ABSTRACT
Purpose: Differentiated thyroid cancer is the most common endocrine malignancy. Recurrences (5–20%) are the main reason for follow-up. Thyroglobulin (Tg) has proven to be an excellent disease marker, but thyroglobulin-antibodies (Tg-Ab) may interfere with Tg measurement, leading to over or underestimation. It is proposed that the Tg-Ab trend can be used as a marker for disease recurrence, yet few studies define trend and have a long-term follow-up. The objective of our study was to investigate the value of a well-defined Tg-Ab trend as a surrogate marker for disease recurrence during long-term follow-up. Methods: We retrospectively studied patients treated at the Nuclear Department of the University Medical Center Utrecht from 1998 to 2010 and the Netherlands Cancer Institute from 2000 to 2009. All patients with Tg-Ab 12 months after treatment were included. The definition of a rise was >50% increase of the Tg-Ab value in a 2 year time period. A decline as >50% decrease of the Tg-Ab value. Results: Twenty-five patients were included. None of the patients with declining or stable Tg-Ab without a concomitant rise in Tg developed a recurrence. Four patients did suffer a recurrence. Three of these patients had a rising Tg-Ab trend, in two of these patients Tg was undetectable. Conclusions: Tg-Ab trend can be used as a crude surrogate marker for long-term follow-up of Tg-Ab patients. A rising trend in Tg-Ab warrants further investigation to detect recurrent disease. Stable or declining Tg-Ab levels do not seem to reflect a risk for recurrence.
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine malignancy. Survival rates are excellent, however, recurrences occur in 5–20% of the patients (Citation1). Recurrences are the main reason for follow-up. The thyroid specific thyroglobulin (Tg) is the primary tumor marker for detecting recurrences and, combined with ultrasound of the neck, the current gold standard for DTC follow-up. However, 20–25% of DTC patients also have thyroglobulin-antibodies (Tg-Ab) present (Citation2,Citation3). These antibodies interfere with Tg measurements by over or underestimating Tg values, depending on the test used (Citation4). Immunoradiometric assays (IRMA) are considered to be consistently more sensitive than radioimmunoassays (RIA) and usually give an underestimation of the Tg level, whereas RIAs can also overestimate the Tg level (Citation5). So any sample with a positive Tg-Ab result is unreliable for measuring serum Tg concentrations and the value of the otherwise excellent tumor marker is of limited use (Citation6). Consequently, Tg-Ab positivity poses a dilemma in follow-up of a significant proportion of DTC patients. Some authors have suggested that Tg-Ab can be used as a surrogate marker, especially in the case of de novo appearance more than one year after diagnosis (Citation6–Citation10), while others found contradictory results (Citation11).
A major limitation of these studies is the absence of a clear definition of ‘trend’. The study of Kim et al. (Citation12) is one of the few studies that does define trend, and it has been incorporated in a consensus meeting regarding this subject. This definition of trend is as follows: a ≥ 50% rise in concentration of measured Tg-Ab 6–12 months after remnant ablation compared to measurements during remnant ablation. A decline was defined as a ≥ 50% decline in Tg-Ab levels. However, the clinical application of this definition in patients at 12 months after primary treatment has not yet been studied.
The aim of the current study was, therefore, to determine whether Tg-Ab trends can serve as a surrogate marker for disease recurrence in patients with well differentiated thyroid cancer during long-term follow-up using the definitions mentioned above.
Materials and methods
Patients
We retrospectively reviewed all patients treated for DTC at the department of Nuclear Medicine of the UMC Utrecht (Utrecht, the Netherlands) between January 1998 and August 2010, and all patients treated at the Department of Nuclear Medicine of the Netherlands Cancer Institute (Amsterdam, the Netherlands) between 2000 and 2009. All patients with detectable Tg-Ab 12 months after primary treatment were included in this study.
Patients were treated with total thyroidectomy, additional lymph node dissection if indicated, and postoperative RAI (3700–7400 MBq). Patient characteristics and follow-up parameters, e.g. laboratory measurements (Tg and Tg-Ab levels) and the results of all imaging modalities were recorded. In addition, tumor characteristics, preoperative and postoperative staging, and results of surgery were registered. Furthermore, data regarding disease status during follow-up and duration of follow-up were recorded.
