ABSTRACT
Colonic contractility normally shows circadian variability regulated by sleep and especially food intake. However, individuals with type 1 diabetes have a reduced or even absent gastrocolic response to a meal, indicating that colonic contractility may be affected by the disease. We hypothesized that individuals with type 1 diabetes and distal symmetric polyneuropathy (DSPN) have decreased motility (expressed as the motility index) and contractility of the colon and a reduced increase in motility index from night to morning compared to healthy controls and individuals with type 1 diabetes without DSPN. Cohorts of 35 individuals with type 1 diabetes and DSPN, 40 individuals with type 1 diabetes without DSPN, and 28 healthy controls were included in this post-hoc, cross-sectional analysis. We investigated, using a wireless motility capsule that measures pH, temperature, and pressure throughout the gastrointestinal tract, whether individuals with type 1 diabetes with and without DSPN, compared to healthy controls, exhibit altered colonic contractility in the evening, night, and morning. Max amplitude, mean peak amplitude, mean contraction, and motility index of the colon were calculated at the afore-designated times. Motility index of the colon tended to be higher in individuals with type 1 diabetes and DSPN compared to controls in the evening (P = .064), but the effect size was small (1.74%). There was no difference in motility index between the groups in the morning or evening. Furthermore, there was no difference in max amplitude, mean peak amplitude, or mean contraction between groups in the morning, evening, and night. As expected, overall contractility increased from night to morning in all groups, but there was no difference between groups in the increase in contractility from night to morning. Colonic contractility generally peaked in the morning, decreased in the evening, and was almost absent at night. Type 1 diabetes and/or DSPN did not impair contractility of the colon at any time point. Contractility and motility increased from morning to night unaffected by type 1 diabetes and/or DSPN.
Introduction
Type 1 diabetes is associated with numerous complications, including polyneuropathy which develops in up to 50% of such individuals. Plausibly, prolonged hyperglycemia causes disruption of coordinated gastrointestinal function (Domènech et al. Citation2011), leading to pleomorphic and nonspecific symptoms including, e.g., nausea, early satiety, abdominal pain, diarrhea, constipation, or fecal incontinence (Meldgaard et al. Citation2018). Intestinal cells contain an intrinsic circadian clock, or ‘gut clock’, which ensures that the processing of food occurs in daily cycles (Asher and Sassone-Corsi Citation2015). The central clock in the suprachiasmatic nucleus of the brain is primarily controlled by light/dark cycles, and the peripheral gut clock is primarily controlled by eating patterns, independent of the central clock (Zarrinpar et al. Citation2016). Normal colonic contractility is regulated by circadian rhythms and especially by sleep and food intake. In diurnally active healthy individuals, the number of colonic pressure waves and periods of colonic activity are reduced in the night compared to the daytime (Rao et al. Citation2001). Furthermore, pressure activity increases rapidly upon wakening and after a meal (Rao et al. Citation2001). In contrast, it has been shown that individuals with type 1 diabetes and mild to severe constipation have a reduced, delayed or even absent gastrocolic response to a meal (Battle et al. Citation1980), indicating that the regulation and coordination of colonic motility may be affected by diabetes and that this may contribute to the diversity of gastrointestinal symptoms reported by people with type 1 diabetes with or without distal symmetric polyneuropathy (DSPN) (Gregersen et al. Citation2016). It is unknown, however, whether circadian variation in colonic contractility is altered in individuals with type 1 diabetes with or without DSPN.
We hypothesized that individuals with type 1 diabetes and DSPN have decreased motility (expressed as the motility index) and contractility of the colon and a reduced increase in motility index from night to morning compared to healthy control individuals and individuals with type 1 diabetes without DSPN. We thus investigated whether colonic motility and contractility is affected in individuals with type 1 diabetes and DSPN, in comparison to individuals with type 1 diabetes without DSPN and healthy controls, in the morning, evening, and night. Furthermore, we assessed whether the increase in colonic motility and contractility from night to morning, previously observed in healthy individuals, is blunted in individuals with type 1 diabetes with or without DSPN.
Materials and methods
Study population
Thirty-five individuals with type 1 diabetes and confirmed DSPN according to the Toronto criteria including clinical signs and nerve testing, (T1D/+DSPN), 40 individuals with type 1 diabetes without any clinical signs of DSPN (T1D/-DSPN), and 28 healthy control individuals (CON) were included in the analyses (). The participants adhered to a routine of diurnal activity and nighttime sleep (). The participants are derived from three different studies (described in the online supplementary material). All participants gave written informed consent before any study procedure was initiated. The studies were approved by the ethical committees of the North Region of Denmark and the Capital Region of Denmark. Data can be provided upon request to the corresponding author.
