Abstract
Filamentous plant pathogens, including fungi and oomycetes, pose significant threats to cultivated crops, impacting agricultural productivity, quality and sustainability. Traditionally, disease control heavily relied on fungicides, but concerns about their negative impacts motivated stakeholders and government agencies to seek alternative solutions. Biocontrol agents (BCAs) have been developed as promising alternatives to minimize fungicide use. However, BCAs often exhibit inconsistent performances, undermining their efficacy as plant protection alternatives. The eukaryotic cell wall of plants and filamentous pathogens contributes significantly to their interaction with the environment and competitors. This highly adaptable and modular carbohydrate armor serves as the primary interface for communication, and the intricate interplay within this compartment is often mediated by carbohydrate-active enzymes (CAZymes) responsible for cell wall degradation and remodeling. These processes play a crucial role in the pathogenesis of plant diseases and contribute significantly to establishing both beneficial and detrimental microbiota. This review explores the interplay between cell wall dynamics and glycan interactions in the phytobiome scenario, providing holistic insights for efficiently exploiting microbial traits potentially involved in plant disease mitigation. Within this framework, the incorporation of glycobiology-related functional traits into the resident phytobiome can significantly enhance the plant’s resilience to biotic stresses. Therefore, in the rational engineering of future beneficial consortia, it is imperative to recognize and leverage the understanding of cell wall interactions and the role of the glycome as an essential tool for the effective management of plant diseases.
Introduction
Filamentous plant pathogens, such as fungi and oomycetes, are major concerns due to their role in causing economically significant plant diseases [Citation1, Citation2]. These organisms grow through thread-like hyphae networks and employ osmotrophy to absorb nutrients after breaking down complex compounds with enzymes [Citation3]. Commonly, phytopathogenic eukaryotes are categorized into necrotrophs (kill host cells and feed on dead tissues), biotrophs (grow on living host tissues while evading plant defenses), and hemibiotrophs (employ both strategies, initially suppressing plant defenses and later transitioning to necrotrophy) [Citation4, Citation5].
The eukaryotic cell wall (CW) in plants and filamentous organisms consists of carbohydrate polymers, glycoproteins, and other compounds [Citation6]. Serving as a dynamic extracellular matrix, the CW provides structural support, protection, and regulates cellular growth and development [Citation6]. Moreover, it also acts as the interface between cells, facilitating intercellular communication and interspecific interspecies [Citation7–10]. For this reason, CW dynamics significantly influence the pathogenesis process. The plant cell wall (PCW) is the first line of defense against filamentous pathogens. Plants have developed sophisticated systems to monitor CW integrity and detect pathogens, triggering CW remodeling or the production of cell wall degrading enzymes (CWDEs) as a defense response [Citation11–13]. Conversely, modifications in the cell walls of fungi (FCW) and oomycetes (OCW) play a crucial role in: facilitating infection, mediating adherence, growth on the host surface, penetration, and invasive growth inside plant tissues. Notably, disrupting CW dynamics proved to be a successful strategy for plant disease control. Indeed, crop protection strategies often involve inhibiting pathogen CW metabolism using specific inhibitors [Citation14] or degrading CW via biocontrol agents (BCAs) [Citation15].
Concerns about the negative impacts of pesticides and the inconsistent performance of BCAs have led to increased interest in developing functional microbiomes for increased resilience toward biotic stresses, including diseases [Citation16]. Recent microbiome research underscores the intricate influence of the plant microbiome (phytobiome) on plant health, challenging traditional paradigms of disease development [Citation17, Citation18] (). Indeed, several studies have emphasized the effective use of artificial microbial consortia to suppress pathogenic organisms [Citation28]. However, establishing such consortia in a dynamic ecosystem like the native microbiome is complex [Citation29]. To enhance crop protection competence, desired functional traits can be introduced within the resident microbiome [Citation30]. Within this framework, the rational engineering of future beneficial consortia cannot overlook understanding and harnessing of CW interactions as an essential tool to manage plant diseases effectively.
Figure 1. A. Illustration of the classical “Disease Triangle,” incorporating the plant, pathogen, and environment as factors influencing diseases. Recent research on the phytobiome suggests a fourth dimension, encompassing microbes involved in symbiotic relationships. In such a perspective, pathogens and symbionts form complex communities termed pathobiome and symbiome. The plant holobiont (plant and associated microbes) acts on three compartments: phyllosphere (above-ground surface), rhizosphere (region influenced by root secretions), and plant endosphere (internal tissues colonized by the endophytic community). B. Carbohydrate composition of cell walls in two different phyla of plant pathogenic fungi (Ascomycota and Basidiomycota) and Oomycetes. The composition values are presented as weight (%) of the dry cell wall [Citation8, Citation19–27].
![Figure 1. A. Illustration of the classical “Disease Triangle,” incorporating the plant, pathogen, and environment as factors influencing diseases. Recent research on the phytobiome suggests a fourth dimension, encompassing microbes involved in symbiotic relationships. In such a perspective, pathogens and symbionts form complex communities termed pathobiome and symbiome. The plant holobiont (plant and associated microbes) acts on three compartments: phyllosphere (above-ground surface), rhizosphere (region influenced by root secretions), and plant endosphere (internal tissues colonized by the endophytic community). B. Carbohydrate composition of cell walls in two different phyla of plant pathogenic fungi (Ascomycota and Basidiomycota) and Oomycetes. The composition values are presented as weight (%) of the dry cell wall [Citation8, Citation19–27].](/cms/asset/bb48955d-1bd3-444b-a542-4a402a751534/ibty_a_2370341_f0001_c.jpg)
Through this review, we aim to comprehensively summarize the interactions involving CW and glycan dynamics in the relationships between filamentous plant pathogens and the phytobiome with a holistic approach. Moreover, we have incorporated illustrative examples of significant phytopathogenic species, providing a perspective for efficiently exploiting microbial traits involved in plant disease mitigation.
Know your enemies: the cell wall of filamentous organisms
Cell wall composition of several species of clinical relevance (e.g., Candida albicans, Aspergillus fumigatus) is well described and constantly updated. Conversely, the detailed CW composition and structure of phytopathogenic filamentous organisms remain limited, also due to methodological challenges in the CW analysis and to significant variations in composition across different species and developmental stages (; ). Overall, polysaccharides contribute up to 90% to the cell wall of filamentous organisms, comprising both soluble and insoluble structures. In fungi, the central core of the cell wall consists of a branched 1,3‐β, 1,6‐β glucan linked to chitin which is a polymer of N-acetylglucosamine units linked by 1,4-β glycosidic bonds [Citation9]. These structural polysaccharides are embedded in an amorphous polysaccharide-protein matrix. The covalent linkage of 1,3-β-glucans and chitin is thought to provide structural rigidity to the FCW. Additionally, in FCW melanin crosslinked with chitin is believed to enhance resistance to turgor pressure and protect cells from environmental stressors, such as radiation. Often an extensive gel-like extracellular polysaccharide (EPS) matrix, mostly composed of α-glucans, surrounds the hyphae [Citation34]. In oomycetes, in addition to 1,3-β-glucans, 1,4-β-glucans represent a major component (30–50%), while chitin and other N-acetylglucosamine residues are present only in minute amounts in some species [Citation8]. Furthermore, the extracellular polysaccharide (EPS) matrix is poorly characterized [Citation35].
Table 1. Main cell wall components of filamentous plant pathogens.
As a critical structure for the growth and development of filamentous organisms, the CW, as well as carbohydrate-active enzymes (CAZymes) involved in its biosynthesis and remodeling, gained attention as attractive targets for drug discovery, and opens avenues for tailoring crop protection strategies. Indeed, understanding how CW variations contribute to pathogenicity and adaptation mechanisms will help to develop targeted interventions against infections thus enhancing the sustainability of agricultural practices.
Glycan chess: the tit-for-tat battle for infection
As a strategic turn-based board game like chess, the battle between plants and pathogens plays out like a series of tit-for-tat strikes. For example, when a soy plant is attacked by Phytophthora spp., it secretes 1,3-β-endoglucanases to target and degrade the pathogen’s cell walls [Citation36]. In response, Phytophthora spp. secretes glucanase inhibitor proteins (GIPs) that counteract the plant’s 1,3-β-endoglucanase activity in the apoplast. Another example involves Phytophthora sojae, which produces xyloglucan-specific endoglucanases to enter the apoplast. Soy plants respond by producing a GIP that binds to the xyloglucan-specific endoglucanases and inactivates them. However, P. sojae counters this by secreting a paralogous decoy of the endoglucanases that binds more tightly to the glucanase inhibitor, indirectly increasing its own activity [Citation37].
The zig-zag plant immunity model describes the plants’ dynamic and sophisticated defense system (). The model consists of two interconnected branches: the Pathogen-Associated Molecular Pattern (PAMP)-Triggered Immunity (PTI) and the Effector-Triggered Immunity (ETI). PAMPs represent exogenous molecules associated with a broad range of pathogens. Glycans, arising from pathogens, such as chitin and β-glucans (1,3-β-glucans, 1,3-/1,6-β-glucans and mixed linkage, 1,3/1,4-β-glucans) represent common PAMPs and are major contributors to PTI induction [Citation38–40]. Similarly, CWDEs secreted by filamentous pathogens contribute to plant immunity, these enzymes are often recognized by plants and act as proteinaceous PAMPs. Additionally, plants recognize endogenous molecules released upon attack as damage-associated molecular patterns (DAMPs), stimulating a further response known as DAMP-triggered immunity (DTI) [Citation41]. PTI and DTI represent the first line of defense that generally prevents microbial colonization, often through the deposition of PCW material to increase resistance at the penetration point [Citation42]. To evade or suppress PTI and/or DTI responses, pathogens have evolved an arsenal of effector proteins during their co-evolution with the host. The second and more robust layer of plant immunity acts against these molecules, and it is known as effector-triggered immunity (ETI), a mechanism which often leads to programmed host cell death to avoid pathogen diffusion [Citation42].
