. 2020;18:50.
- Hay BA, Oberhofer G, Guo M. Engineering the composition and fate of wild populations with gene drive. Annu Rev Entomol. 2021;66:1.
- Li M, Yang T, Kandul NP, et al. Development of a confinable gene drive system in the human disease vector, Aedes aegypti. eLife. 2020;9:e51701.
- Webster SH, Vella MR, Scott MJ. Development and testing of a novel killer-rescue self-limiting gene drive system in Drosophila melanogaster. Proc Royal Soc B. 2020;287:20192994.
- Dhole S, Vella MR, Lloyd AL, et al. Invasion and migration of spatially self‐limiting gene drives: a comparative analysis. Evol Appl. 2018;11(5):794–808.
- Vella MR, Gunning CE, Lloyd AL, et al. Evaluating strategies for reversing CRISPR-Cas9 gene drives. Sci Rep. 2017;7:11038.
- Xu X-RS, Bulger EA, Gantz VM, et al. Active genetic neutralizing elements for halting or deleting gene drives. Mol Cell. 2020;80:246–262.e4.
- Heffel MG, Finnigan GC. Mathematical modeling of self-contained CRISPR gene drive reversal systems. Sci Rep. 2019;9:20050.
- López Del Amo V, Leger BS, Cox KJ, et al. Small-molecule control of super-Mendelian inheritance in gene drives. Cell Rep. 2020;31:107841.
- Marshall JM, Vasquez VN. Field trials of gene drive mosquitoes: lessons from releases of genetically sterile males and Wolbachia-infected mosquitoes. In: Tyagi BK, editor. Eco-bio-social considerations for the safe application of genetically modified vectors to control malaria and dengue. Oxford University Press; 2021.
- Buchman AB, Ivy T, Marshall JM, et al. Engineered reciprocal chromosome translocations drive high threshold, reversible population replacement in Drosophila. ACS Synth Biol. 2018;7:1359–1370.
- Buchman A, Marshall JM, Ostrovski D, et al. Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. Proc Natl Acad Sci USA. 2018;115:4725–4730.
- Edgington MP, Alphey LS. Population dynamics of engineered underdominance and killer-rescue gene drives in the control of disease vectors. PloS Comput Biol. 2018;14:e1006059.
- Edgington MP, Alphey LS. Modeling the mutation and reversal of engineered underdominance gene drives. J Theor Biol. 2019;479:14–21.
- Edgington MP, Harvey‐Samuel T, Alphey L. Split drive killer-rescue provides a novel threshold-dependent gene drive. Sci Rep. 2020;10:20520.
- Noble C, Min J, Olejarz J, et al. Daisy-chain gene drives for the alteration of local populations. Proc Natl Acad Sci USA. 2019;116:8275–8282.
- Oberhofer G, Ivy T, Hay BA. Gene drive and resilience through renewal with next generation Cleave and Rescue selfish genetic elements. Proc Natl Acad Sci USA. 2020;117:9013–9021.
- North AR, Burt A, Godfray HCJ. Modelling the potential of genetic control of malaria mosquitoes at national scale. BMC Biol. 2019;17:26.
- North AR, Burt A, Godfray HCJ. Modelling the suppression of a malaria vector using a CRISPR-Cas9 gene drive to reduce female fertility. BMC Biol. 2020;18:98.
- Pham TB, Phong CH, Bennett JB, et al. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet. 2019;15:e1008440.
- Carballar-Lejarazú R, Ogaugwu C, Tushar T, et al. Next-generation gene drive for population modification of the malaria vector mosquito, Anopheles gambiae. Proc Natl Acad Sci USA. 2020;117(37):22805–22814.
- Champer J, Kim IK, Champer SE, et al. Performance analysis of novel toxin-antidote CRISPR gene drive systems. BMC Biol. 2020;18:27.
- Champer J, Zhao J, Champer SE, et al. Population dynamics of underdominance gene drive systems in continuous space. ACS Synth Biol. 2020;9:779–792.
- Kandul NP, Liu J, Buchman A, et al. Assessment of a split homing based gene drive for efficient knockout of multiple genes. G3 Genes Genom Genet. 2020;10:827–837.
- Sánchez CHM, Wu SL, Bennett JB, et al. MGDrivE: a modular simulation framework for the spread of gene drives through spatially‐explicit mosquito populations. Methods Ecol Evol. 2020;11(2):229–239.
- Long KC, Alphey L, Bloss CS, et al. Core commitments for field trials of gene drive organisms. Sci. 2020;370:1417–1419.
