Abstract
In December 2019, the world observed an unexpected outbreak of an emerging disease named coronavirus (COVID-19) that was first reported in Wuhan city of Hubei province of China. Recent literature has shown the association between COVID-19 infection and derangement in the coagulation profile. In this paper, we are discussing thrombo-genesis, especially the role of the complement system in the immune response against COVID-19 and the pathogenesis associated with tissue inflammation and thrombosis. This role can stipulate a groundwork for further investigation of the pathophysiologic importance of complement in COVID-19, and could propose targets for specific intervention. In addition, we delineated current treatments for thrombosis and the potential therapies by using agents to block the terminal complement pathway. Low molecular weight heparin for all (unless contraindicated) hospitalized COVID-19 patients can be lifesaving. Agents that inhibit the terminal events of the complement cascade might be crucial for ensuring an efficient treatment, decrease clots and permit early discharge in relation to COVID-19.
Communicated by Ramaswamy H. Sarma
Keywords:
1. Introduction
In December 2019, the world observed an unexpected outbreak of an emerging disease named coronavirus (COVID-19) that was first reported in Wuhan city of Hubei province of China (Pant et al., Citation2020).
Three months later, the World Health Organization (WHO) announced that the COVID-19 outbreak has progressed into a global pandemic (Wu et al., Citation2020). Recent literature has shown the association between COVID-19 infection and derangement in the coagulation profile (Wu et al., 2020), that can lead to disseminated intravascular coagulation (DIC) in up to 70% of non-surviving COVID-19 patients (Tang et al., Citation2020). Within this framework, the association between coagulation disorder and the clinical progression to acute respiratory distress syndrome has been a substantial complication with poor outcomes (Wu et al., 2020), including organ dysfunction (Tang et al., Citation2020; Zhu et al., Citation2020).
Main targets of COVID-19 can comprise the vascular endothelial cell, lung epithelial cell, and lymphocytes that can eventually present with severe presentation such as shock, acute respiratory distress syndrome (ARDS) and coagulopathy (Guan et al., Citation2020; Wang, Hu, et al., Citation2020). The pathological observations of bacterial sepsis-associated ARDS are outlined as austere interstitial and alveolar edema with significant neutrophil infiltration, vascular permeability, stenosis of the vascular lumen, vasculature including wall thickening, and micro-thrombus formation (Luo et al., Citation2020).
Despite the overwhelming data portraying the effect of COVID-19 on respiratory failure in up to 20% of symptomatic patients (Marini & Gattinoni, Citation2020), minute attention has been given to endothelial dysfunction and clotting outcomes in severe infection (Hendaus & Jomha, Citation2020; Roumenina et al., Citation2016). Patients with severe COVID-19 can develop extensive microvascular thrombosis and the utilization of coagulation factors. This is revealed by the elevation of D-dimer, thrombocytopenia, decreased fibrinogen levels and prolongation of the prothrombin time (Tang et al., Citation2020). In addition, microangiopathy with schistocytes has been detected on the peripheral smear in COVID-19 patients (Lee et al., Citation2020).
In this paper, we are discussing thrombo-genesis, especially the role of the complement system in the immune response against COVID-19 and the pathogenesis associated tissue inflammation and thrombosis. This role can stipulate a groundwork for further investigation of the pathophysiologic importance of complement in COVID-19, and could propose targets for specific intervention.
In addition, we delineated current treatments for thrombosis and the potential therapies by using agents to block the terminal complement pathway.
2. Clinical studies
Thus far, there have been several studies reporting coagulation activation, particularly in critically ill patients with COVID-19 infection. In three studies, the clinical characteristics of novel coronavirus pneumonia (NCP) patients have been explored, and mortalities ranged from 4.3% to 14.6%, accompanied by organ dysfunction and coagulopathy (Chen et al., Citation2020; Huang et al., Citation2020; Wang, Hu, et al., Citation2020).
In a multicenter prospective cohort study, Helms et al. (Citation2020) assessed the thrombotic risk in severe forms of COVID-19 infection. The study that included 150 COVID-19 patients showed that the majority of patients (> 95%) had elevated D-dimer and fibrinogen, while none of the patients advanced into disseminated intravascular coagulation. Moreover, Von Willebrand (vWF) activity, vWF antigen and factor VIII were elevated, demonstrating inflammation-mediated endothelial triggered pro-coagulant state.
