Abstract
Among the many different types of wound dressings, nanofiber-based materials produced through electrospinning are claimed to be ideal because of their advantageous intrinsic properties and the feasibility of employing several strategies to load bioactive compounds into their structure. Bioactive compounds with antimicrobial properties have been incorporated into different wound dressings to promote healing as well as prevent and treat bacterial infections. Among these, natural products, such as medicinal plant extracts and essential oils (EOs), have proven particularly attractive thanks to their nontoxic nature, minor side effects, desirable bioactive properties, and favorable effects on the healing process. To this end, the present review provides an exhaustive and up-to-date revision of the most prominent medicinal plant extracts and EOs with antimicrobial properties that have been incorporated into nanofiber-based wound dressings. The most common methods used for incorporating bioactive compounds into electrospun nanofibers include: pre-electrospinning (blend, encapsulation, coaxial, and emulsion electrospinning), post-electrospinning (physical adsorption, chemical immobilization, and layer-by-layer assembly), and nanoparticle loading. Furthermore, a general overview of the benefits of EOs and medicinal plant extracts is presented, describing their intrinsic properties and biotechniques for their incorporation into wound dressings. Finally, the current challenges and safety issues that need to be adequately clarified and addressed are discussed.
Introduction
The skin is the outer covering and largest organ of the human body that protects the underlying: muscles, bones, ligaments, and internal organs from external threats [Citation1–7]. However, the structure and function of these components can be affected by several injuries which lead to wounding. Wounds can have an accidental or intentional etiology, such as: cuts, burns, and surgical incisions, or arise from diseases, such as diabetes [Citation3,Citation5,Citation6,Citation8–10]. Hence, under certain conditions, when skin integrity is lost, the structural and functional features of the native skin must be rapidly reestablished to ensure hemostasis and minimize the risk of microbial contamination.
Therefore, developing wound dressings that can prevent bacterial penetration into the wound and subsequent infection is imperative. To achieve this, wound dressings exhibiting antimicrobial activities have been fabricated from different materials and of various shapes using several techniques [Citation1,Citation2,Citation4,Citation5,Citation7,Citation9–13]. Among the many available wound dressings (e.g., films, foams, hydrogels, and hydrocolloids), nanofiber membranes have attracted much attention in recent years. In addition, among the different techniques of nanofiber production, such as: drawing, self-assembly, phase separation, and template synthesis, electrospinning has emerged as the ideal modality to overcome the problems of the: high cost, time consumption, and low efficiency of these methods [Citation2,Citation5,Citation9,Citation13–15]. Based on these advantages, nanofibrous materials produced through electrospinning from naturally derived or synthetic biopolymer blends have been designed as promising materials for promoting the wound healing process while conferring protection against bacterial invasion. These effects result from the remarkable properties of these materials, such as: their three-dimensional structure resembling the extracellular matrix (ECM) of the natural skin, their ability to promote cell adhesion and proliferation, and their capability to deliver therapeutic and/or bioactive compounds to the wound site [Citation1–3,Citation5,Citation7,Citation11–13,Citation15–18]. Among these, bioactive compounds obtained from natural sources, such as medicinal plants, have emerged as attractive alternatives owing to their: health benefits, easy availability, inexpensiveness, and safety [Citation1,Citation11,Citation15,Citation16,Citation18,Citation19]. Medicinal plants possess an enormous potential to overcome certain limitations of the currently available therapeutic products, such as the growing concerns regarding antimicrobial resistance and biofilm-associated infections, which constitute serious global public health threats, estimated to cause 10 million deaths per year by 2050 and incur a cumulative healthcare cost of US$100 trillion [Citation11,Citation15,Citation16,Citation18–20]. Moreover, the immense potential of nanoparticles (NPs) synthesized from plant extracts in the treatment of bacterial, fungal, and skin diseases has been demonstrated, and NPs of a wide range of: sizes, shapes, compositions, and physicochemical and bioactive properties can be obtained [Citation21,Citation22]. In addition, plant-based NP synthesis is biocompatible, nontoxic, and cost-effective but time and energy consuming compared with physical and chemical methods [Citation23].
Herein, we present an overview of the most prominent antimicrobial agents that have thus far been incorporated into electrospun nanofiber materials to both prevent wound infection and promote wound healing. In addition, particular attention has been devoted to natural products, such as medicinal plants and essential oils (EOs), as well as NPs synthesized from plant extracts, given the: ability to prevent bacterial penetration and growth into the wound, low propensity to develop bacterial resistance, and capability to accelerate wound healing.
Electrospun nanofibers used as wound dressings
When the skin tissue is disrupted or wounding compromises skin’s cellular integrity, dressing materials are required to act as a temporary barrier for protecting against external threats and simultaneously promote healing of the damaged skin [Citation2,Citation4,Citation5,Citation7,Citation10,Citation13,Citation16]. An ideal wound dressing must fulfill several inherent requirements to successfully support the healing process and reduce the risk of infection. As such, it must be able to absorb and retain fluids; maintain a moist wound environment; ensure gas exchange and nutrient supply; support cell adhesion, migration, growth, and differentiation; and prevent bacterial growth [Citation1,Citation2,Citation4,Citation9,Citation10,Citation12–16]. In addition, wound dressings should be: comfortable and conformable, biocompatible, nontoxic, and non-allergenic [Citation1,Citation2]. To meet these requirements, diverse types of wound dressings, such as: films, foams, hydrogels, hydrocolloids, and micro/nanofiber membranes, have been developed depending on the severity of the wound and stage of the wound healing process [Citation2,Citation5,Citation9,Citation13–15].
For instance, semipermeable films, such as: Opsite®, TegadermTM, Bioclusive®, and DermafilmTM, are thin pellicles that are impermeable to bacteria and are compatible with gas exchange. They allow air permeation and supply oxygen required for cellular respiration [Citation4,Citation5,Citation15,Citation24–26]. However, these dressings can cause exudate accumulation, leading to tissue maceration and/or infection [Citation4,Citation5,Citation10,Citation15]. On the other hand, several solid foams with different capacity for fluids absorption have emerged as a viable option [Citation15,Citation25,Citation26]. These dressing materials are soft and porous and can easily adapt to wounds. In addition, they can: maintain excellent thermal insulation performance, create an appropriate moist environment, and avoid fluid and exudate accumulation at the wound site [Citation5,Citation10,Citation15,Citation25–27]. However, these materials are generally non-adhesive dressings and consequently require secondary dressing [Citation15]. Some foam-based wound dressings available on the market include: Lyofoam®, Tielle® Plus, Biatain® Adhesive, PermaFoam®, and Mepilex® [Citation28,Citation29]. Furthermore, hydrogels—a comprehensive class of dressings—have been widely used because of their capacity to store large amounts of water inside a three-dimensional polymeric network and/or their ability to absorb excessive amounts of exudate [Citation5,Citation15,Citation25–27]. Typically, a hydrogel contains approximately 96% water, which allows for maintaining a moist environment at the wound surface [Citation4,Citation10,Citation15,Citation25–27]. However, hydrogels naturally display a weak capacity for exudate absorption, resulting in fluid accumulation and skin maceration [Citation15,Citation26,Citation27]. Therefore, dehydrated hydrogels with a higher capacity to absorb excessive exudates from the wound site have been considered an alternative for wounds with high exudate levels [Citation10,Citation25]. Hydrogel-based dressings are available in the form of patches (e.g., Aquaflo™ Hydrogel), or as thick and viscous gels (amorphous) (e.g., Nu-Gel™, Intrasite®, and Solosite®) [Citation29]. Amorphous hydrogels exhibit unique features that ensure optimal hydration of dry wounds because of their ability to act as water donors [Citation10,Citation25]. Nonetheless, hydrogels exhibit weak mechanical properties, necessitating the application of secondary dressings [Citation4,Citation5,Citation15]. More recently, hydrocolloids produced from particles uniformly dispersed in an elastic adhesive matrix have been applied. The adhesive matrix maintains the dressing on the wound, and the hydrocolloid particles absorb and retain fluids, creating a hydrogel suitable for reducing the risk of skin maceration [Citation4,Citation15,Citation25,Citation30]. In addition, the produced hydrogels can ensure a favorable moist environment at the wound site. Thus, these dressings do not adhere to the wounds and are painless for patients [Citation15,Citation25,Citation27]. However, hydrocolloidal gels can develop an unpleasant odor and are easily mistaken for infection [Citation4,Citation5,Citation15,Citation25,Citation27]. Commercially available hydrocolloid dressings include: Alione®, Granuflex®, CombiDERM® ACD®, DuoDERM®, Ultec™, Comfeel®, Hydrocoll®, Nu-Derm™, Granugel®, and Versiva® [Citation4,Citation25,Citation29]. Specifically: alginate (e.g., Kaltostat®, Sorbsan®, and Algisite M), chitosan (CS) (e.g., HemCon® Bandage, Syvek-Patch®, Chitopack C®, Chitopack S®, Beschitin®, Chitodine®, and Trauma DEX®), and collagen (e.g., Alloderm®, Integra®, PuraPly®, FIBRACOL™ Plus, Apligraft®, and Orcel®) dressings have become popular because of their intrinsic properties [Citation4,Citation15,Citation25–27,Citation30,Citation31]. Nevertheless, despite the great diversity of dressings currently available on the market, nanofiber wound dressings have shown promising properties for promoting the healing process.
