?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
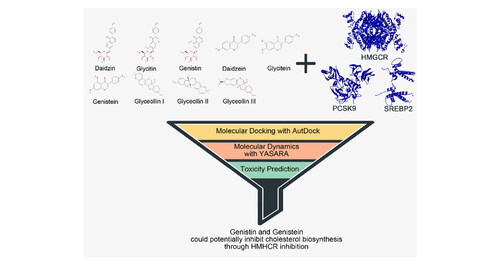
The hypocholesterolemic activity of soy isoflavones has been studied, but the exact mechanism underlying the activity remains unclear. This study reveals the proposed mechanism of the cholesterol-lowering effect of soy isoflavones by computational simulations. Daidzin, Glycitin, Genistin, Daidzein, Glycitein, Genistein, Glyceollin I, Glyceollin II, and Glyceollin III were selected to be analyzed their interaction with 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase (HMGCR) and Sterol Regulatory Element-Binding Protein 2 (SREBP2) as key factors in cholesterol biosynthesis as well as Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) as a common target for hypercholesterolemia. Protein-isoflavones interaction was analyzed using AutoDock. According to binding energy calculations, a total of five out of those nine isoflavones, including Glycitin, Genistin, Genistein, Glyceollin II, and Glyceollin III, were favored to be a HMGCR inhibitor but not with SREBP2 and PCSK9. Those isoflavones were then compared with Simvastatin as known inhibitor of HMGCR. Isoflavone with binding energy lower than Simvastatin then directed to molecular dynamics using YASARA and headed into toxicity estimations. Almost all of those isoflavones could bind with HMGCR with better stability than Simvastatin according to molecular dynamics simulations. Toxicity prediction filtered two out of the five isoflavones mentioned earlier as the proper candidate to be an HMGCR inhibitor. Those isoflavones were Genistin and Genistein. In summary, the hypocholesterolemic activity of soy isoflavones may occur by blocking the cholesterol biosynthesis pathway.
Communicated by Ramaswamy H. Sarma
1. Introduction
Hypercholesterolemia accounts for major public health problems in modern society (Taddei et al., Citation2020). People with hypercholesterolemia have a higher risk of developing cardiovascular diseases (Soran et al., Citation2018; Zárate et al., Citation2016). Besides conventional medicinal therapy, food intervention also mitigates or accompanies medical treatment (Mannu et al., Citation2013). In particular, soybean consumption was discovered to have hypocholesterolemic activity (Ramdath et al., Citation2017; Sirtori et al., Citation1995; Wong et al., Citation1998), suggesting a promising aspect in hypercholesterolemia treatment using food.
Beyond its high amino acid contents, soybeans also have high bioactive compounds, majorly from isoflavones (Wang et al., Citation2013). Dietary isoflavones have been reported to have a protective role in regulating plasma cholesterol levels during the clinical phase (Herwana et al., Citation2020; Taku et al., Citation2007). This result is supported by the experimental evidence that isoflavones reduced LDL cholesterol in blood plasma (Huang et al., Citation2013; Lee et al., Citation2007; Teixeira Damasceno et al., Citation2007). On the other hand, an animal model experiment discovered that isoflavones treatment altered SREBP expression and several of its downstream genes (Mullen et al., Citation2004). Further investigations also tried to uncover the lipid-lowering properties of the isoflavones by altering PPAR, ATP-Binding Cassette super-family G member 5 (ABCG5), and ABCG8 expressions (Huang et al., Citation2013; Mezei et al., Citation2003). Unfortunately, the authors still hypothesized the exact mechanism of the hypolipidemic activity of the isoflavones. Besides, the actual compound from the isoflavones group involved in cholesterol-lowering activity remains unknown. It is still hypothesized that all isoflavones have a beneficial role in maintaining normal plasma cholesterol. Nevertheless, since chemical structure influences its interaction and activity towards biological macromolecules (Lewandowski et al., Citation2020), the mechanism leaves a gap to be filled.
