Abstract
Fisher, M.M., S.J. Miller, A.D. Chapman and L.W. Keenan. 2009. Phytoplankton dynamics in a chain of subtropical blackwater lakes: the Upper St. Johns River, Florida, USA. Lake Reserv. Manage. 25:73–86.
Spatial and seasonal patterns in phytoplankton biovolume and community composition were examined for a chain of lakes in the Upper St. Johns River (USJR), Florida, USA. There was a general downstream trend in both increasing phytoplankton biovolume, and dominance of the algal community by cyanobacteria. Total algal biovolume increased from 0.7 × 106μ m3/ml in the headwaters lake to 5.6 × 106μ m3/ml downstream in Lake Winder. Cyanobacteria dominated the downstream lakes, accounting for approximately 50% of total algal biovolume, yet constituted only 2% of total biovolume in the headwaters lake. The diatom assemblage, as well as water quality data, suggests that these blackwater lakes are mesotrophic to eutrophic and neither nitrogen nor phosphorus limited growth. Fifteen months of cyanobacterial biovolume data were compared to water quality data to determine principal regulating factors. A regression model indicates that the major factors correlated with cyanobacteria in these lakes are temperature, total Kjeldahl nitrogen, water level, and color, with temperature alone accounting for 54% of the variability in cyanobacterial biovolume. This analysis demonstrates that multiple interacting factors need to be considered when attempting to explain spatiotemporal patterns in algal dynamics.
Phytoplankton community composition is an important aspect of surface water quality for aquatic ecosystem mangers due to the influence it has on higher trophic levels, as well as its potential impact on human uses. Community composition, with respect to the relative abundance of major algal divisions, often reflects the extent to which surface waters have been degraded by nutrient enrichment (CitationPearsall 1932, CitationReynolds 1998, CitationHavens et al. 2003). Of particular interest to resource managers is the abundance of blue green algae, or cyanobacteria. Blooms of certain genera of this division have been linked to fish kills, low dissolved oxygen, and odor and taste problems in drinking water. Cyanobacteria are under-utilized by zooplankton grazers, compared to other algal divisions (CitationHavens and East 1997, CitationPaerl et al. 2001). When predominant, they have the potential to reduce transfer of energy and carbon to higher trophic levels in the aquatic food chain (CitationLeonard and Paerl 2005). They may also constitute a public health risk when certain toxin-producing algae are the main genera associated with bloom formation (CitationOliver and Ganf 2000, CitationCarmichael 2001).
Historical information on overall species composition and abundance of phytoplankton in the St. Johns River, including blue-greens, is limited. The most rigorous phytoplankton studies have been confined to the lower river reaches (CitationDeMort and Bowman 1985, CitationLeonard and Paerl 2005). In the upper reaches, few surveys have been conducted. Previous studies were either constrained to a single water body, or conducted intermittently (CitationGoolsby and McPherson 1970, Florida Game and Fresh Water Fish Commission 1977, CitationYount and Belanger 1988, CitationPhlips and Cichra 1999). As a result, these studies only provided a limited view of phytoplankton community in this region of the river.
Currently, phytoplankton and water quality data are collected in the USJR to document the occurrence of blooms and to determine the key factors regulating them. Our purpose was to describe the phytoplankton biovolume, community composition, and seasonal dynamics of approximately three years of phytoplankton data. A secondary objective was to investigate the differences in water quality among lakes that might be responsible for any observed differences in phytoplankton communities and biovolume (particularly cyanobacteria).
Materials and methods
Site description
The St. Johns River is the largest river wholly contained within Florida, USA. Its headwaters are located in east-central Florida, west of Vero Beach, and its mouth is 520 km downstream at Jacksonville. It falls approximately 8 m over this distance (1.6 cm/km), discharging an annual average of 168 m3/sec (CitationMcGrail et al. 1998, CitationMossa 1998). The river drains an area of approximately 24,000 km2, or about one-fifth of the entire state (CitationMossa 1998). It is generally divided into three basins, the upper, middle, and lower, based on surface water quality characteristics and tidal influence (CitationBass and Cox 1985). Very low relief is one of the defining characteristics of the river, and this has important consequences for both flood control and water quality. A system of levees, canals, and water control structures in the upper reaches of the river were constructed to provide flood protection for agriculture and urban development. Low relief, coupled with low spring and early summer rainfall, has a tendency to prolong and intensify nuisance algal blooms, such as the extensive blooms that occurred along the lower St. Johns in summer 2005 and 2006. Increasing concerns about nutrient enrichment has led the United States Environmental Protection Agency to establish Total Maximum Daily Loads (TMDLs) for the Upper St. Johns River. One of the goals of TMDL development was to limit the occurrence of algal blooms, principally blooms of cyanobacteria. However, for the Upper St. Johns River, the linkage between stressor (nutrients) and response (bloom) has not been well documented.
