ABSTRACT
Rotating and permanent night shiftwork schedules typically result in acute and sometimes chronic sleep deprivation plus acute and sometimes chronic disruption of the circadian time structure. Immune system processes and functionalities are organized as circadian rhythms, and they are also strongly influenced by sleep status. Sleep is a vital behavioral state of living beings and a modulator of immune function and responsiveness. Shiftworkers show increased risk for developing viral infections due to possible compromise of both innate and acquired immunity responses. Short sleep and sleep loss, common consequences of shiftwork, are associated with altered integrity of the immune system. We discuss the possible excess risk for COVID-19 infection in the context of the common conditions among shiftworkers, including nurses, doctors, and first responders, among others of high exposure to the contagion, of sleep imbalance and circadian disruption.
Abbreviations
ACE2: Angiotensin-converting enzyme 2; APC: Antigen.-presenting .cells; CCL: Chemokine (C-C motif) ligand; CD+: .Adhesion molecule expression; COVID-19: 2019 coronavirus disease; DCs: Dendritic cells; GH: Growth hormone; HPA: Hypothalamic-pituitary-adrenal; HSF: Heat shock factor; HSP70: Heat shock protein 70; HSP90: Heat shock protein 90; IL: Interleukin; INFγ: Interferon-gamma; LT/LB: T/B lymphocytes; MHC: Major histocompatibility complex; NK: Natural .killer; RAAS: renin–angiotensin–aldosterone system; SARS: .Severe acute respiratory syndrome; SCN: Suprachiasmatic nucleus;SD: Sleep deprivation; SNS: Sympathetic nervous system; Th1/Th2: T helper lymphocytes 1/2; TLR2/TLR4: Toll-like receptor 2/4; TNF-α: Tumor .necrosis .factor alpha; VEGF: Vascular endothelial growth factor
COVID-19 and shiftwork
Coronaviruses are RNA viruses broadly distributed in humans and other mammals (Richman et al. Citation2017). Previously reported human coronaviruses, such as 229E (αHCoV229E), NL63 (HCoV-NL63), OC43 (βHCoV-OC43), and HKU1 (HCoV-HKU1), primarily affected the upper respiratory tract (Chan et al. Citation2012; Tang et al. Citation2017). The new 2019 coronavirus (COVID-19) epidemic (Wang et al. Citation2020), like that of the earlier 2003 severe acute respiratory syndrome (SARS) outbreak, is a beta-coronavirus that can be spread to humans through intermediate hosts such as bats (Paules et al. Citation2020). Human-to-human transmission has been observed via virus-laden respiratory droplets (Chen et al. Citation2020), and severe cases can lead to cardiac injury, respiratory failure, acute respiratory distress syndrome, and death (Holshue et al. Citation2020).
Viruses induce the stress–response reaction in infected cells (Cymerys et al. Citation2009). HSPs (Heat shock proteins) are known as stress proteins that play important roles in physiological activities and also act as molecular chaperonage that stabilizes unfolded protein during the stage of protein synthesis under the influence of stressors, thus, improving cell survival from degradation (Bolhassani and Agi Citation2019). HSPs, especially HSP70, are stimulated by viral infections leading to increase in viral gene expression (Kim and Oglesbee Citation2012). Moreover, when HSP70 is released from cells, it induces innate immune response through toll-like receptor 2/4 (TLR-2 and TLR-4) responses (Bolhassani and Agi Citation2019). Viruses do not inherently possess HSPs; therefore, they rely entirely on the host HSPs for viral protein folding. Thus, processes that regulate host stress proteins are likely targets of strategic manipulation by both invading viruses and already infected hosts (Lee et al. Citation2010).
HSP synthesis is critical for pathogen survival (Goulhen et al. Citation2003). Some viruses can induce the overexpression of HSPs in infected cells, and intensive virus replication is associated with high expression of some HPSs, especially Hsp70 and HSP90. Furthermore, CD11b+ and natural killer (NK) cells, which play major roles in the elimination of infectious agents, show up-regulation of HSP70 initial stages of infection – especially at the peak of virus replication and also show up-regulation of HSP90 during later stages of infection. HSP70 has been identified as a cellular interaction partner of the influenza virus ribonucleoprotein complex (Li et al. Citation2011). In this regard, HSP70 and HSP90 are observed in viral pneumonia and other viral infections (Jesse and Chung Citation2019).
Shiftwork is defined as work done outside the normal hours of the traditional workday, as exemplified by evening, night, early morning, and rotating shift schedules (Almeida and Malheiro Citation2016; National Sleep Foundation, N Citation2016) that are common in essential services (Costa et al. Citation2004). Shiftwork, particularly night work, due to alteration of the sleep-wake cycle from normal plus exposure to artificial light at night, interferes with and disrupts the normal circadian time structure, giving rise to possible psycho-physiological disturbances and compromised neuroimmune-endocrine homeostasis (Akerstedt, Citation1990; Knutsson Citation2003; Reinberg and Ashkenazi Citation2008; Schernhammer et al. Citation2001). The conclusion of the night shift corresponds, in reference to circadian time, with circadian rhythm-driven immune system changes (Kobayashi et al. Citation1996; XU et al. Citation1998), specifically, reduction in NK activity, for example, as observed in nurses (Kobayashi et al. Citation1996).
Sleep and immunomodulatory function
Human circadian rhythms are controlled by a neurological master clock, the suprachiasmatic nucleus (SCN), and also peripheral clocks of almost every cell, including immune system cells (Arjona and Sarkar Citation2006; Duguay and Cermakian Citation2009; Haimovich et al. Citation2010). The SCN thus enables all tissues and cells to anticipate and promptly respond to usual and predictable-in-time cyclic environmental changes and challenges, including pathogens (Labrecque and Cermakian Citation2015). Cells of the innate and adaptive immune system also show circadian expression, for example, as day-night variation in blood count, functioning of peripheral lymphoid organs, lymphocytic proliferation, and blood cytokine levels (Dimitrov et al. Citation2009; Lange et al. Citation2010; Steinman Citation2004; Young et al. Citation1995). In humans adhering to a normal daytime wake and nighttime sleep schedule, the trough of the circadian rhythm in T cell count precedes the peak time (morning) of the circadian rhythm in blood cortisol, while the phase relation these two rhythms in the early evening is reversed (Dimitrov et al. Citation2009; Lange et al. Citation2010), as further described below.