Table 1. Participant characteristics of the control group (CON), type 1 diabetes without DSPN (T1D/-DSPN), and type 1 diabetes with DSPN(T1D/+DSPN). Data are presented as median [Q1, Q3] unless otherwise stated. BMI, body mass index; BPM, beats/min; HbA1c, Hemoglobin A1c; DSPN, distal symmetric polyneuropathy; T1D, type 1 diabetes; CON, control group
Study protocol
In the morning, following an overnight fast of at least 6 h, participants ingested a SmartBar® (Medtronic, Minneapolis, USA, 260 kcal, 3% fat, 21% protein, 75% carbohydrate, 3% fiber) with 200 mL of water followed by a wireless motility capsule (WMC) (Smartpill®, Medtronic, Minneapolis, USA). The WMC technique has previously been described in detail (Farmer et al. Citation2018; Maqbool et al. Citation2009; Rouphael et al. Citation2017). Briefly, the WMC is an ambulatory, noninvasive, radiation-free, indigestible capsule that measures temperature, pH, and pressure as it passes through the gastrointestinal tract. Data are continuously transmitted to an external data receiver worn by the participant.
The time of ingestion was scheduled in the morning between 08:00 and 10:30 h. Participants were instructed to abstain from eating or drinking for 6 h after ingestion of the WMC, after which they could resume their daily diet and activities.
Participants were instructed to register specific events (food ingestion, bowel movement, bedtime, and wake-up time) by pressing a button on the data receiver and reporting the event in a personal diary. Furthermore, participants were instructed to keep the data receiver close to their body until expulsion of the capsule, indicated by loss of signal after a bowel movement, which was verified by a dramatic decrease in temperature on the WMC trace.
Study procedures
Body weight was measured to the nearest 0.1 kg using an electronic scale with the participant wearing only light clothes. Height was measured to the nearest centimeter using a stadiometer. Blood pressure and heart rate were measured on the left arm following 5 min of rest, and clinical biochemical data were analyzed at the local department of clinical biochemistry.
Data analysis
Data were downloaded and analyzed using the MotiliGI software (MotiliGI version 3.0, Medtronic, Minneapolis, USA). A 1 h time frame was analyzed at each of three time points: evening (1 h before bedtime), at night (1 h from the lowest detectable temperature between bedtime and wake-up time), and morning (1 h from wake-up time). Time frames containing >10% signal loss were excluded from the analysis. Observations were excluded from the analysis (Figure S1, online supplementary material) when the bedtime and/or wake-up time were not reported by the participant.
For each of the three time points, four contractility measures were calculated using the MotiliGI software: 1) max amplitude (maximum amplitude within the selected time frame), 2) mean peak amplitude (mean amplitude/contraction), 3) mean contraction (mean number of contractions/min), and 4) motility index. The motility index was calculated by the software as the natural log of the sum of amplitudes*number of contractions + 1 (Farmer et al. Citation2013) and expressed in mmHg*S/min. Furthermore, the difference from night to morning of the four contractility measures was calculated as a morning/night ratio.
Statistical analyses
A one-way ANOVA adjusted for sex and age was used to test the effect of group on each of the outcomes at each time point (evening, night, morning). Sex and age have previously been observed to impact gastrointestinal motility (Wang et al. Citation2015), and since these confounders were not equally distributed among the groups, the analyses were adjusted for these two factors. Data with non-normally distributed residuals or unequal variance were log-transformed before analysis. In case of significant group effects, a Tukey HSD post-hoc test was applied to test between-group differences. For participant characteristics, a Kruskal–Wallis test was used to test the effect of group. Data are presented as medians [Q1;Q3]. Differences between groups are presented as mean (95% confidence interval). Statistical analyses were performed using RStudio version 1.1.463 (RStudio, Boston, MA, USA).
Results
Participant characteristics
Participant characteristics are presented in . Individuals with T1D/-DSPN were younger (P = .005) and had a shorter disease duration (P = <0.001) compared to T1D/+DSPN. The proportion of women (P = .022) as well as mean BMI (P = <0.001) and HbA1c (P = <0.001) were higher in the T1D/+DSPN group compared to the other groups.
