Abstract
Havel, J.E. and R.G. Rhodes. 2009. Spatial disconnection of plankton dynamics in an Ozark reservoir. Lake Reserv. Manage. 25:28–38.
This two-year study examined spatial distribution and seasonal population dynamics of algae and zooplankton in Table Rock Lake (Missouri, USA), a large clear reservoir threatened by increased development in the watershed. Regular samples were collected from a polymictic up-lake site (10 m deep, mean Secchi transparency 1.0 m) and a monomictic down-lake site (37 m deep, 4.0 m Secchi) in the productive James River arm. Average abundance and biovolume of most algae groups showed no statistically-discernable difference between these two sites, and maximum cell densities were similar in magnitude (∼ 105 cells/mL). Exceptional were the chlorophytes, which had their highest abundance up-lake. Based on taxonomic composition, both sites appear to be dominated by pelagic algae, with little evidence of riverine immigrants. Algae and zooplankton showed rapid seasonal changes in total densities and composition, and peak abundances showed no association in time between the two sites. Although no floating or suspended colonies were ever visible in the field, cyanobacteria (particularly Oscillatoria) were common at both study sites, and we found numerous genera linked to nuisance blooms in other lakes. Zooplankton communities differed between sites, with cladocerans common in winter and spring at the down-lake site and rotifers common year round at the up-lake site. Important cladoceran grazers, such as Daphnia, were usually not abundant; however, when they were common, algae abundance was always low.
Excessive algal growth reduces water quality of lakes and reservoirs in a variety of ways. High density of suspended algae decreases water clarity, and decomposition of settling organic matter reduces dissolved oxygen in the hypolimnion (CitationWetzel 2001). Large algal colonies and filaments clog the filters of zooplankton grazers (CitationPorter and Orcutt 1980), reducing the efficiency of food chains. A number of nuisance algae create taste and odor problems for drinking water supplies (CitationVan den Hoek et al. 1995), and numerous strains of cyanobacteria produce toxins that, in high concentration, cause fish and livestock deaths and human illness (CitationCarmichael 1981, CitationChorus and Bartram 1999).
Algal productivity, biomass, and composition are, in turn, controlled by several key environmental factors. Many lakes are limited by the supply rate of macronutrients, such as phosphorus (P; Schindler 1974) and nitrogen (N; CitationVitousek et al. 1997), and low N:P ratios favor growth of cyanobacteria (CitationSmith 1982). In natural lakes, seasonal cycles of solar radiation, heating, and mixing lead to a regular succession of phytoplankton taxa (CitationWetzel 2001, CitationLampert and Sommer 2007). During periods of high abundance of zooplankton grazers (particularly Daphnia), water generally has high water clarity (the spring “clear water phase,” CitationLampert and Sommer 2007). Hydrology is also important. Periods of high river discharge import and resuspend particulates, as well as export plankton. The high population growth rates of phytoplankton allow rapid recovery from such perturbations.
Reservoirs offer an interesting stage for exploring algal dynamics. Large watersheds relative to lake area allow higher natural loading rate in reservoirs than in natural lakes (CitationWetzel 1990). In large reservoirs, strong longitudinal gradients exist in water clarity and productivity. Because high suspended solids cause light limitation of primary producers at the riverine end, and particle settling causes nutrient limitation at the lacustrine end, productivity tends to be highest in the transition zone (CitationKimmel et al. 1990). Trophic state thus depends on location in the reservoir. Flushing rate is highly variable among reservoirs and over time in single reservoirs (Thornton et al. 1990). Because particle settling depends on flow rate and flushing rate (CitationHynes 1970, CitationSøballe and Bachman 1984), the position of the transition zone should move down reservoir during high flow events. In most reservoirs, damming a river provides conditions for increased algae growth over that in the river, where turbidity generally limits light penetration (CitationKimmel et al. 1990). Exceptions with high flushing rates and poor algae growth throughout the reservoir actually show lower densities of suspended algae in the reservoir than in the main-stem river (CitationSøballe and Bachman 1984). The composition of algae in reservoirs should also be strongly tied to hydrology. Intermittent mixing from high flow events should “reset” the conditions favoring particular taxa and riverine algae may serve as important colonists into the community.
In this study, we examined the phytoplankton composition and dynamics at two ends of the James River arm of Table Rock Lake, a large reservoir that has had recent reductions in phosphorus loading. We had three primary questions of interest. First, do seasonal cycles of phytoplankton abundance at each site correspond to regular cycles, such as are observed in natural lakes? In particular, do diatoms dominate only during mixing periods and cyanobacteria only during summer stratification? Second, is algal diversity higher and diatoms more numerous at the up-lake site, such as would occur following settlement from riverine input? Finally, is algae abundance associated with grazer abundance? In particular, is biovolume negatively correlated with density of Daphnia? We also examined whether algae blooms (floating and suspended colonies) continue to occur in the James River arm and if nuisance algae taxa are common.