The St. Johns River has alternated between coastal lagoon and freshwater river over much of its geologic history (White 1958). It is separated from the Kissimmee River system to its west by the relict sand dunes of the Pamlico scarp, and from the Indian River Lagoon estuary to the east by the Atlantic Coastal Ridge. The Upper St. Johns River (USJR) is a mosaic of floodplain marshes, lakes, and river channel (). Six lakes are connected by the river, spanning from Blue Cypress Lake in the extreme southern (headwater) end of the river, to Lake Poinsett, approximately 80 km downstream. The lakes are shallow (approx. mean depth = 2 m) and well mixed, with nutrient concentrations that are consistent with a classification of the lakes as eutrophic (CitationLowe et al. 1984). The region has a semi-tropical climate and receives approximately 142 cm of rainfall annually. Florida Department of Environmental Protection classifies most of the lakes as Class III water bodies, suitable for fishing and swimming. Lake Washington is a Class I water body, suitable for drinking water, and is used by the City of Melbourne, Florida, as a primary source of potable water. The river is generally high in color and dissolved organic carbon and is thus characterized as a blackwater river. This is significant for the ecology of the river because blackwater aquatic ecosystems often do not show the same response to nutrients as typical clear-water lakes and rivers. The Upper St. Johns River receives drainage from several tributaries on the west side of the basin and from predominately agricultural land on the east. Other than extensive citrus groves on the east side, the upper basin is largely unimproved pasture, silvaculture, or wetland. Principal uses of this portion of the river include irrigation, recreation, and water supply. The Upper St. Johns River is the focus of the Upper St. Johns River Basin Project (USJRBP), a joint flood control project co-sponsored by the United States Army Corp of Engineers and the St. Johns River Water Management District. To date, the USJRBP has restored approximately 140 km2 of farmland back to river floodplain. One component of this project is construction of a series of water management areas that provide water quality benefits by segregating agricultural runoff from the St. Johns River.
Figure 1 Location of monthly phytoplankton and water quality sampling stations. BCL = Blue Cypress Lake; HBO = outlet of Lake Hell ′n Blazes; SGO = outlet of Sawgrass Lake; LWC = center of Lake Washington; LWO = outlet of Lake Winder; LPO = outlet of Lake Poinsett.
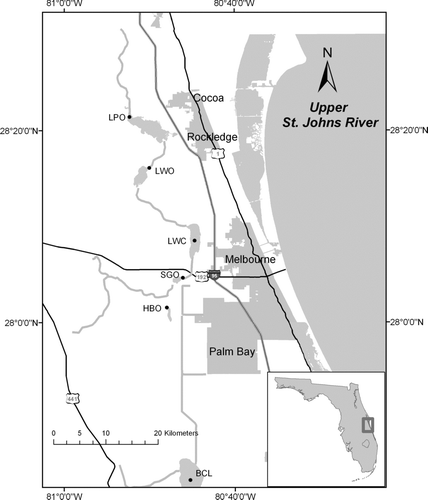
We analyzed 30 months of data from March 2001 through February 2002 and from June 2003 through November 2004. Sampling stations () were in the center of Blue Cypress Lake (BCL), outlet of Lake Hell ′n Blazes (HBO), outlet of Sawgrass Lake (SGO), center of Lake Washington (LWC), outlet of Lake Winder (LWO), and outlet of Lake Poinsett (LPO).
Sample collection and analyses
Surface water grab samples for both phytoplankton and water quality analyses were collected from a depth of 0.5 m. Phytoplankton samples were preserved in the field with Lugols solution at a rate of 12 ml of Lugols solution per liter of sample. After sedimentation in Utermöhl chambers (CitationUtermöhl 1958), phytoplankton counts were conducted on a Nikon Eclipse TE200 inverted microscope following the protocol of CitationLund et al. (1958). A total of 400–600 natural units were enumerated to give a 95% confidence interval of the estimate within ± 10% of the sample mean. A minimum of 15 random fields at 400× and 30 random fields at 200× were counted, as well as a complete scan at 100× to count large or rare taxa. Biovolumes were calculated using the best-fitted geometic shape (CitationHillebrand et al. 1999). Biovolumes were reported for all species making up at least 1% of total biovolume. Average total biovolume of major phytoplankton divisions for each lake is reported as the summation of total biovolume for respective phytoplankton divisions for each lake, divided by the 30-month study period. Biovolume for individual months (for seasonal patterns) was determined by summing biovolume by both division and species, then dividing by the number of collection periods for each reported month. For example, phytoplankton was analyzed for June 2001, June 2003, and June 2004; therefore, data reported for June represent the mean of these three months.
All results presented here are in terms of algal biovolume because individual cell volumes were found to vary over five orders of magnitude. Therefore, reporting data as cell numbers tends to under-represent the importance of large genera, while over-estimating the significance of smaller (though possibly more numerous) genera. Analysis of water quality samples was according to accepted techniques ().
Table 1 Method of analysis of water quality samples. “SM” refers to Standard Methods for Analysis of Water and Wastewater (APHA 1998); “EPA” refers to Methods for the Chemical Analysis of Water and Wastes (USEPA 1983).
