ABSTRACT
Reversibility of frailty in the elderly has been discussed comprehensively and but association between recovery of frail state and rest-activity rhythm (RAR) patterns remains unclear. The aim of the current study was to examine a predictor of RAR patterns at the baseline against change of frail state after the intervention in the elderly community-dwellers. This study was performed during Covid-19 pandemic, at the period from April in 2020 to March in 2022. Participants were publicly recruited from senior’s exercise program hosted by Akita city or Yurihonjo city. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria) was used to assess frail state in each participant before and after the 6-month intervention. To measure the nonparametric RAR parameters consisting of interdaily stability (IS), intra-daily variability (IV), relative amplitude (RA) and average physical activity for the most active 10-h span (M10) or for the least active 5-h span (L5) over the average 24-h profile, an Actiwatch Spectrum Plus device was worn on each participant’s non-dominant wrist for seven continuous days. The final samples were 75 participants except for persons with uncompleted data, classified into the improved group (n = 12), the maintained group (n = 53) and the deteriorated group (n = 10) according to frail alteration after the six-month intervention. As a result of the multinomial logistic regression analysis with the reference of the maintained group, the improvement of frail state associated with a low value of IS and total night-sleep time at the baseline, and M10 and L5 at the initial time were also able to predict worsening of frail state after the six-months intervention. A result of this follow-up study provides grounds for our proposal that alterations of RAR patterns in the elderly could be observed in association with recovery or worsening of frail state after the intervention. The potential finding, however, warrants further longitudinal investigation.
Introduction
Understanding of frailty prevalence in the elderly community-dwellers has been greatly promoted of late years, including physical, cognitive or social aspects comprehensively (Bunt et al. Citation2017; Mehrabi and Beland Citation2020; Qiu et al. Citation2022; To et al. Citation2022; Tolley et al. Citation2021). Of comprehensive elements in frailty, a state of social frailty, which can be defined as a continuum of being at risk of losing social resources and absence of activities or social behaviors (Bunt et al. Citation2017), is associated with health outcomes (mortality, falls or functional limitation) in older community-dwellers (Mehrabi and Beland Citation2020). Thus, further examinations involved in social situations or social environment are focused in frailty researches. The recent trend of relationship between frailty and sleep quality has been well documented in community-based studies (Alqahtani Citation2021; Balomenos et al. Citation2021; Nakakubo et al. Citation2019; Umehara et al. Citation2022). As similarity among these studies, poor sleep quality was independently associated with physical (Alqahtani Citation2021; Umehara et al. Citation2022), cognitive (Zhao et al. Citation2021) or social aspects (Nakakubo et al. Citation2019) of frai state in the elderly. In spite of accumulation of the knowledge related to frailty and sleep disturbance in older adults, there are few literatures available on nonparametric rest-activity rhythm (RAR) patterns in frailty. Our pilot cross-sectional study has reported that prefrail state was significantly associated with a low relative amplitude related to the average physical activity on daytime and night-sleep state over the 24-hour profile (Maekawa and Kume Citation2019). In the additional report of two cases with frailty, a low stability, fragmentation and a low amplitude of nonparametric RAR patterns were potentially observed in activity-plots over the 24-hour profile (Maekawa and Kume. Citation2022). Considering previous findings, the potential association between frailty and the nonparametric RAR patterns cannot be estimated without a further longitudinal study of its association in the elderly community-dwellers.
The aim of this follow-up study was to clarify a predictive factor of rest-activity rhythm at the baseline against change of frail state after the intervention in the elderly community-dwellers. Our starting hypothesis was that low physical activity on daytime and night-sleep state over the 24-hour profile at the baseline time would predict change of frail state after the intervention, since we demonstrated that the elderly community-dwellers participated in senior’s exercise program had positive impact on recovery of physical or cognitive frail state including lower limb performance or cognitive performance, such as memory and information processing speed after the 6-month intervention applied a multi-component exercise program for dementia prevention (Kume et al. Citation2020).