Tang et al. (Citation2020) retrospectively analyzed coagulation results and outcomes of 183 COVID-19 ill patients. The study has shown that the non-survivors had significantly higher D-dimer [2.12 (0.77‐5.27)] and fibrin degradation product (FDP) levels [7.6 (4.0–23.4)], longer prothrombin time [15.5 (14.4–16.3)], and activated partial thromboplastin time compared to survivors [44.8 (40.2‐51.0)].
Han et al. (Citation2020) prospectively collected blood coagulation data in 94 patients with confirmed COVID-19 infection who were admitted in Renmin Hospital of Wuhan University. Data was also collected from 40 healthy controls during the same period. The study showed that the prothrombin time (PT)-activity was lower in the COVID-19 patients compared to control group (81% vs. 97%; p < 0.001). However, the differences were more noticeable in D-dimer and fibrin/fibrinogen degradation products (FDP) testing (10.36 vs. 0.26 ng/L; p < 0.001, and 33.83 vs. 1.55 mg/L; p < 0.001, respectively).
Guan et al. (2020) reviewed data regarding 1099 patients with COVID-19 from 552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China through January 29, 2020.The study showed that the D-dimer levels were more than 0.5 mg/L in 260/560 (46.4%) cases.
In a recent report, Magro et al. (Citation2020) inspected lung and skin tissues from 5 patients with severe COVID-19 presented with respiratory failure (n = 5) and purpuric skin rash (n = 3). Biopsies from the lungs showed substantial deposits of terminal complement components C5b-9 (membrane attack complex), mannose binding lectin (MBL)-associated serine protease (MASP)2, and C4d in the microvasculature. Those findings most likely insinuate persistent, systemic activation of the complement pathways.
3. Pathophysiology
Several reported theories have explained the possibility of thrombosis in COVID-19 illness. Direct viral invasion of endothelial cells (Hendaus & Jomha, Citation2020) or indirect activation mediated by complement (Henry et al., Citation2020) might contribute to cell dysfunction and exocytosis of vWF, as well as platelet activation, leading to micro-thrombogenesis.
Complement system damages endothelium of blood vessels, this leads to contact of blood to subendothelial structures that directly induces activation of blood coagulation cascade (van Hinsbergh, Citation2012).
In addition, the outburst ‘cytokine storm’ leads to dysregulation of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6, which eventually lead to the proliferation of the megakaryocytes causing thrombocytosis (Soy et al., Citation2020). The correlation among a hypercoagulable state, elevated endothelial surrogate markers and the clinical presentation of the COVID-19 respiratory failure, indicates an important role played by endothelial damage and inflammation during severe SARS-CoV-2 infection (Escher et al., Citation2020).
COVID-19 binds with Angiotensin-converting enzyme 2 (ACE2) on the cell membrane of the host cells. This enzyme has been found in venous and arterial endothelial cells in numerous human tissues, including lung, small intestine, colon, skin, the oral and nasal mucosa, bone marrow, spleen, kidney, lymph nodes, thymus, and brain (Hamming et al., Citation2004). ACE2 plays a crucial role in the initiation of COVID-19 infection by attaching to the endothelial cells and hence having the domino effect on the infection and defense processes including the complement system.
