Abstract
Medical technologies are pervasive across women’s health, spanning across obstetric and gynecological care. FemTech, the sector responsible for developing these technologies, is growing at 15.6% per annum. However, there are concerns of disconnects between new product development (NPD) and the care afforded to women in consequence of implementing these innovations. The most crucial stage of NPD involves understanding the clinical need. Without a clear need and clinical use case, innovators risk developing solutions which do not address the issues women and caregivers experience. Thus, the product will miss the market and experience limited uptake. Tools for performing clinical needs assessments and defining the use case are being developed. This review provides an analysis of their strengths and weaknesses to inform FemTech innovators of the available resources. We further discuss concepts for creating a unified approach to assessing unmet needs such that technologies have a higher chance of improving women’s healthcare.
Women constitute half of the world’s population. Yet, matters related to the health of women are considered to be of concern to a meager and often neglected portion of the broader healthcare ecosystem. Even more concerningly, despite evidence that innovation leads to improved outcomes for patients and the healthcare system, women’s health is an area that has experienced few advancements as it is viewed as a niche market by the medical technology industry.
In recent years however, attitudes in healthcare and medical technology are shifting with governments, healthcare providers, and industry seeking to empower women through policy, improved access to quality care, and technology (Barclay & Caulfield, Citation2022). In particular, technology development for women’s health, also referred to as FemTech, is on the rise with breakthrough innovations that improve care delivery, diagnostics and health status monitoring, or addressing conditions affected by taboos and cultural sensitivities already being implemented (Kemble et al., Citation2022). However, as with most areas of medical technology, there are concerns that disconnects between the FemTech industry and the needs of the various stakeholders in the women’s healthcare ecosystem exist.
To reduce the extent of this disconnect, we undertook a narrative literature review of existing frameworks with the aim of understanding how to assess clinical needs and develop the clinical use case for medical device innovation. We provide an analysis to detail the strengths and weaknesses of each model and informs an interdisciplinary framework for guiding the development of medical devices, with special regard to the context of women’s healthcare. This interdisciplinary framework provides a theoretical contribution to the literature and may be applied to real-world FemTech innovations when determining its validity and utility. Our proposed framework will also provide both, innovators and adopters of future FemTech medical devices, with considerations and insights that will help address inequities in women’s healthcare and increase the likelihood of achieving a technological innovation that is acceptable to the market.
Background
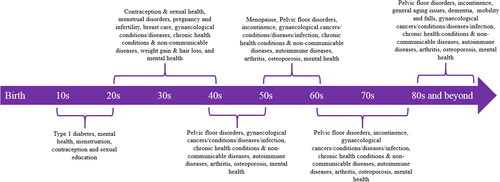
Despite women comprising approximately half of the global population, women’s healthcare has faced a number of systemic inequities which are caused by limited accessibility, lack of funding mechanisms, poor advocacy and lack of policy devoted toward specifically addressing these factors (Amin et al., Citation2021; Australian Institute of Health & Welfare, Citation2019; Kemble et al., Citation2022). This has larger implications, as there is a relationship between slow advances in health and wellbeing, and an individual’s ability to play an active role in society (Marquez, Citation2017). In line with this, one of the United Nation’s Sustainable Development Goals (SGDs) highlighted the value of gender equality and empowerment of all women and described the role that existing healthcare limitations have played against the movement for equality (UN Department of Economic & Social Affairs, Citation2015). Medical technology, and its innovation, stands as a key agent which can be harnessed to contribute to achieving this SGD through improved health outcomes and access to care. The mechanisms to do this are well captured in the quadruple aims of healthcare: (a) improved patient outcomes; (b) improved patient experience; (c) improved provider experience; and (d) lower cost of care. There are numerous areas across the life journey of a woman, that these initiatives can deliver clinical improvements and wider societal impact. These include pregnancy and birth, gynecological conditions, non-communicable diseases including cancer, access to mental health services, sexual and reproduction health, as well as health education (NIH Office of Communications, Citation2016; World Health Organization, Citationn.d.). presents the areas and conditions that require clinical attention along the lifespan of women.
FemTech is the subset of the medical device (MD) industry that is responsible for the development and translation of MDs, software, and services into obstetrics and gynecology. FemTech has potential to not only improve the lives of women, but also inspire positive social changes beyond the healthcare system. With a compounded annual growth rate (CAGR) of 15.6% and a forecasted revenue of 60.01 billion USD by 2027, it is anticipated that FemTech will soon be responsible for a sizeable portion of the MD industry (Emergen Research, Citation2021). However, as the MD industry and FemTech develop, there are concerns about the apparent disconnect between new product development (NPD) and the implementation of innovations into clinical practice (Petkova et al., Citation2010). Understandably, this disconnect affects the sustainability of FemTech ventures since there is a complex, invisible, but yet palpable process which they must navigate to achieve clinical impact.
To address this disparity, techniques and tools which aim to mitigate risk and uncertainty in the early innovation process remain invaluable. The conception and application of these innovation instruments, particularly in the early stages of the medical device innovation (MDI) process, has resulted in the rapid curation of a relatively new field of research, known as early health technology assessment or EHTA. EHTA may be defined as ‘all methods used to inform industry and other stakeholders about the potential value of new medical products in development, including methods to quantify and manage uncertainty’ (M. Ijzerman et al., Citation2017).
When commencing a new MD development project, one of the most crucial steps in the MDI process involves understanding the clinical need for the candidate solution (Yock et al., Citation2015). The term ‘clinical need’ can be defined as ‘a condition for which there exists no satisfactory method of diagnosis, prevention or treatment … or, even if such a method exists, in relation to which the medicinal product concerned will be of major therapeutic advantage to those affected’ (“Commission Regulation (EC) No 507/2006,” Citation2006). Determining the clinical need can be considered an integral part of the EHTA process which involves gathering information that will help the innovator identify gaps in current healthcare practices (Weigl et al., Citation2012). In doing so, innovators can ascertain if there is a true clinical problem from the perspective of multiple stakeholders and if further research and development (R&D) will provide a fitting solution. Additionally, innovators can adjudge whether the R&D investment is worth it, if a better alternative exists, and begin defining the specifications for an innovation so that the technological solution sufficiently fills the gap (M. J. Ijzerman & Steuten, Citation2011; Weigl et al., Citation2012; Yock et al., Citation2015).
In product management, there is a fascinating dynamic between what is known as ‘technology push’ and ‘market pull’ that is now being observed in the discipline of translational medicine (Dixon, Citation2001; Saitman, Citation2019). Both approaches have historically been applied in healthcare, but their differences play a significant role on the innovation pathway (Dixon, Citation2001; Saitman, Citation2019; Steinberger et al., Citation2017). In technology push, the innovator sees a solution for a clinical need the target user (e.g. clinician) faces but cannot see. Thus, the innovator must promote the advantages of their solution and create demand for their product. In the case of market pull, the end user identifies the problem and the innovator responds with a solution that is almost guaranteed to fulfill a market niche based on the end user’s needs and input. When analyzing their impact on innovation processes, the latter approach is less risky, requires less effort and investment, and has a safer pathway to market and return on investment (Dixon, Citation2001). However, a pervasive problem-solution dichotomy in healthcare is that the solution is developed to address a problem, but the clinicians themselves do not believe the problem exists. Conversely, when the problem exists beyond doubt, the solution does not sufficiently fulfill the needs of the clinicians and patients who would use the technology. Hypothetically speaking, validating the clinical need enables the innovator to achieve the benefits of a market pull strategy and hopefully address this dichotomy as they engage with the market to determine if there is a problem and identify requirements for a fitting solution. Particularly if they consider market scalability, cost-tolerance in reimbursement structures, and the wants of stakeholders. These stakeholders usually include clinicians (e.g. obstetricians-gynecologists [ob/gyn], midwives/nurses, etc.), administrators, healthcare organization/providers (HCOs), and patients who are the end-users. Often, these stakeholders influence the adoption process or can provide valuable insights to the venture’s direction (Gold et al., Citation2014; Oliveria et al., Citation2003; Smith et al., Citation2019; Tanenbaum et al., Citation2018; Warty et al., Citation2021).