Exclusion criteria were age under 18 years, missing data regarding the stage of disease, missing follow-up information within the year after treatment, missing laboratory measurements, and/or distant metastases at the time of presentation. Patients with distant metastases were excluded because these patients are not suitable for follow-up with Tg and/or Tg-Ab only and therefore a different follow-up protocol applies.
Laboratory measurement
In the UMC Utrecht, Tg and Tg-Ab levels were measured using the Brahms DYNOtest Tg-pluS and the BRAHMS anti-Tg RIA-kit (Brahms Diagnostica GmBH, Berlin, Germany). The Tg method is a high-sensitivity IRMA. After 2002 the functional sensitivity (FS), defined as the lowest Tg-level that can be measured with a variation less than 20%, for this assay is 0.2 μg/L, and 20 U/mL for the Tg-Ab assay. Up until 2002, the FS was 1.0 μg/L for Tg and 20 U/ml for Tg-Ab, respectively. In the Netherlands Cancer Institute, Tg-Ab levels were assessed by a competitive electrochemiluminescence immunoassay whereby serum TgAb competes for biotinylated human Tg with ruthenium-labeled TgAb. The Tg-TgAb complexes form and bind streptavidin-coated microparticles and are magnetically captured onto the surface of an electrode. Tg levels were measured by a sandwich immunoassay (Cobas6000, Roche Diagnostic GmBH, D-68298 Mannheim).
The FS of this assay for Tg was 0.1 μg/L and 22 U/mL for Tg-Ab. In both centers Tg and Tg-Ab levels were judged positive when patients had a value above the functional sensitivity. TSH levels were measured simultaneously and exceeded 20 mU/L in all patients. All Tg and Tg-Ab levels indicated in the text or tables are TSH stimulated measurements, either by LT4 withdrawal or after rhTSH stimulation.
Tg-Ab trend
For Tg-Ab trend analysis we divided the follow up in periods of 24 months starting from 12 months after primary treatment. Within each period patients were divided in to four groups according to changes in Tg-Ab concentration. The Tg-Ab trend was recorded as increasing when, in one of these periods of 24 months, Tg-Ab levels increased with >50%. When Tg-Ab levels declined >50% or normalized to an undetectable value the trend was recorded as decreasing, and as undetectable, when Tg-Ab levels were below the detection limit. Tg-Ab trend was recorded as persistent stable when Tg-Ab levels were elevated, but fluctuations were <50% (Citation15,Citation16).
Recurrent and suspicion of recurrent disease
Recurrences can occur in patients that have been judged as free of disease, frequently described as ‘no evidence of disease’ (NED). A recurrence had to be proven by cytology, histology or positive post-131I therapy scan. Suspicion of recurrent disease was defined as either 131I therapy was given because of rising biochemical markers (Tg and/or Tg-Ab) or suspicious lesions found at physical examination for which further investigation was initiated.
Imaging modalities
Neck ultrasound was performed at least yearly. In order to provide an anatomic substrate for patients with detectable Tg and/or Tg-Ab additional imaging modalities where used, such as DxWBS, MRI, CT, Iodine-124 (124I), and 18F-FDG PET/CT scanning.
Risk classification
In this study the 2015 ATA risk classification was used (Citation13). Patients with distant metastases (M1) were excluded from analysis. For TNM-classification and stage grouping we used the 7th edition of the TNM-classification (Citation13).
Statistical analysis
Statistical analysis was performed using SPSS 23.0 (Chicago, Illinois, USA). All demographic data are shown in mean values with standard deviation (±SD) unless indicated otherwise.
Results
Patients
In total 443 patients were treated for differentiated thyroid cancer in both hospitals (). A total of 64 (14%) patients had positive Tg-Ab levels at time of diagnosis. One patient died within the year after primary treatment as a result of metastatic thyroid cancer. Twenty-nine patients (7%) had positive Tg-Ab 12 months after primary treatment. Four patients were excluded, two due to pulmonary metastasis, one due to persistent disease diagnosed within one year after primary treatment and one due to non-radical tumor resection without subsequent RAI.
Figure 1. Flowchart of patient selection. Note. DTC = differentiated thyroid cancer; Tg-Ab = thyroglobulin-antibodies.
