ABSTRACT
Light is the main environmental signal synchronizing circadian rhythms to the 24-hour light-dark cycle. Recent research has identified significant inter-individual variability in the sensitivity of the circadian system to light as measured by, among other indicators, melatonin suppression in response to light. These inter-individual differences in light sensitivity could result in differential vulnerability to circadian disruption and related impacts on health. A growing body of experimental evidence points to specific factors which are associated with variability in the melatonin suppression response; however, no review to date has summarized this research to present a comprehensive summary of current knowledge. The aim of this review is to provide an overview of the state of this evidence, which to date spans demographic, environmental, health-related, and genetic characteristics. Overall, we find that there is evidence of inter-individual differences for the majority of the characteristics examined, although research on many factors remains limited. Knowledge of individual factors that are linked to light sensitivity could inform improved lighting personalization, as well as the use of measures of light sensitivity to determine disease phenotypes and treatment recommendations.
Introduction
Beyond vision, light has several important effects on human physiology. Notably, light is the main environmental signal that synchronizes circadian rhythms to the 24-hour light-dark cycle (Cedeño-Laurent et al. Citation2018). The internal circadian clock, which regulates physiological and behavioral rhythms, is synchronized to the Earth’s 24-hour light-dark cycle so that functions such as sleep, physical activity, and energy intake occur at optimal times of day (Czeisler et al. Citation1999; Pittendrigh Citation1993). In addition to regulating the circadian clock, light has a number of important acute effects on physiology. Light increases alertness (Cajochen Citation2007), enhances mood (Terman and Terman Citation2005), alters cognition and thermoregulation (Cajochen et al. Citation1992), impacts sleep architecture (Münch et al. Citation2006), and has impacts on metabolic function (Nelson and Chbeir Citation2018).
The non-visual effects of light are primarily mediated by a specialized group of cells in the retina known as intrinsically photosensitive retinal ganglion cells (ipRGCs), which contain the photopigment melanopsin. Melanopsin has a peak sensitivity to short-wavelength light (~480 nm), and therefore light which contains greater power in this part of the visual spectrum (i.e., more “blue” light) has a larger impact on non-visual responses (Bailes and Lucas Citation2013). These non-visual effects, including effects on the circadian clock, evolved under conditions of very bright light during the day (i.e., sunlight exposure), and darkness at night. However, in modern environments, people experience suboptimal light exposures – spending much of their time under intermediate levels of light (~30–300 lux), and continuing exposure to light well into the evening or night (Cain et al. Citation2020). Given the central role of sleep and circadian rhythms in human health (Allada and Bass Citation2021), understanding the non-visual effects of light has important implications for well-being.
Light at night has become more prevalent due to increased use of electronic devices emitting bright light, which is often blue-enriched (e.g., TVs, smartphones, computers, tablets), in the hours prior to going to sleep (Carter et al. Citation2016; Christensen et al. Citation2016; Lemola et al. Citation2015), and the proliferation of energy-efficient lighting, which often has a greater “blue” light component than traditional lighting and could thus have a greater influence on sleep and circadian rhythms (Cain et al. Citation2020). Exposure to light at night and in the evening can have a dramatic impact on circadian rhythms (Navara and Nelson Citation2007), and leads to suppression of melatonin (Reiter et al. Citation2007), the sleep-related hormone that signals to the body that it is night. Even typical indoor lighting used at night can result in suppression of melatonin by~50% (Phillips et al. Citation2019). Exposure to light at night has been shown to negatively impact sleep outcomes, including greater difficulty falling asleep, increased awakenings, poorer sleep architecture including reduced slow-wave sleep, decreased total sleep time, and difficulty waking up (Chang et al. Citation2015; Chellappa et al. Citation2013; Cho et al. Citation2016; Yang et al. Citation2018). Even exposure to light as low as ~ 5 photopic lux during sleep can result in decreased melatonin production, and reduced sleep quality, in home-based conditions (Stebelova et al. Citation2020).
Inappropriate light exposure at night, and the associated poor sleep and circadian disruption, have been linked to a wide range of adverse health and well-being outcomes. For example, one night of light exposure has been found to alter cardiovascular and metabolic responses (Albreiki et al. Citation2017; Mason et al. Citation2022), and sleeping with the TV or lights on may increase the risk of weight gain or obesity (Obayashi et al. Citation2013; Park et al. Citation2019). Moreover, poor or inadequate sleep is linked to poorer cognitive and academic performance, as well as impairments in emotional functioning such as poorer mood, an impaired ability to regulate emotions, and a reduced threshold for stress response (Killgore and Weber Citation2014; Minkel et al. Citation2012; Shochat et al. Citation2014; Vriend et al. Citation2013). The effects of inadequate sleep are seen across the lifespan, including during early childhood where their impact may be amplified (Berger et al. Citation2012; Sadeh Citation2007). In the long term, sleep disturbances are associated with conditions such as depression, coronary heart disease, and premature mortality (Buysse Citation2014). Finally, long-term circadian disruption is associated with an increased risk for metabolic disorders, cardiovascular disease, and some cancers (Boyce and Barriball Citation2010; Cedeño-Laurent et al. Citation2018; Challet and Kalsbeek Citation2017; Cho et al. Citation2015; Fonken and Nelson Citation2014; Hurley et al. Citation2014; Plano et al. Citation2017).
There is significant inter-individual variability in sensitivity to the non-visual effects of light. Recently, it was found that there can be more than a 50-fold difference between people in sensitivity to evening light as measured by melatonin suppression (Phillips et al. Citation2019). Participants’ responses to a range of different light levels in the evening were assessed to identify the effective dose for 50% melatonin suppression for each participant (ED50). The group-level ED50 was 24.6 lux, but the individual-level ED50s ranged from 6 to 350 lux. Several prior studies also report similar evidence of inter-individual variability (Brainard et al. Citation1988; Gooley et al. Citation2011; Ho Mien et al. Citation2014). These inter-individual differences in light sensitivity may partially explain differential vulnerability to circadian disruption and subsequent impacts on health (Phillips et al. Citation2019). For example, individuals with particularly high light sensitivity may be more vulnerable to circadian disruption and poor sleep outcomes due to exacerbated effects of light at night. Conversely, individuals with low sensitivity could experience abnormal circadian function due to a weaker entraining signal to the circadian clock, resulting in a different phase angle of entrainment or failure to entrain. Due to these individual differences, generalized guidance on light exposure may promote levels that are inappropriate at the individual level. Therefore, it is important to understand factors associated with higher or lower light sensitivity, such that people can avoid potentially unhealthy light exposure.
Individual differences in light sensitivity have been assessed using a number of non-visual light responses. These include shifts in circadian phase, the suppression of melatonin, changes in alertness or related outcomes, and pupillary responses. The most used marker is the melatonin suppression response – likely due to the relative ease of assessment, which is substantially less resource-intensive than assessing circadian phase shifting while still relating to phase shifting within individuals (Lockley et al. Citation2003). Although the use of pupillary markers as a tool for assessing light sensitivity is increasing, they are yet to be related to other standard markers of circadian light sensitivity, such as melatonin suppression or phase-shifting. Therefore, this review will focus on factors associated with differences in the melatonin suppression response.
A growing body of experimental evidence points to specific characteristics which are associated with an altered melatonin suppression response to light and thereby contribute to inter-individual or inter-group variability. However, no review to date has presented a comprehensive summary of current knowledge. While another recent review provided an overview of evidence on individual differences in light sensitivity (Chellappa Citation2020), this overview included papers assessing a range of non-visual responses. Therefore, a full review of the studies within any one measure was beyond the scope of that review. Although some measures may be correlated with one another (e.g., melatonin suppression and phase-shifting), they can be de-coupled (Rahman et al. Citation2018), and it has not yet been determined how different non-visual responses relate to one another within individuals. Therefore, a full review of the factors associated with individual differences in a single response is warranted. The aim of this review is to provide an overview of the state of the evidence with respect to factors associated with individual differences in the melatonin suppression response to light. We have focused on studies with human subjects, given that results in animals may not be directly applicable to humans. However, where evidence with human subjects is sparse, we have noted relevant pre-clinical research.