In association with the circadian system, sleep is known to regulate immune functions (Segerstrom and Miller Citation2004; Weibel et al. Citation1996). Sleep deprivation (SD) is related to substantial alteration of the immune system (Axelsson et al. Citation2013; Born et al. Citation1997; Dinges et al. Citation1994, Citation1995; Fondell et al. Citation2011; Irwin et al. Citation1994, Citation1996; Wilder-Smith et al. Citation2013), in particular, increased production of pro-inflammatory cytokines (Prather et al. Citation2009). SD in the amount of 50–64 h is associated with temporary increase in the activity of TCD4+ lymphocytes, CD8+, and NK (Dinges et al. Citation1994, Citation1995; Wilder-Smith et al. Citation2013). SD also leads to decreased activity of NK cells and CD16+, CD56+, CD57+, and Interleukin (IL)-2 levels (Axelsson et al. Citation2013; Fondell et al. Citation2011; Irwin et al. Citation1994, Citation1996), all being important for the host defense against viruses (Biron et al. Citation1999).
In insomniacs, who routinely experience SD, there is a change in Th1 and Th2 immune balance favoring the Th2 response, with decrease in secretion of interferon-gamma (IFN-γ) and IFN-γ/IL-4 (Sakami et al. Citation2002) and reduction of TCD3+, TCD4+, plus TCD8+ and total lymphocytes (Savard et al. Citation2003). Importantly, the infectivity of rhinovirus has been shown to vary with sleep status. For example, individuals assessed by wrist actigraphy and substantiated to sleep fewer than 6 h before exposure to the contagion were found to be four times more likely to become infected than those who slept more than 7 h (Prather et al. Citation2015). The effect of sleep on the magnitude of the immune response to a viral antigen (Hepatitis B virus) was also evaluated, finding that fewer than 6 h of sleep per night, assessed by sleep diary and wrist actigraphy, was associated with reduction of vaccine protection from the B virus (Prather et al. Citation2012). Importantly, adequate sleep after immunization against the Hepatitis A virus significantly doubled the number of specific Th1 cells, due to the adjuvant effect of slow-wave sleep (Lange et al. Citation2011).
Shiftworker’s sleep and the immune system
Sleep influences two major biological axes/systems that modulate the immune system: the hypothalamic-pituitary-adrenal (HPA) axis mediated by cortisol (D’Aurea et al. Citation2015; Leproult et al. Citation1997; Spiegel et al. Citation1999) and the sympathetic nervous system (SNS) mediated by catecholamines (Molina Citation2005). The mediators of both axes/systems cause reduction of pro-inflammatory cytokines (Barnes Citation1998; Hou et al. Citation2013) and migration and activity of immune cells (Leposavić et al. Citation2008; Sanders Citation2012). Under a night-oriented schedule similar to that of shiftworkers, cytokine release is partly altered in response to change in the sleep-wake cycle (Prather et al. Citation2015). Importantly, COVID-19 affected Chinese persons and revealed a history of chronic illness plus of poor sleep quality – indicated by a global PSQI (Pittsburgh Sleep Quality Index) score of 14, findings that not atypical of the Chinese workforce employed in 30 Chinese occupations (Huang et al. Citation2020).
It is well known that the circadian time structure substantially affects the pharmacokinetics – absortion, distribution, metabolism, and elimination – as well as the pharmacodynamics of medications according to the biological time of their administration (Smolensky et al. Citation2017). In the same way, the handling of and tolerance to chemical, physical, and biological agents, including infectious ones, can differ, often markedly, according to the circadian time of exposure (Smolensky et al. Citation2017). This can be explained by the staging of the circadian system, alone or in combination with the immune system, that based on past evolutionary determinants anticipate environmental challenges and additionally facilitate beneficial adjustment to changes associated with the pro-inflammatory state during nighttime sleep and anti-inflammatory state during daytime activity (Moldofsky Citation1995).
During nighttime sleep, pro-inflammatory hormones and cytokines are synchronized to facilitate the onset of adaptive immune responses, while during daytime activity, anti-inflammatory signals, hormones, and cytokines are supportive of immediate reactions to biological and other environmental challenges (Levi et al. Citation1991). Growth hormone (GH) and prolactin under the usual sleep-wake routine increase to highest levels during the night and decline to lowest levels during the waking state, and this temporal pattern is preserved even in the state of continuous wakefulness. However, in SD, the amplitude of these circadian rhythms is suppressed, such that peak values are attenuated. Additionally, TNF-α, IL-12, and dendritic cells (DCs) ordinarily show peak values during the night in those adhering to a normal sleep-wake routine, but also in those kept awake for 24 h, and without alteration of the period (24 h) of these rhythms. The peak time of the circadian rhythms of cortisol, epinephrine, norepinephrine, and IL10 occurs early in the morning, around the time of awakening from nighttime sleep. In situations of continuous wakefulness, epinephrine lacks a defined rhythm. The peak time of the rhythm in IL10 shifts to the nighttime. Cortisol and norepinephrine present peak value rhythms similar to sleep values, which Nadir values, for both, are higher under conditions of constant wakefulness (Lange et al. Citation2010).
Human beings are a diurnal active species; accordingly, the circadian time structure of humans is organized to efficiently support a routine of activity during the light phase of the day and sleep during the dark phase of the night (Skene and Arendt Citation2006). The circadian structure of shiftworkers when working a night shift of several days duration must be reorganized to accommodate the unnatural routine of activity during the dark phase and sleep during the light phase of the day. The necessary biological adjustments result in a transient state of circadian disturbance, i.e., circadian disruption, that can increase vulnerability to chemical and other xenobiotic stressors (Smolensky et al. Citation2019).
The American Academy of Sleep Medicine proclaims SD has a negative impact on the overall health of the population (Dregan and Armstrong Citation2011), and that shiftworkers are certainly some of the most affected (Dregan and Armstrong Citation2011; Kessler et al. Citation2011; Loef et al. Citation2019). Because immune responsiveness is controlled by circadian processes, they are likely to be affected by shiftwork-schedule-induced disruption (Labrecque and Cermakian Citation2015). Shiftwork can induce progressive SD, stress, and alteration of the natural circadian pattern that as a consequence can compromise immune responsiveness (Mohren et al. Citation2002; Nagai et al. Citation2011; Nakano et al. Citation1982). It is known that shiftworkers show reduction of T lymphocytes (LT), especially in those working fixed night shifts (Axelsson et al. Citation2013; Dinges et al. Citation1994, Citation1995; Irwin et al. Citation1994, Citation1996; Nakano et al. Citation1982; Savard et al. Citation2003; Wilder-Smith et al. Citation2013). It is also well known that IL-1β and TNF-α are associated with sleep quality, which for shiftworkers is depressed because of the requirement to sleep during the daytime light phase, the biological time of greatest alertness for the human species (Dijk D-J Citation1999; Drake et al. Citation2004). Therefore, shiftworkers are often chronically sleep deprived (Honn et al. Citation2016; Smith et al. Citation2009), giving rise to increased risk for diminished health and work accidents (Laugsand et al. Citation2014).