Several pathways have been suggested to regulate plasma cholesterol levels, including blocking de novo cholesterol biosynthesis through inhibition of HMGCR (Boucher & Vogel, Citation2016; Ma et al., Citation2019) or SREBP2 (Tang et al., Citation2011) and preventing Low-Density Lipoprotein Receptor (LDLR) degradation through inhibition of PCSK9 activity (Gustafsen et al., Citation2017; Ma et al., Citation2019). Thus, targeting those proteins may unveil the hypolipidemic activity of the isoflavones. Therefore, this study will explores the mechanism underlying hypocholesterolemic activity of soy-isoflavone as well as complements and comprehends aforementioned proposed mechanism.
2. Materials and method
2.1. Data mining of isoflavone and protein structures
The list of nine isoflavones was selected according to the previously identified from elicited soybean (Atho’illah et al. 2019). HMGCR, SREBP2, and PCSK9 were selected as the target based on the previous studies related to cholesterol metabolism regulation (Mannino et al., Citation2021; Mullen et al., Citation2004; Sung et al., Citation2004). In addition, Simvastatin was used as a control molecule for HMGCR inhibitor, according to the previous study (Marahatha et al., Citation2021). On the other hand, three dimensional (3 D) structures of isoflavones and Simvastatin were retrieved from PubChem, while the protein structure was downloaded from Protein Data Bank (PDB) with the following identity: HMGCR (PDB ID: 1DQ8), PCSK9 (PDB ID: 5OCA), and SREBP2 (PDB ID: 1UKL).
2.2. Binding energy calculation
Binding energy was calculated using AutoDock 4.0 in PyRx 8.0 software using Lamarckian Genetic Algorithm (Dallakyan & Olson, Citation2015; Morris et al., Citation1998, Citation2009). The grid setting was determined according to key residues for each protein (Gustafsen et al., Citation2017; Istvan et al., Citation2000; Lee et al., Citation2003). The grid center position was set as follows: x: 11.8747, y: 17.447, z: −2.9508 for HMGCR; x: 46.3085, y: 10.8373, z: 23.6289 for PCSK9; and x: 23.9692, y: 6.1774, z: 101.417 for SREBP2 with 0.375 Å spacing for all docking simulations.
2.3. Docking analysis and structure visualization
Binding energy, types of the chemistry of residue interaction, and hydrophobicity surface were considered as the parameters of docking results. A complex with binding energy lower than Simvastatin was considered for molecular dynamics simulation. Finally, the complex structure of each protein-ligand was visualized using Biovia Discovery Studio 2019.
2.4. Molecular dynamic simulation and binding energy calculations
YASARA version 19.12.14 software (Krieger & Vriend, Citation2015) with AMBER14 force field (Maier et al., Citation2015) was employed to perform molecular dynamics simulation using the following condition: pH 7.4, 0.9% NaCl concentration, 310°K temperature, 0.997 water density, 1 atm pressure, and 100 ns running time. The Root-Mean-Square Deviation (RMSD) and Root-Mean-Square Fluctuation (RMSF) outputs were used for determining structural stability and rigidity. Also, number of hydrogen bond was analyzed. In addition, free binding energy was calculated using YASARA macros with Boundary Fast method. The ΔEnergy was estimated according to the following sum:
2.5. Toxicity prediction
ProTox II (Banerjee et al., Citation2018) was used to determine the toxicity profile of selected isoflavones. Lethal dose 50 (LD50) and toxicity class were measured, while probability to have hepatotoxic, carcinogenic, immunotoxic, mutagen, and cytotoxic properties were estimated according to the probability score.