Factors associated with differences in melatonin suppression
Demographic factors
Age
There is reason to believe that ocular changes across the lifespan, which are well-known to affect visual perception of light, affect non-visual photoreception as well. Specifically, reduced lens transparency due to increased density and opacity of the lens, as well as the accumulation of yellow pigments and smaller pupil size, among older people can decrease retinal illuminance and light transmission rate (Salvi et al. Citation2006). This may, in turn, lead to reduced stimulation of ipRGCs generating a weaker signal to the circadian clock and other brain areas involved in non-visual light responses. Transmission of short wavelength light (such as “blue” light at 450–490 nm), which has the greatest effect on circadian photoreception, is argued to be particularly reduced by these age-related changes (Kessel et al. Citation2011). In their modelling of age-related losses in crystalline lens transmittance and pupillary area, Turner and Mainster (Citation2008) estimated that 10-year-old children have twice the circadian photoreception of 45-year-old adults and 10 times that of 95-year-old adults. As a result, younger people would be likely to have higher light sensitivity and a correspondingly stronger melatonin suppression response to the same light stimulus.
Indeed, studies typically do find evidence of higher sensitivity among younger people compared to older people. Among the youngest populations studied, Akacem et al. (Citation2018) examined melatonin suppression in preschool-aged children during exposure to ~ 1000 lux around bedtime, finding an average suppression of 88%. Likewise, this research team with additional colleagues (Hartstein et al. Citation2022) subsequently found that children (aged 3.0–4.9 years) experienced 69%-99% melatonin suppression after 1 hour of exposure, spanning 15 light levels from 5 to 5000 lux. Participants experienced consistently high melatonin suppression across this full range of light intensities (i.e., significant suppression occurring even at low intensities). For example, children experienced~82% melatonin suppression in response to light levels of just 5 and 10 lux. However, average melatonin suppression was significantly lower across the lowest quartile of light intensities than the three higher quartiles, so it is possible that a dose-response relationship may exist despite near saturation of the response at very low levels. One study showed that pre-mid pubescent children (9.1–14.7 years old) exhibited significantly greater melatonin suppression when compared to late-post pubertal children (11.5–15.9 years old) (Crowley et al. Citation2015). This suggests that age-related changes in light sensitivity, at least early in life when lens clarity should be relatively stable, may be related in part to hormonal development. Furthermore, children (mean age 9.2 ± 1.5 years) appear to show greater sensitivity to light when compared to their parents. In two experiments exposing participants to light before bedtime (first to 580 lux and then to room light at home), the researchers found that the percentage of melatonin suppression was almost twice as high in the children as in their parents − 88% vs. 46%, and 52% vs. 27%, in the respective experiments (Higuchi et al. Citation2014).
Beyond childhood and adolescence, aging through adulthood also appears to be associated with changes in light sensitivity. Herljevic et al. (Citation2005) found that post-menopausal women (average age of 57 years) had significantly reduced melatonin suppression following exposure to short-wavelength light, compared to pre-menopausal women (average age of 24 years). The authors hypothesized that the differences could be due to age-related changes in lens density; another potential explanation could relate to hormonal development. Likewise, Chellappa et al. (Citation2021) found a 23% attenuation in the increase of melatonin levels (relative to baseline) in younger individuals (aged 18–30 years) after 2 hours of evening exposure to low levels of blue-enriched light (6500K), compared to non-blue-enriched light (2500K-3000K). However, older individuals (aged 55–80 years) did not show such differences in sensitivity across light conditions. Additionally, Duffy et al. (Citation2007) reported that sensitivity among older adults (over 65 years) was lower than those of younger adults (based on data from their previous work) across a range of light intensities.
One recent study evaluated the effect of age-related cataract surgery on light sensitivity, since cataracts contribute to ocular changes that reduce light transmission. Chellappa et al. (Citation2019) assessed changes in melatonin levels during 2 hours of evening light exposure across three combinations of intensity and wavelength, following 3.5 hours of dim-dark adaptation, comparing healthy controls (mean age 63.6 years) with older adults with previous cataracts who had received intraocular cataract lens (IOL) replacement (mean age 69.2 years). During light exposure, the increase in melatonin levels was attenuated, relative to controls, by 23.3% for participants with blue-blocking IOLs and 19.1% for participants with UV lens IOLs – suggesting that IOLs helped restore typical light responses.
Findings relating to age-related changes in melatonin suppression have not been consistent, however. Nathan et al. (Citation1999a) found no significant differences in melatonin suppression across three age groups (18–25, n = 40; 26–35, n = 13; and ≥36 years, n = 10) in response to a 1-hour exposure to 200 lux from midnight to 1:00 AM. Similarly, Najjar et al. (Citation2014) exposed a group of older (55–63 years, n = 8) and younger (24–27 years, n = 5) participants to one hour of monochromatic light at 9 different wavelengths, and found that although the older adults exhibited the expected ocular changes (i.e., expected lens transmittance was reduced at short wavelengths), melatonin suppression overall was not reduced. Younger participants did show a non-significant increase in melatonin suppression in response to long wavelength light. Some authors have suggested that increased lens filtering may not necessarily lead to decreased non-visual light responses, due to possible, not yet determined compensatory mechanisms. (Gabel et al. Citation2017; Najjar et al. Citation2014) A simpler explanation may be that the effects are too small to detect in some comparison groups based on the sample sizes studied. Large inter-individual differences can exist even within a restricted age group, which could mask age-related effects that are of a smaller magnitude (Phillips et al. Citation2019). For example, Nagare et al. (Citation2019) did not find significantly greater melatonin suppression at short-wavelength light exposure compared to longer-wavelength exposure among adolescents than adults (adolescents had a mean age of 15.9 years, n = 18; adults, 42.4 and 38.7 years across two phases of the experiment, n = 23), but did find such differences in a separate study (Nagare et al. Citation2019) with a slightly older comparison group, a mean age of 46 years (n = 12; compared to a younger group with mean age of 16.5, n = 12) – as did Gabel et al. (Citation2017) in a similar study with an also older comparison group (mean age of 63.6 years, n = 12; compared to a younger group with mean age of 25.0 years, n = 26).
To sum, the majority of the evidence points towards increased sensitivity in younger groups, with decreasing sensitivity in older age. These changes are paralleled by age-related ocular changes, which may provide the underlying mechanism. Longitudinal studies using within-subject assessments across the lifespan would be helpful for clarifying how these changes manifest and evolve as we age.
Sex
There is evidence of sex differences in the intrinsic period and the phase angle of circadian entrainment in humans (Cain et al. Citation2010; Duffy et al. Citation2011), as well as evidence of sex differences in the rates of entrainment in response to photic cues in animals (Goel and Lee Citation1995). This raises the possibility of sex differences in light sensitivity and corresponding melatonin suppression as well. However, there is only limited and inconsistent research on this topic to date. Monteleone et al. (Citation1995) found a stronger melatonin suppression response among women (40% greater suppression in plasma melatonin than men, upon exposure to 2000 lux from 2:00–4:00 AM). Nathan et al. (Citation2000) assessed the melatonin suppression response to five light intensities but did not find differences between men and women. Thus, no conclusions can be drawn from the available research.
Menstrual cycle phase
Research examining variability in melatonin suppression across the phases of the menstrual cycle is limited. However, existing research suggests that light sensitivity does not vary with menstrual phase among women who do not have premenstrual dysphoric disorder (PMDD). Differences in women with PMDD will be discussed later. Nathan et al. (Citation1999) exposed participants to dim white light (200 lux) during the menstrual, luteal and follicular phases of their menstrual cycle, and found that there were no significant differences in melatonin suppression across the phases. Similarly, a study examining differences between controls and women with PMDD found that within the healthy control group, melatonin suppression did not differ significantly across phases of the menstrual cycle (Parry et al. Citation2010).
Other factors
One study found differences in light sensitivity between light-eyed “Caucasian” people (blue, green, or light brown irises) and dark-eyed Asian people (dark-brown irises) upon exposing them to 1000 lux for the 2 hours before typical time of peak salivary melatonin concentration (Higuchi et al. Citation2007a). The researchers found that the percentage of melatonin suppression was significantly higher in “Caucasian” (89%) than in Asian participants (73%), but were not able to determine whether the differences were due to eye pigmentation or some other factor related to ethnicity. Additionally, Higuchi et al. (Citation2008) found that a larger pupil size under dim light was associated with greater percentage melatonin suppression after 2 hours of nighttime exposure to 1000 lux (r = 0.658). There was also a slightly weaker relationship between percentage melatonin suppression and pupil size during exposure (r = 0.525). This relationship is likely due at least in part to the fact that larger pupils result in increased retinal light exposure; however, it is not clear whether these pupil dynamics also reflect some underlying mechanism which impacts both pupil size and melatonin suppression.