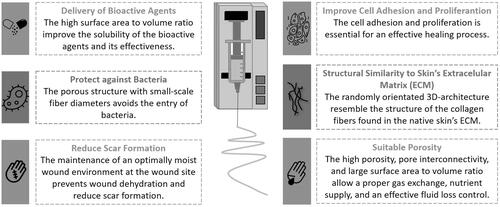
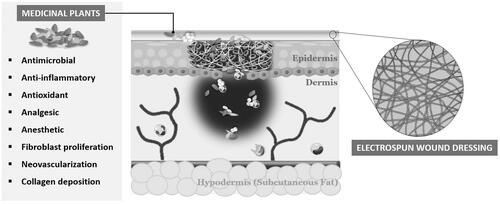
Among the different methods used to fabricate micro-to-nano-scale fibrous wound dressing materials (e.g., phase separation, self-assembly, drawing, and template synthesis), electrospinning has become one of the most desirable and attractive modalities owing to its simplicity, cost-effectiveness, and functional versatility, to produce nanofibrous meshes that can reestablish native skin features () [Citation1–3,Citation11–13,Citation15,Citation16,Citation27,Citation32]. Electrospun nanofibrous membranes can mimic the three-dimensional architecture of the natural skin ECM, which is essential to ensure additional support for cell adhesion and proliferation as well as promote skin regeneration with minimal scarring [Citation1–3,Citation7,Citation11–13,Citation15,Citation16,Citation27,Citation32–35]. Moreover, the inherently high surface-to-volume ratio and porosity of electrospun nanofibers are conducive to hemostasis and allow for: adequate gas permeability, efficient water and nutrient supply, and effective fluid absorption, while maintaining the moisture balance at the wound site [Citation1–3,Citation7,Citation11–13,Citation15,Citation16,Citation27,Citation32–35]. In addition, nonwoven structures are flexible, which ensures their conformability to the wound site and provides an effective physical barrier to protect the wound surface from further injury and bacterial invasion [Citation1,Citation11,Citation36]. Furthermore, several bioactive and/or therapeutic compounds, such as: growth factors, vitamins, analgesics, mineral supplements, antimicrobial, and anti-inflammatory agents, can be efficiently incorporated using different approaches into synthetic and natural polymers and blends; thus, electrospinning is a promising method for controlling drug release and enhancing desirable wound healing properties [Citation2,Citation7,Citation11–13,Citation15,Citation32].
Electrospinning techniques for antimicrobial agent delivery
A wide range of bioactive compounds with antimicrobial activities have been incorporated into the structure of wound dressing materials for preventing bacterial penetration into the wound and simultaneously promote the healing process [Citation2,Citation5,Citation15,Citation16,Citation32,Citation37].
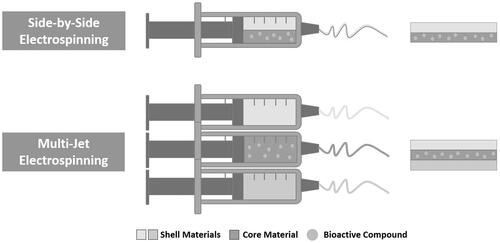
Among the different strategies explored for the incorporation of these compounds into electrospun nanofibrous membranes, pre-spinning methods, such as the blending of polymer solution before spinning and core-shell fibers (from emulsion and coaxial-electrospinning), as well as post-spinning methods, such as: chemical immobilization, physical adsorption, and layer-by-layer (LbL) assembly, have been widely employed to manipulate the release of incorporated compounds from the nanofibers. However, the selection and/or combination of these methods are affected by the roles of bioactive compounds in the healing process as well as by the desirable release rates and profiles [Citation2,Citation11,Citation12,Citation14–16,Citation32,Citation33]. The most common methodologies for the incorporation of these compounds into nanofibers are presented in .
Figure 2. Schematic representation of the most common electrospinning techniques used for the incorporation of bioactive compounds into nanofibers: pre-electrospinning (blend, encapsulation, coaxial, and emulsion electrospinning) and post-electrospinning [physical adsorption, chemical immobilization, and layer-by-layer (LbL) assembly].
![Figure 2. Schematic representation of the most common electrospinning techniques used for the incorporation of bioactive compounds into nanofibers: pre-electrospinning (blend, encapsulation, coaxial, and emulsion electrospinning) and post-electrospinning [physical adsorption, chemical immobilization, and layer-by-layer (LbL) assembly].](/cms/asset/330ee59e-ad78-4c5d-b122-34d0e7de29be/ibty_a_2193859_f0002_b.jpg)
Polymer blend electrospinning
The simplest method to incorporate bioactive compounds into nanofibers is by blending/dispersing them into the polymer solution before electrospinning [Citation13–15,Citation32,Citation38]. However, when this method is applied, the bioactive compounds are immediately released from the nanofiber surface. Moreover, the structural integrity and bioactivity of these compounds can be compromised and potentially harmful to the healing process, particularly when a lasting therapeutic effect on the wound site is desired. Thus, to ensure sustained release and, in particular, to avoid an initial burst effect, it is desirable to absorb and/or encapsulate the bioactive compounds into nanostructures before dispersion in the polymer solution [Citation13,Citation38,Citation39].
