ABSTRACT
Sleep and the sleep-wake rhythm are essential for children’s health and well-being, yet reference values are lacking. This study therefore aimed to assess actigraphic estimates of sleep and the 24-h sleep-wake rhythm, as well as 6-sulfatoxymelatonin (aMT6s) levels in healthy children of different age groups. Additionally, relationships between the outcomes and sex, highest parental educational level (as an indication of socioeconomic status (SES)), and body-mass-index (BMI) were explored. In this cross-sectional study, healthy Dutch children (2–18 years) wore an actigraph (GT3x) for 7 consecutive days, collected first-morning void urine and completed a sleep log and sociodemographic questionnaire. Actigraphically estimated sleep variables were sleep onset latency (SOL), sleep efficiency (SE), total sleep time (TST), and wake after sleep onset (WASO). Non-parametric sleep-wake rhythm variables were intradaily variability (IV); interdaily stability (IS); the activity counts and timing of the least active 5-h period (L5counts and midpoint) and of the most active 10-h period (M10 counts and midpoint); and the relative amplitude (RA), i.e. the ratio of the difference and the sum of M10 and L5 counts. Finally, creatinine-corrected aMT6s levels were obtained by isotope dilution mass spectrometry. Effects of age group (preschool 2–5 years/school-aged 6–12 years/teenager 13–18 years), sex, highest parental educational level and BMI (Z-scores) were explored. Ninety-four children participated, equally divided across age groups (53% boys). Teenagers slept less, but more efficiently, than younger children, while their 24 h sleep-wake rhythm was the least stable and most fragmented (likely due to fragmentation of daytime activity). Additionally, aMT6s levels significantly declined over the age groups. Children from highly educated parents had lower sleep efficiency, but a more stable sleep-wake rhythm. Finally, sex or increase in BMI was not associated with any of the outcomes in this study. In conclusion, this study provides reference values of healthy children across different age groups and different sociodemographic factors. In the future, this information may help to better interpret outcomes in clinical populations.
Introduction
Sleep is important for many health outcomes and essential for optimal childhood development. (El-Sheikh and Sadeh Citation2015) Short sleep duration in children is, for example, associated with overweight and obesity, poorer cognitive, executive and school performance, and behavioral problems (Spruyt Citation2019). Moreover, impaired sleep duration and impaired sleep quality are both related to poorer child psychosocial functioning. (Spruyt Citation2019)
In addition to sleep duration and quality, the circadian sleep-wake rhythm is also of importance. In adults, circadian disruption has been linked to several negative consequences, such as an increased risk of cardiovascular and psychiatric disease, metabolic syndrome, diabetes, and cancer (Baron and Reid Citation2014; Eismann et al. Citation2010; Mitchell et al. Citation2017), in addition to negative effects on cognitive functioning (Luik et al. Citation2015) and fatigue (Pedersen et al. Citation2017). Little is known about the pediatric sleep-wake rhythm (Mitchell et al. Citation2017). However, sleep-wake patterns are not necessarily well correlated with sleep variables, but instead provide additional information (Mitchell et al. Citation2017). For instance, the ability to maintain a robust, non-fragmented rhythm can be seen as an independent health indicator (Garaulet et al. Citation2017; Luik et al. Citation2013; Rogers et al. Citation2014).
Sleep-wake patterns can also provide some insight into biological circadian alignment; however, they are not directly interchangeable with physiological systems (Hofstra and de Weerd Citation2008). The sleep-wake rhythm is synchronized by zeitgebers, the most important being the light-dark cycle. A decrease in light intensity stimulates the secretion of melatonin by the pineal gland (Buber et al. Citation2016; Lewy et al. Citation2010). Melatonin production is not influenced by other external influences than light, unlike other circadian rhythm markers, and is therefore a powerful biomarker in the assessment of circadian dysregulation (Checa-Ros et al. Citation2017; Mirick and Davis Citation2008).
In children, sleep and the sleep-wake rhythm are both preferably measured by actigraphy (Smith et al. Citation2018a, Citation2018b). Actigraphy can be used to calculate several quantitative and qualitative sleep variables, such as sleep onset latency, total sleep time, wake after sleep onset and sleep efficiency. Additionally, there are different approaches to describe the sleep-wake rhythm via actigraphy: cosinor or nonparametric methods (Mitchell et al. Citation2017). Cosinor analysis models the periodicity of the sleep-wake rhythm. The method is parametric; hence the data represent a normal distribution and fit a symmetrical cosinusoid (Calogiuri et al. Citation2013). This means that the rhythm should fit into a pattern with a 12:12-h sleep-wake cycle, with a gradual increase in activity from midnight to mid-day, followed by a gradual decrease in activity until midnight. However, the sleep-wake rhythm is far from sinusoidal. The nonparametric method does not make assumptions about the temporal pattern of the rhythm. Therefore, this method has been proposed as the preferred method of use (Calogiuri et al. Citation2013; Dowling et al. Citation2005).