Environmental factors
Short-term photic history
There is good evidence that recent light history impacts subsequent measures of melatonin suppression. Prior exposure during the day to light which is brighter and/or cooler appears to result in reduced melatonin suppression in response to evening or night light exposure.
A pair of studies by Kozaki et al. found that morning exposure to higher-illuminance and blue-white light, respectively, but not light that was dimmer or contained less light in the blue wavelength range, reduced subsequent melatonin suppression. Participants were exposed to various light intensities (<10, 100, 300, 900 and 2700 lux) from 9:00–12:00 AM, then to 300 lux from 1:00–2:30 AM that night. There were no significant differences in melatonin concentration before and after the nighttime exposure for participants initially exposed to the 900 and 2700 lux conditions. Conversely, participants initially exposed to dimmer conditions experienced significant reductions in melatonin suppression (Kozaki et al. Citation2015). Similarly, a separate experiment participants were exposed to dim, white (100 lux, ~4700 CCT), or blue-white (79 lux, ~9600 CCT) light from 9:00–10:30, then to 300 lux at night from 1:00–2:30. Participants who had been exposed to the daytime dim and white light experienced reductions in melatonin concentration after the nighttime exposure, but those who had been exposed to the blue-white light showed no different in melatonin concentrations during nighttime exposure (Kozaki et al. Citation2016).
Other studies corroborate these findings. For example, Jasser et al. (Citation2006) either allowed participants 2-hour adaptation to 18-lux white light at night or blindfolded them in darkness, then exposed them to 460-nm monochromatic light. They found that prior white light exposure decreased the magnitude of melatonin suppression by up to 46% relative to dark adaptation. Smith et al. (Citation2004) compared melatonin suppression in response to 6.5 hours of nighttime exposure to 200 lux, among participants with lighting histories of either 200 lux or 0.5 lux. Participants with a history of 200 lux demonstrated less suppression: 71% compared to 86% for those with a history of 0.5 lux. Chang et al. (Citation2011) used a similar dimmer photic history but a lower comparison photic history, exposing participants for 3 days to either dim light (1 lux) or typical room light (90 lux) prior to the administration of 6.5 hours of 90 lux at the beginning of the subjective night. Participants in the dim light condition experienced 63% greater melatonin suppression than participants in the typical room light condition. Hébert et al. (Citation2002) likewise examined the effects of exposure over longer periods of time, exposing participants to a “bright” week (~4.3 hours of bright light per day) and subsequently to a “dim” week (wearing dark goggles that limited light transmission and only going outside~1.4 hours per day). The participants were then exposed to 500 lux for 3 hours at night. Significantly more melatonin suppression occurred after the dim week (53%) compared to the bright week (41%).
Season
Related to prior photic history, season affects photoperiod, which in turn affects the amount and timing of available daylight exposure. Reductions in light during the winter, for example, could result in circadian misalignment, impacting sleep/wake cycles and subsequently impacting mood or exacerbating symptoms of affective disorders, including seasonal affective disorder (SAD) or non-seasonal depression. The limited research examining seasonal differences in light sensitivity suggests that light sensitivity is indeed higher in seasons with less daylight.
Higuchi et al. (Citation2007b) examined seasonal differences in the magnitude of melatonin suppression in response to light by exposing participants to 1000 lux for 2 hours in the evening in winter and summer. The percentage of melatonin suppression following exposure was significantly greater in winter (67%) compared to summer (37%), when the duration of daylight was about three times as long as in the winter in the study location. Similarly, Owen and Arendt (Citation1992) exposed groups of men in Antarctica to dim (290–310 lux) and bright (2100–2300 lux) light, either from 1:00–2:00 or 5:00–6:00, in both winter and summer. They found that in the winter, both dim and bright light resulted in a significant reduction in melatonin after exposure (with greater and more significant suppression for the 5:00–6:00 group), whereas in the summer, significant suppression only occurred for the group receiving bright light from 1:00–2:00 AM. The lack of effect for the 5:00–6:00 summer group was potentially due to increased daylight availability in the summer – meaning the 5:00–6:00 exposure did not result in as substantial of a change in the light environment in the summer as compared to the winter.
Health status factors
Altered circadian rhythms and related indicators, such as sleep disturbances, have been implicated in the pathophysiology of a number of psychiatric disorders, particularly mood disorders (Germain and Kupfer Citation2008; Walker et al. Citation2020). For example, circadian rhythm abnormalities are cross-sectionally associated and may be causally involved with bipolar disorder, such as decreased sleep, delayed circadian phase, and diurnal mood variations (Murray and Harvey Citation2010). Given the relationship between light exposure and circadian alignment, research has examined whether altered light sensitivity is also present in these conditions.
Bipolar disorder
Bipolar disorder is characterized by periods of depression and periods of mania or hypomania. Sleep and light are closely linked to mood states in the context of bipolar disorder. Changes in sleep can predict the onset of mood disturbances, and light and sleep interventions can be highly efficacious in stabilizing mood. It has been suggested that this relationship may in part be due to altered light signaling in patients with bipolar disorder. Some evidence indicates an association between bipolar disorder and increased melatonin suppression, and super-sensitivity to light has been proposed as an endophenotypic or trait marker of bipolar disorder (Hallam et al. Citation2009). Heightened light sensitivity has been found even among healthy individuals who are at risk for bipolar disorder, due to a parent with bipolar disorder (Nurnberger et al. Citation1988).
Research by Lewy and colleagues as far back as the early 1980s (Lewy et al. Citation1981) indicated increased melatonin suppression among individuals with bipolar disorder. For example, in one study, Lewy et al. (Citation1985) found 61.5% melatonin suppression among euthymic bipolar patients in comparison to 28% among healthy participants, upon being awoken and exposed to 500 lux from 2:00–4:00 AM. Several subsequent experiments and case studies have supported the finding that individuals with bipolar disorder exhibit supersensitivity (Hallam et al. Citation2005b, Citation2009; Nathan et al. Citation1999b).
Other studies, however, have found no evidence of heightened light sensitivity among individuals with bipolar disorder (Ritter et al. Citation2020; Whalley et al. Citation1991). Nurnberger et al. (Citation2000) found a trend towards increased melatonin suppression in patients with bipolar I disorder only, not those with bipolar II disorder. Furthermore, Lam et al. (Citation1990) found that patients had reduced light suppression compared with healthy controls. It is important to note, however, that mood-stabilizing medications used to treat bipolar disorder may affect light sensitivity, which could mask an actual difference in melatonin suppression. Studies on medication’s effects on sensitivity and their implications are discussed later in this section.
Depression
Although evidence is mixed, some research suggests that people with unipolar depression may have reduced sensitivity to light (in contrast to bipolar disorder). McGlashan et al. (Citation2019) found that patients currently experiencing depression and who had not been taking medications for at least 3 months showed significantly lower levels of melatonin suppression in response to a 100-lux light exposure, compared to both healthy controls and participants who had previously experienced depression and were in remittance. The lack of altered sensitivity among participants in remittance suggests that reduced light sensitivity may be related to experiencing a current depressive episode. Several earlier researchers, however, reported that people with depression do not have altered light sensitivity. For example, Cummings et al. (Citation1989) did not detect differences in melatonin suppression response to 500 lux at night between a group with unipolar depression and a healthy group. However, these studies included patients currently or recently (within the last month) taking antidepressant medications, which may have masked group differences (McGlashan et al. Citation2019).
Seasonal affective disorder
Evidence is mixed regarding whether people with seasonal affective disorder (SAD) experience increased light sensitivity during the winter, when they experience symptoms, and decreased sensitivity in the summer.
