ABSTRACT
Verapamil is the first-line preventive medication for cluster headache, an excruciating disorder with strong circadian features. Whereas second- and third-line preventives include known circadian modulators, such as melatonin, corticosteroids, and lithium, the circadian effects of verapamil are poorly understood. Here, we characterize the circadian features of verapamil using both in vitro and in vivo models. In Per2::LucSV reporter fibroblasts, treatment with verapamil (0.03–10 µM) showed a dose-dependent period shortening of the reporter rhythm which reached a nadir at 1 µM, and altered core clock gene expression at 10 µM. Mouse wheel-running activity with verapamil (1 mg/mL added to the drinking water) also resulted in significant period shortening and activity reduction in both male and female free-running wild-type C57BL6/J mice. The temporal patterns of activity reduction, however, differ between the two sexes. Importantly, piezo sleep recording revealed sexual dimorphism in the effects of verapamil on sleep timing and bout duration, with more pronounced adverse effects in female mice. We also found altered circadian clock gene expression in the cerebellum, hypothalamus, and trigeminal ganglion of verapamil-treated mice. Verapamil did not affect reporter rhythms in ex vivo suprachiasmatic nucleus (SCN) slices from Per2:Luc reporter mice, perhaps due to the exceptionally tight coupling in the SCN. Thus, verapamil affects both peripheral (trigeminal ganglion) and central (hypothalamus and cerebellum) nervous system structures involved in cluster headache pathophysiology, possibly with network effects instead of isolated SCN effects. These studies suggest that verapamil is a circadian modulator in laboratory models at both molecular and behavioral levels, and sex is an important biological variable for cluster headache medications. These observations highlight the circadian system as a potential convergent target for cluster headache medications with different primary mechanisms of action.
Introduction
Cluster headache (CH) is a headache disorder widely regarded as one of the most painful human experiences (Burish et al. Citation2020) with a high rate of suicidal thoughts and attempts (Ji Lee et al. Citation2019; Rozen and Fishman Citation2012; Trejo-Gabriel-Galan et al. Citation2018). The diagnosis of cluster headache is made by defining criteria: 1–8 headache attacks per day of one side of the face, with each attack lasting between 15 min and 3 h, associated with restlessness and/or cranial autonomic features, such as a bloodshot eye and nasal congestion on the same side as the pain (Headache Classification Committee of the International Headache Society (IHS) Citation2018).
In addition to these defining criteria, CH has remarkable circadian features. A total of 82% of the patients have headaches at the same time each day (Rozen and Fishman Citation2012); while there is inter-individual variability, the most common attack in day-active persons time is 02:00 h, regardless of time zone (Barloese et al. Citation2015; Rozen and Fishman Citation2012; Steinberg et al. Citation2018). Anatomical imaging studies in CH have shown enlargement of the anterior hypothalamus, the location of the central pacemaker, the suprachiasmatic nuclei (Arkink et al. Citation2017). Physiology studies have shown alterations in two circadian-related hormones, melatonin and corticosteroids, in CH patients (Bruera et al. Citation2008; Chazot et al. Citation1984; Leone et al. Citation1998; Waldenlind et al. Citation1987). Of note, melatonin and corticosteroids are also effective treatments for CH (May et al. Citation2006).
Molecularly, the circadian system consists of cell-autonomous molecular oscillators which drive a cycle of activation and inhibition of gene expression lasting approximately 24 h. The oscillators can be modulated by external stimuli, including light (Albrecht Citation2012; Hughes et al. Citation2015; LeGates et al. Citation2014), food (Edmonds and Adler Citation1977; Lewis et al. Citation2020), and medications (Chen et al. Citation2018; Tamai et al. Citation2018). These core oscillators are present in most cell types in the body and are regulated by the suprachiasmatic nucleus (SCN) in the anterior hypothalamus. Lymphoblasts from CH patients show reduced expression of the circadian gene Nr1d1 (encoding the nuclear receptor REV-ERBα) compared to control subjects (Costa et al. Citation2015).
Unfortunately, current CH treatments, especially preventive medications, are not particularly effective for many patients: based on patient reports, the most effective preventive treatments are corticosteroids and verapamil, but they are highly effective in only about 50% of the patients (Lademann et al. Citation2015). Due in part to serious side effects of corticosteroids with long-term use, verapamil is considered the first-line preventive medication (May et al. Citation2018; McGeeney Citation2018). Verapamil is a canonical L-type calcium channel antagonist, though it also affects other calcium channels, sodium channels, potassium channels, and p-glycoprotein (Lemma et al. Citation2006; Tfelt-Hansen and Tfelt-Hansen Citation2009). Although the transport of verapamil across the blood–brain barrier is limited by p-glycoprotein (Luurtsema et al. Citation2005; Petersen et al. Citation2019; Römermann et al. Citation2013), verapamil has known binding targets in the SCN (Nahm et al. Citation2005). Verapamil’s mechanism of action in CH is not fully understood (Petersen et al. Citation2019): many second- and third-line CH preventives do not have strong calcium channel inhibition, including melatonin, corticosteroids, lithium, and valproate (Burish et al. Citation2019). However, many second- and third-line CH medications, including all those listed here, have putative effects on the molecular circadian system (Dickmeis et al. Citation2013; Johansson et al. Citation2011; Kandalepas et al. Citation2016; Li et al. Citation2012; Meneses-Santos and Buonfiglio Citation2018; Yin et al. Citation2006). The molecular circadian effects of verapamil are not known, but clinical observations have noted that CH patients taking verapamil have headaches approximately 1 h later than CH patients not taking verapamil (Barloese et al. Citation2018).
Given the lack of a clear common mechanism for preventive medications in CH and the multiple lines of evidence for circadian effects of CH medications and CH, itself, we sought to investigate the effects of the first-line medication verapamil on molecular and behavioral circadian rhythms. To address this, we analyzed the effects of verapamil on circadian reporter cells (Per2::LucSV mouse fibroblasts) and mouse wheel-running and sleep behaviors. We show that verapamil has circadian effects both in vitro and in vivo, shortening the period of PER2 protein oscillation in cells, shortening the period of mice on running wheels, and altering the expression of core circadian genes in reporter cells as well as the hypothalamus, cerebellum, and trigeminal ganglion. Furthermore, verapamil displays sexual dimorphic effects on sleep timing (daytime vs. nighttime) and sleep bout length. These studies demonstrate a clear circadian effect of verapamil on core oscillators and behavioral rhythms.