Core-shell structures
Another strategy to incorporate bioactive compounds is based on the production of core-shell nanofibers through coaxial electrospinning [Citation13,Citation15,Citation40]. Coaxial electrospinning uses two concentrically arranged capillaries instead of a single capillary, as in conventional electrospinning. These concentric capillaries are connected to two separate reservoirs. The outer capillary is attached to the reservoir containing the shell solution and allows the production of nanofibers that provided protection to the core. Meanwhile, the inner capillary is connected to the reservoir containing the core solution, and this is where the bioactive compounds are generally incorporated [Citation15,Citation32,Citation38,Citation40,Citation41]. Hence, core-shell nanofibers provide an added advantage as carriers for: the delivery of bioactive compounds, protecting the native structure of these compounds and their bioactivity from harsh environments during nanofiber fabrication. In addition, the incorporation of bioactive compounds into the core of nanofibers can significantly decrease the initial burst effect and maintain a sustained release, and the nanofiber shell can act as a barrier to the diffusion of incorporated compounds [Citation14,Citation15,Citation38,Citation40,Citation41]. However, although two different and incompatible polymer solutions can be simultaneously applied through coaxial electrospinning, the technique requires a special apparatus and careful selection of operating conditions to ensure desirable results [Citation42,Citation43]. To overcome the limitations associated with coaxial electrospinning, emulsion electrospinning has attracted growing interest for the production of core-shell nanofibers in recent years. This method is similar to conventional electrospinning except that the solution is replaced with a water-in-oil (W/O) or oil-in-water (O/W) emulsion [Citation13,Citation15,Citation32,Citation43–45]. During emulsion electrospinning, the continuous-phase solvent evaporates more swiftly than the dispersed droplets, resulting in a viscosity gradient between the two phases. Subsequently, this gradient guides the emulsion droplets from the surface to the center, and the droplets are stretched into elliptical shapes along the axial region of the fibers under a high voltage [Citation46,Citation47]. Most emulsions used in electrospinning are of the W/O type. These emulsions comprise an aqueous phase composed mainly of water-soluble polymers or bioactive compounds dissolved in an aqueous solution, whereas the oil phase is composed of polymers dissolved in an organic solvent [Citation15,Citation42]. Hence, W/O emulsions are particularly useful for controlled or sustained release of water-soluble bioactive compounds. This is because the compounds encapsulated in the core of the nanofiber structure must pass through the core-shell matrix before being released [Citation43,Citation45]. Furthermore, the core-shell structure of nanofibers produced through emulsion electrospinning may: improve their poor solubility, enhance the affinity of hydrophilic compounds to hydrophobic polymers, and protect bioactive compounds from the harmful effects of the external environment [Citation43,Citation45].
Post-electrospinning treatments
Bioactive compounds can be physically adsorbed on the surface of nanofibers following electrospinning, given that most of these compounds bear functional groups that facilitate their attachment to nanofibers [Citation15,Citation32,Citation38,Citation48]. Generally, when these compounds are adsorbed on the nanofiber surface via physical forces, weak nonspecific intermolecular interactions, such as: electrostatic interactions, hydrogen bonding, hydrophobic interactions, and van der Waals forces, are established between the compounds and electrospun nanofibers [Citation32,Citation48]. Alternatively, these compounds can be covalently immobilized onto the surface of nanofibers using the wet chemical method, plasma treatment, and graft polymerization to obtain more consistent and potent bioactivity [Citation38,Citation39,Citation48]. In this approach, the surface of the nanofibers can be modified using various treatments, and functional groups can be added to their surfaces. Moreover, recently, LbL assembly has been used as a simple, useful, and versatile surface modification method, which allows the formation of surface coatings via successive deposition of polymer layers of opposite charges [Citation32,Citation48]. This alternating deposition of polymers using the LbL technique allows effortless incorporation of bioactive compounds along with the multilayer assembly and accurate control of the desired thickness, which can affect the release profile of these compounds. Electrostatic interactions are the major driving force of assembly [Citation32,Citation48].
In recent years, different electrospinning methods have been developed to provide specific release profiles of bioactive compounds. Among these, two needles placed side-by-side have been applied to produce nanofibers with Janus structures and materials with two distinct layers [Citation13,Citation49]. Meanwhile, multiple-jet electrospinning has been used to simultaneously produce multiple nanofibers, and a modified coaxial electrospinning technique using multiple concentric needles has been explored to ensure sustained release of the incorporated bioactive compounds () [Citation13,Citation49,Citation50].
Incorporation of natural plant extracts with antimicrobial properties into electrospun materials
As previously described, several bioactive compounds have been incorporated into electrospun wound dressings to improve their biological performance [Citation2,Citation6,Citation15,Citation32]. Among these, antimicrobial agents, such as: antibiotics (e.g., gentamicin, tetracycline hydrochloride, ciprofloxacin, and silver sulfadiazine), NPs [e.g., metallic NPs (silver, zinc oxide, titanium dioxide, iron oxide, and copper)], and natural products (e.g., medicinal plant extracts and EOs), have been used to prevent infections at the wound site and further promote the healing process [Citation2,Citation5,Citation6,Citation11,Citation18,Citation32].
Although many studies have reported the beneficial effects of these bioactive agents, natural product-based compounds, particularly plant-derived compounds, have emerged as promising approaches to develop new therapeutic alternatives that can provide effective antimicrobial activity and prevent deleterious effects of infections on the healing process [Citation1,Citation5,Citation11,Citation15,Citation16,Citation18,Citation36,Citation37,Citation51].
Plant extracts
The growing trends of using alternative and complementary medicines to explore innovative and effective wound healing therapies as well as the recognition and awareness of the advantages and feasibility of using medicinal plants as powerful natural supplements for wound management and treatment have garnered much attention from the research community working in the fabrication of wound dressings with antimicrobial properties [Citation11,Citation15,Citation18,Citation51].
Throughout history, medicinal plants have been explored and recognized as a major source of therapeutic agents for improving wound healing. Medicinal plants contain a wide variety of biologically active and effective components, such as: flavonoids, alkaloids, terpenoids, phenolics, fatty acids, and EOs, which confer: antimicrobial, anti-inflammatory, antioxidant, analgesic, anesthetic, antiviral, and anticancer effects [Citation15,Citation18,Citation51]. In addition, medicinal plants promote the wound-healing process by enhancing: fibroblast proliferation, neovascularization, and collagen deposition [Citation15,Citation18,Citation51]. Moreover, these natural healing agents have been proven an interesting approach to overcome certain limitations of various current bioactive compounds incorporated into wound dressing materials owing to their low cost; environmental sustainability; limited adverse effects; and easy availability, extraction, and efficacy [Citation1,Citation11,Citation15,Citation16,Citation51]. In recent years, crude medicinal plant extracts and their derivatives, such as EOs, have gained great interest, achieving higher therapeutic potential for preventing infections and treating infected wounds [Citation11,Citation15,Citation18,Citation51]. Several plant-derived natural compounds with bactericidal activity have been incorporated into wound dressing materials, and a few of these, including the Gentell® Hydrogel Aloe vera wound dressing and Curcuma longa L. (turmeric)-based bandage (patents) used by Johnson &Johnson in Band-Aid®, have been approved by the United States Food and Drug Administration (US FDA) and other drug regulatory agencies [Citation36,Citation52,Citation53]. Likewise, a number of studies have been performed integrating the unique features of electrospun nanofibers with single- and multi-layered structures and the benefits of natural bioactive compounds for effective wound healing () [Citation1,Citation11,Citation15,Citation18,Citation51].
Crude medicinal plant extracts
Crude medicinal plant extracts are ecologically sustainable mixtures of compounds with multiple therapeutic properties, which support their potential role in wound healing [Citation11,Citation15,Citation18,Citation51]. These extracts can be obtained from fresh or milled, dried plants and can be easily extracted using various methods [Citation11,Citation18]. In addition, crude plant extracts produce stronger effects than specific compounds isolated from the same plants owing to the promising synergistic interactions between their bioactive components [Citation54]. These favorable interactions provide better protection against enzymatic degradation and promote transport through cell barriers. Typically, crude plant extracts are considered a promising approach to overcome multi-drug resistance in pathogenic bacteria [Citation15,Citation18,Citation51,Citation54].