Nathan et al. (Citation1999b) compared a group of participants with SAD to a group of healthy controls in the winter, placing participants in a dim room (10–20 lux) starting at 21:00, then exposing them to 200 lux from 12:00–1:00. The participants with SAD exhibited 40% melatonin suppression, compared to 14% suppression among controls. Similarly, Thompson et al. (Citation1990) exposed people with and without SAD to 2000 lux and 300 lux on consecutive nights in the winter and summer. Interestingly, the participants with SAD not only demonstrated increased sensitivity in the winter, they also showed reduced sensitivity in the summer. On the other hand, however, Murphy et al. (Citation1993) compared various responses in participants with and without SAD to full-spectrum artificial daylight (500 lux and 1500 lux), and found that melatonin suppression did not differ significantly between the groups.
Two studies to our knowledge have examined melatonin suppression in relation to phototherapy. In one case study, McIntyre et al. (Citation1990) exposed a woman with SAD to 60 minutes of dim light (200 lux) at midnight, at baseline and after 2 weeks of phototherapy. Her sensitivity decreased in response to the therapy, dropping from 67% melatonin suppression at baseline to 37% after phototherapy. Conversely, Partonen et al. (Citation1997) found that patients with SAD did not differ from controls in melatonin suppression after two weeks of a light treatment consisting of 3300 lux for 5 minutes and 1 hour at 22:00.
Delayed sleep-wake phase disorder (DSWPD)
Some evidence indicates that delayed sleep-wake phase disorder (DSWPD; previously known as delayed sleep phase disorder and delayed sleep phase syndrome) may be associated with heightened light sensitivity. One study on individuals with DSWPD found that they experienced significantly greater melatonin suppression in response to 2 hours’ exposure to 1000 lux beginning 2 hours prior to peak melatonin secretion, relative to healthy controls (Aoki et al. Citation2001). Another study exposed participants with a delayed sleep schedule (a potential sub-clinical indicator of DSWPD) and a control group to 1.5 hours of blue light starting an hour after target bedtime (Moderie et al. Citation2017). The researchers found that among the delayed participants, more severe circadian phase delay was associated with greater melatonin suppression in response to light, although the study did not find differences in percentage of melatonin suppression overall between the delayed and control groups. Finally, a third study found a trend towards melatonin suppression among patients with DSWPD, compared to healthy controls, during exposure to 6.5 hours of ~ 150 lux (Watson et al. Citation2018).
Premenstrual dysphoric disorder (PMDD)
Two studies have assessed whether women with PMDD differ in light sensitivity compared to healthy controls, with different results depending on the type of light. One study exposing women with PMDD and healthy controls to 500 lux from 1:00–3:00 did not find any significant differences in amount or percentage of melatonin suppression between the two groups, or between mid-follicular and late-luteal stages of the menstrual cycle (Parry et al. Citation1997). However, when the same group of researchers conducted a similarly designed study but used 200 lux (Parry et al. Citation2010), they found that overall melatonin suppression was significantly higher among women with PMDD compared to controls (31% vs. 0%). Moreover, among women with PMDD, melatonin suppression was higher during the asymptomatic mid-follicular menstrual cycle phase (31%) compared to the symptomatic late-luteal phase (0%), whereas in control women, melatonin suppression was not significantly different across phases. However, the lack of any suppression in controls, and women with PMDD during the late-luteal phase could be explained by the use of moderate light (50 lux) light as a baseline. This level of light being used at baseline could have resulted in an underestimation of suppression across conditions.
Panic disorder
One study indicated that people with panic disorder may have subsensitivity to light as indicated by decreased melatonin suppression relative to controls. Nathan et al. (Citation1998) assessed melatonin suppression in response to moderate white light (200 lux) among patients with panic disorder, controls, and people with other anxiety disorders. They found that participants with panic disorder did not experience melatonin suppression in response to the light, compared to 10–15% suppression among the other participants.
Medications
Pre-clinical evidence indicates that medications may be able to alter non-visual responses to light in animals (Cuesta et al. Citation2008; Rea et al. Citation1994; Weber et al. Citation1998), raising the question of whether they also change the melatonin suppression response to light in humans. Additionally, some medications may directly influence melatonin production, either increasing or decreasing melatonin levels, which may impact measurements of melatonin suppression. This will be covered in the discussion section of the review. To our knowledge, the effects of three medications on melatonin suppression in humans have been studied: selective serotonin reuptake inhibitors (SSRIs; the most common class of antidepressants), lithium (used to treat bipolar disorder), and sodium valproate (used to treat bipolar disorder among other conditions).
McGlashan et al. (Citation2018) found that a moderate dose of the SSRI citalopram to healthy participants resulted in a 47% increase in melatonin suppression relative to placebo in response to 100 lux. Meanwhile, medications treating bipolar disorder appear to have the reverse effect. With respect to lithium, one study tested the effect of lithium among healthy participants, finding that treatment with lithium for 5 days significantly reduced melatonin suppression in response to 200 lux between 12:00 and 1:00: suppression averaged 55% on the initial control night and −4% after treatment (Hallam et al. Citation2005). Similarly, subchronic (5-day course) administration of sodium valproate significantly reduced melatonin suppression, decreasing from 35% suppression on the control night to 13% suppression after treatment (Hallam et al. Citation2005a). The previously mentioned study by Nurnberger et al. (Citation1988) also found that patients with bipolar I disorder taking no psychotropic meditations for 5 weeks or longer had increased melatonin suppression than those not taking medications for 2 to 5 weeks or currently taking lithium.
As previously mentioned, the effect of medications on melatonin suppression may help to explain the mixed findings in psychiatric populations. Furthermore, alterations in light sensitivity may prove useful in understanding the clinical efficacy of different medications. It may be that achieving an appropriate change in light sensitivity leads to beneficial clinical outcomes. That is, an increase in light sensitivity (e.g., with SSRIs) for those who experience hyposensitivity (e.g., unipolar depression) may allow for improved circadian synchronization and therefore improved sleep and mood. Conversely, a decrease in light sensitivity (e.g., with mood stabilizers) for those with hypersensitivity (e.g., bipolar disorder) may improve clinical outcomes. Prospective work integrating assessments of light sensitivity will be needed to assess this hypothesis.
Genetic factors
Limited studies have examined a potential role of genetic factors in influencing light-induced melatonin suppression. One approach that has been used is assessing similarities in melatonin suppression responses between genetically related individuals. As previously mentioned, healthy individuals who have a parent with bipolar disorder have been found to have higher light sensitivity – suggesting a genetic component (Nurnberger et al. Citation1988). In the general population, one study examined the melatonin suppression response to 500 lux in pairs of monozygotic and dizygotic twins with no psychiatric history (Hallam et al. Citation2006). The researchers found the greatest concordance in melatonin suppression between monozygotic twins, with melatonin suppression in twin A being a significant predictor of melatonin suppression in twin B (R2 = 0.97). Among dizygotic twins, the concordance was not as strong, and suppression in twin A did not predict suppression in twin B (R2 = 0.196).
Two studies have assessed PERIOD (PER) clock genes which could play a role in determining light sensitivity. In one study, researchers conducted genotyping to identify different haplotypes of PER2 and PER3 among participants, then assessed melatonin suppression compared to baseline after exposure to three hours of 5000K light, beginning 3 to 3.5 hours before the time of sleep midpoint (Akiyama et al. Citation2017). There was no association between percentage melatonin suppression and any of the PER3 haplotypes, but the homozygote of one PER2 haplotype, Hap3, showed significantly reduced melatonin suppression. Chellappa et al. (Citation2012) also found evidence of differential sensitivity across PER genes, although in contrast, they found that a PER3 genotype was linked to higher sensitivity. In their experiment, the evening rise in melatonin levels after exposure to blue-enriched 6500K light was attenuated significantly more, compared to pre-exposure baseline, among individuals homozygous for the PER3 5/5 allele (28%), than among those homozygous for the PER3 4/4 allele (50%) (Chellappa et al. Citation2012). Further research is necessary to confirm relationships between specific PER haplotypes and light sensitivity, and explore how such a relationship might operate.