Materials and methods
Animals
Animal husbandry and experiments were carried out under international ethical standards (Portaluppi et al. Citation2010), ARRIVE guidelines (Percie Du Sert et al. Citation2020), and local IACUC guidelines in an animal protocol approved by the University of Texas Health Science Center at Houston (UTHSC-H). A total of 129 mice were used in this study.
Cell culture studies
Adult mouse ear fibroblast cells isolated from Per2::LucSV knock-in mice by replacement of the 3ʹ-UTR with an SV40 late poly(A) sequence (Yoo et al. Citation2017) were used for real-time bioluminescence monitoring. Cells were grown to confluency on 35 mm plates in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin. Cells were synchronized with 200 nM dexamethasone (Sigma–Aldrich) for 1 h, and verapamil (Sigma-Aldrich) was then added at concentrations of 0 (nothing added), 0.03, 0.1, 0.3, 1, 3, and 10 µM, along with luciferin-containing recording media (Yoo et al. Citation2004). The plates were then tightly sealed with vacuum grease and placed in a luminometer (LumiCycle 32, Actimetrics) for continuous bioluminescence monitoring over 6d. The data were detrended using a first-order polynomial, and then best-fit to a sine wave estimated by a Levenberg–Marquardt algorithm for measurement of circadian parameters in the LumiCycle data analysis program (Actimetrics). For real-time qPCR analysis of core clock genes, cells were synchronized with 200 nM dexamethasone (Sigma–Aldrich). Verapamil (Sigma-Aldrich) was then added at concentrations of 3 and 10 µM in recording media. Cells were harvested every 4 h for 28 h (8 time points) and total RNA were extracted.
RNA extraction and real-time RT-PCR analysis
RNA extraction and real-time RT-PCR analysis were carried out as previously described (Nohara et al. Citation2020). Expression levels of 10 genes were analyzed using real-time RT-PCR. Total RNA from Per2::LucSV mouse fibroblasts, cerebellum, hypothalamus, and trigeminal ganglion was isolated using PureXtract RNAsol reagent (GenDEPOT, TX, USA) as indicated by the manufacturer’s protocol. Reverse transcription was performed by cDNA synthesis kit (GenDEPOT, TX, USA). All real-time RT-PCR reactions were performed with SYBR Green PCR Master Mix kits (GenDEPOT, TX, USA) on QuantStudio 7 Flex system (Applied Biosystems). Data were analyzed using Prism 8 software (GraphPad Software, Inc.). Gapdh and beta-actin were used as the housekeeping gene for controls. The primer sets used are shown in .
Table 1. qPCR primer sequences
Mouse wheel-running behavioral study
C57BL6/J mice (Jackson Laboratory) at 9 weeks of age were transferred into individual cages equipped with running wheels. Mice were acclimated for 2 weeks in a 12 h light/12 h dark cycle (LD, light levels 300 lux, room temperature and relative humidity were maintained at 22.6–24.1°C and 38–42%, respectively), followed by 2 weeks in constant darkness (DD) to measure baseline free-running periods in DD. Once a circadian period was established in DD (the free-running period), we measured the effects of verapamil on the free-running period by changing regular water to verapamil (1 mg/mL)-containing water and monitoring mouse activities for an additional 2 weeks. Water intake was measured once per week for measurements of verapamil intake in the first 29 mice. Activity data were recorded continuously by a PC system (Chronobiology Kit, Stanford Software Systems) and analyzed using CLOCKLAB software (Actimetrics). Free-running period was calculated using a periodogram with 6 min resolution (CLOCKLAB). Wheel-running activity level was quantified as a summation every 20 min for each mouse and averaged during the 14d data collection.
Piezo sleep recording
Sleep/wake recording was performed with a noninvasive piezoelectric transducer sleep/wake recording system (Signal Solutions, Inc.) as previously described (Nohara et al. Citation2019). The initial 48 h acclimation period was followed by data recording for 2d. Data were extracted and analyzed by using the Sleepstats software (Signal solutions, Inc).
Real-time bioluminescence measurement from SCN ex vivo cultures
Circadian bioluminescence measurement was performed as previously described (Yoo et al. Citation2004). Briefly, SCNs were dissected and sliced with oscillating tissue slicer (OTS-5000) into 300 um thickness. SCN slices were cultured on Millicell culture membranes (PICMORG50, Millipore) in 35 mm tissue culture dishes containing 2 mL DMEM media (Invitrogen) supplemented with 352.5 μg/ml sodium bicarbonate, 10 mM HEPES (Invitrogen), 2 mM l-Glutamine, 2% B-27 Serum-free supplement (Invitrogen), 25 units/ml penicillin, 25 μg/ml streptomycin (Invitrogen), and 0.1 mM luciferin potassium salt (L-8240, Biosynth AG). DMSO or verapamil (10 uM) was added to the recording media. Bioluminescence was recorded continuously using the LumiCycle luminometer (Actimetrics). Data were analyzed using LumiCycle data analysis program (Actimetrics).
Statistical analysis
Sample size was based on previous studies (Duong et al. Citation2011; Nohara et al. Citation2019). No data were excluded, and no randomization or blinding was performed. Strategies were put in place to minimize confounders, included isolating animals in individual cages and placing all animals in the same cabinet of recording boxes. Data are presented as mean ± standard deviation (cell culture data) or mean ± standard error of the mean (wheel-running and sleep data). Statistical significance was determined by two-tailed Student’s t-test (Excel, Microsoft Office Professional Plus version 2016) and two-way ANOVA with Sidak’s multiple comparison test (GraphPad Prism version 8.20). P < .05 was considered statistically significant.