The period of sleep serves to renew functions related to wakefulness and processes controlling the immunological response to infections (Motivala and Irwin Citation2007; Opp and Krueger Citation2015). The loss of sleep in modern society, as observed among shiftworkers, is associated with increased susceptibility to developing infectious diseases, including the flu (Cohen et al. Citation2009; Patel et al. Citation2012) and other upper airway infections (Prather et al. Citation2015). Thus, the matter of shiftwork-associated SD is of major economic and public health policy interest (Irwin Citation2012).
Chronic stress is correlated with suppression of cellular and humoral immunity (Segerstrom and Miller Citation2004; Tsigos and Chrousos Citation2002). The immune response to shiftwork is highly variable and depends on several factors, in particular, job-related stress that can make one’s job intolerable (Barnes-Farrell et al. Citation2008; Tamagawa et al. Citation2007). In the long term, rotating shiftwork may give rise to chronic adaptive stress, and as shown in , with possible repercussions on the neuroendocrine-immune system (Shields Citation2002) that results in attenuation of immune responsiveness (Amati et al. Citation2007; Boscolo et al. Citation2009; De Gucht et al. Citation1999; Endresen et al. Citation1987; Okamoto et al. Citation2008), evident by increased CD3+ CD16, CD56+ cell numbers, increased IL-6 plasma levels, reduced CD57+, CD8+, CD11b+ cell numbers, reduced LT function (Curti et al. Citation1982; Nakano et al. Citation1982), and attenuated NK activity (Magrini et al. Citation2006; Morikawa et al. Citation2005; XU et al. Citation1998). Accordingly, SD in combination with altered circadian time structure may result in increased vulnerability during night shift not only to industrial contaminants but contagions, such as viruses (Smolensky et al. Citation2019).
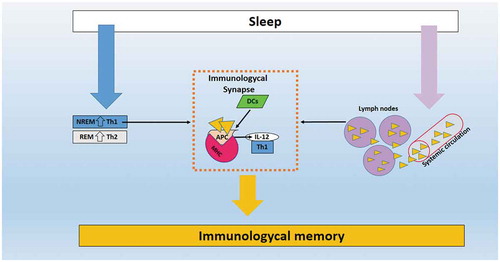
Figure 1. Illustrative image of the role of sleep in immunological memory. (Orange triangles) represents the migration of T/B lymphocytes from systemic circulation IL, Interleukin; APC, antigen-presenting cells; Th1/Th2, T helper lymphocytes 1/2; DCs, dendritic cells.
Importantly, a study of Japanese emergency department physicians demonstrated immune changes at the start of the night shift (Okamoto et al. Citation2008), with the interaction between sleep and immune system adaptive memory (Lange et al. Citation2011; Prather et al. Citation2012). Shiftworkers, especially those working the night shift, experienced a greater number of upper respiratory infections – colds and flu – compared to those working daytime only shifts (Nagai et al. Citation2011). Moreover, the shiftwork schedule of these Japanese emergency department physicians was also associated with depression of the innate immune response, i.e., decreased NK cell activity that was associated with increased fatigue. Reduced sleep (<5 h), as well as poor sleep quality or excessive sleep (>9 h), are linked with risk for pneumonia (Patel et al. Citation2012). In China, COVID-19 positive persons showed leukopenia and lymphopenia, with several or all of the following immune biomarker concentrations being elevated: IL1β, IL7, IL8, IL9, IL10, IL5, IL12p70, IL15, IFNγ, CCL2, CCL3, CCL4, TNF-α, and VEGF (World Health Organization, W Citation2003; Zaki et al. Citation2012), and in association with pulmonary inflammation and extensive lung damage, as also found those affected by the past infectious diseases of SARS and MERS-CoV, and often in conjunction with increased IFNγ, TNF-α, and IL1 (de Groot et al. Citation2013; Mahallawi et al. Citation2018).
Can sleep debt make shiftworkers more vulnerable to the COVID-19?
As discussed above, RNA from viruses is released by stressed or injured cells, as are HPSs (Bianchi Citation2007; Chu and Mazmanian Citation2013; Matzinger Citation2007) and mediators that activate inflammatory signaling pathways like NF-κB. This is followed by the release of acute-phase cytokines, such as IL-1β, TNF, and INFs, with anti-viral activity of vasoactive mediators like prostaglandins (PGs). When a virus evades this first line of defense, the adaptive immune system, consisting of T and B cells, can provide tailored, specific responses that are initiated by CD8+ T cells that recognize the major histocompatibility complex (MHC) and kill the target cell. Depending on the type of infection, antigen-presenting cells (APCs) release certain cytokines, thereby increasing CD4+ and eventually IFNs, IL-12, and Th1 (Iwasaki and Medzhitov Citation2015; Matzinger Citation2007; Vidarsson et al. Citation2014). After elimination of the pathogen in the effector phase, most activated antigen-specific B and T cells die, but some persist as memory cells for the contagion that make possible more efficient response thereafter with renewed exposure (Farber et al. Citation2016; Mueller et al. Citation2013; Weisel and Shlomchik Citation2017).
SD increases the risk of airways’ infection, especially from viruses (Patel et al. Citation2012; Prather et al. Citation2015). As discussed previously, it impairs innate immunity, expressed by reduction of the activity of NK cells and decrease of the Th1 effector cellular response, important for the activation of TCD4+ (Axelsson et al. Citation2013; Fondell et al. Citation2011; Irwin et al. Citation1994, Citation1996; Sakami et al. Citation2002; Savard et al. Citation2003). People positive for CODIV-19 present with higher concentrations of ligand T-cell chemokine-3 (CCL3) and also TNF-α, suggesting the observed cytokine storm of patients severely affected with this virus is responsible for such disease severity. However, COVID-19 also initiates increased secretion of Th2, IL4, and IL10 that suppresses inflammation, which differentiates COVID-19 from SARS-CoV infection (Wong et al. Citation2004).