Discussion & conclusion
The vast differences in the melatonin suppression response seen across participant demographics and patient groups may be explained in a number of ways. Some factors associated with altered sensitivity appear to be directly linked to known physiological changes. For example, changes which occur later in life may be driven by yellowing of the lens or other changes in retinal signaling. This is supported by evidence that intraocular lens replacement can lead to changes to sleep and circadian function (Chellappa et al. Citation2019). Alternatively, differences which are seen earlier in life (e.g., during childhood and puberty) may be linked to hormonal changes regardless of underlying traits. However, the mechanisms for the development of differences between groups with altered sleep and circadian phenotypes are less clear.
Figure 1. A summary of the demographic, environmental, health status, and genetic factors which may affect melatonin suppression.
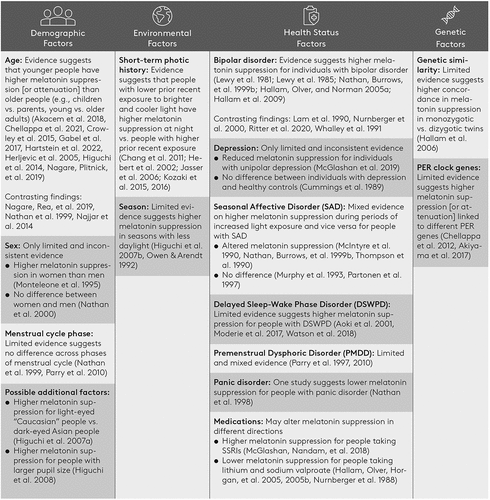
To better understand the mechanisms for inter-individual differences in melatonin suppression, it is necessary to consider how other non-visual light responses may differ in these groups. Although a complete review of these factors is outside the scope of this review, it should be noted that altered light sensitivity has also been observed in other non-visual responses within patient groups reviewed here. For example, patients with SAD also exhibit altered retinal responses to light (Roecklein et al. Citation2013). It is possible that, in this group, differences in melatonin suppression are driven by differences in processing of light information at the level of the retina. However, in other groups, differences in melatonin suppression have been observed where no apparent change in retinal processing is present. For example, during a depressive period, unipolar depression patients show reduced melatonin suppression while retinal responses were consistent with healthy controls (McGlashan et al. Citation2019). In this case, it is possible that altered levels of melatonin suppression are driven by altered processing of light information outside of the retina, for example, at the level of the suprachiasmatic nucleus.
In the context of clinical populations in particular, it is important to consider how potential differences in absolute melatonin production may impact subsequent measures of melatonin suppression. Medications such as beta-blockers are known to profoundly decrease melatonin production (Stoschitzky et al. Citation1999). Alternatively, certain antidepressants, such as selective-serotonin and norepinephrine reuptake inhibitors as well as some SSRIs are known to increase melatonin levels (Carvalho et al. Citation2009; von Bahr et al. Citation2000). These impacts on melatonin production, as well as other changes in melatonin production which occur with age (Sack et al. Citation1986) or other neurological changes, could plausibly impact melatonin suppression. Firstly, this may impact our ability to gain an accurate measure of melatonin suppression, as it may not be possible to distinguish between changes in levels due to medication use, and acute changes due only to light exposure. Secondly, it is not known how changes in melatonin production within an individual may interact with the melatonin suppression response, and it may be that an overall increase or decrease in levels also results in an increase or decrease in light sensitivity which is observed as altered melatonin suppression. Few studies have examined multiple measures of light sensitivity in the same group, or how alternate assessments of light sensitivity relate to one other, particularly in groups who show altered responses to light. It has previously been shown that under laboratory conditions with continuous light exposure, melatonin suppression and circadian phase shifting are linearly correlated with one another (Lockley et al. Citation2003). There is also an association between melatonin suppression and light-induced activity in the suprachiasmatic area (McGlashan et al. Citation2018). However, there are few studies which have included more than two standard measures of circadian light sensitivity, and studies in this field are often in small samples due to them being resource intensive to conduct. Furthermore, no studies have examined changes in light sensitivity (e.g., with the use of a medication, or the onset of a sleep or mood disorder) in more than one measure in the same sample. It is therefore not yet possible to determine which measures, if any, may indicate an overall change in the sensitivity of the non-visual system, and which responses may exhibit changes in sensitivity in isolation from other measures. Further work is needed to determine the relationships between different non-visual light responses, and how these may change under different conditions, to enhance our understanding of the mechanisms underlying these responses.
Beyond understanding how different measures of light sensitivity relate to, or predict, one another, there may be underlying individual factors which result in trait differences between people in one or more of these responses. For example, as outlined above, some studies have linked genetic factors to differences in melatonin suppression (Akiyama et al. Citation2017), or in other measures such as retinal light responses (Roecklein et al. Citation2013). It is possible that genetic factors underlie some of the other observed inter-individual differences in melatonin suppression. Although there is good evidence for a link between genetic factors and sleep and circadian phenotypes (Chang et al. Citation2016; Jones et al. Citation2019; Viola et al. Citation2007), and some evidence for links between genetic haplotypes and individual measures of light sensitivity, it is not known how genetics impact differing measures of non-visual light sensitivity. However, we note that new-gene-by-environment research has identified emerging genetic architecture underlying light sensitivity (Burns et al. Citation2022).
Light has fundamental effects on health and well-being beyond enabling visual processes. These effects are likely mediated by inter-individual differences in sensitivity to light, in part due to differences in melatonin suppression to light. The effects of exposure to artificial light in the evening on sleep and subsequently well-being may be tied to individual differences in melatonin suppression. We have outlined in this review factors linked to altered melatonin suppression, as they pertain to demographics, environmental, health-related, and genetic characteristics (). Overall, there is some evidence of altered light sensitivity for most of the characteristics examined. The evidence for differences in melatonin suppression is most robust for age, short-term photic history, and season. There is also limited evidence in delayed sleep-wake phase disorder and panic disorder patient groups which supports differences in melatonin suppression. Meanwhile, menstrual cycle phase does not appear to be associated with melatonin suppression. For other characteristics studied – such as sex, bipolar disorder, depression, SAD, and PMDD – the evidence is mixed. However, certain medications may affect melatonin suppression and could therefore contribute to the mixed findings for mental health conditions.
The mechanistic basis for inter-individual and inter-group differences in melatonin suppression currently remains unknown and is an important avenue for future research. Furthermore, we note a growing need for studies that include two or more measures of light sensitivity in order to determine the relationships between measures, and how these may differ according to individual factors. Understanding the relationships between different measures of light sensitivity (e.g., melatonin suppression and pupillary markers) will allow for the determination of which measures have the most clinical utility, while still being feasible. Should measures of light sensitivity such as melatonin suppression or pupillary markers prove predictive of risk for health outcomes (e.g., sleep or mood disruption) they could become an important component of clinical practice. For example, to determine when patients may be experiencing hyper- or hypo-sensitivity to light, so that appropriate treatments which consider this underlying phenotype can be selected. Ultimately, this could enable improved treatment outcomes by personalizing circadian-informed treatments.
Acknowledgments
We would like to thank Jessica Riccardi for support with figure production.
Disclosure statement
SR, RV and CC declare current employment, and CS declares prior employment and current consulting work, with Delos Living LLC, a private sector and for-profit company. As part of their employment with Delos, each of them is a named co-inventor on at least one filed patent application related to indoor environments and well-being, some of which pertain specifically to lighting. EMM, AJKP and SWC have received research funding from Delos and Versalux. AJKP and SWC are co-founders and co-directors of Circadian Health Innovations PTY LTD. EMM and SWC have also received research funding from Beacon Lighting, and SWC has consulted for Dyson.
Additional information
Funding
References
- Akacem LD, Wright KP, LeBourgeois MK. 2018. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol Rep [Internet]. 6:e13617. doi:10.14814/phy2.13617
- Akiyama T, Katsumura T, Nakagome S, Lee S-I, Joh K, Soejima H, Fujimoto K, Kimura R, Ishida H, Hanihara T, et al. 2017. An ancestral haplotype of the human PERIOD2 gene associates with reduced sensitivity to light-induced melatonin suppression. PloS One. 12:e0178373.