Melatonin metabolites can be measured in blood, saliva, or urine samples. Melatonin levels show inter-individual variability but intra-individual stability (Ardura et al. Citation2003; Lewy et al. Citation2010). Melatonin is primarily secreted at night, however obtaining nocturnal samples is inconvenient, especially in (young) children. First morning urine level of the melatonin metabolite 6-sulfatoxymelatonin (aMT6s) is highly correlated with the total nocturnal plasma melatonin and the measurement is noninvasive, in contrast to blood or saliva samples. Therefore, measuring first morning aMT6s levels is an appealing alternative to assess the circadian rhythm in children (Cook et al. Citation2000; Graham et al. Citation1998; Mirick and Davis Citation2008). In young children, overnight samples can be collected by the use of diaper gazes or urinary bags. Both overnight samples and first-morning voids adequately represent circulating blood melatonin levels. (Mirick and Davis Citation2008)
Pediatric reference values of the sleep wake-rhythm and melatonin levels are lacking. This study therefore aims to assess these outcomes in healthy children across different age groups. Additionally, the relationships with other sociodemographic variables are explored, i.e. sex, highest attained parental educational level (indication of socioeconomic status (SES)), and body mass index (BMI).
Materials and methods
Participants and procedure
Healthy children aged between 2 and 18 years were recruited through snowball sampling and word of mouth referrals to participate in a cross-sectional study which included actigraphy, a sleep log, aMT6s assessment, and a short survey. Children were not eligible if they visited a health-care provider for sleep disturbances in the preceding 3 months, used any type of sleep medication (including melatonin), or had a medical condition that could potentially affect sleep or the circadian rhythm (epilepsy, blindness, exacerbation of asthma or severe eczema). Additionally, children were excluded if they or their parents were insufficiently fluent in Dutch, causing inability to complete the sleep log and general questionnaire.
Recruitment took place through the professional and social networks of the research team, from Summer 2017 until Spring 2018. Parents of children below 16 years of age and children aged 12 years and over provided informed consent prior to participation. The Institutional Review Board of the VU University medical center Amsterdam approved this study.
Measures
Actigraph
Participants were fitted with a wrist-worn actigraph (ActiGraph wGT3X-BT, Pensacola, FL, USA) for 7 consecutive days. They were requested to wear the actigraph continuously, except while showering or swimming. The actigraph could be placed on the dominant or non-dominant wrist, according to the child’s preference, since previous literature has shown that actigraphic estimates of the dominant and non-dominant wrist yield similar results (Sadeh et al. Citation1994). The child (or parent in case of young participants) kept a sleep-log to document bedtimes, awaking times, naps, and the times that the actigraph was not worn. Actigraphs were set for 1-min epochs.
Actigraphy data were processed with ActiLife version 6.13.3. After visual inspection of the data, the following sleep outcomes were generated by use of the validated Sadeh algorithm (Sadeh et al. Citation1994), and based on the participants’ sleep logs (bedtime): total sleep time (TST), defined as the number of minutes scored asleep during the time spent in bed; sleep onset latency (SOL), defined as the number of minutes between bedtime and the first minute scored as sleep; sleep efficiency (SE), defined as the ratio between TST and time spent in bed; and wake after sleep onset (WASO), defined as the number of minutes awake after sleep onset (Sadeh Citation2011). Based on sleep logs and visual inspection of the data, invalid data were identified and removed from further analysis. Sleep outcomes were only constructed if there were valid data of at least five nights (Acebo et al. Citation1999). To assess the sleep-wake rhythm, raw actigraphy data were extracted from ActiLife and seven non-parametric variables were calculated, based on Van Someren et al. and Gonçalves et al. (Gonçalves et al. Citation2014; Van Someren et al. Citation1999). Intradaily variability (IV) is an estimate of the 24-h rest-activity rhythm and indicates the fragmentation of the rhythm. IV is calculated as “the ratio of the mean squares of the difference between all successive hours (first derivative) and the mean squares around the grand mean (overall variance)” (Van Someren et al. Citation1999, 509). IV ranges between 0 and 2, a higher IV indicates a more fragmented rhythm (Mitchell et al. Citation2017). Interdaily stability (IS) is an estimate of the stability of the rhythm and describes the synchronization to environmental zeitgebers (Gonçalves et al. Citation2014; Van Someren et al. Citation1999). IS is calculated as “the ratio between the variance of the average 24-hour pattern around the mean and the overall variance” (Van Someren et al. Citation1999, 509). IS ranges between 0 and 1, wherein 1 signifies a perfect synchronization. Exact formulas to calculate IS and IV are provided in the research article of Van Someren et al. (Citation1999). An Excel spreadsheet for calculations is available from the authors upon request. L5 counts and L5 onset reflect activity counts and timing, respectively, of the least active consecutive 5 h of the day. M10 counts and M10 onset reflect activity counts and timing of the most active 10 h. The relative amplitude (RA) is the ratio of the difference and the sum of M10 and L5 counts. RA ranges between >0 and <1 wherein a higher amplitude indicates a bigger difference between the least and the most active period during the day, hence a better sleep-wake rhythm (Mitchell et al. Citation2017).