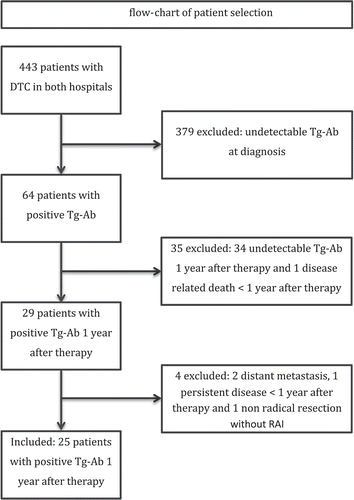
As a result, 25 patients were included. Baseline characteristics are shown in . Most patients were female (76%). The majority of tumors were of papillary origin (88%). The mean follow-up period was 96 months (SD 77.5, range 25–296). Nine-teen patients (76%) had no evidence of disease during the entire follow-up period, four patients (16%) had a recurrence, and two patients (8%) had the suspicion of recurrent disease.
Table 1. Baseline characteristics of patients with positive Tg-Ab (n = 25) at one year after primary treatment.
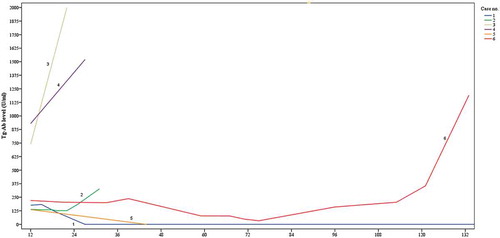
Tg-Ab trend during follow up are shown in . Descriptive case presentations of all patients (n = 6) with recurrent or suspicion of recurrent disease are presented in . A plot of the Tg-Ab trend for these six cases is shown in .
Table 2. Trend analyses of Tg-Ab levels and corresponding disease status during different periods of follow-up.
Table 3. Descriptive case presentation of all patients (n = 6) with recurrent or suspicion of recurrent disease.
Tg-Ab trend
12–36 months follow-up (n = 25)
Eleven patients had a declining trend of Tg-Ab levels. One of these patients (case no. 1) was diagnosed with suspicion of recurrence based on a palpable lesion in the neck detected 31 months after primary treatment. The year before Tg-Ab trend was declining to zero, Tg levels were undetectable and DxWBS was normal. Additional CT showed no lesions indicating metastasis and fine needle aspiration of the palpable lesion showed no malignant cells.
Nonetheless, based on the high clinical suspicion, lymph node extirpation was performed, revealing a follicular proliferation; recurrent disease was therefore not confirmed. During further follow-up (248 months) of this patient Tg-Ab and Tg levels remained undetectable and no recurrences were diagnosed.
Seven patients had an increasing trend of Tg-Ab levels. Two of these patients were diagnosed with recurrent disease (cases no. 2 and 3) and one with suspicion of recurrent disease (case no. 4). In all three cases, Tg levels were undetectable. In case no. 2 18F-FDG PET/CT was performed due to increasing Tg-Ab levels, showing a pathologic lymph node in the neck; histology after resection confirmed metastasis of PTC. During follow-up Tg-Ab and Tg levels remained undetectable and no further recurrence was diagnosed. Case no. 3 presented with a palpable lesion in the neck. Ultrasound and additional CT showed four suspicious lesions. Fine needle aspiration showed lymphogenic PTC metastasis. This was confirmed histologically after additional neck dissection, revealing four metastatic lymph nodes. During subsequent follow-up Tg-Ab levels remained detectable with a persistent stable trend while Tg levels remained undetectable. No further recurrence was found. Case no. 4 was suspected of having recurrence based on increasing Tg-Ab trend with undetectable Tg levels and suspicious uptake on DxWBS at 27 months after primary treatment. The patient was treated with high dose I-131 therapy. However, the post-therapy scan did not show any uptake. Further follow-up revealed no recurrences.
One additional patient with increasing Tg-Ab trend was dismissed from follow-up 27 months after primary treatment based on undetectable Tg levels and normal DxWBS.
Seven patients had a persistent stable Tg-Ab trend. None of these patients developed recurrent or suspicion of recurrent disease.
36–60 months follow-up (n = 21)
Eight patients had declining trends of Tg-Ab levels. Of these eight patients, four declined to zero, one was dismissed from further follow-up. Forty-four months after treatment one patient with declining Tg-Ab trend (case no. 5) was diagnosed with recurrent disease based on a rising Tg level and a palpable lesion in the neck. In this patient the Tg-Ab trend had been declining since primary treatment. The patient was treated with I-131 therapy. Post-therapy scintigraphy showed uptake in a suspicious lesion in the neck thereby confirming recurrent disease.