3. Results and discussion
3.1. Isoflavones interaction with target proteins
Molecular docking simulation revealed that all of the isoflavones interacted with HMGCR at lower binding energy than other target proteins, i.e., PCSK9 and SREBP2. In addition, Glycitin, Genistin, Genistein, Glyceollin II, and Glyceollin III have binding energy lower than Simvastatin as control molecules for HMGCR inhibitor (). The isoflavones with lower binding energy than Simvastatin, except Genistein, also bounded in the hydrophobic area of the HMGCR. Several catalytic residues of HMGCR (Istvan et al., Citation2000) also interacted with these isoflavones, including ARG590, GLU559, LEU853, ASP690, LYS691, ASN755, and ASP767 (). On the other hand, Genistein showed more hydrogen bonds than the other four isoflavones (Supplementary Table 1). These results agree with the previous studies describing the potential of isoflavones as competitive inhibitors of the HMGCR (Sung et al., Citation2004). Accordingly, Glycitin, Genistin, Genistein, Glyceollin II, and Glyceollin III interactions with HMGCR were further analyzed using molecular dynamics simulation.
Figure 1. Amino acid interactions of daidzin (A), Glycitin (B), Genistin (C), Daidzein (D), Glycitein (E), Genistein (F), Glyceollin I (G), Glyceollin II (H), Glyceollin III (I), and Simvastatin (J) with HMGCR.
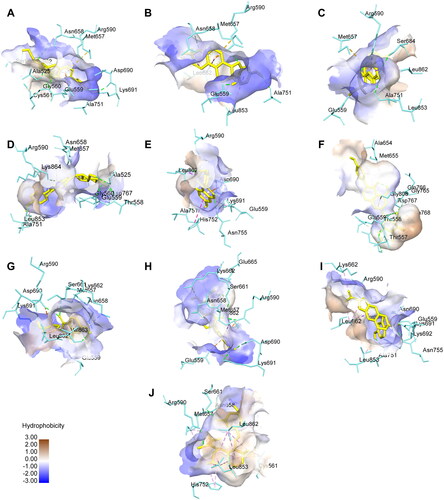
Table 1. The binding energy of each isoflavone with HMGCR, PCSK9, and SREBP2.
To understand the structural dynamics of the selected isoflavones with HMGCR, molecular dynamics were employed. The RMSD of the protein backbone value showed that the interactions of isoflavones and Simvastatin did not alter the structural backbone of HMGCR as all of those ligands have a value of less than 3 Å (). Meanwhile, the structural dynamics of isoflavones were more stable compared to the Simvastatin during the binding to HMGCR (). However, the RMSD of ligand movement described that Glyceollin III might be detached during simulation, indicated by the dramatic escalation of the RMSD at around 50 ns. On the other hand, other isoflavones, particularly Genistein and Glyceollin II, have better interaction stability with the HMGCR than the Simvastatin ().
Figure 2. The RMSD of the selected compounds describes the stability of interaction of HMGCR and Glycitin, Genistin, Genistein, and Simvastatin, respectively. The RMSD of Protein Backbone (A) describes the stability of the protein, the RMSD of Ligand Conformation (B) describes the stability of ligand structure during its interaction with the protein, and the RMSD of Ligand Movement (C) describes the stability of the ligand during the interaction with the protein. Additionally, RMSF values of selected compounds specified on the active site residues (D) and all residues in chain A (E) and chain B (F) of HMGCR. Also, average number of hydrogen bond (G) and its fluctuations during the simulation time (H) were described along with binding energy of selected isoflavones (I).
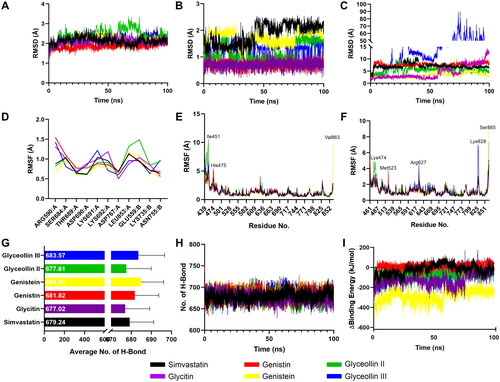
Residual fluctuations represented by RMSF showed no difference among isoflavones and Simvastatin (). Catalytic residues also stayed stable in the complex of HMGCR with isoflavones and Simvastatin, suggesting no alteration of residual fluctuation at the catalytic sites (). RMSF value represented that isoflavones’ binding to the HMGCR did not influence the stability of each residue, particularly catalytic residues of HMGCR. Therefore, all of the isoflavones may bound with great affinity to the HMGCR to act as its inhibitor.