If non-wear time exceeded 3 consecutive hours (Luik et al. Citation2013), a 24-h data period from the non-wear starting time onwards was removed from further analysis. A total number of three valid days were required for analysis (Mitchell et al. Citation2017).
Melatonin
Patients were asked to collect the first-morning void urine on the last day of the one-week measurement. If the child was not yet toilet trained, parents were instructed to apply gauzes in the child’s diaper before the child went to bed and put the wet gauzes in the provided container in which parents were instructed to place the child’s urinary sample next morning. All samples were stored at −80 degrees Celsius until analysis. The aMT6s levels were analyzed by isotope dilution mass spectrometry using online solid-phase extraction in combination with liquid chromatography and tandem mass spectrometry (LC–MS/MS), in the Department of Laboratory Medicine, University Medical Center Groningen. Intra-assay imprecision was below 2.5% and inter-assay imprecision was below 5.4%. The quantification limit for aMT6s was determined at 0.2 nmol/L. Concentrations of aMT6s were adjusted for urinary creatinine levels (Mirick and Davis Citation2008; Schernhammer et al. Citation2006).
Survey
A short survey was used to collect information on the child’s and parents’ sociodemographic variables (age, sex, country of birth, highest attained educational level of the parents), child’s comorbidities and use of medication, and child’s weight and height.
Statistical analysis
We aimed to include 30 children in each age group based on previous studies that used actigraphy to measure sleep or assessed urinary melatonin in healthy children (Belanger et al. Citation2013; Bojkowski and Arendt Citation1990; Paavonen et al. Citation2002; Weiss et al. Citation2010).
Descriptive statistics (mean ± SD or median (min-max)) were used to report on sociodemographic characteristics of the study population and outcomes (sleep, sleep-wake rhythm variables, and aMT6s levels).
These outcomes were also described separately for the three age groups – preschoolers aged 2–5 years, schooled children aged 6–12 years and teenagers aged 13–18 years–, since sleep and sleep-wake patterns change during normal childhood development (Marco et al. Citation2012; Ophoff et al. Citation2018). Differences between age groups were assessed with ANOVA (including a post-hoc test) or Kruskal–Wallis, depending on the distribution of the data. Moreover, sleep and sleep-wake rhythm outcomes were described separately for boys and girls. Differences between sexes were assessed with an independent t-test or Mann–Whitney U test. Additionally, differences in the outcomes regarding parental educational level (low-middle versus high, according to Statistics Netherlands (CBS)) (Centraal Bureau voor de Statistiek Citation2016) were analyzed with linear regression, corrected for the age group (due to the unequal distribution of educational level across ages). Linear regression was only performed if all assumptions were met. Finally, the relationship between the outcome variables and BMI (standardized Z-scores corrected for sex and exact age at date of measurement) (World Health Organization Citation2019) was also analyzed with linear regression. Exact Z-scores were calculated with the LMS values of the Z-score tables of the World Health Organization (Group WMGRS Citation2006). If linearity could not be assumed, BMI was categorized as underweight (Z-score ≤ −1), normal weight (Z-score between −1 and 1) and overweight (Z-score ≥1) and dummy variables were created.
Due to little variation in ethnic background and a low prevalence of comorbidities and use of medication, differences in outcomes in these groups could not be analyzed, but were included in the description of the study sample.
IBM® SPSS® Statistics 26 was used for all statistical analyses. Two-sided p-values of <0.05 were considered statistically significant.
