ABSTRACT
Obesity and type 2 diabetes mellitus are major health concerns worldwide. In obese-type 2 diabetic patients, the function of the central brain clock in the hypothalamus, as well as rhythmicity in white adipose tissue (WAT), are reduced. To better understand how peripheral clocks in white adipose tissue (WAT) are synchronized, we assessed the importance of the central brain clock for daily WAT rhythms. We compared gene expression rhythms of core clock genes (Bmal1, Per2, Cry1, Cry2, RevErbα, and DBP) and metabolic genes (SREBP1c, PPARα, PPARγ, FAS, LPL, HSL, CPT1b, Glut4, leptin, adiponectin, visfatin/NAMPT, and resistin) in epididymal and subcutaneous white adipose tissue (eWAT and sWAT) of SCN-lesioned and sham-lesioned rats housed in regular L/D conditions. Despite complete behavioral and hormonal arrhythmicity, SCN lesioning only abolished Cry2 and DBP rhythmicity in WAT, whereas the other clock gene rhythms were significantly reduced, but not completely abolished. We observed no major differences in the effect of SCN lesions between the two WAT depots. In contrast to clock genes, all metabolic genes lost their daily rhythmicity in WAT, with the exception of NAMPT. Interestingly, NAMPT rhythmicity was even less affected by SCN lesioning than the core clock genes, suggesting that it is either strongly coupled to the remaining rhythmicity in clock gene expression, or very sensitive to other external rhythmic factors. The L/D cycle could be such a rhythmic external factor that generates modulating signals by photic masking via the intrinsic photosensitive retinal ganglion cells in combination with the autonomic nervous system. Our findings indicate that in normal weight rats, gene expression rhythms in WAT can be maintained independent of the central brain clock.
Introduction
The circadian timing system coordinates physiology and behavior and enables an organism to anticipate recurring events during the day-night cycle. In mammals, a molecular clock is found in nearly every cell, consisting of a network of transcriptional translational auto-regulatory feedback loops (TTFL) (recently reviewed in (Takahashi Citation2017). The ‘central pacemaker’ or ‘master clock’ in the mammalian brain resides in the suprachiasmatic nucleus (SCN), a bilateral nucleus in the anterior hypothalamus, located just above the optic chiasm. Its endogenously generated circadian rhythm is synchronized to the exact 24 h rhythm of the external light-dark (LD) cycle by photic input from the retina via the retinohypothalamic tract (RHT) (Canteras et al. Citation2011). Although photic input is the main stimulus for synchronizing the SCN to the external environment, information from many other time cues, such as locomotor activity and arousal, variation in body temperature, local energy availability, circulating nutrients and hormones, as well as social signals contribute to this process. The SCN then uses various signaling pathways to relay this integrated temporal information to the ‘peripheral clocks’ in other brain areas and in the rest of the body (Albrecht Citation2012; Asher and Schibler Citation2011; Mohawk et al. Citation2012).
White adipose tissue (WAT) is an essential metabolic and endocrine tissue, especially given the rise in obesity prevalence worldwide. Circadian disruption disturbs metabolic homeostasis, especially when it is sustained. For example, during shift work the timing of food intake is often not aligned with endogenous clock rhythms in liver, pancreas, and WAT and, therefore, glucose and lipid homeostasis is not ensured. This increases the risk to develop obesity and related metabolic disease (Scheer et al. Citation2009; Stenvers et al. Citation2019). Similarly, in rodents, circadian disruption due to genetic mutations or misaligned environmental time cues (repeated shifts of the LD cycle, time restricted feeding) can induce metabolic dysfunction (Johnston et al. Citation2016; Tsang et al. Citation2017). On the other hand, rodents with obesity or disturbed glucose regulation show decreased WAT rhythmicity (Ando et al. Citation2005). Likewise, we recently found that in obese patients with type 2 diabetes, the number of rhythmic transcripts in subcutaneous WAT was reduced by 80% as compared to lean controls (Stenvers et al. Citation2019). To better understand via which mechanism circadian disturbance induces metabolic disease, it is first necessary to understand how peripheral clocks in WAT are synchronized to ensure energy homeostasis.
Daily rhythms in feeding behavior, as well as hormone release, affect daily gene expression rhythms in WAT. Removal of either the daily rhythm in food intake or that of the adrenal hormones abolished rhythmicity in many clock-controlled metabolic genes in rats. However, clock gene expression rhythms in WAT remained intact. Only when both the feeding and adrenal rhythms were removed, did clock gene expression in WAT (and liver) became arrhythmic (Su et al. Citation2016, Citation2015). These findings indicate that the daily rhythms of many clock-controlled genes are in fact not solely controlled by local clock genes, but also by outputs from the central clock in the SCN, such as rhythmicity in nutrients, hormones, and behavior. Therefore, in the current experiments we set out to assess in further detail the importance of the central brain clock for WAT rhythms in rats.
There are different genetic approaches to assess the role of the SCN in WAT rhythmicity; down-regulating the activating TTFL component Bmal1 (Husse et al. Citation2014; Izumo et al. Citation2014; Lee et al. Citation2015), or upregulating the repressing component Per2 (Lee et al. Citation2015). However, these genetic approaches leave other clock machinery intact (Husse et al. Citation2014; Izumo et al. Citation2014; Lee et al. Citation2015). Although SCN specific Bmal1 ablation severely dampened SCN rhythmicity, the rhythmicity in eWAT clock gene remained, albeit with 30% amplitude reduction and a phase advance of ~4.8 h (Kolbe et al. Citation2016). To exclude that this remaining rhythmicity is an effect of residual SCN function, we employed an alternative approach and used thermal SCN lesions, a well-established method (Kalsbeek et al. Citation1992, Citation2001; La Fleur et al. Citation1999, Citation2001; Moore and Eichler Citation1972; Ruiter et al. Citation2003; Stephan and Zucker Citation1972), which selectively ablates SCN tissue, thus leaving no residual clock gene activity in the SCN. Striving for the smallest lesions possible induces a risk for incomplete lesions. Incomplete lesions often leave the most ventral part of the SCN that is embedded in the optic chiasm intact. If this light recipient part remains intact, this may be sufficient to maintain rhythmicity in L/D conditions, but not constant darkness. Therefore, we housed all animals in L/D conditions. Housing animals in L/D conditions enable discrimination between SCN- and non-SCN mediated effects of light for all rhythms. We hypothesized that thermal lesions of the SCN would remove the 24 h rhythmicity in all clock- and clock-controlled genes in WAT.