Materials and methods
Participants and procedures
The current study was performed at the period from April in 2020 to March in 2022. Participants were publicly recruited to participate in senior’s exercise program hosted by Akita city or Yurihonjo city. The inclusion criteria were persons aged 65 and more, independence of walking in daily life, living at home and completion of all contents in the senior’s exercise program. The exclusion criteria were to have a medical history of dementia, severe hearing or visual impairment, intellectual disability and need for support or care as certified by public long-term care insurance system in Japan. The registrants at the initial time were two hundred three persons (n = 97 in 2020 to 2021 and n = 105 in 2021 to 2022).
The senior’s exercise program applied the dementia prevention exercise program for 90 min, once per 2 weeks for 6 months, depending on a report of Suzuki et al. study (Citation2013). A 90-min exercise program was carried out in all the participants, consisting of 10 min of stretching, 20 min of muscle strength exercise, and 60 min of aerobic exercise, postural balance, and dual-component exercise. One occupational therapist trained in geriatrics supervised for all the sessions.
Demographic data for each participant was measured at the initial time before the above intervention, including age (year), gender (n, female/male), education (year), height (m), weight (kg), body mass index (kg/m2) and cognitive domains of word list memory (WM) (score), trail making test version A (TMT-A) (sec), trail making test version B(TMT-B) (sec) and symbol digit substitution task (SDST) (score). The cognitive measurement of WM, TMT-A&B and SDST used the National Center for Geriatrics and Gerontology functional assessment tool (Makizako et al. Citation2013). This study was approved by the ethics committee of the Faculty of Medicine, Akita University (approval No. 2236), conformed to ethics and methods for biological rhythm research studies (Portaluppi et al. Citation2010).
Classification of frailty status
The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria) was applied to evaluate frailty status in the participants at the initial time and after the 6-month intervention. The revised J-CHS consists of (i)shrinking, (ii)exhaustion, (iii)low level of activity, (iv)weakness and (v)slowness, and each criterion includes (i) unintentional weight loss (e.g., a decrease of 2–3 kg in 6 months), (ii) self-reported exhaustion (e.g., presence of fatigue for 2 weeks), (iii) low physical activity (e.g., no exercise habit for a week), (iv) weakness (e.g., grip strength [GS] is <26/18 kg for male/female), and (v) slow walking speed (WS) (e.g., <1.0 m/s in 5-m walking test). According to pertinent number of (i) to (v),
0 for robust, 1–2 for prefrail and 3–5 for frail were classified for each participant (Satake and Arai Citation2020).
To classify frailty status changing from the initial time to after the 6-month intervention, we defined the improved group, the maintained group and the deteriorated group; the improved group included a person changed from frail to prefrail or from prefrail to robust, the maintained group was people with the frailty status unchanged (robust, prefrail or frail); the deteriorated group contained a parson with change from robust to prefrail or from prefrail to frail.
Measurements
To evaluate the nonparametric rest-activity rhythm (RAR) parameters at the initial time or after the 6-month intervention, each participant was instructed to wear an Actiwatch Spectrum Plus (AW-SP) (Philips Respironics, Inc.) on the non-dominant wrist for 7 to 14 days without removal. AW-SP data (activity counts, AC) recorded by one-min interval was analyzed by Actiware version 6.20 (Philips Respironics, Inc.). The RAR parameters includes interdaily stability (IS), intra-daily variability (IV), and relative amplitude (RA) as reported by Van Someren et al. (Citation1999) and Witting et al. (Citation1990). The IS variable reflects an expression of the RAR synchronization to stable environmental stimuli, ranging in value from zero for normal distribution noise to one for complete synchronization. The IV variable means the fragmented RAR pattern and ranges from zero for no fragmentation to two for fragmented rhythm. The RA variable indicates the relative proportion of average AC between the most active 10-h span (M10) and the least active 5-h span (L5) over the average 24-h profile and ranges in value from zero for low amplitude and one for high amplitude.