Results
Verapamil shortens the reporter period of Per2::LucSV mouse fibroblasts
For cell culture dose determinations, the dose was based on the effective oral dose of verapamil in CH patients of 200–960 mg/d (Blau and Engel Citation2004; A May et al. Citation2006; Petersen et al. Citation2019; Tfelt-Hansen and Tfelt-Hansen Citation2009). We used data from the following studies to help determine our dose range. A single oral dose of verapamil 80 mg in humans results in peak serum plasma levels of 38.4 ng/mL (McAllister and Kirsten Citation1982) or 0.08 µM, while a single oral dose of verapamil 160 mg results in a peak plasma level of 90.2 ng/mL (McAllister and Kirsten Citation1982) or 0.20 µM. Verapamil extended release 240 mg results in a peak serum plasma level between 80 and 164 ng/mL (fda.gov Citation2019) or 0.19–0.36 µM with a nonlinear correlation between dose and plasma level. A chronic 480 mg total daily dose of verapamil (120 mg four times daily) results in plasma levels between 125 and 400 ng/mL (fda.gov Citation2019) or 0.27–0.88 µM. Taken together, we chose doses between 0.03 and 10 µM.
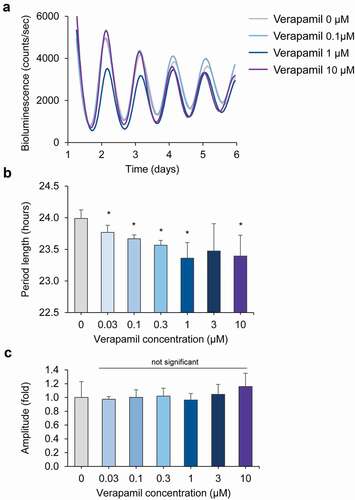
To investigate the effect of the first-line CH medication verapamil on molecular circadian rhythms, we used Per2::LucSV fibroblast cells, which express PER2::LUC fusion proteins from the endogenous Per2 gene promoter to report circadian molecular rhythms with high sensitivity (Chen et al. Citation2012; Yoo et al. Citation2017). We performed whole-field real-time bioluminescence recordings of Per2::LucSV fibroblast cell cultures. Verapamil significantly shortened the period at most concentrations between 0.03 and 10 µM (). There was a dose-dependent period shortening from 0.03 to 1 µM, and the effect reached a nadir of 23.4 h at 1 µM (the period was 24.0 h in controls). Verapamil did not significantly change the reporter amplitude of Per2::LucSV fibroblast cell cultures (). These results provide evidence that verapamil specifically alters circadian periodicity at the oscillator level, without changing its robustness.
Figure 1. Verapamil shortens the circadian period of mouse Per2::lucSV reporter fibroblast cells. (a) Representative PER2::LUC bioluminescence recording of Per2::LucSV fibroblast cells. PER2::LUC fusion protein oscillations show a period shortening effect with verapamil compared to controls. (b) Average circadian period lengths for reporter fibroblast cells treated with increasing concentrations of verapamil (n = 4 for each concentration). Error bars represent standard deviation, * represents a significant (p < .05) change compared to control (“Verapamil 0”). (c) Verapamil does not cause amplitude changes in Per2::LucSV fibroblast cells. Average amplitude values for fibroblasts treated with increasing concentrations of verapamil (n = 4 for each concentration) are shown. Error bars represent standard deviation, p < .05 indicates significance
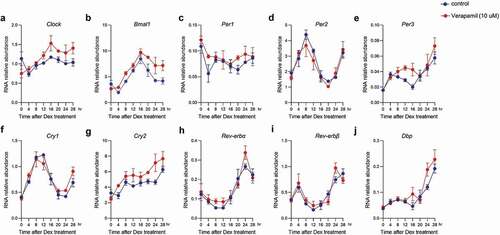
To investigate the molecular basis of verapamil’s effects on the circadian oscillator, we measured clock gene expression in Per2::LucSV fibroblast cells. At 10 µM verapamil concentration, we observed significant effects of verapamil on mRNA expression of Clock, Bmal1, Per1, Per3, Cry2, and Dbp (). There was no significant change in Per2, Cry1, Rev-erbα, and Rev-erbβ. At a lower verapamil concentration of 3 µM, only Clock displayed altered mRNA expression, while all other genes were not significantly different from control (Supplemental ).
Figure 2. Real time qPCR analysis of clock gene expression. (A-J) Circadian expression of Clock, Bmal1, Per1, Per2, Per3, Cry1, Cry2, Rev-erbα, Rev-erbβ, and Dbp in Per2::lucSV reporter cells quantified by qRT-PCR for control (blue) and verapamil 10 uM (red). Data are shown as mean ± SEM every 4 h for 28 h (n = 3). Two-way ANOVA with Sidak’s multiple comparison test showed significant differences between control and Clock (p = .076), Bmal1 (p = .0001), Per1 (p = .0315), Per3 (p = .0199), Cry2 (p = .0004), and Dbp (p = .0345). The other clock genes examined were not significantly different from control
Verapamil shortens the period of free-running C57BL6/J mice
We next studied the effects of verapamil in an animal behavioral model. For mouse behavioral assay dose determinations, the dose of 1 mg/mL verapamil was chosen based on the conversion from human to mouse dosing from the FDA’s “Guidance for Industry for Estimating Safe Starting Doses for Clinical Trials” (DHHS et al. Citation2005). The initial conversion of dosage (mg/kg) to drinking water (mg/mL) assumed a daily water intake of 4 mL and weight of approximately 25 g/20 g (male/female) in 12-week C57BL6/J mice. This dose corresponds to 768 mg (male) and 960 mg (female) daily doses in a 60 kg human. The dose of 1 mg/mL verapamil in the drinking water has also been used in prior mouse studies (Abais et al. Citation2014; Chandra et al. Citation2002; Dong et al. Citation1992; Morris et al. Citation1989).