Materials and methods
Animals
Male Wistar rats (Harlan, Horst, Netherlands) were housed with 4–6 animals per cage in a controlled environment, on a 12/12 h light/dark cycle (ZT0 is lights on at 07:00 h), at room temperature (20 ± 2°C). Food and tap water were provided ad libitum throughout the experiment. The experiment was conducted under approval of the Local Animal Welfare Committee and in accordance with international ethical standards (Portaluppi et al. Citation2010).
SCN lesions
Bilateral thermal SCN lesions were made in rats weighing 180–210 g (n = 91). Rats were anaesthetized with Hypnorm (0.8 ml/kg i.m.) and Dormicum (0.3 ml/kg s.c.), and mounted in a stereotact (David Kopf Instruments, Tujunga, CA), with the toothbar set at 5 mm. After identification of the Bregma, electrodes were introduced bilaterally through a drilled hole in the skull, using the following coordinates: 1.4 mm rostral to Bregma, 1.0 mm lateral to midline, 8.3 mm below the brain surface, at an angle of 6°. The electrode diameter was 0.2 mm and a temperature of 85°C was generated for 1 min (Lesion generator RFG4A by Radionics, Burlington, MA). This temperature was found empirically to result in lesions big enough to eliminate the SCN, yet small enough to leave tissue surrounding the SCN, such as the paraventricular nucleus (PVN), intact, as described and illustrated previously (Kalsbeek et al. Citation1992, Citation2000, Citation2001; La Fleur et al. Citation1999; Palm et al. Citation1999). Sham animals underwent the same procedure, but no current was passed through the electrodes.
After two weeks, when the animals had recovered from the anesthesia and surgical procedure, they were housed individually and effectiveness of the lesions was checked by measuring daily rhythmicity of water intake for 3 weeks. If animals drank >30% of their daily water consumption in the middle 8 h of the light period (ZT2-ZT10), lesions were considered successful and animals were included in the SCNx group (n = 31). This “drinking” measure of arrhythmicity correlates well with immunohistochemical screening of completeness of the SCN lesion by using vasoactive intestinal peptide (VIP) staining as a marker for SCN tissue (Kalsbeek et al. Citation2000; La Fleur et al. Citation1999, Citation2001). Sham animals (n = 31) typically drank only 15% of their daily water intake in these 8 h in the middle of the light period.
Tissue collection
Five weeks after lesioning, animals were anaesthetized with isoflurane and killed by decapitation at a 6 h interval starting at ZT2 (N = 7–8 per group and time point) to obtain WAT tissues and plasma. Epididymal (eWAT) and subcutaneous inguinal (sWAT) white adipose tissues were dissected and snap frozen in liquid nitrogen, and the contralateral WAT depot was dissected for weighing. Furthermore, perirenal- and mesenteric WAT, bilateral adrenals, and thymus were dissected and weighed. Blood was collected in heparinized tubes.
Plasma analyses
Following decapitation, trunk blood was kept on ice until centrifugation for 15 min at 3000 rpm at 4°C. Plasma was transferred to a clean tube and stored at −20°C until use. Plasma glucose was measured using a Biosen apparatus (EKF diagnostics, Cardiff, UK). Plasma insulin, leptin, and corticosterone were measured using a Radio Immuno Assay (Merck Millipore, Billerica, MA, USA).
Gene expression analysis
We studied core clock and metabolic gene expression rhythms in epididymal and subcutaneous white adipose tissue (eWAT and sWAT, respectively) in SCN-lesioned and sham-lesioned rats at 4 time points along the L/D-cycle (ZT2, ZT8, ZT14, and ZT20). Gene expression analysis was performed as previously described in detail (Van Der Spek et al. Citation2018). In short, total RNA was extracted from approximately 100 mg of adipose tissue, using the RNeasy lipid kit including on-column DNAse treatment (Qiagen Benelux, Venlo, Netherlands). cDNA was synthesized with the Transcriptor First Strand cDNA synthesis kit (Roche, Almere, Netherlands). Gene expression was analyzed by real-time RT-qPCR on a Lightcycler 480 system (Roche, Almere, Netherlands). We used additional non-reverse transcriptase samples to control for potential DNA contamination, positive controls, and melting peak analysis for product verification, and analyzed individual sample PCR efficiency to exclude all samples that deviated more than 0.05 from mean efficiency. Hypoxanthine Phosphoribosyl Transferase (HPRT) gene expression was used as a housekeeping gene to control for variation in amount of mRNA input. Primer sequences of clock genes Bmal1, Per2, Cry1, Cry2, RevErbα, and DBP, and metabolic genes SREBP1c, PPARα, PPARγ, FAS, LPL, HSL, CPT1b, Glut4, leptin, visfatin/NAMPT, and resistin, have been published previously (Su et al. Citation2016).
Data analysis and statistics
All PCR data are expressed relative to ZT2, to allow comparison between WAT depots and treatment groups. For identification of outliers, we used Dixon’s Q test with two-tailed values using 95% confidence values (Rorabacher Citation1991). Samples that were determined to be outliers were excluded from further analysis (see supplemental table 4). All data are presented as mean ±SEM unless otherwise stated. P values below 0.05 were considered statistically significant.
Rhythmicity was assessed using Circwave 1.4 (www.hutlab.nl) (Van Der Spek et al. Citation2018). Circwave software fits one or more fundamental sinusoidal curves through the individual data points and compares this with a horizontal line through the data mean (a constant). If the fitted curve differs significantly from the horizontal line, the data set is considered rhythmic. Circwave provides the following information: number of sines in the fitted curve; data mean, the average of all data points with SD; Center of Gravity (COG), representing the general phase of the curve with SD; ANOVA F stat, p-value, and R2; Circwave F stat, p-value and R2. For genes that remained rhythmic after SCN lesioning, a shift in COG was assessed by an unpaired 2-tailed student T-test without assuming consistent SD using GraphPad Prism 7. Amplitudes of Circwave curves were calculated as percentages of data mean to enable comparison of amplitudes between data sets [difference between the zenith (highest point) and nadir (lowest point) and divided by the data mean ((max – min/mean) * 100%)].
Results
After recovery from surgery, SCN lesioned (SCNx, n = 91) and sham operated control rats (SCNsh, n = 31) were housed individually and water consumption was measured for the middle 8 h (ZT2-ZT10) of the light period for 3 weeks. Sham-lesioned control animals drank on average 16 ± 4% (mean±SD) of their daily water consumption during the light period. Successfully SCN lesioned animals (N = 31) drank on average 33 ± 4% during the light period, whereas excluded SCN lesioned animals (n = 60) drank on average 22 ± 4% (One-way Anova P < .0001, R2 = 0.71, T-test SCNsh vs SCNx; p < .0001, Supplemental ). Furthermore, in line with previous findings, the daily peak in plasma corticosterone was absent in the SCN lesioned animals (Supplemental ).