Figure 2. A. Plant Cell Wall (CW) dynamics associated with the zig-zag model of plant immunity. Glycan-related Pathogen-Associated Molecular Patterns (PAMPs), including complex carbohydrates in fungal and oomycete CWs (chitin, β-glucans) and their cell wall degrading enzymes (CWDEs), initiate PAMP-Triggered Immunity (PTI). Additionally, the hydrolytic activity of CWDEs releases endogenous molecules recognized as damage-associated molecular patterns (DAMPs), triggering DAMP-Triggered Immunity (DTI). PTI and/or DTI induce plant CW remodeling and pathogenesis-related (PR) proteins expression (e.g., plant glycosyl hydrolases targeting pathogen structures). However, pathogen-secreted proteins can limit and suppress PTI/DTI responses. In turn, plants can counteract with effector-triggered immunity (ETI) response, inducing lignification and programmed cell death. B Shows CW thickening and callose deposition localization in the plant cell upon PTI/DTI triggering. C Details papilla localization between the plasma membrane and plant CW to block pathogen penetration. Letters represent processes related to degradation (D) and remodeling (R), associated with the plant (green) or the pathogen (red).
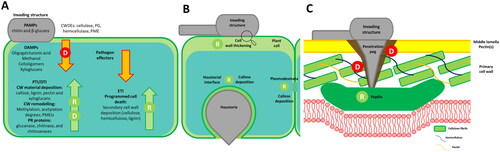
Plant cell wall dynamics
PCWs are primarily composed of cellulose, hemicelluloses, and pectins. In this framework, cellulose provides structural support and rigidity while hemicelluloses and pectins contribute to flexibility and hydration. Moreover, the presence of lignin, a complex organic polymer, in some tissues provides further support and rigidity [Citation43]. Pectins serve as the initial barrier between plants and pathogens, influencing cell adhesion and tissue integrity. When attacked, plants activate gene regulation for pectin remodeling, altering CW properties and susceptibility to pathogen-secreted enzymes [Citation44]. Indeed, a higher degree of esterification (DE) in pectins, is often mediated by the overexpression of pectin methylesterase inhibitor proteins (PMEIs), reducing susceptibility to CWDEs from fungal and oomycete pathogens [Citation45]. Similarly, a reduction in the degree of acetylation of pectins and xylans has also been linked to specific defensive responses in Arabidopsis toward Botrytis cinerea [Citation46, Citation47].
In response to PTI/DTI, papillae represent a common strategy to counteract several filamentous plant pathogens, through the localized reinforcement between the plasma membrane and PCW mediated by the deposition of material at sites of pathogen detection [Citation48]. Although the papillae composition may vary, callose (a 1,3-β-glucan polymer) is one of the most abundant components [Citation49], acting as a mechanical reinforcement toward deformation [Citation50]. In addition, the callose deposition helps to regulate the plasmodesmata’s permeability during biotic stresses to inhibit the trafficking of the pathogen effectors (, [Citation51]). Similarly, lignification of PCW increases resistance to mechanical stress and reduces substrate accessibility to the pathogen CWDEs by increased hydrophobicity. Therefore, upregulation of genes involved in lignin biosynthesis and deposition is a common strategy upon infection with filamentous pathogens. This regulation is triggered by PTI/DTI and ETI mechanisms and regulates programmed cell death in ETI [Citation52]. Besides PCW remodeling, plants can counteract pathogens directly by expressing pathogen-related proteins (PR). Among these, CWDEs belonging to the GH (glycosyl hydrolase) family (e.g., 1,3-β-glucanases, chitinases, and chitosanases) are usually expressed synergistically to the pathogen’s CW degradation.
Fungal and oomycete cell wall dynamics
Adhesion, growth and penetration into the host tissues
Filamentous pathogens typically use infection structures (e.g., conidia, zoospores) to reach and recognize the host. Here, they adhere to the host surface through a secreted glue to increase resilience and infection success and subsequently penetrate the plant tissues. While the complete understanding of adhesion molecules is still debated, proposed mediators include polymers, such as α-linked glucans and glycoproteins like mannoproteins, and adhesins with lectin motifs [Citation53]. For instance, during conidia germination of the rice blast pathogen Magnaporthe oryzae (Fungi – Ascomycota), the release of a glycoprotein-rich mucilage enhances adhesion and morphological development. Additionally, the presence of chitosan further contributes to these processes [Citation54]. On the contrary, wall-less biflagellate oomycete zoospores release mucin-like proteins upon encystment and secrete exopolysaccharides which enhance their adhesion and provide protection from dehydration. Moreover, during germ-tube development, CW depositions (plugs) rich in 1,3-β- and 1,4-β-glucans, which are putatively involved in cytoplasmic streaming, are produced [Citation55].
Appressoria are specialized structures produced by filamentous pathogens to assist plant tissue penetration. Here melanin only plays a role as a virulence agent in fungi, but not in oomycetes which lack melanin in their appressoria-like structures [Citation56]. In M. oryzae increased melanin content allows for sufficient mechanical strength to perforate plant cuticle via turgor pressure [Citation57]. Additionally, increased melanin and chitosan content was observed in infection cushions (multicellular appressoria) produced by the necrotrophic and ubiquitous pathogen Botrytis cinerea [Citation58]. Moreover, the correct assembly of chitin and glucans seems to support appressorium formation. For instance, the correct appressorium assembly and successful penetration in M. oryzae depend on several chitin synthase (CHS) genes (CHS1, CH6, CHS7) [Citation59], and glucan elongation proteins (Gels) belonging to the Glycoside Hydrolase 72 family [Citation19]. In the maize pathogen Colletotrichum graminicola, 1,3-β-glucansynthase (GLS1) expression and increased 1,3-β-glucan contents were detected in appressoria [Citation60]. Analogously, in B. cinerea infection cushions, the overexpression of CW remodeling enzymes, such as: transferases (1,3-β-glucan), hydrolases (mannan) and transglycosylases (chitin/glucan), suggest a substantial remodeling of the FCW [Citation58]. Conversely, cuticle penetration in Phytophthora spp. is orchestrated by the actin cytoskeleton which transports exocytotic vesicles containing CW material to the site of expansion producing a hyphal-slicing effect on plant tissues [Citation61]. In this context, cellulose, analogous to FCW chitin, provides strength and rigidity to the OCWs. Indeed, the pivotal role of the cellulose synthase subunit A (CesA) family in correct appressorial development and pathogenicity was demonstrated for the tomato and potato late blight agent Phytophthora infestans [Citation62]. Moreover, in P. infestans cysts and appressoria, researchers highlighted the abundant presence of CAZymes involved in CW synthesis and remodeling, such as: glycosyl transferases, endo-1,4-β-glucanase and exoglucanase [Citation63].
Invasive growth and feeding on host tissues
Upon penetration into leaf tissues, CWDEs are key elements of the infection process as they are involved in the degradation of the PCW as well as in FCW and OCW remodeling. Thus, they facilitate entry into the host, hyphal ramification, and the formation of invading structures. CWDEs in filamentous pathogens belong mainly to the GH family and can hydrolytically cleave glycosidic bonds in oligo- or polysaccharides. However, the activity of such enzymes can be supported by other CAZymes such as carbohydrate esterases (CE) and Auxiliary Activity (AA) families (e.g., LPMOs) [Citation64, Citation65]. Biotrophic and necrotrophic filamentous pathogens evolved different approaches during invasive growth. Biotrophic fungi exhibit a localized PCW degradation at the entry point of specialized invading structures, such as intracellular hyphae or haustoria (). Usually, the interface between the plant membrane and the invading structure forms an amorphous matrix rich in carbohydrates and proteins, known as an extra haustorial or invasive hyphal matrix [Citation66]. Among secreted proteins during haustorium penetration and development, several CAZymes have been identified. For instance, in P. infestans haustorium the abundant presence of: pectinesterases, glycosyl hydrolases (e.g., 1,3-β-glucosidase) and glycosyl transferases (1,3-β-glucanosyltransferase) have been detected. In particular, the pectinesterase PE1 is highly upregulated and directly secreted at haustoria bases, thus suggesting its role in PCW modification and subsequent degradation [Citation67]. Similarly, haustorial cells in rust fungi synthesize polygalacturonate lyase (PL) and other CWDEs such as cellulases and carbohydrate esterases [Citation68].
Figure 3. A. Localized degradation of the plant cell wall, involving cell wall degrading enzymes (CWDEs) like pectinases (e.g., pectin lyases, pectinesterases), hemicellulases, and cellulases, is crucial for haustorium establishment in biotrophic organisms. Moreover, -omics studies have revealed the role of pathogen CW remodeling mediated by enzymes (1,3-β-glucan transferases, chitin deacetylases and chitin LPMOs) implicated in plant immunity suppression. B. To penetrate the plant cell wall, necrotrophic fungi secrete a diverse array of CWDEs, including glycosyl hydrolases catalyzing glycosidic linkage hydrolysis (e.g., polygalacturonase, xylanases, α-D-glucuronidases, α-L-arabinofuranosidases, α-D-galactosidases, β-xylosidases, and β-mannanases). Esterases (e.g., pectin methylesterases and acetylesterases) often assist these enzymes by removing esterifications, usually from pectins and hemicelluloses. Letters denote processes related to cell wall biosynthesis (B), degradation (D), and remodeling (R), associated with the pathogen (red).
