ABSTRACT
This study aimed to clarify the effect of light exposure during the daytime and nighttime on diet-induced thermogenesis (DIT), which is one kind of energy expenditure, and the contribution of autonomic nervous activities (ANA) to the mechanism behind such effects. We found that the light–dark cycle significantly induced a diurnal rhythm of DIT, with afternoon levels tending to be higher than nighttime levels. By contrast, no such rhythms were observed under constant light or dark conditions. There were also no significant differences in ANA between the light conditions. These findings demonstrate that a diminished light–dark cycle leads to disruption of the diurnal rhythm of metabolism and so the retention of ordinary light–dark cycles may be recommended for health maintenance.
Introduction
The light–dark cycle is known to be the main zeitgeber (time-giver) for the entrainment of the circadian rhythm (Stokkan et al. Citation2001; Waterhouse et al. Citation2007). The light–dark cycle can affect energy expenditure, as light perception information is transmitted via the retinohypothalamic tract to the central circadian clock located in the suprachiasmatic nuclei (SCN) of the brain, and then to peripheral clocks such as that occurring in the liver, intestine and pancreas via signals produced by both the autonomic nervous system and hormones (Cagampang and Bruce Citation2012). The circadian rhythm is generated by endogenous circadian oscillators and persists even under constant environmental conditions. The diurnal rhythm, on the other hand, represents a combination of the endogenous rhythm and other influences such as sleep-wake patterns, activity, meals or light exposure (Klerman Citation2005). A previous study showed that mice experienced an increased body mass under constant light conditions or dim conditions compared with a standard light–dark cycle, despite caloric intake and activity levels being controlled (Fonken et al. Citation2010). In addition, in humans, a positive correlation was found between artificial light at night (ALAN) and the prevalence of obesity and hyperlipidemia (McFadden et al. Citation2014; Obayashi et al. Citation2013). Although the correlation coefficients were adjusted according to sleep duration and physical activities in these studies, information about food intake (time, amount and content) was lacking. Furthermore, some studies showed that melatonin secretion is negatively correlated with the prevalence of diabetes or increment of BMI (Obayashi et al. Citation2014; Schernhammer et al. Citation2006). However, the direct effects of melatonin on diabetes or body mass remain to be studied.
There is likely to be a relationship between increasing ALAN and people’s dietary behavior at night, which results in an excessive caloric intake. Furthermore, unusual eating times have also been shown to contribute to the disruption of the circadian rhythm, resulting in obesity (Froy and Miskin Citation2010; Navara and Nelson Citation2007). Therefore, it remains to be clarified whether ALAN directly induces obesity or whether the observed relationship is, in fact, simply related to food consumption. Furthermore, although some studies have indicated that light exposure affects digestion and absorption in humans (Hirota et al. Citation2003; Sone et al. Citation2003), its direct effects on energy metabolism remain unclear.
One way to determine the effect of light exposure on metabolism is to measure energy expenditure under a controlled food intake. Energy homeostasis is the balance between energy intake and energy expenditure. Under the optimum temperature, which is comfortable and less physical stress, there are generally three types of energy expenditure: basal metabolic rate, activity-induced thermogenesis and diet-induced thermogenesis (DIT). DIT is defined as the increase in energy expenditure above the basal fasting level following food intake, and is associated with the digestion, absorption and storage of nutrients. Although this accounts for a relatively small proportion of the total energy expenditure (c. 10%), DIT is considered an important component of the development and maintenance of obesity (Taheri Citation2006; Westerterp Citation2004). Basic information about the diurnal rhythm of DIT has been reported previously, whereby DIT in the morning is significantly higher than that in the late afternoon and at night (Morris et al. Citation2015; Romon et al. Citation1993). However, the light conditions were not controlled in these studies.
To elucidate the mechanism by which light affects metabolism, it is necessary to understand the relationship between DIT and autonomic nervous activity (ANA). It has been proposed that postprandial activation of the sympathetic nervous system (SNS) plays a role in the cardiovascular and metabolic response to meal ingestion (Acheson et al. Citation1984; Tappy Citation1996; van Baak Citation2008), despite the parasympathetic nervous system (PNS) largely prevailing and causing the heart rate and blood pressure to decrease (Kreier et al. Citation2003). Therefore, in this study, we studied the changes in DIT and ANA under different light conditions to clarify the effect of light exposure during the daytime and nighttime on energy expenditure and the contribution of ANA to the mechanism behind such changes. We hypothesized that the light condition would affect the diurnal rhythm of DIT, and this effect was associated with ANA.