Figure 1. Amplitude, R2, and COG of WAT clock gene rhythms in sham- and SCN-lesioned animals. Amplitudes and R2 of clock gene rhythms were reduced by approximately 50% after SCN lesions. COG was advanced by ~4 h after SCN lesions, with the exception of Bmal1 in sWAT. Cry2 and DBP completely lost rhythmicity. Gray bars indicate the dark phase (ZT12-24). SCNx; SCN lesioned, COG; center of gravity, R2; inter individual variability, eWAT epididymal white adipose tissue, sWAT subcutaneous white adipose tissue
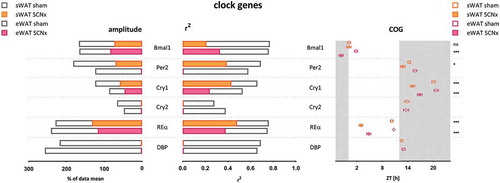
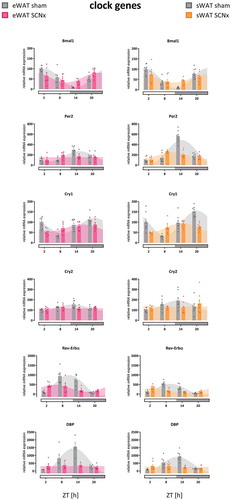
Figure 2. Clock gene rhythms in eWAT and sWAT of sham- and SCN-lesioned animals. Cry2 and DBP lost their rhythmicity after SCN lesions in both WAT compartments, as well as Per2 in eWAT. Bmal1, Per2 in sWAT, Cry1, and RevErbα remained rhythmic after SCN lesions. Gray bars on x-axis indicate the dark phase (ZT12-24). Bar plots represent mean±SEM, scatter plots represent individual data points, area fills represent the curve as fitted by Circwave (www.hutlab.nl)
Body weight gain in SCN lesioned animals was reduced as compared to sham controls (Supplemental , repeated ANOVA; Interaction p < .001; Time p < .001; Group p = .06), as described previously. Sham lesioned animals weighed 6% more at the end of the experiment (BW SCNsh 358 ± 6 g vs. SCNx 337 ± 6 g, T-test p < .05). Total WAT depot weight corrected for body weight did not differ between groups (tWAT: SCNsh 3.9 ± 0.2% vs. SCNx 5.3 ± 1.0%, T-test p = .11). Sham lesioned animals had higher thymus weights (thymus: SCNsh 544 ± 19 mg vs. SCNx 473 ± 17 mg, T-test p < .01), but adrenal weight did not differ between groups (adrenal SCNsh 65 ± 1 mg vs. SCNx 65 ± 2 mg, T-test p = .99) (Supplemenal ).
Figure 3. Amplitude, R2, and COG of WAT metabolic gene expression rhythms in sham- and SCN-lesioned animals. NAMPT gene expression remained rhythmic in WAT after SCN lesions, although the amplitude was reduced by 29% in eWAT and 17% in sWAT, and R2 was reduced by 13% in eWAT and 58% in sWAT. The COG remained unaltered. These results indicate that NAMPT rhythmicity was affected less by SCN lesions than any of the other genes measured, including the core clock genes. Absence of data for FAS, CPT1B, GLUT4, adiponectin, and resistin indicates absence of rhythmicity as calculated by Circwave; it does not indicate absence of gene expression
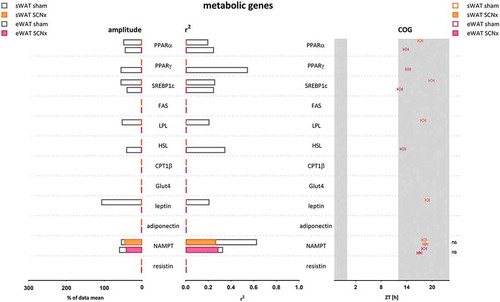
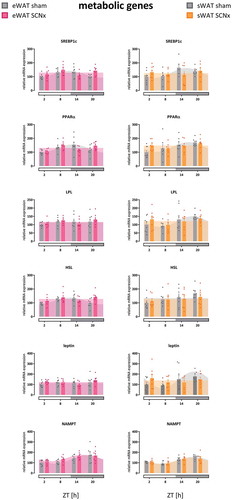
Figure 4. Metabolic gene expression rhythms in eWAT and sWAT of sham- and SCN-lesioned animals. Most metabolic genes lost their daily rhythmicity, with the exception of NAMPT. See supplemental Figure 6 for additional genes. Horizontal gray bars on x-axis indicate the dark phase (ZT12-24). Bar plots represent mean±SEM, scatter plots represent individual data points, area fills represent the curve as fitted by Circwave (www.hutlab.nl)
SCN lesions reduced amplitude and advanced the phase of clock gene rhythms
In sham-operated animals, clock gene expression showed pronounced daily rhythms in both WAT depots ( & ), with amplitudes and peak times comparable to our previous findings in unoperated control animals (Su et al. Citation2015; Van Der Spek et al. Citation2018) and also findings by others in their controls (Ando et al. Citation2005; Kolbe et al. Citation2016; Sukumaran et al. Citation2010; Tdsm et al. Citation2015). Cry2, which showed least robust rhythmicity, and DBP lost their rhythmicity in both WAT compartments after SCN lesions, as well as Per2 in eWAT. Clock genes with more robust rhythms, Bmal1, Per2 in sWAT, Cry1, and RevErbα remained rhythmic after SCN lesions, albeit with lower amplitudes and reduced R2 values ( & ). Amplitudes of clock gene rhythms were reduced by approximately 50% after SCN lesions (Supplemental Table 1). Likewise, R2 values of clock gene rhythms were reduced by approximately 50% after SCN lesions (Supplemental Table 2). The COG of clock gene rhythms was advanced by ~4 hours after SCN lesions (Supplemental Table 3).
Overall, the effect of SCN lesions on clock gene expression in sWAT and eWAT was similar (on average amplitudes reduced by −53% in sWAT vs. −49% in eWAT; R2 reduced by −47% in sWAT vs. −54% in eWAT, and COG advancedby 3.4 h in sWAT vs. 4.4 h in eWAT). Two minor differences between sWAT and eWAT were observed: first, the COG of Bmal1 advanced by 3.5 h in eWAT (p < .001), but did not shift in sWAT (−0.1 h, p = .91). Second, Per2 lost rhythmicity in eWAT, but not in sWAT. Per2 expression in eWAT increased at ZT8 and decreased at ZT14 in SCN lesioned animals compared to controls (thus, peak expression advanced) ( & ), effectively flattening the curve and abolishing the rhythm.