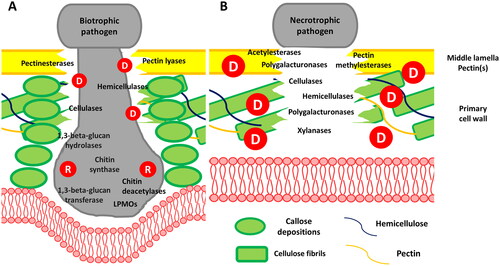
Since biotrophic pathogens do not kill their host, they need to evade plant immunity. In the wheat rust Puccinia, researchers have identified an upregulated expression of chitin deacetylase believed to play a role in preventing the hydrolysis of FCW by host chitinases and suppressing plant immunity [Citation69]. Similarly, in the cucurbit powdery mildew Podosphaera xanthii, a recently discovered haustoria-expressed lytic polysaccharide monooxygenase (LPMO) appears to be involved in suppressing plant immunity by converting immunogenic chitin fragments into chitooligosaccharides [Citation70]. In the hemibiotrophic fungus M. oryzae, the extensive growth of intracellular infection hyphae is observed during the biotrophic phase. To evade the plant immune system, FCW remodeling via the coating of invasive hyphae with 1,3-α-glucans and chitosan is observed. Since plants lack the machinery to degrade 1,3-α-glucans, this polysaccharide acts as a protective layer to mask chitin and 1,3-β-glucans. Furthermore, the presence of chitosan prevents host recognition and increases tolerance toward plant chitinases [Citation31, Citation71]. Interestingly, the infection-specific masking mediated by 1,3-α-glucans deposition seems to be conserved among different plant pathogens, as also suggested by the identification of putative 1,3-α-glucan synthase (AGS1) orthologs in several biotrophic fungi [Citation60]. Moreover, in concert with FCW remodeling, several CWDEs have been proven to trigger infection hyphae development, including endoglucanases targeting hemicelluloses which are indispensable for M. oryzae infection and cell wall integrity [Citation72, Citation73].
On the other hand, the necrotrophic invasion of plants is mostly driven by the extensive degradation of PCW integrity, which ultimately leads to the collapse of plant tissues and subsequent cell death. In this context, necrotrophs are characterized by the ability to utilize complex sugars, with PCW polysaccharides serving as nutrients for their growth [Citation74]. For this reason, the secretion of a diverse array of CWDEs () represents a crucial factor in this lifestyle, which is also reflected in the high number and diversity of CAZymes hosted by necrotrophic genomes [Citation64]. However, several oligomers arise from pathogenic CWDEs activity, which are known to trigger DTI responses in plants. These include methanol and oligogalacturonides, produced by pectin methylesterase (PMEs) and polygalacturonases, cellooligomers derived from cellulose hydrolysis, as well as xyloglucan oligosaccharides from hemicelluloses degradation ([Citation75] and citations therein). Indeed, several studies highlighted the role of GHs like cellulases, xylanases and pectinases in triggering PTI [Citation76], meanwhile, CEs and PLs families are rarely reported [Citation77].
The symbiome-mediated protection against plant pathogens Direct mechanisms of plant protection by beneficial fungi and bacteria
Secretion of extracellular hydrolytic enzymes (mycoparasitism) and antimicrobial small molecules (antibiosis) are regarded as the most effective strategies which the plant symbiome utilizes to protect the plant against pathogens. In addition, competition for space and nutrients between organisms and detoxifying virulence factors benefit plant growth and health [Citation78]. Mycoparasitism, followed by antibiosis is one of the most effective forms of control of filamentous pathogens. Mycoparasitism involves the increased production of CWDEs and effectors [Citation79, Citation80]. The secreted enzymes will: inhibit or modify cell-wall synthesis, perforate cell membrane, or degrade the CW of the pathogens ([Citation81] and citations therein). In contrast, antibiosis is defined mainly by producing antimicrobial small organic compounds, such as antibiotics, which target and interfere with the pathogens’ CW, cell membrane, and protein synthesis [Citation82].
The main targets of the enzymatic repertoire of the symbiome
The contribution of bacterial CWDE in biocontrol agent formulations has been extensively studied in various species (reviewed in [Citation83]). While fungal BCA formulations with several different species are also available on the market [Citation78], studies investigating the exact mode of action or the contribution of CAZymes to protect the plants have mainly been conducted in two closely related fungal species from the order of hypocreales: Trichoderma and Clonostachys [Citation79–81, Citation84] and only limited information on CWDE is available for other fungal BCAs. All plant symbiotic microorganisms use similar strategies to attack plant pathogens, as evidenced by evolutionary conserved enzymes related to mycoparasitism in bacteria and fungi, which function in CW degradation and proteolysis [Citation85, Citation86]. Therefore, carbohydrates and proteins on the pathogen CW are also the primary goal of the mycoparasitic attack. The mycoparasitic enzymes act with extreme precision, facilitating the efficient penetration of the pathogen’s cell by microorganisms, specifically targeting glucans, chitin, and associated CW proteins (Table S1):
Glucans: The major constituents of the fungal plant pathogen CW are glucans with 1,3-β-, 1,4-β- and 1,6-β-linkages. 1,3-β-glucanases are expanded in Trichoderma mycoparasites compared to other fungi [Citation85]. Furthermore, Chaetomium spp. secrete 1,3-β-glucanases with biocontrol activity [Citation87] and bacterial 1,3-β-glucanases are effective against ascomycete plant pathogens (reviewed in [Citation83]). Overexpression of 1,6-β-glucanases and combination with 1,3-β-glucanases in T. harzianum are very effective to promote mycoparasitism (Table S1, [Citation88]). Cellulose degrading β-glucanases have been suggested to be especially important for mycoparasitism of oomycete prey, whose CWs mainly contain large amounts of cellulose (; ). Increased expression of a 1,4-β-endoglucanase in T. longibrachiatum protected cucumber seeds against P. ultimum [Citation89]. A lichenase derived from Bacillus amyloliquefaciens proved promising in the protection of ginseng against Botrytis cinerea and Alternaria panax [Citation82].
Chitin: Chitin is one of the defining characteristics of CWs of fungi. Chitinases and the 1,4-β-glucosaminidases are the signature enzymes for active mycoparasitism in Trichoderma sp. [Citation86]. Within plant beneficial ascomycetes, Trichoderma spp. contain the highest number of chitinases with up to 36 chitinases in T. virens [Citation86]. These high numbers have been associated with specialization to a mycoparasistic lifestyle. Conversely, only little is known about chitinases in other mycoparasitic fungi (Table S1).
Cell wall proteins: Proteases may target CW proteins and glycoproteins. The S8 family protease-encoding gene prb1 of T. atroviride is among the best characterized mycoparasitism-relevant genes [Citation90] with many examples of its effectiveness against plant pathogens (Table S1). Moreover, proteases from bacterial origin have proven effective against several fungal and oomycete plant pathogens ([Citation91], Table S1).
Indirect mechanisms of plant protection by endophytes
Fungal endophytes inhabit plant tissues without causing harm, promoting plant growth, and protecting against various pathogens (Table S2) [Citation92]. Mycorrhizal fungi, such as arbuscular mycorrhizae (AM) and ectomycorrhizae (ECM), play vital roles in: plant growth, nutrient absorption, and disease protection [Citation93]. AM fungi establish intracellular hyphal growth, forming a sophisticated interface with invagination of the host plasma membrane and the deposition of an intercellular matrix, resulting in arbuscules [Citation94, Citation95]. AM and ECM colonization offers indirect plant protection by forming a physical barrier around and within roots, thus limiting pathogen access [Citation96]. This colonization induces systemic resistance, triggering: extensive remodeling, reinforcement, lignification, and accumulation of defense-related proteins [Citation97]. AM induces: fast callose accumulation, modifying plasmodesmata-callose, and thickening the PCW with non-esterified pectins and lignin-like compounds [Citation98,Citation99]. Additionally, AM enhances defense responses by accumulating PR proteins, chitinases, 1,3-β-glucanases, and PCW proteins, such as arabinogalactan-rich proteins and expansins [Citation100]. These proteins act as a physical barrier and strengthen the PCW, providing an immediate response to pathogens before PR protein expression, thereby enhancing plant defense [Citation101].
Value your supporters
In order to mitigate the drawbacks of agrochemical use, as well as to achieve increased sustainability in crop management strategies, over the last 30 years, researchers focused their attention on the selection of BCAs, living organisms including: bacteria, fungi, and oomycetes, that target the pathogen directly or indirectly. Due to the major relevance of previously cited pathogens, a massive amount of data concerning suitable BCAs and their mode of action is available in the literature [Citation15]. Notably, most effective BCAs often employ more than one mode of action to control pathogens, including: competition for resources (e.g., nutrients and space), mycoparasitism, antibiosis (e.g., production of antibiotic molecules and CWDEs) and induced host resistance [Citation15]. However, the low success rate linked to BCAs application is generally attributable to climatic variations, the lack of ecological competence (survival, colonization ability), and its negative interaction with the native microbial community [Citation102]. The successful field application of BCAs requires a comprehensive understanding of plant-microbe and microbe-microbe interactions. Therefore, combining various microbial strains with diverse disease-suppression mechanisms can contribute to the positive outcome of biocontrol [Citation103].
However, the effective exploration of microbial interactions, in natural microbial consortia (i.e., groups of different microbial species that coexist and interact within a particular environment) is often challenged by their high heterogeneity and complexity. For this purpose, artificial microbial assemblies called synthetic communities (SynComs), often created in laboratory settings, are gaining increasing interest. Indeed, SynComs allow researchers to identify approaches to manipulate microbial diversity and microorganism–host relationships while exploring the essential components and processes that constitute natural microbial communities in a more controlled and predictable manner [Citation104].
Interestingly, plant-associated simplistic microbial communities often enhance plant protection compared to single BCA applications. Although mechanisms employed by BCAs for disease control are diverse, CWDEs produced by the microbial community and their induced expression in plant tissues seem to play a vital role in successful pathogen inhibition. For instance, several successful examples of disease incidence and severity mitigation of plants infected by M. oryzae, F. oxysporum and Phytophthora spp. are reported (). However, it must be pointed out that most of the studies focused on bacterial species, underestimating the role of the fungal-associated microbiome. Interestingly, Zhou and collaborators [Citation107], demonstrated that using a cross-kingdom community involving bacteria and fungi performs better than a single-kingdom community. Indeed, AM colonization has been recently proven to alter root exudation, thus influencing beneficial microbiome recruiting and enhancing host plant growth [Citation110]. According to these data, integrating AM fungi into SynComs and consortia in future experiments should be encouraged.
Table 2. Synthetic microbial communities (SynComs) and consortia employed with success in mitigating diseases arising from F. oxysporum, M. oryzae and P. infestans.