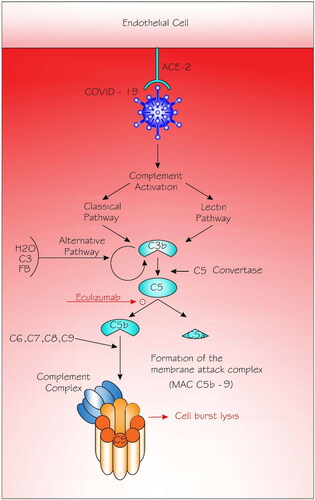
The complement system is the host immune scheme’s initial response to infection. However, uninhibited complement activation furthers cell injury, inflammation, intravascular coagulation, and it eventually results in multiple organ failure and death. The complement system comprise of more than 30 proteins and is responsible for the activation of 3 pathways—the classical, the lectin, and the alternative (). The classical pathway is stimulated by IgM/IgG-antigen complex then binding to the complement component (C) 1 complex, including C1q, C1r, and C1s molecules. As for the lectin pathway, COVID-19 interacts with mannose binding lectin (MBL), a family of proteins differentiated by the presence of collagen-like and lectin-binding domains. MBL is synthesized in the liver and secreted into the blood, and it plays a crucial role in innate immune defense (Ip et al., Citation2005). It actuates the mannose-binding protein–associated serine protease 2 to cleave C4 and C2 and commence the complement lectin pathway, resulting in the formation of the C3 convertase (C4bC2a) of the classical/lectin pathways .The alternative pathway is constantly activated by hydrolysis (tick-over) of C3 that forms C3(H2O). The latter binds to factor B (fB), which is eventually cleaved by factor D (fD) to form the alternative pathway fluid-phase initiation C3 convertase. Later, the C3 convertases cleave C3 into C3a, which is considered an anaphylotoxin, and C3b, buildup on cell surfaces (Thiel et al., Citation2009). Worth mentioning is that C3b synthesized by any of the 3 pathways can impact the formation of the alternative pathway amplification C3 convertase, which cleaves more C3 molecules, causing an amplification loop. Moreover, C3b contributes to the configuration of the C5 convertases that cleave C5, leading to the production of the anaphylatoxin C5a that attracts and activates inflammatory leukocytes, and C5b (Fromell et al., Citation2020). C5a directly reciprocates with its C5aR on endothelial cells (McCullough et al., Citation2018), and produces intense changes of the physiologically thrombo-resistant endothelium, such as upregulation of tissue factor and profound decrease of thrombo-modulin, leading to platelet aggregation and adhesion (Gasque et al.,Citation1997). As for C5b, it instigates the terminal events of complement activation, resulting in the formation of the membrane- attack complex (MAC or C5b-9 complex), which implants itself into cell membranes, leading cell injury, lysis and dysfunction (Kim & Conway, Citation2019; Morgan et al., Citation2017). The terminal pathway which combine the three pathways might be playing a reasonable role in the in the COVID-19–associated tissue inflammatory injury (Noris et al., Citation2020). This is consistent with the results of the study by Magro et al. (Citation2020) showing substantial deposits of terminal complement components C5b-9 (membrane attack complex), mannose binding lectin (MBL)-associated serine protease (MASP)2, and C4d in the microvasculature of the lungs.
Figure 1. Complement activation in COVID-19 infection.
Angiotensin-converting enzyme (ACE2) plays a crucial role in the initiation of COVID-19 infection by attaching to the endothelial cells and hence having the domino effect on the infection and defense processes including the complement system. The classical pathway is triggered by antibody or by direct binding of complement component C1q (not shown) to the pathogen surface; the lectin pathway, which is triggered by mannan-binding lectin binds some encapsulated bacteria; and the alternative pathway is triggered directly on pathogen surfaces. Classical and lectin pathways are activated resulting in the formation of the C3b. The alternative pathway is activated by hydrolysis (tick-over) of C3 that forms C3 (H2O). The latter binds to factor B (fB), which is eventually cleaved by factor D (not shown) to form the alternative pathway fluid-phase initiation C3 convertase. The C3 convertases cleave C3 into C3a and C3b. Eventually, C3b contributes to the configuration of the C5 convertases that cleave C5, leading to the production of C5a and C5b. It is C5b that instigates the terminal events of complement activation, resulting in the formation of the membrane-attack complex (MAC or C5b-9 complex). Eculizumab (a drug, and not part of the complement cascade) inhibits the cleavage of C5 into C5a and C5b and hence prevents the generation of the terminal complement complex C5b-9.
4. Treatment
Many global guidelines recommend that all confirmed or suspected COVID-19 patients admitted to the hospital should be on venous thrombosis prophylaxis, if not contraindicated (Susen et al., Citation2020; Thachil et al., Citation2020).