Statistical analysis
Differences in selected water quality parameters among lakes were examined with ANOVA, followed by post hoc comparisons using Tukey's method and an experiment-wise error rate of 0.05. Water quality data were log-transformed prior to ANOVA, and normality of transformed data was examined using a Kolmogorov-Smirnov test. The distribution of dissolved oxygen data was not improved through several transformations; therefore, analysis was performed on untransformed, non-normally distributed data. Multiple step-wise (forward and reverse) linear regression was used to determine the most important water quality parameters influencing cyanobacteria biovolume. Parameters were included in the model if p < 0.1, and dropped from the model when p > 0.1. Normality of regression residuals was verified with a Kolmogorov-Smirnov test. Seven of 174 water quality samples were identified as outliers and eliminated prior to analysis.
Potential differences in phytoplankton community among lakes that may have been due to varying downriver water chemistry were further examined by forming composite water quality variables using principal components analysis (PCA). The PCA was performed on the correlation matrix and used to select groupings of least correlated water quality parameters that best described differences among the upper St. Johns River lakes. All statistical analyses were performed with the statistics software Minitab, release 14. (Minitab Inc. 2005).
Results
General spatial patterns
Algal taxa representing seven divisions were found in the USJR. Those divisions were Cyanophyta (blue-green), Chlorophyta (green), Xanthophyta (yellow-green), Chrysophyta (golden-brown), Cryptophyta (cryptophytes), Bacillariophyta (diatoms), Pyrrhophyta (dinoflagellates), and Euglenophyta (euglenoids). Xanthophytes were rarely encountered and will not be considered here. In all, 207 genera were identified.
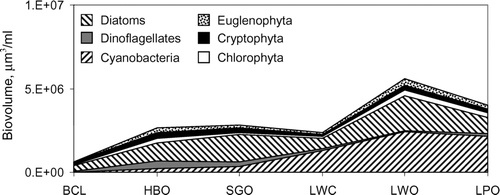
Average monthly total algal biovolume increased dramatically down-river, from 0.7 × 106 μ m3/ml in Blue Cypress Lake to 5.6 × 106 μ m3/ml in Lake Winder (). This corresponds to an increase in average algal cell densities, from 152 cells/ml in Blue Cypress Lake, to 5775 cells/ml in Lake Poinsett. The fact that downstream biovolume increased 8-fold, while cell densities increased 38-fold is due to changes in community composition, from the relatively large cell sizes of green algae and diatoms that constituted the dominant divisions in Blue Cypress Lake, to the much smaller cell sizes of blue-green algae in the downstream lakes. For example, the average diatom cell size was 3200 μ m3, whereas the average blue green cell was 65 μ m3.
Figure 2 Average monthly biovolume in lakes of the Upper St. Johns River. Lakes arranged in order from upstream (BCL = Blue Cypress Lake) to downstream (LPO = Lake Poinsett).
There were major differences in downstream community composition at the division level. For instance, cyanobacteria increased from approximately 2% of the average biovolume in Blue Cypress Lake, to 50% in lakes Washington, Poinsett, and Winder ().
Table 2 Relative phytoplankton community composition in lakes of the Upper St. Johns River. Values represents an average of 30 months of biovolume data.
This increase in cyanobacteria was accompanied by slight declines in other divisions, principally dinoflagellates and cryptophytes.
Average algal biomass in Blue Cypress Lake was approximately one order of magnitude lower than the downstream lakes, with seven genera accounting for 90% of the biomass in the lake (). The downstream lakes were more diverse, with approximately 14 genera comprising 90% of the biomass. For Blue Cypress Lake, a single genus (Aulacoseira) accounted for 65% of the lake's total algal biovolume. Average biovolume in the lakes downstream of Blue Cypress Lake gradually increased, yet the number of genera comprising 90% of each lakes biovolume remained relatively constant. Summed biovolume of diatoms, cyanobacteria, and green algae was greatest in June, mostly due to increases in cyanobacteria, followed by a sharp decline in late summer ().
Figure 3 Relationship between total community average monthly biovolume and community diversity. Left axis refers to the number of genera that comprises 90% of the community biovolume (bars). Line (right axis) depicts total algal biovolume.
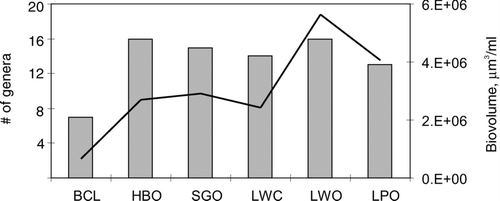
Figure 4 Seasonal variation in biovolume in lakes of the Upper St. Johns River. Data represent average of all six lakes. Discharge data represent mean monthly values from USGS gauging station at US Highway 192, for period of record January 2001 through December 2004.
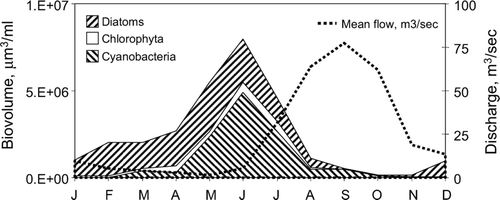
Diatoms, cyanobacteria, and green algae accounted for 58% (Lake Hell ′n Blazes), to 86% (Lake Winder) of total algal biovolume in the USJR lakes. Therefore, description of community composition and seasonal dynamics is restricted to these three divisions.