The ability to determine the clinical need of a MD, and thus define the clinical use case, can be complex. Inadequate definition of the clinical use case often results in NPD failure for an otherwise promising innovation, as the technology has not been developed to address the needs of the end user or the market (Yock et al., Citation2015). This directly affects and informs the rest of the innovation process as well, including resource management, commercialization, and marketing strategies. This can be read in more detail in our previous work (Warty et al., Citation2021). Thus, by defining the clinical need for a technology, innovators can improve the likelihood of successfully implementing their solutions and making better R&D decisions that lead to enhanced patient outcomes (Saitman, Citation2019).
Despite the critical nature of clinical needs assessments (CNA) in the MDI process, there is little discussion by researchers or industry into the development and use of frameworks for evaluating the clinical need of a MD. Often, identifying a clinical gap is achieved using in-depth literature reviews, direct observations, or interviews with key opinion leaders (KOLs) (Smith et al., Citation2019). Whilst these resources are useful in identifying a potential clinical need, more formal methods of evaluation could further reduce the risks associated with the innovation process. Such evaluation may provide consideration to additional variables and ensure a more comprehensive analysis of the clinical opportunity.
Presently, to our knowledge, there is no widely applicable theoretical CNA method that is easy to use for technology innovators, nor has there been any real-world validation of these innovation management processes. Of particular concern is the discernible practice-knowledge gap. There has been little integration of formal CNAs into routine practice, which impedes clinical innovation efforts. In line with this, the aim of this review is to identify and critically evaluate existing CNA frameworks to provide an overview of the MDI landscape. We hope that this review will promote greater awareness of the existing frameworks and encourage innovators to employ those that fit with their practice. Importantly, we attempt to shed light on the multitude of factors that could guide a unified (clinical, technical, and commercial) approach to performing CNAs; such that novel technologies have a higher chance of achieving clinical implementation and improving patient outcomes, particularly in the context of women’s healthcare.
Method
Articles for this traditional review were searched on PubMed, EMBASE, and IEEE Xplore using terms related to ‘clinical needs’ and ‘medical device’ with the AND Boolean operator without further restriction for studies related to performing CNAs during early MDI. Due to limited relevant results from the databases, additional independent searches for grey literature and articles of relevance was performed as well, and a traditional, nonsystematic review methodology was selected. The reference lists of relevant articles were further exploited to identify additional CNA frameworks.
Results: Identified models and frameworks
A summary of the discussed models may be found in .
Table 1. Summary of the existing CNA models.
Stanford biodesign model (Yock et al., Citation2015)
The Stanford Biodesign model, developed in the early 2000s, is part of one of the most reputed and comprehensive programs for MDI in the world. Typically running for one year, this program educates innovative professionals (such as biomedical engineers, clinicians, researchers, and business executives) in MDI. The Stanford Biodesign program has resulted in the founding of over 40 companies and the technologies have been used in more than 2.7 million patients (Stanford Byers Center for Biodesign, Citation2020; Steinberger et al., Citation2017). Its successes have inspired the designs of other Biodesign programs and models (Chaturvedi et al., Citation2015; Grayden et al. Citation2022).
In the Stanford Biodesign model, the development of MDs is divided into three phases.
Identify: Identify and validate a clinical need.
Invent: Brainstorm and screen ideas that address the clinical need and develop the prototypes.
Implement: After selection of the final prototype/concept, begin developing commercialization strategies and business planning.
Understanding the unmet clinical needs form the foundation for focused ideation and development in the Stanford Biodesign model. In doing so, innovators can mitigate the risks associated with a project and maximize chances for successful development (Steinberger et al., Citation2017).
The following discussion summarizes key concepts from the in-depth analysis that is provided by the authors of Biodesign: The Process of Innovating Medical Technologies—the modality in which the Stanford Biodesign program is presented (Yock et al., Citation2015).
As per the Biodesign model, the identification phase can be classified into needs finding and needs screening. Each stage consists of a number of substages which detail how to perform a clinical needs assessment. Needs Finding involves developing a strategic focus, understanding the various elements of a clinical problem (typically through direct observation in clinical environments and challenging the status quo), and developing a needs statement around this. Mokarram et al. have developed a guide for creating such needs statements (Mokarram et al., Citation2021). Needs Screening involves undertaking disease state research, evaluating existing solutions, and analyzing the market and stakeholders, before filtering down to a specific need to pursue. This is elaborated further in the Supplementary Materials.
From this, a needs specification can be created, dividing the requirements into ‘must-have’ or ‘nice-to-have’ specifications which then influence the Invention stages. A difficulty with this occurs when the line between ‘must-have’ and ‘nice-to-have’ is blurred for particular requirements, or when the requirements are categorized insufficiently to either over-constrain or under-constrain the needs specification. As such, the expertise and experience of the multi-disciplinary team and advisors become vital as they guide the innovators through this determination process.
Identifying multiple clinical needs without introducing solution bias, and systematically filtering down to select a commercially viable need that can be technologically resolved, mimics the ‘market pull’ approach. It encourages innovators to attempt to become the market through ethnographic engagement and participant observation. This is because the multidisciplinary team often immerses themselves in the clinical environment (e.g. maternity ward, birth suite, gynecology clinics, etc.) as part of the CNA process, and in doing so, develops an intimate understanding of the market. With this understanding, the team can then follow a refined innovation pathway that is safe, de-risked, and is likely to have an assured pathway to market.
As with any method, there are disadvantages. One challenge is that the needs finding process may result in a number of unmet needs being identified but later discarded as a result of the needs screening process. Given that the entire program duration is one year, often clinical needs that are easier to resolve tend to be addressed, due to time constraints. Due to this, many unmet and potentially viable needs remain as unaddressed clinical challenges. Another challenge is that the Biodesign program typically requires a year of training to which many innovators, particularly clinician-innovators, are not able or willing to commit (Chaturvedi et al., Citation2015; Yang et al., Citation2016). Innovators who employ this model without having undergone the program may face challenges using the resources or following specific processes which are specially developed. Another possible risk is that clinical teams who are being observed will subconsciously change their behavior simply because they are being observed (Hawthorne effect) (McCambridge et al., Citation2014). Lastly, in practicing this model, the multidisciplinary team may perform ethnographic observation to identify unmet needs in only one clinic (though the model itself advocates for visits to multiple facilities) or are championed by a single influential clinician, creating a vulnerability due to the sample size being n = 1. Similarly, while the model advocates for observing the entire timeline of care to properly comprehend the status quo, this may not be executed sufficiently due to logistical issues such as gaining access to relevant clinical areas. These are problematic because the need may not exist in other clinical environments, for example, in rural vs urban clinics, or high resources vs low resourced healthcare systems. In other words, confirmation bias can be introduced as these issues may skew the innovators understanding of the problem and the viability of their solution as they attempt to extrapolate their findings to other clinical settings.
Chaturvedi model – A sample Stanford biodesign derivative (Chaturvedi et al., Citation2015)
The Chaturvedi model is a derivative of the Stanford Biodesign model, but uses the experiences of the authors to develop a method that is suited for a newly industrialized country such as India. As part of this model, additional consideration is provided to the stakeholders, reimbursement system, regulatory standards, and the broader Indian market.
The method described by Chaturvedi follows the Stanford Biodesign model phases of Identify, Invent, and Implement. However, the model includes deviations that are tailored to the newly industrialized market of India. It could thus be applicable to other developing or newly industrialized countries such as China, Turkey, Brazil, Mexico, and South Africa, as they build their domestic MDI capabilities and infrastructure for fitting in global value chains (Boddin, Citation2016). These deviations include the provision of training to engineers, designers, business graduates and clinicians on the team on the mindset and concepts required to create a startup for enabling translation into the Indian healthcare system. The opinions of clinicians from the local clinical environment can be insightful. Given India’s MedTech industry is still developing, clinicians do not need to manage impacts to their clinical careers by committing full-time to MDI. The complexity of this must be considered however, given the benefits associated with close clinician collaboration and co-designing (Smith et al., Citation2019). Next, a novel needs filtering method which facilitates the identification of needs which, if fulfilled, could result in successful implementation within the Indian system. Lastly, the model includes provisions of additional considerations into the needs specification for incomplete clinical data due to poor documentation practices, as well as for the interests of stakeholders and the Indian company.