Statistical analysis
For analysis in the current study, the one-way analysis of variance (ANOVA) was, firstly, performed to compare demographic data, RAR and night sleep parameters at the initial period or at the period after the intervention among the groups (the improved group, the maintained group and the deteriorated group). For analysis of the nominal scale, the Kruskal-Wallis test was also applied to compare demographics, such as gender, weight loss for the past 6 months, exhaustion and low levels of activity. The multinomial logistic regression analysis was then applied to examine predictive factors of RAR parameters at the initial time against change of frail classification after the intervention. For the multiple logistic regression analysis with a dependent variable of change of frailty status before or after the intervention, the maintained group was included into the reference group (dummy variable, improved group = 1; maintained group = 2; deteriorated group = 3 for each category of independent variable). Firstly, the variables with a reference value of p < .20 according to a result of the ANOVA were put into the crude model, with adjusted factors of age, gender and education. Secondly, we put the variables with a reference value of p < .20 into Model I. The final model (Model II) was reanalyzed inputting the independent variables with a reference value of p < .10 in Model I, and adding all the RAR variables (IS, IV and RA) depending on previous findings of association between frailty indicator and the RAR parameters in elderly community-dwellers (Maekawa et al. Citation2019). In addition, the numerical conversion of ×100 or ×1/100 was performed towards each RAR variable, due to avoidance of the extremely high or low odds ratio (Dutkiewicz et al. Citation2020). The likelihood-ratio test or Nagelkerke’s R2 value was applied to examine adaptation or contribution rate of each regression model.
Finally, the activity-plots for each group were made to visually clarify the average 24-h profile at the initial time or after the intervention. The Kruskal-Wallis test was also used to compare with activity-plots among the groups at the initial time or in the 6-month intervention. For statistical analysis, the SPSS Version 27.0 for Windows (SPSS Inc., Chicago. IL, USA) was used, and the level of significance was set at p = .05.
Result
Final samples were 75 participants except for 128 persons with uncompleted data, dividing into the improved group (n = 12), the maintained group (n = 53) and the deteriorated group (n = 10) (). lists demographic data for overall and each group. As a result of the comparison test among the groups, demographic data including age, the WM score and the SDST score had significant difference (). Likewise, the total sleep time during the night was significantly different among the groups at the initial time (p < .05). Other parameters had no significance among the group (p > .05).
Table 1. Demographic data of overall or each group.
indicated each regression model (Crude model, Model I or Model II) with adjustment of age, gender and education. In the Model I, the improved group had significant association with total sleep time during the night (odds ratio, 1.02; 95% confidence interval [95%CI], 1.00 to 1.04; p = .03), with high adaptation of the regression model (Model I: Likelihood-ratio test, P = .001, Nagelkerke’s R2 = 0.498). Thereafter, the final model (Model II) showed that the improved group was significantly associated with IS×100 (odds ratio, 0.84; 95%CI, 0.72 to 0.97; p = .02), total sleep time during the night (odds ratio, 1.03; 95%CI, 1.01 to 1.05; p = .01) and age (odds ratio, 1.57; 95%CI, 1.13 to 2.16; p = .01), and the deteriorated group had significant association with L5/100 (odds ratio, 1.74; 95%CI, 1.01 to 3.02; p = .047) and M10/100 (odds ratio, 0.96; 95%CI, 0.93 to 0.996; p = .03), depending on the reference of the maintained group. Additionally, adaptation of the estimated regression model was better statistically (Model: Likelihood-ratio test, P = .004, Nagelkerke’s R2 = 0.467).
Table 2. Model of the multinomial logistic regression analysis.
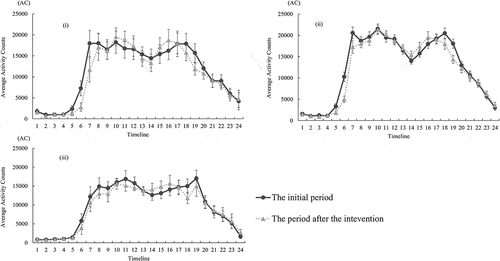
Lastly, or displayed the activity-plots over the 24-h profile for the groups at the initial time or in the 6-month intervention. According to a result of the Kruskal-Wallis test, the average AC at timeline 9 was significantly different among the groups (p = .036) and the plots of timeline 8, 10 and 11 tended to be different among the groups (p < .1) in the 6-month intervention. However, significant group difference of the activity-plots at the initial time was not observed, as well as the other timelines of the activity-plots after the intervention (p > .05).
Figure 2. Comparison of activity – plots among improved, maintained group and deteriorated group at the initial period (a) and at the period after the intervention (b) each error bar indicates the standard error of the mean (SEM) accordingly to the hourly average of activity counts. The number of horizontal axis (Timeline) shows along the axis of time from 0:00AM to 23:59PM (e.g. the number of 1 means 0:00–0:59AM and the number of 24 indicates 23:00–23:59PM). AC, activity counts.