Materials and methods
Participants
Twelve female Japanese students (21–22 years old) from Fukuoka Women’s University in Japan were recruited between June 2014 and June 2015, 10 of whom completed the experiments. shows the characteristics of the participants. The body mass of each participant was measured using a scale (Inner Scan BC-600; Tanita Co., Tokyo, Japan) at 18:00 on the first day of each experiment and height information was collected from the annual health checkup at the University. There was no significant change in the body mass or body mass index (BMI) of any of the participants during the experiment (one-way analysis of variance (ANOVA). The average BMI (19.0) was slightly lower than the national average of 21.1 for this generation in Japan (data from Japan Ministry of Health, Labour and Welfare in Citation2014), but still within the normal range (18.5–24.9) classified by the Japan Society for the Study of Obesity.
Table 1. Subjects’ characteristics.
Study protocol
The experiments were performed in a temperature- and humidity-controlled chamber (TBR-4AX; Tabai Espec Co., Osaka, Japan) that was at 26 ± 1 C and 60 ± 2% RH to eliminate any effect of temperature on energy expenditure. The temperature of 26 ± 1°C is assumed to be optimum, since participants’ physical activity was low (1.0 Met) and clothing was light (0.5 clo) in the chamber. For 1 week prior to the experiments, the participants were asked to retire at 24:00 ± 1 h and get up at 7:00 ± 1 h at home. During this period, they refrained from taking sleeping pills, and avoided excessive amounts of exercise, alcohol and caffeine. Their adherence to this schedule was checked by inspecting their sleep diaries.
The time schedule for the experiments is shown in . Each subject spent 3 days and 2 nights in the experimental room for each of three experiments, which were conducted under different light conditions: a dim light condition during the daytime (7:00–18:00) and nighttime (18:00–24:00) (Dim–Dim, DD); a bright light condition during the daytime and a dim light condition during the nighttime (Bright–Dim, BD); and a bright light condition during both the daytime and nighttime (Bright–Bright, BB). Subjects spent the time from 24:00 to 07:00 under the dim light condition. For the dim light condition, an incandescent lamp was used to provide indirect lighting in one corner of the room, giving a horizontal illuminance level on the floor in the center of the room of <10 lx, as measured with an illuminometer (LM-8000; Fuso Co., Tokyo, Japan). For the bright light condition, fluorescent lamps (FHF32EX-D-H, 6700K, 32W; Panasonic Co., Osaka, Japan) installed in the ceiling were used as a light source, which provided approximately 7,000 lx. The order of the light condition treatments was randomly chosen for each subject and there was an interval of at least 5 days between successive experiments.
Figure 1. The time schedule for each experiment.
The bars are colored according to the time of day: white, daytime; light gray, nighttime and dark gray, sleep time. The slim, horizontal shaded bars indicate the measurement times for resting energy expenditure (REE) and autonomic nervous activity (ANA) around mealtimes.
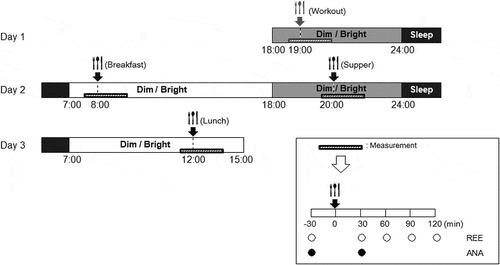
The first day of each experiment was set aside for practicing and acclimatizing the participants to the experimental setup and controlling their condition. An identical meal () was provided at 8:00 as a breakfast and at 20:00 as a supper on day 2, and at 12:00 as a lunch on day 3. These meals consisted of retort pouch foods and boiled eggs. Information about the energy and nutrient contents of the pouch foods were provided by the manufacturers, while those of the eggs were calculated according to the Standard Tables of Food Composition (Ministry of Education, Culture, Sports, Science and Technology of Japan, 2010. The standard tables of food composition.). There was at least a 12-h interval between each meal to eliminate any effects of the previous meal. The subjects were free to drink only water between meals.