Because shiftwork can present increased vulnerability to infectious diseases (Adams et al. Citation1984), and adaptive and innate immune system display circadian rhythms, disruption of immune responsiveness is likely to enhance susceptibility to infection (Ananthakrishnan Citation2015; Anderson et al. Citation2017). Shiftworkers, who typically are sleep deprived, report higher incidence and severity of respiratory infections (Archer and Oster Citation2015; Archer et al. Citation2018), and these observations suggest they, incontrast to non-shiftworkers, may be more susceptible to COVID-19. The higher incidence and severity to respiratory and conceivably other infections may be associated with SD, i.e., associated decreased slow-wave NREM sleep, when there is increase of IL-12 and DCs, the main precursors of APCs. Concomitant reduction of IL-10 and IL-4 levels facilitates the Th1/Th2 balance in favor of the Th1 response (Dimitrov et al. Citation2004; Lange et al. Citation2006).
Production of IL-12 by APCs is essential for the activation of Th cells (Abbas et al. Citation1994). The pro-inflammatory pattern during the early portion of sleep is balanced by Th2 response during the final portion of sleep when REM sleep tends to be most prevalent (Dimitrov et al. Citation2004). The induction of Th1 cells and the migration of immune cells from the systemic circulation to the secondary lymphoid organs during sleep may increase the interaction between the APCs and naïve T cells and B lymphocytes, like a immunological synapse, and these could be mechanisms through which sleep would collaborate on a more efficient immunological memory (Bollinger et al. Citation2010; Born et al. Citation1997; Dimitrov et al. Citation2004; Reis et al. Citation2011) as illustrated in .
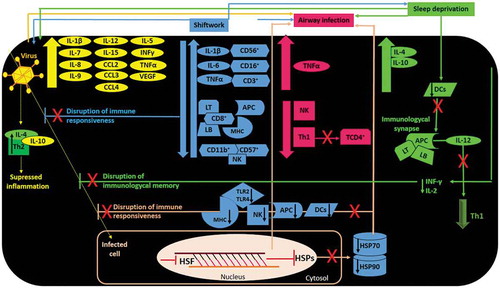
We postulate that these mechanisms of immunological memory may not be efficient in shiftworkers. Furthermore, considering that the HSP70 and HSP90 elevation is responsible for modulating the level of pathology and cell death (Morimoto Citation1998), it is possible that shiftworkers, who are constantly under stress and sleep deprived, may have attenuated expression of HSPs due lower production of heat shock transcription factor (HSF) and suppressed induction of NK and DCs (Bolhassani and Agi Citation2019). Consequently, in shiftworkers who are also highly to be likely acutely or chronically circadian disrupted, MHC-antigen processing and presentation (Call and Wucherpfennig Citation2005) and toll-like receptors (TLRs) processes may not be efficiently synchronized in time upon exposure to viral antigens (Vyas et al. Citation2008) and thus inefficient in activating potent immune response (Bolhassani and Agi Citation2019), thereby increasing susceptibility to COVID-19 ().
Figure 2. Illustrative scheme of the relationship between immune response, shiftwork, sleep debt, airway infections, and COVID-19. (Orange arrow) Virus induces stress responses mediated by inflammatory markers, leading to airway infections. (Blue arrows) Shifworkers who are sleep deprived, experience frequent airway infections, and are more susceptible to show failure of immune response against COVID-19 and other viruses than those working the normal daytime schedule. (Green arrows) Sleep deprivation induces airway infection to viruses by contributing to immune response failure. (Pink arrows) Infected cells of shiftworkers may fail to produce HSP70 and HSP90 efficiently, which may potentiate risk for airway infection, impact negatively on anti-viral defense by reduction of DCs, APCs, NK, TLRs and MHC. IL, interleukin; CCL, CCL motif chemokine ligand; INFγ, interferon-gamma; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; CD+, adhesion molecule expression; LT/LB, T/B lymphocytes; MHC, major histocompatibility complex; APC, antigen-presenting cells; NK, natural killer; Th1/Th2, T helper lymphocytes 1/2; DCs, dendritic cells; TLR2/TLR4, toll-like receptor 2/4; HSP70, heat shock protein 70; HSP90, heat shock protein 90; HSF: heat shock factor.
Evidence of the importance of HSPs is supported by the findings that HSP90 is involved in other human respiratory diseases of vial origin, e.g., the 2013 Caprine Parainfluenza Virus Type 3 Infection that affected goats in China (Zhong et al. Citation2019), and that HSP70 is associated with suppression of the avian infectious bronchitis virus (IBV) (Zhang et al. Citation2017). Thus, given the extensive knowledge of the disruptive and potentially compromising effects of shiftwork with regards to circadian rhythms in the vulnerability to viral contagions, which may be further exacerbated by SD plus associated disruption of the immune and neuroendocrine among other processes and systems, mandates greater research into both the more complete understanding of the postulated potential for elevated risk for COVID-19 infection in permanent nightshift and rotating shiftworkers as well as preventative strategies, including ones linked to circadian (predictable-in-time) vulnerabilities as so-called chronopreventation interventions.
Another area of requiring future exploration regarding risk for COVID-19 virus infection and severity of infection is the circadian rhythm of the renin-angiotensin-aldosterone system (RAAS) and its constituents. COVID-19 virus invades human alveolar epithelial cells mainly through ACE2 (angiotensin-converting enzyme 2) (Liu et al. Citation2014). Several approaches address ACE2-mediated COVID-19, such as spike protein-based vaccine (ACE2 as a COVID-19 receptor), inhibition of transmembrane protease activity (essential for entry through interaction with ACE2 receptor), blocking ACE2 receptor, and delivering the soluble form of ACE2. The RAAS predictably activates during nighttime sleep in those routinely adhering to the normal daytime activity/nighttime sleep pattern; however, it is instable in SD individuals (Stumpe et al. Citation1976) and most likely permanent night and rotating shiftworkers. The peak of the 24 h temporal pattern in angiotensin 2, peaks toward the end of sleep (Hermida et al. Citation2011, Citation2007). The role of this rhythm and its interruption due to shiftwork in affecting the risk of developing more severe symptoms of diseases is unknown and deserves investigation.
