Abstract
Mango (Mangifera indica L.) is one of the most important fruit crops in the world with yields of approximately 40 million tons annually and its production continues to decrease every year as a result of the attack of certain pathogens i.e. Colletotrichum gloeosporioides, Erythricium salmonicolor, Amritodus atkinsoni, Idioscopus clypealis, Idioscopus nitidulus, Bactrocera obliqua, Bactrocera frauenfeldi, Xanthomonas campestris, and Fusarium mangiferae. So F. mangiferae is the most harmful pathogen that causes mango malformation disease in mango which decreases its 90% yield. Nanotechnology is an eco-friendly and has a promising effect over traditional methods to cure fungal diseases. Different nanoparticles possess antifungal potential in terms of controlling the fungal diseases in plants but applications of nanotechnology in plant disease managements is minimal. The main focus of this review is to highlight the previous and current strategies to control mango malformation and highlights the promising applications of nanomaterials in combating mango malformation. Hence, the present review aims to provide brief information on the disease and effective management strategies.
Communicated by Ramaswamy H. Sarma
1. Introduction
1.1. Background of mango malformation disease
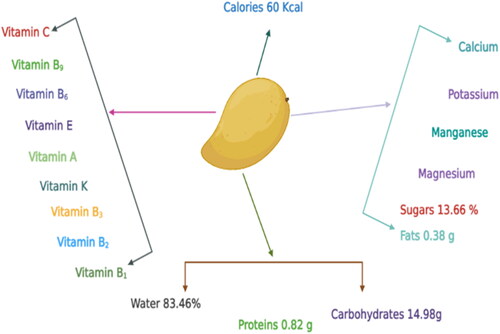
Mango (Mangifera indica L.) belongs to the family Anacardiaceae and highly nutritive fruit crop (Shivran et al., Citation2023). Its pulp is a rich source of vitamins A and C along with sugars, proteins, organic acids, carbohydrates, ascorbic acid, and minerals (Zafar & Sidhu, Citation2017). Mangoes are packed with essential nutrients, including vitamins, minerals, and dietary fiber. They are an excellent source of vitamin C, delivering more than 50% of the recommended daily intake per serving (Kabeer et al., Citation2023). The abundance of vitamin C in mangoes supports immune function and collagen synthesis, promoting overall health (Naliyadhara et al., Citation2023). Moreover, mangoes are a good source of dietary fiber, aiding digestion and potentially contributing to weight management (Tariq et al., Citation2023). Mangoes boast a wealth of antioxidants, such as phenolic compounds, beta-carotene, and lutein. These antioxidants play a vital role in protecting the body’s cells from oxidative damage caused by free radicals, thereby reducing the risk of chronic diseases (Naliyadhara et al., Citation2023). Furthermore, research suggests that mango extracts exhibit potential anticancer properties due to their antioxidant activity (Adin et al., Citation2023).
Mangoes are renowned for their delightful taste and juiciness, but they also offer a wealth of antioxidants that confer numerous health benefits. Enzymes and vitamins found in mangoes play a crucial role in antioxidant activity (Dumitru Veleșcu et al., Citation2023). Mangoes contain significant levels of the antioxidant enzyme superoxide dismutase (SOD), which protects cells from oxidative stress caused by free radicals (Shaban et al., Citation2023). Furthermore, it possesses an excellent source of vitamin C, a potent antioxidant that bolsters the immune system and shields cells from damage. Nutrient research indicates that a single medium-sized mango can supply approximately 70 milligrams of vitamin C, a substantial portion of the recommended daily intake (Yahia et al., Citation2023) (). Mangoes also boast high levels of vitamin A and various carotenoids, including beta-carotene, alpha-carotene, and beta-cryptoxanthin, which contribute to their antioxidant properties (D’Souza et al., Citation2023). Consumption of mangoes has been associated with various health benefits. Studies indicate that regular intake of mangoes may contribute to lowering blood pressure and reducing the risk of cardiovascular diseases (Tulp et al., Citation2023). Bioactive compounds found in mangoes, such as mangiferin, show promise in managing conditions like diabetes and inflammation (Gupta et al., Citation2022).
Mangoes offer versatility in culinary applications and find their way into a wide range of dishes. They are used in the production of beverages, desserts, sauces, and savory recipes. The distinctive flavor profile of mangoes adds depth and sweetness, making them a prized ingredient in the food industry (Shahbaz et al., Citation2023a). Mangoes make a substantial contribution to the global food industry, both in domestic consumption and international trade (Mukhametzyanov et al., Citation2023). The cultivation and trade of mangoes create employment opportunities, support rural economies, and generate significant revenue for producing countries. For instance, India relies on mangoes as a major agricultural export, driving substantial economic benefits (De Weerdt et al., Citation2023).
Pakistan ranks as the sixth largest mango producer country in the world. The mango industry is the second main fruit industry in Pakistan, with an annual report production of 1.72 million tons in a range of approximately 171.7 thousand hectares. Pakistan exports approximately 85,000 tons of mango valued at around $36.66 million per year (Zahid et al., Citation2023).
The Indo-Burma region is especially famous for the origin of mangos and then its production trend accelerated to other regions of the world (Yadav et al., Citation2022). This plant was cultivated in the Indian sub-continent about 4,000 years ago. The Mango malformation disease was first reported by Watt 1891 in India (Sankaran et al., Citation2021). This disease is spread around the world in mango-producing countries around the world and causes loss in its production. It is spread in many countries such as Pakistan, Malaysia, Switzerland, Africa, Uganda, Sudan, Cuba, Central America, Australia, and UAE (Sahoo et al., Citation2023). There are more than 1000 varieties of mangoes in the world, but only a few are cultivated for commercial gain (Shankar et al., Citation2023). India holds the distinction of being the largest global producer of mangoes, contributing approximately 40% to the total mango production worldwide (Thao et al., Citation2023). Its cultivation spans across diverse states such as Uttar Pradesh, Andhra Pradesh, and Bihar, encompassing a wide range of mango varieties. China has emerged as a prominent mango-producing nation in Asia, with its mango production steadily increasing in recent years, establishing itself as a significant player in the global mango market (Raza et al., Citation2023).
Mexico, renowned for its exquisite mango varieties, notably excels in mango production. The state of Chiapas significantly contributes to Mexico’s mango production and exports substantial quantities to the United States (Gómez-Ollé et al., Citation2023). Thailand has garnered a reputation for its superior mangoes, particularly the highly popular ‘Nam Dok Mai’ variety. The country has witnessed remarkable growth in mango production, boasting an annual output of approximately 3 million metric tons (Bharatkumar et al., Citation2023). Pakistan emerged as another notable mango producer, known for its delectable varieties such as Sindhri and Anwar Ratol (Javaid et al., Citation2012). The country’s mango production has been steadily expanding, facilitating substantial exports to global markets (El‐Sayed & Fitzsimmons, Citation2023). Pakistan ranked seventh in mango exports, behind India, Brazil, Mexico, Thailand, Peru, and the Netherlands. Mangoes are imported from Pakistan by 60 countries around the world. The main nations that import Pakistani mangoes are Iran, Malaysia, the Netherlands, and the UK, the United Arab Emirates (UAE), Saudi Arabia, and other Middle Eastern and European countries (Zahid et al., Citation2023).
1.2. Significance of addressing mango malformation disease
In every stage of its existence, the mango is affected by a variety of diseases (Khan & Korban, Citation2022). A large number of pathogens attack every fragment of the plant, containing the leaf, stem, roots, twig, petiole, flower, and fruits. However, only a few diseases are economically significant. Rot, dieback, mildew, necrosis, scab, leaf spot, wilt, canker, and deformity are all symptoms of these diseases (Gaytán et al., Citation2022). Approximately 83 diseases are identified in mango trees all over the world, 27 of them being particularly serious in Pakistan. Controlling the disease will always be a difficult task for cultivars to achieve maximum yield due to tolerance to pesticides. As a result, several recently developed fungicide chemicals remain vulnerable (Shahbaz et al., Citation2023). Resistance to fungicides is a major problem in today’s plant disease management. In most cases, resistance of plant disease to fungicides can be automatically produced by applying many newly produced fungicides that it considered a threat to disease crops (Azam et al., Citation2020). Different abiotic and biotic factors have had a substantial impact on mango production in Pakistan in recent years (Zhang et al., Citation2022). Abiotic causes include thermal regimes, floods, droughts, rainfall, windstorms, food deficiencies, and poor cultural practices (Malik et al., Citation2018). Changes in temperature and heavy rains not only alter mango tree-bearing habits, but also predispose them to various diseases, the most important of which is mango malformation (Ramírez & Kallarackal, Citation2018). Both the vegetative shoot and the inflorescence develop hypertrophied tissue due to the disease. Floral malformation causes economic loss because the malformed inflorescence does not produce fruits, while vegetative malformation slows the growth of the canopy (Katoch et al., Citation2019).
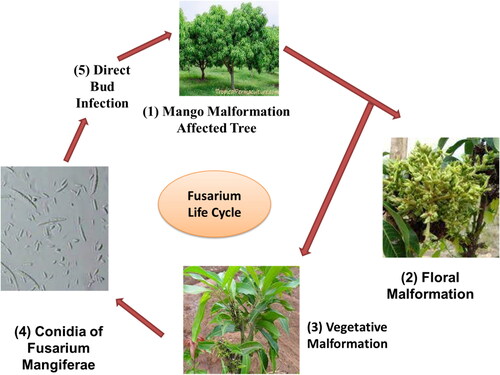
The floral form of mango malformation refers to a form that occurs in inflorescence and is more severe than the vegetative malformation (Alvi et al., Citation2022). Rachises (primary, secondary, and tertiary) become short, dense, and strongly branched, or become hypertrophied. With more branching, the panicles become greener and heavier in weight. Disease-affected panicles have closed flowers that cannot open (Kumar et al., Citation2021). Flowers are rarely bisexual and are usually male. This form of ovary or flowers having both sexes is abnormally swollen and without function. The flower shaving of both sexes can show low pollen capacity. The ovary can be fertilized under some conditions but later disintegrate after becoming dry masses (Kaur & Kaur, Citation2018).
Malformation diseases panicles are said to have one to three times the number of flowers as healthy panicles. On the same panicle or shoot, healthy and malformed flowers will co-exist. Malformed panicles are classified as heavy, medium, or light depending on the nature of the disease and the compactness of the panicle (Alvi et al., Citation2022). Flowers with large masses appear in heavy form, become dry, and remain with branches as brown, discolored bunches, on the other hand; branches without malformation develop until the next season (Venkataravanappa & Sonavane, Citation2022). In nurseries, vegetative malformation frequently affects young seedlings. Malformation involves the presence of small, deformed, rough leaves that form a cluster on the upper side of the shoot. In nurseries, small seedlings are mainly affected that are under already disease attack trees these new seedlings are already distressed. The malformation is found in plants as young as three to four months old (Katoch et al., Citation2019). New densely bunched hypertrophied shoots with swollen upper and flower buds are among the symptoms of the disease (Chakruno et al., Citation2022). Hypertrophied tissues are made up of several swollen vegetative buds. Shoot, developments hampered as apical supremacy is lost. Bunchy Top and Witch’s Broom are multibranched shoot apexes with rough leaves and twisted terminals bearing small internodes (Malik et al., Citation2018). The leaves are thin and hard and are prone to bend into the support system. Since the shoot does not fully extend, it gives the appearance of a tightly packed bunch. Seedlings that are infected early in the disease’s life cycle are stunted and die, but seedlings infected later in the disease’s life cycle may carry unusual growth on the upper side of the affected area. The trees four to eight years old, there would be 90% chances of vegetative malformation (Zakaria, Citation2023).
The disease affects young plants. As a result, there are no plants for base or propagation. This condition motivates researchers to work on it and discover management strategies that will protect new plants while improving the health of orchards. The causative agent F. moniliforme was first used to replicate the vegetative deformity in 1966 (Aldrich et al., Citation2021). MMD spreads from one location to another and from infected nurseries and planted material (Garcia-Lopez et al., Citation2023).
Fusarium mangiferae is the causing agent of mango malformation disease that is the main limitation of its cultivation, a quantity of mango declining with thoughtful economic consequences (Adikaram et al., Citation2023). Mycelia persisted indirectly interacting with the surface of a plant without penetrating the tissue, indicating that the positioning of the fungal is epiphytic. The fungus is limited to sheltered zones among scales, immature leaf bases, and buds with vegetative deformities and immature inflorescences (Sarkar et al., Citation2022).