Effect on colonic contractility in type 1 diabetes with or without DSPN
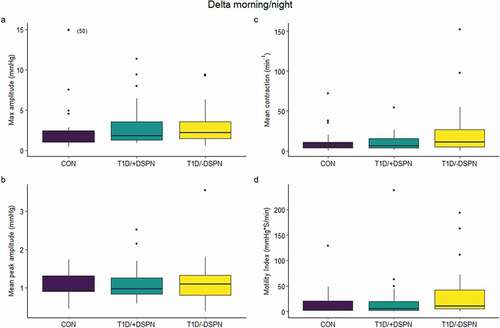
There was a group-dependent effect on motility index in the evening (P = .042; ). The post-hoc test showed that the motility index tended to be higher in T1D/+DSPN compared to CON in the evening, with a mean difference of 1.74% (95% CI: 1.08; 2.81%, P = .064) (). There was no difference in motility index between groups in the morning or night. Furthermore, there was no difference between groups in max amplitude, mean peak amplitude, or mean contraction at any of the three time points (). Finally, there was no difference between groups in the delta of the morning vs. night values of any of the contractility measures ().
Figure 1. Box and whisker plots demonstrating max amplitude (a), mean peak amplitude (b), mean contraction (c), and motility index (d) in the control group (CON) (evening: n = 25, night: n = 28, morning: n = 23), type 1 diabetes without DSPN (T1D/-DSPN) (evening: n = 36, night: n = 36, morning: n = 26), and type 1 diabetes with DSPN (T1D/+DSPN) (evening: n = 30, night: n = 32, morning: n = 30). Purple boxes = CON, teal boxes = T1D/+DSPN, yellow boxes = T1D/-DSPN. Effect of group adjusted for sex and age was assessed by one-way ANOVA at each time-point, and a Tukey post-hoc test was applied to asses between-group differences. Overall effect of group is marked by a line with p-value. DSPN, distal symmetric polyneuropathy; T1D, type 1 diabetes; CON, control group
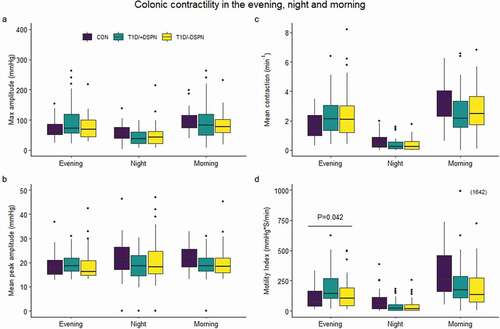
Figure 2. Box and whisker plots demonstrating delta values calculated as morning/night of max amplitude (a), mean peak amplitude (b), mean contraction (c), and motility index (d) in the control group (CON: n = 23), type 1 diabetes without DSPN (T1D/-DSPN: n = 22), and type 1 diabetes with DSPN (T1D/+DSPN: n = 27). Purple boxes = CON, teal boxes = T1D/+DSPN, yellow boxes = T1D/-DSPN. Effect of group adjusted for sex and age was assessed by one-way ANOVA at each time-point. DSPN, distal symmetric polyneuropathy; T1D, type 1 diabetes; CON, control group
Temporal variation in colonic contractility
In general, max amplitude, mean contraction, and motility index peaked in the morning, decreased in the evening, and was lowest at night (), whereas mean peak amplitude was comparable between the three time points ().
Discussion
Colonic contractility was measured by the WMC technique in three cohorts, and we found that the presence of type 1 diabetes and DSPN, with reference to CON, did not seem to change colonic contractility in the morning, evening, or at night. A tendency toward a slightly higher motility index in individuals with T1D/+DSPN compared to CON in the evening was observed; however, this is presumably clinically irrelevant due to the small effect size. This notion is supported by the fact that our T1D/+DSPN cohort had markedly prolonged colonic transit compared to healthy controls (Farmer et al. Citation2017), which – at least in healthy individuals – seems contradictory to an increased motility index. Consequently, the combination of decelerated colonic transit and equal or even increased motility index in individuals with T1D/+DSPN suggests that the neuronal support of colonic contractility and propulsion may be disrupted, uncoordinated, and less effective in the presence of type 1 diabetes and DSPN.