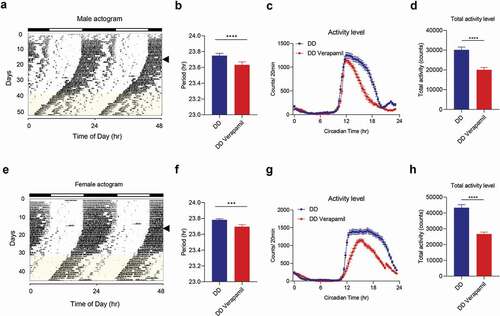
C57BL6/J wild-type mice were individually housed in cages with running wheels. To establish baseline circadian rhythms, mice were given food and water ad libitum for 2 weeks in a 12 h light/12 h dark cycle (LD), followed for up to 4 weeks in constant darkness (DD). Once a circadian period was established in DD (the free-running period), we measured the effects of verapamil on the free-running period by changing regular water to 1 mg/mL verapamil-containing water. The mice continued in DD and had access to food and verapamil water ad libitum for an additional 2 weeks. Water intake averages (across the first 29 mice) were 7.4 ml (LD), 7.9 ml (DD), and 6.5 ml (DD/Verapamil water) per day, which are within the normal range (Bachmanov et al. Citation2002). The free-running period was found to be significantly shortened after treatment with verapamil in both male (23.75 ± 0.03 h vs. 23.63 ± 0.04 h, p < .0001) and female mice (23.78 ± 0.01 h vs. 23.70 ± 0.02 h, p < .001) ( for males and E and F for females). Likewise, verapamil significantly reduced activity levels in both sexes (males: 30,162 ± 1425 counts/20 min vs. 20,071 ± 1191 counts/20 min; p < .0001; females: 43,295 ± 1991 counts/20 min vs. 26,695 ± 1215 counts/20 min; p < .0001) ( for males and G and H for females). In male mice, verapamil did not affect the activity onset (which occurred around Circadian Time 12), but it decreased the subsequent activity. Interestingly, however, activities of female mice were reduced throughout the active period to a greater extent, suggesting a sexual dimorphic effect on the temporal pattern of activity between males and females.
Figure 3. Verapamil treatment shortens circadian wheel-running periods in mice. (a–d) Male mice (n = 29). (e–h) Female mice (n = 30). (a and e) Representative actograms are shown for C56BL6/J male and female mice. Arrowheads indicate the LD (light:dark) to DD (dark:dark) transition. Water containing Verapamil (1 mg/ml) was administered during the interval indicated by yellow shading on the actogram. (b and f) Free-running period of C56BL6/J male and female mice under DD (constant darkness) for normal water. Error bars represent ± SEM. (c and g) Average wave plots summarizing wheel-running activity during DD for normal water, and verapamil water. (d and h) Daily total wheel-running activity during DD for normal water and verapamil water. Data are presented as mean ± SEM. T-test shows the significant statistical differences between normal water and verapamil water (***, p < .001; ****, p < .0001)
Verapamil displays sex-specific effects on sleep
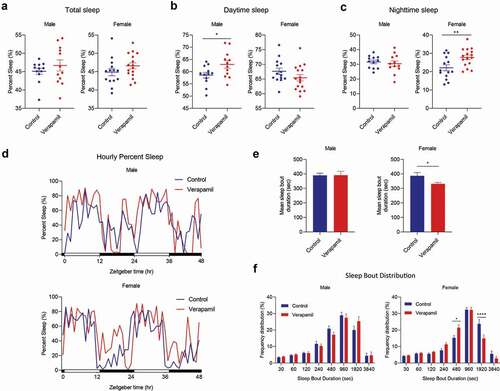
Next, we employed a piezo noninvasive sleep assay (Nohara et al. Citation2019) to investigate whether verapamil affects sleep. Male and female mice were pretreated with verapamil for two to three weeks prior to sleep measurements and compared to control mice receiving regular water. Although the total amount of sleep was not altered by verapamil (), male and female mice exhibited distinct sleep changes in response to verapamil exposure (sss). Whereas verapamil-treated male mice showed significantly more daytime sleep (58.6 ± 1.2% vs. 63.0 ± 1.6%, p < .05), in female mice, verapamil increased sleep amount during the nighttime (22.1 ± 1.7% vs. 27.8 ± 1.2%, p < .01), the active phase for mice. Mean sleep bout duration time was significantly reduced by verapamil treatment only in female mice (). Furthermore, histogram analysis of sleep bout distribution revealed a trend toward shorter bouts in female mice, while male mice did not exhibit significant changes (). These observations indicate strong sexual dimorphic effects of verapamil on mouse sleep, with adverse effects in female mice, including increased nighttime sleep and shorter bout duration.
Figure 4. Verapamil shows sex-specific effects on sleep timing and quality in C57BL6/J mice. (a) Total percent sleep time of male and female mice with normal water (blue) and verapamil water (red). (b) Percent daytime sleep of male and female mice with normal water (blue; male n = 12, female n = 14) and verapamil water (red; male n = 12, female n = 16). (c) Percent nighttime sleep of male and female mice with normal water (blue) and verapamil water (red). T-test shows the significant statistical difference in percent sleep time between normal and verapamil water (*, p < .05; **, p < .01). (d) Representative sleep recording for hourly sleep percentage male and female mice with normal water (blue) and verapamil water (red). (e) Mean sleep bout duration for male and female mice with normal water (blue) and verapamil water (red). Data are presented as mean ± SEM. (f) Sleep bout length distribution of male and female mice with normal water (blue) and verapamil water (red). Data are presented as mean ± SEM. Two-way ANOVA with Sidak’s multiple comparison test shows the significant statistical difference between percent sleep of normal and verapamil water (*, p < .05; ****, p < .0001)
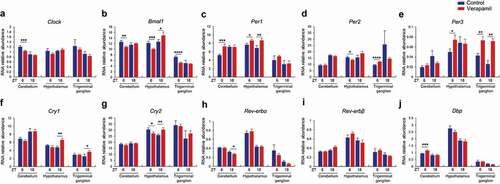
Verapamil alters clock gene expression in central and peripheral regions relevant for CH
The hypothalamus and cerebellum are central nervous system structures known to have pronounced circadian oscillations and may be important structures in the pathophysiology of cluster headache (Arkink et al. Citation2017; Clelland et al. Citation2014; May et al. Citation1998; Naegel et al. Citation2014; Teepker et al. Citation2012; Yang et al. Citation2015). The trigeminal ganglion is an important peripheral nervous system structure for cluster headache pain (Jarrar et al. Citation2003; May et al. Citation2018; McGeeney Citation2018), though its circadian oscillations are not well studied. To examine whether verapamil treatment can affect the expression of clock genes in these areas, we measured mRNA expression levels of 10 core clock genes collected at ZT6 and ZT18 from verapamil-treated and control mice (). Verapamil altered circadian expression of Clock, Bmal1, Per1, Rev-erbα, and Dbp at ZT6 significantly in the cerebellum from verapamil-treated mice compared to control mice. In the hypothalamus, verapamil altered expression of Bmal1, Per1, and Cry2 at both time points, changed expression of Per2 and Per3 at ZT6, and changed Cry1 expression at ZT18. Furthermore, in the trigeminal ganglion, we found altered mRNA expression of Per3 at both time points, altered mRNA expression of Bmal1 and Per2 at ZT6, and altered mRNA expression of Cry1 at ZT18. Taken together, these results show that verapamil can alter circadian clock gene expression in brain regions and in the trigeminal ganglion. Interestingly, Bmal1 mRNA expression was consistently downregulated by verapamil at ZT6 in all tissues as compared to control.