The antimicrobial properties of crude medicinal plant extracts are mainly attributed to their active metabolites, which can alter the permeability of bacterial membranes, leading to cell wall disruption and lysis. Moreover, plant extracts can interfere with fundamental cellular processes and metabolic pathways [Citation55,Citation56].
Crude extracts with a high antibacterial activity of several medicinal plants have been applied as electrospun dressing materials using different strategies. Among these: Aloe vera, Azadirachta indica, Calendula officinalis, Centella asiatica, Chamomilla recutita, Curcuma longa, Garcinia mangostana, Lawsonia inermis, and Tridax procumbens, are commonly used for the production of bioactive plant-based wound dressings [Citation1,Citation5,Citation15,Citation18,Citation36,Citation51,Citation57].
For instance, Yao et al. [Citation58] produced gelatin/polyvinyl alcohol (PVA) nanofibers containing Centella asiatica extract for application as a dressing material to treat skin wounds. The incorporation of Electrospun gelatin/PVA nanofibers incorporated with Centella asiatica extract significantly increased cell growth and proliferation as well as enhanced antibacterial activity compared with neat gelatin/PVA membranes. Moreover, in vivo assays revealed that after 7 days of treatment, animals treated with gelatin/PVA membranes containing Centella asiatica extract displayed a slightly higher wound recovery rate and as collagen synthesis rate than those treated with neat gelatin/PVA membranes, gauze (control), and a commercial wound dressing (Comfeel®, Peterborough, United Kingdom). Further histopathological assessments supported the results of the animal experimentation [Citation58].
In addition, Pourhojat et al. [Citation59] assessed the performance of the electrospun polylactic-co-glycolic acid (PLGA) nanofibers containing Hypericum perforatum as antimicrobial coverage for wounds. The developed membranes better controlled the water vapor transmission rate (WVTR) and presented a greater exudate absorption capacity and higher burst drug release kinetics, which followed the Higuchi kinetic model. Moreover, in vitro evaluation of the biological properties of the membranes revealed their capacity to inhibit the growth of bacteria at the wound site as well as their biocompatibility [Citation59]. Shokrollahi et al. [Citation60] developed patches based on Chamomilla recutita L. (chamomile)-loaded carboxyethyl chitosan (CECS)/PVA and polycaprolactone (PCL). Compared with the commercial Ag coating, the developed multilayered nanofibrous patches exhibited: increased antibacterial efficiency, antioxidant activity, and biocompatibility and enhanced cell viability with increasing culture time [Citation60].
Recently, Mouro et al. [Citation61] incorporated Chelidonium majus L., a medicinal plant known to display a broad spectrum of pharmacological activities, into electrospun nanofibers composed of PCL, PVA, and pectin (PEC) using emulsion electrospinning. The developed membranes exhibited suitable characteristics for application as wound dressings. As such, they presented enhanced bactericidal activity against Staphylococcus aureus and Pseudomonas aeruginosa and did not cause cytotoxicity in normal human dermal fibroblasts (NHDFs).
Various medicinal plant extracts with antibacterial activity that have been incorporated in electrospun wound dressings to prevent bacterial infections and enhance healing are listed in .
Table 1. Examples of plant extracts that have been incorporated into electrospun wound dressing materials.
Essential oils
EOs are secondary metabolites typically extracted from aromatic plants, and they exhibit a wide range of therapeutic properties, including: antimicrobial, antioxidant, anti-inflammatory, anti-allergic, anticancer, and antiviral properties, as well as repellent effects [Citation5,Citation9,Citation18,Citation37,Citation51]. However, these volatile natural mixtures require multi-step preparation methods and special laboratory facilities as well as a large amount of raw material. In addition, their effectiveness is limited by the fact that EOs are easily degraded and more susceptible to losses by volatilization or thermal decomposition [Citation11,Citation104,Citation105].
The antimicrobial activity of EOs is attributed to their phenolic compounds, which are typically hydrophobic. In this context, the mechanisms of action are based on their partitioning into the phospholipid bilayer present in the bacterial cell membrane and the lipids on the cell wall. The establishment of such different interactions increases membrane permeability to ions and other cellular contents, causing cytoplasmic leakage and pH decrease and inhibiting vital cellular processes, such as ATP biosynthesis, DNA transcription, and protein synthesis, which ultimately lead to the disruption of cell structure and cell death. Moreover, EOs can interfere with the function of the cytoplasmic membrane by blocking the transfer of nutrients through the cell membrane and coagulation of bacterial cell constituents [Citation4,Citation5,Citation9,Citation18,Citation37].
Among the known EOs, cinnamon, lavender, olive, peppermint, tea tree, and thymol (THY) oils have been used in electrospun materials for antibacterial purposes () [Citation5,Citation15,Citation18,Citation37,Citation118].
Table 2. Examples of electrospun wound dressing materials incorporated with essential oils (Eos).
For instance, Hajiali et al. [Citation119] produced alginate–lavender nanofibers for burn management. In both in vitro and in vivo assays, the nanofiber dressings of sodium alginate and lavender oil exhibited promising antibacterial and anti-inflammatory activities, highlighting their potential for improving the burn-healing process. Furthermore, Gámez-Herrera et al. [Citation120] electrosprayed THY-loaded PLGA microparticles onto electrospun PCL-based nanofibers. The dressings successfully inhibited Staphylococcus aureus growth but did not compromise cell viability. In addition, when infected wounds inoculated with Staphylococcus aureus were treated with the dressings, these materials could minimize the growth of bacteria at the wound site [Citation120]. In a recent study, Zare et al. [Citation121] prepared core-shell nanofibers composed of gelatin/PVA/Trachyspermum ammi (ajwain) EO (core) and Aloe vera/arabinose/polyvinylpyrrolidone (PVP) (shell) using coaxial electrospinning. The produced membranes exhibited excellent antioxidant and antimicrobial properties through prolonged release of ajwain EO and accelerated bacteria-induced wound healing [Citation121].
Plant-based nanoparticles
NPs, particularly metallic NPs (silver, zinc oxide, titanium dioxide, iron oxide, and copper), have attracted much attention for wound healing applications because of their ability to act as antimicrobial agents and nanocarriers to deliver different bioactive agents [Citation9,Citation32,Citation51]. Among these, particular emphasis has been offered to Ag NPs, since the positive charges on Ag+ ions released from Ag NPs can bind to the negative charges on the bacterial cell wall, resulting in the destabilization or disruption of cell wall membranes and subsequent penetration inside the cells, which ultimately leads to the leakage of intracellular constituents and cell death [Citation5,Citation37,Citation122].
The antimicrobial efficacy of Ag NPs and their great potential to overcome the emerging issues caused by antibiotic-resistant bacteria have encouraged many studies, and the utility of some Ag NP-based products as alternatives to antibiotics has already been demonstrated [Citation15,Citation51,Citation122]. To date, several clinical studies on Ag-containing dressings have been conducted, and some US FDA-approved biocomposites modified with Ag NPs are already available on the market for wound dressing applications [Citation5,Citation122]. For instance, Acticoat™, Bactigras™ (Smith & Nephew), Aquacel™ (ConvaTec), PolyMem Silver™ (Aspen), and Tegaderm™ (3 M) are promising therapeutic alternatives to conventional antibiotics [Citation5,Citation122,Citation123].