2. Pathogen biology
2.1. Pathogen and its identification
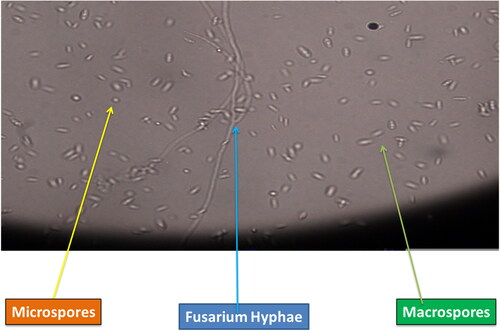
The pathogen after isolation from disease affected inflorescence was identified on the basis of morphological and molecular techniques (Bibi et al., Citation2023). The morphological characteristics of F. mangiferae were white cottony colonies and the presence of micro conidia in oval, ellipsoidal, and non-Septat abundances that confirm that the pathogen is F. mangiferae (Koffi et al., Citation2021). F. mangiferae sometimes has both micro and macro conidia with three septa after the 10th day of inoculation. Short macro conidia with medium-length falcate straight length with spherical ends were also found in this pathogen (Mirghasempour et al., Citation2022). The morphological basis for the identification of the fungal pathogen cannot fulfill the relationship of the isolated strain to the species level. Therefore, molecular techniques were performed for the identification of the species level (Bhunjun et al., Citation2021). To resolve phylogenetic relationships, the DNA sequence of ITS1 and ITS2 (rRNA transcription unit) has a promising role. The closely related taxa information was obtained through many copies of genes located in rDNA due to its closely related spontaneous evolution rate. ITS1 and ITS4 were considered universal primers for fungal identification. The amplification band was in the range of 550-600 bp, as observed by Setlem and Ramlal (Citation2022). Cutting-edge technology has revolutionized the identification of Fusarium mangiferae, a pathogenic fungus that affects mango trees. DNA-based molecular techniques, such as Polymerase Chain Reaction (PCR), have been instrumental in the accurate and rapid detection of this fungal species (Khan et al., Citation2023). Next-Generation Sequencing (NGS) has also played a significant role in identifying Fusarium species, including F. mangiferae, from infected mango trees, providing insights into the pathogenicity and genetic diversity of the fungus (Chaturvedi et al., Citation2022). Another advanced technology, Loop-Mediated Isothermal Amplification (LAMP), offers rapid and sensitive detection of Fusarium mangiferae, providing quick and reliable results for disease diagnosis and surveillance (Kumari et al., Citation2022) (). These cutting-edge technologies, including PCR, NGS, and LAMP, have significantly enhanced the identification and detection of F. mangiferae, enabling timely interventions and effective disease management strategies to preserve the health and productivity of mango trees (Soroka et al., Citation2021).
2.2. Host-pathogen interaction
Interactive regulation of genes between host and F. mangiferae causes mango malformation disease in M. indica (Usha et al., Citation2022). Hormonal changes in infected buds may be caused by F. mangiferae. In fungi, genes responsible for producing secondary metabolites are often grouped in a gene cluster. Genes for polyketide synthases, nonribosomal peptide synthetases, or terpene cyclase, which convert primary metabolites into molecules that act as precursors for the synthesis of biologically active secondary metabolites or families of structurally related secondary metabolites, such as GA, are frequently found in these clusters (Yang et al., Citation2023) ().
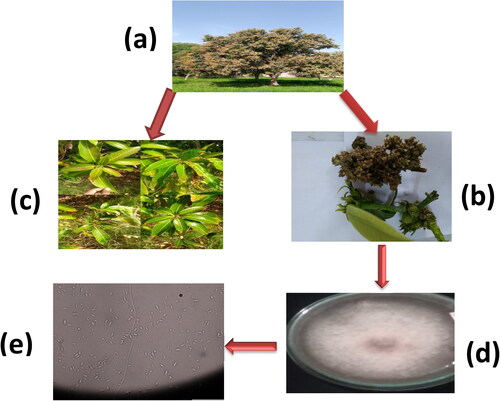
In recent years, other Fusarium types have been implicated in malformation, including F. tuppence from Brazil, F. maximum from Mexico, and F. sterihyphosum from Brazil and South Africa (Uemura et al., Citation2022). Koch’s postulates, on the other hand, verified F. subglutinans and F. oxysporum as the causative agents of malformation. The fungus, which causes this vast spectrum of plant syndromes, especially MMD, has been considered all F. subglutinans for many years, leading to great misunderstanding. F. mangiferae has been discovered in Sri Lanka, the USA, Oman, India, Israel, Egypt, Spain, South Africa, Malaysia, and China, which seem to be the most frequent cause of MMD in the world (Vignassa et al., Citation2021). shows the various stages (a) Mango Tree bearing mango malformation disease (b) Vegetative Malformation, (c) Floral Malformation, (d) Isolated Fusarium culture, (e) Isolated culture under microscopic study showing microspores and macrospores of F. mangiferae
3. Conventional methods for mango malformation disease control
3.1. Cultural practices
Proper sanitation measures, including the removal and destruction of infected plant mango malformation materials, are important for preventing the spread of mango malformation disease. Pruning infected branches and maintaining good orchard hygiene are essential cultural practices for disease control (Ploetz, Citation2007).
3.2. Chemical treatments
Chemical control measures involving the use of fungicides or growth regulators can be employed to manage mango malformation disease. Fungicides such as triazole derivatives or copper-based compounds can help suppress the growth and spread of the causal fungus. Additionally, growth regulators like paclobutrazol can be applied to regulate vegetative growth and enhance tree vigor, contributing to disease management (Rana et al., Citation2021).
3.3. Pruning techniques
Pruning plays a vital role in mango malformation disease management. Removing diseased branches and affected plant parts can significantly reduce the incidence and severity of the disease (Chakruno et al., Citation2022). Pruning should be conducted during the dormant season, and proper disposal of pruned materials is crucial to prevent further disease spread (Das & Pattanayak, Citation2020).
3.4. Disease-free planting material
The use of disease-free planting material is essential for preventing the introduction and spread of mango malformation disease. Planting mango varieties that are resistant or tolerant to the disease can help mitigate its impact (Ghosh et al., Citation2022).
3.5. Monitoring and early detection
Regular monitoring of mango orchards for signs and symptoms of mango malformation disease is critical for early detection and timely management. Early detection enables the prompt implementation of control measures such as pruning or chemical treatments (Li et al., Citation2021). Training farmers and orchard workers to recognize the disease symptoms contributes to effective disease management (Islam et al., Citation2020).
4. Limitations and challenges associated with current treatment approaches
The effectiveness of chemical treatments in managing mango malformation disease is often restricted. The causal fungus, Fusarium mangiferae, has demonstrated resistance to commonly used fungicides, making it difficult to achieve satisfactory disease suppression (Kumari et al., Citation2021). Sharma et al. (Citation2021) stated that the challenge posed by the limited efficacy of chemical control measures in managing mango malformation disease. Chemical treatments employed for mango malformation disease control often lack specificity, leading to potential adverse effects on non-target organisms and the environment (Molina-Cárdenas et al, Citation2021). Many fungicides used to manage the disease have broad-spectrum activity, impacting beneficial microorganisms and natural enemies of pests (Azeem et al., Citation2022).
The intricate mechanisms underlying mango malformation disease are still not fully understood. The interactions between the causal fungus and mango trees, as well as the factors influencing disease development and progression, remain insufficiently elucidated (Nazarov et al., Citation2020). The significant limitation in current treatment approaches for mango malformation disease due to the limited understanding of disease mechanisms (Pierson et al., Citation2023). Current treatment approaches for mango malformation disease heavily rely on chemical interventions, which may not align with sustainable agricultural practices. There is a need for integrated disease management strategies that incorporate cultural, biological, and chemical control measures (Datir & Regan, Citation2022; Fatima et al., Citation2023; Mehak et al., Citation2023).
Implementing existing treatment approaches for mango malformation disease can pose economic challenges, particularly for small-scale farmers. The costs associated with chemical inputs, equipment, and labor required for disease management can be substantial (Leakey et al., Citation2022).
5. Causes and factors contributing to mango malformation disease
5.1. Fungal infection
mango malformation disease is primarily caused by the fungal pathogen F. mangiferae, which infects the floral tissues of mango trees, resulting in the abnormal development of inflorescences and vegetative shoots. F.mangiferae is widely recognized as the main causal agent of mango malformation disease (Gautam et al., Citation2017).
5.2. Environmental factors
Various environmental factors contribute to the occurrence and severity of mango malformation disease. Temperature and humidity play a crucial role in creating favorable conditions for fungal growth and infection. High temperatures and relative humidity during the flowering season promote the dissemination of the pathogen, leading to an increased incidence of the disease. Environmental factors significantly influence the development of mango malformation disease (Noriega-Cantú et al., Citation1999).
5.3. Nutritional imbalances
Imbalances in nutrient availability, particularly nitrogen and potassium, can make mango trees more susceptible to mango malformation disease. Inadequate or excessive levels of these nutrients can disrupt plant physiology and weaken the tree’s defense mechanisms, increasing its vulnerability to fungal infection. Nutritional imbalances contribute to the development of mango malformation disease (Haq et al., Citation2023; Umar et al., Citation2022).
5.4. Genetic factors
The susceptibility of mango cultivars to mango malformation disease varies based on their genetic makeup. Some cultivars are more prone to the disease, while others exhibit varying degrees of resistance. Genetic factors, including the expression of defense-related genes, play a crucial role in determining a plant’s ability to withstand fungal infection. Genetic factors contribute to the variability in mango malformation disease incidence among different mango cultivars (Raj et al., Citation2017).
5.5. Insect vectors
Insects, such as thrips and aphids, can act as vectors for the transmission of mango malformation disease. These sap-feeding insects carry fungal spores from infected plant tissues to healthy ones, facilitating the spread of the disease within the orchard. Insect vectors significantly contribute to the transmission and dissemination of mango malformation disease (Kumar et al., Citation1993).
6. Previous strategies for the management of mango malformation disease control
The most successful disease prevention involves avoiding inoculums, selecting resistant varieties, and pursuing disease control options that aim to eradicate the causative agent. Disease occurrence was reduced and yield increased as a result of physical changes accompanied by chemical treatments such as Prochloraz and benomyl spray. A powerful antifungal fabrication made from various extracts from a plant was found to be advantageous. Since malformed mango plants lack some PGRs, researchers discovered that spraying PGRs on them could help increase production by decreasing disease occurrence. Different bacteria have been discovered to secrete an antagonist substance to defeat the growth of fungi linked to this disease, and several pathologists have tried to combat the occurrence of the disease; however, none of the results has been proven to be 100% successful and the disease remains a mystery (Katoch et al., Citation2019). To date, the disorder has been primarily managed by sanitation and continuous spraying of synthetic fungicides on the foliar (Soto-Plancarte et al., Citation2015). These types of fungicides have been identified as destructive to the ecosystem and their continued use may lead to the resilience of the fungus. As a result, the development of new disease management technologies has become crucial (Sharma et al., Citation2009). Shahbaz et al. (Citation2023) reported that around 83 diseases have been reported in mango trees and fruits around the world, with 27 diseases recognized as more significant in Pakistan.
Malformation is one of diseases that is damaging without destroying trees. The vegetative form of the disease obstructs canopy growth during the vegetative phase of the host plant, and therefore the floral form significantly decreases average fruit production with hibernating inoculums during the dormant phase of the host plant (Malik et al., Citation2018). F. mangiferae is a causal agent of mango malformation, which is the main restriction to mango production, resulting in a substantial decrease in yield and serious effects on GDP (Cohen et al., Citation2017). In an experiment, the application of humic acid as potassium humate; 0.15%, 0.30%, and 0.45%, boron as boric acid; 300, 600 mg l−1 was used during the 2017 and 2018 seasons using a combined and single foliar spray. The result showed a 25% malformation in trees with the best concentration of 0.30% HA + 600 mg l−1 of BA (El-Hoseiny et al., Citation2020). The researchers experimented with single and l-combined applications of benomyl 50 WP spray at 2.0 g l−1 water in the particular treatments evaluated. Applications with clipping at a distance of 45 cm monitored by the spray of benomyl revealed the best consequences, giving a 70.37% reduction over the count of last year (Iqbal et al., Citation2011).
Nanoparticles such as ZnO are stated with concentrations of 50, 100, and 150 mg/L, nano silicon with concentrations of 150 and 300 mg/L as foliar spray on mango trees. Floral malformations are significantly reduced by using this spray. The foliar application of 100 mg/L of nanozinc oxide and 150 mg/L of nanosilicon proved to be more useful than other concentrations to control floral malformations (Elsheery et al., Citation2020). The occurrence and duration of flowering in plants were investigated as related to malformation disease that was minimized by the application of chemicals on the plant leaves, which slows or advances the start of flowering. When added at the flower bud differentiation level, auxin and gibberellins were shown to minimize panicle malformation. Healthy panicles were formed when anti-metformin was applied, also such as ascorbic acid, silver nitrate, glutathione, potassium met bisulfate, and naphthalene acetic acid (Kumar et al., Citation2018). Rymbai and Rajesh (Citation2011) reported that in October a foliar application of naphthalene acetic acid (100 ppm or 200 ppm) and the incidence of use of benomyl to an acceptable degree to control the disease. The use of naphthalene acetic acid (100 ppm) and indole-3-butyric acid (200 ppm) before flower bud differentiation condensed the appearance of floral malformation. If foliar application to the infected area of the malformed plant is carried out, cheats such as mangiferin Zn++ and mangiferin Cu++ are recommended to decrease the concentration of mangiferin and restore biochemical function, thus controlling the mango malformation. However, the use of these chemicals should be limited, since they can be harmful in high concentrations and over long periods. Potassium metabisulfite is toxic to human health and causes extreme eye irritation, skin irritation, and respiratory irritation. Researchers used the most general treatment to eradicate branches that have mango malformation disorder. Pruning removes the principle-accumulating shoot tip that causes malformations (Kumar et al., Citation1993). It was stated that in the Dashehari variety, moderate pruning of 20 cm shoots that have malformed panicles in January (in the panicle emergence state) decreases the disease (Sirohi et al., Citation2009). It was shown that pruning typically involves removing and burning infected terminal parts, as well as subtending three nodes. Ploetz et al. (Citation2002), Kumar et al. (Citation2018), and Freeman et al. (Citation2014) stated that sanitation activity minimizes MMD by decreasing inoculums.