Two patients had an increasing trend and ten patients a persistent stable Tg-Ab trend. No events occurred in both groups and from the persistent stable group six patients were dismissed from follow-up. During this period Tg-Ab levels of one patient remained undetectable.
60–132 months follow-up (n = 13)
No events occurred during this period and seven patients were dismissed from follow-up, four due to persistent stable trend and three due to undetectable trend.
132–156 months follow-up (n = 6)
One patient (case no. 6) had an increasing Tg-Ab trend and was diagnosed with recurrence 133 months after primary treatment. Besides rising Tg-Ab levels, Tg levels were rising and a palpable lesion in the neck was found during physical examination. Lymph node extirpation confirmed solitary PTC metastasis and the patient was treated additionally with I-131 which also showed uptake in pulmonary metastasis. Tg-Ab trend analysis for this patient showed a rise of less than 50% from 84 months onwards, with an increasing trend of over 50% from 120 months after primary treatment. No cases were dismissed follow-up during this follow-up period.
156 months follow-up (n = 5)
After more than 156 months of follow-up no events occurred.
Total of Tg-Ab trends and corresponding events
Over the total follow-up period 25 declining trends were recorded in which one recurrence (with concurrent increasing Tg level) and one suspicion of recurrent disease occurred (which proved not to be a lymph node metastasis). Fifteen increasing trends were registered with 3 recurrences (one with concurrent increasing Tg level and two with undetectable Tg level) and 1 suspicion of recurrent disease (post-therapy 131I scan showed no uptake). Furthermore, no events occurred in 32 stable and 31 undetectable trends ().
Table 4. Cumulative trend analysis of Tg-Ab levels and corresponding disease status.
Discussion
This study evaluates the use of the trend of Tg-Ab level as a marker for disease status during the follow-up of DTC patients with detectable Tg-Ab levels 12 months after primary treatment using predefined clear definitions for trends in Tg-Ab levels. The current study shows that if Tg-Ab levels are declining or stable, the risk of recurrence is negligible. Thus, in these cases no further imaging is warranted. However, when Tg levels are rising concurrently with declining or undetectable Tg-Ab levels, this presents an indication for additional imaging to search for a possible recurrence. In case of rising Tg-ab levels, we recommend that the treating physician should actively try to diagnose recurrence, although one should be aware that no recurrence will be found in the majority of the patients.
Of the patients with detectable Tg-Ab 12 months after primary treatment, four (15%) were diagnosed with a recurrence during further follow-up. Three of the four recurrences occurred during the first three years after initial therapy. One patient had a recurrence after 133 months precipitated by rising Tg-Ab and Tg-levels. Three out of four patients showed a rise in Tg-Ab before recurrence was confirmed, while the other patient had a declining Tg-Ab level, with a concomitant rise in the Tg level. In our cohort no patients were diagnosed with recurrent disease when Tg-Ab levels were stable or undetectable.
Our data are in concordance with the published clinical position statement by Verburg et al. on Tg-Ab positive patients that changes in serum TgAb levels can be used as an imprecise surrogate marker and that the trend is of greater value than the absolute level (Citation14). Most studies report a low recurrence rate in patients with Tg-Ab levels that eventually become negative (Citation3,Citation6). By some authors it is proposed that Tg-Ab persistence alone for more than 3 years after initial treatment may suggest recurrence or persistent disease (Citation15). Our study, however, does not support these results. The presence of Tg-Ab, in some patients for as long as 156 months after primary treatment, was not found to be a predictor for recurrent disease as long as Tg-Ab level remained stable.
If Tg-Ab levels are rising, additional diagnostic modalities such as US of the neck, DxWBS, 18F-FDG and 124I PET/CT are available to the clinician for further evaluation of this patient group (Citation9,Citation16–Citation22). This applies, off course, to patients with a rising Tg level independently of the Tg-Ab trend.
A limitation of our study is the relatively small number of patients. However, it is similar to the number of patients presented in other studies investigating Tg-Ab positive patients (Citation12,Citation23,Citation24). This underlines that the number of patients in clinical practice is also low. Statistical significance could not be assessed due to this limited number of events and therefore limits the strength of our conclusion.