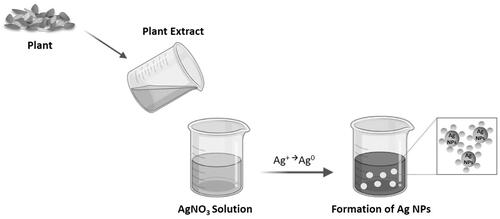
However, Ag NP production requires chemical or physicochemical synthetic processes, which are environmentally unfriendly, hazardous to the human body, and require time-consuming protocols [Citation51,Citation122,Citation124]. Thus, to overcome these limitations and to efficiently and safely prepare Ag NPs, biological synthesis approaches, or the so-called “green” methods, based on the use of: bacteria, fungi, algae, and plants, are being explored due to their high: biocompatibility, biodegradability, nontoxicity, cost-effectiveness, and green nature [Citation51,Citation122,Citation124]. As such, these compounds act as reducing agents of Ag+ ions to produce Ag NPs () [Citation51].
Among the different agents explored to date for the synthesis of Ag NPs, plant extracts have gained significant attention owing to their: eco-friendly nature, easy availability, low cost, execution simplicity, sustainability, and potential for large-scale production [Citation51,Citation124]. In addition, plant extracts are rich in phytochemical constituents and medicinal properties, rendering them suitable for promoting wound healing and preventing infections [Citation125].
Paul et al. [Citation126] prepared Ag NPs through green synthesis from Pongamia pinnata seed extract. The developed Ag NPs exhibited bactericidal activity against Staphylococcus aureus, Escherichia coli, and Bacillus subtilis as well as antioxidant and wound healing properties suitable for the treatment of wound infections. Moreover, Zangeneh et al. [Citation127] proposed an eco-friendly and useful method to synthesize Ag NPs from Stachys lavandulifolia flower extract. The Ag NPs produced using this protocol exhibited higher antibacterial activity than all standard antibiotics as well as produced excellent antioxidant and cutaneous wound healing effects.
In addition, Ag NPs synthesized from plant extracts have been successfully incorporated into electrospun nanofibers for wound healing. Augustine et al. [Citation23,Citation128] incorporated Ag NPs synthesized from Piper nigrum leaf extract into electrospun PCL membranes. The produced membranes controlled the invasion and colonization of pathogens at the wound site, and the biosynthesized Ag NPs showed superior biocompatibility to chemically synthesized NPs [Citation23,Citation128]. Moreover, in another study, Augustine et al. [Citation129] produced electrospun PVA membranes containing biosynthesized Ag NPs from Mimosa pudica and demonstrated their suitability for application as antimicrobial wound dressings [Citation129].
Furthermore, Kohsari et al. [Citation130] developed electrospun CS/polyethylene oxide (PEO) nanofiber mats containing Ag NPs synthesized from Filipendula vulgaris extract. The fabricated nanofiber mats inhibited the growth of both Staphylococcus aureus and Escherichia coli and exhibited suitable properties to ensure effective wound healing [Citation130]. Furthermore, Alippilakkotte et al. [Citation131] fabricated poly(lactic acid) (PLA)/Ag NPs nanofibers using a green synthesis method with Momordica charantia fruit extract and assessed their antibacterial activity against Staphylococcus aureus and Escherichia coli using the agar disk diffusion method. The PLA/Ag NPs nanofibers exhibited potent antibacterial properties without inducing any cytotoxic effects on fibroblasts [Citation131].
In addition to extensive research into Ag NPs biosynthesized from plant extracts, some studies have reported similar therapeutic properties of other metallic NPs from plant extracts [Citation21,Citation132–134]. For instance, Zhao et al. [Citation132] reported that Cu NPs synthesized from Allium eriophyllum Boiss leaf aqueous extract exhibited antioxidant, antibacterial, and antifungal properties as well as wound-healing potential. Moreover, Seydi et al. [Citation133] synthesized Ti NPs using green synthesis from Ziziphora clinopodioides Lam leaves. The obtained NPs were non-cytotoxic and showed: antioxidant, antibacterial, antifungal, and cutaneous wound healing effects [Citation133]. In another study, Zangeneh et al. [Citation21] explored the beneficial effects of Fe NPs synthesized from Allium eriophyllum Boiss extract on wound healing and demonstrated their antibacterial, antioxidant, and antifungal properties. In addition, Hui et al. [Citation134] prepared Au NPs and combined them with tetracycline [Citation134], which could be used to treat skin wound infections.
Therefore, these findings attest to the intrinsic bioactive properties of metallic NPs and the feasibility of incorporating them into electrospun nanofibers for antimicrobial wound-dressing applications. For instance, zinc oxide NPs synthesized from Ilex paraguariensis leaf extract were successfully loaded into electrospun polyacrylic acid (PAA)/polyallylamine hydrochloride (PAH) fiber mats, which were proven to be effective as wound dressing materials because of their antimicrobial properties and resemblance to the skin ECM [Citation135].
Conclusions
Despite the efforts devoted, the susceptibility of wounds to bacterial growth and subsequent infection risk remains a concern, particularly among people with weakened immune systems. To date, numerous studies have been conducted to develop different types of dressing materials that can act as a physical barrier to protect the wound from microbial invasion and simultaneously boost the healing process. Among the available wound dressings, nanofibers produced through electrospinning—simple, cost-effective, versatile, and easy scale-up technique—are one of the most efficient materials that can reproduce the native three dimensional structure of the skin ECM, and further enhance healing. Thus, electrospun nanofibers represent a biocompatible and biodegradable option with unique properties that fulfill the requirements of an ideal wound dressing materials. In addition, the plausibility of incorporating several bioactive compounds, particularly: eco-friendly, inexpensive, nontoxic, and efficient alternatives against drug-resistant bacteria, such as natural agents (e.g., plant extracts and EOs), into electrospun nanofibers using diverse strategies (e.g., blend, co-axial, emulsion, LbL, and multi-jet electrospinning) highlights their potential to both prevent bacterial colonization at the wound site and promote the reestablishment of the structural and functional integrity of the damaged skin.
Nevertheless, despite the efforts devoted to date, several challenges in the application of electrospun nanofibers for wound dressing remain to be addressed. For instance, some of the available materials cannot precisely reproduce the structure and function of the native skin. Therefore, the orientation of the nanofibers should be optimized and their arrangement and porosity controlled to improve performance. Likewise, different techniques should be integrated and diverse natural antimicrobial agents should be loaded into electrospun nanofibers to control their release profiles for specific applications.
Additionally, in the near future, the incorporation of sensors in electrospun nanofiber materials containing natural bioactive agents must be explored to successfully control their release from nanofibers as well as detect bacteria at the wound site, avoid bacterial growth in wounds, and ensure a noninfectious healing environment.
Finally, efforts are warranted to transfer electrospun wound dressing materials containing natural products from the laboratory and clinical scale to the industrial and even commercial scale. For this purpose, additional assays and validation processes are essential to ensure the quality, safety, and functional performance of these dressing materials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data that support the findings of this study are included in the article.
Additional information
Funding
References
- Adamu BF, Gao J, Jhatial AK, et al. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater Des. 2021;209:109942.
- Azimi B, Maleki H, Zavagna L, et al. Bio-based electrospun fibers for wound healing. J Func Biomater. 2020;11:67.
- Juncos Bombin AD, Dunne NJ, McCarthy HO. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C. 2020;114:110994.
- Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23:2392.
- Simões D, Miguel SP, Ribeiro MP, et al. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130–141.
- Sousa Coelho D, Veleirinho B, Alberti T, et al. Electrospinning technology: designing nanofibers toward wound healing application. In Nanomaterials – toxicity, human health and environment. London: IntechOpen; 2018. p. 1–19.
- Wang F, Hu S, Jia Q, et al. Advances in electrospinning of natural biomaterials for wound dressing. J Nanomater. 2020;2020:1–14.
- Chouhan D, Dey N, Bhardwaj N, et al. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials. 2019;216:119267.
- Mihai MM, Dima MB, Dima B, et al. Nanomaterials for wound healing and infection control. Materials. 2019;12:2176.
- Sarheed O, Ahmed A, Shouqair D, et al. Antimicrobial dressings for improving wound healing. In Wound healing – new insights into ancient challenges. London: IntechOpen; 2016. p. 373–398.
- Fatehi P, Abbasi M. Medicinal plants used in wound dressings made of electrospun nanofibers. J Tissue Eng Regen Med. 2020;14:1527–1548.
- Jeckson TA, Neo YP, Sisinthy SP, et al. Delivery of therapeutics from layer-by-layer electrospun nanofiber matrix for wound healing: an update. J Pharm Sci. 2021;110:635–653.
- Liu X, Xu H, Zhang M, et al. Electrospun medicated nanofibers for wound healing: review. Membranes. 2021;11:770.
- Ambekar RS, Kandasubramanian B. Advancements in nanofibers for wound dressing: a review. Eur Polym J. 2019;117:304–336.
- Pilehvar-Soltanahmadi Y, Dadashpour M, Mohajeri A, et al. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini-Rev Med Chem. 2018;18:414–427.
- Croitoru AM, Ficai D, Ficai A, et al. Nanostructured fibers containing natural or synthetic bioactive compounds in wound dressing applications. Materials. 2020;13:2407.
- Gizaw M, Thompson J, Faglie A, et al. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering. 2018;5:9–28.
- Zhang W, Ronca S, Mele E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials. 2017;7:42–17.
- Mulat M, Pandita A, Khan F. Medicinal plant compounds for combating the multi-drug resistant pathogenic bacteria: a review. Curr Pharm Biotechnol. 2019;20:183–196.
- O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. 2016; [cited 2022 February 28]. Available from: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
- Zangeneh MM, Zangeneh A. Biosynthesis of iron nanoparticles using Allium eriophyllum Boiss extract: chemical characterization, antioxidant, cytotoxicity, antibacterial, antifungal, and cutaneous wound healing effects. Appl Organomet Chem. 2020;34:e5304.
- Zangeneh MM. Green synthesis and chemical characterization of silver nanoparticles from aqueous extract of Falcaria vulgaris leaves and assessment of their cytotoxicity and antioxidant, antibacterial, antifungal and cutaneous wound healing properties. Appl Organomet Chem. 2019;33:e4963.
- Veeraraghavan VP, Periadurai ND, Karunakaran T, et al. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929). Saudi J Biol Sci. 2021;28:3633–3640.
- Ding J, Zhang J, Li J, et al. Electrospun polymer biomaterials. Prog Polym Sci. 2019;90:1–34.
- Qin Y. Medical textile materials. Amsterdam: Elsevier Inc.; 2015.
- Rajendran S. Advanced textiles for wound care. Sawston (UK): Advanced Textiles for Wound Care; 2009.
- Abrigo M, McArthur SL, Kingshott P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol. Biosci. 2014;14:772–792.
- Echague CG, Hair PS, Cunnion KM. A comparison of antibacterial activity against Methicillin-Resistant Staphylococcus aureus and gram-negative organisms for antimicrobial compounds in a unique composite wound dressing. Adv Skin Wound Care. 2010;23:406–413.
- Kadunc B, Palermo E, Addor F, et al. Tratado de cirurgia dermatológica, cosmiatria e laser: da sociedade Brasileira de dermatologia. Amsterdam: Elsevier Health Sciences; 2013.
- Vasconcelos A, Cavaco-Paulo A. Wound dressings for a proteolytic-rich environment. Appl Microbiol Biotechnol. 2011;90:445–460.
- Jayakumar R, Prabaharan M, Sudheesh Kumar PT, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337.
- Miguel SP, Figueira DR, Simões D, et al. Electrospun polymeric nanofibres as wound dressings: a review. Colloid Surf B. 2018;169:60–71.
- Goh YF, Shakir I, Hussain R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J Mater Sci. 2013;48:3027–3054.
- Sell SA, Wolfe PS, Garg K, et al. The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers. 2010;2:522–553.
- Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomed. 2013;8:2997–3017.
- Bhullar SK, Buttar HS. Perspectives on nanofiber dressings for the localized delivery of botanical remedies in wound healing. AIMS Mater Sci. 2017;4:370–382.
- Mohammadi MA, Rostami M, Beikzadeh S, et al. Electrospun nanofibers as advanced antibacterial platforms: a review of recent studies. Int J Pharm Sci. 2018;10:463–473.
- Gao Y, Bach Truong Y, Zhu Y, et al. Electrospun antibacterial nanofibers: production, activity, and in vivo applications. J Appl Polym Sci. 2014;131:40797.
- Wade RJ, Burdick JA. Advances in nanofibrous scaffolds for biomedical applications: from electrospinning to self-assembly. Nano Today. 2014;9:722–742.
- Kriegel C, Arrechi A, Kit K, et al. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci Nutr. 2008;48:775–797.
- Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2018;11:1165–1188.
- Hu J, Wei J, Liu W, et al. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a drug delivery system by emulsion electrospinning. J Biomater Sci – Polym Ed. 2013;24:972–985.
- Wang X, Yuan Y, Huang X, et al. Controlled release of protein from core-shell nanofibers prepared by emulsion electrospinning based on green chemical. J Appl Polym Sci. 2015;132:1–9.
- Hu J, Prabhakaran MP, Ding X, et al. Emulsion electrospinning of polycaprolactone: influence of surfactant type towards the scaffold properties. J Biomater Sci – Polym Ed. 2015;26:57–75.
- Viry L, Moulton SE, Romeo T, et al. Emulsion-coaxial electrospinning: designing novel architectures for sustained release of highly soluble low molecular weight drugs. J Mater Chem 2012;22:11347–11353.
- Shahriar SMS, Mondal J, Hasan MN, et al. Electrospinning nanofibers for therapeutics delivery. Nanomaterials. 2019;9:532.
- Zhang C, Feng F, Zhang H. Emulsion electrospinning: fundamentals, food applications and prospects. Trends Food Sci Technol. 2018;80:175–186.
- Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033–1042.
- Teixeira MA, Amorim MTP, Felgueiras HP. Poly(vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers. 2019;12:7.
- Li Y, Zhu J, Cheng H, et al. Developments of advanced electrospinning techniques: a critical review. Adv Materials Technologies. 2021;6:2100410.
- Hajialyani M, Tewari D, Sobarzo-Sánchez E, et al. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomed. 2018;13:5023–5043.
- Agarwal R, Sarwar Alam M, Gupta B. Preparation of curcumin loaded poly(Vinyl Alcohol)-poly(Ethylene Oxide)-carboxymethyl cellulose membranes for wound care application. J Biomat Tissue Engng. 2013;3:273–283.