POCTRN (point-of-care technologies research network) models (Weigl et al., Citation2012)
The focus of the paper produced by Weigl et al., is on the value CNA performance possesses for developers of point-of-care (POC) diagnostic devices (Weigl et al., Citation2012). It highlights the importance of maintaining CNA documentation which can inform market and product requirements at the initiation of product development and their subsequent use by developers as reference points for when a product concept is changed or refined. The paper also discusses the activities and purpose of needs assessments before providing a basic methodology on how to create a CNA, and the considerations such as the design (qualitative vs quantitative vs hybrid), eligibility criteria of participants, and data analysis methods. The authors argue that CNAs can facilitate NPD decisions and influence strategies for commercialization and marketing.
Weigl’s framework provides considerations (existing gap, potential solutions to gap, stakeholder needs, and barriers to uptake) for inclusion in a CNA and recommends an iterative approach to the assessment. Ideally, a CNA should be performed at conceptualization where needs identification and assessment occurs, during planning, and periodically repeated to confirm that the product conforms to the CNA’s findings.
The structure proposed by Weigl et al. is quite generalist. This is because the ascribed model is simply the one employed by POCTRN and is one of many methodologies. Commonalities between these methodologies include defining a research question or purpose for the CNA and identifying key stakeholders. The authors express that a CNA can be developed using a logic framework which prescribes the inputs (e.g. staff and time spent performing CNA), outputs (data collection methods such as surveys, interviews, etc.), and outcomes (analysis of findings from outputs). For the data collection strategy (or outputs), the type of method depends on the research question. For example, observational studies, literature reviews, and focus groups/opinion leader interviews are qualitative tools that can inform the design of quantitative surveys which enable innovators to develop generalizations, predictions, and calculations of the likelihood of an outcome occurring. From this, developers can understand the impact of the research question and assessment methods on the data that is collected and used to develop conclusions about the clinical need.
An important point is ensuring that the respondents to any data collection exercise are credible sources of information. In one section, the authors describe the Warfare Analysis Laboratory Exercise (WALEx) where clinician-users (nurses/midwives, medical practitioners [such as ob/gyns], allied health, and physician assistants), biomedical engineers, patients of the target demographic, industry, and regulators are isolated in a room to discuss what qualities they want in a product. These specialists should be screened by some eligibility criteria and recruited using a strong sampling plan. However, performing such exercises can be difficult due to the time commitment and fiscal resources that may be required but often not available in a very early stage venture or project.
This model focuses on CNAs rather than also providing a means of developing the clinical use case. It intends to guide the design of CNA protocols rather than provide a formal structure like the Stanford model or other models detailed below. As a result, it provides little guidance on how to focus the research question to validate an identified need. It also lacks detail on how the barriers to uptake may factor into the CNA. However, the framework demonstrates the importance of a high-quality, well defined protocol which is built around the research question, and the value of ensuring that investigators are trained in data collection. Thus, it provides interesting perspectives on how a CNA may be developed before it is factored into developing a clinical case.
Ijzerman and Steuten model (M. J. Ijzerman & Steuten, Citation2011)
The Ijzerman and Steuten model focuses on providing an overview of the MDI process. This model proposes that performing a clinical case analysis as an additional step to clinical needs finding and needs validation can provide additional information for decision making by innovators.
As part of this clinical case analysis, innovators are expected to ask questions along the lines of:
What is the intended application/product?
What are the advantages of the new product?
What is the target group (size)?
What are the comparator interventions?
What is the expected clinical outcome?
By answering these questions, innovators are able to gain an improved understanding of the standard of care and develop awareness of potential competition and alternative options that could impact adoption. Furthermore, innovators will be able to determine how to optimize the market potential of their product and predict how their proposed solutions could be implemented.
In a recent review by Smith et al., the Ijzerman and Steuten model was expanded upon to demonstrate the value of involving clinicians in the needs assessment process (Smith et al., Citation2019). Smith et al. identified that the position occupied by clinicians in healthcare places them uniquely to assist in formulating a statement of clinical need. Clinicians also can demarcate points that are potentially useful for developing the clinical use case, for example, how the innovation could fit within existing clinical guidelines and the potential effect on clinical management (Smith et al., Citation2019). The authors highlight how poor engagement with clinicians could result in: a problem being inadequately explored (which impacts NPD); limited implementation/adoption of new technologies (if the product does not fit the healthcare paradigm); an ineffective regulatory strategy (in the presence of deficient trial design strategies); and an inability to draw upon KOLs to endorse the technology once mature (Smith et al., Citation2019; Warty et al., Citation2021).
Markiewicz’s iterative stakeholder engagement model (Markiewicz, Citation2017)
The Markiewicz model is based on the premise that iterative and continuous engagement of stakeholders throughout the NPD process can ensure that the innovation fulfills the users’ needs, addresses concerns with the learning curve, and identifies the fit with the clinical environment and regulatory infrastructure (Markiewicz, Citation2017).
The Markiewicz model is closely associated with the Ijzerman and Steuten model. It proposes that stakeholders are engaged when developing the product specifications and during the early proof-of-concept phases (M. J. Ijzerman & Steuten, Citation2011; Markiewicz, Citation2017). At this first juncture of specification development, stakeholders are approached to provide recommendations for manufacturers to consider during NPD. A systematic review is performed to guide the questions that are asked of stakeholders. Then, at the proof-of-concept phase, interviews with KOLs and patients are used to inform manufacturers of alternative clinical use cases for the device under development and confirm the existing clinical use case before the evaluation processes commence. Performing interviews at the proof-of-concept stage also informs the manufacturers which potential clinical use cases had the highest/lowest potentials for implementation as well.
Turner framework (Turner et al., Citation2020)
The COVID-19 pandemic resulted in a global shortage of medical equipment and supplies, leading to reductions in the quality of clinical care that was provided (Bown, Citation2021; US Food and Drug Administration, Citation2022). In response, workaround solutions for these shortages were developed through custom-made devices intended for local use. However, the enthusiasm of these innovators to fill a gap may result in bias and uncontrolled use of potentially detrimental solutions (Duggan et al., Citation2019). The Turner framework aims to address this by allowing innovators to develop their solutions in a stepwise manner.
Targeted toward innovative clinicians, this framework involves:
Clearly defining the problem that the device is intended to resolve.
Defining which safety indices are relevant to the device and using those as benchmarks that must be met. This step involves the performance of literature reviews, consultations with experts, analysis of performance measures from manufacturing standards, clinical guidelines, and MD regulators.
Seeking feedback on the design’s utility, potential pitfalls, and identifying any existing solutions. Turner et al. suggest that consultations with biomedical engineers will mitigate risks with the innovation process. Such specialists can advise on topics ranging from the technology itself to the overall lifecycle of the device (including aspects of the supply chain, training, manufacturer needs, construction, use, maintenance, and discontinuation).
Performing extensive laboratory and in situ simulations.
From step 4 onwards, the Turner Framework focuses on the steady and safe introduction of these custom devices into clinical settings. Turner et al. recommends doing so by first implementing the solution in a low-risk setting (subject to local clinical and ethics approval), and then transitioning to a higher-risk setting where the users are trained on a standardized protocol. Finally, undergoing an iterative cycle of feedback, review, re-design, and improvement to critically evaluate the performance of the solution.
Ocampo and Kaminski model (Ocampo & Kaminski, Citation2019)
This model focuses on the MDI process for medium-risk MDs and is broken down into 3 key phases: Pre-development, Development, and Post-development. Only the first two phases will be discussed in this review as post-development is associated with post-market surveillance and obsolescence.
