The protein folding problem (PFP), which is still considered to be one of the most daunting challenges for scientists, is the question of how a protein’s amino acid sequence dictates its three-dimensional protein structure. The notion of a folding “problem” first emerged around 1960, with the appearance of the first atomic-resolution protein structures (Dill, Ozcan, Shell, & Weikl, Citation2008). The major milestones in PFP solving were Anfinsen’s thermodynamic hypothesis and Levinthal paradox, which made in the late of 1960s and early of 1970s (Anfinsen, Citation1973; Haber & Anfinsen, Citation1962; Levinthal, Citation1968). During the last 40 years, besides the traditional molecular biological techniques, many new theoretical and computational approaches have arisen, such as multiple-sequence alignments, molecular dynamics simulations, and the Critical Assessment of Techniques for Protein Structure Prediction (CASP) community-wide event for protein structure prediction. Current computer algorithms are now predicting native structures of small proteins with remarkable accuracy. Especially, for the once seemingly intractable Levinthal puzzle, there is a viable hypothesis now: A protein can fold quickly and solve its big global optimization puzzle by piecewise solutions of smaller component puzzles (Dill et al., Citation2008; Fiebig & Dill, Citation1993).
Recently, Ben-Naim made attempts to resolve the Levinthal puzzle from a new perspective (Ben-Naim, Citation2012). In his opinion, the searching for a solution to the PFP as formulated by Levinthal and reformulated by Kennedy and Norman (Citation2005) has gone astray twice: (i) in searching for target-based solutions; and (ii) by adherence to the hydrophobic dominance dogma. Instead, he believed that the protein folding is cause-biased at each stage of the folding process, and the most important part of the “cause” is the hydrophilic force (Ben-Naim, Citation2012).
For a decade, our group has been constantly devoting to the studies on protein misfolding diseases. The conformational conversion of amyloid proteins is associated with numerous protein aggregation pathologies and infectious properties. We will comment on this issue based on the data obtained from molecular biological and molecular dynamic studies on amyloid-prone cystatin.
Mutations in the hydrophobic core of cystatin lead to amyloidosis
The cystatins are tight and reversible binding inhibitors of the papain-like cysteine proteinases. They form a superfamily of homologous proteins, of which human cystatin C (hCC) and chicken cystatin (cC, Figure ) are the representative ones. Human cystatin C amyloid angiopathy is a dominantly inherited disorder characterized by tissue deposition of amyloid in blood vessels that leads to recurrent hemorrhagic stroke (Gudmundsson, Hallgrimsson, Jonasson, & Bjarnason, Citation1972). Especially, the hCC L68Q variant, a mutation located in the hydrophobic core of the protein, is the causative agent of hCC amyloid angiopathy (Abrahamson, Citation1996).
Figure 1 The cC monomeric structure with selected residues Ile66, Ile108 in the hydrophobic core, and Val55 in Loop 1.
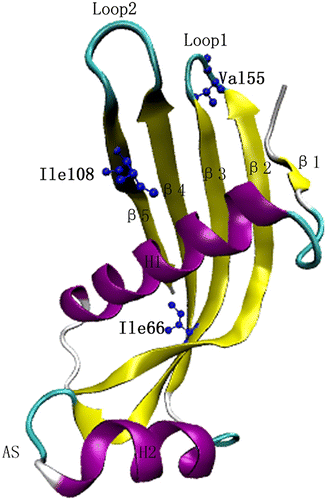
In our previous research, cC I66Q (corresponding to hCC L68Q) was successfully secreted by yeasts Pichia pastoris and Saccharomyces cerevisiae (He, Song, Ueyama, Azakami, & Kato, Citation2005). The large amounts of insoluble aggregate and polymeric form cC I66Q besides the monomer and dimer forms were observed in the culture medium. During storage of cC I66Q monomer under physiological and acidic conditions, typical binding with Congo red and thioflavin T and the formation of amyloid fibrils in the electron microscopy (EM) were observed, whereas the characteristic of similar amyloidosis was hardly detected for the wild-type recombinant cystatin (He et al., Citation2005). Interestingly, our study also revealed that the nonglycosylated form of I108T, another mutant in the hydrophobic core in cC, showed similar effects to amyloid mutant cC I66Q (He et al., Citation2006). The thioflavin T fluorescence value of I108T showed remarkable increase after 18 days of incubation, and typical amyloid fibrils were clearly observed for the nonglycosylated form of I108T in the EM after 35 days of incubation (He et al., Citation2006). These data suggest that the interior hydrophobic core of cystatin provides the structural stability favoring the native state during the protein folding.