- Gupta B, Agarwal R, Sarwar Alam M. Aloe vera loaded poly(vinyl alcohol)-poly(ethylene oxide)-carboxymethyl cellulose-polyester nonwoven membranes. J Biomat Tissue Engng. 2013;3:503–511.
- El-Hamid MIA. A new promising target for plant extracts: inhibition of bacterial quorum sensing. J Mol Biol Biotech. 2016;1:1.
- Gonelimali FD, Lin J, Miao W, et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018;9:1639.
- Omojate GC, Enwa FO, Jewo AO, et al. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens – a review. J Pharm Chem Biol Sci. 2014;2:77–85.
- Firdous SM, Sautya D. Medicinal plants with wound healing potential. Bangladesh J Pharmacol. 2018;13:41–52.
- Yao CH, Yeh JY, Chen YS, et al. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J Tissue Eng Regen Med. 2017;11:905–915.
- Faegheh P, Shahab S, Mahmoodreza S, et al. Preparation of antibacterial electrospun Poly lactic-co–glycolic acid nanofibers containing Hypericum Perforatum with bedsore healing property and evaluation of its drug release performance. Int J Nano Dimen. 2018;9:286–297.
- Shokrollahi M, Bahrami SH, Nazarpak MH, et al. Multilayer nanofibrous patch comprising chamomile loaded carboxyethyl chitosan/poly(vinyl alcohol) and polycaprolactone as a potential wound dressing. Int J Biol Macromol. 2020;147:547–559.
- Mouro C, Gomes AP, Ahonen M, et al. Chelidonium majus L. incorporated emulsion electrospun PCL/PVA_PEC nanofibrous meshes for antibacterial wound dressing applications. Nanomaterials. 2021;11:1785.
- Ribeiro AS, Costa SM, Ferreira DP, et al. Chitosan/nanocellulose electrospun fibers with enhanced antibacterial and antifungal activity for wound dressing applications. React Funct Polym. 2021;159:104808.
- Jenifer P, Kalachaveedu M, Viswanathan A, et al. Fabricated approach for an effective wound dressing material based on a natural gum impregnated with Acalypha indica extract. J Bioact Compat Polym. 2018;33:612–628.
- Suryamathi M, Viswanathamurthi P, Seedevi P. Herbal plant leaf extracts immobilized PCL nanofibrous mats as skin-inspired anti-infection wound healing material. Regen Eng Transl Med. 2021;8(1):9.
- Mouro C, Dunne CP, Gouveia IC. Designing new antibacterial wound dressings: development of a dual layer cotton material coated with poly(vinyl alcohol)_chitosan nanofibers incorporating Agrimonia eupatoria L. Extract Mol. 2020;26:83.
- Aghamohamadi N, Sanjani NS, Majidi RF, et al. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater Sci Eng C. 2019;94:445–452.
- Miguel SP, Ribeiro MP, Coutinho P, et al. Electrospun polycaprolactone/Aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers. 2017;9:183.
- Aruan NM, Sriyanti I, Edikresnha D, et al. Polyvinyl alcohol/soursop leaves extract composite nanofibers synthesized using electrospinning technique and their potential as antibacterial wound dressing. Procedia Eng. 2017;170:31–35.
- Mirbehbahani FS, Hejazi F, Najmoddin N, et al. Artemisia annua L. as a promising medicinal plant for powerful wound healing applications. Prog Biomater. 2020;9:139–151.
- Ali A, Shahid MA, Hossain MD, et al. Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int J Biol Macromol. 2019;138:13–20.
- Pedram Rad Z, Mokhtari J, Abbasi M. Preparation and characterization of Calendula officinalis-loaded PCL/gum arabic nanocomposite scaffolds for wound healing applications. Iran Polym J. 2019;28:51–63.
- Kharat Z, Amiri Goushki M, Sarvian N, et al. Chitosan/PEO nanofibers containing Calendula officinalis extract: preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int J Pharm. 2021;609:121132.
- Sadri M, Arab-Sorkhi S, Vatani H, et al. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015;16:1742–1750.
- Zhu P, Zhang X, Wang Y, et al. Electrospun polylactic acid nanofiber membranes containing Capparis spinosa L. extracts for potential wound dressing applications. J Appl Polym Sci. 2021;138:50800.
- Mouro C, Fangueiro R, Gouveia IC. Preparation and Characterization of Electrospun Double-layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.). Polymers. 2020;12:2653–2618.
- Motealleh B, Zahedi P, Rezaeian I, et al. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ɛ-caprolactone)/polystyrene blends. J. Biomed. Mater. Res. 2014;102:977–987.
- Sriyanti I, Marlina L, Jauhari J. Optimization of the electrospinning process for preparation of nanofibers from poly (vinyl alcohol) (pva) and Chromolaena odorata L. extract (COE). J Pendidik Fis Indones. 2020;16:47–56.
- Ravichandran S, Radhakrishnan J, Jayabal P, et al. Antibacterial screening studies of electrospun Polycaprolactone nano fibrous mat containing Clerodendrum phlomidis leaves extract. Appl Surf Sci. 2019;484:676–687.
- Bui HT, Chung OH, Dela Cruz J, et al. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol. Res. 2014;22:1288–1296.
- Nourmohammadi J, Hadidi M, Nazarpak MH, et al. Physicochemical and antibacterial characterization of nanofibrous wound dressing from silk fibroin-polyvinyl alcohol- Elaeagnus angustifolia extract. Fibers Polym. 2020;21:456–464.
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, et al. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm. 2013;452:333–343.
- Suwantong O, Pankongadisak P, Deachathai S, et al. Electrospun poly(l-lactic acid) fiber mats containing crude Garcinia mangostana extracts for use as wound dressings. Polym. Bull. 2014;71:925–949.
- Amina M, Al-Youssef HM, Amna T, et al. Poly(urethane)/G. mollis composite nanofibers for biomedical applications. J Nanoengng Nanomfg. 2012;2:85–90.
- Ramalingam R, Dhand C, Leung CM, et al. Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater Sci Eng C. 2019;98:503–514.
- Akşit NN, Gürdap S, İşoğlu SD, et al. Preparation of antibacterial electrospun poly(D, L-lactide-co-glycolide)/gelatin blend membranes containing Hypericum capitatum var. capitatum. Int J Polym Mater Polym Biomat. 2021;70:797–809.
- Mouro C, Gomes AP, Gouveia IC. Double-layer PLLA/PEO_Chitosan nanofibrous mats containing Hypericum perforatum L. as an effective approach for wound treatment. Polym Adv Technol. 2021;32:1493–1506.
- Eakwaropas P, Ngawhirunpat T, Rojanarata T, et al. Fabrication of electrospun hydrogels loaded with Ipomoea pes-caprae (L.) R. Br extract for infected wound. J Drug Deliv Sci Technol. 2020;55:101478.
- Kim JH, Lee H, Jatoi AW, et al. Juniperus chinensis extracts loaded PVA nanofiber: enhanced antibacterial activity. Mater Lett. 2016;181:367–370.
- Avci H, Monticello R, Kotek R. Preparation of antibacterial PVA and PEO nanofibers containing Lawsonia Inermis (henna) leaf extracts. J Biomater Sci – Polym Ed. 2013;24:1815–1830.
- Yousefi I, Pakravan M, Rahimi H, et al. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater Sci Eng C. 2017;75:433–444.
- Hadisi Z, Nourmohammadi J, Nassiri SM. The antibacterial and anti-inflammatory investigation of Lawsonia inermis-gelatin-starch nano-fibrous dressing in burn wound. Int J Biol Macromol. 2018;107:2008–2019.