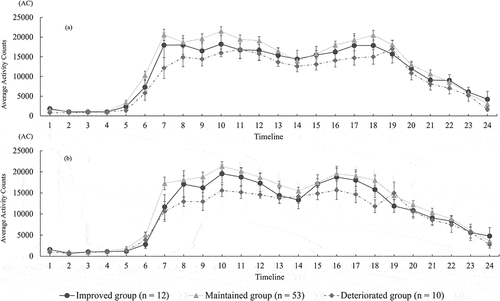
Figure 3. Comparison of activity – plots at the initial period and at the period after the intervention within the improved (i) the maintained group (ii) and the deteriorated group (iii). Each error bar indicates the standard error of the mean (SEM) accordingly to the hourly average of activity counts. The number of horizontal axis shows along the axis of time from 0:00AM to 23:59PM (e.g. the number of 1 means 0:00–0:59AM and the number of 24 indicates 23:00–23:59PM). AC, activity counts.
Discussion
Our result of the multinomial logistic regression analysis with adjustment of age, gender and education showed that the improvement of frail state associated with stability (IS) of the circadian rest-activity patterns and total night-sleep time of sleep parameters at the baseline, based on a reference group of the maintained group after the six-month intervention. On the other hand, low physical activity over the most active span (M10) and high activity in the most inactive span (L5) at the initial time were also able to predict worsening of frail condition after the six-months intervention.
A result yielded in the follow-up study has disclosed that stability (IS), low physical activity on daytime (M10) or high activity during the night (L5) of the RAR patterns and the night-sleep status might be predictable against change (recovery or worsening) of frail state in the elderly community-dwellers. So far, no direct comparison has been made of whether nonparametric RAR patterns in the elderly at the baseline could be a predictor against change of frail classification, and but some studies related to physical activity or sleep quality in frailty has been reported as the follow; Regarding association between frailty and physical activity level, some studies focused on sedentary behavior in older adults with frailty (da Silva et al. Citation2019; Kehler et al. Citation2020; Manas et al. Citation2019). Studies applied accelerometry showed a significant effect of sedentary time on frailty (Manas et al. Citation2019) and Low physical activity level with excessive time spent in sedentary behavior (physical activity level < 150 min/wk. and sedentary behavior ≧ 540 min/day) was associated with frailty (Kehler et al. Citation2020). Moreover, prolonged sedentary time was significantly observed worse frail state in females rather than males (da Silva et al. Citation2019). On the other hand, a 2-year follow-up study focused on transition of frail state reported that, within older adults with robust at baseline, transitioning into prefrail/frail (beta coefficient = 3.04, p < .001) was associated with a higher score of depressive symptoms and its association was accelerated by short sleep duration (Liu et al. Citation2021). Recent advances have led to a better understanding of poor sleep quality in frailty (Pourmotabbed et al. Citation2020; Wai and Yu Citation2020) or low physical activity level in frailty (da Silva et al. Citation2019; Kehler et al. Citation2020; Manas et al. Citation2019), and a early study of Nóbrega et al. (Citation2014) highlighted the influence of sleep latency, which means a prolonged time between lying down and falling asleep, in the elderly with frailty. Successively, they suggested that deficient mechanisms to induce sleep would be associated with frailty. Of these compounds, Gonçalves et al. (Citation2015) reviewed that the functional meaning of non-parametric RAR variables would be clinical perspective which is different from the homeostatic component of sleep regulation. Considering the above findings, predictable factors of the RAR patterns or the night-sleep state against change (recovery or worsening) of frail state in older adults is controversial and warrants longitudinal investigation.
Interestingly, the group comparison of activity-plots at the period of 6-month following up demonstrated difference of the average physical activity throughout the timeline 8 to 11 (e.g. a timeline of AM7:00 to AM10:59). The timeline of AM7:00 to AM10:59 falls under morning activity, and participation of morning social or physical activity sessions (AM9:00 to AM10:30) improves subjective sleep quality in the elderly, as well as improvement of neuropsychological performance (Benloucif et al. Citation2004). However, the effect of sleep parameters as assessed by actigraphy and polysomnography did not improve in the Benloucif’s report (Citation2004). Not much has been done to clarify effect of morning hour activity in transition of frail state after the intervention, and but this raised the possibility that recovery or worsening of frail state after the intervention morning might have impact on difference of morning hour activity in the elderly.