Figure 2. Changes in diet-induced thermogenesis (DIT) following meal intake under three light conditions.
Changes in DIT with (a) the DD treatment (dim light exposure throughout the experiment); (b) the BD treatment (bright light exposure during the daytime and dim light exposure during the nighttime) and (c) the BB treatment (bright light exposure throughout the experiment except for sleep time). †: p < 0.1.
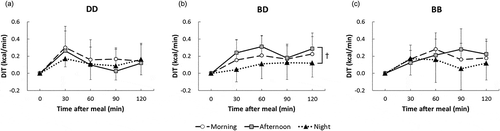
Table 2. Contents and nutrient composition of the meal.
To calculate DIT, the resting energy expenditure (REE) of each participant was measured over a 5-min period at 30 min before and 30, 60, 90 and 120 min after each meal using a respiratory analyzer with an indirect and open-circuit method (VO2000; MGC Diagnostics Co., MN, USA). To determine ANA, the heart rate variability of each participant was measured over a 5-min period at 30 min before and after each meal using electrocardiography (Activetracer AC-301A; GMS Co., Tokyo, Japan). Prior to each measurement, the subject had been resting on a chair for 5 min. The measurement times for both DIT and ANA were considered as representing the morning and night on day 2, and the afternoon on day 3. During the experiments, the participants refrained from exercise and watching any display monitors, including personal computers and cell phones.
All participants provided written informed consent prior to the experiment, and the study protocol was conducted in accordance with the ethics committee of Fukuoka Women’s University and met the ethical standards of the Journal (Portaluppi et al. Citation2010).
Calculation of DIT and ANA
Weir’s equation (Weir Citation1949) was used to calculate REE based on the amounts of inhaled oxygen (VO2) and exhaled carbon dioxide (VCO2), which were measured with the computerized respiratory analyzer. DIT was then obtained by calculating the difference between REE before and after each meal:
The area under the curve (AUC) for a 2-h period was also calculated using the trapezoidal method.
ANA was determined using electrocardiography through the analysis of heart rate variability (HRV). HRV was analyzed using a power spectral analysis (MemCalc ver2.0; GMS Co., Tokyo, Japan), which calculated the areas of three frequency ranges: very low frequency (VLF; 0.007–0.035 Hz), which is thought to reflect activation of the SNS in relation to energy metabolic regulation (Matsumoto et al. Citation2001, Citation1999); low frequency (LF; 0.04–0.15 Hz), which reflects both SNS and PNS, with LF/HF being considered to represent SNS; and high frequency (HF; 0.15–0.4 Hz), which reflects only PNS. Thus, three parameters of ANA (VLF, LF/HF and HF) were considered when comparing the recorded values before and after each meal:
ANA difference = ANA (30 min after meal)–ANA (30 min before meal)
Statistical analyses
The data in all graphs are presented as means ± SD (standard deviation). We investigated the effect of Light (DD, BD and BB), Period (morning, afternoon and night) and Time (every 30 min for 2 h after the meal) on DIT, and the interactions between these using three-way ANOVA. In addition, the effect of Period and Time under each light condition was analyzed to observe diurnal rhythms of DIT using a two-way ANOVA. AUCs for DIT were analyzed using two-way ANOVA and summed AUCs (total AUCs) for each light condition were analyzed using one-way ANOVA.
We also studied the effect of Light (DD, BD and BB), Period (morning, afternoon and night) and Time (at 30 min before and after the meal) on ANA, and the interactions between these using three-way ANOVA. In addition, the effect of Light and Period on ANA was analyzed using two-way ANOVA, and the effect of Period alone on ANA was analyzed using one-way ANOVA.
The Bonferroni method was used to correct for multiple comparisons using ANOVA. All analyses were conducted in SPSS (Ver. 22; IBM, Tokyo, Japan) and p < 0.05 was considered to be statistically significant.