Structural and dynamic properties of amyloid mutant cC I66Q and I108T
To explore the detailed structural and dynamic properties of the amyloid mutant cC I66Q and I108T, molecular dynamic (MD) simulations of the I66Q, I108T mutant, and wild-type cCs were performed under conditions that enable forming amyloid fibrils in our previous biological experiments (He et al., Citation2005, Citation2006). The MD results showed that both the I66Q and I108T mutants exhibited relatively large secondary structure changes and obvious expanding tendency of hydrophobic core compared to wild-type cC (Yu et al., Citation2010, Citation2012). More importantly, the appendant structure (AS) of the typical amyloid mutant cC I66Q showed a large displacement and distortion toward its hydrophobic core (Yu et al., Citation2012). The structural analysis on cystatin monomer suggested that structural changes in the AS might make the hydrophobic core expand more easily. In addition, analysis on docking dimer has shown that the distorted AS was in favor of intermolecular interactions between two cystatin monomers. Data from an independent theoretical derived algorithm as well as biological experiments also support this hypothesis (Yu et al., Citation2012).
Effect of Val55 mutations on the structural stability of amyloid mutant cC I66Q
Staniforth et al. have reported that cC V55D mutant, a hydrophilic mutant in the Loop 1, can keep monomer stage following refolding from 7 M GdnHCl then storage for 24 h at 20 °C and after heat treatment at 90 °C for 30 min (Staniforth et al., Citation2001). Residue 55 in cC corresponds to residue 57 in hCC. Using MD simulation with a constructed β2-L1-β3 peptide, Rodziewicz-Motowidlo et al. have found that the Asp57 and Asn57 mutations in the Loop 1 of hCC could stabilize the closed form of hCC, whereas the Pro57 mutation could lead to the opening of the hCC structure and then to dimer/oligomer formation (Rodziewicz-Motowidlo et al., Citation2009). These results suggested that beside the hydrophobic factors, hydrophilic factors are also related to the folding and stability maintenance of cystatin protein.
To probe the detailed relationship between hydrophilic and hydrophobic factors, we performed MD simulations to investigate the effect of Val55 mutation on the structural stability of amyloid mutant cC I66Q. The RMSD profiles indicated that the conformation of the protein appeared to be equilibrated after 3 ns and the RMSD curves of V55D/I66Q, and V55 N/I66Q mutants were similar with wild-type cC (Figure ), indicating that both V55 N and V55D could enhance the structural stability of cC I66Q, especially in the case of V55 N. Remarkably, both second-site mutations obviously stabilize the RMSD curves of the hydrophobic core comparing with I66Q mutant (Figure ). Since the intramolecular hydrogen bond (HB) is most obvious in several kinds of hydrophilic forces, we further move on to analyze the intramolecular HBs. As shown in Table , there were more HBs in V55 N/I66Q and V55D/I66Q mutants compared with I66Q and even wild-type cC. This indicated that the HBs significantly contribute to the stability of the cC and might inhibit the process of domain swapping of cC I66Q. Together, our data suggested that the instability induced by the mutant in hydrophobic core could be rescued by its hydrophilic mutants in Loop 1. In other words, although the hydrophobic force is important on protein folding, the hydrophilic force seems more important in the case of cC protein.
Figure 2 Time dependence of the RMSD from the total amino acid of cC for the Cα (A) and the hydrophobic core of cC for the backbone (B) in the 10 ns MD simulation.
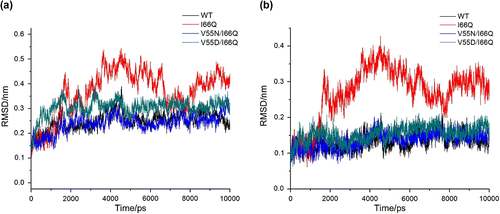
Table 1. The intramolecular HBs number in mutants and wild-type cC.