Therefore, enforcing huge trees with difficult-to-reach panicles is a challenge. This method is used economically in South Africa and the United States to prevent mango malformation disease. Pruning with the foliar application of fungicides, insecticides, and other plant regulators was described as an effective way to minimize the effect of mango malformation. Iqbal et al. (Citation2011) and Freeman et al. (Citation2014) stated that fungicides such as benzimidazoles and Topsin-M were found to reduce mango malformation when used, although statistical consequences were not proven. An experiment was carried out in which chemicals including cobalt sulfates, nickel sulfates, and cadmium sulfates were used, which were shown to slightly suppress the disease but were unsafe to use in food (Singh et al., Citation1994). The use of Prochloraz and benomyl sprays, as well as physical changes, was shown to reduce the severity of mango malformation disorder. They also found that the spraying of PGRs helped reduce the impact of the disease because malformed tissues lack some PGRs (Katoch et al., Citation2019). Similarly, Veldman et al. (Citation2018) published the experimental results carried out in the northern region of South Africa, in which 35 fungal isolates were identified from infected orchards, 32 of which were classified as F. mangiferae. Antifungal characteristics of 77 bacterial isolates from mango orchards and other settings investigated. Among all the isolated bacteria, the Alcaligenes faecalis genes were able to stop the progression of the pathogen. An experiment was conducted with Dashehari mango, pruning newborn panicles to reduce the effect of mango malformation by eliminating deformed panicles measuring 5, 10, 15, 20, and 25 cm. After shoot pruning, a paste of copper oxychloride and linseed oil was spread on the cut surface. As a result, 25 cm pruning of malformed panicles exhibited a significant reduction in disease frequency compared to other treatments (Prakash et al., Citation2016). An experiment was carried out in which various N, P, and K combinations (M.indica) were applied. To assess the time of flush emergence, the frequency of malformations and morphological characteristics, soil treatments with N (1000 g), P (750 g), and K (750 g) were applied between February and August. The lowest and highest number of malformed panicles was discovered. Furthermore, the NPK-treated plant had longer and healthy panicles.
Furthermore, compared to other fertiliser therapies, NPK treatment had the lowest percentage of malformation rate. The results of the regrowth pattern of the shoots after pruning revealed that the majority of such terminals 93.59% remained pruned, while some of them developed vegetative flushes at 2.19%. However, using a single fertiliser or a combination of fertilisers will not be sufficient to control this problem (Azam et al., Citation2020). An experiment in which Prochloraz was spraying on malformed panicles was described, including the removal of the malformed inflorescence several times. Removal of malformed inflorescence showed a significant result in one year from 19.9% to 3.55% in four years, but no authentic result was found between treatments. The spraying of Prochloraz-Zn at three-week intervals and the removal of deformed inflorescence had a substantial effect on mango malformation disease (Schoeman et al., Citation2019).
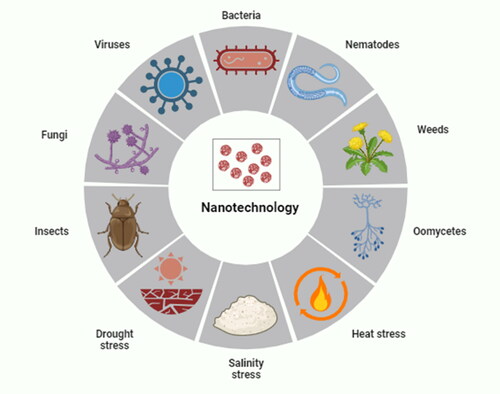
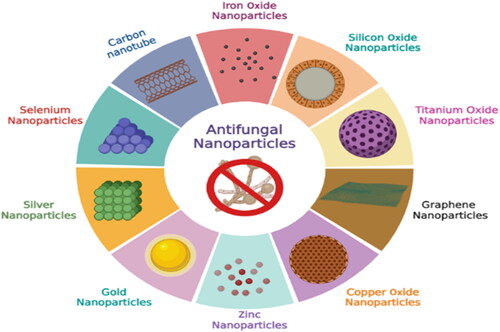
Helaly et al. (Citation2018) experimented with two mango cultivars named ‘Keitt and Ewais’ using treated wastewater + salicylic acid. While treated wastewater did not influence malformed panicles, salicylic acid worked well to reduce malformed panicles. Furthermore, combining the effects of treated wastewater with salicylic acid in a concentration of 50% reduced the percentage of panicle malformation. Plant pests and diseases are responsible for 20–40% of crop losses each year. Toxic pesticides are used to treat plant diseases, which are potentially harmful to humans and the environment. Nanotechnology has the potential to improve pesticides by lowering toxicity, extending shelf life, and improving the solubility of poorly water-soluble pesticides, all of which could have a good influence on the environment (Worrall et al., Citation2018). Elmer & White (Citation2018) described that metalloids, metallic oxides, non-metals, and carbon nanomaterials, as well as functionalized dendrimers, liposomes, and quantum dots, are examples of engineered nanoparticles with sizes ranging from 1 to 100 nm. They can be used as bactericides/fungicides and nanofertilizers because of their small size, large surface area, and strong reactivity. Nanoparticles can be used to build biosensors to detect plant diseases and transport vehicles for genetic material, probes, and agrichemicals (Umair Raza et al., Citation2023). Nanotechnology plays an important role in alleviating the biotic and abiotic stress in plants shown in .
Nanotechnology plays a revolutionary role in science and technology by managing materials at the nanoscale. Infect, nanotechnology refers to any technology based on nanosized materials having many applications in the actual world by encompassing the fabrication and use of physical, chemical, and biological systems from individual molecules to atoms into nanosized materials and uses this submicron-sized material into these systems. This technology solves global problems and meets our needs and aspirations (Nasrollahzadeh et al., Citation2019).
7. Application of nanotechnology in agriculture
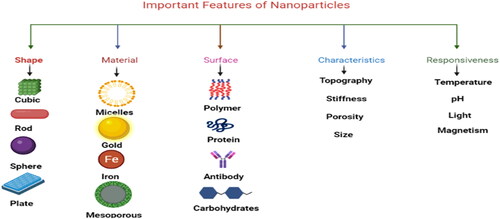
Nanomaterials play an important role in the field of agriculture due to their size, surface area, and characteristics ().
7.1. Enhanced nutrient delivery and efficiency
Nanotechnology holds promise in augmenting nutrient delivery systems for plants. Nanoscale nutrient carriers, such as nano-fertilizers, exhibit the potential to enhance nutrient absorption and mitigate nutrient losses. DeRosa et al. (Citation2010) demonstrated that nanoscale zinc oxide and iron oxide particles augment nutrient uptake in tomato plants, thereby promoting growth and development while concurrently reducing environmental ramifications.
7.2. Controlled release of pesticides
Nanotechnology offers the prospect of precise and controlled pesticide release, thus mitigating the adverse environmental consequences and curtailing pesticide resistance. Encapsulation of pesticides within nanocarriers enables gradual and sustained release, ensuring effective pest management. Kah et al. (Citation2019) developed a nanopesticide formulation using chitosan nanoparticles loaded with a pesticide, which exhibited controlled release, heightened efficacy, and diminished environmental contamination.
7.3. Disease detection and monitoring
Nanotechnology-based biosensors provide the means for rapid and sensitive detection of plant diseases, facilitating early intervention and disease management. Wang et al. (Citation2019) engineered a nanobiosensor for the detection of the devastating citrus greening bacterium, exhibiting remarkable sensitivity, specificity, and speed in pathogen detection, enabling timely disease mitigation strategies.
7.4. Soil remediation and environmental sustainability
Nanoparticles have exhibited potential in soil remediation by adsorbing or degrading pollutants, ameliorating soil fertility, and promoting plant growth. Khodakovskaya et al. (Citation2018) investigated the utilization of cerium oxide nanoparticles for remediating contaminated soils, revealing their efficacy in reducing heavy metal toxicity and fostering plant growth in polluted soil, thereby underscoring their potential for environmental sustainability.
Nanotechnology offers a wide range of uses in agriculture, pharmacology, medicine, and other domains (Balaure et al., Citation2017; Sinha et al., Citation2017). Nanotechnology improves seed germination, transfers target genes, nano biosensors, hormone delivery, and nano barcoding in agriculture, as well as reduces the emission of chemicals associated with agriculture (Hayles et al., Citation2017). This technology is an art and science that manipulates matter to nanoscale sizes, characterizes them, and then uses them for the desired purpose by manipulating their shape and structure (Abasi et al., Citation2022; Abobatta, Citation2018; Hassan et al., Citation2022; Shahbaz et al., Citation2022a; Citation2022b). Plant-based nanoparticles are cost-effective and eco-friendly and are used as stress alleviators in plants (Shahbaz et al., Citation2023b) ( and ).
Table 1. Examples of nanoparticles previously used to control fungal diseases in plants.
Sarojini et al. (Citation2020) reported the antimicrobial activity of SeNPs against S. mutans and Lactobacillus that produced a zone of inhibition higher than usual. Similarly, antifungal activity indicated a zone of inhibition against Candida albicans that was almost identical to the standard used. SeNPs were synthesized from Psidium guajava by using the agar well diffusion method to find the antibacterial effectiveness against E. faecalis (Miglani, & Tani-Ishii, Citation2021).
8. Applications of nanotechnology in plant disease management
8.1. Targeted delivery and controlled release
Nanotechnology enables the engineering of nanoparticles to encapsulate fungicides or antimicrobial agents, facilitating their targeted delivery to specific plant tissues. This approach ensures precise and localized delivery, thereby minimizing off-target effects and reducing the overall amount of chemicals required. Gour et al. (Citation2019) demonstrated the successful utilization of chitosan nanoparticles as a vehicle for targeted delivery of a fungicide against Alternaria solani, a causative agent of early blight in tomato plants.
8.2. Enhanced efficacy against drug-resistant pathogens
Nanoparticles exhibit inherent antifungal properties and can enhance the effectiveness of fungicides, even against pathogens that have developed resistance to conventional treatments. Notably, silver nanoparticles (AgNPs) have demonstrated potent antifungal activity against Candida albicans, including strains resistant to fluconazole, a commonly used antifungal drug (Rai et al., Citation2009).
8.3. Early disease detection through nanobiosensors
Nanotechnology facilitates the development of highly sensitive nanobiosensors for early detection of plant diseases. These nanobiosensors are designed to detect pathogen-specific molecules or plant biomarkers that serve as indicators of disease presence. Zhan et al. (Citation2018) successfully developed a nanobiosensor utilizing gold nanoparticles for the detection of Phytophthora infestans, the causal agent of late blight in potato plants.
8.4. Safer andenvironmentally nriendly nanopesticides
Nanotechnology offers the creation of nanopesticides with improved safety profiles, addressing concerns related to environmental contamination. Due to their enhanced efficacy and targeted delivery, nanopesticides necessitate lower application rates, resulting in reduced chemical usage and potential environmental risks. For instance, Kah et al. (Citation2019) demonstrated the efficacy of zinc oxide nanoparticles as nanopesticides against Botrytis cinerea, the causative agent of gray mold in plants.
8.5. Nanocarriers for biocontrol agents
This technology provides a platform for the development of nanocarriers that protect and enhance the efficacy of biocontrol agents, such as beneficial microbes or natural compounds. These nanocarriers offer controlled release mechanisms, improving the stability and prolonging the shelf life of biocontrol agents. Riseh et al. (Citation2022) developed nanocarriers based on chitosan nanoparticles loaded with a biocontrol agent for the management of Fusarium wilt in tomato plants, effectively enhancing the biocontrol agent’s efficacy.
9. Advantages of using nanotechnology for disease management
9.1. Targeted delivery of therapeutics
Nanotechnology enables precise and targeted delivery of therapeutic agents, such as antimicrobial compounds or drugs, to specific sites, enhancing treatment efficacy while minimizing side effects on healthy tissues. Zhang et al. (Citation2021) developed a targeted nanocarrier system to deliver antimicrobial peptides for treating bacterial infections. The nanocarriers exhibited improved antimicrobial activity and reduced toxicity compared to free antimicrobial peptides.
9.2. Enhanced penetration and bioavailability
Nanoscale drug delivery systems improve the penetration and bioavailability of therapeutic agents by overcoming biological barriers and delivering drugs to specific locations, including intracellular targets. Waghule et al. (Citation2020) developed lipid-based nanoparticles for delivering antifungal drugs to treat invasive fungal infections. These nanoparticles demonstrated enhanced drug penetration into biofilms and improved antifungal activity.
9.3. Diagnostic sensitivity and specificity
Nanotechnology-based diagnostic tools offer increased sensitivity and specificity in disease detection. Nanosensors and nanobiosensors can detect disease markers or pathogens at low concentrations, enabling early and accurate diagnosis. Roointan et al. (Citation2019) developed a nanobiosensors for detecting cancer biomarkers, demonstrating high sensitivity and specificity in identifying the target biomarkers for potential applications in early cancer diagnosis.
9.4. Real-time monitoring and imaging
Nanotechnology enables real-time monitoring and imaging of disease progression, facilitating improved disease management. Nanoparticles can be engineered with imaging or contrast agents to visualize disease sites and monitor treatment response. Ernst et al. (Citation2015) developed magnetic nanoparticles for real-time monitoring of bacterial infections using magnetic resonance imaging (MRI), allowing accurate tracking and monitoring of bacterial growth in vivo.