We did confirm that colonic motility exhibits temporal variation, with the difference between the night and morning values being the most pronounced. This is in accordance with previous studies, showing that pressure activity increased rapidly (threefold) upon wakening (Rao et al. Citation2001). The increase in contractility and motility from night to morning previously observed in healthy individuals was, however, also seen in individuals with T1D/+DSPN. Therefore, our hypothesis that individuals with T1D/+DSPN have a reduced increase in motility index of the colon from night to morning compared to CON and T1D/-DSPN was not confirmed. This implies that even though the gut is in fact affected by diabetes as shown previously (Farmer et al. Citation2017), the intrinsic circadian gut clock may not be affected. In contrast, mice with streptozotocin-induced type 1 diabetes expressed a decrease in amplitude and a phase delay in their mRNA expression of clock genes per2 and per3 in the colon (Bostwick et al. Citation2010). In addition, it has previously been demonstrated that per1per2 double knockout mice lack the natural circadian rhythmicity in intracolonic pressure changes and colonic circular muscle contractility compared to wildtype mice (Hoogerwerf et al. Citation2010), suggesting that normal rhythmicity of these clock genes is necessary to maintain circadian rhythmicity. However, it is unknown whether these findings also apply to humans.
Individuals with type 1 diabetes experience a wide range of gastrointestinal symptoms, ranging from severe constipation to chronic diarrhea or fecal incontinence (Meldgaard et al. Citation2018) that are characterized by different colonic motility patterns. An absent gastrocolic response to a meal has been observed in individuals with type 1 diabetes with severe constipation, whereas individuals with type 1 diabetes with mild constipation and healthy controls had increased colonic contractility in response to a meal (Battle et al. Citation1980). Thus, symptom severity may reflect the degree of neuronal damage. Furthermore, slow transit constipation causes impaired contractility measured with the WMC in the morning compared to normal transit constipation and healthy bowel habits (Surjanhata et al. Citation2016). In the present study, we did not characterize gastrointestinal symptoms of the participants, and, therefore, we cannot rule out that a large variation in participant phenotypes may explain our findings.
There are several limitations to this study. Firstly, data for the present study represent secondary analyses; however, the WMC procedures were similar between the studies, and only 13 of the 103 participants ingested the WMC approximately 0.5–1.5 h later in the morning than specified. The WMC measures pressure, but it cannot distinguish between propagating or retrograde peristalsis, or if the pressure is simply a result of uncoordinated static muscle tone. Therefore, we were not able to distinguish the effectiveness and propulsion of colonic peristalsis between the groups. Furthermore, others have shown that pressure activity of the transverse/descending colon is significantly higher than in the rectosigmoid colon within the first hour after meal ingestion (Rao et al. Citation2000). Since it is not possible to distinguish the different segments of the colon using the WMC, the segmental difference in colonic pressure activity may have impacted the results of our study.
We did not control the food intake of the participants prior to or during the study, which may have influenced our results. Especially the dietary fiber content of food prior to the test day affects colonic motility dramatically, e.g., eating an additional 9 g of dietary fiber for 3d has been shown to decrease colonic transit time by 10.8 h (Timm et al. Citation2011). Also, food consumption in proximity to the time frames of interest would elicit a gastrocolic response and affect the measurement (Battle et al. Citation1980). However, standardizing the timing and composition of food intake could disturb the natural circadian rhythm of the participants’ gastrointestinal tract, and, therefore, we aimed at maintaining the natural rhythm of the individuals.
In summary, the presence of T1D/+DSPN did not seem to affect contractility of the colon in the morning, evening, or night in the present study. We did, however, confirm that colonic contractility is in general greatest in the morning and almost absent at night.
Acknowledgements
Parts of this study were presented in abstract form at the Biennial Meeting of the European Society of Neurogastroenterology and Motility, 5th to 7th September 2019, Lisbon, Portugal and the 10th Annual Center for Circadian Biology Meeting, 4th to 6th March, San Diego, USA.
MMJ wrote the manuscript and researched data. ALW researched data, contributed to discussion and reviewed/edited the manuscript. SLJ, PSS, IMNW and VSZ researched data and reviewed/edited the manuscript. KF, JSQ and CB contributed to discussion and reviewed/edited the manuscript. All authors read and approved the final version of the manuscript and declare no conflicts of interest.