During the pre-development phase, the organization first aims to align the product development objectives with its defined enterprise strategy by analyzing their product portfolio and their fit within the market. This alignment is based on product trends and findings from the market analysis and is classed as part of the strategy planning activities. The next set of activities entail defining the product, clarifying the project scope, and creating a project plan.
The development phase can be broken down into six subphases, as follows: 3. Feasibility study, 4. System design, 5. Detailed design, 6. Production process, 7. Production support, and 8. Product launch. Ocampo and Kaminski identify that subphases 3, 4, and 5 are used to set up the project, whilst the latter 3 subphases are associated with production and product launch. The authors of this review construe Subphases 3, 4, and 5 as relevant for influencing the clinical use case definition.
Ocampo and Kaminski describe the conduct of a feasibility study (Subphase 3) as crucial to the NPD process. The purpose of a feasibility study is to verify that the product is feasible technically, economically, financially, and that there is a potential market. From this, the project team can develop strategies for translation, and a project plan that will define the product. The considerations and activities which are performed are elaborated in Supplementary Materials. The creators of this model then propose a gate (G3) which takes into consideration the findings of the feasibility study activities to determine the overall feasibility of the project.
Subphase 4 System design then details how the final concept is developed and selected using the outputs of the feasibility study to mitigate risk during the EHTA stages of MDI. The final concept is then defined by a proposed system architecture, parameters that influence product design, component/subsystem compatibility, ability to be transferred to manufacturing, and compatibility with intended user environments. This definition is then evaluated to determine the stability or anticipated pace of innovation/reinvention, costs, and user considerations (Cain & Mittman, Citation2002; Ocampo & Kaminski, Citation2019). In doing so, the detailed design (Subphase 5) can be produced, tested, and clinically validated before being transferred to manufacturing.
Ocampo and Kaminski is one of the more comprehensive models of MDI in its description of CNA and clinical use case considerations. This model provides consideration to several of the barriers to uptake, which strengthens its value as a product development guide (Warty et al., Citation2021). However, as this model was conceived only recently, there is no data validating its utility to our knowledge.
World Health Organization (WHO) model (World Health Organization, 2011)
The WHO model aims to provide member states (national governments) with a framework for performing CNAs when identifying which health areas could benefit from technological intervention and how this could be achieved. Due to the diverse socioeconomic statuses of these member states, the framework is generic and set toward identifying systemic/HCO deficiencies that could be addressed through the development or acquisition of medical technologies. As such, this model could prove useful for innovators in identifying needs in a provider/state-centric manner. This is because following concepts defined in this model will help align the innovators’ understanding of the clinical need with that of the HCO or policy maker. This could facilitate implementation of technologies by ensuring that the developed MDs fulfill a clinical need as is potentially defined by health services, and more adequately fulfill the requirements of HCOs. This has the additional benefit of providing insights for innovators on how to market their devices to policy makers to facilitate technology diffusion.
When interpreting this model, it is evident that there is a general approach and a specific approach with the latter being used to supplement the former. This strengthens the value of the CNA activity and better influences the MDI process to ensure clinical implementation.
As part of the general approach, the first step involves identifying what health services are needed by analyzing the target population and associated epidemiological data. This is followed by identifying the existing conditions and availability of HCOs, MDs, and human resources/personnel. After this, the existing standards and practices for health service delivery, MDs, and personnel such as clinical engineers for operation, maintenance, and management of medical equipment; are assessed to see how they could be applied or modified. From this data, an overall gap can be identified to produce a list of general needs which can finally be ranked by priority based on resources constraints such as budget and personnel limitations.
The model then focuses on the specific aspects of needs assessment in a series of 7 steps, as summarized here:
Identify the health service requirements: This step involves understanding the local geographic and public health conditions with a focus on the population of the target area, size of region, number of individuals, population density, and disease burden. By providing considerations to the epidemiological needs, population-based challenges, practice guidelines/standards/recommendations, and local healthcare priorities; innovators can tailor a solution that is appropriate to the requirements of target HCOs.
Identify the availability of health services: Services such as maternal and child health, reproductive medicine, gynecologic oncology, surgical services, etc.; facilities such as hospitals or clinics; and skilled personnel are not always available or accessible. By providing consideration to the availability and accessibility of HCOs, the perceptions of HCOs and the target population on the services provided, the conditions and types of facilities, and the staffing levels; it is possible to develop an overview of the available services and their providers to gain an understanding of what systems exist.
MD situation: The goal of this step is to identify the existing MDs and the associated infrastructure (building, electricity, water, waste disposal) by evaluating medical equipment inventories and the condition of the infrastructure and management systems around them.
Qualification and number of human resources required to cover the required healthcare demand: This step aims to evaluate the availability, capacity, and capability of current human resources. For example, the availability of magnetic resonance imaging (MRI) equipment in the northern hemisphere is at a ratio of 25 MRIs to 1 million inhabitants versus 1 MRI to 25 million in sub-Saharan Africa (Dechambenoit, Citation2016). For neurologists and neurosurgeons, there is 1 neurologist per 3,000,000 people and 1 neurosurgeon per 3,000,000 people in sub-Saharan Africa (versus ratios of 1:40,000 for neurologists per capita and 1:200,000 neurosurgeon/capita in the Northern Hemisphere) (Dechambenoit, Citation2016). This is relevant to planning the development of staffing and training plans.
Financial situation: This step involves evaluating the capacity of an HCO or 3rd-party payer to finance the overall operations including delivery of health service, health technology management, and associated infrastructure.
Analysis and interpretation: Once all this information is gathered, it is possible to interpret the data to draw conclusions that can shape how the population needs are addressed, and if the MD fulfills the clinical gap.
Prioritization and appraisal of options: Using the findings and conclusions of the CNA, the needs must be prioritized. With continually decreasing healthcare budgets, it is likely that there will be insufficient resources to fulfill all the identified needs. This process should also consider the potential solutions of HCOs and patients as the decisions that will be made will depend on local circumstances, although national priorities will play a role. The goal is often to prioritize needs that will produce the greatest impact for the lowest investment of resources. This step is known as option appraisal. Once this is done, an implementation plan should be developed, ideally in collaboration with HCOs to ensure that the plan is realistic and feasible.
Guidance and impact tracking system (GAITS) tool (Consortia for improving medicine with innovation and technology)
Developed by the Consortium for Improving Medicine with Innovation and Technology (CIMIT), GAITS is a tool for tracking progress through innovation processes for medical devices, digital medicine, and biomarker diagnostics. The tool evaluates all stages from identifying a need to when an innovation becomes the standard of care (Consortia for Improving Medicine with Innovation and Technology). It provides resources to help innovators to progress through each of these steps. Many of these resources were sourced from other reputable models including Stanford Biodesign. In the GAITS methodology, each task that is tracked is classified into one of four domains, which help to build a sophisticated unified framework. These domains are clinical, market/business, regulatory, and technology.
In relation to developing insights into unmet clinical needs and available solutions, the GAITS methodology prescribes that the clinical domain consists of designing the unmet needs statement and disease state characterization (Consortia for Improving Medicine with Innovation and Technology). Under the business domain, there is emphasis on needs screening and selection, as well as a characterization of the existing solutions. The regulatory domain requires that innovators familiarize themselves with the relevant regulations, providing resources that can help identify the optimal regulatory pathway. In order to fulfill the requirements of the technology domain, innovators should summarize the state-of-the-art for care and alternative solutions.