9.5. Synergistic therapy approaches
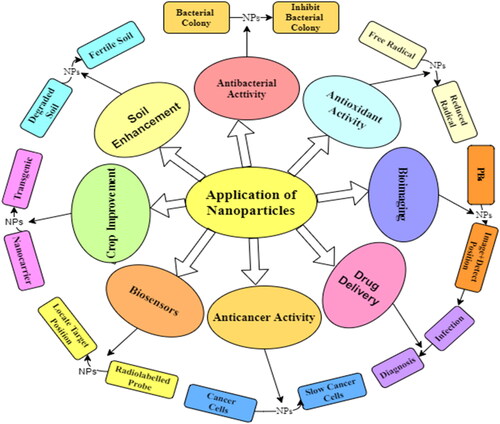
Nanotechnology allows for the development of combination therapies by incorporating multiple therapeutic agents or modalities into a single nanosystem, leading to synergistic effects and improved therapeutic outcomes. Jokerst et al. (Citation2017) developed a nanosystem that combined photothermal therapy and chemotherapy for cancer treatment, resulting in enhanced tumor regression compared to individual treatments alone. Ruparelia et al. (Citation2008) reported the antimicrobial activity of silver and copper nanoparticles against different strains of Escherichia coli (four strains), Bacillus subtilis and Staphylococcus aureus (three strains) using the disc diffusion method and the average size of silver and copper nanoparticles were reported to be 3 and 9 nm, respectively, using different characterization techniques. Similarly, Dizaj et al. (Citation2015) also reported the antimicrobial activity of carbon-based nanoparticles. The antimicrobial activity of CeONPs was evaluated using different bacterial and fungal pathogens and concluded that CeONPs also have antimicrobial activity (Roy et al., Citation2013). The plant based silver nanoparticles synthesized by the Streptacidiphilus durhamensis strain comprised antimicrobial activity and also possesses physicochemical and biochemical characteristics shown in (Buszewski et al., Citation2018). Silver nanoparticles have been used to control fungal and bacterial infection in the management of plant disease and have strong antimicrobial activity and enhance the physiological and biochemical attributes of the plant (Cao et al., Citation2010).
Venkataraju et al. (Citation2014) described that zinc oxide nanoparticles synthesized from Aloe vera leaf extract contain antimicrobial activity. Similarly, the antimicrobial activity of zinc oxide nanoparticles was also reported by (Elumalai & Velmurugan, Citation2015; Jesline et al., Citation2015; Manna, Citation2012; Pasquet et al., Citation2014). Cerium oxide nanoparticles also contain antimicrobial activity (Firmino et al., Citation2017). Similarly, the antimicrobial activity of titanium oxide nanoparticles was investigated by (Azizi-Lalabadi et al., Citation2019; Nadeem et al., Citation2018). The effective antimicrobial activity of silver nanoparticles decorated with silica nanoparticles was described by Otari et al., (Citation2019).
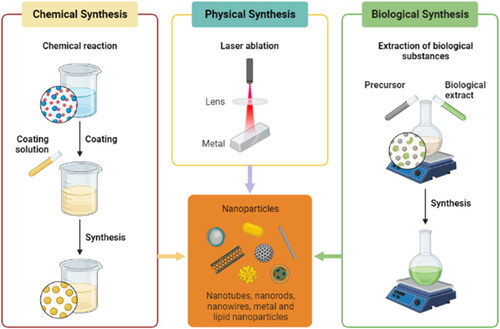
10. Methods of synthesizing nanoparticles
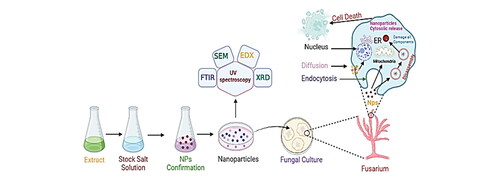
Nanoparticles synthesized by various methods are shown in .
10.1. Chemical reduction method
The chemical reduction method is a widely used approach for synthesizing nanoparticles. It involves the reduction of metal precursors in a solution to produce nanoparticles. The process typically utilizes a reducing agent that reacts with the metal ions, leading to the formation of nanoparticles. This method offers flexibility in controlling the size, shape, and composition of the nanoparticles by adjusting reaction conditions and precursor concentrations and the use of a chemical reduction approach to synthesize gold nanoparticles with controlled sizes and shapes (Harish et al., Citation2023).
10.2. Sol-gel method
The sol-gel method is another commonly employed technique for nanoparticle synthesis. It involves the formation of a colloidal solution (sol) that undergoes a gelation process to form nanoparticles. The sol-gel process typically starts with the hydrolysis and condensation of precursor molecules, which eventually leads to the formation of a gel network. The gel can then be processed to obtain nanoparticles. This method allows for the synthesis of nanoparticles with controlled particle sizes and offers the ability to incorporate various dopants and functionalization agents. The sol-gel method was employed to produce silica nanoparticles with controlled particle sizes (Priyan et al., Citation2023). The versatility of the sol-gel method makes it suitable for applications ranging from catalysis to biomedical fields (Rai & Chauhan, Citation2023).
10.3. Hydrothermal/solvothermal method
The hydrothermal or solvothermal method involves the synthesis of nanoparticles under high-pressure and high-temperature conditions. By subjecting a precursor solution to such conditions, the reaction rate is enhanced, leading to the formation of well-defined nanoparticles with controlled morphologies and enhanced crystallinity (Yadav et al., Citation2023). This method is particularly useful for the synthesis of metal oxide nanoparticles researchers utilized a solvothermal approach to synthesize metal oxide nanoparticles with specific properties (Soni et al., Citation2023). The hydrothermal/solvothermal method allows for the precise control of nanoparticle size, shape, and crystallinity, which is essential for tailoring their properties for various applications (Barhoum et al., Citation2023).
10.4. Electrochemical method
The electrochemical method is a versatile technique for synthesizing nanoparticles with precise control over their size, shape, and morphology. This method involves the electrodeposition or electrolysis of metal ions in an electrochemical cell. By controlling the electrode potential and other experimental parameters, nanoparticles can be fabricated directly onto conductive substrates or collected from solution (Kamble & Mane, Citation2023). Electrochemical synthesis offers advantages such as scalability, compatibility with various substrates, and the ability to produce nanoparticles with well-defined structures. Researchers utilized the electrochemical method to synthesize silver nanoparticles on conductive substrates (Harish et al., Citation2023).
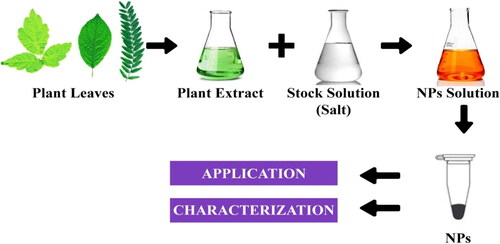
10.5. Green synthesis
Green synthesis methods have gained significant attention due to their eco-friendly and sustainable nature (Rani et al., Citation2023) (). These methods utilize natural extracts, biomolecules, or microorganisms as reducing and stabilizing agents for nanoparticle synthesis. By employing green synthesis approaches, the need for harsh chemicals and toxic solvents can be minimized. Additionally, green synthesis methods often offer advantages such as cost-effectiveness, biocompatibility, and the ability to produce nanoparticles with unique properties. It employs a green synthesis approach using plant extracts to synthesize silver nanoparticles with antimicrobial properties (Malik et al., Citation2023). Green synthesis methods provide a greener alternative for nanoparticle synthesis, making them appealing for various applications, including biomedical and environmental fields. The formation of nanoparticles using the green method highlights the use of natural vegetation, microbes, microalgae, enzymes, plants, and plant extracts, which gives a dependable, easy, low-cost, friendly behavior with an ecosystem, nontoxic and stable nature, and eco-friendly results (Menon et al., Citation2020).
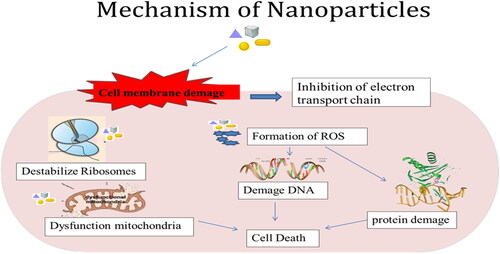
Because plants function as both stabilizing and reducing agents, synthesis mediated by green sources allows fine control of nanoparticle size and form (Wadhwani et al., Citation2016). Chemically produced nanoparticles have a substantially lower inhibitory effect than green nanoparticles (Vijayakumar et al., Citation2018). Plants play a key role in nanoparticle-green synthesis. It has a large role to play in it. Plants produce metabolites that help break down precursor chemicals. It also acts as a catalyst and stabilizer in the creation of nanoparticles (Muthamil Selvan et al., Citation2018). Nanoparticles enter cells, activate transcription factors and reduce the ROS production that causing due to fungal stress shown in .
11. Challenges in the practical implementation of nanotechnology
The safety of nanomaterials and their potential environmental impact is a significant concern in the practical application of nanotechnology in agriculture. It is essential to thoroughly evaluate the potential risks associated with the use of nanomaterials and ensure their safe handling and disposal. According to Handy et al. (Citation2008), comprehensive studies on the environmental fate and toxicity of nanoparticles are necessary to minimize any adverse effects on ecosystems. The regulatory framework for nanotechnology in agriculture is still evolving, and clear guidelines and regulations governing the use of nanomaterials are needed. Adequate regulations are necessary to ensure the safe development, production, and use of nanotechnology-based products in agriculture. Stone et al. (Citation2018) emphasize the need for a coordinated and adaptive regulatory approach to address the unique challenges posed by nanotechnology. The cost of nanotechnology-based solutions can be a barrier to their practical implementation in agriculture. The production and application of nanomaterials often involve complex processes and specialized equipment, leading to higher costs compared to conventional approaches. Additionally, scaling up nanotechnology processes to meet the demands of large-scale agricultural operations can be challenging. Castillo-Henríquez et al. (Citation2020) highlighted the importance of carefully evaluating the high cost of nanotechnology applications against their potential benefits in disease control and crop productivity.
There is still much to learn about the interactions between nanomaterials and plants, including their uptake, translocation, and potential effects on plant physiology and growth. More research is needed to understand the mechanisms of nanomaterial uptake by plants and their impacts on plant health. Servin et al. (Citation2012) stress the significance of a comprehensive understanding of nanomaterial-plant interactions for the safe and effective implementation of nanotechnology in agriculture. The practical application of nanotechnology-based solutions for disease control in agriculture, including Mango malformation disease, may face challenges related to formulation stability, dosage optimization, and effective delivery to target sites. DeRosa et al. (Citation2010) discuss these challenges in the context of using nanotechnology for plant disease management, emphasizing the need to address formulation and delivery issues.
12. Conclusions and future directions
This study signifies the importance of mango and emphasizes the mango malformation disease that affects the mango crop and decreases its production. Primarily, control was attempted using fungicides that have toxic effects on the environment and humans. Nanotechnology is the most advanced technology in the twenty first century and has been used to control fungal diseases in plants. Nanoparticles are biocompatible, inexpensive, and non-toxic and cannot affect human health. It is concluded that there is less use of nanotechnology for the treatment of mango diseases, especially mango malformations since only zinc and silicon nanoparticles were used to control floral malformations. As is known, nanotechnology produces a promising effect in controlling plant diseases and ultimately increasing its yield, so the use of nanoparticles to control mango malformations will be fruitful. Metallic nanoparticles possess antifungal properties and some properties allow them to destroy fungal cells and produce immunity in plants that enables them to fight any incoming pathogen. The fame of green nanotechnology is increasing tremendously and is being used in all biological fields. Metallic nanoparticles such as selenium, silver, titanium, zinc, chitosan, silicon, copper, iron, manganese, gold, and cerium oxide nanoparticles act as a strong antifungal potential compared to ordinary fungicides. According to a previous study on the management of mango malformations using ordinary fungicides, it was clearly revealed that proper management of this disease is needed. Reviewing the literature on nanotechnology also plays a revolutionary role in the identification, diagnosis, and proper control of plant diseases. The researchers clearly indicate direct application of nanoparticles in the control of plant diseases. Conducting research on mango malformation can help scientists and researchers gain a deeper understanding of the causes, mechanisms, and factors contributing to the disease. This knowledge can lead to the development of effective management strategies and treatments to control or prevent mango malformation. Nanotechnology offers innovative approaches such as nano encapsulation of bioactive compounds or genetic material delivery systems, which can enhance plant resistance to diseases. Nanoparticles can be used to deliver targeted treatments or genetic material to the mango trees, promoting resistance against pathogens associated with malformation. Collaborative research in nanotechnology can aid in the development of rapid and accurate diagnostic tools for mango malformation. Nanotechnology can contribute to the development of sustainable and eco-friendly approaches for disease management in mangoes. Nanomaterials can be utilized to create environmentally friendly fungicides or biopesticides that are highly effective against pathogens causing mango malformation. This can reduce the dependence on conventional chemical pesticides, promoting environmentally friendly agricultural practices. Collaboration among scientists, researchers, and experts in the field of nanotechnology and mango pathology can foster knowledge exchange and interdisciplinary approaches. Overall, further research on mango malformation and collaboration in the field of nanotechnology can bring about significant advancements in disease management, the development of disease-resistant varieties, diagnostic tools, sustainable approaches, and overall improvement in mango production and quality. Therefore, the use of nanotechnology for the management of mango malformation is needed.
Author contributions
All authors of this review article have contributed significantly to the literature study, and critically revised the review article. M.S and J.S.S.S: Conceptualization/conceived the study idea, planned and designed the research structure, wrote the first draft of the manuscript, data validation, visualization, customized images, and final draft. N.I.R, supervised research, drafting process, and revised the first draft. A.M & N.F & M.U.R: Formal analysis, Validation, Resources, suggestions. F.A: helped with data validation and interpretation, Conceptualization, Data curation, Review editing. M.I Methodology, Visualization, Validation, and final editing of the manuscript. J.P: helped with data validation and interpretation, guided the draft write-up, and carried out a critical revision of the final draft. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We are grateful to the Nanobiotechnology Lab, Department of Botany, PMAS-Arid Agriculture University, Rawalpindi, and Punjab, Pakistan for their support in the successful completion of this experimental work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the obtained data are presented in this article.