Low molecular weight heparin, or unfractionated heparin should be favored over direct oral anticoagulants due to possible drug‐drug interactions associated with the oral route. Such treatments interaction can affect CYP3A4, CYP450 and/or the transporter permeability glycoprotein (P-gp), augmenting the bleeding risk or reduce the antithrombotic effect (Thachil et al., Citation2020).The suggested dose for anticoagulation agent as prophylaxis varies among centers. Some institutions used successfully ‘intermediate’ dose such as 0.5 mg/kg twice a day of enoxaparin, utilizing a risk-adapted strategy based on levels of fibrinogen, D-dimer, and co-morbidities (Bikdeli et al., Citation2020), while others have used full-dose anticoagulation in COVID-19 patients for preventing microvascular thrombosis (Susen et al.,Citation2020). So far, there is not enough data to support either approach. Furthermore, heparin displays non-anticoagulant properties as capabilities to impede neutrophil chemotaxis and leukocyte migration through the endothelium, to attach to inflammatory cytokines, to confiscate acute phase reactants and to counteract C5a (Li & Vlodavsky, Citation2009; Young, Citation2008).
Fibrinolytic agents have also been used. Wang, Hajizadeh, et al. (Citation2020) have used tissue plasminogen activator (tPA) for the management of COVID-19 associated acute respiratory distress syndrome in 3 critically ill patients. The authors reported a temporally related amelioration in their respiratory status, with one of them has had a lasting response (Wang, Hajizadeh, et al., Citation2020).
Complement inhibition could be efficient potential candidate as a therapeutic step in treating COVID-19 (Risitano et al., Citation2020). The literature has shown that complement inhibition agents as efficient therapeutic targets in neuro-inflammatory and hematological diseases (Olson et al., Citation2018; Pittock et al., Citation2019).
Eculizumab is a long-acting humanized monoclonal antibody targeted against complement protein C5. It inhibits the cleavage of C5 into C5a and C5b and hence prevents the generation of the terminal complement complex C5b-9 (), which is involved in cell lysis (Jodele et al., Citation2020). Eculizumab has also been successfully used to protect against microvascular thrombosis in patients with atypical hemolytic uremic syndrome (Legendre et al., Citation2013)
Diurno et al. (Citation2020) presented a case series aimed at reporting preliminary data obtained with anti-complement C5 therapy with eculizumab in COVID-19 patients admitted to intensive care unit with severe pneumonia or acute respiratory distress syndrome in Napoli, Italy. All patients effectively recuperated after treatment with eculizumab, in addition to a mean drop in C-reactive protein levels from 14.6 mg/dl to 3.5 mg/dl.
Currently, several studies (Clinical trials NCT04288713, NCT04346797 and NCT04355494) are investigating eculizumab in Covid-19 infection, hypothesizing that modulating the activity of the distal complement can prevent the formation of the membrane attack complex, and hence decrease mortality (Clinical Trials, Citation2020).
5. Conclusion
Low molecular weight heparin for all (unless contraindicated) hospitalized COVID-19 patients can be lifesaving. Agents that inhibit the terminal events of the complement cascade might be crucial for ensuring an efficient treatment, decrease clots and permit early discharge in relation to COVID-19.
Acknowledgements
Special appreciation for architect Riad Younes for developing the figures. Open Access funding provided by the Qatar National Library.
Disclosure statement
The authors have no potential conflicts of interest relevant to this article to disclose.
Funding
The authors have no financial relationships relevant to this article to disclose.
References
- Bikdeli, B., Madhavan, M. V., Jimenez, D., Chuich, T., Dreyfus, I., Driggin, E., Nigoghossian, C., Ageno, W., Madjid, M., Guo, Y., Tang, L. V., Hu, Y., Giri, J., Cushman, M., Quéré, I., Dimakakos, E. P., Gibson, C. M., Lippi, G., Favaloro, E. J., Fareed, J., … Lip, G. Y. H. (2020). Global COVID-19 Thrombosis Collaborative Group, endorsed by the ISTH, NATF, ESVM, and the IUA, supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. Journal of the American College of Cardiology, 75(23), 2950–2973. https://doi.org/https://doi.org/10.1016/j.jacc.2020.04.031
- Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., Qiu, Y., Wang, J., Liu, Y., Wei, Y., Xia, J., Yu, T., Zhang, X., & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395(10223), 507–513. https://doi.org/https://doi.org/10.1016/S0140-6736(20)30211-7
- Clinical Trials. (2020). https://clinicaltrials.gov.