Materials and methods
Site description
Table Rock Lake is a deep-storage reservoir (area = 174 km2) located in the Ozark highlands of southwestern Missouri. Table Rock is one of four impoundments on the White River and follows another deep-storage reservoir (Beaver Lake; ). In addition to the main stem (length 92 km), Table Rock Lake has several major arms including the James River (55 km), Kings River, and Indian River. At conservation pool (278 m a.s.l.), Table Rock Lake has average and maximum depths of 18.9 m and 66 m, respectively (CitationKnowlton and Jones 1989b). The 10,407 km2 watershed lies in steep hills of oak-hickory forest, with limestone and dolomite substrate. The thin, well-buffered soils, support livestock grazing. Table Rock Lake is the least fertile of Missouri reservoirs, with 4 μ g/L chlorophyll and 9 μ g/L total phosphorus measured at the dam (CitationJones and Knowlton 1993) and is the only reservoir in the state to have meta-limnetic algal peaks (CitationKnowlton and Jones 1989a). Built in 1958 for hydropower and flood control, Table Rock Lake also supports a large amount of recreational uses (CitationUSACE 2008), and subsequently has had rapid development of lake communities and the nearby city of Branson.
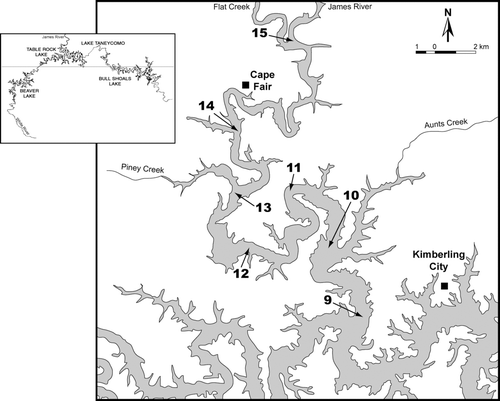
Figure 1 Sample sites on the James River arm of Table Rock Lake, in southwestern Missouri, USA. The James River arm meets the main stem (White River) near site 9. Inset map of the upper White River lakes courtesy of W.R. Green. The horizontal line represents the Missouri-Arkansas border.
The James River arm has historically been the most productive arm of the lake. With most nutrient loading coming from sewage treatment plant (STP) effluent, the James River contributed up to 27% of the phosphorus loading to the lake as a whole (CitationObrecht et al. 2005). During 1995–2000, total phosphorus levels (summer median) over a longitudinal gradient on the James River arm (: sites 15 and 9) ranged from 98 to 16 μ g/L (CitationObrecht et al. 2005). Occasional algae blooms appeared as large patches of highly turbid green water during the summers of 1998–2000, attracting attention of the general public and regulatory agencies (DNR 1999, CitationDeslatte 2002). Recent upgrades to the Springfield STP (completed in 2001) reduced phosphorus loading from this source by 89%, resulting in greatly reduced concentrations of total phosphorus in the river and in the water column at up-lake sites. During 2001–2003, median summer phosphorus concentrations near sites 15 and 9 were 58 and 10 μ g/L, respectively (CitationObrecht et al. 2005). Nevertheless, chlorophyll levels showed only a modest decrease (6–17%) over the same period, and this arm of the lake continues to maintain a strong gradient in fertility (CitationObrecht et al. 2005).
Hydrology
We obtained discharge data during 2002–2003 from the U.S. Geological Survey (http://waterdata.usgs.gov) for the James River at Galena (20 km upstream from site 15). Total discharge into the James River arm (Q) was estimated by multiplying these data by 1.25 to account for other inflows (CitationKnowlton and Jones 1989b). Total lake volume on each date was obtained from the U.S. Army Corps of Engineers, Little Rock District (http://www.swl-wc.usace.army.mil/). Volume of the James River arm (V) was estimated by multiplying the total lake volume by 15.4% (CitationKnowlton and Jones 1989b). Hydraulic residence time for the James River arm (T w ) was then estimated by: Tw = V ÷ D.
Although an earlier study of water residence time made the assumption that the James River water mixes with the epilimnion during stratified periods (CitationKnowlton and Jones 1989b), more-recent work suggests that, during low discharge periods in summer, an interflow occurs along the metalimnion, with little down-lake movement of the epilimnion (M. Knowlton, University of Missouri, pers. comm., 22 January 2008). Thus, our calculations of water residence time for the epilimnion during stratified periods are probably underestimates.
Sampling
During January 2002 to December 2003, we visited seven sites along the James River arm (: sites 9–15), sampling every other week during the eight warm months (Mar–Oct) and monthly during the four cold months (Nov–Feb). We also collected additional field measures during January–May 2004. On each sampling date, we monitored Secchi transparency at each of the seven sites and depth profiles of temperature and dissolved oxygen with a YSI model 57 oxygen meter at the two main sites (sites 15 and 9). These two sites are 42 river km apart and represent up-lake (riverine/transition) and down-lake (lacustrine) conditions on the James River arm. At conservation pool, sites 15 and 9 have maximum depths of 10 and 37 m, respectively. These sites differ considerably in fertility (see Study site above).
Plankton collections and analysis
On each sample date during the main study period, phytoplankton samples were collected from sites 9 and 15. A tube sampler, which integrated water from the surface to 5-m depth, was twice extended into the water, withdrawn, and emptied into a 2-L bottle. After mixing, a single 100-mL sample from each site was preserved in Lugol's iodine. Later, a single 5–10-mL subsample was settled in an Utermöhl chamber and analyzed under an Olympus inverted microscope at 400×. Algae were identified to genus by the keys in CitationPrescott (1978). The total number of cells in 15–30 randomly-chosen squares of a Whipple disk were enumerated and converted to density (CitationVollenweider 1969). From the algae counts, we assembled taxonomic lists and also determined density of major groups over time at the two main sites. For display purposes, we lump three algal groups (chlorophytes, cryptomonads, and euglenoids) into a single group called “greens.”