Additional information
Funding
References
- Abasi, F., Raja, N. I., Mashwani, Z. U. R., Amjad, M. S., Ehsan, M., Mustafa, N., Haroon, M., & Proćków, J. (2022). Biogenic silver nanoparticles as a stress alleviator in plants: A mechanistic overview. Molecules (Basel, Switzerland), 27(11), 3378. https://doi.org/10.3390/molecules27113378
- Abdelaziz, A. M., Dacrory, S., Hashem, A. H., Attia, M. S., Hasanin, M., Fouda, H. M., Kamel, S., & ElSaied, H. (2021). Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatalysis and Agricultural Biotechnology, 35, 102083. https://doi.org/10.1016/j.bcab.2021.102083
- Abobatta, W. F. (2018). Development growth and productivity of orange orchards (Citrus sinensis L) in Egypt (delta region). Advances in Agricultural Technology & Plant Sciences, 1, 180003.
- Adikaram, N. K. B., Maharachchikumbura, S. S. N., Yakandawala, D. M. D., Manawadu, L. N., Dissanayake, D. M. S., & Jayasinghe, L. (2023). Postharvest stem-end browning (SEB) disease in ripe mango (Mangifera indica L.) cultivar TomEJC. European Journal of Plant Pathology, 165(3), 447–464. https://doi.org/10.1007/s10658-022-02616-5
- Adin, S. N., Gupta, I., Aqil, M., Mujeeb, M., & Ahad, A. (2023). BBD driven optimization of extraction of therapeutically active xanthanoid mangiferin from Mangifera indica L. leaves and its antioxidant activity. Pharmacognosy Research, 15(1), 84–93. https://doi.org/10.5530/097484900279
- Aldrich, J. A., Kebreab, E., Fouts, J., Reed, K. F., Tate, B. N., Deys, M. M., & Chase, L. E. (2021). 2021 Cornell Nutrition Conference-complete proceedings of manuscripts [Paper presentation].
- Alvi, A. R., Chohan, S., Abid, M., Malik, M. T., Riaz, H. M., & Mudassar, S. (2022). Flower induction and control of inflorescence diseases in mangoes using selected fungicides and chemical fertilizers. International Journal of Phytopathology, 11(1), 01–08. https://doi.org/10.33687/phytopath.011.01.3555
- Azam, M., Qadri, R., Khan, M. I., Khan, M., Akhtar, N., Khan, N. H., & Ayyub, C. M. (2020). Impact of fertilizer combinations on malformation physiology of mango panicles (Mangifera indica. L) cv. Dusheri. Pure and Applied Biology, 9(1), 626–634. https://doi.org/10.19045/bspab.2020.90068
- Azeem, S., Agha, S. I., Jamil, N., Tabassum, B., Ahmed, S., Raheem, A., Jahan, N., Ali, N., & Khan, A. (2022). Characterization and survival of broad-spectrum biocontrol agents against phytopathogenic fungi. Revista Argentina de Microbiologia, 54(3), 233–242. https://doi.org/10.1016/j.ram.2021.10.005
- Azizi-Lalabadi, M., Ehsani, A., Divband, B., & Alizadeh-Sani, M. (2019). Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Scientific Reports, 9(1), 17439. https://doi.org/10.1038/s41598-019-54025-0
- Balaure, P. C., Gudovan, D., & Gudovan, I. (2017). Nanopesticides: A new paradigm in crop protection. In Alexandru Mihai Grumezescu (Ed.), New pesticides and soil sensors (pp. 129–192). Academic Press. https://doi.org/10.1016/B978-0-12-804299-1.00005-9
- Barhoum, A., Meftahi, A., Kashef Sabery, M. S., Momeni Heravi, M. E., & Alem, F. (2023). A review on carbon dots as innovative materials for advancing biomedical applications: Synthesis, opportunities, and challenges. Journal of Materials Science, 58(34), 13531–13579. https://doi.org/10.1007/s10853-023-08797-6
- Bharatkumar, D. P., Khatun, P., Kumar, C., & Yadav, A. K. (2023). Role of agriculture processing in export growth of agricultural products. Journal of Current Research in Food Science, 4(1), 49–56. https://doi.org/10.22271/foodsci.2023.v4.i1a.91
- Bhunjun, C. S., Phillips, A. J., Jayawardena, R. S., Promputtha, I., & Hyde, K. D. (2021). Importance of molecular data to identify fungal plant pathogens and guidelines for pathogenicity testing based on Koch’s Postulates. Pathogens (Basel, Switzerland), 10(9), 1096. https://doi.org/10.3390/pathogens10091096
- Bibi, G., Gagosh Nayyar, B., Ajmal, M., Mehak, A., Seerat, W., Shahbaz, M., Mukhtar, T., & Akram, A. (2023). Effect of culture filtrates of Alternaria alternata on seed germination and seedling growth of sesame. Archives of Phytopathology and Plant Protection, 56(8), 625–635. https://doi.org/10.1080/03235408.2023.2212417
- Buszewski, B., Railean-Plugaru, V., Pomastowski, P., Rafińska, K., Szultka-Mlynska, M., Golinska, P., Wypij, M., Laskowski, D., & Dahm, H. (2018). Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. Journal of Microbiology, Immunology, and Infection = Wei Mian yu Gan Ran za Zhi, 51(1), 45–54. https://doi.org/10.1016/j.jmii.2016.03.002
- Cao, X. L., Cheng, C., Ma, Y. L., & Zhao, C. S. (2010). Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. Journal of Materials Science. Materials in Medicine, 21(10), 2861–2868. https://doi.org/10.1007/s10856-010-4133-2
- Castillo-Henríquez, L., Alfaro-Aguilar, K., Ugalde-Álvarez, J., Vega-Fernández, L., Montes de Oca-Vásquez, G., & Vega-Baudrit, J. R. (2020). Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials (Basel, Switzerland), 10(9), 1763. https://doi.org/10.3390/nano10091763
- Chakruno, P., Banik, S., & Sumi, K. (2022). Status and strategies for managing mango (Mangifera indica L.) diseases. In Sarivastava and Singh (Ed.), Diseases of horticultural crops: Diagnosis and management: Volume 1: Fruit crops (p. 355). CRC Press.
- Chaturvedi, K., Singh, P., & Mehrotra, R. (2022). Application of’omics technologies in tropical and subtropical fruit crops. In Gyana Ranjan Rout and K.V. Peter (Ed.), Omics in horticultural crops (pp. 119–145). Academic Press. https://doi.org/10.1016/B978-0-323-89905-5.00027-6
- Cohen, Y., Belausov, E., Maymon, M., Elazar, M., Shulman, I., Saada, D., Shtienberg, D., & Freeman, S. (2017). Fusarium mangiferae localization in planta during initiation and development of mango malformation disease. Plant Pathology, 66(6), 924–933. https://doi.org/10.1111/ppa.12650
- D’Souza, S., Udavant, P., Kadam, J., Khairnar, S., Ahire, E. D., & Sable, R. (2023). Role of vitamins in metabolic diseases. Vitamins as Nutraceuticals: Recent Advances and Applications., 205–233. https://doi.org/10.1002/9781394175543.ch9
- Darwesh, O. M., & Elshahawy, I. E. (2021). Silver nanoparticles inactivate sclerotial formation in controlling white rot disease in onion and garlic caused by the soil borne fungus Stromatinia cepivora. European Journal of Plant Pathology, 160(4), 917–934. https://doi.org/10.1007/s10658-021-02296-7
- Das, S., & Pattanayak, S. (2020). Integrated disease management on grapes–a pioneer of a reformed movement towards sustainability. International Journal of Current Microbiology and Applied Sciences, 9(5), 993–1005. https://doi.org/10.20546/ijcmas.2020.905.109
- Datir, S., & Regan, S. (2022). Advances in physiological, transcriptomic, proteomic, metabolomic, and molecular genetic approaches for enhancing Mango fruit quality. Journal of Agricultural and Food Chemistry, 71(1), 20–34. https://doi.org/10.1021/acs.jafc.2c05958
- De Weerdt, J., Pienaar, L., Hami, E., & Durand, W. (2023). Leveraging urbanization for inclusive development in Malawi: Anchoring the secondary city development of Salima and Chipoka in a modernizing fruit value chain. International Food Policy Research Institute.
- Derbalah, A., Shenashen, M., Hamza, A., Mohamed, A., & El Safty, S. (2018). Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. Egyptian Journal of Basic and Applied Sciences, 5(2), 145–150. https://doi.org/10.1016/j.ejbas.2018.05.002
- DeRosa, M. C., Monreal, C., Schnitzer, M., Walsh, R., & Sultan, Y. (2010). Nanotechnology in fertilizers. Nature Nanotechnology, 5(2), 91–91. https://doi.org/10.1038/nnano.2010.2
- Dizaj, S. M., Mennati, A., Jafari, S., Khezri, K., & Adibkia, K. (2015). Antimicrobial activity of carbon-based nanoparticles. Advanced Pharmaceutical Bulletin, 5(1), 19. https://doi.org/10.5681/apb.2015.003.
- Dumitru Veleșcu, I., Nicoleta Rațu, R., Arsenoaia, V. N., Roșca, R., Marian Cârlescu, P., & Țenu, I. (2023). Research on the process of convective drying of apples and apricots using an original drying installation. Agriculture, 13(4), 820. https://doi.org/10.3390/agriculture13040820
- El-Batal, A. I., Sidkey, N. M., Ismail, A., Arafa, R. A., & Fathy, R. M. (2016). Impact of silver and selenium nanoparticles synthesized by gamma irradiation and their physiological response on early blight disease of potato. Journal of Chemical and Pharmaceutical Research, 8(4), 934–951.
- El-Gazzar, N., & Ismail, A. M. (2020). The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatalysis and Agricultural Biotechnology, 27, 101708. https://doi.org/10.1016/j.bcab.2020.101708
- El-Hoseiny, H. M., Helaly, M. N., Elsheery, N. I., & Alam-Eldein, S. M. (2020). Humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. HortScience, 55(7), 1026–1037. https://doi.org/10.21273/HORTSCI15053-20
- Elmer, W., & White, J. C. (2018). The future of nanotechnology in plant pathology. Annual Review of Phytopathology, 56(1), 111–133. https://doi.org/10.1146/annurev-phyto-080417-050108
- El-Saadony, M. T., Saad, A. M., Najjar, A. A., Alzahrani, S. O., Alkhatib, F. M., Shafi, M. E., Selem, E., Desoky, E.-S M., Fouda, S. E. E., El-Tahan, A. M., & Hassan, M. A. A. (2021). The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi Journal of Biological Sciences, 28(8), 4461–4471. https://doi.org/10.1016/j.sjbs.2021.04.043
- El‐Sayed, A. F. M., & Fitzsimmons, K. (2023). From Africa to the world—The journey of Nile tilapia. Reviews in Aquaculture, 15(S1), 6–21. https://doi.org/10.1111/raq.12738
- Elsheery, N. I., Helaly, M. N., El-Hoseiny, H. M., & Alam-Eldein, S. M. (2020). Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy, 10(4), 558. https://doi.org/10.3390/agronomy10040558
- Elumalai, K., & Velmurugan, S. (2015). Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Applied Surface Science, 345, 329–336. https://doi.org/10.1016/j.apsusc.2015.03.176
- Ernst, T. M., Fehling, H., Bernin, H., Zaruba, M. D., Bruchhaus, I., Adam, G., Ittrich, H., & Lotter, H. (2015). Magnetic resonance imaging of pathogenic protozoan parasite Entamoeba histolytica labeled with superparamagnetic iron oxide nanoparticles. Investigative Radiology, 50(10), 709–718. https://doi.org/10.1097/RLI.0000000000000175
- Fatima, N., Sarwar, S., Shahbaz, M., Hanif, M., Youosaf, N., & Abrar, A. (2023). Characterization of mycoflora associated with the rhizosphere of rice crop from selected sites in district Gujranwala, Punjab, Pakistan. Plant Protection, 7(2), 245–254.
- Fernández, J. G., Fernández-Baldo, M. A., Berni, E., Camí, G., Durán, N., Raba, J., & Sanz, M. I. (2016). Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochemistry, 51(9), 1306–1313. https://doi.org/10.1016/j.procbio.2016.05.021
- Firmino, H. C. T., Nascimento, E. P., Neves, G. A., & Menezes, R. R. (2017). Antimicrobial activity of cerium oxide nanoparticles. Revista Eletronica de Materiais e Processos, 12(2), 64–95.
- Freeman, S., Shtienberg, D., Maymon, M., Levin, A. G., & Ploetz, R. C. (2014). New insights into mango malformation disease epidemiology lead to a new integrated management strategy for subtropical environments. Plant Disease, 98(11), 1456–1466. https://doi.org/10.1094/PDIS-07-14-0679-FE
- Garcia-Lopez, E., Batista-Marte, C. M., Serra, C. A., Sosa-Natta, A. S., Villegas-Monter, A., Hernandez-Castro, E., Camacho-Tapia, M., & Mora-Aguilera, J. A. (2023). Mango malformation: Etiology, symptoms, distribution and cultivar susceptibility in the Dominican Republic. Canadian Journal of Plant Science, 103(3), 300–311. https://doi.org/10.1139/cjps-2022-0005
- Gautam, P., Shukla, A., & Sharma, S. (2017). First report of Fusarium mangiferae causing mango malformation disease in India. Journal of Plant Pathology & Microbiology, 8(1), 394.