Results
shows the changes in DIT that occurred over 120 min after the meal intake in each period of the day (morning, afternoon and night) under each of the three light conditions (DD, BD and BB). There was a significant effect of Period on DIT (p < 0.05), as well as a significant interaction between light conditions and time (p < 0.05), indicating that DIT significantly changed with time after the last meal, but the nature of this effect varied between light treatments. In addition, there was a significant diurnal rhythm of DIT for the BD treatment (Period, p < 0.05; ), with DIT tending to be higher in the afternoon than at night (p = 0.056), while such differences were not found for the DD and BB treatments ( and ). For the DD treatment, Time had a significant effect on DIT (p < 0.05), which means that changes in DIT following a meal were almost identical regardless of the time of day.
shows the AUCs for DIT over 2 h for each light treatment and period, and DIT and ANA representing differences between the values obtained 30 min before and after each meal. Period had a significant effect on AUC (p < 0.05). However, further analysis showed that AUC only had a tendency to differ between periods for the BD treatment (p = 0.053), with DIT tending to be higher in the afternoon than at night (p = 0.076), which matches the findings shown in . The AUCs in the morning, afternoon, and night were summed for each treatment to determine the overall effect of light exposure on DIT (total AUC). There were no significant differences in the total AUCs between the three light conditions.
Table 3. Diet-induced thermogenesis (DIT) and autonomic nerve activities (ANA) at different times of the day under different light conditions.
also compares the findings for DIT and the three measures of ANA (VLF, LF/HF and HF), based on differences between the values 30 min before and after each meal. VLF decreased significantly after the meal (Time, p < 0.05), while the change in HF after the meal differed between periods (Period × Time interaction, p < 0.05) due to the different behavior of HF in the afternoon compared with morning and night. There were no significant differences in LF/HF. DIT only tended to differ between periods (p = 0.071) and between afternoon and night (p = 0.087) for the BD treatment, again matching the previous findings (). By contrast, there was no significant difference in VLF, LF/HF or HF between light conditions. HF significantly differed between periods (p < 0.05) and was significantly higher in the morning than in the afternoon (p < 0.05), however, indicating that diurnal changes in HF were not affected by light conditions.
Discussion
A diurnal rhythm of DIT has previously been reported in the absence of light control, with DIT being significantly higher in the morning than in the late afternoon and at night (Morris et al. Citation2015; Romon et al. Citation1993). In the present study, a significant diurnal rhythm of DIT was only found with the BD treatment (), with DIT being higher at lunchtime than at dinner time. This finding clearly demonstrates that it is the light–dark cycle that induces the diurnal rhythm of DIT, and so, if people remain in an environment with little light change during a day (such as occurred with the DD and BB treatments), the diurnal rhythm becomes unclear. Therefore, since disruption of the circadian rhythm has been reported as having harmful effects on human health (Davis et al. Citation2001; Morikawa et al. Citation2005; Schernhammer et al. Citation2001), retaining a diurnal rhythm of DIT may be helpful for maintaining overall health and a healthy body mass.
In order to consider the total DIT during the course of a day under different light conditions, we summed the morning, afternoon, and night DIT values (, total AUC). This showed that there were no significant differences in DIT among the light treatments. Although it has been found that ALAN is positively correlated with obesity and hyperlipidemia (McFadden et al. Citation2014; Obayashi et al. Citation2013), the lack of difference between the BD and BB conditions in our study indicates that light exposure at night is unlikely to decrease DIT at night. Thus, the observed correlation between ALAN and obesity and hyperlipidemia may be attributed to circadian rhythm disruption rather than a decrease in DIT. However, although there was no significant difference in the total DIT, DIT was found to behave differently under the BD and BB treatments (). This may have been due to ALAN causing phase delays in the circadian rhythm (Khalsa et al. Citation2003). It should be noted that since DIT was measured for only 2 h in this study and increases in DIT can last for 5–8 h (Verboeket-van de Venne et al. Citation1996), measurements need to be taken over a longer period to determine the relationships between ALAN and phase delays in DIT, and DIT and body mass gains/losses.