Diatoms
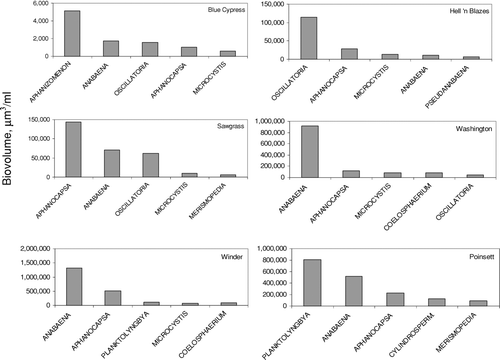
Diatom biovolume generally increased downriver, from an average of 7 × 105 μ m3/ml in Blue Cypress Lake to 5.6 × 106 μ m3/ml in Lake Winder. Diatoms dominated the algal community in Blue Cypress Lake, with cyanobacteria playing a minor role. Aulacoseira spp. comprised 82% of the diatom biomass in Blue Cypress Lake (). Aulacoseira is one of the most common diatom genera of all centric diatoms in freshwater systems (CitationStoermer and Julius 2003). The average biovolume of Aulacoseira spp. ranged from approximately 200,000 μ m3/ml in Lake Hell ′n Blazes, to 800,000 μ m3/ ml in Sawgrass Lake. Other dominant diatom genera for the USJR lakes included Thalassiosira, Skeletonema, Cyclotella, andSurirella ().
Table 3 Top 10 species of diatom, cyanobacteria, and green algae in the lakes of the Upper St. Johns River. Ranking based on total biovolume of all algal divisions.
Figure 5 Top five diatom genera in USJRB lakes. Bars represent the average monthly biovolume of 30 months of data, from March 2001 through November 2004. Note variable y-axis scaling.
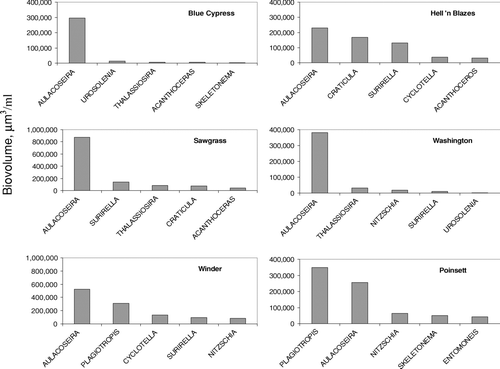
Plagiotropis lepidoptera seemed to dominate in Lake Poinsett and was the second most dominant diatom in Lake Winder; however, this was biased by very high biovolume of this species in spring of 2001. P. lepidoptera is often associated with brackish water (CitationStoermer and Julius 2003). In fact, the bloom of P. lepidoptera coincided with the end of the extreme drought in 2001, when conductivity in Lake Poinsett was approximately 2500 μ S/cm and chloride concentration was 500 mg/L. This species has not been observed since May 2001. Diatom biovolume was lowest in late fall, increased steadily throughout the winter, and was maximum in May and June.
Cyanobacteria
Average monthly biovolume of cyanobacteria increased dramatically with distance downstream, from 0.012 × 106 μ m3/ml in Blue Cypress Lake, to approximately 2 × 106 μ m3/ml in Lake Winder, an almost 200-fold increase. The trend in downstream increase in biovolume was mirrored in increasing cyanobacteria cell counts (data not shown). Principal genera include Anabaena, Oscillatoria, Aphanocapsa, and Microcystis (). All of these have either been shown to produce or are suspected of producing toxins (CitationCarmichael 2001). The cyanobacterial community in Blue Cypress Lake also differed qualitatively from the other USJR lakes. The largest contributor to total cyanobacteria biovolume in Blue Cypress Lake was Aphanizomenon flos-aquae, yet this species was of only minor importance in the other lakes, constituting less than 2% of the cyanobacteria biovolume. Cyanobacteria biovolume showed a pronounced seasonal pattern, peaking in June at approximately 5 × 106 μ m3/ml (average of all lakes). The months of October through February typically had very low cyanobacteria algal biovolume, in contrast to the more even seasonal distribution seen in diatom biovolume.