- Gaytán, Á., Moreira, X., Castagneyrol, B., Van Halder, I., De Frenne, P., Meeussen, C., Timmermans, B. G. H., Ten Hoopen, J. P. J. G., Rasmussen, P. U., Bos, N., Jaatinen, R., Pulkkinen, P., Söderlund, S., Covelo, F., Gotthard, K., & Tack, A. J. M. (2022). The co‐existence of multiple oak leaf flushes contributes to the large within‐tree variation in chemistry, insect attack and pathogen infection. The New Phytologist, 235(4), 1615–1628. https://doi.org/10.1111/nph.18209
- Ghosh, D., Kokane, S., Savita, B. K., Kumar, P., Sharma, A. K., Ozcan, A., Kokane, A., & Santra, S. (2022). Huanglongbing pandemic: Current challenges and emerging management strategies. Plants (Basel, Switzerland), 12(1), 160. https://doi.org/10.3390/plants12010160
- Gómez-Ollé, A., Bullones, A., Hormaza, J. I., Mueller, L. A., & Fernandez-Pozo, N. (2023). MangoBase: A genomics portal and gene expression atlas for Mangifera indica. Plants (Basel, Switzerland), 12(6), 1273. https://doi.org/10.3390/plants12061273
- Gour, N., Upadhyaya, P., & Patel, J. (2019). Nanomaterials as therapeutic and diagnostic tool for controlling plant diseases. In Sandeep Kumar Verma and Ashok Kumar Das (Eds.), Comprehensive analytical chemistry (Vol. 84, pp. 225–261). Elsevier. https://doi.org/10.1016/bs.coac.2019.04.003
- Gupta, A. K., Gurjar, P. S., Beer, K., Pongener, A., Ravi, S. C., Singh, S., Verma, A., Singh, A., Thakur, M., Tripathy, S., & Verma, D. K. (2022). A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food Bioscience, 48, 101783. https://doi.org/10.1016/j.fbio.2022.101783
- Handy, R. D., von der Kammer, F., Lead, J. R., Hassellöv, M., Owen, R., & Crane, M. (2008). The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology (London, England), 17(4), 287–314. https://doi.org/10.1007/s10646-008-0199-8
- Haq, E. U., Hassan, F. U., Zhou, F., Gong, X., Manaf, A., Shabbir, G., … & Shamsi, I. H. (2023). Nitrogen Fertilization Improves the Agro-Morphological and Yield Attributes of Sinapis alba L. Agronomy, 13(6), 1621.https://doi.org/10.3390/agronomy13061621.
- Harish, V., Ansari, M. M., Tewari, D., Yadav, A. B., Sharma, N., Bawarig, S., García-Betancourt, M.-L., Karatutlu, A., Bechelany, M., & Barhoum, A. (2023). Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. Journal of the Taiwan Institute of Chemical Engineers, 149, 105010. https://doi.org/10.1016/j.jtice.2023.105010
- Hashem, A. H., Abdelaziz, A. M., Askar, A. A., Fouda, H. M., Khalil, A., Abd-Elsalam, K. A., & Khaleil, M. M. (2021). Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in Faba Bean Plants. Journal of Fungi (Basel, Switzerland), 7(3), 195. https://doi.org/10.3390/jof7030195
- Hassan, H. U., Raja, N. I., Abasi, F., Mehmood, A., Qureshi, R., Manzoor, Z., Shahbaz, M., & Proćków, J. (2022). Comparative study of antimicrobial and antioxidant potential of olea ferruginea fruit extract and its mediated selenium nanoparticles. Molecules (Basel, Switzerland), 27(16), 5194. https://doi.org/10.3390/molecules27165194
- Hayles, J., Johnson, L., Worthley, C., & Losic, D. (2017). Nanopesticides: A review of current research and perspectives. In Alexandru Mihai Grumezescu (Ed.), New pesticides and soil sensors (pp. 193–225). https://doi.org/10.1016/B978-0-12-804299-1.00006-0
- Helaly, M. N., El-Sheery, N. I., El-Hoseiny, H., Rastogi, A., Kalaji, H. M., & Zabochnicka-Świątek, M. (2018). Impact of treated wastewater and salicylic acid on physiological performance, malformation and yield of two mango cultivars. Scientia Horticulturae, 233, 159–177. https://doi.org/10.1016/j.scienta.2018.01.001
- Iqbal, Z., Akhtar, N., Ghazanfar, M. U., Shehzad, S. M., Ahmad, S., Asif, M., & Pervez, M. A. (2011). Management of mango malformation through physical alteration and chemical spray. African Journal of Agricultural Research, 6(7), 1897–1901.
- Islam, A. H. M. S., Schreinemachers, P., & Kumar, S. (2020). Farmers’ knowledge, perceptions and management of chili pepper anthracnose disease in Bangladesh. Crop Protection, 133, 105139. https://doi.org/10.1016/j.cropro.2020.105139
- Ismail, A. W. A., Sidkey, N. M., Arafa, R. A., Fathy, R. M., & El-Batal, A. I. (2016). Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against Alternaria solani caused early blight disease on potato. British Biotechnology Journal, 12(3), 1–11. https://doi.org/10.9734/BBJ/2016/24155
- Javaid, A., Shoaib, A., & Khan, S. N. (2012). Chapter 25. Mango cultivation in Pakistan. In S. G. Valavi, R. Mohan, J. N. Govil, K. V., & Peter, G. Thottappilly (Eds.), Mango cultivation in different countries (Vol. 2, pp. 385–394). Studium Press LLC.
- Jesline, A., John, N. P., Narayanan, P. M., Vani, C., & Murugan, S. (2015). Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Applied Nanoscience, 5(2), 157–162. https://doi.org/10.1007/s13204-014-0301-x
- Jo, Y. K., Kim, B. H., & Jung, G. (2009). Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Disease, 93(10), 1037–1043. https://doi.org/10.1094/PDIS-93-10-1037
- Jokerst, J. V., Lobovkina, T., Zare, R. N., & Gambhir, S. S. (2017). Nanoparticle PEGylation for imaging and therapy. Nanomedicine (London, England), 6(4), 715–728. https://doi.org/10.2217/nnm.11.19
- Joshi, S. M., De Britto, S., & Jogaiah, S. (2021). Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. Journal of Biotechnology, 325, 196–206. https://doi.org/10.1016/j.jbiotec.2020.10.023
- Kabeer, S., Govindarajan, N., Radhakrishnan, P., Alharbi, H. F., Essa, M. M., & Qoronfleh, M. W. (2023). Formulation of fortified instant weaning food from Musa paradisiaca (banana) and Eleusine coracana. Frontiers in Nutrition, 10, 1203955. https://doi.org/10.3389/fnut.2023.1203955
- Kah, M., Kookana, R. S., Gogos, A., & Bucheli, T. D. (2019). A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature Nanotechnology, 13(8), 677–684. https://doi.org/10.1038/s41565-018-0131-1
- Kamble, C., & Mane, R. S. (2023). Introduction to wet chemical methods and metal oxide nanostructures. In Rajaram Mane, Vijaykumar Jadhav and Abdullah Al-Enizi (Eds.), Solution methods for metal oxide nanostructures (pp. 3–16). Elsevier. https://doi.org/10.1016/B978-0-12-824353-4.00002-6
- Khan, A., & Korban, S. S. (2022). Breeding and genetics of disease resistance in temperate fruit trees: Challenges and new opportunities. Theoretical and Applied Genetics, 135(11), 3961-3985. https://doi.org/10.1007/s00122-022-04093-0.
- Katoch, P., Katoch, A., & Dangi, B. (2019). An overview on mango malformation and the potential approaches to their management. Journal of Pharmacognosy and Phytochemistry, 8(4), 621–626.
- Kaur, A., & Kaur, N. (2018). Mango malformation: A fungal disease, physiological disorder or malady of stress. Journal of Applied and Natural Science, 10(1), 403–409. https://doi.org/10.31018/jans.v10i1.1638
- Kaur, P., Thakur, R., Duhan, J. S., & Chaudhury, A. (2018). Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer arietinum). Journal of Chemical Technology & Biotechnology, 93(11), 3233–3243. https://doi.org/10.1002/jctb.5680
- Khan, H. A., Mukhtar, M., & Bhatti, M. F. (2023). Mycovirus-induced hypovirulence in notorious fungi Sclerotinia: A comprehensive review. Brazilian Journal of Microbiology: [Publication of the Brazilian Society for Microbiology], 54(3), 1459–1478. https://doi.org/10.1007/s42770-023-01073-4
- Khodakovskaya, M. V., de Silva, K., Biris, A. S., Dervishi, E., & Villagarcia, H. (2018). Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano, 6(3), 2128–2135. https://doi.org/10.1021/nn204643g
- Krishnaraj, C., Ramachandran, R., Mohan, K., & Kalaichelvan, P. T. (2012). Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 93, 95–99. https://doi.org/10.1016/j.saa.2012.03.002
- Koffi, D., Bonouman, I. V., Toure, A. O., Kouadjo, F., N’gou, M. R. E., Sylla, K., & Denning, D. W. (2021). Estimates of serious fungal infection burden in Côte d’Ivoire and country health profile. Journal of Medical Mycology, 31(1), 101086.
- Kumar, A., Bhuj, B. D., Ram, S., & Singh, C. P. (2021). Mango malformation: Etiology and preventive measures. Annals of the Romanian Society for Cell Biology, 25(6), 8025–8058.
- Kumar, J., Singh, U. S., & Beniwal, S. P. S. (1993). Mango malformation: One hundred years of research. Annual Review of Phytopathology, 31(1), 217–232. https://doi.org/10.1146/annurev.py.31.090193.001245
- Kumar, N., Krishnani, K. K., & Singh, N. P. (2018). Comparative study of selenium and selenium nanoparticles concerning acute toxicity, biochemical attributes, and histopathological response in fish. Environmental Science and Pollution Research International, 25(9), 8914–8927. https://doi.org/10.1007/s11356-017-1165-x
- Kumari, R., Sharma, N., Samurailatpam, S., Rai, A. K., & Singh, S. P. (2022). Advancements in molecular techniques for the detection of foodborne pathogens. In Pranjal Chandra and Parmjit S. Panesar (Eds.), Nanosensing and bioanalytical technologies in food quality control (pp. 195–224). Springer Singapore. https://doi.org/10.1007/978-981-16-7029-9_9
- Kumari, S., Singh, A. K., Kumar, A., Singh, K. P., & Bains, G. (2021). Evaluating the efficacy of chitosan and salicylic acid on photosynthetic pigments and antioxidant enzymes towards resistance of mango malformation. Scientia Horticulturae, 285, 110160. https://doi.org/10.1016/j.scienta.2021.110160
- Lashin, I., Hasanin, M., Hassan, S. A., & Hashem, A. H. (2023). Green biosynthesis of zinc and selenium oxide nanoparticles using callus extract of Ziziphus spina-christi: Characterization, antimicrobial, and antioxidant activity. Biomass Conversion and Biorefinery, 13(11), 10133–10146. https://doi.org/10.1007/s13399-021-01873-4
- Leakey, R., Tientcheu Avana, M.-L., Awazi, N., Assogbadjo, A., Mabhaudhi, T., Hendre, P., Degrande, A., Hlahla, S., & Manda, L. (2022). The future of food: Domestication and commercialization of indigenous food crops in Africa over the third decade (2012–2021). Sustainability, 14(4), 2355. https://doi.org/10.3390/su14042355
- Li, D., Song, Z., Quan, C., Xu, X., & Liu, C. (2021). Recent advances in image fusion technology in agriculture. Computers and Electronics in Agriculture, 191, 106491. https://doi.org/10.1016/j.compag.2021.106491
- Malik, A. Q., Mir, T. U. G., Kumar, D., Mir, I. A., Rashid, A., Ayoub, M., & Shukla, S. (2023). A review on the green synthesis of nanoparticles, their biological applications, and photocatalytic efficiency against environmental toxins. Environmental Science and Pollution Research International, 30(27), 69796–69823. https://doi.org/10.1007/s11356-023-27437-9
- Malik, M. T., Naqvi, S. A., Bakhsh, M. A., & Tariq, T. (2018). Field case investigations of mango malformation disease in five districts of southern Punjab and its biological mediated management under controlled conditions. Pakistan Journal of Phytopathology, 30(2), 191–197. https://doi.org/10.33866/phytopathol.030.02.0462
- Malik, M. T., Sahu, Z., Tariq, T., Khan, A. H., Ullah, H., Zainab, A., & Ammar, M. (2018). Impact of environmental variables on spore dispersal trend of Fusarium mangiferae causing mango malformation disease in Pakistan. Pakistan Journal of Phytopathology, 30(1), 83–90. https://doi.org/10.33866/phytopathol.030.01.0448
- Manna, A. C. (2012). Synthesis, characterization, and antimicrobial activity of zinc oxide nanoparticles. In Adhar C. Manna (Ed.), Nano-antimicrobials (pp. 151–180). Springer. https://doi.org/10.1007/978-3-642-24428-5_5
- Mehak, A., Ajmal, M., Shahbaz, M., Nayyar, B. G., Seerat, W., Fatima, N., & Mukhtar, T. (2023). Antifungal and antibacterial activity of oilseed cakes against soil-borne phytopathogens. Pure and Applied Biology, 12(2), 1084–1092. https://doi.org/10.19045/bspab.2023.120111
- Menon, S., Agarwal, H., Rajeshkumar, S., Jacquline Rosy, P., & Shanmugam, V. K. (2020). Investigating the antimicrobial activities of the biosynthesized selenium nanoparticles and its statistical analysis. BioNanoScience, 10(1), 122–135. https://doi.org/10.1007/s12668-019-00710-3
- Miglani, S., & Tani-Ishii, N. (2021). Biosynthesized selenium nanoparticles: Characterization, antimicrobial, and antibiofilm activity against Enterococcus faecalis. PeerJ. 9, e11653. https://doi.org/10.7717/peerj.11653
- Mirghasempour, S. A., Michailides, T., Chen, W., & Mao, B. (2022). Fusarium Spp. associated with Dendrobium officinale dieback disease in China. Journal of Fungi (Basel, Switzerland), 8(9), 919. https://doi.org/10.3390/jof8090919
- Molina-Cárdenas, L., López-Urquídez, G. A., Amarillas-Bueno, L. A., Vega-Gutierrez, T. A., Tirado-Ramírez, M. A., Velázquez-Alcaraz, T. D J., Velarde-Félix, S., & López-Orona, C. A. (2021). Mango malformation disease caused by Fusarium neocosmosporiellum in Mexico. Canadian Journal of Plant Pathology, 43(5), 714–721. https://doi.org/10.1080/07060661.2021.1880483
- Mukhametzyanov, R. R., Ostapchuk, T. V., Dzhancharov, T. M., Ivantsova, N. N., & Vasileva, E. N. (2023). Changes in global production and trade of major tropical fruits. In Elena (Ed.), Digital agriculture for food security and sustainable development of the agro-industrial complex (pp. 147–153). Springer International Publishing.