The next milestone revolves around the idea or potential solution to the unmet need. The solution to the unmet need is described, evaluated, and then selected for the next milestones which take the solution from proof-of-concept to recognition as the standard of care (Consortia for Improving Medicine with Innovation and Technology). In the milestone of ‘Idea’, the clinical domain consists of understanding the context in which the solution is going to be used (the clinical workflow), updating the needs statement with any new information that arises from any domain. Feedback is obtained from at least 5 clinical stakeholders (for example, clinicians, users, support personnel), and lastly a statement of how the solution can benefit the patient, user, or system is developed. Under the business domain, the way in which each stakeholder will influence the uptake of the solution is identified. A value proposition statement can then be developed for key stakeholders. Then, the methodology requires an evaluation of the competitive landscape to determine where the solution will fit in the market, and how it will aid with defining the venture’s business model. Under the regulatory domain, understanding similar products on the market will determine the type and extent of testing that is required. It is also important to determine if the solution meets the regulatory definition of a MD. Finally, the technology domain requires innovators to design experiments around various hypotheses. The purpose of this is to de-risk major technical challenges, screen ideas and provide rationale for the options that will advance to development, manage IP disclosures with relevant institutions, and develop paper prototypes (for example, descriptions and drawings of solution which also discuss specifications, pricing, indications for use, etc.).
A unique feature of the GAITS cycle is that it categorically defines MDI processes into the clinical, technology, regulatory, and market/business domains to demonstrate the unified nature of all MDI processes. Because of its simplicity and comprehensive content, the checklists created by CIMIT are being adopted by developers, investors and organizations that provide innovation support. However, it has been noted that the checklist content provided can be vague and the resources are targeted primarily to the US market. As such, industry stakeholders often have to develop or adapt the resources to their respective contexts.
Discussion
Comparative discussion of existing models
When comparing these models (Supplementary Materials), we find that most support early performance of CNAs, with the exceptions of Weigl et al. and Markiewicz et al. who both further advocate for an iterative approach. The data collection techniques that were recommended included the use of ethnography or observation (Turner et al., Citation2020; Weigl et al., Citation2012; Yock et al., Citation2015), interviews (Markiewicz, Citation2017; Weigl et al., Citation2012), literature reviews (Weigl et al., Citation2012), or answering sets of questions (M. J. Ijzerman & Steuten, Citation2011; Ocampo & Kaminski, Citation2019; World Health Organization, Citation2011).
Table 2. Potential inputs into a skeleton unified CNA framework as identified from studies in this literature review.
We can see differences in the scope of these models with only Ijzerman and Steuten, Stanford Biodesign, GAITS, and Markiewicz models being designed in a fashion that permits the model to be applied for various MDs (though Markiewicz was applied to a POC setting in their case study). The POCTRN model, though providing basic principles to developing CNAs, has a specific focus on POC devices as well, whilst Turner focuses on custom devices for emergency situations where there is a supply shortage (i.e. COVID-19 pandemic). Ocampo and Kaminski focus on medium risk (Class 1–2) MDs for their model. Though the WHO model is provider-centric (created for healthcare policy makers and HCOs), it provides considerations that could be used by MD developers to gain insights into the condition of the health services of their target markets, around which they can tailor their commercialization strategies. This focus distinguishes the WHO model from others that were designed for developers, who may not otherwise provide consideration to the health services requirements and their influence on the design and commercialization strategies. In its essence, it will ensure compatibility of an innovation with existing environment which is strongly correlated with its diffusion and integration (Cain & Mittman, Citation2002; Rogers, Citation2003). Positively, several frameworks recognized the patient as a stakeholder which is in line with present trends toward patient-centered care and could inspire future frameworks for co-designing with patients as they are the ultimate beneficiary of healthcare (Coulter & Oldham, Citation2016; Mallik, Citationn.d.). Unfortunately, innovators often believe ‘that the needs of the patient do not originate from the patient themselves, and that patients’ needs are better articulated through a hierarchy of health professionals’ which limits the patients’ ability to voice any concerns or expectations (voice of the customer), potentially impeding innovative efforts (Money et al., Citation2011).
When examining the utility of these existing models, we found that only the Stanford Biodesign program collected data describing venture outcomes due to the program, as well as the extent to which the products have been clinically applied (Stanford Byers Center for Biodesign, Citation2020; Wall et al., Citation2017). One study demonstrated a 72% venture survival rate and 20% acquisition/exit rate for companies that were founded during the program. Additionally, 36% of these companies were implemented on the market within the 1–14-year window from whence the program alumni responded to the survey (Wall et al., Citation2017).
This deserves recognition as it is stark contrast to industry metrics of NPD/innovation where data shows only 56% of companies that survive the first four years and the estimated 75–90% of health technology startups that ultimately fail (Shah, Citation2018; Shepherd, Citation2018; Spink, Citation2018). Furthermore, even though new products can be created, fewer than 7–9% of these achieve clinical adoption - a lengthy process that can take up to 17 years - for a variety of factors (Balas & Boren, Citation2000; Leng et al., Citation2018; Morris et al., Citation2011; National Innovation and Science Agenda (Australia,), Citation2015; Science and Technology Committee (UK,), Citation2013; Scott et al., Citation2015). This suggests that CNAs could improve venture success and clinical implementation if conducted rigorously. Particularly if the CNA has been used to guide, not only MD development, but also the associated commercialization strategies.
Wall et al. further implies the benefit of a unified approach versus a model which is primarily focused on only one of the domains - clinical, technical, or business - of a CNA (see Supplementary Materials—Table S3) (Wall et al., Citation2017). The clinical domain was discussed by all the models except one, Ijzerman and Steuten, which employed a clinical case analysis. When comparing considerations for the clinical domain, most models recommend involving healthcare workers (including clinicians, clinical engineers, and administrators) as part of the data collection process to understand user needs and how they perceive the fit in the healthcare system. For the technical domain (discussed by Stanford Biodesign, POCTRN, GAITS, Turner, and Ocampo and Kaminski), consultations with engineers were recommended to understand the impact on system-requirements, technical feasibility, relevant technical standards, and manufacturer needs. The business domain was considered by the Stanford Biodesign, POCTRN, GAITS, Ijzerman and Steuten, and Ocampo and Kaminski models. Each emphasized a different commercial concept that could provide preliminary guidance to innovators. For example, in Ijzerman and Steuten, the clinical case analysis focuses on developing the product-market fit for the target group, whilst Stanford Biodesign focuses on an analysis of the market and financial stakeholders during the needs identification stage. POCTRN and Ocampo and Kaminski focus on economic viability, market requirements, and business sustainability, though the latter also discusses IP management. Interestingly, GAITS was the only model to provide consideration to regulators as a separate, specific domain, rather than a subunit of either the clinical, technical, or business domains.
A unified approach to CNAs
The models presented above highlight the existing theoretical tools for determining the clinical need for a medical innovation. However, only the Stanford model, GAITS, Ocampo and Kaminski, and aspects of the POCTRN guide present a truly unified (clinical, technical, and business) approach to performing CNAs utilizing the tenets of EHTA. Despite the presence of comprehensive models, there is scope to optimize the process. This may be done through the development of a model or framework for determining unmet clinical needs that are commercially sustainable and that will support decision making for developing the clinical use case, to minimize the need for venture pivots due to poor problem-solution fit. Of particular interest is if such a model can be applied in an easy-to-use manner for interpretation by a broader range of innovators including clinicians, who may lack training or comprehension of MDI processes.
We believe that a unified approach is necessary because the data collected during the CNA process not only validates the existence of a clinical problem, but contributes toward developing and commercializing the MD. In relation to the clinical aspects, a good CNA will help identify and understand the clinical problem, stakeholder requirements for implementation and use, and provide a basic understanding into the state of the health services and surrounding infrastructure. The more technical concerns of a properly conducted CNA can then help analyze existing solutions, evaluate the technical feasibility of the proposal, identify requirements and specifications of the proposed solution, and guide research and development strategy which will mitigate risk during the product development and testing processes. Technical considerations as part of the CNA process can further guide the transfer process from the bench to the manufacturing as design considerations need to evaluate manufacturer needs against user needs for feasibility and benefit. From a business perspective, the CNA will not only identify the market and provide considerations for the market analysis; it will provide several input considerations for the various commercialization strategies; facilitate economic/financial analyses; and help determine venture sustainability to help the product achieve its market fit.