SCN lesions abolished diurnal rhythmicity of metabolic genes, NAMPT remains rhythmic
Metabolic genes showed less pronounced 24 h patterns in both WAT depots than observed in clock genes, as observed previously (Van Der Spek et al. Citation2018). Five out of the 12 metabolic genes measured in sWAT were rhythmic in controls, while 4 of these 5 lost their rhythmicity after SCN lesions (NAMPT remained rhythmic, while PPARα, SREBP1c, LPL, and leptin lost rhythmicity). Similarly, also in eWAT 5 out of the 12 metabolic genes measured were rhythmic in controls, while 4 out of the 5 genes lost their rhythmicity after SCN lesions (NAMPT remained rhythmic, while PPARα, PPARγ, SREBP1c, and HSL lost rhythmicity). Gene expression of FAS, CPT1β, Glut4, adiponectin, and resistin was not rhythmic in controls in either WAT depot. For amplitudes, R2 and COG see , and Supplement Tables 1–3. For PCR data per gene see and Supplemental Figure 5.
Interestingly, NAMPT gene expression remained rhythmic in both sWAT and eWAT after SCN lesions, albeit with reduced amplitude In sWAT, the amplitude was reduced from 57% to 47% of the data mean (Δ-17%), and in eWAT from 61% to 43% of the data mean (Δ-29%). R2 was reduced from 0.63 to 0.27 (−58%) in sWAT, and it was reduced from 0.33 to 0.29 (−13%) in eWAT. COG did not change in either WAT depot (sWAT +0.2 h, p = .76; eWAT −1.1 h, p = .18). These results indicate that NAMPT rhythmicity was affected less by SCN lesions than any of the other genes measured, including the core clock genes.
Discussion
Aim and findings
In line with previous work we observed pronounced daily rhythmicity in core clock genes in WAT of sham-lesioned animals, and less pronounced rhythms in metabolic genes (Ando et al. Citation2005; Kolbe et al. Citation2016; Su et al. Citation2016, Citation2015; Sukumaran et al. Citation2010; Tdsm et al. Citation2015; Zvonic et al. Citation2006). Despite hormonal and behavioral arrhythmicity, SCN lesions induced loss of rhythmicity in only two out of the six core clock genes investigated (Cry2, DBP) ( & ). Unlike Cry1, Cry2 gene expression is only modestly rhythmic in WAT, and it may, therefore, be more susceptible to lose statistically significant rhythmicity (Anafi et al. Citation2014; Khan et al. Citation2012). In contrast, DBP gene expression is highly rhythmic in WAT, as well as in many other tissues, but it lost rhythmicity completely in eWAT and sWAT after SCN lesions. Together, these findings suggest Cry2 and DBP do not form part of the most robust feedback loops in the core clock in WAT, corresponding with their absence in a proposed top 20 ‘core clock genes’ (Anafi et al. Citation2014).
The other four clock genes that we investigated retained statistically significant rhythmicity; however, amplitudes and R2 were both reduced by approximately 50%, and their phase was advanced by ~4 h. These findings are in line with the study by Kolbe et al. (Kolbe et al. Citation2016), reporting a 30% reduction in the amplitude of the eWAT clock genes and a mean phase advance of ~5 h. We found a more pronounced effect, including complete loss of rhythmicity of Cry2 and DBP, which might be explained by the fact that the mice in the study by Kolbe et al. were released into DD 2 days before measuring gene expression (Husse et al. Citation2014; Kolbe et al. Citation2016).
We previously showed that gene expression rhythms in intra-abdominal and subcutaneous WAT depots only show minor differences (Van Der Spek et al. Citation2018). In the current study, we also found no differential effects of SCN lesions on WAT depots. Only minor differences were detected in the response to SCN lesions between sWAT and eWAT. For example, Bmal1 advanced by 3.5 h in eWAT, but showed no significant phase change in sWAT, and Per2 lost rhythmicity in eWAT, but not sWAT. The latter is in line with our previous study and our current observation that Per2 expression shows stronger daily rhythmicity in sWAT than in intra-abdominal WAT depots (Van Der Spek et al. Citation2018).
In line with our findings in WAT, other peripheral tissues have been shown to retain core clock gene rhythmicity after SCN lesions as well (Akhtar et al. Citation2002; Husse et al. Citation2014; Izumo et al. Citation2014; Tahara et al. Citation2012; Yoo et al. Citation2004). For example, in mice, thermal SCN lesions merely reduced the amplitude of clock gene expression rhythms in liver for Bmal1 and Per2 (Akhtar et al. Citation2002). Genetic SCN lesions reduced the amplitude and phase advanced Bmal1, Per1, Per2, RevErbα, and DBP expression rhythms in adrenal, liver, kidney, and heart in mice (Husse et al. Citation2014). Furthermore, BMAL1, PER2, and REVERBα bioluminescence remained rhythmic in several peripheral tissues after thermal SCN lesions, both ex vivo (Yoo et al. Citation2004) and in vivo (Tahara et al. Citation2012). Equally, after genetic SCN lesions, daily rhythms in PER2 bioluminescence showed a decreased amplitude and increased phase distribution in several peripheral tissues (Izumo et al. Citation2014). Some earlier studies reported that SCN lesioning completely abolished the rhythm of Per2 in the liver of rats (Sakamoto et al. Citation1998) and mice (Hara et al. Citation2001), but these studies only compared two time points, which is insufficient to assess rhythmicity accurately. Together, these studies provide accumulating evidence that after SCN lesions peripheral tissues do retain some rhythmicity, although these rhythms are more variable due to decreased amplitudes and increased phase distribution. Thus, despite behavioral arrhythmicity and contrary to the general assumption, SCN lesions do not abolish all rhythmicity in gene expression.
What mechanism may cause peripheral core clock rhythms to remain after SCN lesions?
Light is the main Zeitgeber for the circadian timing system, and in our current study all animals were housed under regular L/D conditions. Several studies have shown that an L/D-cycle can synchronize peripheral clocks in the absence of a functioning genetic clock in the SCN (Husse et al. Citation2014; Izumo et al. Citation2014; Koronowski et al. Citation2019; Lee et al. Citation2015; Welz et al. Citation2019). These data indicate that either the intact neuronal structures within the SCN, or extra-SCN pathways, enable photic masking to modulate peripheral rhythms.