- Diurno, F., Numis, F. G., Porta, G., Cirillo, F., Maddaluno, S., Ragozzino, A., De Negri, P., Di Gennaro, C., Pagano, A., Allegorico, E., Bressy, L., Bosso, G., Ferrara, A., Serra, C., Montisci, A., D’Amico, M., Schiano Lo Morello, S., Di Costanzo, G., Tucci, A. G., … Facchini, G. (2020). Eculizumab treatment in patients with COVID-19: Preliminary results from real life ASL Napoli 2 Nord experience. European Review for Medical and Pharmacological Sciences, 24(7), 4040–4047. https://doi.org/https://doi.org/10.26355/eurrev_202004_20875
- Escher, R., Breakey, N., & Lämmle, B. (2020). Severe COVID-19 infection associated with endothelial activation. Thrombosis Research, 190, 62. https://doi.org/https://doi.org/10.1016/j.thromres.2020.04.014
- Fromell, K., Adler, A., Åman, A., Manivel, V. A., Huang, S., Dührkop, C., Sandholm, K., Ekdahl, K. N., & Nilsson, B. (2020). Assessment of the role of C3(H2O) in the alternative pathway. Frontiers in Immunology, 11, 530. https://doi.org/https://doi.org/10.3389/fimmu.2020.00530
- Gasque, P., Singhrao, S. K., Neal, J. W., Götze, O., & Morgan, B. P. (1997). Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. The American Journal of Pathology, 150(1), 31–41.
- Guan, W.-J., Ni, Z.-Y., Hu, Y., Liang, W.-H., Ou, C.-Q., He, J.-X., Liu, L., Shan, H., Lei, C.-L., Hui, D. S. C., Du, B., Li, L.-J., Zeng, G., Yuen, K.-Y., Chen, R.-C., Tang, C.-L., Wang, T., Chen, P.-Y., Xiang, J., … Zhong, N.-S. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicne, 382(18), 1708–1720. https://doi.org/https://doi.org/10.1056/NEJMoa2002032
- Jodele, S., Medvedovic, M., Luebbering, N., Chen, J., Dandoy, C. E., Laskin, B. L., & Davies, S. M. (2020). Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Advances, 4(6), 1166–1177. https://doi.org/https://doi.org/10.1182/bloodadvances.2020001515
- Hamming, I., Timens, W., Bulthuis, M. L., Lely, A. T., Navis, G., & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631–637. https://doi.org/https://doi.org/10.1002/path.1570
- Han, H., Yang, L., Liu, R., Liu, F., Wu, K. L., Li, J., Liu, X. H., & Zhu, C. L. (2020). Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical Chemistry and Laboratory Medicine (CCLM), 58(7), 1116–1120. https://doi.org/https://doi.org/10.1515/cclm-2020-0188
- Helms, J., Tacquard, C., Severac, F., Leonard-Lorant, I., Ohana, M., Delabranche, X., Merdji, H., Clere-Jehl, R., Schenck, M., Fagot Gandet, F., Fafi-Kremer, S., Castelain, V., Schneider, F., Grunebaum, L., Anglés-Cano, E., Sattler, L., Mertes, P. M., & Meziani, F. (2020). High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Medicine, 46(6), 1089–1098. https://doi.org/https://doi.org/10.1007/s00134-020-06062-x
- Hendaus, M. A., & Jomha, F. A. (2020). Covid-19 induced superimposed bacterial infection. Journal of Biomolecular Structure & Dynamics, 1–7. Advance online publication. https://doi.org/https://doi.org/10.1080/07391102.2020.1772110
- Henry, B. M., Vikse, J., Benoit, S., Favaloro, E. J., & Lippi, G. (2020). Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clinica Chimica Acta; International Journal of Clinical Chemistry, 507, 167–173. https://doi.org/https://doi.org/10.1016/j.cca.2020.04.027
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. https://doi.org/https://doi.org/10.1016/S0140-6736(20)30183-5 https://doi.org/https://doi.org/10.1016/S0140-6736(20)30183-5