- Brossard D, Belluck P, Gould F, et al. Promises and perils of gene drives: navigating the communication of complex, post-normal science. Proc Natl Acad Sci USA. 2019;116:7692–7697.
- Deplazes-Zemp A, Grossniklaus U, Lefort F, et al. Gene drives: benefits, risks, and possible applications. Swiss Academies Factsheets. 2020;15:4.
- Roberts A, Paes de Andrade P, Okumu F, et al. Results from the workshop “problem formulation for the use of gene drive in mosquitoes. Am J Trop Med Hyg. 2017;96:530–533.
- Hayes KR, Hosack GR, Dana GV, et al. Identifying and detecting potentially adverse ecological outcomes associated with the release of gene-drive modified organisms. J Responsible Innov. 2018;5(sup1):S139–S158.
- Simon S, Otto M, Engelhard M. Synthetic gene drive: between continuity and novelty. EMBO Rep. 2018;19(5):e45760.
- Collins CM, Bonds JA, Quinlan MM, et al. Effects of the removal or reduction in density of the malaria mosquito, Anopheles gambiae s.l., on interacting predators and competitors in local ecosystems. Med Vet Entomol. 2019;33:1–15.
- Rode NO, Estoup A, Bourguet D, et al. Population management using gene drive: molecular design, models of spread dynamics and assessment of ecological risks. Conserv Genet. 2019;20(4):671–690.
- Rode NO, Courtier-Orgogozo V, Débarre F. Can a population targeted by a CRISPR-based homing gene drive be rescued? G3: Genes, Genom Genet. 2020;10:3403–3415.
- Teem JL, Ambali A, Glover B, et al. Problem formulation for gene drive mosquitoes designed to reduce malaria transmission in Africa: results from four regional consultations 2016–2018. Malar J. 2019;18:347.
- Dolezel M, Lüthi C, Gaugitsch H. Beyond limits – the pitfalls of global gene drives for environmental risk assessment in the European Union. BR. 2020;15:1–29.
- Smets G, Rüdelsheim P. Study on risk assessment application of annex I of decision CP 9/13 to living modified organisms containing engineered gene drives, on behalf of the Secretariat of the Convention on Biological Diversity. CBD/CP/RA/AHTEG/2020/1/4. 2020. Available from: https://www.cbd.int/doc/c/f22d/a5d7/850597e99231b7d0dd194c7f/cp-ra-ahteg-2020-01-04-en.pdf.
- Then C, Kawall K, Valenzuela N. Spatio‐temporal controllability and environmental risk assessment of genetically engineered gene drive organisms from the perspective of EU GMO regulation. Integr Environ Assess Manag. 2020;16:555–568.
- Raybould A. Problem formulation and hypothesis testing for environmental risk assessments of genetically modified crops. Environ Biosafety Res. 2006;5:119–125.
- Raybould A. Hypothesis-led ecological risk assessment of GM crops to support decision-making about product use. In Chaurasia A, Hawksworth DL, Pessoa de Miranda M, editors. GMOs. Topics in biodiversity and conservation. Vol 19. Switzerland (Cham): Springer International Publishing; 2020. p. 305–342.
- Wolt JD, Keese P, Raybould A, et al. Problem formulation in the environmental risk assessment for genetically modified plants. Transgenic Res. 2010;19:425–436.
- Devos Y, Craig W, Devlin RH, et al. Using problem formulation for fit‐for‐purpose pre‐market environmental risk assessments of regulated stressors. EFSA J. 2019;17:e170708.
- Adelman Z, Akbari O, Bauer J, et al. Rules of the road for insect gene drive research and testing. Nat Biotechnol. 2017;35:716–718.
- Ad Hoc Technical Expert Group (AHTEG). Report of the Ad Hoc Technical Expert Group. CBD/CP/RA/AHTEG/2020/1/5. 2020. Available from: https://www.cbd.int/doc/c/a763/e248/4fa326e03e3c126b9615e95d/cp-ra-ahteg-2020-01-05-en.pdf.
- Keiper F, Atanassova A. Regulation of synthetic biology: developments under the convention on biological diversity and its protocols. Front Bioeng Biotechnol. 2020;8:310.
- EFSA Panel on Genetically Modified Organisms. Scientific Opinion on the Guidance on the risk assessment of food and feed from genetically modified animals and animal health and welfare aspects. Efsa J. 2012;10:1–43.
- EFSA Panel on Genetically Modified Organisms. Guidance on the environmental risk assessment of genetically modified animals. Efsa J. 2013;11:1–190.