- Muthamil Selvan, S., Vijai Anand, K., Govindaraju, K., Tamilselvan, S., Kumar, V. G., Subramanian, K. S., Kannan, M., & Raja, K. (2018). Green synthesis of copper oxide nanoparticles and mosquito larvicidal activity against dengue, zika and chikungunya causing vector Aedes aegypti. IET Nanobiotechnology, 12(8), 1042–1046. https://doi.org/10.1049/iet-nbt.2018.5083
- Nadeem, M., Tungmunnithum, D., Hano, C., Abbasi, B. H., Hashmi, S. S., Ahmad, W., & Zahir, A. (2018). The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chemistry Letters and Reviews, 11(4), 492–502. https://doi.org/10.1080/17518253.2018.1538430
- Naliyadhara, N., Kumar, A., Kumar Gangwar, S., Nair Devanarayanan, T., Hegde, M., Alqahtani, M. S., Abbas, M., Sethi, G., & Kunnumakkara, A. (2023). Interplay of dietary antioxidants and gut microbiome in human health: What has been learnt thus far? Journal of Functional Foods, 100, 105365. https://doi.org/10.1016/j.jff.2022.105365
- Nasrollahzadeh, M., Sajadi, S. M., Sajjadi, M., & Issaabadi, Z. (2019). An introduction to nanotechnology. In Mahmoud Nasrollahzadeh (Ed.), Interface science and technology (Vol. 28, pp. 1–27). Elsevier. https://doi.org/10.1016/B978-0-12-813586-0.00001-8
- Nazarov, P. A., Baleev, D. N., Ivanova, M. I., Sokolova, L. M., & Karakozova, M. V. (2020). Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Naturae, 12(3), 46–59. https://doi.org/10.32607/actanaturae.11026
- Noriega-Cantú, D. H., Téliz, D., Mora-Aguilera, G., Rodríguez-Alcazar, J., Zavaleta-Mejía, E., Otero-Colinas, G., & Campbell, C. L. (1999). Epidemiology of mango malformation in Guerrero, Mexico, with traditional and integrated management. Plant Disease, 83(3), 223–228. https://doi.org/10.1094/PDIS.1999.83.3.223
- Otari, S. V., Patel, S. K., Kalia, V. C., Kim, I. W., & Lee, J. K. (2019). Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Indian Journal of Microbiology, 59(3), 379–382. https://doi.org/10.1007/s12088-019-00812-2
- Pasquet, J., Chevalier, Y., Pelletier, J., Couval, E., Bouvier, D., & Bolzinger, M. A. (2014). The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 457, 263–274. https://doi.org/10.1016/j.colsurfa.2014.05.057
- Peng, F., Wang, X., Zhang, W., Shi, X., Cheng, C., Hou, W., Lin, X., Xiao, X., & Li, J. (2022). Nanopesticide formulation from pyraclostrobin and graphene oxide as a nanocarrier and application in controlling plant fungal pathogens. Nanomaterials (Basel, Switzerland), 12(7), 1112. https://doi.org/10.3390/nano12071112
- Pierson, S. K., Lim, M. S., Srkalovic, G., Brandstadter, J. D., Sarmiento Bustamante, M., Shyamsundar, S., Mango, N., Lavery, C., Austin, B., Alapat, D., Lechowicz, M. J., Bagg, A., Li, H., Casper, C., van Rhee, F., & Fajgenbaum, D. C. (2023). Treatment consistent with idiopathic multicentric castleman disease guidelines is associated with improved outcomes. Blood Advances, 7(21), 6652–6664. https://doi.org/10.1182/bloodadvances.2023010745
- Ploetz, R. C. (2007). Diseases of tropical perennial crops: challenging problems in diverse environments. Plant Disease, 91(6), 644–663.
- Ploetz, R., Zheng, Q. I., Vázquez, Á., & Sattar, M. A. A. (2002). Status and impact of mango malformation in Egypt. International Journal of Pest Management, 48(4), 279–285. https://doi.org/10.1080/09670870210149817
- Prakash, S., Singh, M. K., Kumar, A., & Kumar, M. (2016). Response of mango malformation to stage and severity of pruning of shoot-bearing malformed panicles. Journal of Hill Agriculture, 7(1), 156–158. https://doi.org/10.5958/2230-7338.2016.00028.8
- Priyan, S. R., Kumar, G. S., Surendhiran, S., & Shkir, M. (2023). Size‐controlled synthesis of mesoporous silica nanoparticles using rice husk by microwave‐assisted sol–gel method. International Journal of Applied Ceramic Technology, 20(5), 2807–2816. https://doi.org/10.1111/ijac.14444
- Quiterio-Gutiérrez, T., Ortega-Ortiz, H., Cadenas-Pliego, G., Hernández-Fuentes, A. D., Sandoval-Rangel, A., Benavides-Mendoza, A., Cabrera-de la Fuente, M., & Juárez-Maldonado, A. (2019). The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. International Journal of Molecular Sciences, 20(8), 1950. https://doi.org/10.3390/ijms20081950
- Rai, M., Yadav, A., & Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27(1), 76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
- Raj, R. S., Thakur, S. V., Vyas, Y. S., Patel, K. M., Patel, P. V., Joshi, M. N., Tyagi, S. N., & Bagatharia, S. B. (2017). Diversity analysis, pathogen load and gene expression studies of mango cultivars infected by mango malformation disease. Plant Growth Regulation, 81(1), 117–130. https://doi.org/10.1007/s10725-016-0194-7
- Ramírez, F., & Kallarackal, J. (2018). Phenological growth stages of Feijoa [Acca sellowiana (O. Berg) Burret] according to the BBCH scale under tropical Andean conditions. Scientia Horticulturae, 232, 184–190. https://doi.org/10.1016/j.scienta.2017.12.059
- Rana, R., Siddiqui, M., Skalicky, M., Brestic, M., Hossain, A., Kayesh, E., Popov, M., Hejnak, V., Gupta, D., Mahmud, N., & Islam, T. (2021). Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae, 7(10), 332. https://doi.org/10.3390/horticulturae7100332
- Rani, N., Singh, P., Kumar, S., Kumar, P., Bhankar, V., & Kumar, K. (2023). Plant-mediated synthesis of nanoparticles and their applications: A review. Materials Research Bulletin, 163, 112233. https://doi.org/10.1016/j.materresbull.2023.112233
- Raza, S. T., Khan, A. H., Hameed, A., Muhammad, N., Grewal, A. G., Malik, M. T., Imran, M., Mustafa, G., & Iqbal, A. (2023). A review on white mango scale biology, ecology, distribution and management. Agriculture, 13(9), 1770. https://doi.org/10.3390/agriculture13091770
- Riseh, R. S., Hassanisaadi, M., Vatankhah, M., Soroush, F., & Varma, R. S. (2022). Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. International Journal of Biological Macromolecules, 222(Pt A), 1589–1604. https://doi.org/10.1016/j.ijbiomac.2022.09.278
- Roointan, A., Mir, T. A., Wani, S. I., Hussain, K. K., Ahmed, B., Abrahim, S., Savardashtaki, A., Gandomani, G., Gandomani, M., Chinnappan, R., & Akhtar, M. H. (2019). Early detection of lung cancer biomarkers through biosensor technology: A review.Journal of Pharmaceutical and Biomedical Analysis, 164, 93–103. https://doi.org/10.1016/j.jpba.2018.10.017
- Roy, A., Gauri, S. S., Bhattacharya, M., & Bhattacharya, J. (2013). Antimicrobial activity of CaO nanoparticles. Journal of Biomedical Nanotechnology, 9(9), 1570–1578. https://doi.org/10.1166/jbn.2013.1681
- Ruparelia, J. P., Chatterjee, A. K., Duttagupta, S. P., & Mukherji, S. (2008). Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomaterialia, 4(3), 707–716. https://doi.org/10.1016/j.actbio.2007.11.006
- Rymbai, H., & Rajesh, A. M. (2011). 5. Mango malformation a review by H. Rymbai and Rajesham AM. Life Sciences Leaflets, 22, 1079.
- Rai, N., & Chauhan, I. (2023). Multifunctional Aerogels: A comprehensive review on types, synthesis and applications of aerogels. Journal of Sol-Gel Science and Technology, 105(2), 324–336. https://doi.org/10.1007/s10971-022-06026-1
- Sahoo, A., Dwivedi, A., Madheshiya, P., Kumar, U., Sharma, R. K., & Tiwari, S. (2023). Insights into the management of food waste in developing countries: With special reference to India. Environmental Science and Pollution Research International, 30(2), 1–27. https://doi.org/10.1007/s11356-023-27901-6
- Salem, M. F., Abd-Elraoof, W. A., Tayel, A. A., Alzuaibr, F. M., & Abonama, O. M. (2022). Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. Journal of Nanobiotechnology, 20(1), 182. https://doi.org/10.1186/s12951-022-01393.-x
- Sankaran, M., Dinesh, M. R., & Ravishankar, K. V. (2021). Classical genetics and breeding. In Chittaranjan (Ed.), The mango genome (pp. 111–130). Springer Link. https://doi.org/10.1007/978-3-030-47829-2_7
- Sarkar, M., Chakraborty, B., & Srivastava, J. N. (2022). Key diseases of cucurbits and their management. In Srivastava and Singh (Ed.), Diseases of horticultural crops: Diagnosis and management: Volume 1: Fruit crops (p. 153). CRC Press.
- Sarojini, K., Arivarasu, L., Rajeshkumar, S., & Lakshmi, T. (2020). Green synthesis of Solanum trilobatum mediated selenium nanoparticles and its anti-inflammatory and anti-microbial activity. Plant Cell Biotechnology aAnd Molecular Biology, 33(2), 75–81.
- Sathiyabama, M., & Charles, R. E. (2015). Fungal cell wall polymer based nanoparticles in protection of tomato plants from wilt disease caused by Fusarium oxysporum f. sp. lycopersici. Carbohydrate Polymers, 133, 400–407. https://doi.org/10.1016/j.carbpol.2015.07.066
- Schoeman, M. H., Zulu, N. B., Botha, F. A., & Calitz, F. J. (2019). Integrated control of mango blossom malformation in South Africa. South African Journal of Plant and Soil, 36(1), 51–56. https://doi.org/10.1080/02571862.2018.1484190
- Servin, A. D., Morales, M. I., Castillo-Michel, H., Hernandez-Viezcas, J. A., Munoz, B., Zhao, L., Nunez, J. E., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2012). Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environmental Science & Technology, 47(20), 11592–11598. https://doi.org/10.1021/es403368j
- Setlem, S. K., & Ramlal, S. (2022). Isolation, extraction and identification of aflatoxin producing Aspergillus fungi by HPLC analysis and ITS sequencing. Clinical Case Reports International, 6, 1393.
- Shahbaz, M., Akram, A., Raja, N. I., Mukhtar, T., Mehak, A., Fatima, N., & Abasi, F. (2023). Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS One, 18(2), e0274679. https://doi.org/10.1371/journal.pone.027467.