Since hydrogen bond has a vital role in protein-ligand stability, the number of hydrogen bond will influence the binding of isoflavones to the HMGCR (Fu et al., Citation2018; Patil et al., Citation2010). Genistin, Genistein and Glyceollin II have greater number of formed hydrogen bond than other isoflavones and Simvastatin (). This data also supported by the RMSD of ligand movement where Genistein and Glyceollin II remains stable and better than Simvastatin as control inhibitor. However, binding energy calculations described that Genistin was the most stable one even compared to the Simvastatin. Among all of the complexes, Genistein had lowest energy than other complex (, ).
Table 2. Calculations on free binding energy of HMGCR with every selected ligands.
Despite many experiments and clinical studies conducted to evaluate the hypolipidemic activity of soy-isoflavones, the exact mechanism is still poorly understood. This study revealed that Genistin, Genistein, and Glyceollin II might perform as HMGCR inhibitors through their interaction with HMGCR catalytic residues. The interaction remains stable compared to Simvastatin, suggesting that those compounds have excellent potential to block uncontrolled cholesterol biosynthesis in hyperlipidemia. Isoflavones, mainly Genistein, have been evaluated as an HMGCR inhibitor in vitro as Statin (Sung et al., Citation2004). As a rate-limiting factor of cholesterol biosynthesis, HMGCR inhibition alleviated plasma cholesterol levels (Lee et al., Citation2007). The HMGCR blocking activity was also proven to have an atheroprotective role in vivo (Mansouri et al., Citation2021). Advance shrinking of cholesterol levels leads to upregulation of LDLR (Notarnicola et al., Citation2008) through the SREBP2 pathway at the transcription scale (Lu et al., Citation2019), thus allowing plasma cholesterol clearance for cellular metabolic utilization (Goldstein & Brown, Citation2009). Therefore, soy isoflavones may have hypolipidemic activity through alterations of HMGCR expression and activity at the transcriptomic and proteomic levels.
3.2. Toxicity profile of selected isoflavones
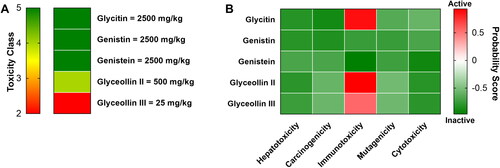
Toxicity prediction revealed that Glycitin, Genistin, and Genistein were the less toxic isoflavones among selected isoflavones. Glyceollin III became the most toxic isoflavone with LD50 25 mg/kg according to the ProTox II estimations (Banerjee et al., Citation2018). Glyceollin II was classified as class 4 with slight toxicity profile based on LD50 calculation (). Further, Glycitin, Glyceollin II, and Glyceollin III showed high probability to have immunotoxic effect (). In view of that, Genistin and Genistein were suggested as the best HMGCR inhibitors with the lowest toxicity profile.
4. Conclusion
Genistin and Genistein may have excellent properties as HMGCR competitive inhibitors with the highest affinities and low toxicity profiles. The cholesterol-regulating mechanism of soy isoflavones may be achieved by inhibiting cholesterol biosynthesis by blocking HMGCR activity.
Supplemental Material
Download MS Word (22.7 KB)Acknowledgement
The authors thank to Dr. Dinia R. Dwijayanti for her constructive comments and suggestions during the manuscript arrangement.
Disclosure statement
The authors declare no competing interest in this work.