A potential weakness of the study needs to be mentioned as the follow; Firstly, final samples was smaller than we expected due to limited social participation during Covid-19 pandemic. Further, difference of sample size for each group according to frail state before and after the intervention should be considered carefully for interpretation of the regression model in this study. Second, sex difference of samples was observed in the present study, with a bias of female. Given that female with frailty has longer sedentary time than male with frail state (da Silva et al. Citation2019), this confounder must be reconsidered in the future examination. Finally, it is not possible to establish causal relationships between alterations of frail state and group difference of activity-plots in the morning activity’s span and but we believe its difference of morning hour activity according to frail recovery after the intervention was beyond the aims of the study.
In conclusion, this follow-up study provides potential information on association between change of frail state after the intervention and RAR patterns in the elderly community-dwellers. Additionally, a following-up care for frailty prevention should be potentially the focus for reducing the morning hour activity in the elderly.
Description of authors’ roles
Dr. Yu Kume designed this study, analyzed the collected data for the study, and wrote the article. Dr. Ayuto Kodama analyzed the collected data and revised the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alqahtani BA. 2021. Association between physical frailty and sleep quality among saudi older adults: A community-based, cross-sectional study. Int J Environ Res Public Health. 18:12741. doi:10.3390/ijerph182312741
- Balomenos V, Ntanasi E, Anastasiou CA, Charisis S, Velonakis G, Karavasilis E, Tsapanou A, Yannakoulia M, Kosmidis MH, Dardiotis E, et al. 2021. Association between sleep disturbances and frailty: Evidence from a population-based study. J Am Med Dir Assoc. 22:551–558 e551. doi:10.1016/j.jamda.2020.08.012
- Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J, Zee PC. 2004. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 27:1542–51. doi:10.1093/sleep/27.8.1542
- Bunt S, Steverink N, Olthof J, van der Schans CP, Hobbelen JSM. 2017. Social frailty in older adults: A scoping review. Eur J Ageing. 14:323–34. doi:10.1007/s10433-017-0414-7
- da Silva VD, Tribess S, Meneguci J, Sasaki JE, Garcia-Meneguci CA, Carneiro JAO, Virtuoso JS Jr. 2019. Association between frailty and the combination of physical activity level and sedentary behavior in older adults. BMC Public Health. 19:709. doi:10.1186/s12889-019-7062-0
- Dutkiewicz R, Zetterberg H, Andreasson U, Blennow K, Nellgard B. 2020. Dementia and CSF-biomarkers for Alzheimer’s disease predict mortality after acute hip fracture. Acta Anaesthesiol Scand. 64:93–103. doi:10.1111/aas.13472
- Gonçalves BS, Adamowicz T, Louzada FM, Moreno CR, Araujo JF. 2015. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev. 20:84–91. doi:10.1016/j.smrv.2014.06.002
- Kehler DS, Clara I, Hiebert B, Stammers AN, Hay JL, Schultz A, Arora RC, Tangri N, Duhamel TA. 2020. Sex-differences in relation to the association between patterns of physical activity and sedentary behavior with frailty. Arch Gerontol Geriatr. 87:103972. doi:10.1016/j.archger.2019.103972
- Kume Y, Fujita T, Uemura S, Inomata S, Tsugaruya M, Sato A, Nakamura Y, Itakura Y, Ota H. 2020. Effect of a dual-task exercise to motor and memory function for Japanese older individuals in depopulated rural districts: Preliminary intervention research from 2016 to 2019. Int Psychogeriatr. 32:285–86. doi:10.1017/S1041610219000711
- Liu H, Li D, Zhao X, Fang B, Zhang Q, Li T. 2021. Longitudinal impact of frailty states and sleep duration on subsequent depressive symptoms of older adults. J Am Geriatr Soc. 69:1003–11. doi:10.1111/jgs.16999