On most dates, only single subsamples were counted from a single sample site. We therefore assessed sampling variation at two levels. Results from a previous study using the same method for counting algae indicated that variation of total algae counts among triplicate subsamples to be small (coefficient of variation, CV = 15%; Pattinson 2001). To assess sampling variation in the field, we collected triplicate samples on six dates from random pelagic locations at each of the two study sites. Counts of total algal abundance indicated a CV of ∼ 20% (range 9–113%). Thus, smaller differences in density over time or between sites could be attributed to sampling variation. Nevertheless, larger differences between sites and among sampling dates were apparent (see Results below).
In addition to density, we also estimated biovolume of the algae from each site and date. Converting to biovolume accounts for 104-fold differences in the size of individual cells and filaments and gives a truer picture of the dominant groups of phytoplankton. This estimation involved taking measurements (in μ m) of 10–30 representative algae from each genus on a single sample date. Because cells from other samples were not measured, we must assume that these dimensions are similar among dates. We then calculated the volume of individual cells or filaments (in μ m3) using formulas for standard geometric shapes. Biovolume for each sample was then calculated by multiplying cell density for each genus by its individual volume and then summing across all genera, either within a particular group (e.g., cyanobacteria) or for the total algae.
On the same dates as other collections, we sampled zooplankton from each of the two main sites, using an 80-μ m mesh Wisconsin net (25-cm diameter mouth), which integrated water from the sample depth to the surface. This mesh size undoubtedly missed many of the smaller rotifers (CitationLevchuk 2007), so the diversity and abundance of the rotifers is likely larger than that reported below. This mesh size could not be reduced because clogging would reduce sampling efficiency. At the deeper site 9, a single tow was taken from 10 m depth; at site 15, two tows from 1 m above the bottom to the surface were pooled into one sample. The samples were preserved in sugar-buffered formalin (CitationPrepas 1978), and then later analyzed under a Wild-Leitz dissecting microscope (at 25×). Zooplankton were identified to genus (CitationThorp and Covich 2001), and subsamples enumerated by standard techniques (CitationDowning and Rigler 1984) until at least 200 individuals were counted. From these data, we tracked density of major groups over time. To assess sampling variation, we also collected triplicate samples from each site on six dates. Total zooplankton counts showed a variation among replicates (as CV) of ∼ 35% (range 22–47%).
Results
Limnological trends
Hydrology
Discharge in the James River (at Galena) during the study period ranged from 2.8 to 835.9 m3/s, with maximum discharge occurring during May 2002. This month was unusually wet, with all of the last three weeks having higher flows than long-term daily means over this period (50.6 m3/s; waterdata.usgs.gov). The last 6 months of 2002 had low-moderate flows (all < 20 m3/s), typical of this region during summer and fall. Discharges were much lower in 2003 (maximum 78 m3/s) than in 2002 and showed much less variability over the year than did flows in 2002 (F = 22.18, p < 0.001). Hydraulic residence time (Tw) for the James River arm during 2002 varied from 6 days during the May high-water period to 1,684 days in October, with a median of 465 days. In 2003, T w ranged from 57–1,221 days. During the 2-year study period, water level of Table Rock Lake varied over a range of 4.5 m. Highest levels occurred during 2002, remaining above power pool from April until August.
Stratification and mixing
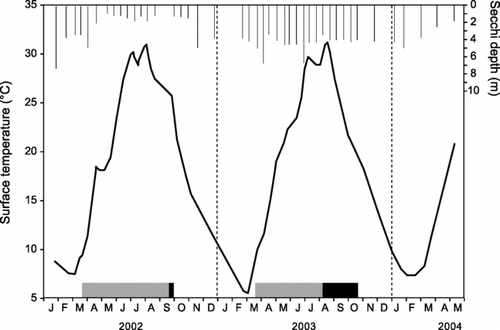
Depth profiles of temperature and oxygen revealed the annual cycles of circulation and mixing in the two main sample sites. The deep down-lake site 9 had a warm monomictic pattern, being stratified from late March until early October and circulating through the winter in both years. Maximum summertime temperatures in the epilimnion commonly reached 30°C, with winter minima of 6–7°C (). During the study period, the hypolimnion at site 9 became hypoxic (dissolved oxygen < 1 ppm) for a brief period in September 2002 and a longer period (Aug–Oct) in 2003, being restored with lake turnover in October. In contrast, the shallow up-lake site 15 had a polymictic circulation pattern, with short periods of stratification punctuated by frequent mixing events throughout the summer. This site warmed quickly in spring and the hypolimnion became hypoxic each year during intermittent stratified periods during June–August. Stratification at site 15 was interrupted during the May 2002 rainy period, during which time site 9 remained stratified.
Figure 2 Limnology of Table Rock Lake, James River arm, site 9, January 2002–May 2004. Temperature is shown as the smooth line and Secchi depths as the lines extending from the top. Stratified periods are shown as boxes at the bottom, with low oxygen (< 1 ppm) hypolimnion indicated by darker shading.