- Ip, W. K., Chan, K. H., Law, H. K., Tso, G. H., Kong, E. K., Wong, W. H., To, Y. F., Yung, R. W., Chow, E. Y., Au, K. L., Chan, E. Y., Lim, W., Jensenius, J. C., Turner, M. W., Peiris, J. S., & Lau, Y. L. (2005). Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. The Journal of Infectious Diseases, 191(10), 1697–1704. https://doi.org/https://doi.org/10.1086/429631
- Kim, H., & Conway, E. M. (2019). Platelets and complement cross-talk in early atherogenesis. Frontiers in Cardiovascular Medicine, 6, 131. https://doi.org/https://doi.org/10.3389/fcvm.2019.00131
- Lee, A. Y. Y., Connors, J. M., & Baumann Kreuziger, L. (2020). Online COVID-19 resources of the American Society of Hematology. https://www.hematology.org/covid-19/covid-19-and-coagulopathy
- Legendre, C. M., Licht, C., Muus, P., Greenbaum, L. A., Babu, S., Bedrosian, C., Bingham, C., Cohen, D. J., Delmas, Y., Douglas, K., Eitner, F., Feldkamp, T., Fouque, D., Furman, R. R., Gaber, O., Herthelius, M., Hourmant, M., Karpman, D., Lebranchu, Y., … Loirat, C. (2013). Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. New England Journal of Medicine, 368(23), 2169–2181. https://doi.org/https://doi.org/10.1056/NEJMoa1208981
- Li, J. P., & Vlodavsky, I. (2009). Heparin, heparan sulfate and heparanase in inflammatory reactions. Thrombosis and Haemostasis, 102(5), 823–828. https://doi.org/https://doi.org/10.1160/TH09-02-0091
- Luo, W., Yu, H., Gou, J., Li, X., Sun, Y., Li, J., & Liu, L. (2020). Clinical pathology of critical patient with Novel Coronavirus pneumonia (COVID-19). Preprints, 2020, 2020020407.
- Magro, C., Mulvey, J. J., Berlin, D., Nuovo, G., Salvatore, S., Harp, J., Baxter-Stoltzfus, A., & Laurence, J. (2020). Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Research, 220, 1–13. https://doi.org/https://doi.org/10.1016/j.trsl.2020.04.007
- Morgan, B. P., Boyd, C., & Bubeck, D. (2017). Molecular cell biology of complement membrane attack. Seminars in Cell & Developmental Biology, 72, 124–132. https://doi.org/https://doi.org/10.1016/j.semcdb.2017.06.009
- Marini, J. J., & Gattinoni, L. (2020). Management of COVID-19 respiratory distress. JAMA, 323(22), 2329. https://doi.org/https://doi.org/10.1001/jama.2020.6825
- McCullough, R. L., McMullen, M. R., Poulsen, K. L., Kim, A., Medof, M. E., & Nagy, L. E. (2018). Anaphylatoxin receptors C3aR and C5aR1 are important factors that influence the impact of ethanol on the adipose secretome. Frontiers in Immunology, 9, 2133. https://doi.org/https://doi.org/10.3389/fimmu.2018.02133
- Noris, M., Benigni, A., & Remuzzi, G. (2020). The case of complement activation in COVID-19 multiorgan impact. Kidney International, 98(2), 314–322. https://doi.org/https://doi.org/10.1016/j.kint.2020.05.013
- Olson, S. R., Lu, E., Sulpizio, E., Shatzel, J. J., Rueda, J. F., & DeLoughery, T. G. (2018). When to stop eculizumab in complement-mediated thrombotic microangiopathies. American Journal of Nephrology, 48(2), 96–107. https://doi.org/https://doi.org/10.1159/000492033
- Pant, S., Singh, M., Ravichandiran, V., Murty, U., & Srivastava, H. K. (2020). Peptide-like and small-molecule inhibitors against Covid-19. Journal of Biomolecular Structure & Dynamics, 1–10. https://doi.org/https://doi.org/10.1080/07391102.2020.1757510
- Pittock, S. J., Berthele, A., Fujihara, K., Kim, H. J., Levy, M., Palace, J., Nakashima, I., Terzi, M., Totolyan, N., Viswanathan, S., Wang, K. C., Pace, A., Fujita, K. P., Armstrong, R., & Wingerchuk, D. M. (2019). Eculizumab in Aquaporin-4-positive neuromyelitis optica spectrum disorder. New England Journal of Medicine, 381(7), 614–625. https://doi.org/https://doi.org/10.1056/NEJMoa1900866