Because of the dysfunction of the renin-angiotensin system seen in COVID-19 patients, there is great interest in the therapeutic potential against COVID-19 involving medications that target ACE2 (Tikellis et al. Citation2011; Zhong et al. Citation2010). Although there is clinical evidence demonstrating that RAS inhibitors improve the clinical outcomes of COVID-19 patients with hypertension (Meng et al. Citation2020), it is important to consider that various circadian rhythms are involved in the regulation of blood pressure during the 24 h (Portaluppi Citation2000). Similarly, several endogenous circadian rhythms affect the pharmacokinetics and pharmacodynamics of antihypertensive treatments (Hermida and Smolensky Citation2004), and the role of circadian time on the efficacy of prophylactic medications against viruses and also therapies to manage infection is also unknown and worthy of study.
References
- Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Elsevier Health Sciences; 1994
- Adams F, Quesada JR, Gutterman JU. 1984. Neuropsychiatric manifestations of human leukocyte interferon therapy in patients with cancer. JAMA. 252(7):938–941. doi:10.1001/jama.1984.03350070056026.
- Akerstedt T. 1990. Psychological and psychophysiological effects of shift work. Scand J Work Environ Health. 16(Suppl 1):67–73. doi:10.5271/sjweh.1819.
- Almeida CMOD, Malheiro A. 2016. Sleep, immunity and shift workers: A review. Sleep Sci. 9(3):164–168. doi:10.1016/j.slsci.2016.10.007.
- M Amati 1, M Tomasetti, L Mariotti, L M Tarquini, M Ciuccarelli, M Poiani..., L Santarelli. 2007. [Study of a population exposed to occupational stress: correlation among psychometrics tests and biochemical-immunological parameters]. G Ital Med Lav Ergon. 29(3 Suppl):356–358. PMID: 3393871.
- Ananthakrishnan AN. 2015. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 12(4):205–217. doi:10.1038/nrgastro.2015.34.
- Jason R Anderson, Ian Carroll, M Andrea Azcarate-Peril, Amber D Rochette, Leslie J Heinberg, Christine Peat..., John Gunstad. 2017. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 38:104–107. doi:10.1016/j.sleep.2017.07.018.
- Archer AE, Von Schulze AT, Geiger PC. 2018. Exercise, heat shock proteins and insulin resistance. 373(1738). doi:10.1098/rstb.2016.0529.
- Archer SN, Oster H. 2015. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res. 24(5):476–493. doi:10.1111/jsr.12307.
- Arjona A, Sarkar DK. 2006. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 20(5):469–476. doi:10.1016/j.bbi.2005.10.002.
- John Axelsson, Javaid-ur Rehman, Torbjorn Akerstedt, Rolf Ekman, Gregory E Miller, Caroline Olgart Höglund, Mats Lekander 2013. Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/T helper 2 balance in humans. PLoS One. 8:e82291. doi:10.1371/journal.pone.0082291.
- Barnes PJ. 1998. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 94(6):557–572. doi:10.1042/cs0940557.
- Janet Barnes-Farrell, Kimberly Davies-Schrils, Alyssa McGonagle, Benjamin Walsh, Lee Di Milia, Frida Marina Fischer..., Donald Tepas. 2008. What aspects of shiftwork influence off-shift well-being of healthcare workers? Appl Ergon. 39(5):589–596. doi:10.1016/j.apergo.2008.02.019.
- Bianchi ME. 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 81(1):1–5. doi:10.1189/jlb.0306164.
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. 1999.Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 17:189–220. doi:10.1146/annurev.immunol.17.1.189.
- Bolhassani A, Agi E. 2019. Heat shock proteins in infection. Clin Chim Acta. 498:90–100. doi:10.1016/j.cca.2019.08.015.
- Bollinger T, Bollinger A, Naujoks J, Lange T, Solbach W. 2010. The influence of regulatory T cells and diurnal hormone rhythms on T helper cell activity. Immunology. 131(4):488–500. doi:10.1111/j.1365-2567.2010.03320.x.
- Born J, LangeT, Hansen K, Mölle M, Fehm HL. 1997. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 158(9):4454–4464. PMID: 9127011.
- Boscolo P, Di Donato A, Di Giampaolo L, Forcella L, Reale M, Dadorante V..., Fattorini E. 2009. Blood natural killer activity is reduced in men with occupational stress and job insecurity working in a university. Int Arch Occup Environ Health. 82(6):787–794. doi:10.1007/s00420-008-0374-5.
- Call ME, Wucherpfennig KW. 2005. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 23:101–125. doi:10.1146/annurev.immunol.23.021704.115625.
- Chan JF, Kenneth SM, Kelvin KW, Cheng CC, Chen H, Yuen KY. 2012. Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? J Infect. 65(6):477–489. doi:10.1016/j.jinf.2012.10.002.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y..., Li Zhang. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7.
- Chu H, Mazmanian SK. 2013. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 14(7):668–675. doi:10.1038/ni.2635.
- Cohen S, Doyle W, Alper C, Janicki-Deverts D, Turner R. 2009. Sleep habits and susceptibility to the common cold. Arch Intern Med. 169(1):62–67. doi:10.1001/archinternmed.2008.505.
- Costa G, Akerstedt T, Nachreiner F, Baltieri F, Carvalhais J, Folkard S..., Jorge Silvério. 2004. Flexible working hours, health, and well-being in Europe: some considerations from a SALTSA project. Chronobiol Int. 21(6):831–844. doi:10.1081/cbi-200035935.
- Curti R, Radice L, Cesana G, Zanettini R, Grieco A. 1982. Work stress and immune system: lymphocyte reactions during rotating shift work. Preliminary results. Med Lav. 73(6):564–569. PMID: 6984725.
- Cymerys J, Krzyżowska M, Spohr I, Winnicka A, Niemiałtowski M. 2009. Hsp-27, hsp-70 and hsp-90 expression and apoptosis in macrophages during ectromelia (mousepox) virus infection. Centr Eur J Immunol. 34:20–28.
- D'Aurea C, Poyares D, Piovezan R, Passos G, Tufik S, de Mello M. 2015. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr. 73(6):516–519. doi:10.1590/0004-282X20150053.
- de Groot R, Baker S, Baric R, Brown C, Drosten C, Enjuanes L..., Ziebuhro J. 2013. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 87(14):7790–7792. doi:10.1128/JVI.01244-13.
- De Gucht V, Fischler B, Demanet C. 1999. Immune dysfunction associated with chronic professional stress in nurses. Psychiatry Res. 85(1):105–111. doi:10.1016/s0165-1781(98)00131-0.
- Dijk D-J ED. 1999. Circadian and homeostatic control of wakefulness and sleep. Reg Sleep Wakefullness. doi:10.1177/0748730405278292.