By using thermal ablation of the SCN, we excluded the possibility of photic masking via neuronal structures within the SCN. However, extra-SCN pathways are unaffected by these lesions. The discovery of the melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs) provided evidence for the existence of such a light-sensitive non-SCN neural pathway, with direct projections to many brain areas, including both SCN target and non-SCN target areas (Canteras et al. Citation2011; Hattar et al. Citation2006; Noseda et al. Citation2017). The autonomic nervous system (ANS) could then serve to communicate these light signals from the brain to peripheral tissues, including white adipose tissue (Bartness and Song Citation2007). Indeed, several examples exist of light-induced changes in peripheral tissues that are mediated via the ANS (Aras et al. Citation2019; Cailotto et al. Citation2005; Fan et al. Citation2018; Ishida et al. Citation2005; Zhang et al. Citation2020). Exposure to light may directly increase lipolysis and decrease leptin production in subcutaneous white adipose tissue, indicating that the L/D cycle may also provide a direct rhythmic input to sWAT (Nayak et al. Citation2020; Ondrusova et al. Citation2017). Moreover, light might also entrain peripheral clocks via non-ANS pathways as recently we showed that nocturnal light exposure affects the liver transcriptome via both ANS and non-ANS pathways (Opperhuizen et al. Citation2019). Notable, however, all the above experiments were performed in SCN-intact animals.
Interestingly, whereas behavioral and humoral rhythms desynchronize quickly in SCN lesioned animals in D/D, this occurs at a much slower rate for the peripheral tissue clocks, as illustrated by animals that were kept in DD for 2 days (Akhtar et al. Citation2002; Kolbe et al. Citation2016), 1 week (Husse et al. Citation2014), 3 weeks (Yoo et al. Citation2004), or up to 1 month (Izumo et al. Citation2014; Tahara et al. Citation2012); they and still showed core clock rhythmicity in peripheral tissues. This has been explained by the fact that in absence of the master clock, autonomous feedback loops in organs and individual cells within tissues persist. Over time their amplitude reduces, and they increasingly run out of phase with each other, but, thereby, their rhythms only slowly desynchronize.
Systemic factors, such as locomotor activity, food intake and humoral factors can entrain and synchronize rhythmicity. Locomotor activity, however, is completely arrhythmic in SCN lesioned animals (Akhtar et al. Citation2002; Izumo et al. Citation2014; Stephan and Zucker Citation1972; Tahara et al. Citation2012; Yoo et al. Citation2004), as is body temperature (Refinetti et al. Citation1994), food intake (Janssen et al. Citation1994; Stoynev et al. Citation1982, Citation1986), and as we show here, drinking behavior (Supplemental ). Additionally, we did not observe rhythmicity in plasma concentrations of insulin, corticosterone, or glucose, which can directly influence clock gene expression (Balsalobre et al. Citation2000; So et al. Citation2009) (Supplemental ). In conclusion, behavioral or humoral time cues are unlikely to preserve daily rhythmicity in peripheral clocks in the absence of the master clock, but in L/D conditions, photic masking via retinal projections to the ANS might keep peripheral clocks cycling.
Most metabolic gene expression rhythms are abolished after SCN lesions
We found that SCN lesions abolished daily rhythmicity in metabolic output genes ( & ). In intact animals, rhythmicity of metabolic genes is generally modest and less pronounced than that of clock genes (Schwanhüusser et al. Citation2011; Van Der Spek et al. Citation2018). Daily rhythms of metabolic genes are regulated by clock genes (Asher and Schibler Citation2011), as well as feeding behavior, circulating nutrients, and hormone fluctuations. Since all of these cues are arrhythmic in SCN lesioned animals, this likely explains most of the effect of SCN lesions on the loss of metabolic gene expression rhythms. Moreover, core clock proteins (directly or indirectly) function as transcription factors for many metabolic genes; thus, since the amplitudes of clock gene expression rhythms were reduced after SCN lesions, this will further contribute to the diminished rhythmicity of metabolic genes.
NAMPT retains more rhythmicity than the core clock after SCN lesions
Surprisingly, NAMPT remained rhythmic in both sWAT and eWAT, with only a modest amplitude reduction (−17% in sWAT and −29% in eWAT) and without phase shift ( & ). Since SCN lesioning did alter clock gene rhythmicity more severely, this raises the question of which factor(s) maintained the rhythmicity of NAMPT.
NAMPT is the rate-limiting enzyme in the NAD+ salvage pathway; reduction of NAD+ to NADH is essential for transfer of energy from nutrients to ATP. NAMPT expression is regulated directly by BMAL1, and forms an additional TTFL through Sirtuin1 (SIRT1) (Nakahata et al. Citation2009; Ramsey et al. Citation2009), thus linking cellular metabolism to the circadian clock. In our dataset, Bmal1 rhythms were reduced and phase advanced in eWAT, but not sWAT. NAMPT amplitude was slightly more affected in eWAT than in sWAT; thus, the reduced Bmal1 rhythm may still be sufficient to drive the rhythm in NAMPT gene expression. Indeed, in arrhythmic Bmal1-null mice with reconstituted Bmal1 expression in the liver specifically, oscillation of redox-related metabolites and hepatic NAMPT expression was partly restored (Koronowski et al. Citation2019). This suggests that NAMPT is either very sensitive to the rhythmic regulation by BMAL1, and this loop keeps cycling through SIRT1, or NAMPT is regulated by other rhythmic factors in addition to BMAL1.
NAMPT is, indeed, regulated by several more factors involved in cellular energy metabolism. Firstly, AMPK is a key sensor of ATP resources in the cell, and is essential for rhythmicity of NAMPT in WAT. Furthermore, the ratio NAD+/NADH determines the production of reactive oxygen species (ROS), and oxidative stress increases NAMPT release (Lin et al. Citation2015; Lu et al. Citation2019). Consequently, NAMPT inhibition induces susceptibility to oxidative stress (Hong et al. Citation2016; Xu et al. Citation2017). Anti-oxidant proteins that eliminate ROS, such as peroxiredoxins, may generate rhythmicity in absence of the TTFL (Bass and Takahashi Citation2011; Edgar et al. Citation2012; Neill et al. Citation2011), and may even couple the light signal to the molecular clock (Bodvard et al. Citation2017). Surprisingly, sWAT contains light sensitive melanopsin channels (Nayak et al. Citation2020; Ondrusova et al. Citation2017), raising the suggestion that light could, indeed, reach sWAT. Therefore, the direct effects of light on cellular metabolism pathways could provide an explanation for the remaining rhythmicity in NAMPT, suggesting it is rhythmicity in NAMPT that keeps the molecular clock ticking. On the other hand, this mechanism would not explain the retained rhythmicity in eWAT.