Previous studies have reported that activation of SNS (LF/HF and VLF) plays a role in thermogenesis following meal ingestion. However, such a relationship was not detected in this study. On the other hand, there was a significant diurnal rhythm of HF, which exhibited much higher levels in the morning than in the afternoon (). This supports the previous findings of Vandewalle et al. (Citation2007), who showed that HF peaked in the early morning and fell to a low level in the afternoon and night under constant routines with controlled light, food, activity, and sleep. Our results showed that the diurnal change in HF was not significantly affected by light condition, indicating that the circadian rhythm in ANA may have a strong impact on ANA measurements, and so it is necessary to consider the time of day when taking these. It should be noted, however, that the participants in our study remained in a sitting position for most of the course of the experiments, which may have enhanced PNS above levels that would be encountered during normal daily life that involves exercise and physical activity.
To clarify the mechanism by which light affects DIT, additional studies are required that use more precise methods to measure ANA, since this is known to vary with the time of day, and is easily affected by various external and internal changes. Also, what mediated the diurnal change of DIT, modification of metabolism and/or heat loss, needs to be clarified. In addition, future studies could consider different routes for transmitting light information from SCN to peripheral clocks, such as hormonal regulation. For example, the melatonin signal changes the plasma levels of insulin and leptin (Nishida et al. Citation2003; Wolden-Hanson et al. Citation2000), which are associated with the risk of diabetes (Lyssenko et al. Citation2009). In addition, brown adipose tissue (BAT) has the potential to mediate light effects on DIT, as it affects whole-body metabolism, and may alter insulin sensitivity (Lowell et al. Citation1993; Yang et al. Citation2003) and modify susceptibility to mass gain (Almind et al., Citation2007). It has been suggested that BAT plays a role in DIT and that obesity is characterized by reduced thermogenesis in animals (Trayhurn Citation2017). However, it remains unclear whether melatonin and/or BAT are associated with energy expenditure in humans.
It should be noted that this study had some limitations. Firstly, the periods of light exposure differed with time of day, with the morning in particular possibly having been too short to affect the measurements of DIT and ANA. Secondly, all the subjects were young females who were moderately lean compared with the average female in Japan, which may have led to higher DIT values than other members of the population (de Jonge and Bray Citation1997). Finally, the experiments were performed throughout the year, and so seasonal changes in DIT may have affected the results. Seasonal variation in unabsorbed dietary carbohydrate has been reported in Japanese subjects (Tsumura et al. Citation2005) and so seasonal variation in DIT may need to be considered.
Our study demonstrated that light exposure affects DIT and so a diminished light–dark cycle in our life leads to disruption of the diurnal rhythm of metabolism. Therefore, retention of a normal light–dark cycle may be a recommended course of action for overall health maintenance.
Declaration of interest statement
The authors report no conflicts of interest.
Funding
This work was supported by JSPS KAKENHI Grant Number JP26440262.
Additional information
Funding
References
- Acheson KJ, Ravussin E, Wahren J, Jéquier E. 1984. Thermic effect of glucose in man. Obligatory and facultative thermogenesis. J Clin Invest. 74:1572–80. doi:10.1172/JCI111573.
- Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. 2007. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci USA. 104:2366–71. doi:10.1073/pnas.0610416104.
- Cagampang FR, Bruce KD. 2012. The role of the circadian clock system in nutrition and metabolism. Br J Nutr. 108:381–92. doi:10.1017/S0007114512002139.
- Davis S, Mirick DK, Stevens RG. 2001. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 93:1557–62. doi:10.1093/jnci/93.20.1557.
- de Jonge L, Bray GA. 1997. The thermic effect of food and obesity: a critical review. Obes Res. 5:622–31. doi:10.1002/j.1550-8528.1997.tb00584.x.
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 107:18664–69. doi:10.1073/pnas.1008734107.
- Froy O, Miskin R. 2010. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY). 2:7–27. doi:10.18632/aging.100116.
- Hirota N, Sone Y, Tokura H. 2003. Effect of evening exposure to dim or bright light on the digestion of carbohydrate in the supper meal. Chronobiol Int. 20:853–62. doi:10.1081/CBI-120024216.
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. 2003. A phase response curve to single bright light pulses in human subjects. J Physiol. 549:945–52. doi:10.1113/jphysiol.2003.040477.
- Klerman EB. 2005. Clinical aspects of human circadian rhythms. J Biol Rhythms. 20:375–86. doi:10.1177/0748730405278353.
- Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, Buijs RM. 2003. Hypothesis: shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes. 52:2652–56. doi:10.2337/diabetes.52.11.2652.
- Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. 1993. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 366:740–42. doi:10.1038/366740a0.
- Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, et al. 2009. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 41:82–88. doi:10.1038/ng.288.
- Matsumoto T, Miyawaki C, Ue H, Kanda T, Yoshitake Y, Moritani T. 2001. Comparison of thermogenic sympathetic response to food intake between obese and non-obese young women. Obes Res. 9:78–85. doi:10.1038/oby.2001.10.
- Matsumoto T, Miyawaki T, Ue H, Kanda T, Zenji C, Moritani T. 1999. Autonomic responsiveness to acute cold exposure in obese and non-obese young women. Int J Obes Relat Metab Disord. 23:793–800. doi:10.1038/sj.ijo.0800928.
- McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. 2014. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the breakthrough generations study. Am J Epidemiol. 180:245–250. pii: kwu117.
- Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. 2005. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health. 31:179–83. doi:10.5271/sjweh.867.
- Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. 2015. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring). 23:2053–58. doi:10.1002/oby.21189.
- Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 43:215–24. doi:10.1111/jpi.2007.43.issue-3.
- Nishida S, Sato R, Murai I, Nakagawa S. 2003. Effect of pinealectomy on plasma levels of insulin and leptin and on hepatic lipids in type 2 diabetic rats. J Pineal Res. 35:251–56. doi:10.1034/j.1600-079X.2003.00083.x.
- Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. 2014. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol Int. 31:394–400. doi:10.3109/07420528.2013.864299.
- Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. 2013. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 98:337–44. doi:10.1210/jc.2012-2874.
- Portaluppi F, Smolensky M, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27:1911–29. doi:10.3109/07420528.2010.516381.
- Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. 1993. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr. 57:476–80.
- Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE. 2006. Urinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res. 40:116–24. doi:10.1111/jpi.2006.40.issue-2.
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 93:1563–68. doi:10.1093/jnci/93.20.1563.
- Sone Y, Hyun KJ, Nishimura S, Lee YA, Tokura H. 2003. Effects of dim or bright-light exposure during the daytime on human gastrointestinal activity. Chronobiol Int. 20:123–33. doi:10.1081/CBI-120017688.
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. 2001. Entrainment of the circadian clock in the liver by feeding. Science. 291:490–93. doi:10.1126/science.291.5503.490.
- Taheri S. 2006. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 91:881–84. doi:10.1136/adc.2005.093013.
- Tappy L. 1996. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 36:391–97. doi:10.1051/rnd:19960405.
- Trayhurn P. 2017. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 134:62–70. pii: S0300-9084.
- Tsumura Y, Hirota N, Tokura H, Rutkowska D, Sone Y. 2005. Seasonal variation in amount of unabsorbed dietary carbohydrate from the intestine after breakfast in Japanese subjects. Chronobiol Int. 22:1107–19. doi:10.1080/07420520500398080.
- van Baak MA. 2008. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol Behav. 94:178–86. doi:10.1016/j.physbeh.2007.12.020.
- Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, Dijk DJ. 2007. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res. 16:148–55. doi:10.1111/j.1365-2869.2007.00581.x.
- Verboeket-van de Venne WP, Westerterp KR, Hermans-Limpens TJ, de Graaf C, van het Hof KH, Weststrate JA. 1996. Long-term effects of consumption of full-fat or reduced-fat products in healthy non-obese volunteers: assessment of energy expenditure and substrate oxidation. Metabolism. 45:1004–10. doi:10.1016/S0026-0495(96)90271-1.
- Waterhouse J, Reilly T, Atkinson G, Edwards B. 2007. Jet lag: trends and coping strategies. Lancet. 369:1117–29. doi:10.1016/S0140-6736(07)60529-7.
- Weir JB. 1949. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 109:1–9. doi:10.1113/jphysiol.1949.sp004363.
- Westerterp KR. 2004. Diet induced thermogenesis. Nutr Metab (Lond). 1:5. doi:10.1186/1743-7075-1-5.
- Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD. 2000. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 141:487–97. doi:10.1210/endo.141.2.7311.
- Yang X, Enerbäck S, Smith U. 2003. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res. 11:1182–91. doi:10.1038/oby.2003.163.