- Dimitrov S, Lange T, Tieken S, Fehm H, Born J. 2004. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 18(4):341–348. doi:10.1016/j.bbi.2003.08.004.
- Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T. 2009. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 113(21):5134–5143. doi:10.1182/blood-2008-11-190769.
- Dinges D, Douglas S, Zaugg L, Campbell D, McMann J, Whitehouse W...,Orne M. 1994. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 93(5):1930–1939. doi:10.1172/JCI117184.
- Dinges D, Douglas S, Hamarman S, Zaugg L, Kapoor S. 1995. Sleep deprivation and human immune function. Adv Neuroimmunol. 5(2):97–110. doi:10.1016/0960-5428(95)00002-j.
- Drake C, Roehrs T, Richardson G, Walsh J, Roth T. 2004. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 27(8):1453–1462. doi:10.1093/sleep/27.8.1453.
- Dregan A, Armstrong D. 2011. Cross-country variation in sleep disturbance among working and older age groups: an analysis based on the European social survey. Int Psychoger. 23(9):1413–1420. doi:10.1017/S1041610211000664.
- Duguay D, Cermakian N. 2009. The crosstalk between physiology and circadian clock proteins. Chronobiol Int. 26(8):1479–1513. doi:10.3109/07420520903497575.
- Endresen I, Værnes R, Ursin H, Tinde O. 1987. Psychological stress-factors and concentration of immunoglobulins and complement components in Norwegian nurses. Work Stress. 1(4):365–375. doi:10.1080/02678378708258527.
- Farber D, Netea M, Radbruch A, Rajewsky K, Zinkernagel R. 2016. Immunological memory: lessons from the past and a look to the future. Nat Rev Immunol. 16(2):124–128. doi:10.1038/nri.2016.13.
- Fondell E, Axelsson J, Franck K, Ploner A, Lekander M, Bälter K, Gaines H. 2011. Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behav Immun. 25(7):1367–1375. doi:10.1016/j.bbi.2011.04.004.
- Goulhen F, Grenier D, Mayrand D. 2003. Oral microbial heat-shock proteins and their potential contributions to infections. Crit Rev Oral Biol Med. 14(6):399–412. doi:10.1177/154411130301400603.
- Haimovich B, Calvano J, Haimovich A, Calvano S, Coyle S, Lowry S. 2010. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 38(3):751–758. doi:10.1097/CCM.0b013e3181cd131c.
- Hermida R, Ayala D, Fernández J, Portaluppi F, Fabbian F, Smolensky M. 2011. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens. 24(4):383–391. doi:10.1038/ajh.2010.217.
- Hermida RC, Ayala DE, Portaluppi F. 2007. Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev. 59(9–10):904–922. doi:10.1016/j.addr.2006.08.003.
- Hermida RC, Smolensky MH. 2004. Chronotherapy of hypertension. Curr Opin Nephrol Hypertens. 13(5):501–505. doi:10.1097/00041552-200409000-00004.
- Holshue M, DeBolt C, Lindquist S, Lofy S, Wiesman J, Bruce H..., Pillai S. 2020. First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 382(10):929–936. doi:10.1056/NEJMoa2001191.
- Honn K2, Garde A4, Fischer F, Dongen H. 22nd international symposium on shiftwork and working time: challenges and solutions for healthy working hours. 2016. 581–588. doi:10.1080/07420528.2016.1195632.
- Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, Huan Cg. 2013. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. 439(4):471–476. doi:10.1016/j.bbrc.2013.08.101.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y...,Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5.
- Irwin M, Mascovich A, Gillin J, Willoughby R, Pike J, Smith T. 1994. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 56(6):493–498. doi:10.1097/00006842-199411000-00004.
- Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin J. 1996. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 10(5):643–653. doi:10.1096/fasebj.10.5.8621064.
- Irwin MR. 2012. Sleep and infectious disease risk. Sleep. 35:1025–1026. doi:10.5665/sleep.1976.
- Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol. 16(4):343–353. doi:10.1038/ni.3123.
- Jesse FFA, Chung ELT. 2019. Establishment of lung auscultation scoring method and responses of acute phase proteins and heat shock proteins in vaccinated and non-vaccinated goats. 51(2):289–295. doi:10.1007/s11250-018-1683-7.
- Kessler R, Berglund P, Coulouvrat C, Hajak G, Roth T, Shahly V, Shillington A, Stephenson J, Walsh J. 2011. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 34(9):1161–1171. doi:10.5665/SLEEP.1230.
- Kim MY, Oglesbee M. 2012. Virus-heat shock protein interaction and a novel axis for innate antiviral immunity. Cells. 1(3):646–666. doi:10.3390/cells1030646.
- Knutsson A. 2003. Health disorders of shift workers. Occup Med. 53(2):103–108. doi:10.1093/occmed/kqg048.
- Kobayashi F, Furu Hi, Akamatsu Y, Watanabe T, Horibe H. 1996. Changes in psychophysiological functions during night shift in nurses. Int Arch Occup Environ Health. 69(2):83–90. doi:10.1007/s004200050120.
- Labrecque N, Cermakian N. 2015. Circadian clocks in the immune system. J Biol Rhythms. 30(4):277–290. doi:10.1177/0748730415577723.
- Lange T, Dimitrov S, Fehm H, Westermann J, Born J. 2006. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 166(16):1695–1700. doi:10.1001/archinte.166.16.1695.
- Lange T, Dimitrov S, Bollinger T, Diekelmann S, Born J. 2011. Sleep after vaccination boosts immunological memory. J Immunol. 187(1):283–290. doi:10.4049/jimmunol.1100015.
- Lange T, Dimitrov S, Born J. 2010. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 1193:48–59. doi:10.1111/j.1749-6632.2009.05300.x.
- Laugsand L, Strand L, Vatten L, Janszky I, Bjørngaard J. 2014. Insomnia symptoms and risk for unintentional fatal injuries–the HUNT Study. Sleep. 37(11):1777–1786. doi:10.5665/sleep.4170.
- Lee H, Ock C, Kim S, Hahm K. 2010. Heat shock protein: hard worker or bad offender for gastric diseases. Int J Proteomics. 2010:259163. doi:10.1155/2010/259163.
- Leposavić G, Pilipović I, Radojević K, Pesić V, Perisić M, Kosec D. 2008. Catecholamines as immunomodulators: a role for adrenoceptor-mediated mechanisms in fine tuning of T-cell development. Auton Neurosci. 144(1–2):1–12. doi:10.1016/j.autneu.2008.09.003.