- Albreiki MS, Middleton B, Hampton SM. 2017. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr Connect. 6:100–110. doi:10.1530/EC-16-0097
- Allada R, Bass J. 2021. Circadian mechanisms in medicine. N Engl J Med. 384:550–561. doi:10.1056/NEJMra1802337
- Aoki H, Ozeki Y, Yamada N. 2001. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 18:263–271. doi:10.1081/CBI-100103190
- Bailes HJ, Lucas RJ. 2013. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 280:20122987. doi:10.1098/rspb.2012.2987
- Berger RH, Miller AL, Seifer R, Cares SR, Lebourgeois MK. 2012. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J Sleep Res. 21:235–246. doi:10.1111/j.1365-2869.2011.00962.x
- Boyce P, Barriball E. 2010. Circadian rhythms and depression. Aust Fam Physician. 39:307–310.
- Brainard GC, Lewy AJ, Menaker M, Fredrickson RH, Miller LS, Weleber RG, Cassone V, Hudson D. 1988. Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 454:212–218. doi:10.1016/0006-8993(88)90820-7
- Burns AC, Phillips AJK, Rutter MK, Saxena R, Cain SW, Lane JM. 2022. Genome-wide gene by environment study of time spent in daylight and chronotype identifies emerging genetic architecture underlying light sensitivity [Internet]. doi:10.1101/2022.06.29.22277078
- Buysse DJ. 2014. Sleep health: Can we define it? Does it matter? Sleep. 37:9–17. doi:10.5665/sleep.3298
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SBS, Santhi N, Schoen MW, Czeisler CA, Duffy JF. 2010. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 25:288–296. doi:10.1177/0748730410374943
- Cain SW, McGlashan EM, Vidafar P, Mustafovska J, Curran SPN, Wang X, Mohamed A, Kalavally V, Phillips AJK. 2020. Evening home lighting adversely impacts the circadian system and sleep. Sci Rep. 10:19110. doi:10.1038/s41598-020-75622-4
- Cajochen C. 2007. Alerting effects of light. Sleep Med Rev. 11:453–464. doi:10.1016/j.smrv.2007.07.009
- Cajochen C, Dijk DJ, Borbély AA. 1992. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep. 15:337–343.
- Carter B, Rees P, Hale L, Bhattacharjee D, Paradkar MS. 2016. Association between portable screen-based media device access or use and sleep outcomes: A systematic review and meta-analysis. JAMA Pediatr. 170:1202–1208. doi:10.1001/jamapediatrics.2016.2341
- Carvalho L, Gorenstein C, Moreno R, Pariante C, Markus R. 2009. Effect of antidepressants on melatonin metabolite in depressed patients. J Psychopharmacol (Oxf). 23:315–321. doi:10.1177/0269881108089871
- Cedeño-Laurent JG, Williams A, MacNaughton P, Cao X, Eitland E, Spengler J, Allen J. 2018. Building evidence for health: Green buildings, current science, and future challenges. Annu Rev Public Health. 39:291–308. doi:10.1146/annurev-publhealth-031816-044420
- Challet E, Kalsbeek A. 2017. Editorial: Circadian rhythms and metabolism. Front Endocrinol Internet. 8:8. doi:10.3389/fendo.2017.00201
- Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci. 112:1232–1237. doi:10.1073/pnas.1418490112
- Chang A-M, Bjonnes A, Aeschbach D, Buxton OM, Gooley JJ, Anderson C, Van Reen E, Cain SW, Czeisler CA, Duffy JF, et al. 2016. Circadian gene variants influence sleep and the sleep electroencephalogram in humans. Chronobiol Int. 33:561–573.
- Chang A-M, Scheer FAJL, Czeisler CA. 2011. The human circadian system adapts to prior photic history. J Physiol. 589:1095–1102. doi:10.1113/jphysiol.2010.201194
- Chellappa SL. 2020. Individual differences in light sensitivity affect sleep and circadian rhythms. Sleep. 44:zsaa214. doi:10.1093/sleep/zsaa214
- Chellappa SL, Bromundt V, Frey S, Cajochen C. 2021. Age-related neuroendocrine and alerting responses to light. GeroScience. 43:1767–1781. doi:10.1007/s11357-021-00333-1
- Chellappa SL, Bromundt V, Frey S, Steinemann A, Schmidt C, Schlote T, Goldblum D, Cajochen C. 2019. Association of intraocular cataract lens replacement with circadian rhythms, cognitive function, and sleep in older adults. JAMA Ophthalmol. 137:878. doi:10.1001/jamaophthalmol.2019.1406
- Chellappa SL, Steiner R, Oelhafen P, Lang D, Götz T, Krebs J, Cajochen C. 2013. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 22:573–580. doi:10.1111/jsr.12050
- Chellappa SL, Viola AU, Schmidt C, Bachmann V, Gabel V, Maire M, Reichert CF, Valomon A, Götz T, Landolt H-P, et al. 2012. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 97:E433–437. doi:10.1210/jc.2011-2391
- Cho C-H, Lee H-J, Yoon H-K, Kang S-G, Bok K-N, Jung K-Y, Kim L, Lee E-I. 2016. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol Int. 33:117–123. doi:10.3109/07420528.2015.1108980
- Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. 2015. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 32:1294–1310. doi:10.3109/07420528.2015.1073158
- Christensen MA, Bettencourt L, Kaye L, Moturu ST, Nguyen KT, Olgin JE, Pletcher MJ, Marcus GM. 2016. Direct measurements of smartphone screen-time: Relationships with demographics and sleep. PloS One. 11:e0165331. doi:10.1371/journal.pone.0165331
- Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. 2015. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 100:4067–4073. doi:10.1210/jc.2015-2775
- Cuesta M, Mendoza J, Clesse D, Pévet P, Challet E. 2008. Serotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodent. Exp Neurol. 210:501–513. doi:10.1016/j.expneurol.2007.11.026
- Cummings MA, Berga SL, Cummings KL, Kripke DF, Haviland MG, Golshan S, Gillin JC. 1989. Light suppression of melatonin in unipolar depressed patients. Psychiatry Res. 27:351–355. doi:10.1016/0165-1781(89)90149-2
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. 1999. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 284:2177–2181.