- Devos Y, Gallani B, European Food Safety Authority, et al. Stakeholder workshop “Problem formulation for the environmental risk assessment of gene drive modified insects” (15 May 2019, Brussels). EFSA Support Publ. 2020;17:1–16.
- Devos Y, Bonsall MB, European Food Safety Authority, et al. Outcome of a public consultation on the draft adequacy and sufficiency evaluation of existing EFSA guidelines for the molecular characterisation, environmental risk assessment and post-market environmental monitoring of genetically modified insects containing engineered gene drives. EFSA Support Publ. 2020;17:1–315.
- World Health Organization (WHO). Guidance framework for testing of genetically modified mosquitoes. 2014. Available from: https://www.who.int/tdr/publications/year/2014/guide-fmrk-gm-mosquit/en/.
- Redford KH, Brooks TM, Macfarlane NBW, et al., editors. Genetic frontiers for conservation: an assessment of synthetic biology and biodiversity conservation. Technical assessment. Gland (CH): IUCN; 2019. Available from: https://portals.iucn.org/library/node/48409.
- High-Level African Panel on Emerging Technologies (APET). Gene drives for malaria control and elimination in Africa. 2018. Available from: https://www.nepad.org/publication/gene-drives-malaria-control-and-elimination-africa.
- Devos Y, Bonsall MB, Firbank LG, et al. Gene drive-modified organisms: developing practical risk assessment guidance. Trends Biotechnol. 2020. DOI:https://doi.org/10.1016/j.tibtech.2020.11.015
- Reynolds JL. Governing new biotechnologies for biodiversity conservation: gene drives, international law, and emerging politics. Glob Environ Polit. 2020;20(3):28–48.
- Hokanson KE. When policy meets practice: the dilemma for guidance on risk assessment under the Cartagena Protocol on Biosafety. Front Bioeng Biotechnol. 2019;7:82.
- Connolly JB, Mumford JD, Fuchs S, et al. Systematic identification of plausible pathways to potential harm via problem formulation for investigational releases of a population suppression gene drive to control the human malaria vector Anopheles gambiae in West Africa. Malar J. 2021;20(1):170.
- EFSA Panel on Genetically Modified Organisms. Applicability of the EFSA opinion on site‐directed nucleases type 3 for the safety assessment of plants developed using site‐directed nucleases type 1 and 2 and oligonucleotide‐directed mutagenesis. EFSA J. 2020;18:6299.
- Golnar AJ, Ruell E, Lloyd AL, et al. Embracing dynamic models for gene drive management. Trends Biotechnol. 2021;39:211–214.
- Wu SL, Bennett JB, Sánchez CHM, et al. MGDrivE 2: a simulation framework for gene drive systems incorporating seasonality and epidemiological dynamics. PLoS Comput Biol. 2021;17:e1009030.
- Callaway E. ‘Gene drive’ moratorium shot down at UN biodiversity meeting. Nat Biotechnol. 2016. DOI:https://doi.org/10.1038/nature.2016.21216
- Callaway E. Ban on ‘gene drives’ is back on the UN’s agenda — worrying scientists. Nature. 2018;563:454–455.
- Brown Z. Economic, regulatory and international implications of gene drives in agriculture. Choices. 2017;32:1–8.
- Hartley S, Thizy D, Ledingham K, et al. Knowledge engagement in gene drive research for malaria control. PLoS Negl Trop Dis. 2019;13:e0007233.
- Thizy D, Emerson C, Gibbs J, et al. Guidance on stakeholder engagement practices to inform the development of areawide vector control methods. PLoS Negl Trop Dis. 2019;13:e0007286.
- Kelsey A, Stillinger D, Pham TB, et al. Global governing bodies: a pathway for gene drive governance for vector mosquito control. Am J Trop Med Hyg. 2020;103:976–985.
- Santos MR. Evaluating gene drive approaches for public benefit. In: Chaurasia A, Hawksworth DL, Pessoa de Miranda M, editors. GMOs. Topics in biodiversity and conservation. Vol. 19. Switzerland (Cham): Springer International Publishing; 2020. p. 421–437.
- Warmbrod KL, Kobokovich K, West R, et al. Gene drives: pursuing opportunities, minimizing risk. Baltimore (MD): Johns Hopkins University; 2020. (Johns Hopkins University Report on Responsible Governance (Johns Hopkins Bloomberg School of Public Health, Center for Health Security). Available from: https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2020/200518-Gene-Drives-Report.pdf.
- Annas GJ, Beisel CL, Clement K, et al. A code of ethics for gene drive research. CRISPR J. 2021;4:19–24.