Spatial and seasonal variation in water clarity
Water clarity showed a strong gradient from up-lake to down-lake sites. The average Secchi transparency (median among dates) increased from 1.0 m at site 15 to 4.0 m at site 9, with intermediate values at intervening sites (). Down-lake sites also showed the greatest seasonal variation in water clarity. For instance, during the 2-year study period, Secchi transparency at site 9 ranged from 1.1 to 7.5 m, with minimum values during summer and early fall of 2002. In contrast, transparency at up-lake site 15 ranged from 0.5 to 4.1 m, with most dates between 0.8 and 1.2 m transparency (). On every date, Secchi transparency at site 9 was greater than that at site 15.
Figure 3 Transparency at 7 sites, arranged from down-lake to up-lake, on Table Rock Lake, James River arm. Each site was visited on 41 dates, January 2002–May 2004. Variation at each site (among dates) is indicated with Box-Whisker plots: box shows median ± first and third quartiles, whiskers show 1.5 times the inter-quartile range, and dots are outliers.
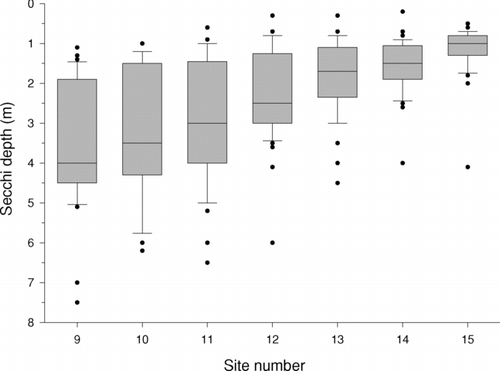
Although minimum transparencies occurred during and following the high flows period in spring 2002, over all dates, water clarity showed no significant association with discharge (correlation between Secchi depth and log discharge, r = 0.022, p = 0.84). Thus, turbidity was generally not dominated by suspended material carried by flows, but was instead generated by phytoplankton growing in the lake.
Phytoplankton composition and dynamics
Algal composition
The algae community in Table Rock Lake is quite diverse. Taxonomic diversity was explored most thoroughly with the 2003 samples, and during this period we identified a total of 69 genera from the two main study sites (). Of these, 94% were identified at the up-lake site 15 and 80% at down-lake site 9 (). With the exception of two benthic diatoms (Cocconeis and Rhoicosphenia), the algae were dominated by genera typically found in the phytoplankton of lakes ().
Table 1 Algal genera and frequency of occurrence in the James River Arm of Table Rock Lake, January-December 2003. Total sample dates = 20, total genera = 69. Those genera labeled with an asterisk are known to cause nuisance blooms or taste and odor problems (CitationCarmichael 1981, CitationChorus and Bartram 1999, current study).
The algae included 10 genera with species known to cause nuisance blooms or taste and odor problems (). We found nine genera of cyanobacteria. Of these, we frequently detected three genera with widespread reputations for toxin-forming strains (CitationChorus and Bartram 1999): Anabaena, Aphanizomenon, and Oscillatoria. Site 15, in particular, had frequent occurrences, with Anabaena and Oscillatoria appearing on 58% and 71% of sample dates, respectively. Oscillatoria was abundant (> 104 cells/mL) on numerous dates during the summer and fall at each site.
Algal dynamics
Over all 41 sample dates (2002–2003), the algae showed large seasonal fluctuations. At down-lake site 9, total densities ranged from ∼ 103–105 cells per mL, with density lowest during winter and early spring (, top). Algae at up-lake site 15 showed similar variation, although the seasonal pattern was not as strong (, bottom). Secchi transparency had a significant negative correlation with both algae density and biovolume (Spearman rank correlations, r = −0.359 and r = −0.372, respectively, both p < 0.001). At densities above 30,000 cells per mL, transparency was always low (< 2 m). Thus, suspended algae contributed a significant amount of turbidity to Table Rock Lake. However, no visible masses (as floating colonies) were ever observed at either site during the course of this study.
Figure 4 Algae population dynamics (as density), by major group, at Table Rock Lake, James River arm. Note different scales for the two graphs. To simplify display, chlorophytes, cryptomonads, and euglenoids are pooled into a single group (“Greens”).
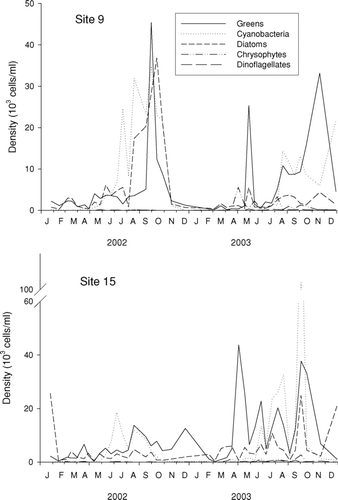
Algal communities at each site were dominated by greens, diatoms, and cyanobacteria (). Periods of peak abundance for these groups showed considerable overlap. At site 9, diatoms were most abundant during August–November in 2002 and had only minor peaks in 2003. Cyanobacteria reached high abundances in June–November in 2002 and August–December in 2003. Greens were highest during September–October in 2002 and May and August–December 2003 (, top). On most dates, the greens were dominated by chlorophyte cells or colonies, although single-celled cryptophytes were occasionally abundant (a peak of Cryptomonas in May 2003). Euglenoids and dinoflagellates were never abundant. Chrysophytes showed short periods of abundance (e.g., May 2003), but they were never dominant.