Results
Study population
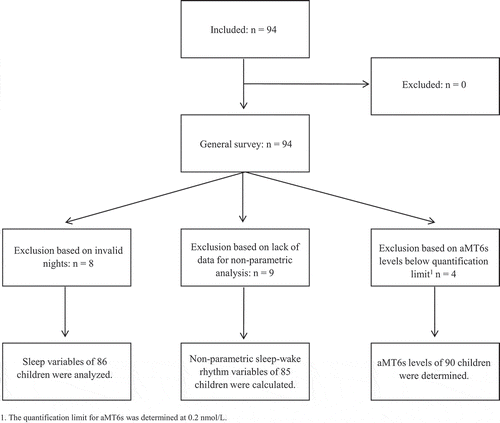
In total, 94 children of different ages (32% preschoolers, 35% school-aged, 33% teenagers) participated in this study, of whom 44 girls (47%) and 50 boys (53%) (equally divided across age groups). A flowchart of the study population is provided in and children’s characteristics are described in . Reported comorbidities (6.4%) included mild eczema, congenital heart disease, Attention Deficit Disorder (ADD), mild asthma, rheumatism, and clubfoot. Additionally, the reported use of medication (9.6%) included xylomethazoline, triamcinolonacetonide, inhalation corticosteroids, naproxen, and the contraceptive pill. These children were not excluded from this study, because there were no parent-reported sleep difficulties.
Table 1. Descriptive statistics of the study population (N = 94).
Sleep variables
There were enough valid nights of actigraphy in 86 children. The overall SE was 78 ± 6.9%, with significantly higher SE in teenagers (82 ± 8.4%) compared to preschool (76 ± 4.8%) and school-age children (77 ± 5.8%). TST, SOL, and WASO were significantly lower in teenagers compared to preschool and school-age children. There were no significant differences between boys and girls. The sleep efficiency of children of low- to middle-educated parents was better than in children from highly educated parents, even after correction for age group (difference 3%, p = .049). Results on sleep variables are summarized in –. Additionally, no significant associations were found between sleep variables and an increase in BMI (data not shown).
Table 2. Sleep variables, by age group.
Table 3. Sleep variables, by sex.
Table 4. Sleep variables, by parental education.
Non-parametric sleep-wake rhythm variables
Sleep-wake rhythm variables could be calculated for 85 children. Sleep-wake rhythm variables differed across age groups: IS, M10 counts and RA decreased in older children, whereas IV increased. There were no differences in sleep-wake variables between boys and girls. Regarding parental educational level, IS was significantly higher in children from highly educated parents compared to children from low- to middle-educated parents (corrected for age group). See –. No significant associations were found between sleep-wake rhythm variables and BMI (data not shown).
Table 5. Non-parametric sleep-wake rhythm variables, by age group.
Table 6. Non-parametric sleep-wake rhythm variables, by sex.
Table 7. Non-parametric sleep-wake rhythm variables, by parental education.
Urinary 6-sulfatoxymelatonin (aMT6s) levels
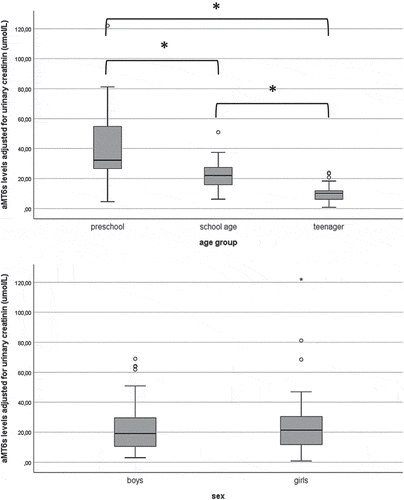
All children returned urine samples to determine aMT6s levels; however, the urine of four participants had creatinine or aMT6s levels below the quantification limit. There were significant differences in aMT6s levels between all age groups, with highest levels in toddlers (32.2 (range 4.6–122.0) µmol/molcreat), lowest levels in teenagers (10.2 (0.8–23.9) umol/molcreat) and in-between levels in school-aged children (22.0 (6.2–50.9) umol/molcreat). There were no significant differences between boys (19.1 (3.0–69.0) umol/molcreat) and girls (21.4 (0.8–122.0) umol/molcreat). See . Additionally, no significant associations were found between parental education and aMT6s levels (corrected for age group). Finally, no significant association was found between BMI and aMT6s levels (data not shown).
Discussion
In the current study, we provide reference values of sleep, the sleep-wake rhythm and aMT6s levels of healthy children, and assessed the relationships between these outcomes and several demographic characteristics.