Phytobiome engineering for sustainable control
Functional core microbiomes refer to groups of microorganisms associated with a given host plant, that provide microbial functions at the plant and ecosystem levels. These functions result from activities coded by genes (replicators) within organisms considered as vehicles (or chassis) [Citation16, Citation111]. In this context, SynComs engineered to provide enhanced microbiome-associated traits to the host genotype could increase plant resilience toward stresses, and thus benefit disease management strategies [Citation112].
Mono-association and small SynCom studies indicate that even modest complexity, involving one or a few strains, can enhance disease resistance in plant hosts against specific pathogens. Therefore, in increasing SynCom or microbial consortia in general (involving both naturally and artificially introduced organisms) in plant protection, microbes in the community should possess several beneficial traits and act through synergistic interactions among themselves [Citation29]. In this context, harnessing the information on glycan-related processes and players involved in plant pathogenesis and disease suppression can help design and improve microbial-based solutions for plant protection.
A roadmap to harness cell wall dynamics in the phytobiome context
Phytobiome engineering is driven by two approaches: “bottom-up” and “top-down” [Citation113]. The complexity of top-down methods has led to a shift in favor of bottom-up approaches, which are more controllable and logistically simpler [Citation30]. In the bottom-up approach, plant-associated microbes are isolated, cultured, modified to introduce desired traits, and reintroduced into the phytobiome [Citation113]. Therefore, identifying desired functional traits for integration into selected microbes is crucial to harness the potential benefits of engineered consortia fully.
The extensive experience gained from biocontrol studies in past years demonstrates that CAZymes involved in CW degradation and remodeling often represent a fundamental trait for the successful outcome of biocontrol.
It is important to note that CWDEs from BCAs may trigger a PTI response by acting against the PCW, potentially contributing to systemic plant resistance. However, most CWDEs from BCAs are specifically targeted against the pathogen’s CW, reducing their growth and infectivity. We suggest prioritizing efforts to identify new targets in pathogen CWs and CWDEs, as current knowledge is limited to chitinases and 1,3-β-glucanases in fungi or 1,4-β-glucanases in oomycetes. [Citation83]. Targeting main FCW components often results in compensating mechanisms activated by the target organism to maintain their CW integrity. For instance, increased content (e.g., chitin or cellulose) or cross-linking between CW polysaccharides (e.g., mannose and the glucan matrix) have been proven to impact CW integrity in fungi (). Although 1,6-β-glucan represents a minor constituent of fungal and oomycete CWs, the crosslinking with other CW components (e.g., mannoproteins and 1,3-β-glucan/chitin matrix) seems to play a vital role in maintaining the rigid structure of the CW [Citation34]. Similarly, although limited in content, mixed linkage glucan polymers (MLGs) with both 1,3-β- and 1,4-β-linkages have been reported in FCWs. They seem indispensable for the survival of the human pathogen A. nidulans [Citation129]. A 1,3-1,4-β-glucanase (lichenase) derived from Bacillus amyloliquefaciens FS6 proved promising in protecting ginseng against B. cinerea and Alternaria panax [Citation82], while treatment with MLGs have been proved to confer disease resistance against fungal and oomycete pathogens [Citation130].
Table 3. Potential microbial traits of interest for plant-protection phytobiome engineering.
Recently, an outer-membrane 1,6-β-glucanase (GluM) isolated from Corallococcus spp. was proven to inhibit several phytopathogens, with enhanced activity compared to analogous proteins previously reported [Citation114, Citation115]. Moreover, GluM showed increased inhibition compared to a commercial 1,3-β-glucanase, suggesting that 1,6-β-glucan may be an interesting antifungal target. Similarly, 1,3-1,4-β-glucanases of bacterial origin could represent an interesting trait for biocontrol, as previous studies reported their effect for the enhanced inhibition of several phytopathogenic fungi [Citation116, Citation117]. In addition, the microbial antagonism mediated by members of the GH25 family with lysozyme activity appears promising for the control of fungal and oomycete pathogens [Citation122, Citation131], however, since the biological function of such enzymes is poorly characterized, further studies on this GH family are required.
As previously reported, 1,3-α-glucans surrounding FCW and chitin deacetylation to chitosan are mechanisms employed by pathogens to evade host immunity [Citation31, Citation71]. For this reason, microbial degradation of such polysaccharides could provide desirable traits for their control. Indeed, expression of 1,3-α-glucanases in fungal BCAs seems to support their antagonistic action toward fungal pathogens [Citation123, Citation124]. Moreover, a new subgroup of 1,3-α-glucanases belonging to GH87 has been isolated from bacterial sources [Citation125]. Chitin deacetylases (CE4) and chitosanases (GH75) are also interesting for biocontrol. Mycoparasisitc Trichoderma spp. have an expanded set of chitin deacetylases and chitosanases [Citation132]. In the presence of B. cinerea, S. sclerotiorum or R. solani, expression of a subset of both enzyme families was highly upregulated in T. atroviride and single knock out strains of the respective genes showed reduced mycoparasitic potential in vitro [Citation121]. Moreover, antifungal chitosanases resulted in positive inhibition of several phytopathogens [Citation118–120] ().
Interestingly, an acetyl xylan esterase and a swollenin were strongly induced in T. reesei in confrontation with P. capsici [Citation84]. Both proteins contain a cellulose binding domain (CBD) and the acetyl xylan esterases deacetylate xylan and xylooligosaccharides and play an important role in the degradation of cellulosic plant material [Citation84]. From their high expression (more than 100-fold) in the presence of P. capsici it is intriguing to speculate that these enzymes act together to degrade the cellulosic CW parts of the oomycetes.
Proteins containing conserved carbohydrate-binding modules (CBMs), but no catalytic domain, are also interesting targets for plant protection. Multiple LysM domains (CBM50) with a length of approximately 50 amino acids can be present in such proteins. The LysM domains are highly specific for chitin and chitin-like compounds, such as peptidoglycan [Citation133]. T. atroviride Tal6 contains 7 LysM domains and contributes to the mycoparasitic capacity of the fungus [Citation126]. Although ceratoplatanins are non-classical carbohydrate binding proteins [Citation134], with no conserved CBM, the T. atroviride and T. virens cystein rich ceratoplatanins exhibit a plant protective mechanism by triggering systemic resistance in plants [Citation127, Citation128].
In addition to their biocontrol role, CWDEs involved in PCW remodeling are fundamental in the relationships between phytobiome associated fungi and the host plant. The role of endophytic fungi colonization on plant defense enhancement is well-established. Investigating the contribution of CAZymes in endosymbiosis establishment could provide a foundation for future selection of traits and phytobiome associated fungi useful for SynCom development. Recently, putative essential factors for endophytic root colonization have been described in Arabidopsis root mycobiome, where several CWDEs seem to play a vital role in endophytism [Citation135]. Similarly, ectomycorrhizal symbiosis development seems to correlate with several CWDEs’ expression. On the contrary, arbuscular mycorrhiza are less dependent on such enzymes, however, an endo-mannanase possibly acting on hemicelluloses has been recently identified in Glomeromycota [Citation136] (Table S3).
Comparative -omics and machine learning for trait selection
Changes in the native phytobiome composition are often linked to increased plant resilience toward biotic and abiotic stresses, therefore monitoring the stress-induced changes in functional phytobiome composition through -omic approaches (e.g., metagenomics, transcriptomics, proteomics) represents a valuable source of information to drive the rational selection of traits useful for plant protection under the “DefenseBiome” concept. This concept involves isolating stress-induced microbes from infected plants, culturing them axenically, and reconstructing them as a SynCom for plant application [Citation137].
In particular, the comparative analysis of CAZyme families (i.e., CAZyme characterization or CAZy-typing) representation in phytobiome under “healthy” or “disease” phenotypes, can provide a novel framework to investigate disease biomarkers and traits of interest. To the best of our knowledge, no specific studies focused on comparative CAZy-typing of phytobiomes under healthy and disease conditions have been reported in the literature. However, some studies highlighted patterns associated with CAZymes representation under different phenotypes, thus suggesting the potential use of such data for trait identification [Citation138–141]. Moreover, the recent CAZy-typing of the human gut and rumen microbiome provides promising workflow examples [Citation142, Citation143].
In addition, machine learning (ML) models hold promise for future enhancement of microbiome data to link phenotypes with taxonomic and functional characteristics. By combining planta transcriptomics with a ML algorithm (Random Forest Classifier), Mesny and collaborators [Citation135] defined a conserved set of CAZymes as potential determinants for endophytism. Similarly, a machine learning approach to predict fungal lifestyles was recently employed, revealing that CAZYme profiles represent a powerful predictive feature [Citation144]. Indeed, a Classification and Regression Tree (CRT) algorithm was recently employed to correlate CW composition with disease resistance and fitness phenotypes in Arabidopsis mutants [Citation145].
Synthetic biology to drive phytobiome engineering
The transition from a traditional disease triangle model to a pathobiome-host-symbiome association framework can be conceptually associated with a dynamic and strategic battle between a defending host, plant-associated allies, and multiple invading pathogens (). However, the high dynamism, heterogeneity and complexity make assembly and stability of microbial communities challenging, thus undermining potential applications.
Figure 4. A. “Smart” symbiome role in the pathogenesis process. The proposed model of the plant disease pyramid highlights the crucial role of the "smart" symbiome in the pathogenesis process. It considers glycome biosynthesis (B), degradation (D), and remodeling (R) in each organism cluster (pyramid corner), directly influencing plant glycome and health. Positive traits induced in symbiotic organisms can counteract pathogen traits, benefiting plant health and enhancing immunity. The "smart" symbiome operates on two levels: inducing resistance (e.g., mediated by endophytic fungi) and activating functional traits (e.g., Cell Wall Degrading Enzymes – CWDE) in response to pathogen-related compounds. Circle sizes indicate health representation, with larger circles indicating overrepresentation and increased health, while smaller circles signify underrepresentation and reduced health in each organism group. B. Simplified representation of bacterial circuit(s) able to detect plant or pathogen-derived signals to readily tune transcription (promoter) and translation (ribosome binding site) level to control expression of target genes. C. Overview on future research “hotspots” in glycopathobiome and phytobiome engineering for enhanced biocontrol.