Remarkably, in SCN-lesioned animals on a feeding schedule, only hepatic NAMPT levels lost their rhythmicity; whereas, all of the other clock genes investigated remained rhythmic in these conditions (Sabath et al. Citation2014). These results show NAMPT levels are not directly regulated by food intake; in fact, the enforced feeding schedule seems to disturb its rhythmicity, since in fasted conditions NAMPT levels in SCN-lesioned animals retained their rhythmicity. Moreover, in intact animals NAMPT rhythms were also lost when feeding times were not in line with the L/D cycle (Salgado-Delgado et al. Citation2013). Since SCN-lesioned animals in the Sabath et al. (Sabath et al. Citation2014) study were also housed in L/D conditions, these results agree with the idea that the L/D cycle determines NAMPT rhythmicity, instead of feeding rhythms or the molecular clock.
Study limitations
Although the regular L/D cycle may seem a limitation, as it provides rhythmic input, the housing in L/D conditions does allow differentiation between the pathways responsible for the endogenous SCN-controlled rhythms and those responsible for the non-SCN masking effects of the L/D cycle. Furthermore, we mainly focussed on gene expression, whereas within every regulatory stage from gene expression to protein activity additional layers of circadian control have been revealed (Lee et al. Citation2015). Nevertheless, Janich et al. concluded that whenever both mRNA abundance and ribosome occupancy cycled, they globally did so in synchrony (Janich et al. Citation2015). In the current study, we have sampled every 6 h over a 24 h period to assess rhythmicity. This limited sampling frequency could cause underestimation of rhythmicity. However, the rhythmicity we found in sham lesioned animals corresponded well to the rhythmicity in our earlier study that sampled every 3 h (Van Der Spek et al. Citation2018). Finally, Circwave recognizes wave forms using Fourier transformation, thus assuming rhythms consist of (multiple) sine waves, and could thereby underestimate spiky or saw-tooth shaped wave forms (Thaben and Westermark Citation2014), as previously discussed (Van Der Spek et al. Citation2018), causing underestimation of rhythmicity.
Conclusions
We found that SCN lesions, despite behavioral and hormonal arrhythmicity, reduced but did not abolish clock gene rhythms in rat eWAT and sWAT, with decreased amplitudes and advanced phase. We observed no major differences in the effect of the SCN lesions between WAT depots. Cry2 and DBP completely lost their rhythm, suggesting they are part of less robust, or more adaptable, feedback loops in the core clock in WAT. Metabolic genes lost their modest rhythmicity in WAT, with the exception of NAMPT, which was even less affected than the core clock genes, suggesting that it is either strongly controlled by the remaining rhythmicity in clock gene expression, or it is regulated by an unknown other rhythmic factor. The ANS could provide such an extra-SCN pathway that generates entrainment signals by photic masking, and, thereby, may have contributed to the persisting WAT rhythmicity as observed in our study.
Supplemental Material
Download PDF (172.2 KB)Acknowledgements
The authors would like to thank Elisa Gritsch for assistance with animal experiments, Jeffrey Schaap for assistance with PCRs, Roelof Hut for developing Circwave software, and JM Ruijter and C Ramakers for developing the LinRegPCR software.
Disclosure statement
The authors report no conflict of interest.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. 2002. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 12(7):540–550. doi:10.1016/S0960-9822(02)00759-5
- Albrecht U. 2012. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron [Internet]. 74(2):246–260. doi:10.1016/j.neuron.2012.04.006.
- Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, Hughes ME, Baggs JE, Growe J, Liu AC, et al. 2014. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 12(4):e1001840. doi:10.1371/journal.pbio.1001840
- Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. 2005. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 146(12):5631–5636. doi:10.1210/en.2005-0771
- Aras E, Ramadori G, Kinouchi K, Liu Y, Ioris RM, Brenachot X, Ljubicic S, Veyrat-Durebex C, Mannucci S, Galié M, et al. 2019. Light entrains diurnal changes in insulin sensitivity of skeletal muscle via ventromedial hypothalamic neurons. Cell Rep. 27(8):2385–2398.e3. doi:10.1016/j.celrep.2019.04.093
- Asher G, Schibler U. (2011). Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab [Internet]. 13:125–137. doi:10.1016/j.cmet.2011.01.006.
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science (80-). 289(5488):2344–2347. doi:10.1126/science.289.5488.2344
- Bartness TJ, Song CK. 2007. Brain-adipose tissue neural crosstalk. Physiol Behav. 91(4):343–351. doi:10.1016/j.physbeh.2007.04.002
- Bass J, Takahashi JS. 2011. Redox redux. Nature [Internet]. 469(7331):476–478. http://www.nature.com/articles/469476a.
- Bodvard K, Peeters K, Roger F, Romanov N, Igbaria A, Welkenhuysen N, Palais G, Reiter W, Toledano MB, Käll M, et al. 2017. Light-sensing via hydrogen peroxide and a peroxiredoxin. Nat Commun. 8(1). doi:10.1038/ncomms14791
- Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pévet P, Buijs RM. 2005. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 22(10):2531–2540. doi:10.1111/j.1460-9568.2005.04439.x
- Canteras NS, Ér R-B, Goto M, Cipolla-Neto J, Swanson LW. 2011. The Retinohypothalamic tract: comparison of axonal projection patterns from four major targets. Brain Res Rev. 65(2):150–183. doi:10.1016/j.brainresrev.2010.09.006. [Internet].
- Edgar RS, Green EW, Zhao Y, Van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al. 2012. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 485(7399):459–464. doi:10.1038/nature11088
- Fan SMY, Chang YT, Chen CL, Wang WH, Pan MK, Chen WP, Huang WY, Xu Z, Huang HE, Chen T, et al. 2018. External light activates hair follicle stem cells through eyes via an ipRGC–SCN–sympathetic neural pathway. Proc Natl Acad Sci U S A. 115(29):E6880–E6889. doi:10.1073/pnas.1719548115
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. 2001. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 6(3):269–278. doi:10.1046/j.1365-2443.2001.00419.x
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau K-W, Berson DM. 2006. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol [Internet]. 497(3):326–349. http://www.ncbi.nlm.nih.gov/pubmed/16736474 .