First, we investigated several sleep variables. We can conclude that, in comparison with younger children, older children have a shorter SOL, a decreased WASO, and hence an increased SE. This might be explained by a higher sleep pressure in the teenager group, caused by later bedtimes but (too) early wake-up times – as observed in the sleep diaries and supported by previous literature (Carskadon et al. Citation2004). Regarding sex, we did not find differences in sleep outcomes between boys and girls. This is consistent with previous findings of a systematic review on sleep in children aged 4–12 years (Belmon et al. Citation2019). In contrast, sleep differences between adult men and women have been well recognized. These differences are probably related to hormonal factors and become apparent during puberty (Mallampalli and Carter Citation2014). We did not find gender-specific differences in any of the sleep variables, and this might be due to the fact that our sample included too small a number of post-pubertal girls. Regarding parental educational level, we found that SE was better in children from low- to middle-educated parents compared to children from highly educated parents, even after correction for a potential confounding of age. This is in contrast with findings from previous studies, which found that children from higher SES families had better SE (Bagley et al. Citation2015; El-Sheikh et al. Citation2013). However, highest parental educational level only gives an indication of SES; we could not include employment or family income. Since we had no information on this, results should be interpreted with caution. Additionally, it might be that our study findings were influenced by the over-representation of highly educated families in our sample. Regarding BMI, we found no associations between an increased BMI and any of the sleep variables, although it is known that sleep is related to obesity (Li et al. Citation2017). However, only a few children in our sample were overweight, and only one girl met the criteria for obesity (Z-score ≥2), which can explain the inconsistency.
Regarding sleep duration, the average actigraphy-measured TST of children in our study population (8.6 h, 8.3 h, and 7.2 h, respectively, for preschoolers, school-aged children, and teenagers) was lower than the recommended sleep duration per age group (approximately 10–13 h for preschoolers, 9–11 h for school-aged children and 8–10 h for teenagers) (Hirshkowitz et al. Citation2015). It could well be that children nowadays do not meet the recommended sleep duration (Matricciani et al. Citation2012). On the other hand, our findings might also be explained by an overestimation of wakefulness during sleep (WASO) by newer actigraph devices, especially in young children (Meltzer et al. Citation2012). Proper sleep assessment is a function of both the hardware and interpretative algorithm technologies (Haghayegh et al. Citation2019, Citation2020). A previous study in 13 older children (10–14 years) that used the same actigraph (ActiGraph wGT3X-BT, Pensacola, FL, USA) and algorithm (Sadeh) as we did also show an underestimation of TST by an average of 49 min, an overestimation of WASO by an average of 59 min, and hence an 11.6% lower SE, compared to the golden standard (PSG) (Quante et al. Citation2018). Thus, it is important to measure sleep the same way as was done to generate reference values when studying other pediatric populations, in order to justify a comparison. Additionally, it is recommended to use the same actigraph device across populations, since different devices might elucidate different results.
Second, we examined non-parametric sleep-wake rhythm variables. The sleep-wake rhythm variables were highly dependent on age, similar to the sleep variables. Older children had a less stable rhythm than younger children. It is known from the literature that the sleep-wake rhythm changes during adolescence, due to both behavioral and biological factors (Carskadon et al. Citation2004). Timing of sleep tends to delay, which causes problems with insufficient sleep on week (school) days and ‘catch-up’ sleep on weekend days. This could explain the more instable pattern of the rhythm that we found.
Additionally, the sleep-wake rhythm of older children was more fragmented than the rhythm of younger children; hence, transitions between rest and activity appear more frequently. This is consistent with previous research (Mitchell et al. Citation2017) and with findings that teenagers are (periodically) inactive during the day, in our sample reflected by distinctly lower M10 counts. Higher fragmentation could also be caused by more frequent nighttime awakenings. Yet, since teenagers comprised the group with the highest SE and lowest WASO, this might not be the most likely explanation for an increased fragmentation in this population.
We did not find differences in sleep-wake rhythm variables between boys and girls, in contrast to the report of Mitchell et al., which found that girls had a less fragmented rhythm compared to boys (Mitchell et al. Citation2017). Future research regarding the influence of sex on the sleep-wake rhythm is warranted.
Regarding parental education, we observed a more stable rhythm in children from highly educated parents, compared to that of low- to middle-educated parents. It might be that higher educated parents pay more attention to healthy sleep hygiene (Bathory and Tomopoulos Citation2017), which is crucial in the synchronization of the sleep-wake rhythm. However, as stated before, conclusions regarding parental education in our study should be drawn with caution. Finally, regarding BMI, we found no associations between an increased BMI and any of the sleep-wake variables, which could be explained by the low prevalence of overweight/obese children in our sample.