Green algae
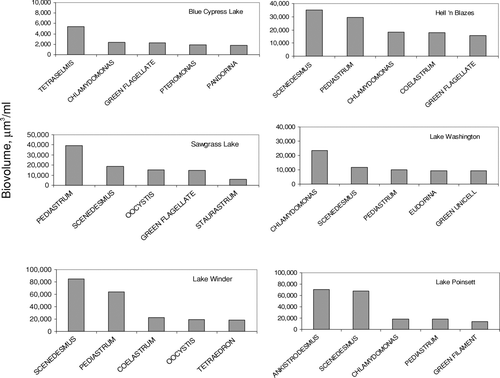
Green algae represented from 3% (Blue Cypress Lake) to 11% (Lake Hell ′n Blazes) of the total community biovolume. Unlike cyanobacteria, there was no clear trend in downriver green algal biovolume. Biovolume was greatest in Lake Winder, with an average monthly biovolume of 0.35 × 106 μ m3/ml, and lowest in Blue Cypress Lake, with an average monthly biovolume of 0.021 × 106 μ m3/ml. Dominant green genera for the Upper St. Johns River lakes included Scenedesmus, Pediastrum, and Chlamydomonas (). As with diatom and cyanobacteria algal data, Blue Cypress Lake differed both quantitatively and qualitatively from the other Upper Basin lakes, with lower total green algal biovolume and a different community composition. The motile green alga, Tetraselmis cordiformis, was the dominant green algae in Blue Cypress Lake and constituted a minor fraction of the green algal community in Lakes Washington, Winder, and Poinsett. Tetraselmis cordiformis is the only freshwater species of this genus (CitationBecker and Hickisch 2005). It has a large cell size and high lipid content. Marine species are commercially produced as a food source for shellfish and shrimp hatcheries; thus, this species may constitute an important food source to higher trophic levels in USJRB lakes. Tetraselmis reached maximum biovolume in Lake Hell ′n Blazes (0.014 × 106 μ m3/ml) and minimum biovolume in Lake Washington (0.0005 × 106 μ m3/ml). Green algae tended to be the most diverse division, even though they did not predominate in most of the lakes. Green algae showed a steady increase in biovolume from December through July, peaking at an average of 0.6 × 106 μ m3/ml and then declined abruptly in August and September.
Relationships between water quality and cyanobacteria biovolume
The lakes and river channel of the Upper St. Johns are best characterized as blackwater, with high color and high dissolved organic carbon, averaging 242 (± 121 SD) PCU and 27 (± 3 SD) mg/L, respectively ( and ).
Table 4 Average value of selected physical and chemical characteristics of Upper St. Johns River lakes. Summary statistics cover period of record from March 2001 through November 2005. Values in parentheses represent one standard deviation. Values with same superscript letter are not significantly different at P = 0.05.
Table 5 Average value of selected water quality constituents for Upper St. Johns River lakes. Summary statistics cover period of record from March 2001 through November 2005. Values in parentheses represent one standard deviation. Values with same superscript letter are not significantly different at P = 0.05.
The lakes are relatively high in nutrients. Total phosphorus (P) concentration was not significantly different among lakes, averaging approximately 0.1 mg/L. Nitrogen (N) species varied significantly among lakes, with total Kjeldahl nitrogen TKN and NH4-N lower and NOx -N higher in Blue Cypress Lake than most of the other lakes. Both chloride and conductivity increased significantly downriver, probably as a result of inputs of saline groundwater and well-derived irrigation water.
Blue Cypress Lake is dramatically different than the other lakes, with lower total phytoplankton biomass and a different community composition. This is expected, given the differences in water quality between Blue Cypress Lake and the other lakes. Principal component analyses revealed some of the underlying differences among lakes (). The highest weightings on the first principal component (x-axis) were on color, calcium, conductivity, and chloride ().
Table 6 Results of principal components analysis showing coefficients on each standardized water quality variable, as well as the percent of cumulative variance explained by the first three components.
Figure 8 Principal component analysis of Upper St. Johns River water quality data. Each point represents a single water quality sample. The ellipse in the lower left of the graph encircles samples collected primarily from Blue Cypress Lake.
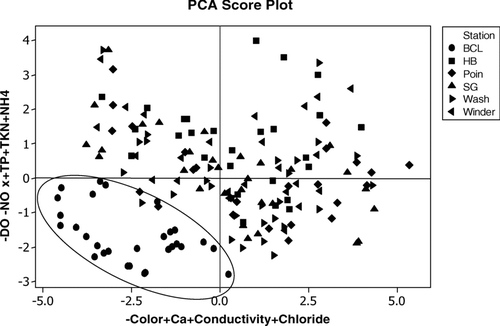
This axis can be thought of as color and ionic strength axis, with Blue Cypress Lake having lower ionic composition and high color. The second component axis is a dissolved oxygen and nutrient axis, with high dissolved oxygen, high nitrate, and low total P, TKN, and ammonium in Blue Cypress Lake, relative to the downstream lakes. Together, these first two components explained 56% of the underlying variation among the lakes.
As stated previously, cyanobacteria blooms threaten the ecology of aquatic ecosystems, diminish their recreational value, and pose a threat to potable water supply. The remainder of this discussion will therefore focus on relationships between cyanobacteria and water quality in the USJR. The best correlates to cyanobacteria biovolume were factors that are related to river flow and stage. For example, calcium, chloride, and conductivity were all significantly related to biovolume () and were also correlated with river stage (surface water elevation, relative to mean sea level) (p < 0.001; data not shown). Increases in calcium, chloride, and conductivity occur due to concentration of solutes in the river during periods of low flow, as well as increased inputs of groundwater. For the upper St. Johns River, higher stage results in greater color, and lower chloride, conductivity, and biovolume. It is possible that there is no causal relationship with these analytes and cyanobacteria biovolume, they are simply related to flow in the river. Total kjeldahl nitrogen was the nutrient most significantly correlated with blue-green biovolume (r2 = 0.554; p < 0.001)
Table 7 Pearson correlation coefficients (r) and significance level (p) for the relationship between cyanobacteria algal biovolume and selected water quality parameters for lakes in the Upper St. Johns River.
Many interacting factors exert control over blooms of cyanobacteria. Multiple step-wise regression revealed four primary physico-chemical factors that were related to cyanobacteria biovolume ().