- Maekawa H, Kume Y. 2019. Imbalance of nonparametric rest-activity rhythm and the evening-type of chronotype according to frailty indicators in elderly community dwellers. Chronobiol Int. 36:1208–16. doi:10.1080/07420528.2019.1626416
- Makizako H, Shimada H, Park H, Doi T, Yoshida D, Uemura K, Tsutsumimoto K, Suzuki T. 2013. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr Gerontol Int. 13:860–66. doi:10.1111/ggi.12014
- Manas A, Pozo-Cruz BD, Rodriguez-Gomez I, Losa-Reyna J, Rodriguez-Manas L, Garcia-Garcia FJ, Ara I. 2019. Can physical activity offset the detrimental consequences of sedentary time on frailty? A moderation analysis in 749 older adults measured with accelerometers. J Am Med Dir Assoc. 20:634–638 e631. doi:10.1016/j.jamda.2018.12.012
- Mehrabi F, Beland F. 2020. Effects of social isolation, loneliness and frailty on health outcomes and their possible mediators and moderators in community-dwelling older adults: A scoping review. Arch Gerontol Geriatr. 90:104119. doi:10.1016/j.archger.2020.104119
- Nakakubo S, Doi T, Makizako H, Tsutsumimoto K, Kurita S, Kim M, Ishii H, Suzuki T, Shimada H. 2019. Association of sleep condition and social frailty in community-dwelling older people. Geriatr Gerontol Int. 19:885–89. doi:10.1111/ggi.13734
- Nóbrega PV, Maciel AC, de Almeida Holanda CM, Oliveira Guerra R, Araújo JF. 2014. Sleep and frailty syndrome in elderly residents of long-stay institutions: A cross-sectional study. Geriatr Gerontol Int. 14:605–12. doi:10.1111/ggi.12144
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27:1911–29. doi:10.3109/07420528.2010.516381
- Pourmotabbed A, Boozari B, Babaei A, Asbaghi O, Campbell MS, Mohammadi H, Hadi A, Moradi S. 2020. Sleep and frailty risk: A systematic review and meta-analysis. Sleep Breath. 24:1187–97. doi:10.1007/s11325-020-02061-w
- Qiu Y, Li G, Wang X, Zheng L, Wang C, Wang C, Chen L. 2022. Prevalence of cognitive frailty among community-dwelling older adults: A systematic review and meta-analysis. Int J Nurs Stud. 125:104112. doi:10.1016/j.ijnurstu.2021.104112
- Satake S, Arai H. 2020. The revised Japanese version of the cardiovascular health study criteria (revised J-CHS criteria). Geriatr Gerontol Int. 20:992–93. doi:10.1111/ggi.14005
- Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Ito K, Shimokata H, Washimi Y, Endo H, Kato T. 2013. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 8:e61483. doi:10.1371/journal.pone.0061483
- To TL, Doan TN, Ho WC, Liao WC. 2022. Prevalence of frailty among community-dwelling older adults in Asian Countries: A systematic review and meta-analysis. Healthcare (Basel). 10. doi:10.3390/healthcare10050895
- Tolley APL, Ramsey KA, Rojer AGM, Reijnierse EM, Maier AB. 2021. Objectively measured physical activity is associated with frailty in community-dwelling older adults: A systematic review. J Clin Epidemiol. 137:218–30. doi:10.1016/j.jclinepi.2021.04.009
- Umehara T, Kaneguchi A, Yamasaki T, Matsuura A, Kito N, Tanaka H, Yamaoka K. 2022. Interactive effects of exercise and sleep on frailty severity in community-dwelling older adults: A cross-sectional study. J Rural Med. 17:21–28. doi:10.2185/jrm.2021-041
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. 1999. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 16:505–18. doi:10.3109/07420529908998724
- Wai JL, Yu DS. 2020. The relationship between sleep-wake disturbances and frailty among older adults: A systematic review. J Adv Nurs. 76:96–108. doi:10.1111/jan.14231
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. 1990. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 27:563–72. doi:10.1016/0006-3223(90)90523-5
- Zhao Y, Lu Y, Zhao W, Wang Y, Ge M, Zhou L, Yue J, Dong B, Hao Q. 2021. Long sleep duration is associated with cognitive frailty among older community-dwelling adults: Results from West China Health and Aging Trend study. BMC Geriatr. 21:608. doi:10.1186/s12877-021-02455-9