- Duffy JF, Cain SW, Chang A-M, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk D-J, Wright KP, Czeisler CA. 2011. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci. 108:15602–15608. doi:10.1073/pnas.1010666108
- Duffy JF, Zeitzer JM, Czeisler CA. 2007. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 28:799–807. doi:10.1016/j.neurobiolaging.2006.03.005
- Fonken LK, Nelson RJ. 2014. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 35:648–670. doi:10.1210/er.2013-1051
- Gabel V, Reichert CF, Maire M, Schmidt C, Schlangen LJM, Kolodyazhniy V, Garbazza C, Cajochen C, Viola AU. 2017. Differential impact in young and older individuals of blue-enriched white light on circadian physiology and alertness during sustained wakefulness. Sci Rep. 7:7620. doi:10.1038/s41598-017-07060-8
- Germain A, Kupfer DJ. 2008. Circadian rhythm disturbances in depression. Hum Psychopharmacol Clin Exp. 23:571–585. doi:10.1002/hup.964
- Goel N, Lee TM. 1995. Sex differences and effects of social cues on daily rhythms following phase advances in octodon degus. Physiol Behav. 58:205–213. doi:10.1016/0031-9384(95)00051-J
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SMW, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. 2011. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 96:E463–472. doi:10.1210/jc.2010-2098
- Hallam KT, Begg DP, Olver JS, Norman TR. 2009. Abnormal dose-response melatonin suppression by light in bipolar type I patients compared with healthy adult subjects. Acta Neuropsychiatr. 21:246–255. doi:10.1111/j.1601-5215.2009.00416.x
- Hallam KT, Olver JS, Chambers V, Begg DP, McGrath C, Norman TR. 2006. The heritability of melatonin secretion and sensitivity to bright nocturnal light in twins. Psychoneuroendocrinology. 31:867–875. doi:10.1016/j.psyneuen.2006.04.004
- Hallam KT, Olver JS, Horgan JE, McGrath C, Norman TR. 2005. Low doses of lithium carbonate reduce melatonin light sensitivity in healthy volunteers. Int J Neuropsychopharmacol. 8:255–259. doi:10.1017/S1461145704004894
- Hallam KT, Olver JS, Norman TR. 2005a. Effect of sodium valproate on nocturnal melatonin sensitivity to light in healthy volunteers. Neuropsychopharmacology. 30:1400–1404. doi:10.1038/sj.npp.1300739
- Hallam KT, Olver JS, Norman TR. 2005b. Melatonin sensitivity to light in monozygotic twins discordant for bipolar I disorder. Aust N Z J Psychiatry. 39:947. doi:10.1111/j.1440-1614.2005.01705_1.x
- Hartstein LE, Behn CD, Akacem LD, Stack N, Wright KP Jr., Mk L. 2022. High sensitivity of melatonin suppression response to evening light in preschool-aged children. J Pineal Res. 72:e12780. doi:10.1111/jpi.12780
- Hébert M, Martin SK, Lee C, Eastman CI. 2002. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 33:198–203. doi:10.1034/j.1600-079X.2002.01885.x
- Herljevic M, Middleton B, Thapan K, Skene DJ. 2005. Light-induced melatonin suppression: Age-related reduction in response to short wavelength light. Exp Gerontol. 40:237–242. doi:10.1016/j.exger.2004.12.001
- Higuchi S, Ishibashi K, Aritake S, Enomoto M, Hida A, Tamura M, Kozaki T, Motohashi Y, Mishima K. 2008. Inter-individual difference in pupil size correlates to suppression of melatonin by exposure to light. Neurosci Lett. 440:23–26. doi:10.1016/j.neulet.2008.05.037
- Higuchi S, Motohashi Y, Ishibashi K, Maeda T. 2007a. Influence of eye colors of caucasians and Asians on suppression of melatonin secretion by light. Am J Physiol-Regul Integr Comp Physiol. 292:R2352–2356. doi:10.1152/ajpregu.00355.2006
- Higuchi S, Motohashi Y, Ishibashi K, Maeda T. 2007b. Less exposure to daily ambient light in winter increases sensitivity of melatonin to light suppression. Chronobiol Int. 24:31–43. doi:10.1080/07420520601139805
- Higuchi S, Nagafuchi Y, Lee S, Harada T. 2014. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 99:3298–3303. doi:10.1210/jc.2014-1629
- Ho Mien I, Chua E-P, Lau P, Tan L-C, Lee I-G, Yeo S-C, Tan SS, Gooley JJ. 2014. Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PloS One [Internet]. 9:e96532. doi:10.1371/journal.pone.0096532
- Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, Reynolds P. 2014. Light at night and breast cancer risk among California teachers. Epidemiol Camb Mass. 25:697–706. doi:10.1097/EDE.0000000000000137
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. 2006. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 21:394–404. doi:10.1177/0748730406292391
- Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, Jeffries AR, Dashti HS, Hillsdon M, Ruth KS, et al. 2019. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 10:343.
- Kessel L, Siganos G, Jørgensen T, Larsen M. 2011. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 34:1215–1219. doi:10.5665/SLEEP.1242
- Killgore WDS, Weber M. 2014. Sleep deprivation and cognitive performance. In: Bianchi M, editor. Sleep deprivation dis eff body brain behav [Internet]. New York, NY: Springer. p. 209–29. doi:10.1007/978-1-4614-9087-6_16
- Kozaki T, Kubokawa A, Taketomi R, Hatae K. 2015. Effects of day-time exposure to different light intensities on light-induced melatonin suppression at night. J Physiol Anthropol. 34:27. doi:10.1186/s40101-015-0067-1
- Kozaki T, Kubokawa A, Taketomi R, Hatae K. 2016. Light-induced melatonin suppression at night after exposure to different wavelength composition of morning light. Neurosci Lett. 616:1–4. doi:10.1016/j.neulet.2015.12.063
- Lam RW, Berkowitz AL, Berga SL, Clark CM, Kripke DF, Gillin JC. 1990. Melatonin suppression in bipolar and unipolar mood disorders. Psychiatry Res. 33:129–134. doi:10.1016/0165-1781(90)90066-e
- Lemola S, Perkinson-Gloor N, Brand S, Dewald-Kaufmann JF, Grob A. 2015. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 44:405–418. doi:10.1007/s10964-014-0176-x
- Lewy AJ, Nurnberger JI, Wehr TA, Pack D, Becker LE, Powell RL, Newsome DA. 1985. Supersensitivity to light: Possible trait marker for manic-depressive illness. Am J Psychiatry. 142:725–727. doi:10.1176/ajp.142.6.725
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Rosenthal NE. 1981. Manic-depressive patients may be supersensitive to light. Lancet Lond Engl. 317:383–384. doi:10.1016/s0140-6736(81)91697-4
- Lockley SW, Brainard GC, Czeisler CA. 2003. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 88:4502–4505. doi:10.1210/jc.2003-030570
- Mason IC, Grimaldi D, Reid KJ, Warlick CD, Malkani RG, Abbott SM, Zee PC. 2022. Light exposure during sleep impairs cardiometabolic function. Proc Natl Acad Sci. 119:e2113290119. doi:10.1073/pnas.2113290119
- McGlashan EM, Coleman MY, Vidafar P, Phillips AJK, Cain SW. 2019. Decreased sensitivity of the circadian system to light in current, but not remitted depression. J Affect Disord. 256:386–392. doi:10.1016/j.jad.2019.05.076
- McGlashan EM, Nandam LS, Vidafar P, Mansfield DR, Rajaratnam SMW, Cain SW. 2018. The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacol (Berl). 235:3201–3209. doi:10.1007/s00213-018-5019-0
- McGlashan EM, Poudel GR, Vidafar P, Drummond SPA, Cain SW. 2018. Imaging individual differences in the response of the human suprachiasmatic area to light. Front Neurol [ Internet]. 9. doi:10.3389/fneur.2018.01022
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. 1990. Melatonin supersensitivity to dim light in seasonal affective disorder. Lancet Lond Engl. 335:488. doi:10.1016/0140-6736(90)90732-k
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Simpson NS, Dinges DF. 2012. Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 12:1015–1020. doi:10.1037/a0026871
- Moderie C, Van der Maren S, Dumont M. 2017. Circadian phase, dynamics of subjective sleepiness and sensitivity to blue light in young adults complaining of a delayed sleep schedule. Sleep Med. 34:148–155. doi:10.1016/j.sleep.2017.03.021
- Monteleone P, Esposito G, La Rocca A, Maj M. 1995. Does bright light suppress nocturnal melatonin secretion more in women than men? J Neural Transm Gen Sect. 102:75–80. doi:10.1007/BF01276567
- Münch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. 2006. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol-Regul Integr Comp Physiol. 290:R1421–1428. doi:10.1152/ajpregu.00478.2005
- Murphy DG, Murphy DM, Abbas M, Palazidou E, Binnie C, Arendt J, Campos Costa D, Checkley SA. 1993. Seasonal affective disorder: Response to light as measured by electroencephalogram, melatonin suppression, and cerebral blood flow. Br J Psychiatry J Ment Sci. 163:327–337. doi:10.1192/bjp.163.3.327
- Murray G, Harvey A. 2010. Circadian rhythms and sleep in bipolar disorder: Circadian rhythms and sleep in BD. Bipolar Disord. 12:459–472. doi:10.1111/j.1399-5618.2010.00843.x
- Nagare R, Plitnick B, Figueiro MG. 2019. Effect of exposure duration and light spectra on nighttime melatonin suppression in adolescents and adults. Light Res Technol Lond Engl [ 2001]. 51:530–543. doi:10.1177/1477153518763003
- Nagare R, Rea MS, Plitnick B, Figueiro MG. 2019. Nocturnal melatonin suppression by adolescents and adults for different levels, spectra, and durations of light exposure. J Biol Rhythms. 34:178–194. doi:10.1177/0748730419828056
- Najjar RP, Chiquet C, Teikari P, Cornut P-L, Claustrat B, Denis P, Cooper HM, Gronfier C. 2014. Aging of non-visual spectral sensitivity to light in humans: Compensatory mechanisms? PloS One. 9:e85837. doi:10.1371/journal.pone.0085837
- Nathan PJ, Burrows GD, Norman TR. 1998. Subsensitive melatonin suppression by dim white light: Possible biological marker of panic disorder. Int J Neuropsychopharmacol. 1:115–120. doi:10.1017/S1461145798001254
- Nathan PJ, Burrows GD, Norman TR. 1999a. The effect of age and pre-light melatonin concentration on the melatonin sensitivity to dim light. Int Clin Psychopharmacol. 14:189–192. doi:10.1097/00004850-199905030-00008
- Nathan PJ, Burrows GD, Norman TR. 1999b. Melatonin sensitivity to dim white light in affective disorders. Neuropsychopharmacology. 21:408–413. doi:10.1016/S0893-133X(99)00018-4
- Nathan PJ, Norman TR, Burrows GD. 1999. Effect of the menstrual cycle stage on the melatonin suppression by dim white light. Psychoneuroendocrinology. 24:193–200. doi:10.1016/s0306-4530(98)00075-4
- Nathan PJ, Wyndham EL, Burrows GD, Norman TR. 2000. The effect of gender on the melatonin suppression by light: A dose response relationship. J Neural Transm Vienna Austria [ 1996]. 107:271–279. doi:10.1007/s007020050022
- Navara KJ, Nelson RJ. 2007. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J Pineal Res. 43:215–224. doi:10.1111/j.1600-079X.2007.00473.x
- Nelson RJ, Chbeir S. 2018. Dark matters: Effects of light at night on metabolism. Proc Nutr Soc. 77:223–229. doi:10.1017/S0029665118000198
- Nurnberger JI, Adkins S, Lahiri DK, Mayeda A, Hu K, Lewy A, Miller A, Bowman ES, Miller MJ, Rau NL, et al. 2000. Melatonin Suppression by Light in Euthymic Bipolar and Unipolar Patients. Arch Gen Psychiatry. 57:572–579.