Third, we investigated aMT6s levels, which gives information on the alignment of the circadian time structure. We found strong differences among age groups, with a significant decrease in aMT6s levels in older children. This might reflect the less stable sleep-wake patterns that we found in the teenager group. Additionally, there are some reports of a decline in melatonin levels from young childhood to adolescence; yet, this probably indicates an increase in body size rather than a real decline in melatonin production (Bojkowski and Arendt Citation1990; Griefahn et al. Citation2003; Wada et al. Citation2013). We did not find differences regarding sex, parental educational level, or BMI. To date, little data had been published on aMT6s levels of healthy children, so the specific clinical implications warrant further research.
Limitations
The first limitation in this research is the procedure of recruiting subjects. They were recruited through snowball sampling and word of mouth referrals, whereby a completely representative sample of the Dutch population could not be generated. For example, there was barely any variance in ethnicity and most of the parents were highly educated. Due to this overrepresentation of highly educated families, we needed to dichotomize educational level into low-middle and high. Besides, highest parental educational level was the only information that we had on children’s SES, which is a limitation as well.
Second, several children and parents perceived difficulties in reporting on their (child’s) exact sleep and wake-up times and wearing times of the actigraph, as confirmed by occasionally observed discordance between actigraphic activity counts and patient-reported behavior. We decided to rely on the information recorded in the sleep diaries to avert incorrect assumptions. However, this might have led to some bias in our results, for example, an over- or underestimation of SOL and SE, since these variables are particularly dependent on patient-reported bedtime.
Conclusions and future directions
In this research, we assessed sleep variables, the sleep-wake rhythm, and aMT6s levels in healthy children. The evaluation of the sleep-wake rhythm is complementary to sleep variables because it assesses not only activity during sleep but during the entire day; hence, it provides a more comprehensive view. In addition, in contrast to cosinor analysis, the use of nonparametric analysis evaluates the sleep-wake rhythm without making unjustifiable assumptions about the sinusoidal pattern of the rhythm. Since we have provided reference values of healthy children, future studies can use our method to assess sleep-wake patterns in pediatric illness populations, taking into account (possible) differences caused by age, sex, parental education, and BMI.
Declaration of interest statement
The authors report no conflict of interest.
Data availability statement
The data of this study are available from the corresponding author (RvL), upon reasonable request.
Additional information
Funding
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, Carskadon MA. 1999. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 22(1):95–103. DOI:10.1093/sleep/22.1.95.
- Ardura J, Gutierrez R, Andres J, Agapito T. 2003. Emergence and evolution of the circadian rhythm of melatonin in children. Horm Res Paediatr. 59(2):66–72. DOI:10.1159/000068571.
- Bagley EJ, Kelly RJ, Buckhalt JA, El-Sheikh M. 2015. What keeps low-SES children from sleeping well: the role of presleep worries and sleep environment. Sleep Med. 16(4):496–502. DOI:10.1016/j.sleep.2014.10.008.
- Baron KG, Reid KJ. 2014. Circadian misalignment and health. Int Rev Psychiatry. 26(2):139–154. DOI:10.3109/09540261.2014.911149.
- Bathory E, Tomopoulos S. 2017. Sleep regulation, physiology and development, sleep duration and patterns, and sleep hygiene in infants, toddlers, and preschool-age children. Curr Probl Pediatr Adolesc Health Care. 47(2):29–42. DOI:10.1016/j.cppeds.2016.12.001.
- Belanger ME, Bernier A, Paquet J, Simard V, Carrier J. 2013. Validating actigraphy as a measure of sleep for preschool children. J Clin Sleep Med. 9(7):701–706. DOI:10.5664/jcsm.2844.
- Belmon LS, van Stralen MM, Busch V, Harmsen IA, Chinapaw MJM. 2019. What are the determinants of children’s sleep behavior? A systematic review of longitudinal studies. Sleep Med Rev. 43:60–70. DOI:10.1016/j.smrv.2018.09.007.
- Bojkowski CJ, Arendt J. 1990. Factors influencing urinary 6-sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin Endocrinol. 33(4):435–444. DOI:10.1111/j.1365-2265.1990.tb03882.x.
- Buber A, Cakaloz B, Isildar Y, Unlu G, Bostanci HE, Aybek H, Herken H. 2016. Increased urinary 6-hydroxymelatoninsulfate levels in attention deficit hyperactivity disorder diagnosed children and adolescent. Neurosci Lett. 617:195–200. DOI:10.1016/j.neulet.2016.02.016.
- Calogiuri G, Weydahl A, Carandente F. 2013. Methodological issues for studying the rest–activity cycle and sleep disturbances: a chronobiological approach using actigraphy data. Biol Res Nurs. 15(1):5–12. DOI:10.1177/1099800411416224.