There are potential benefits in employing a unified framework (). For example, by gathering data that forms the basis of the clinical use case and design strategy and guides the development of commercialization strategies, innovators undergo a more efficient translation process. This would be due to the evidence-based approach associated with decision making that will mitigate risk within the NPD process (thereby highlighting the importance of CNAs as an EHTA activity in MDI). This unified framework can be further strengthened by employing a team of clinicians, engineers, and business-minded individuals to perform the data collection and analysis. Each team member can provide expert analyses in their respective domains (clinical, technical, and business) whilst providing inputs into other domains that arise from differences in thought, training, and experience. Validation data on the Stanford Biodesign model is in favor of such multidisciplinary approaches (Wall et al., Citation2017). Hence, it is anticipated that such a framework will increase venture sustainability, as well as the likelihood of diffusion and clinical implementation of medical technology innovations.
Applying the skeleton unified framework to devices in obstetrics and gynecology
When focusing on technologies for women’s health, there are a number of additional considerations for innovators when performing the CNA activity. For example, who the stakeholders are, challenges with providing care, and the barriers to uptake (especially social barriers and stakeholder perceptions) which can restrict the ability to develop innovative solutions for unmet needs.
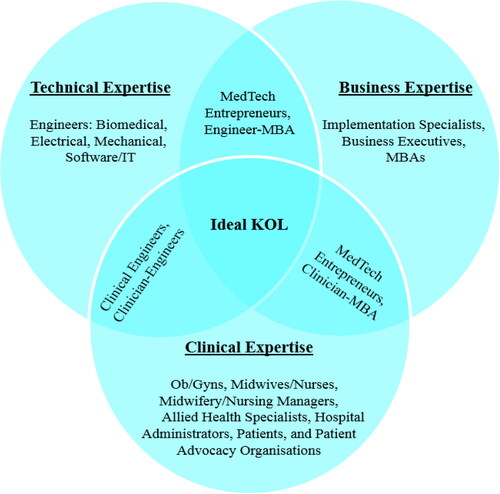
In FemTech, there are a number of stakeholders who can provide valuable insights for a unified CNA. These stakeholders are summarized in classified by their domain in the unified framework. Engineers, IT professionals, and technologists are usually the best positioned professionals to address the technological aspects of the CNA and development of the clinical use case, whilst implementation specialists, or individuals with a business background can address the considerations of the business domain. When seeking clinical input, it should be considered that there are a variety of clinicians who are qualified to provide insights into specific aspects of a patient’s care cycle, and that a clinical problem may affect each one differently. For example, whilst a urogynecologist may be involved in the initial care of a pelvic floor dysfunction patient or perform the surgery, pelvic floor nurses may assist in pelvic floor assessments and pessary management. Meanwhile, physiotherapists will have primary responsibility for the patient’s pelvic floor rehabilitation. Administrators will be able to advise on reimbursement and other health service-related topics as well; whilst patients and patient advocacy groups can provide immense insights based on their experiences as receivers of clinical care. Lastly, interdisciplinary individuals as KOLs, advisors, or innovators can simplify the CNA process by providing valuable knowledge across multiple domains.
Figure 2. Stakeholders who can provide insights into the clinical, technical, and business domains. Note: MBAs refers to graduates of the Master of Business Administration degree.
Another consideration for innovators is associated with the challenges with providing care to women. Whilst women are more likely to seek treatment than men, live longer, and are more likely to face poorer health than men; historically, they have faced issues with accessing healthcare (Heise et al., Citation2019). There are a number of reasons for this, including socioeconomic status, lower education/literacy, issues of gender inequity, social and/or cultural norms, etc. (Heise et al., Citation2019). As such, innovators must provide consideration to how the technology will be implemented so that issues associated with accessibility and cost of care can be addressed. An additional challenge with caring for women is associated with the ability to generate direct rapport with the patient as well as respect their right to bodily autonomy. This, in part is because, in many societies, women lack the independence to make medical decisions and have limited control over their health (only 55% of females worldwide are able to experience this autonomy) (Baker et al., Citation2021). The need for respecting patient autonomy is widely valued, particularly as it is a core tenet in bioethics, and because it has helped improve patient-clinician relationships. However, innovators should understand that it is not only the clinician who can infringe upon the patients’ autonomy, but also potentially their solution and the practices associated with the solution’s use as well (Entwistle et al., Citation2010). This understanding is vital as poor ability to build trust, amongst female patients especially, has been associated with poor health service utilization as well (Thapa & Niehof, Citation2013).
There are also a number of barriers to clinical uptake that innovators must consider in the early MDI stages (Warty et al., Citation2021). However, technologies targeting women’s health face additional social barriers which are often associated with the perception around a technology. A prime example of this is the well-publicized impact of the transvaginal mesh, where historical failures associated with the technology have heightened public, clinician, and patient risk aversions to future mesh technologies and other gynecological interventions (CBC News, Citation2019; Devlin, Citation2017, Citation2018; Hooton, Citation2019; US Food & Drug Administration, Citation2021a, Citation2021b). As a result, the outdated Burch colposuspension has returned the gynecological practice and is now the current gold standard surgical intervention for stress urinary incontinence (Veit-Rubin et al., Citation2019). Another example is in obstetrics, where clinicians are often averse to anything that could result in harm to the fetus, sometimes at the expense of the woman’s well-being. As such, research and interventions are applied to stricter standards to protect: (a) the fetus from any harm, (b) the mothers’ ability to produce future healthy offspring and/or (c) the mothers’ quality of life after her pregnancy (Mastroianni et al., Citation1999). Indeed, there has been growing interest amongst bioethicists and technological philosophers about issues in pregnancy management, including concerns regarding over-medicalization, the impact of antenatal screening and testing, and technologized birthing practices (Kukla & Wayne, Citation2016; Topçu & Brown, Citation2019; Verbeek, Citation2008). Lastly, there is the impact of cultural taboos or sensitivities that are related to gynecological conditions, with a commonly touted example being menstrual hygiene (Gottlieb, Citation2020). To varying degrees, these cultural sensitivities are present in most societies around the world, and this is something FemTech innovators must consider.
Conclusion
In industry, one of the key issues in relation to CNAs is that there is little integration of formal methods into routine practice, that signifies a potential practice-knowledge gap. Additionally, with the exception of the Stanford Biodesign model which has basic and limited data on the success rates of the program, there is no real-world assessment or validation method of these innovation frameworks. This raises concern, as it is unclear whether these frameworks are being implemented and to what effect. Furthermore, it is likely that, without the visibility and successful application of such frameworks, innovators of medical technologies see little value in employing or developing such tools or alternatively, are not aware of their existence. In this review, we provided a critical analysis of existing CNA frameworks to identify potential considerations for FemTech innovators to include as part of their CNAs. Additionally, these same considerations can form the basis of a unified framework which could be used to mitigate clinical, technical, and business risks and decision uncertainty along the translation journey. Additionally, we describe the use of such frameworks in women’s healthcare, where inequities in healthcare and innovation exist. If such a unified framework were to be developed and validated, we may be able to provide FemTech innovators with an EHTA tool for use in early MDI that could result in novel medical technologies efficiently and effectively addressing current and future unmet clinical needs.
Supplemental Material
Download MS Word (54.3 KB)Acknowledgments
The authors acknowledge Prof Sally McArthur for their expertise and assistance throughout this study.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Amin, A., Remme, M., Allotey, P., & Askew, I. (2021). Gender equality by 2045: Reimagining a healthier future for women and girls. BMJ (Clinical Research ed.), 373, n1621. https://doi.org/10.1136/bmj.n1621
- Australian Institute of Health and Welfare. (2019). The health of Australia’s females. Australian Institute of Health and Welfare. Australian Government.
- Baker, D., Behrendt, A., Baric, S., Devillé, M., Ferguson, L., Luchsinger, G., & Roseman, M. (2021). My body is my own: Claiming the right to autonomy and self-determination. United Nations Population Fund.