- Nurnberger JI, Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E. 1988. Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A preliminary report. Neuropsychopharmacol off Publ Am Coll Neuropsychopharmacol. 1:217–223. doi:10.1016/0893-133x(88)90020-6
- Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. 2013. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 98:337–344. doi:10.1210/jc.2012-2874
- Owen J, Arendt J. 1992. Melatonin suppression in human subjects by bright and dim light in Antarctica: Time and season-dependent effects. Neurosci Lett. 137:181–184. doi:10.1016/0304-3940(92)90399-r
- Park YM, White AJ, Jackson CL, Weinberg CR, Sandler DP. 2019. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med [Internet]. 179:1061. doi:10.1001/jamainternmed.2019.0571
- Parry BL, Meliska CJ, Sorenson DL, Lopez A, Martínez LF, Hauger RL, Elliott JA. 2010. Increased sensitivity to light-induced melatonin suppression in premenstrual dysphoric disorder. Chronobiol Int. 27:1438–1453. doi:10.3109/07420528.2010.503331
- Parry BL, Udell C, Elliott JA, Berga SL, Klauber MR, Mostofi N, LeVeau B, Gillin JC. 1997. Blunted phase-shift responses to morning bright light in premenstrual dysphoric disorder. J Biol Rhythms. 12:443–456. doi:10.1177/074873049701200506
- Partonen T, Vakkuri O, Lönnqvist J. 1997. Suppression of melatonin secretion by bright light in seasonal affective disorder. Biol Psychiatry. 42:509–513. doi:10.1016/S0006-3223(96)00376-9
- Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, Lockley SW, Cain SW. 2019. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci. 116:201901824. doi:10.1073/pnas.1901824116
- Pittendrigh CS. 1993. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 55:16–54. doi:10.1146/annurev.ph.55.030193.000313
- Plano SA, Casiraghi LP, García Moro P, Paladino N, Golombek DA, Chiesa JJ. 2017. Circadian and metabolic effects of light: Implications in weight homeostasis and health. Front Neurol [ Internet]. 8. doi:10.3389/fneur.2017.00558
- Rahman SA, St Hilaire MA, Gronfier C, Chang A-M, Santhi N, Czeisler CA, Klerman EB, Lockley SW. 2018. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol. 596:2147–2157. doi:10.1113/JP275501
- Rea MA, Glass JD, Colwell CS. 1994. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci off J Soc Neurosci. 14:3635–3642. doi:10.1523/JNEUROSCI.14-06-03635.1994
- Reiter RJ, Tan D-X, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. 2007. Light at night, chronodisruption, melatonin suppression, and cancer risk: A review. Crit Rev Oncog. 13:303–328. doi:10.1615/CritRevOncog.v13.i4.30
- Ritter P, Wieland F, Skene DJ, Pfennig A, Weiss M, Bauer M, Severus E, Güldner H, Sauer C, Soltmann B, et al. 2020. Melatonin suppression by melanopsin-weighted light in patients with bipolar I disorder compared to healthy controls. J Psychiatry Neurosci. 45:79–87. doi:10.1503/jpn.190005
- Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M, Wood-Vasey WM, Franzen P. 2013. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 210:150–158. doi:10.1016/j.psychres.2013.05.023
- Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM. 1986. Human melatonin production decreases with age. J Pineal Res. 3:379–388. doi:10.1111/j.1600-079X.1986.tb00760.x
- Sadeh A. 2007. Consequences of sleep loss or sleep disruption in children. Sleep Med Clin. 2:513–520. doi:10.1016/j.jsmc.2007.05.012
- Salvi SM, Akhtar S, Currie Z. 2006. Ageing changes in the eye. Postgrad Med J. 82:581–587. doi:10.1136/pgmj.2005.040857
- Shochat T, Cohen-Zion M, Tzischinsky O. 2014. Functional consequences of inadequate sleep in adolescents: A systematic review. Sleep Med Rev. 18:75–87. doi:10.1016/j.smrv.2013.03.005
- Smith KA, Schoen MW, Czeisler CA. 2004. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 89:3610–3614. doi:10.1210/jc.2003-032100
- Stebelova K, Roska J, Zeman M. 2020. Impact of dim light at night on urinary 6-sulphatoxymelatonin concentrations and sleep in healthy humans. Int J Mol Sci. 21:7736. doi:10.3390/ijms21207736
- Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, Lindner W. 1999. Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol. 55:111–115. doi:10.1007/s002280050604
- Terman M, Terman JS. 2005. Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectr. 10:647–663. doi:10.1017/S1092852900019611
- Thompson C, Stinson D, Smith A. 1990. Seasonal affective disorder and season-dependent abnormalities of melatonin suppression by light. The Lancet. 336:703–706. doi:10.1016/0140-6736(90)92202-S
- Turner PL, Mainster MA. 2008. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br J Ophthalmol. 92:1439–1444. doi:10.1136/bjo.2008.141747
- Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, von Schantz M, Dijk D-J. 2007. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 17:613–618. doi:10.1016/j.cub.2007.01.073
- von Bahr C, Ursing C, Yasui N, Tybring G, Bertilsson L, Röjdmark S. 2000. Fluvoxamine but not citalopram increases serum melatonin in healthy subjects – an indication that cytochrome P450 CYP1A2 and CYP2C19 hydroxylate melatonin. Eur J Clin Pharmacol. 56:123–127. doi:10.1007/s002280050729
- Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, McLaughlin EN. 2013. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol. 38:1058–1069. doi:10.1093/jpepsy/jst033
- Walker WH, Walton JC, DeVries AC, Nelson RJ. 2020. Circadian rhythm disruption and mental health. Transl Psychiatry. 10:1–13. doi:10.1038/s41398-020-0694-0
- Watson LA, Phillips AJK, Hosken IT, McGlashan EM, Anderson C, Lack LC, Lockley SW, Rajaratnam SMW, Cain SW. 2018. Increased sensitivity of the circadian system to light in delayed sleep–wake phase disorder. J Physiol. 596:6249–6261. doi:10.1113/JP275917
- Weber ET, Gannon RL, Rea MA. 1998. Local administration of serotonin agonists blocks light-induced phase advances of the circadian activity rhythm in the hamster. J Biol Rhythms. 13:209–218. doi:10.1177/074873098129000057
- Whalley LJ, Perini T, Shering A, Bennie J. 1991. Melatonin response to bright light in recovered, drug-free, bipolar patients. Psychiatry Res. 38:13–19. doi:10.1016/0165-1781(91)90048-t
- Yang M, Ma N, Zhu Y, Su Y-C, Chen Q, Hsiao F-C, Ji Y, Yang C-M, Zhou G. 2018. The acute effects of intermittent light exposure in the evening on alertness and subsequent sleep architecture. Int J Environ Res Public Health. 15:524. doi:10.3390/ijerph15030524