- Risitano, A. M., Mastellos, D. C., Huber-Lang, M., Yancopoulou, D., Garlanda, C., Ciceri, F., & Lambris, J. D. (2020). Complement as a target in COVID-19? Nature Reviews. Immunology, 20(6), 343–344. https://doi.org/https://doi.org/10.1038/s41577-020-0320-7
- Roumenina, L. T., Rayes, J., Frimat, M., & Fremeaux-Bacchi, V. (2016). Endothelial cells: Source, barrier, and target of defensive mediators. Immunological Reviews, 274(1), 307–329. https://doi.org/https://doi.org/10.1111/imr.12479
- Soy, M., Keser, G., Atagündüz, P., Tabak, F., Atagündüz, I., & Kayhan, S. (2020). Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clinical Rheumatology, 39(7), 2085–2094. https://doi.org/https://doi.org/10.1007/s10067-020-05190-5
- Susen, S., Tacquard, C. A., Godon, A., Mansour, A., Garrigue, D., Nguyen, P., Godier, A., Testa, S., Levy, J. H., Albaladejo, P., & Gruel, Y. (2020). Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Critical Care, 24(1), 364. https://doi.org/https://doi.org/10.1186/s13054-020-03000-7
- Tang, N., Li, D., Wang, X., & Sun, Z. (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 18(4), 844–847. https://doi.org/https://doi.org/10.1111/jth.14768
- Thachil, J., Tang, N., Gando, S., Falanga, A., Cattaneo, M., Levi, M., Clark, C., & Iba, T. (2020). ISTH interim guidance on recognition and management of coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis: JTH, 18(5), 1023–1026. https://doi.org/https://doi.org/10.1111/jth.14810
- Thiel, S., Kolev, M., Degn, S., Steffensen, R., Hansen, A. G., Ruseva, M., & Jensenius, J. C. (2009). Polymorphisms in mannan-binding lectin (MBL)-associated serine protease 2 affect stability, binding to MBL, and enzymatic activity. Journal of Immunology (Baltimore, MD: 1950), 182(5), 2939–2947. https://doi.org/https://doi.org/10.4049/jimmunol.0802053
- van Hinsbergh, V. W. (2012). Endothelium-role in regulation of coagulation and inflammation. Seminars in Immunopathology, 34(1), 93–106. https://doi.org/https://doi.org/10.1007/s00281-011-0285-5
- Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., Zhao, Y., Li, Y., Wang, X., & Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA, 323(11), 1061–1069. https://doi.org/https://doi.org/10.1001/jama.2020.1585
- Wang, J., Hajizadeh, N., Moore, E. E., McIntyre, R. C., Moore, P. K., Veress, L. A., Yaffe, M. B., Moore, H. B., & Barrett, C. D. (2020). Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. Journal of Thrombosis and Haemostasis: JTH, 18(7), 1752–1755. https://doi.org/https://doi.org/10.1111/jth.14828
- Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., Huang, H., Zhang, L., Zhou, X., Du, C., Zhang, Y., Song, J., Wang, S., Chao, Y., Yang, Z., Xu, J., Zhou, X., Chen, D., Xiong, W., … Song, Y. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 911–934. https://doi.org/https://doi.org/10.1001/jamainternmed.2020.0994
- Young, E. (2008). The anti-inflammatory effects of heparin and related compounds. Thrombosis Research, 122(6), 743–752. https://doi.org/https://doi.org/10.1016/j.thromres.2006.10.026
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., & Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine, 382(8), 727–733. https://doi.org/https://doi.org/10.1056/NEJMoa2001017