CB conceptualized the design and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Asher G, Sassone-Corsi P. 2015. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 161:84–92. doi:https://doi.org/10.1016/j.cell.2015.03.015
- Battle WM, Snape WJ, Alavi A, Cohen S, Braunstein S. 1980. Colonic dysfunction in diabetes mellitus. Gastroenterology. 79:1217–1221. doi:https://doi.org/10.1016/0016-5085(80)90916-6
- Bostwick J, Nguyen D, Cornélissen G, Halberg F, Hoogerwerf WA. 2010. Effects of acute and chronic STZ-induced diabetes on clock gene expression and feeding in the gastrointestinal tract. Mol Cell Biochem. 338:203–213. doi:https://doi.org/10.1007/s11010-009-0354-4
- Domènech A, Pasquinelli G, De Giorgio R, Gori A, Bosch F, Pumarola M, Jiménez M. 2011. Morphofunctional changes underlying intestinal dysmotility in diabetic RIP-I⁄hIFNb transgenic mice. Int J Exp Path. 92:400–412. doi:https://doi.org/10.1111/j.1365-2613.2011.00789.x
- Farmer AD, Pedersen AG, Brock B, Jakobsen PE, Karmisholt J, Mohammed SD, Scott SM, Drewes AM, Brock C. 2017. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia. 60:709–718. doi:https://doi.org/10.1007/s00125-016-4199-6
- Farmer AD, Scott SM, Hobson AR. 2013. Gastrointestinal motility revisited: the wireless motility capsule. United European Gastroenterol J. 1:413–421. doi:https://doi.org/10.1177/2050640613510161
- Farmer AD, Wegeberg AL, Brock B, Hobson AR, Mohammed SD, Scott SM, Bruckner-Holt CE, Semler JR, Hasler WL, Hellström PM, et al. 2018. Regional gastrointestinal contractility parameters using the wireless motility capsule: inter-observer reproducibility and influence of age, gender and study country. Alimen Pharmacol Ther. 47:391–400. doi:https://doi.org/10.1111/apt.14438
- Gregersen H, Liao D, Drewes AM, Drewes AM, Zhao J. 2016. Ravages of diabetes on gastrointestinal sensory-motor function: implications for pathophysiology and treatment. Curr Gastroenterol Rep. 18:1–10. doi:https://doi.org/10.1007/s11894-015-0481-x
- Hoogerwerf WA, Shahinian VB, Cornélissen G, Halberg F, Bostwick J, Timm J, Bartell PA, Cassone VM. 2010. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol. 298:143–150. doi:https://doi.org/10.1152/ajpgi.00402.2009
- Maqbool S, Parkman HP, Friedenberg FK. 2009. Wireless capsule motility: comparison of the smartPill® GI monitoring system with scintigraphy for measuring whole gut transit. Digest Dis Sci. 54:2167–2174. doi:https://doi.org/10.1007/s10620-009-0899-9
- Meldgaard T, Olesen SS, Farmer AD, Krogh K, Wendel AA, Brock B, Drewes AM, Brock C. 2018. Diabetic enteropathy: from molecule to mechanism-based treatment. J Diabetes Res. doi:https://doi.org/10.1155/2018/3827301.
- Rao SSC, Kavelock R, Beaty J, Ackerson K, Stumbo P. 2000. Effects of fat and carbohydrate meals on colonic motor response. Gut. 46:205–211. doi:https://doi.org/10.1136/gut.46.2.205
- Rao SSC, Sadeghi P, Beaty J, Kavlock R, Ackerson K. 2001. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 280:629–639. doi:https://doi.org/10.1152/ajpgi.2001.280.4.G629
- Rouphael C, Arora Z, Thota PN, Lopez R, Santisi J, Funk C, Cline M. 2017. Role of wireless motility capsule in the assessment and management of gastrointestinal dysmotility in patients with diabetes mellitus. Neurogastroenterol Motil. 29:1–7. doi:https://doi.org/10.1111/nmo.13087
- Surjanhata B, Guay L, Kuo B. 2016. Blunted contractility in slow transit constipation during awakening compared to healthy and normal transit constipation subjects as evaluated by wireless motility capsule. Gastroenterology. 150:S535. Abstract. doi:https://doi.org/10.1016/S0016-5085(16)31842-X.
- Timm D, Willis H, Thomas W, Sanders L, Boileau T, Slavin J. 2011. The use of a wireless motility device (SmartPill) for the measurement of gastrointestinal transit time after a dietary fibre intervention. Br J Nutr. 105:1337–1342. doi:https://doi.org/10.1017/S0007114510004988.
- Wang YT, Mohammed SD, Farmer AD, Wang D, Zarate N, Hobson AR, Hellström PM, Semler JR, Kuo B, Rao SS, et al. 2015. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther. 42:761–772.
- Zarrinpar A, Chaix A, Panda S. 2016. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 27:69–83. doi:https://doi.org/10.1016/j.tem.2015.11.007