- Hong SM, Park CW, Kim SW, Nam YJ, Yu JH, Shin JH, Yun CH, Im SH, Kim KT, Sung YC, et al. 2016. NAMPT suppresses glucose deprivation-induced oxidative stress by increasing NADPH levels in breast cancer. Oncogene. 35(27):3544–3554. doi:10.1038/onc.2015.415
- Husse J, Leliavski A, Tsang AH, Oster H, Eichele G. 2014. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 28(11):4950–4960. doi:10.1096/fj.14-256594
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. 2005. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2(5):297–307. doi:10.1016/j.cmet.2005.09.009
- Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, Takahashi JS. 2014. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife. 3:1–27. doi:10.7554/eLife.04617
- Janich P, Arpat AB, Castelo-Szekely V, Lopes M, Gatfield D. 2015. Ribosome profiling reveals the rhythmic liver translatome and circadian clock regulation by upstream open reading frames. Genome Res. 25(12):1848–1859. doi:10.1101/gr.195404.115
- Janssen BJA, Tyssen CM, Duindam H, Rietveld WJ. 1994. Suprachiasmatic lesions eliminate 24-h blood pressure variability in rats. Physiol Behav. 55(2):307–311. doi:10.1016/0031-9384(94)90138-4
- Johnston JD, Ordovás JM, Scheer FA, Turek FW. 2016. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr. 7(2):399–406. doi:10.3945/an.115.010777
- Kalsbeek A, Buijs RM, Van Heerikhuize JJ, Arts M, Van Der Woude TP. 1992. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 580(1–2):62–67. doi:10.1016/0006-8993(92)90927-2
- Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. 2000. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology [Internet]. 141(10):3832–3841. http://www.ncbi.nlm.nih.gov/pubmed/11014240 .
- Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. 2001. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 142(6):2677–2685. doi:10.1210/endo.142.6.8197
- Khan SK, Xu H, Ukai-Tadenuma M, Burton B, Wang Y, Ueda HR, Liu AC. 2012. Identification of a novel cryptochrome differentiating domain required for feedback repression in circadian clock function. J Biol Chem. 287(31):25917–25926. doi:10.1074/jbc.M112.368001
- Kolbe I, Husse J, Salinas G, Lingner T, Astiz M, Oster H. 2016. The SCN clock governs circadian transcription rhythms in murine epididymal white adipose tissue. J Biol Rhythms. 31(6):577–587. doi:10.1177/0748730416666170
- Koronowski KB, Kinouchi K, Welz PS, Smith JG, Zinna VM, Shi J, Samad M, Chen S, Magnan CN, Kinchen JM, et al. 2019. Defining the Independence of the Liver Circadian Clock. Cell. 177(6):1448–1462.e14. doi:10.1016/j.cell.2019.04.025. [Internet].
- La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. 1999. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 11(8):643–652. doi:10.1046/j.1365-2826.1999.00373.x
- La Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. 2001. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 50(6):1237–1243. doi:10.2337/diabetes.50.6.1237
- Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld JE, et al. (2015). Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron [Internet]. 85(5):1086–1102. doi:10.1016/j.neuron.2015.02.006
- Lin YC, Wu HC, Liao CC, Chou YC, Pan SF, Chiu CM. 2015. Secretion of one Adipokine Nampt/Visfatin suppresses the inflammatory stress-induced NF-κ B activity and affects Nampt-dependent cell viability in Huh-7 cells. Mediators Inflamm. 2015:1–9. doi:10.1155/2015/392471
- Lu YB, Chen CX, Huang J, Tian YX, Xie X, Yang P, Wu M, Tang C, Zhang WP. 2019. Nicotinamide phosphoribosyltransferase secreted from microglia via exosome during ischemic injury. J Neurochem. 150(6):723–737. doi:10.1111/jnc.14811
- Mohawk JA, Green CB, Takahashi JS. 2012. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 35(1):445–462. doi:10.1146/annurev-neuro-060909-153128
- Moore RY, Eichler VB. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res [Internet]. 42(1):201–206. https://linkinghub.elsevier.com/retrieve/pii/0006899372900546 .
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. 2009. Circadian control of the NAD+ Salvage pathway by CLOCK-SIRT1. Science (80-). 324(5927):654–657. doi:10.1126/science.1170803
- Nayak G, Zhang KX, Vemaraju S, Odaka Y, Buhr ED, Holt-Jones A, Kernodle S, Smith AN, Upton BA, D’Souza S, et al. 2020. Adaptive thermogenesis in mice is enhanced by opsin 3-dependent adipocyte light sensing. Cell Rep. 30(3):672–686.e8. doi:10.1016/j.celrep.2019.12.043
- Neill JSO, Van OG, Dixon LE, Troein C, Bouget F, Reddy AB, Millar AJ. 2011. UKPMC funders group circadian rhythms persist without transcription in a eukaryote. Nature [Internet]. 469(7331):554–558. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3040569&tool=pmcentrez&rendertype=abstract .
- Noseda R, Lee AJ, Nir RR, Bernstein CA, Kainz VM, Bertisch SM, Buettner C, Borsook D, Burstein R. 2017. Neural mechanism for hypothalamic-mediated autonomic responses to light during Migraine. Proc Natl Acad Sci U S A. 114(28):E5683–E5692. doi:10.1073/pnas.1708361114
- Ondrusova K, Fatehi M, Barr A, Czarnecka Z, Long W, Suzuki K, Campbell S, Philippaert K, Hubert M, Tredget E, et al. 2017. Subcutaneous white adipocytes express a light sensitive signaling pathway mediated via a melanopsin/TRPC channel axis. Sci Rep [Internet]. 7(1):1–9. doi:10.1038/s41598-017-16689-4.
- Opperhuizen A-L, Foppen E, Jonker M, Wackers P, Van Faassen M, Van Weeghel M, Van Kerkhof L, Fliers E, Kalsbeek A. (2019). Effects of light-at-night on the rat liver – a role for the autonomic nervous system. Front Neurosci [Internet]. 13: https://www.frontiersin.org/article/10.3389/fnins.2019.00647/full
- Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. 1999. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience [Internet]. 93(2):659–666. http://www.ncbi.nlm.nih.gov/pubmed/10465449
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27(9–10):1911–1929. doi:10.3109/07420528.2010.516381
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. 2009. Circadian clock feedback cycle through NAMPT-Mediated NAD+ biosynthesis. Science (80-). 324(5927):651–654. doi:10.1126/science.1171641
- Refinetti R, Kaufman CM, Menaker M. 1994. Complete suprachiasmatic lesions eliminate circadian rhythmicity of body temperature and locomotor activity in golden hamsters. J Comp Physiol A [Internet]. 175(2):223–232. http://link.springer.com/10.1007/BF00215118 .