PFP is still a complicated puzzle in biology. In our previous comment “The yeast prion case: Could there be a uniform concept underlying complex protein folding?”, we commented that special amino acids should be crucial for the structural features of certain type of proteins, and it might only be in a limited fixed type of proteins that the occurrence of amino acids (stoichiometry) determines the structural features (Song, Song, & Chen, Citation2011). This is a sequel to that one –and in the present article, our opinion for supporting the thesis of Ben-Naim on one kind of specific protein. However, as pointed out by Ben-Naim himself, any successful simulation of protein folding provides a specific answer to a specific protein, and it can never offer a general solution to the PFP (Ben-Naim, Citation2012). Thus, the results obtained here cannot be employed to prove that all the protein folding features of all proteins are in accordance with this principle. This means it is difficult to disprove that hydrophobic interactions play a major role in protein folding from current limited data. Nevertheless, “Ben-Naim’s answer” is worthy of being studied deeply but not criticized arbitrarily.
Acknowledgment
This work was supported by grants from National Natural Science Foundation of China (No. 30970152) and partially sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China (No. 2010-1561). We are grateful to Mengyuan Zhang for technical assistance in MD simulations.
References
- Abrahamson , M. 1996 . Molecular basis for amyloidosis related to hereditary brain hemorrhage . Scandinavian Journal of Clinical and Laboratory Investigation , 56 : 47 – 56 .
- Anfinsen , C. B. 1973 . Principles that govern the folding of protein chains . Science , 181 : 223 – 230 .
- Ben-Naim , A. 2012 . Levinthal’s question revisited, and answered . Journal of Biomolecular Structure & Dynamics , 30 : 113 – 124 .
- Dill , K. A. , Ozcan , S. B. , Shell , M. S. and Weikl , T. R. 2008 . The protein folding problem . Annual Review Biophysics , 37 : 289 – 316 .
- Fiebig , K. M. and Dill , K. A. 1993 . Protein core assembly processes . Journal of Chemical Physics , 98 : 3475 – 3487 .
- Gudmundsson , G. , Hallgrimsson , J. , Jonasson , T. A. and Bjarnason , O. 1972 . Hereditary cerebral hemorrhage with amyloidosis . Brain , 95 : 387 – 404 .
- Haber , E. and Anfinsen , C. B. 1962 . Side-chain interactions governing the pairing of half-cystine residues in ribonuclease . Journal of Biological Chemistry , 237 : 1839 – 1844 .
- He , J. , Song , Y. , Ueyama , N. , Azakami , H. and Kato , A. 2005 . Characterization of recombinant amyloidogenic chicken cystatin mutant I66Q in yeast . Journal of Biochemistry , 137 : 477 – 485 .
- He , J. , Song , Y. , Ueyama , N. , Saito , A. , Azakami , H. and Kato , A. 2006 . Prevention of amyloid fibril formation of amyloidogenic chicken cystatin by site-specific glycosylation in yeast . Protein Science , 15 : 213 – 222 .
- Kennedy , D. and Norman , C. 2005 . What don’t we know? . Science , 309 : 75
- Levinthal , C. 1968 . Are there pathways for protein folding . Journal de Chimie Physique , 65 : 44 – 45 .
- Rodziewicz-Motowidlo , S. , Iwaszkiewicz , J. , Sosnowska , R. , Czaplewska , P. , Sobolewski , E. , Szymanska , A. , … and Liwo , A. 2009 . The role of the Val57 amino-acid residue in the hinge Loop of the Human cystatin C conformational studies of the Beta2-L1-Beta3 segments of wild-type Human cystatin C and its mutants . Biopolymers , 91 : 373 – 383 .
- Song , Y. , Song , Y. and Chen , X. 2011 . The yeast prion case: Could there be a uniform concept underlying complex protein folding? . Journal of Biomolecular Structure & Dynamics , 28 : 663 – 665 .
- Staniforth , R. , Giannini , S. , Higgins , L. , Conroy , M. , Hounslow , A. , Jerala , R. , … and Waltho , J. 2001 . Three-dimensional domain swapping in the folded and molten-globule states of cystatins, an amyloid-forming structural superfamily . EMBO Journal , 20 : 4774 – 4781 .
- Yu , Y. , Wang , Y. , He , J. , Liu , Y. , Li , H. , Zhang , H. and Song , Y. 2010 . Structural and dynamic properties of a new amyloidogenic chicken cystatin mutant I108T . Journal of Biomolecular Structure & Dynamics , 27 : 641 – 649 .
- Yu , Y. , Liu , X. , He , J. , Zhang , M. , Li , H. , Wei , D. and Song , Y. 2012 . Appendant structure plays an important role in amyloidgenic cystatin dimerization prior to domain swapping . Journal of Biomolecular Structure & Dynamics , 30 : 102 – 112 .