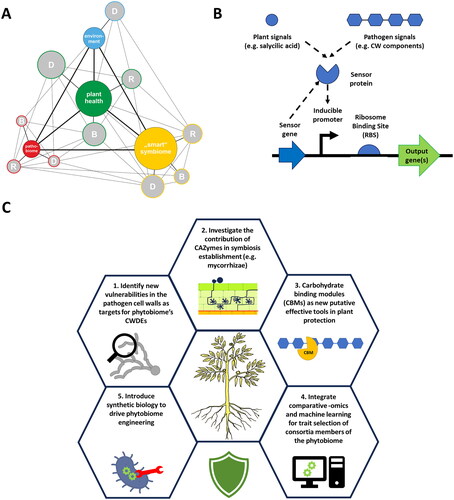
Once useful microbial traits are identified, microbial vectors engineered to carry them require reintroduction into the phytobiome. These vehicles are expected to maintain traits stably, requiring genome-level engineering. Indeed, improving single BCAs by genetic engineering technologies dates back to 2000, when the first Trichoderma biocontrol strains were engineered to overexpress (mostly) chitinases. This strategy successfully increased mycoparasitic activity against plant pathogens [Citation146, Citation147]. Moreover, engineering the putative enzymes to be more efficient seems to increase biocontrol activity. A chitinase, overexpressed in two T. harzianum strains as chimera with an additional chitin binding domain enhanced ISR in Phaseolus vulgaris L. next to its increased in vitro biocontrol activity against R. solani [Citation148]. In recent years, the ability to engineer non-model microbes greatly improved, thanks to: single-step integration of large DNA constructs (e.g., >50 kb in length) and broad host-range (BHR) plasmid strategies (e.g., phage integrases, integrative and conjugative elements, chassis-independent recombinase-assisted genome engineering) (extensively reviewed by Ke et al. [Citation113]).
Controlling gene expression of beneficial traits in microbes or transgenic plants is crucial, as constant production may have unintended consequences on the plant or compromise microbial adaptability to the phytobiome environment due to metabolic costs. Ideally, the heterologous expression of traits, such as CWDEs, should be linked to the presence of the pathogen. In nature, natural regulatory genetic circuits, such as inducible expression systems, control interactions with the environment, inter-cell communication, and metabolic dynamics [Citation149]. Synthetic genetic circuits (SGCs), defined as sets of genetic parts including coding and regulatory DNA, can be introduced into an organism to perform a specific function (). In the context of microbiomes, SGCs can sense fluctuating environmental signals and modulate gene expression spatiotemporally [Citation150]. While SGC development initially focused on model organisms, advances in high-throughput sequencing enable the construction of diverse bioparts for SGC assembly (e.g., promoters, ribosome binding sites, and terminators) based on accurate genome-wide information. Computational design and prediction of complex SGCs are accomplished using targeted softwares (e.g., Cello) [Citation151].
An extensive collection of suitable genetic parts for SGC construction in rhizobacteria has been recently described [Citation152]. Since some molecules act as biomarkers for various plant stresses, including pathogen infection (e.g., sucrose and salicylic acid), inducible promoter and regulator genes represent interesting parts to construct SGCs [Citation153, Citation154]. Moreover, Polysaccharide Utilization Loci (PUL), typical gene clusters employed by Bacteroidetes species which encode for recognition, import, and degradation of a specific class of complex polysaccharides could also act as sensors to design SGC [Citation155]. Bacteroidetes are ubiquitous bacteria, prevalent in soil ecosystems and often associated with eukaryotic microbiomes, including plants and animals [Citation156]. Recently, the combination of regulatory genes and inducible promoters linked to complex glycans (e.g., mannan, xylan and dextran) in gut microbiota have been developed for strain engineering purposes [Citation157].
While it remains to be verified, developing inducible promoters related to CW components specific to fungal and oomycete pathogens holds potential for the advancement of smart SynComs. Utilizing genetic elements from Polysaccharide Utilization Loci (PUL) could facilitate the creation of carbohydrate-inducer systems. Recently, FCW utilization loci potentially activated by fungal 1,3-β-glucans and 1,6-β-glucan have been identified in bacteria from the soil and human gut microbiota [Citation157, Citation158]. While no reports of PUL specifically targeting oomycete-borne polysaccharides exist, advancements in PUL prediction capabilities are expected to grow in the next few years. This growth is supported by a fully automated approach integrated into a dedicated database hosted by cazy.org [Citation159].
Conclusions
Within the dynamic and multifaceted processes shaping plant diseases, a groundbreaking synergy between phytobiome and glycosciences emerges. This review provides a conclusive, holistic overview of the state-of-the-art knowledge on glycan-related processes involved in plant – pathogen interactions, the glycopathobiome. We summarize the interplay between CW and glycan interactions, including direct and indirect plant protection mechanisms, pathogen attack and the role of plant-beneficial microbial consortia. Moreover, we provide insights for the efficient selection and exploitation of microbial traits potentially involved in plant disease mitigation through comparative -omics and machine learning approaches. Our roadmap outlines strategies for leveraging CW dynamics, serving as a comprehensive guide for designing stable SynComs, and employing synthetic biology tools to advance phytobiome engineering (). The idea is to step forward in biocontrol while increasing the overall sustainability of agricultural systems.
Supplemental Material
Download PDF (171.8 KB)Acknowledgment
We apologize to many authors whose contributions could not be cited in this review due to space limitations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kamoun S, Furzer O, Jones JD, et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol. 2015;16:413–434. doi: 10.1111/mpp.12190.
- Dean R, Kan JA, Pretorius ZA, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x.
- Richards TA, Dacks JB, Jenkinson JM, et al. Evolution of filamentous plant pathogens: gene exchange across eukaryotic kingdoms. Curr Biol. 2006;16:1857–1864. doi: 10.1016/j.cub.2006.07.052.
- Hardham AR. Cell biology of fungal and Oomycete infection of plants. Biol Fungal Cell. Berlin, Heidelberg: Springer; 2007. p. 251–289.
- McCombe CL, Greenwood JR, Solomon PS, et al. Molecular plant immunity against biotrophic, hemibiotrophic, and necrotrophic fungi. Essays Biochem. 2022;66:581–593. doi: 10.1042/EBC20210073.
- Sakurai N. Cell wall functions in growth and development-a physical and chemical point of view. Bot Mag Tokyo. 1991;104:235–251. doi: 10.1007/BF02489456.
- Srivastava V, McKee LS. V. Bulone Plant cell walls eLS. 2017. p. 1–17.
- Mélida H, Sandoval-Sierra JV, Diéguez-Uribeondo J, et al. Analyses of extracellular carbohydrates in oomycetes unveil the existence of three different cell wall types. Eukaryot Cell. 2013;12:194–203. doi: 10.1128/EC.00288-12.
- Gow NA, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5:10–1128. doi: 10.1128/microbiolspec.FUNK-0035-2016.
- Gow NA, Lenardon MD. Architecture of the dynamic fungal cell wall. Nat Rev Microbiol. 2023;21:248–259. doi: 10.1038/s41579-022-00796-9.
- Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front Plant Sci. 2014;5:228. doi: 10.3389/fpls.2014.00228.
- Bacete L, Mélida H, Miedes E, et al. Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J. 2018;93:614–636. doi: 10.1111/tpj.13807.
- Bacete L, Schulz J, Engelsdorf T, et al. THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc Natl Acad Sci. 2022;119:e2119258119.
- Gisi U, Lamberth C, Mehl A, et al. Carboxylic acid amide (CAA) fungicides. Mod Crop Prot Compd. 2019;2:845–869.
- Köhl J, Kolnaar R, Ravensberg WJ. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci. 2019;10:845. doi: 10.3389/fpls.2019.00845.
- Toju H, Peay KG, Yamamichi M, et al. Core microbiomes for sustainable agroecosystems. Nat Plants. 2018;4:247–257. doi: 10.1038/s41477-018-0139-4.
- Bernardo-Cravo AP, Schmeller DS, Chatzinotas A, et al. Environmental factors and host microbiomes shape host-pathogen dynamics. Trends Parasitol. 2020;36:616–633. doi: 10.1016/j.pt.2020.04.010.
- Mannaa M, Seo YS. Plants under the attack of allies: moving towards the plant pathobiome paradigm. Plants. 2021;10:125. doi: 10.3390/plants10010125.
- Samalova M, Mélida H, Vilaplana F, et al. The β-1, 3-glucanosyltransferases (Gels) affect the structure of the rice blast fungal cell wall during appressorium-mediated plant infection. Cell Microbiol. 2017;19:12659.
- Ruiz-Herrera J, Leon CG, Carabez-Trejo A, et al. Structure and chemical composition of the cell walls from the haploid yeast and mycelial forms of Ustilago maydis. Fungal Genet Biol. 1996;20:133–142. doi: 10.1006/fgbi.1996.0028.
- Barbosa IP, Kemmelmeier C. Chemical composition of the hyphal wall from fusarium graminearum. Exp Mycol. 1993;17:274–283. doi: 10.1006/emyc.1993.1026.
- Schoffelmeer EAM, Klis FM, Sietsma JH, et al. The cell wall of fusarium oxysporum. Fungal Genet Biol. 1999;27:275–282. doi: 10.1006/fgbi.1999.1153.
- Pham TAT, Schwerdt JG, Shirley NJ, et al. Composition and biosynthetic machinery of the Blumeria graminis f. sp. hordei conidia cell wall. Cell Surf. 2019;5:100029. doi: 10.1016/j.tcsw.2019.100029.
- Wang MC, Bartnicki-Garcia S. Structure and composition of walls of the yeast form of verticillium albo-atrum. J Gen Microbiol. 1970;64:41–54. doi: 10.1099/00221287-64-1-41.
- Cantu D, Greve C, Labavitch L, et al. Characterization of the cell wall of the ubiquitous plant pathogen Botrytis cinerea. Mycol Res. 2009;113:1396–1403. doi: 10.1016/j.mycres.2009.09.006.