- Carskadon MA, Acebo C, Jenni OG. 2004. Regulation of adolescent sleep. Ann NY Acad Sci. 1021:276–291.DOI:10.1196/annals.1308.032.
- Centraal Bureau voor de Statistiek. 2016. Standaard Onderwijsindeling 2016 [Standard educational classification]. Den Haag/Heerlen, The Netherlands: Centraal Bureau voor de Statistiek [Statistics Netherlands] [accessed 2018 Aug 22]. https://www.cbs.nl/nl-nl/onze-diensten/methoden/classificaties/onderwijs-en-beroepen/standaard-onderwijsindeling–soi–/standaard-onderwijsindeling-2016.
- Checa-Ros A, Munoz-Hoyos A, Molina-Carballo A, Munoz-Gallego A, Narbona-Galdo S, Jerez-Calero A, Augustin-Morales MDC. 2017. Analysis of different melatonin secretion patterns in children with sleep disorders: melatonin secretion patterns in children. J Child Neurol. 32(12):1000–1008. DOI:10.1177/0883073817726680.
- Cook MR, Graham C, Kavet R, Stevens RG, Davis S, Kheifets L. 2000. Morning urinary assessment of nocturnal melatonin secretion in older women. J Pineal Res. 28(1):41–47. DOI:10.1034/j.1600-079x.2000.280106.x.
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ. 2005. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatrics. 17(2):221–236. DOI:10.1017/s1041610205001584.
- Eismann EA, Lush E, Sephton SE. 2010. Circadian effects in cancer-relevant psychoneuroendocrine and immune pathways. Psychoneuroendocrinology. 35(7):963–976. DOI:10.1016/j.psyneuen.2009.12.011.
- El-Sheikh M, Bagley EJ, Keiley M, Elmore-Staton L, Chen E, Buckhalt JA. 2013. Economic adversity and children’s sleep problems: multiple indicators and moderation of effects. Health Psychol. 32(8):849–859. DOI:10.1037/a0030413.
- El-Sheikh M, Sadeh A. 2015. Sleep and development: introduction to the monograph. Monogr Soc Res Child Dev. 80(1):1–14. DOI:10.1111/mono.12141.
- Garaulet M, Martinez-Nicolas A, Ruiz JR, Konstabel K, Labayen I, Gonzalez-Gross M, Marcos A, Molnar D, Widhalm K, Casajus JA, et al. 2017. Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: the HELENA study. Clin Nutr. 36(6):1558–1566. DOI:10.1016/j.clnu.2016.09.026.
- Gonçalves BS, Cavalcanti PR, Tavares GR, Campos TF, Araujo JF. 2014. Nonparametric methods in actigraphy: an update. Sleep Sci. 7(3):158–164. DOI:10.1016/j.slsci.2014.09.013.
- Graham C, Cook MR, Kavet R, Sastre A, Smith DK. 1998. Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 24(4):230–238. DOI:10.1111/j.1600-079x.1998.tb00538.x.
- Griefahn B, Bröde P, Blaszkewicz M, Remer T. 2003. Melatonin production during childhood and adolescence: a longitudinal study on the excretion of urinary 6-hydroxymelatonin sulfate. J Pineal Res. 34(1):26–31. DOI:10.1034/j.1600-079x.2003.02931.x.
- Haghayegh S, Khoshnevis S, Smolensky MH, Diller KR, Castriotta RJ. 2019. Accuracy of wristband fitbit models in assessing sleep: systematic review and meta-analysis. J Med Internet Res. 21(11):e16273. DOI:10.2196/16273.
- Haghayegh S, Khoshnevis S, Smolensky MH, Diller KR, Castriotta RJ. 2020. Performance assessment of new-generation fitbit technology in deriving sleep parameters and stages. Chronobiol Int. 37(1):47–59. DOI:10.1080/07420528.2019.1682006.
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, et al. 2015. National sleep foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 1(1):40–43. DOI:10.1016/j.sleh.2014.12.010
- Hofstra WA, de Weerd AW. 2008. How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav. 13(3):438–444. DOI:10.1016/j.yebeh.2008.06.002.
- Lewy A, Songer J, Yuhas K, Emens J. 2010. Circadian function and therapeutic potential of melatonin in humans. In: Encyclopedia of neuroscience. Oxford, UK: Elsevier Ltd, pp. 893–900.
- Li L, Zhang S, Huang Y, Chen K. 2017. Sleep duration and obesity in children: a systematic review and meta-analysis of prospective cohort studies. J Paediatr Child Health. 53(4):378–385. DOI:10.1111/jpc.13434.