Additional information
Funding
References
- Atho'illah, M. F., Safitri, Y. D., Nur’aini, F. D., Savitri, R. U., Rahayu, S., Widyarti, S., & Rifa'i, M. (2019). Evaluation of glyceollin accumulation and antioxidant properties on soybean (Glycine max L.) through combination of different biotic elicitor and light. Scientific Study & Research Chemistry & Chemical Engineering, Biotechnology, Food Industry 20, 199–208. https://doi.org/10.13140/RG.2.2.19614.28483
- Banerjee, P., Eckert, A. O., Schrey, A. K., & Preissner, R. (2018). ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Research, 46(W1), W257–W263. https://doi.org/10.1093/nar/gky318
- Boucher, P., & Vogel, H. G. (2016). Inhibition of cholesterol biosynthesis. In F. J. Hock (Ed.), Drug discovery and evaluation: Pharmacological assays (pp. 2247–2271). Springer International Publishing.
- Dallakyan, S., & Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. Methods in molecular biology (Clifton, N.J.), 1263, 243–250. https://doi.org/10.1007/978-1-4939-2269-7_19
- Fu, Y., Zhao, J., & Chen, Z. (2018). Insights into the molecular mechanisms of protein-ligand interactions by molecular docking and molecular dynamics simulation: A case of oligopeptide binding protein. Computational and Mathematical Methods in Medicine, 2018, 3502514. https://doi.org/10.1155/2018/3502514
- Goldstein, J. L., & Brown, M. S. (2009). History of discovery: The LDL receptor. Arteriosclerosis, Thrombosis, and Vascular Biology, 29(4), 431–438. https://doi.org/10.1161/ATVBAHA.108.179564
- Gustafsen, C., Olsen, D., Vilstrup, J., Lund, S., Reinhardt, A., Wellner, N., Larsen, T., Andersen, C. B. F., Weyer, K., Li, J., Seeberger, P. H., Thirup, S., Madsen, P., & Glerup, S. (2017). Heparan sulfate proteoglycans present PCSK9 to the LDL receptor. Nature Communications, 8(1), 503. https://doi.org/10.1038/s41467-017-00568-7
- Herwana, E., Pusparini, P., & Graciela, A. (2020). High dietary daidzein intake lowers cholesterol levels among post-menopausal women. Universa Med, 39(1), 47–54. https://doi.org/10.18051/UnivMed.2020.v39.47-54
- Huang, H., Xie, Z., Boue, S. M., Bhatnagar, D., Yokoyama, W., Yu, L. L., & Wang, T. T. Y. (2013). Cholesterol-lowering activity of soy-derived glyceollins in the golden Syrian hamster model. Journal of Agricultural and Food Chemistry, 61(24), 5772–5782. https://doi.org/10.1021/jf400557p
- Istvan, E. S., Palnitkar, M., Buchanan, S. K., & Deisenhofer, J. (2000). Crystal structure of the catalytic portion of human HMG-CoA reductase: Insights into regulation of activity and catalysis. The EMBO Journal, 19(5), 819–830. https://doi.org/10.1093/emboj/19.5.819
- Krieger, E., & Vriend, G. (2015). New ways to boost molecular dynamics simulations. Journal of Computational Chemistry, 36(13), 996–1007. https://doi.org/10.1002/jcc.23899
- Lee, S.-O., Renouf, M., Ye, Z., Murphy, P. A., & Hendrich, S. (2007). Isoflavone glycitein diminished plasma cholesterol in female golden Syrian hamsters. Journal of Agricultural and Food Chemistry, 55(26), 11063–11067. https://doi.org/10.1021/jf070972r
- Lee, S. J., Sekimoto, T., Yamashita, E., Nagoshi, E., Nakagawa, A., Imamoto, N., Yoshimura, M., Sakai, H., Chong, K. T., Tsukihara, T., & Yoneda, Y. (2003). The structure of importin-beta bound to SREBP-2: Nuclear import of a transcription factor. Science (New York, N.Y.), 302(5650), 1571–1575. https://doi.org/10.1126/science.1088372