- Leproult R, Copinschi G, Buxton O, Cauter E. 1997. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 20(10):865–870. PMID: 9415946.
- Lévi F, Canon C, Dipalma M, Florentin I, Misset J. 1991. When should the immune clock be reset? From circadian pharmacodynamics to temporally optimized drug delivery. Ann N Y Acad Sci. 618:312–329. doi:10.1111/j.1749-6632.1991.tb27251.x.
- Li G, Zhang J, Tong X, Liu W, Ye X. 2011. Heat shock protein 70 inhibits the activity of Influenza A virus ribonucleoprotein and blocks the replication of virus in vitro and in vivo. PLoS One. 6(2):e16546–e16546. doi:10.1371/journal.pone.0016546.
- Liu X, Liu X, Huang W, Leo S, Li Y, Liu M, Yuan H. 2014. Evening -versus morning- dosing drug therapy for chronic kidney disease patients with hypertension: a systematic review. Kidney Blood Press Res. 39(5):427–440. doi:10.1159/000368456.
- Loef B, Nanlohy N, Jacobi R, de Ven C, Mariman R, der Beek A...Baarle D. 2019. Immunological effects of shift work in healthcare workers. Sci Rep. 9(1):18220. doi:10.1038/s41598-019-54816-5.
- Magrini A, Pietroiusti A, Coppeta L, Babbucci A, Barnaba E, Papadia C..., A Bergamaschi A. 2006. Shift work and autoimmune thyroid disorders. Int J Immunopathol Pharmacol. 19(4 Suppl):31–36. PMID: 17291404.
- Mahallawi W, Khabour O, Zhang Q, Makhdoum H, Suliman B. 2018. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 104:8–13. doi:10.1016/j.cyto.2018.01.025.
- Matzinger P. 2007. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 8(1):11–13. doi:10.1038/ni0107-11.
- Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R..., Zhang G. 2020. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 9(1):757–760. doi:10.1080/22221751.2020.1746200.
- Mohren D, Jansen N, Kant I, Galama J, den Brandt P, Swaen G. 2002. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 44(11):1003–1011. doi:10.1097/00043764-200211000-00005.
- Moldofsky H. 1995. Sleep and the immune system. Int J Immunopharmacol. 17(8):649–654. doi10.1016/0192-0561(95)00051-3.
- Molina PE. 2005. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 24(1):3–10. doi:10.1097/01.shk.0000167112.18871.5c.
- Morikawa Y, Kitaoka-Higashiguchi K, Tanimoto C, Hayashi M, Oketani R, Miura K..., Nakagawa H. 2005. A cross-sectional study on the relationship of job stress with natural killer cell activity and natural killer cell subsets among healthy nurses. J Occup Health. 47(5):378–383. doi:10.1539/joh.47.378.
- Morimoto RI. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12(24):3788–3796. doi:10.1101/gad.12.24.3788.
- Motivala SJ, Irwin MR. 2007. Sleep and immunity: cytokine pathways linking sleep and health outcomes. Curr Dir Psychol Sci. 16(1):21–25. doi:10.1146/annurev-psych-010213-115205.
- Mueller S, Gebhardt T, Carbone F, Heath W. 2013. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 31:137–161. doi:10.1146/annurev-immunol-032712-095954.
- Nagai M, Morikawa Y, Kitaoka K, Nakamura K, Sakurai M, Nishijo M, ...,Hideaki Nakagawa. 2011. Effects of fatigue on immune function in nurses performing shift work. J Occup Health. 53(5):312–319. doi:10.1539/joh.10-0072-oa.
- Nakano Y, Miura T, Hara I, Aono H, Miyano N, Miyajima K..., Kosaka H. 1982. The effect of shift work on cellular immune function. J Hum Ergol. 11(Suppl):131–137. PMID: 6985367.
- National Sleep Foundation, N. What is shift work?; 2016 30 March 2020. Available from: https://www.sleepfoundation.org/shift-work-disorder/what-shift-work.
- Okamoto H, Tsunoda T, Teruya K, Takeda N, Uemura T, Matsui T..., Takashima Y. 2008. An occupational health study of emergency physicians in Japan: health assessment by immune variables (CD4, CD8, CD56, and NK cell activity) at the beginning of work. J Occup Health. 50(2):136–146. doi:10.1539/joh.l6084.
- Opp MR, Krueger JM. 2015. Sleep and immunity: A growing field with clinical impact. Brain Behav Immun. 47:1–3. doi:10.1016/j.bbi.2015.03.011.
- Patel S, Malhotra A, Gao X, Hu F, Neuman M, Fawzi W. 2012. A prospective study of sleep duration and pneumonia risk in women. Sleep. 35(1):97–101. doi:10.5665/sleep.1594.
- Paules CI, Marston HD, Fauci AS. 2020. Coronavirus infections-more than just the common cold. Jama. doi:10.1001/jama.2020.0757
- Portaluppi FSM. 2000. Circadian rhythm and environmental determinants of blood pressure regulation in normal and hypertensive conditions. Blood Pre Mon Cardiovasc Med Ther. 79–118. doi:10.1007/978-1-59259-004-9_5.
- Aric A Prather, Anna L Marsland, Martica Hall, Serina A Neumann, Matthew F Muldoon, Stephen B Manuck. 2009. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 82(1):12–17. doi:10.1016/j.biopsycho.2009.04.008.
- Aric A Prather, Martica Hall, Jacqueline M Fury, Diana C Ross, Matthew F Muldoon, Sheldon Cohen, Anna L Marsland. 2012. Sleep and antibody response to hepatitis B vaccination. Sleep. 35(8):1063–1069. doi:10.5665/sleep.1990.
- Prather A, Janicki-Deverts D, Hall M, Cohen S. 2015. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 38(9):1353–1359. doi:10.5665/sleep.4968.
- Reinberg A, Ashkenazi I. 2008. Internal desynchronization of circadian rhythms and tolerance to shift work. Chronobiol Int. 25(4):625–643. doi:10.1080/07420520802256101.
- Reis E, Lange T, Köhl G, Herrmann A, Tschulakow A, Naujok Js..., Köhl J. 2011. Sleep and circadian rhythm regulate circulating complement factors and immunoregulatory properties of C5a. Brain Behav Immun. 25(7):1416–1426. doi:10.1016/j.bbi.2011.04.011.