- Balas, E., & Boren, S. (2000). Managing Clinical Knowledge for Health Care Improvement. Yearbook of Medical Informatics, 1, 65–70. https://doi.org/10.1055/s-0038-1637943
- Barclay, S., & Caulfield, M. (2022). Women’s Health Strategy for England. HH Associates ltd. on Behalf of the Controller of Her Majesty’s Stationery Office, CP 736.
- Boddin, D. (2016). IMF Working Paper (WP/16/207): The Role of Newly Industrialized Economies in Global Value Chains. International Monetary Fund https://doi.org/10.5089/9781475545456.001
- Bown, C. P. (2021). How COVID-19 Medical Supply Shortages Led to Extraordinary Trade and Industrial Policy. Asian Economic Policy Review, 17(1), 114–135. https://doi.org/10.1111/aepr.12359
- Cain, M., & Mittman, R. (2002). Diffusion of Innovation in Healthcare. California Healthcare Foundation.
- News, C. B. C, CBC. News. (2019). Transvaginal mesh pulled from Canadian market following safety review. https://www.cbc.ca/news/health/prolapse-mesh-pulled-canada-1.5226794
- Chaturvedi, J., Logan, A., Narayan, G., & Kuttappa, S. (2015). A structured process for unmet clinical need analysis for medical device innovation in India: Early experiences. BMJ Innovations, 1(3), 81–87. https://doi.org/10.1136/bmjinnov-2014-000010
- Commission Regulation (EC.) No 507/ (2006). Article 4, Paragraph 2 C.F.R. (2006).
- Consortia for Improving Medicine with Innovation and Technology. GAITS - Guidance and Impact Tracking System. Consortia for Improving Medicine with Innovation and Technology (CIMIT) https://www.gaits.org/fr/
- Consortia for Improving Medicine with Innovation and Technology. Milestone: Idea. Consortia for Improving Medicine with Innovation and Technology https://www.gaits.org/fr/home/-/project/milestone/4102
- Consortia for Improving Medicine with Innovation and Technology. Milestone: Need. Consortia for Improving Medicine with Innovation and Technology https://www.gaits.org/fr/home/-/project/milestone/4101
- Coulter, A., & Oldham, J. (2016). Person-centred care: What is it and how do we get there? Future Hospital Journal, 3(2), 114–116. https://doi.org/10.7861/futurehosp.3-2-114
- Dechambenoit, G. (2016). Access to health care in sub-Saharan Africa. Surgical Neurology International, 7, 108. https://doi.org/10.4103/2152-7806.196631
- Devlin, H. (2017). New Zealand bans vaginal mesh implants. The Guardian. https://www.theguardian.com/science/2017/dec/12/new-zealand-bans-vaginal-mesh-implants
- Devlin, H. (2018). Government halts vaginal mesh surgery in NHS hospitals. The Guardian. https://www.theguardian.com/society/2018/jul/09/government-halts-vaginal-mesh-surgery-in-nhs-hospitals
- Dixon, J. C. (2001). The “Market Pull” versus “Technology Push” Continuum of Engineering Education. 2001 American Society for Engineering Education Annual Conference & Exposition New Mexico US.
- Duggan, L. V., Marshall, S. D., Scott, J., Brindley, P. G., & Grocott, H. P. (2019). The MacGyver bias and attraction of homemade devices in healthcare. Canadian Journal of Anaesthesia = Journal Canadien D’anesthesie, 66(7), 757–761. https://doi.org/10.1007/s12630-019-01361-4
- Emergen Research. (2021). Femtech Market By Type (Devices, Software, Services), By End-Use (Direct-to-Consumer, Hospitals, Fertility Clinics, Surgical Centers, Diagnostic Centers), By Application (Reproductive Health, Pregnancy & Nursing Care, Pelvic & Uterine Healthcare), By Region, Forecasts to 2027. E. Research. https://www.emergenresearch.com/industry-report/femtech-market
- Entwistle, V. A., Carter, S. M., Cribb, A., & McCaffery, K. (2010). Supporting patient autonomy: The importance of clinician-patient relationships. Journal of General Internal Medicine, 25(7), 741–745. https://doi.org/10.1007/s11606-010-1292-2
- Gold, H. T., Pitrelli, K., Hayes, M. K., & Murphy, M. M. (2014). Decision to adopt medical technology: Case study of breast cancer radiotherapy techniques. Medical Decision Making: An International Journal of the Society for Medical Decision Making, 34(8), 1006–1015. https://doi.org/10.1177/0272989X14541679
- Gottlieb, A. (2020). Menstrual taboos: Moving beyond the curse. In C. Bobel, I. T. Winkler, B. Fahs, K. A. Hasson, E. A. Kissling, & T. A. Roberts (Eds.), The Palgrave Handbook of Critical Menstruation Studies. (pp. 143–162). Palgrave Macmillan. https://doi.org/10.1007/978-981-15-0614-7_14
- Grayden, D., Lim, K., & Vitale, M. (2022). Biodesign Innovation Melbourne. University of Melbourne.
- Heise, L., Greene, M. E., Opper, N., Stavropoulou, M., Harper, C., Nascimento, M., Zewdie, D., Darmstadt, G. L., Greene, M. E., Hawkes, S., Heise, L., Henry, S., Heymann, J., Klugman, J., Levine, R., Raj, A., & Rao Gupta, G, Gender Equality, Norms, and Health Steering Committee. (2019). Gender inequality and restrictive gender norms: Framing the challenges to health. Lancet (London, England), 393(10189), 2440–2454. https://doi.org/10.1016/S0140-6736(19)30652-X
- Hooton, A. (2019). The ‘eight-minute’ cure: How transvaginal mesh sentenced thousands of women to a life of pain. The Sydney Morning Herald. https://www.smh.com.au/lifestyle/health-and-wellness/the-eight-minute-cure-how-transvaginal-mesh-sentenced-thousands-of-women-to-a-life-of-pain-20190611-p51whn.html
- Ijzerman, M., Koffijberg, H., Fenwick, E., & Krahn, M. (2017). Emerging Use of Early Health Technology Assessment in Medical Product Development: A Scoping Review of the Literature. PharmacoEconomics, 35(7), 727–740. https://doi.org/10.1007/s40273-017-0509-1
- Ijzerman, M. J., & Steuten, L. M. (2011). Early assessment of medical technologies to inform product development and market access: A review of methods and applications. Applied Health Economics and Health Policy, 9(5), 331–347. https://doi.org/10.2165/11593380-000000000-00000
- Kemble, E., Perez, L., Sartori, V., Tolub, G., & Zheng, A. (2022). The dawn of the FemTech revolution McKinsey & Company https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/the-dawn-of-the-femtech-revolution
- Kukla, R., & Wayne, K. (2016). Pregnancy, Birth, and Medicine. Stanford University https://plato.stanford.edu/entries/ethics-pregnancy/
- Leng, G., Williams, S., Hung, I., Partridge, G., & Sanghvi, S. (2018). Uptake of medical devices approved by NICE. BMJ Innovations, 4(4), 178–184. https://doi.org/10.1136/bmjinnov-2018-000273
- Mallik, S. (n.d.). Patient centric Innovation That Delivers More than Wellness. Infosys https://www.infosys.com/insights/industry-stories/patient-centric-innovation.html
- Markiewicz, K. (2017). Health technology assessment of medical devices during development., University of Twente.
- Marquez, P. (2017). Healthy women are the cornerstone of healthy societies. World Bank https://blogs.worldbank.org/health/healthy-women-are-cornerstone-healthy-societies
- Mastroianni, A., Faden, R., & Federman, D. (1999). Women and Health Research. Ethical and Legal Issues of Including Women in Clinical Studies: Volume 2: Workshop and Commissioned Papers. National Academies Press.