- O'Connell RJ, Ride JP. Chemical detection and ultrastructural localization of chitin in cell walls of Colletotrichum lindemuthianum. Physiol Mol Plant Pathol. 1990;37:39–53. doi: 10.1016/0885-5765(90)90084-B.
- Kido Y, Nagasato T, Ono K, et al. Change in a cell-wall component of Rhizoctonia solani Inhibited by Validamycin. Agric Biol Chem. 1986;50:1519–1525. doi: 10.1271/bbb1961.50.1519.
- Shayanthan A, Ordoñez PAC, Oresnik IJ. The role of synthetic microbial communities (syncom) in sustainable agriculture. Front. Agron. 2022;4:58. doi: 10.3389/fagro.2022.896307.
- Martins SJ, Pasche J, Silva HAO, et al. The use of synthetic microbial communities to improve plant health. Phytopathology®. 2023;113:1369–1379. doi: 10.1094/PHYTO-01-23-0016-IA.
- Pradhan S, Tyagi R, Sharma S. Combating biotic stresses in plants by synthetic microbial communities: principles, applications and challenges. J Appl Microbiol. 2022;133:2742–2759. doi: 10.1111/jam.15799.
- Fujikawa T, Sakaguchi A, Nishizawa Y, et al. Surface α-1, 3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants [Internet]. PLoS Pathog. 2012;8:e1002882. doi: 10.1371/journal.ppat.1002882
- Eck WHV. Chemistry of cell walls of Fusarium solani and the resistance of spores to microbial lysis. Soil Biol Biochem. 1978;10:155–157.
- Manocha MS, Colvin JR. Structure of the cell wall of Pythium debaryanum. J Bacteriol. 1968;95:1140–1152. doi: 10.1128/jb.95.3.1140-1152.1968.
- Garcia-Rubio R, Oliveira HC, Rivera J, et al. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol. 2019;10:2993. doi: 10.3389/fmicb.2019.02993.
- Wanke A, Malisic M, Wawra S, et al. Unraveling the sugar code: the role of microbial extracellular glycans in plant-microbe interactions. J Exp Bot. 2021;72:15–35. doi: 10.1093/jxb/eraa414.
- Saraiva M, Ściślak ME, Ascurra YT, et al. The molecular dialog between oomycete effectors and their plant and animal hosts. Fungal Biol Rev. 2023;43:100289. doi: 10.1016/j.fbr.2022.10.002.
- Ma Z, Zhu L, Song T, et al. A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science. 2017;355:710–714. doi: 10.1126/science.aai7919.
- Klarzynski O, Plesse B, Joubert J-M, et al. Linear β-1, 3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000;124:1027–1038. doi: 10.1104/pp.124.3.1027.
- Liu T, Liu Z, Song C, et al. Chitin-induced dimerization activates a plant immune receptor. Science. 2012;336:1160–1164. doi: 10.1126/science.1218867.
- Mélida H, Sopeña-Torres S, Bacete L, et al. Non-branched β-1,3-glucan oligosaccharides trigger immune responses in Arabidopsis. Plant J. 2018;93:34–49. doi: 10.1111/tpj.13755.
- De Lorenzo G, Cervone F. Plant immunity by damage-associated molecular patterns (DAMPs). Essays Biochem. 2022;66:459–469. doi: 10.1042/EBC20210087.
- Ngou BPM, Ding P, Jones JD. Thirty years of resistance: zig-zag through the plant immune system. Plant Cell. 2022;34:1447–1478. doi: 10.1093/plcell/koac041.
- Zhong R, Cui D, Ye ZH. Secondary cell wall biosynthesis. New Phytol. 2019;221:1703–1723. doi: 10.1111/nph.15537.
- Kumar R, Meghwanshi GK, Marcianò D, et al. Sequence, structure and functionality of pectin methylesterases and their use in sustainable carbohydrate bioproducts: a review. Int J Biol Macromol. 2023;244:125385. doi: 10.1016/j.ijbiomac.2023.125385.
- Wormit A, Usadel B. The multifaceted role of pectin methylesterase inhibitors (PMEIs. Int J Mol Sci. 2018;19:2878. doi: 10.3390/ijms19102878.
- Pogorelko G, Lionetti V, Fursova O, et al. Arabidopsis and Brachypodium distachyon transgenic plants expressing Aspergillus nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiol. 2013;162:9–23. doi: 10.1104/pp.113.214460.
- Wan J, He M, Hou Q, et al. Cell wall associated immunity in plants. Stress Biol. 2021;1:3. doi: 10.1007/s44154-021-00003-4.
- Kaur S, Samota MK, Choudhary M, et al. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol Mol Biol Plants. 2022;28:485–504. doi: 10.1007/s12298-022-01146-y.
- Underwood W. The plant cell wall: a dynamic barrier against pathogen invasion. Front Plant Sci. 2012;3:85. doi: 10.3389/fpls.2012.00085.
- Abou-Saleh RH, Hernandez-Gomez MC, Amsbury S, et al. Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures. Nat Commun. 2018;9:4538. doi: 10.1038/s41467-018-06820-y.
- Iswanto ABB, Vu MH, Pike S, et al. Pathogen effectors: what do they do at plasmodesmata? Mol Plant Pathol. 2022;23:795–804. doi: 10.1111/mpp.13142.
- Yadav S, Chattopadhyay D. Lignin: the building block of defense responses to stress in plants. J Plant Growth Regul. 2023;42:6652–6666. doi: 10.1007/s00344-023-10926-z.
- Epstein L, Nicholson R. Adhesion and adhesives of fungi and oomycetes. In Smith A, editor. Biological Adhesives. Cham: Springer; 2016. p. 25–55.
- Geoghegan IA, Gurr SJ. Chitosan mediates germling adhesion in Magnaporthe oryzae and is required for surface sensing and germling morphogenesis. PLoS Pathog. 2016;12:e1005703. doi: 10.1371/journal.ppat.1005703.
- Kots K, Meijer HJ, Bouwmeester K, et al. Filamentous actin accumulates during plant cell penetration and cell wall plug formation in Phytophthora infestans. Cell Mol Life Sci. 2017;74:909–920. doi: 10.1007/s00018-016-2383-y.
- Chethana KT, Jayawardena RS, Chen YJ, et al. Diversity and function of appressoria. Pathogens. 2021;10:746. doi: 10.3390/pathogens10060746.
- Ryder LS, Talbot NJ. Regulation of appressorium development in pathogenic fungi. Curr Opin Plant Biol. 2015;26:8–13. doi: 10.1016/j.pbi.2015.05.013.
- Choquer M, Rascle C, Gonçalves IR, et al. The infection cushion of Botrytis cinerea: a fungal ‘weapon’ of plant-biomass destruction. Environ Microbiol. 2021;23:2293–2314. doi: 10.1111/1462-2920.15416.
- Lu K, Chen R, Yang Y, et al. Involvement of the cell wall integrity pathway in signal recognition, cell wall biosynthesis and virulence in Magnaporthe oryzae [Internet]. Mol Plant-Microb Interact. 2023;36:608–622. doi: 10.1094/MPMI-11-22-0231-CR.
- Oliveira-Garcia E, Valent B. How eukaryotic filamentous pathogens evade plant recognition. Curr Opin Microbiol. 2015;26:92–101. doi: 10.1016/j.mib.2015.06.012.
- Bronkhorst J, Kots K, Jong D, et al. An actin mechanostat ensures hyphal tip sharpness in Phytophthora infestans to achieve host penetration. Sci Adv. 2022;8:eabo0875. doi: 10.1126/sciadv.abo0875.
- Grenville-Briggs LJ, Anderson VL, Fugelstad J, et al. Cellulose synthesis in Phytophthora infestans is required for normal appressorium formation and successful infection of potato. Plant Cell. 2008;20:720–738. doi: 10.1105/tpc.107.052043.
- Resjö S, Brus M, Ali A, et al. Proteomic analysis of Phytophthora infestans reveals the importance of cell wall proteins in pathogenicity. Mol Cell Proteomics. 2017;16:1958–1971. doi: 10.1074/mcp.M116.065656.
- Kubicek CP, Starr TL, Glass NL. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol. 2014;52:427–451. doi: 10.1146/annurev-phyto-102313-045831.
- Rodriguez-Moreno L, Ebert MK, Bolton MD, et al. Tools of the crook-infection strategies of fungal plant pathogens. Plant J. 2018;93:664–674. doi: 10.1111/tpj.13810.
- Perfect SE, Green JR. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol. 2001;2:101–108. doi: 10.1046/j.1364-3703.2001.00055.x.
- Wang S, Welsh L, Thorpe P, et al. The Phytophthora infestans haustorium is a site for secretion of diverse classes of infection-associated proteins. MBio. 2018;9:10–1128. doi: 10.1128/mBio.01216-18.
- Mafa MS, Visser B, Boshoff WH, et al. Flagging defensive roles of carbohydrate-active enzymes (CAZymes) and carbohydrates during Puccinia triticina-wheat interactions. Physiol Mol Plant Pathol. 2023;124:101947. doi: 10.1016/j.pmpp.2023.101947.
- Xiao M, Chen D, Liu S, et al. A chitin deacetylase Ps CDA2 from Puccinia striiformis f. sp. tritici confers disease pathogenicity by suppressing chitin-triggered immunity in wheat. Mol Plant Pathol. 2023.
- Polonio Á, Fernández-Ortuño D, Vicente A, et al. A haustorial-expressed lytic polysaccharide monooxygenase from the cucurbit powdery mildew pathogen Podosphaera xanthii contributes to the suppression of chitin-triggered immunity. Mol Plant Pathol. 2021;22:580–601. doi: 10.1111/mpp.13045.
- Fujikawa T, Kuga Y, Yano S, et al. Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol Microbiol. 2009;73:553–570. doi: 10.1111/j.1365-2958.2009.06786.x.