Biovolume takes into account the differences in the size of individual cells and colonies, and, because of changes in composition over time, the seasonal patterns for major taxa showed some differences from the patterns seen for density (, compare ). For instance, at site 9 in late April 2002, even though densities of diatoms were only moderate (, top), they showed a large peak in biovolume (, top) because of the large individual cell sizes of the dominant genera (Synedra and Fragillaria). Similarly, the dynamics of greens showed a different pattern in 2003 for biovolume than for density. The brief May 2003 peak in cell density disappeared when expressed as biovolume ( and ), because of the small size of the dominant Cryptomonas. In contrast, the peak biovolume in early October was more pronounced than density because the assemblage was dominated by the large colonial chlorophyte Dictyosphaerium ( and ).
Figure 5 Algae population dynamics (as biovolume), by major group, at Table Rock Lake, James River arm.
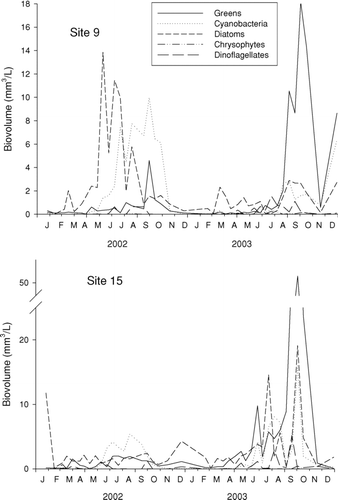
At the shallower up-lake site 15, the same three groups dominated the algal community, but seasonal patterns were weak for most groups (, bottom). Greens were frequently common and diatoms persisted year round. Cyanobacteria were abundant only during the warm months (Jun–Oct 2002 and Jun–Nov 2003). A possible “bloom” occurred in early October 2003, when cyanobacteria dominated numerically (, bottom). However, closer inspection revealed nine different cyanobacteria genera. Furthermore, because of the large differences in sizes of individual cells and filaments, expressing algae as biovolume indicates that, on this date, site 15 was dominated by greens (, bottom).
Peak densities of algae at the two sites were generally not synchronous and also did not show consistent time lags. For instance, the fall 2002 peaks of both diatoms and greens observed at site 9 were missing at site 15. Multiple peaks of greens appeared during 2003 at site 15, whereas only two peaks appeared at site 9 (). Another difference between sites was the timing of high densities (> 104 filaments/mL) of Oscillatoria. At site 9, these episodes were limited to six dates in 2002; at site 15, episodes occurred only in 2003 (four dates).
Despite the large difference between sites in algal dynamics, algal abundance was generally similar. Across all dates, total algae showed no significant difference between sites for either density (Wilcoxon matched pairs test, n = 41, p = 0.11) or biovolume (p = 0.26). Although we predicted that diatom densities would be higher at site 15 because of river input and resuspension, total diatom densities were not significantly different between sites (Wilcoxon matched pairs, p = 0.141). Peak densities of cyanobacteria were high in 2003 at site 15; however, over all dates there was no significant difference between sites in either cyanobacteria densities (p = 0.69) or biovolume (p = 0.09). The only group clearly different between sites was the greens (chlorophytes, cryptomonads, and euglenoids), for which both total density (p = 0.002) and biovolume (p < 0.001) were about double at site 15 than site 9. In sum, although the algae data reveal large differences between sites in the timing of algae peaks, there was generally little consistent difference in their overall abundances.
Zooplankton composition, dynamics, and association with algae density
We identified 30 zooplankton genera, distributed equally among the copepods, cladocerans, and rotifers. Most genera that are benthic in habit were restricted to the shallower site 15 and were generally not common. Pelagic forms dominated the zooplankton at both sites. Although the rotifers were likely under-represented in our samples by the mesh size of collecting nets, this group was very common in samples from both sites.
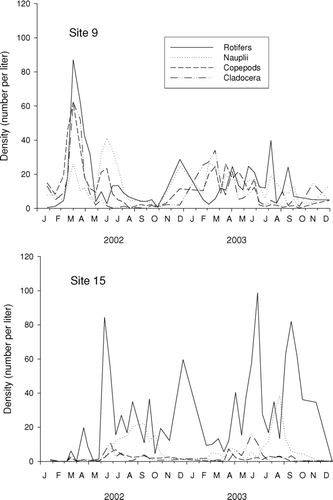
Like the algae, zooplankton showed large fluctuations in abundance at both sites (). At site 9, a peak occurred in April 2002 and consisted of high densities of all major groups (cladocerans, copepods, and rotifers; , top). Nauplii (larval copepods) were common all year. Total cladocerans were in low abundance during July–September 2002. In contrast, cladocerans remained high throughout 2003 (, top), the same period when water clarity was high at site 9 (). The cladoceran Daphnia was generally abundant during spring, but rare in the water column both years from July through November.