- Lewandowski, W., Lewandowska, H., Golonko, A., Świderski, G., Świsłocka, R., & Kalinowska, M. (2020). Correlations between molecular structure and biological activity in “logical series” of dietary chromone derivatives. PLoS ONE, 15(8), e0229477. https://doi.org/10.1371/journal.pone.0229477
- Lu, R., Zheng, Z., Yin, Y., & Jiang, Z. (2019). Effect of Genistein on cholesterol metabolism-related genes in HepG2 cell. Journal of Food Science, 84(8), 2330–2336. https://doi.org/10.1111/1750-3841.14725
- Ma, S., Sun, W., Gao, L., & Liu, S. (2019). Therapeutic targets of hypercholesterolemia: HMGCR and LDLR. Diabetes, Metabolic Syndrome and Obesity : targets and Therapy, 12, 1543–1553. https://doi.org/10.2147/DMSO.S219013
- Maier, J. A., Martinez, C., Kasavajhala, K., Wickstrom, L., Hauser, K. E., & Simmerling, C. (2015). ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. Journal of Chemical Theory and Computation, 11(8), 3696–3713. https://doi.org/10.1021/acs.jctc.5b00255
- Mannino, G., Iovino, P., Lauria, A., Genova, T., Asteggiano, A., Notarbartolo, M., Porcu, A., Serio, G., Chinigò, G., Occhipinti, A., Capuzzo, A., Medana, C., Munaron, L., & Gentile, C. (2021). Bioactive triterpenes of protium heptaphyllum gum resin extract display cholesterol-lowering potential. International Journal of Molecular Sciences, 22(5), 2664. https://doi.org/10.3390/ijms22052664
- Mannu, G. S., Zaman, M. J. S., Gupta, A., Rehman, H. U., & Myint, P. K. (2013). Evidence of lifestyle modification in the management of hypercholesterolemia. Current Cardiology Reviews, 9(1), 2–14. https://doi.org/10.2174/157340313805076313
- Mansouri, A., Vahdati, A., Nematbakhsh, M., & Moshtaghian, J. (2021). Hypercholesterolemia role of vitamin D3 and genistein effect in reducing atherosclerosis in rat. Hormozgan Med J, 19, 306–314.
- Marahatha, R., Basnet, S., Bhattarai, B. R., Budhathoki, P., Aryal, B., Adhikari, B., Lamichhane, G., Poudel, D. K., & Parajuli, N. (2021). Potential natural inhibitors of xanthine oxidase and HMG-CoA reductase in cholesterol regulation: In silico analysis. BMC Complementary Medicine and Therapies, 21(1), 1. https://doi.org/10.1186/s12906-020-03162-5
- Mezei, O., Banz, W. J., Steger, R. W., Peluso, M. R., Winters, T. A., & Shay, N. (2003). Soy Isoflavones exert antidiabetic and hypolipidemic effects through the PPAR Pathways in obese zucker rats and murine RAW 264.7 cells. The Journal of Nutrition, 133(5), 1238–1243. https://doi.org/10.1093/jn/133.5.1238
- Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785–2791. https://doi.org/10.1002/jcc.21256
- Morris, G. M., Goodsell, D. S., Halliday, R. S., Huey, R., Hart, W. E., Belew, R. K., & Olson, A. J. (1998). Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
- Mullen, E., Brown, R. M., Osborne, T. F., & Shay, N. F. (2004). Soy Isoflavones Affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. The Journal of Nutrition, 134(11), 2942–2947. https://doi.org/10.1093/jn/134.11.2942
- Notarnicola, M., Messa, C., Orlando, A., D'Attoma, B., Tutino, V., Rivizzigno, R., & Caruso, M. G. (2008). Effect of genistein on cholesterol metabolism-related genes in a colon cancer cell line. Genes & Nutrition, 3(1), 35–40. https://doi.org/10.1007/s12263-008-0082-5