- Yang C, Liu R, Pang J, et al. Poaceae-specific cell wall-derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice. Nat Commun. 2021;12:2178. doi: 10.1038/s41467-021-22456-x.
- Shabbir A, Batool W, Yu D, et al. Magnaporthe oryzae chloroplast targeting endo-β-1, 4-Xylanase I MoXYL1A Regulates conidiation, appressorium maturation and virulence of the rice blast fungus. Rice (N Y). 2022;15:44. doi: 10.1186/s12284-022-00584-2.
- Derbyshire MC, Raffaele S. Till death do us pair: co-evolution of plant-necrotroph interactions. Curr Opin Plant Biol. 2023;76:102457. doi: 10.1016/j.pbi.2023.102457.
- Hou S, Liu Z, Shen H, et al. Damage-associated molecular pattern-triggered immunity in plants. Front Plant Sci. 2019;10:646. doi: 10.3389/fpls.2019.00646.
- Rafiei V, Vélëz H, Tzelepis G. The role of glycoside hydrolases in phytopathogenic fungi and oomycetes virulence. Int J Mol Sci. 2021;22:9359. doi: 10.3390/ijms22179359.
- Guo J, Cheng Y. Advances in fungal elicitor-triggered plant immunity. Int J Mol Sci. 2022;23:12003. doi: 10.3390/ijms231912003.
- Thambugala KM, Daranagama DA, Phillips AJL, et al. Fungi vs. fungi in biocontrol: an overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infect Microbiol. 2020;10:604923. doi: 10.3389/fcimb.2020.604923.
- Steyaert JM, Ridgway HJ, Elad Y, et al. Genetic basis of mycoparasitism: a mechanism of biological control by species of Trichoderma. N Z J Crop Hortic Sci. 2003;31:281–291. doi: 10.1080/01140671.2003.9514263.
- Karlsson M, Atanasova L, Jensen DF, et al. Necrotrophic mycoparasites and their genomes. Microbiol Spectr. 2017;5. doi: 10.1128/microbiolspec.funk-0016–2016.
- Mukherjee PK, Mendoza-Mendoza A, Zeilinger S, et al. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol Rev. 2022;39:15–33. doi: 10.1016/j.fbr.2021.11.004.
- Wang H, Liu R, You MP, et al. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): role of bacterial diversity. Microorganisms. 2021;9:1988. doi: 10.3390/microorganisms9091988.
- Mishra P, Mishra J, Dwivedi SK, et al. Microbial enzymes in biocontrol of phytopathogens. In: Arora N, Mishra J, Mishra V, editors. Microbial enzymes: roles and applications in industries. Microorganisms for sustainability; vol 11. Singapore: Springer; 2020.
- Reithner B, Ibarra-Laclette E, Mach RL, et al. Identification of mycoparasitism-related genes in Trichoderma atroviride. Appl Environ Microbiol. 2011;77:4361–4370. doi: 10.1128/AEM.00129-11.
- Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011;12:R40. doi: 10.1186/gb-2011-12-4-r40.
- Seidl-Seiboth V, Ihrmark K, Druzhinina IS, et al. Molecular evolution of Trichoderma chitinases. In: Gupta VK, Schmoll M, Herrera-Estrella A, et al. editors. Biotechnol Biol Trichoderma. Oxford, UK: Elsevier; 2014. p. 67–78.
- Jiang C, Miao G, Li J, et al. Identification and characterization of two novel extracellular β-Glucanases from Chaetomium globosum against Fusarium sporotrichioides. Appl Biochem Biotechnol. 2023;196:3199–3215. doi: 10.1007/s12010-023-04698-1.
- Djonović S, Pozo MJ, Kenerley CM. Tvbgn3, a β-1,6-glucanase from the biocontrol fungus trichoderma virens, is involved in mycoparasitism and control of Pythium ultimum. Appl Environ Microbiol. 2006;72:7661–7670. doi: 10.1128/AEM.01607-06.
- Migheli Q, González-Candelas L, Dealessi L, et al. Transformants of Trichoderma longibrachiatum overexpressing the beta-1,4-endoglucanas gene egl1 show enhanced biocontrol of Pythium ultimum on cucumber. Phytopathology. 1998;88:673–677. doi: 10.1094/PHYTO.1998.88.7.673.
- Geremia RA, Goldman GH, Jacobs D, et al. Molecular characterization of the proteinase-encoding gene, prb1, related to mycoparasitism by Trichoderma harzianum. Mol Microbiol. 1993;8:603–613. doi: 10.1111/j.1365-2958.1993.tb01604.x.
- Jangir M, Pathak R, Sharma S, et al. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum F. Sp. Lycopersici. Biol Control. 2018;123:60–70. doi: 10.1016/j.biocontrol.2018.04.018.
- Grabka R, d’Entremont TW, Adams SJ, et al. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants. 2022;11:384. doi: 10.3390/plants11030384.
- Wang F, Zhang L, Zhou J, et al. Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant Soil. 2022;481:1–22. doi: 10.1007/s11104-022-05621-z.
- Bonfante P. At the interface between mycorrhizal fungi and plants: the structural organization of cell wall, plasma membrane and cytoskeleton. Fungal Assoc. Berlin, Heidelberg: Springer; 2001. p. 45–61.
- Balestrini R, Bonfante P. Cell wall remodeling in mycorrhizal symbiosis: a way towards biotrophism. Front Plant Sci. 2014;5:237. doi: 10.3389/fpls.2014.00237.
- Ghorbanpour M, Omidvari M, Abbaszadeh-Dahaji P, et al. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol Control. 2018;117:147–157. doi: 10.1016/j.biocontrol.2017.11.006.
- German L, Yeshvekar R, Benitez-Alfonso Y. Callose metabolism and the regulation of cell walls and plasmodesmata during plant mutualistic and pathogenic interactions. Plant Cell Environ. 2023;46:391–404. doi: 10.1111/pce.14510.
- Cordier C, Pozo MJ, Barea JM, et al. Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. MPMI. 1998;11:1017–1028. doi: 10.1094/MPMI.1998.11.10.1017.
- Marquez N, Giachero ML, Gallou A, et al. Transcriptional changes in mycorrhizal and nonmycorrhizal soybean plants upon infection with the fungal pathogen Macrophomina phaseolina. Mol Plant Microbe Interact. 2018;31:842–855. doi: 10.1094/MPMI-11-17-0282-R.
- Song Y, Chen D, Lu K, et al. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front Plant Sci. 2015;6:786. doi: 10.3389/fpls.2015.00786.
- Rashid A. Defense responses of plant cell wall non-catalytic proteins against pathogens. Physiol Mol Plant Pathol. 2016;94:38–46. doi: 10.1016/j.pmpp.2016.03.009.
- Marrone PG. Pesticidal natural products-status and future potential. Pest Manag Sci. 2019;75:2325–2340. doi: 10.1002/ps.5433.
- Maciag T, Kozieł E, Rusin P, et al. Microbial consortia for plant protection against diseases: more than the sum of its parts. Int J Mol Sci. 2023;24:12227. doi: 10.3390/ijms241512227.
- Trivedi P, Leach JE, Tringe SG, et al. Plant-microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18:607–621. doi: 10.1038/s41579-020-0412-1.
- Feng S, Jin L, Tang S, et al. Combination of rhizosphere bacteria isolated from resistant potato plants for biocontrol of potato late blight. Pest Manag Sci. 2022;78:166–176. doi: 10.1002/ps.6618.
- Sharma R, Chauhan A, Shirkot CK. Characterization of plant growth promoting Bacillus strains and their potential as crop protectants against Phytophthora capsici in tomato. Biol Agric Hortic. 2015;31:230–244. doi: 10.1080/01448765.2015.1009860.
- Zhou X, Wang J, Liu F, et al. Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat Commun. 2022;13:7890. doi: 10.1038/s41467-022-35452-6.
- Li Z, Bai X, Jiao S, et al. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome. 2021;9:217. doi: 10.1186/s40168-021-01169-9.
- Thapa S, Prasanna R, Ramakrishnan B, et al. Microbial inoculation elicited changes in phyllosphere microbial communities and host immunity suppress Magnaporthe oryzae in a susceptible rice cultivar. Physiol Mol Plant Pathol. 2021;114:101625. doi: 10.1016/j.pmpp.2021.101625.
- Xu Y, Chen Z, Li X, et al. Mycorrhizal fungi alter root exudation to cultivate a beneficial microbiome for plant growth. Funct Ecol. 2023;37:664–675. doi: 10.1111/1365-2435.14249.
- Lemanceau P, Blouin M, Muller D, et al. Let the core microbiota be functional. Trends Plant Sci. 2017;22:583–595. doi: 10.1016/j.tplants.2017.04.008.
- Oyserman BO, Medema MH, Raaijmakers JM. Road MAPs to engineer host microbiomes. Curr Opin Microbiol. 2018;43:46–54. doi: 10.1016/j.mib.2017.11.023.
- Ke J, Wang B, Yoshikuni Y. Microbiome engineering: synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 2021;39:244–261. doi: 10.1016/j.tibtech.2020.07.008.
- Li Z, Ye X, Liu M, et al. A novel outer membrane β-1, 6-glucanase is deployed in the predation of fungi by myxobacteria. ISME J. 2019;13:2223–2235. doi: 10.1038/s41396-019-0424-x.
- Ye X, Xu C, Xie T, et al. Myxobacterial outer membrane β-1, 6-glucanase induced the cell death of fusarium oxysporum by destroying the cell wall integrity. Appl Environ Microbiol. 2023;89:e0123622. doi: 10.1128/aem.01236-22.
- Li J, Liu W, Luo L, et al. Expression of Paenibacillus polymyxa β-1, 3-1, 4-glucanase in Streptomyces lydicus A01 improves its biocontrol effect against Botrytis cinerea. Biol Control. 2015;90:141–147. doi: 10.1016/j.biocontrol.2015.06.008.
- Xu T, Zhu T, Li S. gene from Bacillus velezensis ZJ20 exerts antifungal effect on plant pathogenic fungi. World J Microbiol Biotechnol. 2016;32:26. β-1, 3-1:4-. doi: 10.1007/s11274-015-1985-0.