Figure 5. Verapamil alters circadian expression of clock genes. (a–j) Circadian expression of Clock, Bmal1, Per1, Per2, Per3, Cry1, Cry2, Rev-erbα, Rev-erbβ, and Dbp in the cerebellum, hypothalamus, and trigeminal ganglion of control (blue bar, n = 4 for ZT 6, n = 3 for ZT18) and verapamil treated male mice (red bar, n = 4 for ZT6, n = 3 for ZT18). The data are shown as mean ± SEM (*, p < .05; **, p < .01; ***, p < .001; ****, p < .0001). Two-tailed unpaired t-test, p < .05 was considered significant
We next sought to understand the effect of verapamil on the central clock of the circadian system, using SCN ex vivo cultures from Per2::Luc mice. Compared with control (DMSO), SCN cultures treated with verapamil 10 µM did not show a significant difference in PER2::LUC oscillation patterns (Supplemental Figure 2A). Accompanying analyses of circadian parameters revealed that the period length (Supplemental Figure 2B; Mean value, 24.6 ± 0.15 h for DMSO vs. 24.67 ± 0.38 h for 10 µM verapamil) and fold amplitudes (Supplemental Figure 2C; Mean value, 1.15 ± 0.2 for DMSO vs 1.09 ± 0.12 for 10 µM verapamil) of the bioluminescence oscillations were not significantly altered in SCN cultures treated with verapamil compared with control.
Discussion
In this study, we show that verapamil, the first-line preventive medication for CH, has circadian effects at both the cellular and behavioral levels. Specifically, verapamil altered the core circadian oscillators by shortening the period in Per2::LucSV fibroblasts. This core circadian alteration also had a behavioral correlate, as verapamil also shortened the free-running period of C57BL6/J wild-type mice. Importantly, verapamil displayed sex-specific effects, including differential wheel-running activities and sleep patterns, between male and female mice. The exaggerated nighttime sleep and reduced sleep bout lengths, indicative of dysregulated sleep timing and consolidation, suggest sleep perturbation by verapamil in female mice.
As the central pacemaker is believed to be primarily responsible for driving behavioral rhythms (Moore and Eichler Citation1972; Ralph et al. Citation1990; Stephan and Zucker Citation1972), our results suggest a central action of verapamil consistent with our current understanding of CH as a central nervous system disorder (May et al. Citation2018). The hypothalamus is considered a potential site for the initiation of a cluster headache attack (May et al. Citation2018; McGeeney Citation2018), and verapamil alters core circadian genes in the hypothalamus (specifically Bmal1, Per1, Per2, Per3, Cry1, and Cry2 in our experiments). In SCN ex vivo slices, however, there was no change in PER2::LUC reporter oscillations, perhaps due to the exceptionally tight coupling of the SCN clock known to be resistant to genetic and pharmacological manipulation (Chen et al. Citation2012; Liu et al. Citation2007). Of note, when verapamil was administered to mice systemically via drinking water, Bmal1 expression was broadly altered by verapamil in all tissues tested. Bmal1 was also altered by verapamil 10 uM in our experiments on fibroblasts cultures. These findings suggest general effects of verapamil on Bmal1, including structures relevant to cluster headache.
While our data suggest a period-shortening effect, a human observational study suggests that CH patients display a 1 h phase delay in the timing of attacks if they take verapamil (Barloese et al. Citation2018). Typically, phase delays in humans are associated with period lengthening (not shortening) in rodent models, as is seen in delayed sleep phase disorder and mutations in CRY1 (Patke et al. Citation2017). To our knowledge, no circadian free-running experiments in humans have been performed with verapamil to reconcile the human phase delay with our findings of period-shortening. With our current lack of information on the molecular circadian alterations of cluster headache patients, it is difficult to directly compare the findings of clinical and laboratory studies. Furthermore, there is currently no animal model of CH that includes a circadian timing of attacks (only animal models that explore the trigeminal pain system (Harriott et al. Citation2019) or the autonomic system (Akerman et al. Citation2009)). However, verapamil’s circadian effects appear to extend to humans, and additional studies are warranted to investigate the specific circadian effect of verapamil in humans.
In humans, CH is more common in males at a ratio of 4.3:1 (Fischera et al. Citation2008). It has been suggested that verapamil is less effective in women, though no systematic studies have been performed (Petersen et al. Citation2019). In our study, we found that verapamil caused more pronounced disturbances in female sleep, specifically sleep timing (more sleep during the active phase) and quality (decreased sleep bout length). In addition, verapamil also altered the temporal pattern of wheel-running activity in a sex-specific manner, again with more significant changes in female mice. Sleep disturbances are a rare but occasionally documented side effect of verapamil in humans (PDR Citation2020), though sex-specific differences in sleep disturbances are not clear. Future studies are needed to investigate whether this sexual dimorphism is related to sex-dependent CH disease manifestations and treatment efficacy in humans.
In the treatment of CH, one issue limiting drug development is the lack of a common mechanism amongst preventive medications. Our study reveals an interesting circadian modulatory mechanism shared by verapamil, corticosteroids, melatonin, lithium, and valproate because of the prominent circadian features of this disease. Additional studies are needed to determine the importance of the circadian effects of these medications in CH and to investigate the specific shared mechanism: these medications might have a common circadian target or might have different targets that result in a convergent effect on the core circadian oscillators.