- Shahbaz, M., Fatima, N., Mashwani, Z.-U.-R., Akram, A., Haq, E. U., Mehak, A., Abasi, F., Ajmal, M., Yousaf, T., Raja, N. I., UlHassan, H., & Pérez de la Lastra, J. M. (2022a). Effect of phytosynthesized selenium and cerium oxide nanoparticles on wheat (Triticum aestivum L.) against stripe rust disease. Molecules (Basel, Switzerland), 27(23), 8149. https://doi.org/10.3390/molecules27238149
- Shahbaz, M., Akram, A., Raja, N. I., Mukhtar, T., Mashwani, Z. U. R., Mehak, A., Fatima, N., Sarwar, S., Haq, E. U., & Yousaf, T. (2022b). Green synthesis and characterization of selenium nanoparticles and its application in plant disease management: A review. Pakistan Journal of Phytopathology, 34(1), 189–102. https://doi.org/10.33866/phytopathol.034.01.0739
- Shahbaz, M., Akram, A., Raja, N. I., Mukhtar, T., Mehak, A., Fatima, N., Ajmal, M., Ali, K., Mustafa, N., & Abasi, F. (2023a). Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PloS One, 18(2), e0274679. https://doi.org/10.1371/journal.pone.0274679
- Shahbaz, M., Akram, A., Mehak, A., Haq, E. U., Fatima, N., Wareen, G., Fitriatin, B. N., Sayyed, R. Z., Ilyas, N., & Sabullah, M. K. (2023b). Evaluation of selenium nanoparticles in inducing disease resistance against spot blotch disease and promoting growth in wheat under biotic stress. Plants, 12(4), 761. https://doi.org/10.3390/plants12040761
- Shaban, N. Z., El-Rashidy, F. H., Adam, A. H., Beltagy, D. M., Ali, A. E., Abde-Alaziz, A. A., & Talaat, I. M. (2023). Anticancer role of mango (Mangifera indica L.) peel and seed kernel extracts against 7, 12-dimethylbenz [a] anthracene-induced mammary carcinogenesis in female rats. Scientific Reports, 13(1), 7703. https://doi.org/10.1038/s41598-023-34626-6
- Shankar, S., Kumar, G., Singh, A., & Mishra, P. K. (2023). Revealed comparative advantage (RCA) and its application to evaluate India’s performance of fresh mangoes, mangosteen & guavas during the period 1991-2020: An analysis with respect to trade. Journal of Contemporary Issues in Business and Government, 29(1), 105–111.
- Sharma, A., Sharma, I. M., Sharma, M., Sharma, K., & Sharma, A. (2021). Effectiveness of fungal, bacterial and yeast antagonists for management of mango anthracnose (Colletotrichum gloeosporioides). Egyptian Journal of Biological Pest Control, 31(1), 11. https://doi.org/10.1186/s41938-021-00480-9
- Sharma, R. R., Singh, D., & Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control, 50(3), 205–221. https://doi.org/10.1016/j.biocontrol.2009.05.001
- Shivran, M., Sharma, N., Sharma, N., Muthusamy, V., Dubey, A. K., Singh, S. K., Singh, B. P., Kumar, N., Sevanthi, A. M., Singh, N., & Singh, N. K. (2023). Development of ripening gene-specific markers and their association with shelf-life in mango (Mangifera indica L.) varieties. National Academy Science Letters, 46(3), 179–184. https://doi.org/10.1007/s40009-023-01207-0
- Singh, Z., Singh, L., Arora, C. L., & Dhillon, B. S. (1994). Effect of cobalt, cadmium, and nickel as inhibitors of ethylene biosynthesis on floral malformation, yield, and fruit quality of mango. Journal of Plant Nutrition, 17(10), 1659–1670. https://doi.org/10.1080/01904169409364838
- Sinha, K., Ghosh, J., & Sil, P. C. (2017). New pesticides: A cutting-edge view of contributions from nanotechnology for the development of sustainable agricultural pest control. In Alexandru Mihai Grumezescu (Ed.), New pesticides and soil sensors (pp. 47–79). Academic Press. https://doi.org/10.1016/B978-0-12-804299-1.00003-5
- Sirohi, S. C., Prakash, S., Rana, P., & Singh, R. (2009). Response of mango malformation to the severity of malformed panicle bearing shoot pruning. Indian Journal of Horticulture, 66(3), 393–395.
- Soni, A., Bhandari, M. P., Tripathi, G. K., Bundela, P., Khiriya, P. K., Khare, P. S., Kashyap, M. K., Dey, A., Vellingiri, B., Sundaramurthy, S., Suresh, A., & Pérez de la Lastra, J. M. (2023). Nano‐biotechnology in tumour and cancerous disease: A perspective review. Journal of Cellular and Molecular Medicine, 27(6), 737–762. https://doi.org/10.1111/jcmm.17677
- Soroka, M., Wasowicz, B., & Rymaszewska, A. (2021). Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells, 10(8), 1931. https://doi.org/10.3390/cells10081931
- Soto-Plancarte, A., Santillán-Mendoza, R., Fernández-Pavía, S. P., Ploetz, R. C., Freeman, S., Ortega-Arreola, R., Osuna-Ávila, P., Velázquez-Monreal, J. J., & Rodríguez-Alvarado, G. (2015). Mango nurseries as sources of Fusarium mexicanum, cause of mango malformation disease in central western Mexico. Phytoparasitica, 43(4), 427–435. https://doi.org/10.1007/s12600-015-0471-4
- Stone, V., Führ, M., Feindt, P. H., Bouwmeester, H., Linkov, I., Sabella, S., Murphy, F., Bizer, K., Tran, L., Ågerstrand, M., Fito, C., Andersen, T., Anderson, D., Bergamaschi, E., Cherrie, J. W., Cowan, S., Dalemcourt, J.-F., Faure, M., Gabbert, S., … Poortvliet, P. M. (2018). The essential elements of a risk governance framework for current and future nanotechnologies. Risk Analysis: An Official Publication of the Society for Risk Analysis, 38(7), 1321–1331. https://doi.org/10.1111/risa.12954
- Tariq, A., Sahar, A., Usman, M., Sameen, A., Azhar, M., Tahir, R., Younas, R., & Issa Khan, M. (2023). Extraction of dietary fiber and polyphenols from mango peel and its therapeutic potential to improve gut health. Food Bioscience, 53, 102669. https://doi.org/10.1016/j.fbio.2023.102669
- Thao, B. T. T., Vo, T. T. K., Tran, T. Y. N., Le, D. T., Tran, T. T., Bach, L. G., & Dao, T. P. (2023). Application of mathematical techniques to study the moisture loss kinetics and polyphenol degradation kinetics of mango (Mangifera indica L.) slices during heat pump drying by pilot equipment. LWT, 176, 114454. https://doi.org/10.1016/j.lwt.2023.114454
- Tulp, O. L., Rizvi, S. A., & Sciranka, A. A. (2023). The use of natural products as an adjunct in the treatment of hypertension: A case study with mango-ginger tea. International Journal of Complementary and Internal Medicine, 3(1), 85–91.
- Uemura, E. V. G., Barbosa, M. D. S., Simionatto, S., Al-Harrasi, A., Al-Hatmi, A. M., & Rossato, L. (2022). Onychomycosis caused by Fusarium species. Journal of Fungi (Basel, Switzerland), 8(4), 360. https://doi.org/10.3390/jof8040360
- Umair Raza, M., Abasi, F., Shahbaz, M., Ehsan, M., Seerat, W., Akram, A., Raja, N. I., Mashwani, Z. U.-R., Hassan, H. U., & Proćków, J. (2023). Phytomediated silver nanoparticles (AgNPs) embellish antioxidant defense system, ameliorating HLB-diseased ‘Kinnow’Mandarin plants. Molecules (Basel, Switzerland), 28(5), 2044. https://doi.org/10.3390/molecules28052044
- Umar, U. D., Ahmed, N., Zafar, M. Z., Rehman, A., Naqvi, S. A. H., Zulfiqar, M. A., Malik, M. T., Ali, B., Saleem, M. H., & Marc, R. A. (2022). Micronutrients foliar and drench application mitigate mango sudden decline disorder and impact fruit yield. Agronomy, 12(10), 2449. https://doi.org/10.3390/agronomy12102449
- Usha, K., Nagaraja, A., Sahoo, R. N., Singh, B., & Kumar, N. (2022). Predicting the probability of occurrence of floral malformation in mango (Mangifera indica L) under climate change scenario. International Journal of Phytology Research, 2(2), 06–13.
- Vanti, G. L., Masaphy, S., Kurjogi, M., Chakrasali, S., & Nargund, V. B. (2020). Synthesis and application of chitosan-copper nanoparticles on damping off causing plant pathogenic fungi. International Journal of Biological Macromolecules, 156, 1387–1395. https://doi.org/10.1016/j.ijbiomac.2019.11.179
- Veldman, W. M., Regnier, T., & Augustyn, W. A. (2018). Biocontrol of Fusarium mangiferae responsible for mango malformation using bacterial isolates. Scientia Horticulturae, 230, 186–195. https://doi.org/10.1016/j.scienta.2017.10.039
- Venkataraju, J. L., Sharath, R., Chandraprabha, M. N., Neelufar, E., Hazra, A., & Patra, M. (2014). Synthesis, characterization and evaluation of antimicrobial activity of zinc oxide nanoparticles. Journal of Biochemical Technology, 3(5), 151–154.
- Venkataravanappa, V., & Sonavane, P. S. (2022). Major diseases of coorg mandarin (Citrus Reticulata) and their Management. In Srivastava, A. K. Singh (Ed.), Diseases of horticultural crops: Diagnosis and management: Volume 1: Fruit crops (p. 299). CRC Press.
- Vignassa, M., Meile, J. C., Chiroleu, F., Soria, C., Leneveu-Jenvrin, C., Schorr-Galindo, S., & Chillet, M. (2021). Pineapple mycobiome related to fruitlet core rot occurrence and the influence of fungal species dispersion patterns. Journal of Fungi (Basel, Switzerland), 7(3), 175. https://doi.org/10.3390/jof7030175
- Vijayakumar, S., Krishnakumar, C., Arulmozhi, P., Mahadevan, S., & Parameswari, N. (2018). Biosynthesis, characterization, and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmi spentaphylla (Retz.) DC. Microbial Pathogenesis, 116, 44–48. https://doi.org/10.1016/j.micpath.2018.01.003
- Wadhwani, S. A., Shedbalkar, U. U., Singh, R., & Chopade, B. A. (2016). Biogenic selenium nanoparticles: Current status and future prospects. Applied Microbiology and Biotechnology, 100(6), 2555–2566. https://doi.org/10.1007/s00253-016-7300-7
- Waghule, T., Sankar, S., Rapalli, V. K., Gorantla, S., Dubey, S. K., Chellappan, D. K., Dua, K., & Singhvi, G. (2020). Emerging role of nanocarriers based topical delivery of anti‐fungal agents in combating growing fungal infections. Dermatologic Therapy, 33(6), e13905. https://doi.org/10.1111/dth.13905
- Wang, H., Ramnani, P., Pham, T., Villarreal, C. C., Yu, X., Liu, G., & Mulchandani, A. (2019). Gas biosensor arrays based on single-stranded DNA-functionalized single-walled carbon nanotubes for the detection of volatile organic compound biomarkers released by huanglongbing disease-infected citrus trees. Sensors (Basel, Switzerland), 19(21), 4795. https://doi.org/10.3390/s19214795
- Worrall, E. A., Hamid, A., Mody, K. T., Mitter, N., & Pappu, H. R. (2018). Nanotechnology for plant disease management. Agronomy, 8(12), 285. https://doi.org/10.3390/agronomy8120285
- Yadav, A., Kumar, H., Sharma, R., & Kumari, R. (2023). Synthesis, processing, and applications of 2D (nano) materials: A sustainable approach. Surfaces and Interfaces, 39, 102925. https://doi.org/10.1016/j.surfin.2023.102925
- Yadav, S., Yadav, S. P. S., Adhikari, N., Sah, R. K., & Gupta, S. (2022). Effects of Gibberellic acid (GA3) on shelf life and physiochemical properties of mango (Mangifera indica L. var Bombay green). Archives of Agriculture and Environmental Science, 7(4), 541–548. https://doi.org/10.26832/24566632.2022.0704010
- Yahia, E. M., de Jesús Ornelas-Paz, J., Brecht, J. K., García-Solís, P., & Celis, M. E. M. (2023). The contribution of mango fruit (Mangifera indica L.) to human nutrition and health. Arabian Journal of Chemistry, 16(7), 104860. https://doi.org/10.1016/j.arabjc.2023.104860
- Yang, J., Li, J. X., Zhang, F., & Zhao, X. Q. (2023). Global regulation of fungal secondary metabolism in Trichoderma reesei by the transcription factor Ypr1, as revealed by transcriptome analysis. Engineering Microbiology, 3(2), 100065. https://doi.org/10.1016/j.engmic.2022.100065
- Zafar, T. A., & Sidhu, J. S. (2017). Composition and nutritional properties of mangoes. In Muhammad Siddiq (Ed.), Handbook of mango fruit: Production, postharvest science, processing technology and nutrition (pp. 217–236). Wiley. https://doi.org/10.1002/9781119014362.ch11
- Zahid, M., Sharif, M. S., Ahmed, R., & Abbas, T. (2023). “Direction and destination pattern of mango export from Pakistan” A Markov Chain approach. Review of Education, Administration & Law, 6(1), 75–82. https://doi.org/10.47067/real.v6i1.301
- Zakaria, L. (2023). Fusarium species associated with diseases of major tropical fruit crops. Horticulturae, 9(3), 322. https://doi.org/10.3390/horticulturae9030322
- Zhan, F., Wang, T., Iradukunda, L., & Zhan, J. (2018). A gold nanoparticle-based lateral flow biosensor for sensitive visual detection of the potato late blight pathogen, Phytophthora infestans. Analytica Chimica Acta, 1036, 153–161. https://doi.org/10.1016/j.aca.2018.06.083
- Zhang, C., Yi, H., Gao, X., Bai, T., Ni, Z., Chen, Y., Wang, M., Zhang, Y., Pan, J., Yu, W., & Xie, D. (2022). Effect of different altitudes on morpho-physiological attributes associated with mango quality. Diversity, 14(10), 876. https://doi.org/10.3390/d14100876
- Zhang, W., Hu, E., Wang, Y., Miao, S., Liu, Y., Hu, Y., Liu, J., Xu, B., Chen, D., & Shen, Y. (2021). Emerging antibacterial strategies with application of targeting drug delivery system and combined treatment. International Journal of Nanomedicine, 16, 6141–6156. https://doi.org/10.2147/IJN.S311248