Site 15 showed different dynamics (, bottom). For instance, during 2002, the spring peak observed in site 9 never occurred in site 15, and, during the late summer depression at site 9, zooplankton (primarily nauplii and rotifers) persisted in site 15. Cladocera and adult copepods were usually in low abundance at site 15. Across all dates, these groups had 4–8 times lower densities (respectively) at this site than site 9 (), differences that were highly significant (Wilcoxon matched-pairs test, p = 0.001 for cladocerans and p < 0.001 for copepods).
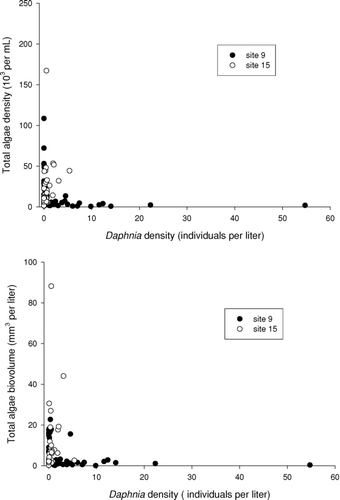
We were interested in determining if some of the seasonal variation in algal abundance in Table Rock Lake could be attributed to variation in the abundance of zooplankton, particularly large grazing Daphnia. If algae are controlled by zooplankton, then we would expect a negative association between their densities. Plotting both total algal density and biovolume against Daphnia density indicates that algal abundance was highly variable when Daphnia were in low densities (< 6/L), but, when Daphnia were common, algae were always in low abundance (). This negative association is statistically discernable (Spearman rank correlation, r = −0.316, p = 0.004).
Discussion
Steep longitudinal gradients and rapid changes in hydrology make large reservoirs interesting systems to explore seasonal dynamics and control of phytoplankton. The current study of the productive James River arm of Table Rock Lake revealed strong longitudinal structure. Over the 42 km between site 15 (up-lake) and site 9 (down-lake), the James River arm changes from a shallow, polymictic, and turbid water body to one that is deep, monomictic, and generally clear. These two sites are examples of lacustrine and transition regions of reservoirs (Thornton et al. 1990). Based on prior measures of nutrients and chlorophyll (CitationObrecht et al. 2005), site 15 would be classified as eutrophic and site 9 oligo-mesotrophic (CitationDodds 2002). The current study of algal composition and dynamics provides a perspective into the biological mechanisms behind the spatial and temporal variation in algal biomass (as chlorophyll) in reservoirs. In particular, we can explore the differences between seasonal cycles at up-lake and down-lake sites and the potential role of riverine input of phytoplankton. Our data can also be used to test the association between phytoplankton and the abundance of grazing zooplankton.
Seasonal cycles of phytoplankton
Our results reveal substantial seasonal variation in algal density and biovolume in this moderately-productive reservoir. Over the 2-year period of study, total algae densities varied over 100-fold among dates. Generally, densities and biovolume were maximal during summer and fall and minimal in winter, although this seasonal pattern was less pronounced at up-lake site 15 ( and ). Peaks of algae abundance at sites 15 and 9 were generally disconnected in time. We also found no evidence of staggering of algal peaks in time with downstream flow, such as was observed in the main-stem of another reservoir (CitationHavel and Pattinson 2004). The slow flushing rate during most dates in Table Rock Lake allows plankton dynamics to be driven primarily by local events.
Seasonal patterns of dominance by major taxa were also quite different between sites. For instance, the fall 2002 diatom peak at site 9 was missing at site 15 and greens showed many more peaks at site 15 than at site 9. The causes of seasonal succession of algae in natural lakes are well known (CitationPorter 1977). For instance, the diatoms, with their silicious and heavy cell walls, typically decline in summer but are favored during mixing periods when cells are resuspended and silica concentrations high. Shallow regions of reservoirs, such as site 15, have frequent mixing events that should be sufficient to redistribute nutrients and cells. Cyanobacteria (blue-green algae) are typically favored during the warm months, when nitrogen fixers can grow in nitrogen depleted conditions (CitationDodds 2002). In the current study, cyanobacteria followed this trend, being most abundant in summer and fall. Dinoflagellates are typically most common when dissolved organic matter is abundant (CitationDodds 2002), but were never abundant at either of our two study sites.
Lack of riverine control of algal abundance
According to CitationKimmel et al. (1990), if flushing rate exceeds the doubling time of phytoplankton, algal productivity is limited by advective losses. This situation is probably rare in the James River arm of Table Rock Lake. Our estimates indicate hydraulic residence time was usually about 1–3 years, which far exceeds the replacement rate of algae (1–3 days; Fogg 1965 ). The rare exception (May 2002 high flow periods) had a minimal residence time of 6 days. During this period, phytoplankton density was low at both sites.
Riverine influence may also be detected by comparing algal density and diversity at our two sites. Tributary inputs during high flow events may carry benthic diatoms sloughed from the stream bottom (CitationAllan 1995). In some reservoir systems, rapidly-sinking riverine algae are replaced by lacustrine algae (CitationSøballe and Bachman 1984). If such conditions were important in the current study system, then we would expect diatom density to be higher at the up-lake site (15) than at the down-lake site (9). However, over all dates, diatom density and biovolume were not significantly higher at site 15 than at site 9. Furthermore, algal diversity was not higher at site 15 and diatom taxa that are strictly benthic (Cocconeis and Rhoicosphenia) were not restricted to this site (). In general, the algal community at both sites was dominated by planktonic genera. As a final check, we compared cell sizes at the two sites. Because, by Stokes' Law, large cells generally sink faster than small cells (CitationDodds 2002), we would predict that cell sizes would be generally larger at site 15. Size frequency distributions of algae on each of several dates indicated no appreciable difference between sites. In sum, phytoplankton diversity and abundance in the James River arm of Table Rock Lake appears to be controlled primarily by in situ processes, with little influence of immigration from riverine communities.