Table 8 Results of step-wise linear regression of fourteen water quality parameters on log-transformed cyanobacterial biovolume. All model terms significant at p < 0.01.
Temperature alone explained 54% of the variability in biovolume, with TKN, stage, and color explaining an additional 9% of the variability. The model coefficient on TKN was positive, indicating that increases in TKN seem to have favored blue-greens. Inorganic N was not a significant regressor in the model, although correlation analysis indicated that declining inorganic N was associated with increased cyanobacteria. We expected total blue-green biovolume was related to stage given the earlier finding that total algal biovolume declined sharply during periods of increased river flow.
From a management perspective, little can be done concerning altering hydrology or temperature to control blue green blooms. If the regression analysis is repeated using only water quality data (except for chloride), NO3 was the most important parameter in the model, explaining 28% of the variability in blue-green biovolume. Its coefficient was negative, which is expected because low inorganic N tends to confer a competitive advantage to blue-green algae. Other significant regressors were dissolved O2, conductivity, alkalinity, with TKN, pH and Secchi transparency explaining a minor proportion of the total variability. Because stage and temperature were eliminated from the second regression analysis, parameters highly correlated to stage and river flow were selected by the step-wise regression for inclusion in the model, even though they probably are not causative factors for bloom formation. For example, dissolved O2 is highly related to temperature, due to the effect of temperature on both solubility and respiration. Conductivity is related to hydrology because low flow tends to concentrate solutes in the river. Therefore, midsummer conditions such as low flows, low dissolved O2, and concentration of solutes occur contemporaneously with increased blue-green biovolume, yet they probably are not causative.
Discussion
Phytoplankton biovolumes for the USJR lakes are comparable to Lake Okeechobee, another subtropical lake in south Florida. CitationCichra et al. (1995) reported biovolumes of approximately 4 × 106 μ m3/ml for center lake regions and 8 × 106 μ m3/ml for the western littoral area of the lake. Biovolumes of the center of that lake resemble USJR lakes Sawgrass and Hell ′n Blazes, whereas biomass in the western littoral area of Lake Okeechobee, an area known for frequent algal blooms, is quite similar to levels observed in Lakes Poinsett and Winder. CitationCichra et al. (1995) also found some zonation in Lake Okeechobee with respect to relative divisional abundance. The center of the lake tended toward dominance by diatoms, whereas the western side of the lake was dominated by cyanobacteria. This is analogous to the longitudinal zonation in the Upper St. Johns, with diatoms dominating upstream and declining in importance with distance downriver.
Blue Cypress Lake had much lower total algal biovolume, as well as lower overall community diversity than the downstream lakes. CitationAgusti et al. (1991) in a survey of 165 Florida lakes found that as total algal biomass increased, total number of taxa increased until a maximum richness is reached, then declines with further increases in biomass. CitationProulx et al. (1996) in a mesocosm study of the combined effects of herbivory and nutrient additions found a similar relationship between nutrients, algal biomass, and community richness. Unlike those studies, lower diversity in Blue Cypress Lake compared to the downstream lakes did not seem to be driven by differences in macronutrients among lakes because total P and TKN levels were similar among these lakes. Also, previous studies of the response of USJR algal communities to nutrients revealed that nutrients levels were sufficient to support significantly greater algal biomass than is present (CitationAldridge and Schelske 2000). Other factors, such as hydrology, may be a more important determinant of community biomass and diversity in these lakes. CitationConnell (1978) observed that very stable ecosystems tend to have low diversity, whereas ecosystems with occasional or moderate disturbances foster communities with relatively high diversity. In the case of the USJR, the disturbances that led to higher diversity in the downstream lakes may be the greater annual variation in downstream river flow, as well as intermittent nutrient subsidies from farms that are downstream of Blue Cypress Lake. Abrupt declines in late summer/early fall may have been due to high seasonal rainfall, resulting in advection of phytoplankton from the USJR. A second potential cause of the dramatic decline in biovolume was a sudden increase in lake volume (i.e., dilution) associated with increased fall rainfall. However, total biovolume of blue-green, diatoms, and green algae declined to less than 2% of their mid-summer maximums, therefore dilution alone could not account for the reduction.
Cyanobacterial biovolume increased dramatically downriver, with an attendant decline in dominance by diatoms. Also, the downstream lakes had greater diatom diversity than Blue Cypress Lake. CitationDuarte et al. (1992) found that, for Florida lakes of varying trophic status, those lakes whose total algal biomass was dominated by diatoms tended toward mesotrophy. Declining diatom species diversity is sometimes regarded as an indication of nutrient impacts (CitationLotter 1998). However, for the USJR chain of lakes, differences in trophic condition are minor and probably do not account for the downstream changes in relative abundances among algal divisions.
CitationWhitmore (1989) examined diatom valves from surficial sediments of 30 Florida lakes and related the diatom community to lake trophic status. A comparison of the predominant diatoms found in the USJR lakes to those characterized in the CitationWhitmore (1989) survey is consistent with water quality data that indicate that the USJR lakes are mesotrophic to eutrophic. Aulacoseira ambigua, a dominant species in all USJR lakes, is often associated with eutrophication (CitationSchelske et al. 1996, CitationCanter and Haworth 1991).