In conclusion, our work reveals verapamil as a clock-altering drug, shortening the circadian period at both the molecular and behavioral levels. Importantly, we observed significant sex-specific effects of verapamil on sleep and wheel-running behaviors, consistent with the notion that sex is an important biological variable for CH and its medications. Verapamil is the first-line preventive medication for CH, a disorder with strong circadian features, and several CH medications share a circadian-altering effect but are otherwise unrelated mechanistically. Additional studies are needed to understand whether these medications share an ability to modulate circadian rhythms, which will facilitate future chronotherapeutic developments against CH.
Declaration of Conflicting Interests
The authors declare no conflicting interests.
Supplemental Material
Download Zip (612.5 KB)Acknowledgements
We thank Joseph Takahashi for insights into circadian medication effects. We thank Brian Lam and Jiah Yang for assistance with cell culture work. This work was supported by The National Headache Foundation and The Will Erwin Headache Research Foundation (to M.J.B.) and the Welch Foundation (AU-1971-20180324 and AU-1731-20190330 to S.-H.Y. and Z.C., respectively).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Abais JM, Xia M, Li G, Chen Y, Conley SM, Gehr TWB, Boini KM, Li PL. 2014. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J Biol Chem. 289(39):27159–27168. doi:https://doi.org/10.1074/jbc.M114.567537
- Akerman S, Holland PR, Lasalandra MP, Goadsby PJ. 2009. Oxygen inhibits neuronal activation in the trigeminocervical complex after stimulation of trigeminal autonomic reflex, but not during direct dural activation of trigeminal afferents: harold g. wolff lecture award winner. Headache. 49(8):1131–1143. doi:https://doi.org/10.1111/j.1526-4610.2009.01501.x
- Albrecht U. 2012. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 74(2):246–260. doi:https://doi.org/10.1016/j.neuron.2012.04.006
- Arkink EB, Schmitz N, Schoonman GG, Van Vliet JA, Haan J, Van Buchem MA, Ferrari MD, Kruit MC. 2017. The anterior hypothalamus in cluster headache. 37. 11:1039–1050. doi:https://doi.org/10.1177/0333102416660550
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 32(6):435–443. doi:https://doi.org/10.1023/A:1020884312053
- Barloese M, Lund N, Petersen A, Rasmussen M, Jennum P, Jensen R. 2015. Sleep and chronobiology in cluster headache. Cephalalgia. 35(11):969–978. doi:https://doi.org/10.1177/0333102414564892
- Barloese M, Haddock B, Lund NT, Petersen A, Jensen R. 2018. Chronorisk in cluster headache: a tool for individualised therapy? Cephalalgia. 38(14):2058–2067. doi:https://doi.org/10.1177/0333102418769955.
- Blau JN, Engel HO. 2004. Individualizing treatment with verapamil for cluster headache patients. Headache J Head Face Pain. 44(10):1013–1018. doi:https://doi.org/10.1111/j.1526-4610.2004.04196.x
- Bruera O, Sances G, Leston J, Levin G, Cristina S, Medina C, Barontini M, Nappi G, Figuerola MDL. 2008. Plasma melatonin pattern in chronic and episodic headaches. Evaluation during sleep and waking. Funct Neurol. 23(2):77–81.
- Burish MJ, Chen Z, Yoo SH 2019. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiologica. 225(1). doi:https://doi.org/10.1111/apha.13161
- Burish MJ, Pearson SM, Shapiro RE, Zhang W, Schor LI. 2020. Cluster headache is one of the most intensely painful human conditions: results from the International Cluster Headache Questionnaire. Headache. doi:https://doi.org/10.1111/head.14021
- Chandra M, Shirani J, Shtutin V, Weiss LM, Factor SM, Petkova SB, Rojkind M, Dominguez-Rosales JA, Jelicks LA, Morris SA, et al. 2002. Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil Strain): an echocardiographic study. Int J Parasitol. 32(2):207–215. doi:https://doi.org/10.1016/S0020-7519(01)00320-4
- Chazot G, Claustrat B, Brun J, Jordan D, Sassolas G, Schott B. 1984. A chronobiological study of melatonin, cortisol growth hormone and prolactin secretion in cluster headache. Cephalalgia. 4(4):213–220. doi:https://doi.org/10.1046/j.1468-2982.1984.0404213.x
- Chen Z, Yoo S-H, Takahashi JS. 2018. Development and Therapeutic potential of small-molecule modulators of circadian systems. Annu Rev Pharmacol Toxicol. 58(1):231–252. doi:https://doi.org/10.1146/annurev-pharmtox-010617-052645
- Chen Z, Yoo S-HS-H, Park Y-SY-S, Kim K-HK-H, Wei S, Buhr E, Ye Z-Y, Pan H-LH-L, Takahashi JS. 2012. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A. 109(1):101–106. doi:https://doi.org/10.1073/pnas.1118034108
- Clelland CD, Zheng Z, Kim W, Bari A, Pouratian N. 2014. Common cerebral networks associated with distinct deep brain stimulation targets for cluster headache. 34. 3:224–230. doi:https://doi.org/10.1177/0333102413509431
- Costa M, Squassina A, Piras IS, Pisanu C, Congiu D, Niola P, Angius A, Chillotti C, Ardau R, Severino G, et al. 2015. Preliminary transcriptome analysis in lymphoblasts from cluster headache and bipolar disorder patients implicates dysregulation of circadian and serotonergic genes. J Mol Neurosci. 56(3):688–695. doi:https://doi.org/10.1007/s12031-015-0567-9
- DHHS, U. S. D. of H. and H. S., FDA, F. and D. A., & CDER, C. for D. E. and R. 2005. Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf
- Dickmeis T, Weger BD, Weger M. 2013. The circadian clock and glucocorticoids – interactions across many time scales. Mol Cell Endocrinol. 380(1–2):2–15. doi:https://doi.org/10.1016/j.mce.2013.05.012
- Dong R, Liu P, Wee L, Butany J, Sole MJ. 1992. Verapamil ameliorates the clinical and pathological course of murine myocarditis. J Clin Invest. 90(5):2022–2030. doi:https://doi.org/10.1172/JCI116082
- Duong HA, Robles MS, Knutti D, Weitz CJ. 2011. A molecular mechanism for circadian clock negative feedback. Science. 332(6036):1436–1439. doi:https://doi.org/10.1126/science.1196766
- Edmonds SC, Adler NT. 1977. Food and light as entrainers of circadian running activity in the rat. Physiol Behav. 18(5):915–919. doi:https://doi.org/10.1016/0031-9384(77)90201-3
- fda.gov. 2019. CALAN SR (verapamil hydrochloride) sustained-release oral caplets: labeling-package insert, suppl-44, reference ID: 4512000. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/019152s044lbl.pdf
- Fischera M, Marziniak M, Gralow I, Evers S. 2008. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. 28. 6:614–618. doi:https://doi.org/10.1111/j.1468-2982.2008.01592.x
- Harriott AM, Strother LC, Vila-Pueyo M, Holland PR. 2019. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J Headache Pain. 20(1):91. doi:https://doi.org/10.1186/s10194-019-1043-7
- Headache Classification Committee of the International Headache Society (IHS). 2018. The international classification of headache disorders, 3rd edition. Cephalalgia. 38(1):1–211. doi:https://doi.org/10.1177/0333102417738202
- Hughes S, Jagannath A, Hankins MW, Foster RG, Peirson SN. 2015. Photic regulation of clock systems. Methods Enzymol. 552:125–143. doi:https://doi.org/10.1016/bs.mie.2014.10.018
- Jarrar RG, Black DF, Dodick DW, Davis DH. 2003. Outcome of trigeminal nerve section in the treatment of chronic cluster headache. Headache. 17(1):39–40. doi:https://doi.org/10.1212/01.wnl.0000055902.23139.16
- Ji Lee M, Cho S-J, Wook Park J, Kyung Chu M, Moon H-S, Chung P-W, Myun Chung J, Sohn J-H, Kim B-K, Kim B-S, et al. 2019. Increased suicidality in patients with cluster headache. Cephalalgia. 033310241984566. doi:https://doi.org/10.1177/0333102419845660
- Johansson AS, Brask J, Owe-Larsson B, Hetta J, Lundkvist GBS. 2011. Valproic acid phase shifts the rhythmic expression of PERIOD2::LUCIFERASE. J Biol Rhythms. 26(6):541–551. doi:https://doi.org/10.1177/0748730411419775
- Kandalepas PC, Mitchell JW, Gillette MU. 2016. Melatonin signal transduction pathways require e-box-mediated transcription of Per1 and Per2 to reset the SCN Clock at Dusk. PLoS One. 11(6):e0157824. doi:https://doi.org/10.1371/journal.pone.0157824
- Lademann V, Jansen J-P, Evers S, Frese A. 2015. Evaluation of guideline-adherent treatment in cluster headache. Cephalalgia. 36(8):760–764. doi:https://doi.org/10.1177/0333102415612774
- LeGates TA, Fernandez DC, Hattar S. 2014. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 15(7):443–454. doi:https://doi.org/10.1038/nrn3743
- Lemma GL, Wang Z, Hamman MA, Zaheer NA, Gorski JC, Hall SD. 2006. The effect of short- and long-term administration of verapamil on the disposition of cytochrome P450 3A and P-glycoprotein substrates. Clin Pharmacol Ther. 79(3):218–230. doi:https://doi.org/10.1016/j.clpt.2005.11.001
- Leone M, Lucini V, D’Amico D, Grazzi L, Moschiano F, Fraschini F, Bussone G. 1998. Abnormal 24-hour urinary excretory pattern of 6-sulphatoxymelatonin in both phases of cluster headache. Cephalalgia. 18(10):664–667. doi:https://doi.org/10.1046/j.1468-2982.1998.1810664.x.
- Lewis P, Oster H, Korf HW, Foster RG, Erren TC. 2020. Food as a circadian time cue — evidence from human studies. Nat Rev Endocrinol. 16(4):213–223. doi:https://doi.org/10.1038/s41574-020-0318-z
- Li J, Lu W-Q-Q, Beesley S, Loudon ASII, Meng Q-J-J. 2012. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One. 7(3):1–8. doi:https://doi.org/10.1371/journal.pone.0033292
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. 2007. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 129(3):605–616. doi:https://doi.org/10.1016/j.cell.2007.02.047
- Luurtsema G, Molthoff CFM, Schuit RC, Windhorst AD, Lammertsma AA, Franssen EJF. 2005. Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood-brain barrier: kinetics and metabolism in the rat. Nucl Med Biol. 32(1):87–93. doi:https://doi.org/10.1016/j.nucmedbio.2004.06.007
- May A, Leone M, Afra J, Linde M, Sándor PS, Evers S, Goadsby PJ. 2006. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 13(10):1066–1077. doi:https://doi.org/10.1111/j.1468-1331.2006.01566.x
- May A, Bahra A, Büchel C, Frackowiak RSJ, Goadsby PJ. 1998. Hypothalamic activation in cluster headache attacks. Lancet. 352(9124):275–278. doi:https://doi.org/10.1016/S0140-6736(98)02470-2
- May A, Schwedt TJ, Magis D, Pozo-rosich P, Evers S. 2018. Cluster headache. Nat Rev Dis Prim. 4:1–17. doi:https://doi.org/10.1038/nrdp.2018.6
- McAllister RG, Kirsten EB. 1982. The pharmacology of verapamil. IV. Kinetic and dynamic effects after single intravenous and oral doses. Clin Pharmacol Ther. 31(4):418–426. doi:https://doi.org/10.1038/clpt.1982.54
- McGeeney BE. 2018. Cluster Headache and Other Trigeminal Autonomic Cephalalgias. Semin Neurol. 38(6):603–607. doi:https://doi.org/10.1055/s-0038-1673682
- Meneses-Santos D, Buonfiglio D. 2018. Chronic treatment with dexamethasone alters clock gene expression and melatonin synthesis in rat pineal gland at night. Nat Sci Sleep. 10:203–215. https://doi.org/10.2147/NSS.S158602