- Rorabacher DB. 1991. Statistical treatment for rejection of deviant values: critical values of Dixon’s “Q” parameter and related subrange ratios at the 95% confidence level. Anal Chem. 63(2):139–146. doi:10.1021/ac00002a010
- Ruiter M, La Fleur SE, Van Heijningen C, Van Der Vliet J, Kalsbeek A, Buijs RM. 2003. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes [Internet]. 52(7):1709–1715. http://www.ncbi.nlm.nih.gov/pubmed/12829637 .
- Sabath E, Salgado-Delgado R, Guerrero-Vargas NN, Guzman-Ruiz MA, Del Carmen Basualdo M, Escobar C, Buijs RM. 2014. Food entrains clock genes but not metabolic genes in the liver of suprachiasmatic nucleus lesioned rats. FEBS Lett [Internet]. 588(17):3104–3110. doi:10.1016/j.febslet.2014.06.045.
- Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, et al. 1998. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 273(42):27039–27042. doi:10.1074/jbc.273.42.27039
- Salgado-Delgado RC, Saderi N, Basualdo MDC, Guerrero-Vargas NN, Escobar C, Buijs RM. 2013. Shift work or food intake during the rest phase promotes metabolic disruption and desynchrony of liver genes in male rats. PLoS One. 8(4):e60052. doi:10.1371/journal.pone.0060052
- Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 106(11):4453–4458. doi:10.1073/pnas.0808180106
- Schwanhüusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. 2011. Global quantification of mammalian gene expression control. Nature. 473(7347):337–342. doi:10.1038/nature10098
- So AYL, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. 2009. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A. 106(41):17582–17587. doi:10.1073/pnas.0909733106
- Stenvers DJ, Scheer FAJL, Schrauwen P, La Fleur SE, Kalsbeek A. (2019). Circadian clocks and insulin resistance. Nat Rev Endocrinol [Internet]. 15(2):75–89. http://www.ncbi.nlm.nih.gov/pubmed/30531917
- Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A [Internet]. 69(6):1583–1586. http://www.ncbi.nlm.nih.gov/pubmed/4556464 .
- Stoynev AG, Ikonomov OC, Usunoff KG. 1982. Feeding pattern and light-dark variations in water intake and renal excretion after suprachiasmatic nuclei lesions in rats. Physiol Behav [Internet]. 29(1):35–40. http://www.ncbi.nlm.nih.gov/pubmed/7122732 .
- Stoynev AG, Ikonomov OC, Vrabchev NC, Usunoff KG. 1986. Suprachiasmatic nuclei lesions do not eliminate the circadian rhythms of electrolyte excretion in the rat. Physiol Behav [Internet]. 38(5):657–662. http://www.ncbi.nlm.nih.gov/pubmed/3823179 .
- Su Y, Foppen E, Zhang Z, Fliers E, Kalsbeek A. 2016. Effects of 6-meals-a-day feeding and 6-meals-a-day feeding combined with adrenalectomy on daily gene expression rhythms in rat epididymal white adipose tissue. Genes Cells [Internet]. 21(1):6–24. http://www.ncbi.nlm.nih.gov/pubmed/26567532 .
- Su Y, Van Der Spek R, Foppen E, Kwakkel J, Fliers E, Kalsbeek A. 2015. Effects of adrenalectomy on daily gene expression rhythms in the rat suprachiasmatic and paraventricular hypothalamic nuclei and in white adipose tissue. Chronobiol Int. 32(2):211–224. doi:10.3109/07420528.2014.963198
- Sukumaran S, Xue B, Jusko WJ, DuBois DC, Almon RR. 2010. Circadian variations in gene expression in rat abdominal adipose tissue and relationship to physiology. Physiol Genomics. 42 A(2):141–152. doi:10.1152/physiolgenomics.00106.2010
- Tahara Y, Kuroda H, Saito K, Nakajima Y, Kubo Y, Ohnishi N, Seo Y, Otsuka M, Fuse Y, Ohura Y, et al. 2012. In vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol [Internet]. 22(11):1029–1034. doi:10.1016/j.cub.2012.04.009.
- Takahashi JS. 2017. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet [Internet]. 18(3):164–179. doi:10.1038/nrg.2016.150.
- Tdsm DF, De Oliveira AC, Andreotti S, Do Amaral FG, Chimin P, Ara DP, Torres Leal FL, Ral S, Campana AB, Lopes AB, et al. 2015. Pinealectomy interferes with the circadian clock genes expression in white adipose tissue. J Pineal Res. 58(3):251–261. doi:10.1111/jpi.12211
- Thaben PF, Westermark PO. 2014. Detecting rhythms in time series with rain. J Biol Rhythms. 29(6):391–400. doi:10.1177/0748730414553029
- Tsang AH, Astiz M, Leinweber B, Oster H. 2017. Rodent models for the analysis of tissue clock function in metabolic rhythms research. Front Endocrinol (Lausanne). 8(FEB):1–7. doi:10.3389/fendo.2017.00027
- Van Der Spek R, Fliers E, La Fleur SE, Kalsbeek A. (2018). Daily gene expression rhythms in rat white adipose tissue do not differ between subcutaneous and intra-abdominal depots. Front Endocrinol (Lausanne) [Internet]. 9: http://journal.frontiersin.org/article/10.3389/fendo.2018.00206/full
- Welz P-S, Zinna VM, Symeonidi A, Koronowski KB, Kinouchi K, Smith JG, Guillén IM, Castellanos A, Furrow S, Aragón F, et al. (2019). BMAL1-driven tissue clocks respond independently to light to maintain homeostasis. Cell [Internet]. 177(6):1436–1447.e12. https://linkinghub.elsevier.com/retrieve/pii/S0092867419305070
- Xu R, Yuan Z, Lijuan Y, Li L, Li D, Lv C. 2017. Inhibition of NAMPT decreases cell growth and enhances susceptibility to oxidative stress. Oncol Rep. 38(3):1767–1773. doi:10.3892/or.2017.5793
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. 2004. PERIOD2::LUCIFERASEreal-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 101(15):5339–5346. doi:10.1073/pnas.0308709101
- Zhang KX, D’Souza S, Upton BA, Kernodle S, Vemaraju S, Nayak G, Gaitonde KD, Holt AL, Linne CD, Smith AN, et al. 2020. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature. 585(7825):420–425. doi:10.1038/s41586-020-2683-0
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. 2006. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 55(4):962–970. doi:10.2337/diabetes.55.04.06.db05-0873