Green algae, cryptophytes, and euglenophytes constituted a minor proportion of the algal community. This is in contrast to a previous study of USJR phytoplankton community structure that found the Upper Basin lakes to be numerically dominated by green algae such as Chlorella and Chlorococcum (CitationYount and Belanger 1988). Chlorococcum is typically found in moist soil (not in the plankton community) and dominance by green algae in Florida lakes is suggestive of oligotrophic conditions (CitationDuarte et al. 1992); therefore, their results seem somewhat anomalous.
Regression analyses revealed that temperature was the principal factor regulating cyanobacteria biovolume in the USJR, consistent with other research showing the importance of temperature as a regulator of cyanobacteria (CitationRobarts and Zohary 1987, CitationGrover and Chrzanowski 2006). For the USJR, when water temperature was below 25°C, total biovolume was low. Temperature-related metabolic controls on algal growth clearly play a large role in determining cyanobacterial standing crop; however, other factors coincident with cooler temperatures in Florida, such as increased wind speed, probably also act to minimize bloom formation. Conversely, low early and mid-summer river flow and lower average wind speed are favorable for the development of summer blooms, such as occurred in the lower St. Johns River in 2005 and 2006. Lower summer wind speeds and reduced turbulence favor cyanobacteria because they have adaptations that allow them to maintain optimal vertical position in the water column under conditions of low turbulence (CitationPaerl et al. 2001).
Nutrients are often targeted as primary means of controlling algal blooms. For example, previous analyses aimed at determining pollutant loading goals for the Upper St. Johns River showed that, even though there was not a statistically significant relationship between cyanobacterial biovolume and phosphorus, there was a marked increase in the potential for high biovolume when total P exceeded 0.09 mg/L. In the current analysis, phosphorus explained only 1% of the variability in cyanobacteria biovolume (r2 = 0.011; p = 0.169). As expected, water column P was not a dominant factor influencing cyanobacteria because the N:P ratio in USJR is approximately 20:1 (w/w), which suggests that primary production is neither N nor P limited. In this study, TKN was much more correlated with cyanobacteria biovolume than P, possibly due to increased presence of N2-fixing biomass. Results from limiting nutrient enrichment bioassays for USJR lakes have demonstrated little or no response of the phytoplankton community to either nutrients or light (CitationAldridge and Schelske 2000). Also, dissolved nutrients are typically present in concentrations that should not pose a limitation to phytoplankton growth, with mean lake concentration NO3, NH4, and SRP of 0.068, 0.065, and 0.048 mg/L, respectively. Experiments on the lower St. Johns River and Lake George (middle St. Johns River) have demonstrated both N and P limitation, and that diazotrophic cyanobacteria blooms can represent a significant source of nitrogen to the river (CitationPaerl et al. 2005). Under conditions of low N and reduced flow, cyanobacteria have a competitive advantage due to the ability of some genera to fix atmospheric N and to utilize dissolved inorganic nitrogen at very low concentration (CitationSmith 1983). This has a high cellular energy cost and is done under conditions of nitrogen limitation.
The negative correlation between river stage and biovolume was most likely due to hydraulic residence times that were lower than phytoplankton regeneration rates (i.e., the standing crop of algae was flushed downstream during high flows). Water residence time has been shown to be an important regulator of phytoplankton standing crop in other ecosystems (CitationPhlips et al 2002, CitationBonilla et al. 2005). Intense hydrologic events, such as late summer early fall tropical storms, can trigger “reset events” that can suddenly cause major changes in both community composition and total biomass (CitationRyder and Pesendorfer 1989).
In summary, there was a general down-river trend in increasing phytoplankton biovolume and dominance of the algal community by cyanobacteria. Cyanobacteria dominated the algal community for the three downstream lakes (Washington, Poinsett, and Winder), whereas diatoms predominated in the upstream lakes (Blue Cypress, Hell ′n Blazes, and Sawgrass). The diatom assemblage, as well as water quality data, suggested that the lakes have characteristics similar to lakes classified as mesotrophic to eutrophic, though the Upper Basin lakes are less productive and thus also share some characteristics of dystrophic lakes. The principal factors associated with cyanobacteria biovolume in these lakes seem to be river flow, temperature, and nitrate. These findings likely apply to other riverine lakes; hydrologic factors need to be considered when analyzing spatiotemporal patterns in phytoplankton dynamics.
Acknowledgments
We are grateful for the hard work of Michael Hein (Water and Air Research, Inc., Gainesville, Florida) who performed approximately half of the phytoplankton identifications described in this study. The suggestions of two anonymous reviewers greatly improved a previous version of this manuscript.
Notes
1From Lowe et al. 1984.
References
- Agusti , S. , Duarte , C. M. and Canfield , D. E. 1991 . Biomass partitioning in Florida phytoplankton communities . J. Plankton Res. , 13 : 239 – 245 .
- Aldridge , F. J. and Schelske , C. L. 2000 . Study of light and nutrient limitation in Lake Washington Draft final report to the St. Johns River Water management District
- APHA, AWWA and WEF . 1998 . American Public Health Association. Standard Methods for the Examination of Water and Wastewater, , 20th ed.