- Vakilian S, Norouzi M, Soufi-Zomorrod M, et al. L. inermis-loaded nanofibrous scaffolds for wound dressing applications. Tissue Cell. 2018;51:32–38.
- Shahrousvand M, Haddadi-Asl V, Shahrousvand M. Step-by-step design of poly (ε-caprolactone)/chitosan/Melilotus officinalis extract electrospun nanofibers for wound dressing applications. Int J Biol Macromol. 2021;180:36–50.
- Hashmi M, Ullah S, Kim IS. Electrospun Momordica charantia incorporated polyvinyl alcohol (PVA) nanofibers for antibacterial applications. Mater Today Commun. 2020;24:101161.
- Ali A, Mohebbullah M, Shahid M, et al. PVA-Nigella sativa nanofibrous mat: antibacterial efficacy and wound healing potentiality. J Text Inst. 2021;112:1611–1621.
- Kwak HW, Kang MJ, Bae JH, et al. Fabrication of Phaeodactylum tricornutum extract-loaded gelatin nanofibrous mats exhibiting antimicrobial activity. Int J Biol Macromol. 2014;63:198–204.
- Suganya S, Senthil Ram T, Lakshmi BS, et al. Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: an excellent matrix for wound dressings. J. Appl. Polym. Sci. 2011;121:2893–2899.
- Ganesan P, Pradeepa P. Development and characterization of nanofibrous mat from PVA/Tridax Procumbens (TP) leaves extracts. Wound Med. 2017;19:15–22.
- Suryamathi M, Ruba C, Viswanathamurthi P, et al. Tridax Procumbens extract loaded electrospun PCL nanofibers: a novel wound dressing material. Macromol. Res. 2019;27:55–60.
- Farahani H, Barati A, Arjomandzadegan M, et al. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int J Biol Macromol. 2020;162:762–773.
- Maver T, Kurečič M, Pivec T, et al. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose. 2020;27:4487–4508.
- Avci H, Gergeroglu H. Synergistic effects of plant extracts and polymers on structural and antibacterial properties for wound healing. Polym. Bull. 2019;76:3709–3731.
- Jin G, Prabhakaran MP, Kai D, et al. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 2013;34:724–734.
- Bilia AR, Guccione C, Isacchi B, et al. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid-Based Complement Altern Med. 2014;2014:1–14.
- Moure A, Cruz JM, Franco D, et al. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171.
- Mouro C, Simões M, Gouveia IC. Emulsion electrospun fiber mats of PCL/PVA/chitosan and eugenol for wound dressing applications. Adv Polym Technol. 2019;2019:1–11.
- Eğri Ö, Erdemir N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artificial Cells Nanomed Biotechnol. 2019;47:1404–1415.
- Sofi HS, Akram T, Tamboli AH, et al. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int J Pharm. 2019;569:118590.
- Avci H, Ghorbanpoor H, Nurbas M. Preparation of Origanum minutiflorum oil-loaded core–shell structured chitosan nanofibers with tunable properties. Polym. Bull. 2018;75:4129–4144.
- Zarghami A, Irani M, Mostafazadeh A, et al. Fabrication of PEO/chitosan/PCL/olive oil nanofibrous scaffolds for wound dressing applications. Fibers Polym. 2015;16:1201–1212.
- Unalan I, Slavik B, Buettner A, et al. Physical and antibacterial properties of peppermint essential oil loaded poly (ε-caprolactone) (PCL) electrospun fiber mats for wound healing. Front Bioeng Biotechnol. 2019;7:346.
- Bai MY, Chang YT, Tsai JC, et al. Tea tree oil-Containing chitosan/polycaprolactone electrospun nonwoven mats: a systematic study of its anti-bacterial properties in vitro. Int J Nanotechnol. 2013;10:959–972.
- Ardekani NT, Khorram M, Zomorodian K, et al. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int J Biol Macromol. 2019;125:743–750.
- Rafiq M, Hussain T, Abid S, et al. Development of sodium alginate/PVA antibacterial nanofibers by the incorporation of essential oils. Mater Res Express. 2018;5:035007.
- Lee K, Lee S. Electrospun nanofibrous membranes with essential oils for wound dressing applications. Fibers Polym. 2020;21:999–1012.
- Barzegar S, Zare MR, Shojaei F, et al. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int J Pharm. 2021;597:120288.
- Zhang W, Huang C, Kusmartseva O, et al. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React Funct Polym. 2017;117:106–111.
- Liakos I, Rizzello L, Scurr DJ, et al. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int J Pharm. 2014;463:137–145.
- Hajiali H, Summa M, Russo D, et al. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B. 2016;4:1686–1695.
- Gámez-Herrera E, García-Salinas S, Salido S, et al. Drug-eluting wound dressings having sustained release of antimicrobial compounds. Eur J Pharm Biopharm. 2020;152:327–339.
- Zare MR, Khorram M, Barzegar S, et al. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int J Pharm. 2021;603:120698.
- Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials. 2019;12:2540.
- Uttayarat P, Jetawattana S, Suwanmala P, et al. Antimicrobial electrospun silk fibroin mats with silver nanoparticles for wound dressing application. Fibers Polym. 2012;13:999–1006.
- Ivanova N, Gugleva V, Dobreva M, et al. Silver nanoparticles as multi-functional drug delivery systems. In Nanomedicines. London: IntechOpen; 2019.
- Burdușel A-C, Gherasim O, Grumezescu AM, et al. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018;8:681.
- Paul M, Londhe VY. Pongamia pinnata seed extract-mediated green synthesis of silver nanoparticles: preparation, formulation and evaluation of bactericidal and wound healing potential. Appl Organometal Chem. 2019;33:e4624.
- Zangeneh MM, Joshani Z, Zangeneh A, et al. Green synthesis of silver nanoparticles using aqueous extract of Stachys lavandulifolia flower, and their cytotoxicity, antioxidant, antibacterial and cutaneous wound‐healing properties. Appl Organomet Chem. 2019;33:e5016.
- Augustine R, Kalarikkal N, Thomas S. Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl Nanosci. 2016;6:337–344.
- Augustine R, Hasan A, Yadu Nath VK, et al. Electrospun polyvinyl alcohol membranes incorporated with green synthesized silver nanoparticles for wound dressing applications. J Mater Sci Mater Med. 2018;29:163.
- Kohsari I, Shariatinia Z, Pourmortazavi SM. Antibacterial electrospun chitosan-polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydr Polym. 2016;140:287–298.
- Alippilakkotte S, Kumar S, Sreejith L. Fabrication of PLA/Ag nanofibers by green synthesis method using Momordica charantia fruit extract for wound dressing applications. Colloids Surf A. 2017;529:771–782.
- Zhao H, Su H, Ahmeda A, et al. Biosynthesis of copper nanoparticles using Allium eriophyllum Boiss leaf aqueous extract; characterization and analysis of their antimicrobial and cutaneous wound-healing potentials. Appl Organomet Chem. 2020;2020:e5587.
- Seydi N, Mahdavi B, Paydarfard S, et al. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl Organomet Chem. 2019;33:e5009.
- Zheng H, Sun S, Ma X, et al. Tamarindus indica leaf extract mediated Au NPs for antimicrobial treatment of infectious skin wounds in postoperative wound care applications. Sci Adv Mater. 2021;13:1012–1018.
- Bandeira M, Chee BS, Frassini R, et al. Antimicrobial PAA/PAH electrospun fiber containing green synthesized zinc oxide nanoparticles for wound healing. Materials. 2021;14:2889.