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Ikram MA, Tiemeier H. 2015. Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep Med. 16(7):850–855. DOI:10.1016/j.sleep.2015.03.012.
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. 2013. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 30(10):1223–1230. DOI:10.3109/07420528.2013.813528.
- Mallampalli MP, Carter CL. 2014. Exploring sex and gender differences in sleep health: a society for women’s health research report. J Womens Health (Larchmt). 23(7):553–562. DOI:10.1089/jwh.2014.4816.
- Marco CA, Wolfson AR, Sparling M, Azuaje A. 2012. Family socioeconomic status and sleep patterns of young adolescents. Behav Sleep Med. 10(1):70–80. DOI:10.1080/15402002.2012.636298.
- Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. 2012. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 129(3):548–556. DOI:10.1542/peds.2011-2039.
- Meltzer LJ, Walsh CM, Traylor J, Westin AM. 2012. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 35(1):159–166. DOI:10.5665/sleep.1608.
- Mirick DK, Davis S. 2008. Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomarkers Prev. 17(12):3306–3313. DOI:10.1158/1055-9965.EPI-08-0605.
- Mitchell JA, Quante M, Godbole S, James P, Hipp JA, Marinac CR, Mariani S, Cespedes Feliciano EM, Glanz K, Laden F. 2017. Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int. 34(8):1042–1056. DOI:10.1080/07420528.2017.1337032.
- Ophoff D, Slaats M, Boudewyns A, Glazemakers I, Van Hoorenbeeck K, Verhulst S. 2018. Sleep disorders during childhood: a practical review. Eur J Pediatr. 177(5):641–648. DOI:10.1007/s00431-018-3116-z.
- Paavonen EJ, Fjällberg M, Steenari M, Aronen ET. 2002. Actigraph Placement and Sleep Estimation in Children. Sleep. 25(2):235–237. DOI:10.1093/sleep/25.2.235.
- Pedersen M, Ekstedt M, Smastuen MC, Wyller VB, Sulheim D, Fagermoen E, Winger A, Pedersen E, Hrubos-Strom H. 2017. Sleep-wake rhythm disturbances and perceived sleep in adolescent chronic fatigue syndrome. J Sleep Res. 26(5):595–601. DOI:10.1111/jsr.12547.
- Quante M, Kaplan ER, Cailler M, Rueschman M, Wang R, Weng J, Taveras EM, Redline S. 2018. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 10:13–20. DOI:10.2147/NSS.S151085.
- Rogers VE, Zhu S, Ancoli-Israel S, Hinds PS. 2014. Impairment in circadian activity rhythms occurs during dexamethasone therapy in children with leukemia. Pediatr Blood Cancer. 61(11):1986–1991. DOI:10.1002/pbc.25147.
- Sadeh A. 2011. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 15(4):259–267. DOI:10.1016/j.smrv.2010.10.001.
- Sadeh A, Sharkey KM, Carskadon MA. 1994. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 17(3):201–207. DOI:10.1093/sleep/17.3.201.
- Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE. 2006. Urinary 6‐sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res. 40(2):116–124. DOI:10.1111/j.1600-079X.2005.00285.x.
- Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. 2018a. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 14(7):1231–1237. DOI:10.5664/jcsm.7230.
- Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. 2018b. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 14(7):1209–1230. DOI:10.5664/jcsm.7228.
- Spruyt K. 2019. A review of developmental consequences of poor sleep in childhood. Sleep Med. 60:3–12. DOI:10.1016/j.sleep.2018.11.021.
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. 1999. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 16(4):505–518. DOI:10.3109/07420529908998724.
- Wada K, Nakamura K, Tamai Y, Tsuji M, Masue T, Watanabe K, Ando K, Nagata C. 2013. Associations of urinary 6-sulfatoxymelatonin with demographics, body mass, sex steroids, and lifestyle factors in preschool Japanese children. Ann Epidemiol. 23(2):60–65.DOI:10.1016/j.annepidem.2013.05.013.
- Weiss AR, Johnson NL, Berger NA, Redline S. 2010. Validity of activity-based devices to estimate sleep. J Clin Sleep Med. 6(4):336–342. DOI:10.5664/jcsm.27874.
- WHO Multicentre Growth Reference Study Group. 2006. Computation of centiles and Z-scores for length/height-for-age, weight-for-age, weight-for-length, weight-for-height and BMI-for-age. WHO child growth standards: methods and development. Geneva, Switzerland: World Health Organization; p. 301–304.
- World Health Organization. 2019. WHO child growth standards. https://www.who.int/childgrowth/standards/bmi_for_age/en/.