- Richman DD, Whitley RJ, Hayden FG. 2017. Clinical Virology. Fourth Edition ed. American Society of Microbiology.
- Sakami S, Ishikawa T, Kawakami N, Haratani T, Fukui A, Kobayashi F..., Kawamura N. 2002. Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th2 dominance. Neuroimmunomodulation. 10(6):337–343. doi:10.1159/000071474.
- Sanders VM. 2012. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 26(2):195–200. doi:10.1016/j.bbi.2011.08.001.
- Savard J, Laroche L, Simard S, Ivers H, Morin C. 2003. Chronic insomnia and immune functioning. Psychosom Med. 65(2):211–221. doi:10.1097/01.psy.0000033126.22740.f3.
- Schernhammer E, Laden F, Speizer F, Willett W, Hunter D, Kawachi I, Colditz G. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 93(20):1563–1568. doi:10.1093/jnci/93.20.1563.
- Segerstrom SC, Miller GE. 2004. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 130(4):601–630. doi:10.1037/0033-2909.130.4.601.
- Shields M. 2002. Shift work and health. Health Rep. 13(4):11–33. PMID: 15069802
- Skene DJ, Arendt J. 2006. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 43(Pt 5):344–353. doi:10.1258/000456306778520142.
- Smith MR, Fogg LF, Eastman CI. 2009. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 32(11):1481–1489. doi:10.1093/sleep/32.11.1481.
- Smolensky MH, Reinberg AE, Fischer FM. 2019. Working time society consensus statements: circadian time structure impacts vulnerability to xenobiotics-relevance to industrial toxicology and nonstandard work schedules. Ind Health. 57(2):158–174. doi:10.2486/indhealth.SW-2.
- Smolensky MH, Reinberg AE, Sackett-Lundeen L. 2017. Perspectives on the relevance of the circadian time structure to workplace threshold limit values and employee biological monitoring. Chronobiol Int. 34(10):1439–1464. doi:10.1080/07420528.2017.1384740.
- Spiegel K, Leproult R, Van Cauter E. 1999. Impact of sleep debt on metabolic and endocrine function. Lancet. 354(9188):1435–1439. doi:10.1016/S0140-6736(99)01376-8.
- Steinman L. 2004. Elaborate interactions between the immune and nervous systems. Nat Immunol. 5(6):575–581. doi:10.1038/ni1078.
- K.O. Stumpe, M.D. R. Kolloch, M.D.H. Vetter, M.D.W. Gramann, M.D.F. Krück, M.D.Ch. ResselM. Higuchi. 1976. Acute and long-term studies of the mechanisms of action of beta-blocking drugs in lowering blood pressure. Am J Med. 60(6):853–865. doi:10.1016/0002-9343(76)90905-0.
- Tamagawa R, Lobb B, Booth R. 2007. Tolerance of shift work. Appl Ergon. 38(5):635–642. doi:10.1016/j.apergo.2006.05.003.
- Tang J, Lam T, Zaraket H, Lipkin W, Drews S, Hatchette T..., Koopmans M. 2017. Global epidemiology of non-influenza RNA respiratory viruses: data gaps and a growing need for surveillance. Lancet Infect Dis. 17(10):e320–e326. doi:10.1016/S1473-3099(17)30238-4.
- Tikellis C, Bernardi S, Burns WC. 2011. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens. 20(1):62–68. doi:10.1097/MNH.0b013e328341164a.
- Tsigos C, Chrousos GP. 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 53(4):865–871. doi:10.1016/s0022-3999(02)00429-4.
- Vidarsson G, Dekkers G, Rispens T. 2014. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 5:520. doi:10.3389/fimmu.2014.00520.
- Vyas JM, Van der Veen AG, Ploegh HL. 2008. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 8(8):607–618. doi:10.1038/nri2368.
- Peter C, Frederick W, George G, Gao F. 2020. A novel coronavirus outbreak of global health concern. Lancet. 395(10223):470–473. doi:10.1016/S0140-6736(20)30185-9.
- Weibel L, Spiegel K, Follenius M, Ehrhart J, Brandenberger G. 1996. Internal dissociation of the circadian markers of the cortisol rhythm in night workers. Am J Physiol. 270(4 Pt 1):E608–13. doi:10.1152/ajpendo.1996.270.4.E608.
- Weisel F, Shlomchik M. 2017. Memory B cells of mice and humans. Annu Rev Immunol. 35:255–284. doi:10.1146/annurev-immunol-041015-055531.
- Smith A, Mustafa F, Earnest A, Gen L, Macary P. 2013. Impact of partial sleep deprivation on immune markers. Sleep Med. 14(10):1031–1034. doi:10.1016/j.sleep.2013.07.001.
- Wong C, Lam C, Wu A, Ip W, Lee N, Chan I..., Sung J. 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 136(1):95–103. doi:10.1111/j.1365-2249.2004.02415.x.
- World Health Organization, W. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003; 2003 30 March 2020]. Available from: https://www.who.int/csr/sars/country/table2004_04_21/en/.
- Xu M, Miura Y, Nagao F, Muto T, Okumura K. 1998. NK cell activity and subsets of truck drivers along with related factors. Nippon Eiseigaku Zasshi. 53(2):456–462. doi:10.1111/imm.12224.
- Young M, Matthews J, Kanabrocki E, Sothern R, Johnson B, Scheving L. 1995. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor in men. Chronobiol Int. 12(1):19–27. doi:10.3109/07420529509064496.
- Zaki A, Boheemen S, Bestebroer T, Osterhaus A, Fouchier R. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 367(19):1814–1820. doi:10.1056/NEJMoa1211721.
- Zhang Z, Yang X, Xu P, Wu X, Zhou L, Wang H. 2017. Heat shock protein 70 in lung and kidney of specific-pathogen-free chickens is a receptor-associated protein that interacts with the binding domain of the spike protein of infectious bronchitis virus. Arch Virol. 162(6):1625–1631. doi:10.1007/s00705-017-3280-x.
- Zhong C, Li J, Mao L, Liu M, Zhu X, Li W L..., Liao. 2019. Proteomics analysis reveals heat shock proteins involved in caprine parainfluenza virus type 3 infection. BMC Vet Res. 15(1):151. doi:10.1186/s12917-019-1897-6.
- Zhong J, et al. 2010. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 122(7):717–728. doi:10.1161/CIRCULATIONAHA.110.955369. 18 p following 728. doi:10.1161/CIRCULATIONAHA.110.955369.