- Pang Y, Yang J, Chen X, et al. An antifungal chitosanase from Bacillus subtilis SH21. Molecules. 2021;26:1863. doi: 10.3390/molecules26071863.
- Song YS, Seo DJ, Jung WJ. Characterization and antifungal activity of chitosanase produced by Pedobacter sp. PR-M6. Microb Pathog. 2019;129:277–283. doi: 10.1016/j.micpath.2019.02.026.
- Gupta V, Prasanna R, Srivastava AK, et al. Purification and characterization of a novel antifungal endo-type chitosanase from Anabaena fertilissima. Ann Microbiol. 2012;62:1089–1098. doi: 10.1007/s13213-011-0350-2.
- Kappel L, Münsterkötter M, Sipos G, et al. Chitin and chitosan remodeling defines vegetative development and Trichoderma biocontrol. PLoS Pathog. 2020;16:e1008320. doi: 10.1371/journal.ppat.1008320.
- Eitzen K, Sengupta P, Kroll S, et al. A fungal member of the Arabidopsis thaliana phyllosphere antagonizes Albugo laibachii via a GH25 lysozyme. Elife. 2021;10:65306. doi: 10.7554/eLife.65306.
- Ait-Lahsen H, Soler A, Rey M, et al. An antifungal exo-α-1, 3-glucanase (AGN13. 1) from the biocontrol fungus Trichoderma harzianum. Appl Environ Microbiol. 2001;67:5833–5839. doi: 10.1128/AEM.67.12.5833-5839.2001.
- Sanz L, Montero M, Redondo J, et al. Expression of an α-1, 3-glucanase during mycoparasitic interaction of Trichoderma asperellum. FEBS J. 2005;272:493–499. doi: 10.1111/j.1742-4658.2004.04491.x.
- Suyotha W, Yano S, Itoh T, et al. Characterization of α-1, 3-glucanase isozyme from Paenibacillus glycanilyticus FH11 in a new subgroup of family 87 α-1, 3-glucanase. J Biosci Bioeng. 2014;118:378–385. doi: 10.1016/j.jbiosc.2014.03.008.
- Romero-Contreras YJ, Ramírez-Valdespino CA, Guzmán-Guzmán P, et al. Tal6 from Trichoderma atroviride is a LysM effector involved in mycoparasitism and plant association. Front Microbiol. 2019;10:2231. doi: 10.3389/fmicb.2019.02231.
- Vargas WA, Djonović S, Sukno SA, et al. Dimerization controls the activity of fungal elicitors that trigger systemic resistance in plants. J Biol Chem. 2008;283:19804–19815. doi: 10.1074/jbc.M802724200.
- Gaderer R, Lamdan NL, Frischmann A, et al. Sm2, a paralog of the Trichoderma cerato-platanin elicitor Sm1, is also highly important for plant protection conferred by the fungal-root interaction of Trichoderma with maize. BMC Microbiol. 2015;15:2. doi: 10.1186/s12866-014-0333-0.
- Chang SC, Saldivar RK, Liang PH, et al. Structures, Biosynthesis, and Physiological Functions of (1,3;1,4)-β-D-Glucans. Cells [Internet]. 2021;10:510. doi: 10.3390/cells10030510.
- Rebaque D, Del Hierro I, López G, et al. Cell wall-derived mixed-linked β-1, 3/1, 4-glucans trigger immune responses and disease resistance in plants. Plant J. 2021;106:601–615. doi: 10.1111/tpj.15185.
- Wang S, Ng TB, Chen T, et al. First report of a novel plant lysozyme with both antifungal and antibacterial activities. Biochem Biophys Res Commun. 2005;327:820–827. doi: 10.1016/j.bbrc.2004.12.077.
- Kappel L, Yu L, Escobar C, et al. A comparative cell wall analysis of Trichoderma spp. confirms a conserved polysaccharide scaffold and suggests an important role for chitosan in mycoparasitism. Microbiol Spectr. 2024;e03495–23. doi: 10.1128/spectrum.03495-23.
- Akcapinar GB, Kappel L, Sezerman OU, et al. Molecular diversity of LysM carbohydrate-binding motifs in fungi. Curr Genet. 2015;61:103–113. doi: 10.1007/s00294-014-0471-9.
- de Oliveira AL, Gallo M, Pazzagli L, et al. The structure of the elicitor Cerato-platanin (CP), the first member of the CP fungal protein family, reveals a double psibeta-barrel fold and carbohydrate binding. J Biol Chem. 2011;286:17560–17568. doi: 10.1074/jbc.M111.223644.
- Mesny F, Miyauchi S, Thiergart T, et al. Genetic determinants of endophytism in the Arabidopsis root mycobiome. Nat Commun. 2021;12:7227. doi: 10.1038/s41467-021-27479-y.
- Gong Y, Lebreton A, Zhang F, et al. Role of carbohydrate-active enzymes in mycorrhizal symbioses. Essays Biochem. 2023;67:471–478. doi: 10.1042/EBC20220127.
- Liu H, Brettell LE, Qiu Z, et al. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020;25:733–743. doi: 10.1016/j.tplants.2020.03.014.
- Kwak MJ, Kong HG, Choi K, et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol. 2018;36:1100–1109. doi: 10.1038/nbt.4232.
- Li PD, Zhu ZR, Zhang Y, et al. The phyllosphere microbiome shifts toward combating melanose pathogen. Microbiome. 2022;10:56. doi: 10.1186/s40168-022-01234-x.
- Carrión VJ, Perez-Jaramillo J, Cordovez V, et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366:606–612. doi: 10.1126/science.aaw9285.
- Liao LB, Chen XX, Xiang J, et al. Zanthoxylum bungeanum root-rot associated shifts in microbiomes of root endosphere, rhizosphere, and soil. PeerJ. 2022;10:e13808. doi: 10.7717/peerj.13808.
- Onyango SO, Juma J, Paepe K, et al. Oral and gut microbial carbohydrate-active enzymes landscape in health and disease. Front Microbiol. 2021;12:653448. doi: 10.3389/fmicb.2021.653448.
- Neves AL, Yu J, Suzuki Y, et al. Accelerated discovery of novel glycoside hydrolases using targeted functional profiling and selective pressure on the rumen microbiome. Microbiome. 2021;9:229. doi: 10.1186/s40168-021-01147-1.
- Dort E, Layne E, Feau N, et al. Large-scale genomic analyses with machine learning uncover predictive patterns associated with fungal phytopathogenic lifestyles and traits [Internet]. Sci Rep. 2023;13:17203. doi: 10.21203/rs.3.rs-2778162/v1.
- Molina A, Miedes E, Bacete L, et al. Arabidopsis cell wall composition determines disease resistance specificity and fitness. Proc Natl Acad Sci. 2021;118:e2010243118.
- Limón MC, Pintor-Toro JA, Benítez T. Increased antifungal activity of Trichoderma harzianum transformants that overexpress a 33-kDa chitinase. Phytopathology. 1999;89:254–261. doi: 10.1094/PHYTO.1999.89.3.254.
- Limón MC, Chacón MR, Mejías R, et al. Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Appl Microbiol Biotechnol. 2004;64:675–685. doi: 10.1007/s00253-003-1538-6.
- Eslahi N, Kowsari M, Zamani M, et al. The profile change of defense pathways in Phaseouls vulgaris L. by biochemical and molecular interactions of Trichoderma harzianum transformants overexpressing a chimeric chitinase. Biol Control. 2021;152:104304. doi: 10.1016/j.biocontrol.2020.104304.
- Jones TS, Oliveira SM, Myers CJ, et al. Genetic circuit design automation with Cello 2.0. Nat Protoc. 2022;17:1097–1113. doi: 10.1038/s41596-021-00675-2.
- Stoof R, Wood A, Goñi-Moreno Á. A model for the spatiotemporal design of gene regulatory circuits. ACS Synth Biol. 2019;8:2007–2016. doi: 10.1021/acssynbio.9b00022.
- Kang M, Choe D, Kim K, et al. Synthetic biology approaches in the development of engineered therapeutic microbes. Int J Mol Sci. 2020;21:8744. doi: 10.3390/ijms21228744.
- Dundas CM, Dinneny JR. Genetic circuit design in rhizobacteria. Biodes Res. 2022;2022:9858049. Available from: doi: 10.34133/2022/9858049.
- Miller WG, Brandl MT, Quiñones B, et al. Biological sensor for sucrose availability: relative sensitivities of various reporter genes. Appl Environ Microbiol. 2001;67:1308–1317. doi: 10.1128/AEM.67.3.1308-1317.2001.
- Ryu MH, Zhang J, Toth T, et al. Control of nitrogen fixation in bacteria that associate with cereals. Nat Microbiol. 2020;5:314–330. doi: 10.1038/s41564-019-0631-2.
- Grondin JM, Tamura K, Déjean G, et al. Polysaccharide utilization loci: fueling microbial communities. J Bacteriol. 2017;199:10–1128. doi: 10.1128/JB.00860-16.
- Pan X, Raaijmakers JM, Carrión VJ. Importance of Bacteroidetes in host-microbe interactions and ecosystem functioning. Trends Microbiol. 2023;31:959–971. doi: 10.1016/j.tim.2023.03.018.
- Tan Y, Liang J, Lai M, et al. Advances in synthetic biology toolboxes paving the way for mechanistic understanding and strain engineering of gut commensal Bacteroides spp. Clostridium Spp. Biotechnol Adv. 2023;69:108272. doi: 10.1016/j.biotechadv.2023.108272.
- Temple MJ, Cuskin F, Baslé A, et al. A Bacteroidetes locus dedicated to fungal 1, 6-β-glucan degradation: unique substrate conformation drives specificity of the key endo-1, 6-β-glucanase. J Biol Chem. 2017;292:10639–10650. doi: 10.1074/jbc.M117.787606.
- Terrapon N, Lombard V, Drula E, et al. PULDB: the expanded database of polysaccharide utilization loci. Nucleic Acids Res. 2018;46:677–683.