Concluding remarks
Although total algal abundance and its composition are initially controlled by the supply rate of nutrients (CitationWetzel 2001), zooplankton grazers also affect the algae community and water clarity. In a classic example, following recovery of Lake Washington to reduced phosphorus inputs, water clarity continued to improve when large grazing Daphnia became numerous enough to further depress algal densities (CitationEdmondson 1991). Numerous field surveys and experiments have confirmed the importance of zooplankton grazers for controlling phytoplankton abundance and composition, as well as lake water clarity (CitationVanni and Findlay 1990, CitationLathrop et al. 1996, CitationLampert and Sommer 2007). Grazer control of algae may occasionally be important in Table Rock Lake. Our data indicate a negative association between high Daphnia abundance and both algal abundance and biovolume (), consistent with such control when Daphnia were numerous. A preliminary experiment using the algae community from Table Rock Lake indicated that Daphnia grazers at high densities (50/L) did indeed significantly reduce chlorophyll levels, as well as abundance of small chlorophytes and cyanobacteria (Litvan and Havel, unpublished data). Such an effect was not present at lower grazer densities (5/L). The importance of such grazer effects in reservoirs such as Table Rock should be sensitive to the structure of the fish community, as well as nutrient loading rates. Teasing apart the relative importance of these top- and bottom-up effects requires additional experiments (CitationLampert and Sommer 2007).
Although Table Rock Lake is generally very clear, the James River arm has had a reputation for occasional summer algae blooms, driven by phosphorus loading from wastewater treatment plants. Algal blooms in 1999 (seen as a sudden increase in turbidity, primarily from Cryptomonas) brought much attention to deteriorating water clarity in the lake (DNR 1999; CitationDeslatte 2002). CitationObrecht et al. (2005) reported a sizable reduction of phosphorus levels in the James River and in this arm of the lake following treatment plant upgrades in 2001. However, reduction in chlorophyll levels has been more modest, suggesting that algal growth is sustained by internal loading. Are intermittent algae blooms still a problem? During the current study, we observed no floating masses of algae and no complaints were reported by the general public (D. Casaletto, Table Rock Water Quality, Inc., Kimberling City, MO, pers. comm., June 26, 2007). Furthermore, in our algae counts, we never observed excessive numbers (> 105 cells/mL) dominated by a single taxonomic group. However, we did detect a number of potential nuisance genera () and the cyanobacteria Oscillatoria was sometimes abundant. Thus, under the right conditions, noxious blooms and odors could occur. Because Table Rock Lake continues to be threatened by rapid increases in lakeside developments and confined animal operations in its watershed, future monitoring of this lake is warranted.
Acknowledgments
We thank K. Pattinson and K. Medley for technical assistance. Additional help in the field was provided by L. Kelsay, J. Kimmons, and M. Litvan. We appreciate the helpful comments by J. Graham, E. Davis-Bowles, J. Jones, and three anonymous reviewers on earlier drafts of the manuscript. Funding was provided by the United States Environmental Protection Agency, through the Missouri Department of Natural Resources, under section 319 of the Clean Water Act (grant number G02-NPS-08).
References
- Allan , J. D. 1995 . Stream ecology: structure and function of running waters , London , UK : Chapman and Hall .
- Carmichael , W. , ed. 1981 . The water environment: algal toxins and health , 491 New York : Plenum Press .
- Chorus , I. and Bartram , J. , eds. 1999 . Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management , 416 London : E & FN Spon .
- DNR . 1999 . Poster display: Aerial photographs of algae bloom in Table Rock Lake , Branson , MO : Missouri Department of Natural Resources. White River Basin Water Quality Forum .
- Deslatte , A. 2002 . “ Tourism vs. agriculture: states spar over water ” . In Springfield News Leader Springfield , MO
- Dodds , W. K. 2002 . Freshwater ecology: concepts and environmental applications , San Diego , CA : Academic Press .
- Downing , J. A. and Rigler , F. H. 1984 . A manual on methods for the assessment of secondary productivity in fresh waters , Oxford , UK : Blackwell Scientific Publications . IBP handbook 17
- Edmondson , W. T. 1991 . The uses of ecology: Lake Washington and beyond , Seattle , WA : University of Washington Press .
- Fogg , G. E. 1965 . Algal cultures and phytoplankton ecology , Madison : University of Wisconsin Press .
- Havel , J. E. and Pattinson , K. R. 2004 . Spatial distribution and seasonal dynamics of plankton in a terminal multiple-series reservoir . Lake Reserv. Manage. , 20 : 14 – 26 .
- Hynes , H. B. N. 1970 . Ecology of running waters , Toronto : University of Toronto Press .