- Moore RY, Eichler VB. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42(1):201–206. doi:https://doi.org/10.1016/0006-8993(72)90054-6
- Morris SA, Weiss LM, Factor S, Bilezikian JP, Tanowitz H, Wittner M. 1989. Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J Am Coll Cardiol. 14(3):782–789. doi:https://doi.org/10.1016/0735-1097(89)90126-5
- Naegel S, Holle D, Desmarattes N, Theysohn N, Diener H-C-C, Katsarava Z, Obermann M. 2014. Cortical plasticity in episodic and chronic cluster headache. NeuroImage Clin. 6:415–423. doi:https://doi.org/10.1016/j.nicl.2014.10.003
- Nahm SS, Farnell YZ, Griffith W, Earnest DJ. 2005. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci. 25(40):9304–9308. doi:https://doi.org/10.1523/JNEUROSCI.2733-05.2005
- Nohara K, Kim E, Wirianto M, Mileykovskaya E, Dowhan W, Chen Z, Yoo SH. 2020. Cardiolipin Synthesis in Skeletal Muscle Is Rhythmic and Modifiable by Age and Diet. Oxid Med Cell Longev. doi:https://doi.org/10.1155/2020/5304768
- Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, Sun Y, Han L, Esser KA, Mileykovskaya E, et al. 2019. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun. 10(1):1–15. doi:https://doi.org/10.1038/s41467-019-11926-y
- Patke A, Murphy PJ, Onat OE, Krieger AC, Özçelik T, Campbell SS, Young MW. 2017. Mutation of the human circadian clock Gene CRY1 in familial delayed sleep phase disorder. Cell. 169(2):203–215.e13. doi:https://doi.org/10.1016/j.cell.2017.03.027
- PDR L 2020. verapamil hydrochloride - Drug Summary. PDR: Prescribers’ Digital Reference. https://www.pdr.net/drug-summary/Verelan-verapamil-hydrochloride-960.2852
- Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, MT A, Baker M, WJ B, Clark A, IC C, Dirnagl U, et al. 2020. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18(7):e3000410. doi:https://doi.org/10.1371/journal.pbio.3000410
- Petersen AS, Barloese MCJJ, Snoer A, Soerensen AMS, Jensen RH. 2019. Verapamil and cluster headache: still a mystery. Narrative Rev Efficacy Mech Perspect. 59:8. doi:https://doi.org/10.1111/head.13603
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27(9–10):1911–1929. doi:https://doi.org/10.3109/07420528.2010.516381
- Ralph MR, Foster RG, Davis FC, Menaker M. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science. 247(4945):975–978. doi:https://doi.org/10.1126/science.2305266
- Römermann K, Wanek T, Bankstahl M, Bankstahl JP, Fedrowitz M, Müller M, Löscher W, Kuntner C, Langer O. 2013. (R)-[11C]verapamil is selectively transported by murine and human P-glycoprotein at the blood-brain barrier, and not by MRP1 and BCRP. Nucl Med Biol. 40(7):873–878. doi:https://doi.org/10.1016/j.nucmedbio.2013.05.012
- Rozen TD, Fishman RS. 2012. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 52(1):99–113. doi:https://doi.org/10.1111/j.1526-4610.2011.02028.x
- Steinberg A, Fourier C, Ran C, Waldenlind E, Sjöstrand C, Belin AC. 2018. Cluster headache – clinical pattern and a new severity scale in a Swedish cohort. 38. 7:1286–1295. doi:https://doi.org/10.1177/0333102417731773
- Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 69(6):1583–1586. doi:https://doi.org/10.1073/pnas.69.6.1583
- Tamai TK, Nakane Y, Ota W, Kobayashi A, Ishiguro M, Kadofusa N, Ikegami K, Yagita K, Shigeyoshi Y, Sudo M, et al. 2018. Identification of circadian clock modulators from existing drugs. EMBO Mol Med. 10(5):e8724. doi:https://doi.org/10.15252/emmm.201708724
- Teepker M, Menzler K, Belke M, Heverhagen JT, Voelker M, Mylius V, Oertel WH, Rosenow F, Knake S. 2012. Diffusion tensor imaging in episodic cluster headache. Headache. 52(2):274–282. doi:https://doi.org/10.1111/j.1526-4610.2011.02000.x
- Tfelt-Hansen P, Tfelt-Hansen J. 2009. Verapamil for cluster headache. Clinical pharmacology and possible mode of action. Headache J Head Face Pain. 49(1):117–125. doi:https://doi.org/10.1111/j.1526-4610.2008.01298.x
- Trejo-Gabriel-Galan JM, Aicua-Rapún I, Cubo-Delgado E, Velasco-Bernal C. 2018. Suicide in primary headaches in 48 countries: a physician-survey based study. Cephalalgia. 38(4):798–803. doi:https://doi.org/10.1177/0333102417714477.
- Waldenlind E, Gustafsson SA, Ekbom K, Wetterberg L. 1987. Circadian secretion of cortisol and melatonin in cluster headache during active cluster periods and remission. J Neurol Neurosurg Psychiatry. 50(2):207–213. doi:https://doi.org/10.1136/jnnp.50.2.207
- Yang F-C-C, Chou K-H-H, Fuh J-L-L, Lee P-L-L, Lirng J-F-F, Lin -Y-Y-Y, Lin C-P-P, Wang S-J-J. 2015. Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg Psychiatry. 86(4):437–445. doi:https://doi.org/10.1136/jnnp-2014-308122
- Yin L, Wang J, Klein PS, Lazar MA. 2006. Nuclear receptor Rev-erbα is a critical lithium-sensitive component of the circadian clock. Science. 311(5763):1002–1005. doi:https://doi.org/10.1126/science.1121613
- Yoo S-H, Kojima S, Shimomura K, Koike N, Buhr ED, Furukawa T, Ko CH, Gloston G, Ayoub C, Nohara K, et al. 2017. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci. 114(42):E8855–E8864. doi:https://doi.org/10.1073/pnas.1706611114
- Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-KH-K, Oh WJ, Yoo OJ, et al. 2004. PERIOD2::LUCIFERASEreal-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 101(15):5339–5346. doi:https://doi.org/10.1073/pnas.0308709101