- Patil, R., Das, S., Stanley, A., Yadav, L., Sudhakar, A., & Varma, A. K. (2010). Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PloS One, 5(8), e12029. https://doi.org/10.1371/journal.pone.0012029
- Ramdath, D. D., Padhi, E. M. T., Sarfaraz, S., Renwick, S., & Duncan, A. M. (2017). Beyond the cholesterol-lowering effect of soy protein: A review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients, 9(4), 324. https://doi.org/10.3390/nu9040324
- Sirtori, C. R., Lovati, M. R., Manzoni, C., Monetti, M., Pazzucconi, F., & Gatti, E. (1995). Soy and cholesterol reduction: Clinical experience. The Journal of Nutrition, 125(3 Suppl), 598S–605S. https://doi.org/10.1093/jn/125.suppl_3.598S
- Soran, H., Adam, S., Mohammad, J. B., Ho, J. H., Schofield, J. D., Kwok, S., Siahmansur, T., Liu, Y., Syed, A. A., Dhage, S. S., Stefanutti, C., Donn, R., Malik, R. A., Banach, M., & Durrington, P. N. (2018). Hypercholesterolaemia – practical information for non-specialists. Archives of Medical Science : AMS, 14(1), 1–21. https://doi.org/10.5114/aoms.2018.72238
- Sung, J. H., Lee, S.-J., Park, K. H., & Moon, T. W. (2004). Isoflavones inhibit 3-hydroxy-3-methylglutaryl coenzyme a reductase in vitro. Bioscience, Biotechnology, and Biochemistry, 68(2), 428–432. https://doi.org/10.1271/bbb.68.428
- Taddei, C., Zhou, B., Bixby, H., Carrillo-Larco, R. M., Danaei, G., Jackson, R. T., Farzadfar, F., Sophiea, M. K., Di Cesare, M., Iurilli, M. L. C., Martinez, A. R., Asghari, G., Dhana, K., Gulayin, P., Kakarmath, S., Santero, M., Voortman, T., Riley, L. M., Cowan, M. J., … Ezzati, M. (2020). Repositioning of the global epicentre of non-optimal cholesterol. Nature, 582(7810), 73–77. https://doi.org/10.1038/s41586-020-2338-1
- Taku, K., Umegaki, K., Sato, Y., Taki, Y., Endoh, K., & Watanabe, S. (2007). Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. The American Journal of Clinical Nutrition, 85(4), 1148–1156. https://doi.org/10.1093/ajcn/85.4.1148
- Tang, J.-J., Li, J.-G., Qi, W., Qiu, W.-W., Li, P.-S., Li, B.-L., & Song, B.-L. (2011). Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metabolism, 13(1), 44–56. https://doi.org/10.1016/j.cmet.2010.12.004
- Teixeira Damasceno, N. R., Apolinário, E., Dias Flauzino, F., Fernandes, I., & Abdalla, D. S. P. (2007). Soy isoflavones reduce electronegative low-density lipoprotein (LDL(-)) and anti-LDL (-) autoantibodies in experimental atherosclerosis. European Journal of Nutrition, 46(3), 125–132. https://doi.org/10.1007/s00394-006-0640-9
- Wang, Q., Ge, X., Tian, X., Zhang, Y., Zhang, J., & Zhang, P. (2013). Soy isoflavone: The multipurpose phytochemical (Review). Biomedical Reports, 1(5), 697–701. https://doi.org/10.3892/br.2013.129
- Wong, W. W., Smith, E. O., Stuff, J. E., Hachey, D. L., Heird, W. C., & Pownell, H. J. (1998). Cholesterol-lowering effect of soy protein in normocholesterolemic and hypercholesterolemic men. The American Journal of Clinical Nutrition, 68(6 Suppl), 1385S–1389S. https://doi.org/10.1093/ajcn/68.6.1385S
- Zárate, A., Manuel-Apolinar, L., Saucedo, R., Hernández-Valencia, M., & Basurto, L. (2016). Hypercholesterolemia as a risk factor for cardiovascular disease: Current controversial therapeutic management. Archives of Medical Research, 47(7), 491–495. https://doi.org/10.1016/j.arcmed.2016.11.009