- Jones , J. R. and Knowlton , M. F. 1993 . Limnology of Missouri Reservoirs: An analysis of regional patterns . Lake Reserv. Manage. , 8 : 17 – 30 .
- Kimmel , B. L. , Lind , O. T. and Paulson , L. J. 1990 . “ Reservoir primary production ” . In Reservoir limnology: ecological perspectives , Edited by: Thornton , K. W. , Kimmel , B. L. and Payne , F. E. 133 – 193 . New York : John Wiley & Sons .
- Knowlton , M. F. and Jones , J. R. 1989a . Comparison of surface and depth-integrated composite samples for estimating algal biomass and phosphorus values and notes on the vertical distribution of algae and photosynthetic bacteria in Midwestern lakes . Arch. Hydrobiol. Suppl. , 83 : 175 – 196 .
- Knowlton , M. F. and Jones , J. R. 1989b . Summer distribution of nutrients, phytoplankton, and dissolved oxygen in relation to hydrology in Table Rock Lake, a large Midwestern reservoir . Arch. Hydrobiol. Suppl. , 83 : 197 – 225 .
- Lampert , W. and Sommer , U. 2007 . Limnoecology: the ecology of lakes and streams, , 2nd ed , Oxford , UK : Oxford University Press .
- Lathrop , R. C. , Carpenter , S. R. and Rudstam , L. G. 1996 . Water clarity in Lake Mendota since 1900: responses to differing levels of nutrients and herbivory . Can. J. Fish. Aquat. Sci. , 53 : 2250 – 2261 .
- Levchuk , A. P. 2007 . Zooplankton of the upper Mississippi River: patterns of community structure and sampling methodology , Master's thesis Urbana-Champaign : University of Illinois .
- Obrecht , D. , Thorpe , A. P. and Jones , J. R. 2005 . Responses in the James River arm of Table Rock Lake, Missouri (USA) to point-source phosphorus reduction . Verh. Internat. Verein. Limnol. , 29 : 1043 – 1048 .
- Pattinson , K. R. 2001 . Blue-green algae and the seasonal succession of Daphnia , Master's thesis Springfield, Missouri : Missouri State University .
- Porter , K. G. 1977 . The plant-animal interface in freshwater ecosystems . Am. Sci. , 65 : 159 – 170 .
- Porter , K. G. and Orcutt , J. D. Jr. 1980 . “ Nutritional adequacy, manageability, and toxicity as factors that determine the food quality of green and blue-green algae for Daphnia ” . In Evolution and ecology of zooplankton communities , Edited by: Kerfoot , W. C. 268 – 281 . Hanover , NH : University Press of New England .
- Prepas , E. 1978 . Sugar-frosted Daphnia: an improved fixation technique for Cladocera . Limnol. Oceanogr. , 23 : 557 – 559 .
- Prescott , G. W. 1978 . How to know the freshwater algae , Boston : WCB/McGraw-Hill .
- Schindler , D. W. 1974 . Eutrophication and recovery in experimental lakes: implications for lake management . Science , 184 : 897 – 899 .
- Smith , V. H. 1982 . The nitrogen and phosphorus dependence of algal biomass in lakes: An empirical and theoretical analysis . Limnol. Oceanogr. , 27 : 1101 – 1112 .
- Søballe , D. M. and Bachmann , R. W. 1984 . Influence of reservoir transit on riverine algal transport and abundance . Can. J. Fish. Aquat. Sci. , 41 : 1803 – 1813 .
- Thornton , K. W. , Kimmel , B. L. and Payne , F. E. , eds. 1990 . Reservoir limnology: ecological perspectives , New York , NY : John Wiley & Sons, Inc. .
- Thorp , J. H. and Covich , A. P. , eds. 2001 . Ecology and classification of North American freshwater invertebrates, , 2nd ed , San Diego , CA : Academic Press .
- USACE . 2008 . < http://www.swl.usace.army.mil/parks/tablerock/index.htm>Table Rock Lake. U. S. Army Corps of Engineers, Little Rock District (accessed October 8, 2008)
- Van den Hoek , C. , Mann , D. G. and Jahns , H. M. 1995 . Algae: an introduction to Phycology , Cambridge , UK : Cambridge University Press .
- Vanni , M. J. and Findlay , D. L. 1990 . Trophic cascades and phytoplankton community structure . Ecology , 71 : 921 – 937 .
- Vitousek , P. M. , Aber , J. , Howarth , R. W. , Likens , G. E. , Matson , P. A. , Schindler , D. W. , Schlesinger , W. H. and Tilman , G. D. 1997 . Human alteration of the global nitrogen cycle: causes and consequences . Issues Ecol. , 1 : 1 – 15 .
- Vollenweider , R. A. 1969 . A manual on methods for measuring primary production in aquatic environments , Oxford , UK : Blackwell Scientific Publications . IBP Handbook No. 12
- Wetzel , R. G. 1990 . “ Reservoir ecosystems: conclusions and speculations ” . In Reservoir limnology: ecological perspectives , Edited by: Thornton , K. W. , Kimmel , B. L. and Payne , F. E. 227 – 238 . New York : John Wiley & Sons .
- Wetzel , R. G. 2001 . Limnology: lake and river ecosystems, , 3rd edition , San Diego , CA : Academic Press .