The retrospective nature of our study could introduce detection bias. Where patients with a rising Tg-Ab level would have additional investigations, and patients with stable or declining levels would not.
Main strengths of our study are the long mean follow-up period and the use of a well-defined definition for trend, making the reliability and reproducibility of our data more robust.
Conclusion
The trend in Tg-Ab levels can be used as a crude surrogate marker for Tg. In patients with declining or stable Tg-Ab levels, without a concomitant rise in Tg, the risk of recurrence is negligible. In contrast, a rise in the Tg-Ab trend >50% in a 2 year time period in patients without detectable Tg warrants the need for additional diagnostic work-up to detect possible recurrent disease.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Notes on contributors
Siegrid G. A. de Meer
Siegrid de Meer G. A and Wessel M.C.M. Vorselaars contributed equally to the manuscript
References
- Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506.
- Ringel M, Nabhan F. Approach to follow-up of the patient with differentiated thyroid cancer and positive anti-thyroglobulin antibodies. J Clin Endocrinol Metab. 2013;98:3104–3110. doi: 10.1210/jc.2013-1412.
- Pacini F, Mariotti S, Formica N, et al. Thyroid autoantibodies in thyroid cancer: Incidence and relationship with tumour outcome. Acta Endocrinol. 1988;119:373–380.
- Spencer CA, Bergoglio LM, Kazarosyan M, et al. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:5566–5575. doi: 10.1210/jc.2005-0671.
- Giovanella L, Clark P, Chiovato L, et al. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: A clinical position paper. Eur J Endocrinol. 2014;171:R33–R46. doi: 10.1530/EJE-14-0148.
- Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: Prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127. doi: 10.1210/jcem.83.4.4683.
- Chung J, Park YJ, Kim TY, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf). 2002;57:215–221.
- Görges R, Maniecki M, Jentzen W, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153:49–55. doi: 10.1530/eje.1.01940.
- Seo J, Lee S, Ahn B, et al. Recurrence detection in differentiated thyroid cancer patients with elevated serum level of antithyroglobulin antibody: Special emphasis on using (18)F-FDG PET/CT. Clin Endocrinol (Oxf). 2010;72:558–563. doi: 10.1111/j.1365-2265.2009.03693.x.
- Hsieh C, Wang P. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid. 2014;24:488–493. doi: 10.1089/thy.2012.0611.
- Smooke Praw S, Ro K, Levin O, et al. Thyroglobulin antibody levels do not predict disease status in papillary thyroid cancer. Clin Endocrinol (Oxf). 2014;81:271–275. doi: 10.1111/cen.12421.
- Yoon J, Kim W, Kim T, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:4683–4689. doi: 10.1210/jc.2008-0962.
- Haugen B, Alexander E, Bible K, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020.
- Verburg F, Luster M, Cupini C, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: A clinical position statement. Thyroid. 2013;23:1211–1225. doi: 10.1089/thy.2012.0606.
- Chiovato L, Latrofa F, Braverman L, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351.
- Cooper D, Doherty G, Haugen B, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110.
- Pacini F, Castagna MG, Brilli L, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5): v214–9. doi: 10.1093/annonc/mdq190.
- Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158.
- Pitoia F, Ward L, Wohllk N, et al. Recommendations of the Latin American Thyroid Society on diagnosis and management of differentiated thyroid cancer. Arq Bras Endocrinol Metabol. 2009;53:884–887.
- Gharib H, Papini E, Valcavi R, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63.
- Freudenberg L, Jentzen W, Stahl A, et al. Clinical applications of 124I-PET/CT in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2011; 38(Suppl1): S48–56. doi: 10.1007/s00259-011-1773-5.
- Van Nostrand D, Khorjekar G, O’Neil J, et al. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in the identification of metastasis in differentiated thyroid cancer with 131I planar whole-body imaging and 124I PET. J Nucl Med. 2012;53:359–362. doi: 10.2967/jnumed.111.096016.
- Ozkan E, Soydal C, Araz M, et al. The additive clinical value of 18F-FDG PET/CT in defining the recurrence of disease in patients with differentiated thyroid cancer who have isolated increased antithyroglobulin antibody levels. Clin Nucl Med. 2012;37:755–758. doi: 10.1097/RLU.0b013e31825ae77b.
- Pacini F, Molinaro E, Castagna MG, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925.