- McCambridge, J., Witton, J., & Elbourne, D. R. (2014). Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. Journal of Clinical Epidemiology, 67(3), 267–277. https://doi.org/10.1016/j.jclinepi.2013.08.015
- Mokarram, N., Denend, L., Lyon, J., Rait, D., Brinton, T., Makower, J., & Yock, P. (2021). Need Statements in Healthcare Innovation. Annals of Biomedical Engineering, 49(7), 1587–1592. https://doi.org/10.1007/s10439-021-02782-3
- Money, A. G., Barnett, J., Kuljis, J., Craven, M. P., Martin, J. L., & Young, T. (2011). The role of the user within the medical device design and development process: Medical device manufacturers’ perspectives. BMC Med Inform Decis Mak, 11, 15. https://doi.org/10.1186/1472-6947-11-15
- Morris, Z. S., Wooding, S., & Grant, J. (2011). The answer is 17 years, what is the question: Understanding time lags in translational research. Journal of the Royal Society of Medicine, 104(12), 510–520. https://doi.org/10.1258/jrsm.2011.110180
- National Innovation and Science Agenda [Australia. (2015)]. National Innovation and Science Agenda Commonwealth of Australia.
- NIH Office of Communications. (2016). What health issues or conditions are specific to women only?. National Institute of Child Health and Human Development, National Institute of Health. https://www.nichd.nih.gov/health/topics/womenshealth/conditioninfo/whatconditions
- Ocampo, J. U., & Kaminski, P. C. (2019). Medical device development, from technical design to integrated product development. Journal of Medical Engineering & Technology, 43(5), 287–304. https://doi.org/10.1080/03091902.2019.1653393
- Oliveria, S. A., Sachs, D., Belasco, K. T., & Halpern, A. C. (2003). Adoption of new technologies for early detection of melanoma in dermatologic practice. Journal of the American Academy of Dermatology, 49(5), 955–959. https://doi.org/10.1067/S0190-9622(03)02464-2
- Petkova, H., Schanker, B., Samaha, D., & Hansen, J. (2010). Barriers to innovation in the field of medical devices - Background Paper 6. W. H. Organisation.
- Rogers, E. (2003). Diffusion of Innovations. (Fifth ed.). Free Press.
- Saitman, A. (2019). Translational Medicine: A Clinical Pull Or A Technological Push?
- Science and Technology Committee [UK. (2013)]. Bridging the valley of death: Improving the commercialisation of research - Eighth Report of Session 2012–13. The Stationery Office by Order of the House.
- Scott, A., Pasichnyk, D., Harstall, C., & Chojecki, D. (2015). Optimizing adoption and diffusion of medical devices at the system level.
- Shah, A. (2018). 9 in 10 Digital Health Start-ups Fail. Here’s how You can Avoid being One of Them Entrepreneur India. https://www.entrepreneur.com/article/307595
- Shepherd, M. (2018). Medtech Funding: Emerging from the Valley of Death? MPO - Medical Product Outsourcing. https://www.mpo-mag.com/issues/2018-10-01/view_columns/medtech-funding-emerging-from-the-valley-of-death/
- Smith, V., Warty, R., Nair, A., Krishnan, S., Sursas, J. A., da Silva Costa, F., Vollenhoven, B., & Wallace, E. M. (2019). Defining the clinician’s role in early health technology assessment during medical device innovation - A systematic review. BMC Health Serv Res, 19(1), 514. https://doi.org/10.1186/s12913-019-4305-9
- Spink, D. (2018). The Top 7 Reasons Why Medical Devices Fail. Boyd Technologies https://www.boydtech.com/articles/the-top-7-reasons-why-medical-devices-fail
- Stanford Byers Center for Biodesign. (2020). Our Impact. Stanford Byers Center for Biodesign. https://biodesign.stanford.edu/our-impact/trainee-outcomes.html
- Steinberger, J. D., Denend, L., Azagury, D. E., Brinton, T. J., Makower, J., & Yock, P. G. (2017). Needs-Based Innovation in Interventional Radiology: The Biodesign Process. Techniques in Vascular and Interventional Radiology, 20(2), 84–89. https://doi.org/10.1053/j.tvir.2017.04.006
- Tanenbaum, M. L., Adams, R. N., Lanning, M. S., Hanes, S. J., Agustin, B. I., Naranjo, D., & Hood, K. K. (2018). Using Cluster Analysis to Understand Clinician Readiness to Promote Continuous Glucose Monitoring Adoption. Journal of Diabetes Science and Technology, 12(6), 1108–1115. https://doi.org/10.1177/1932296818786486
- Thapa, D. K., & Niehof, A. (2013). Women’s autonomy and husbands’ involvement in maternal health care in Nepal. Social Science & Medicine (1982), 93, 1–10. https://doi.org/10.1016/j.socscimed.2013.06.003
- Topçu, S., & Brown, P. (2019). The impact of technology on pregnancy and childbirth: Creating and managing obstetrical risk in different cultural and socio-economic contexts. Health, Risk & Society, 21(3-4), 89–99. https://doi.org/10.1080/13698575.2019.1649922
- Turner, M. C., Duggan, L. V., Glezerson, B. A., & Marshall, S. D. (2020). Thinking outside the (acrylic) box: A framework for the local use of custom-made medical devices. Anaesthesia, 75(12), 1566–1569. https://doi.org/10.1111/anae.15152
- UN Department of Economic and Social Affairs. (2015). Gender equality and women’s empowerment. United Nations.
- US Food and Drug Administration. (2021a). FDA’s Activities: Urogynecologic Surgical Mesh. US Food and Drug Administration. https://www.fda.gov/medical-devices/urogynecologic-surgical-mesh-implants/fdas-activities-urogynecologic-surgical-mesh
- US Food and Drug Administration. (2021b). Urogynecologic Surgical Mesh Implants. US Food and Drug Administration. https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants
- US Food and Drug Administration. US Food and Drug Administration. (2022). Medical Device Shortages During the COVID-19 Public Health Emergency. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/medical-device-shortages-during-covid-19-public-health-emergency
- Veit-Rubin, N., Dubuisson, J., Ford, A., Dubuisson, J. B., Mourad, S., & Digesu, A. (2019). Burch colposuspension. Neurourology and Urodynamics, 38(2), 553–562. https://doi.org/10.1002/nau.23905
- Verbeek, P.-P. (2008). Obstetric Ultrasound and the Technological Mediation of Morality: A Postphenomenological Analysis. Human Studies, 31(1), 11–26. https://doi.org/10.1007/s10746-007-9079-0
- Wall, J., Hellman, E., Denend, L., Rait, D., Venook, R., Lucian, L., Azagury, D., Yock, P. G., & Brinton, T. J. (2017). The Impact of Postgraduate Health Technology Innovation Training: Outcomes of the Stanford Biodesign Fellowship. Annals of Biomedical Engineering, 45(5), 1163–1171. https://doi.org/10.1007/s10439-016-1777-1
- Warty, R. R., Smith, V., Salih, M., Fox, D., McArthur, S. L., & Mol, B. W. (2021). Barriers to the Diffusion of Medical Technologies Within Healthcare: A Systematic Review. IEEE Access. 9, 139043–139058. https://doi.org/10.1109/ACCESS.2021.3118554
- Weigl, B. H., Gaydos, C. A., Kost, G., Beyette, F. R., Jr., Sabourin, S., Rompalo, A., de Los Santos, T., McMullan, J. T., & Haller, J. (2012). The Value of Clinical Needs Assessments for Point-of-Care Diagnostics. Point of Care, 11(2), 108–113. https://doi.org/10.1097/POC.0b013e31825a241e
- World Health Organisation. (n.d.). Women’s Health. World Health Organisation (WHO). https://www.who.int/health-topics/women-s-health
- World Health Organisation. (2011). Needs assessment for medical devices. World Health Organisation.
- Yang, M. Y., Gemba, K., & Tamada, S. (2016). Training innovators at the stanford biodesign program and its implications [Paper presentation].2016 Portland International Conference on Management of Engineering and Technology (PICMET)., https://doi.org/10.1109/PICMET.2016.7806570
- Yock, Zenios, Brinton, Kumar, Watkins, Denend, & Krummel, Kurihara. (2015). Biodesign: The process of innovating medical technologies. (Second ed.). Cambridge University Press.