- Bass , D. G. and Cox , D. T. 1985 . “ River habitat and fishery resources of Florida ” . In Florida Aquatic Habitat and Fishery Resources , Edited by: Seaman , W. J. Jr. 121 – 187 . American Fisheries Society . Florida Chapter
- Becker , B. and Hickisch , A. 2005 . Inhibition of Contractile Vacuole Function by Brefeldin A . Plant Cell Physiol. , 46 : 201 – 212 .
- Bonilla , S. , Conde , D. , Aubrioy , L. and Perez , M. C. 2005 . Influence of hydrology on phytoplankton species composition and life strategies in a subtropical costal lagoon periodically connected with the Atlantic Ocean . Estuaries. , 28 : 884 – 895 .
- Canter , H. M. and Haworth , E. Y. 1991 . The occurrence of two new planktonic diatoms in the English Lake District. A. islandica subspecies helvetica A. ambigua . Freshwater Forum , 1 : 39 – 48 .
- Carmichael , W. W. 2001 . Health effects of toxin-producing cyanobacteria: “the cyanoHABs.” . Hum. Ecol. Risk Assess. , 7 : 1393 – 1407 .
- Cichra , M. F. , Badylak , S. , Henderson , N. , Reuter , B. H. and Phlips , E. J. 1995 . Phytoplankton community structure in the open water zone of a shallow, subtropical lake (Lake Okeechobee, Florida, USA) . Arch. Hydrobiol. Spec. Issues Adv. Limnol. , 45 : 157 – 175 .
- Connell , J. H. 1978 . Diversity in tropical rain forests and coral reefs . Science. , 199 : 1302 – 1310 .
- DeMort , C. E. and Bowman , R. D. 1985 . Seasonal cycles of phytoplankton populations and total chlorophyll of the lower St. Johns River Estuary, Florida . Florida Scientist , 48 : 96 – 107 .
- Duarte , C. M. , Agusti , S. and Canfield , D. E. 1992 . Patterns in phytoplankton community structure in Florida lakes . Limnol. Oceanogr. , 37 : 155 – 161 .
- Florida Game and Fresh Water Fish Commission . 1977 . 1976-1977 Annual progress report for federal aid in fish restoration Dingell-Johnson project F-33-1 St Johns River fishery resources
- Goolsby , D. A. and McPherson , B. F. 1970 . Preliminary evaluation of chemical and biological characteristics of the upper St. Johns River Basin, Florida , U. S. Department of the Interior . Geological Survey Open-file report No. 71001
- Grover , J. P. and Chrzanowski , T. H. 2006 . Seasonal dynamics of phytoplankton in two warm temperate reservoirs: association of taxonomic composition with temperature . J. Plank. Res. , 28 : 1 – 17 .
- Havens , K. E. and East , T. 1997 . In situ responses of Lake Okeechobee (Florida, USA) phytoplankton to nitrogen, phosphorus, and Everglades Agricultural Area canal water . J. Lake Reserv. Manage. , 13 : 26 – 37 .
- Havens , K. E. , James , R. T. , East , T. and Smith , V. H. 2003 . N:P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution . Environ. Pollut. , 122 : 379 – 390 .
- Hillebrand , H. , Dürselen , C-D , Kirschtel , D. , Pollingher , U. and Zohary , T. 1999 . Biovolume calculation for pelagic and benthic microalgae . J. Phycol. , 35 : 403 – 424 .
- Leonard , J. A. and Paerl , H. W. 2005 . Zooplankton community structure, micro-zooplankton grazing impact, and seston energy content in the St. Johns river system, Florida as influenced by the toxic cyanobacterium Cylindrospermopsis raciborskii . Hydrobiol. , 537 : 89 – 97 .
- Lotter , A. F. 1998 . The recent eutrophication of Baldeggersee (Switzerland) as assessed by fossil diatom assemblages . The Holecene. , 8 : 395 – 405 .
- Lowe , E. F. , Brooks , J. E. , Fall , C. J. , Gerry , L. R. and Hall , G. B. 1984 . U. S. EPA clean lakes program, Phase I diagnostic-feasibility study of the Upper St. Johns River chain of lakes Vol. 1-Diagnostic study , Palatka, Florida : St. Johns River Water Management District . Technical Publication SJ 84-15
- Lund , J. W. G. , Kipling , G. and Le Cren , E. D. 1958 . The inverted microscope method for estimating algae numbers and statistical basis of estimating by counting . Hydrobiologia , 11 : 143 – 170 .
- McGrail , L. , Berk , K. , Brandes , D. , Munch , D. , Neubauer , C. , Osburn , W. , Rao , D. , Thompson , J. and Toth , D. 1998 . “ St. Johns River Water Management District ” . In Water Resources Atlas of Florida , Edited by: Fernald , E. A. and Purdum , E. D. 214 – 237 . Tallahassee : Florida State University, Institute of Science and Public Affairs .
- Mossa , J. 1998 . “ Surface Water ” . In Water Resources Atlas of Florida , Edited by: Fernald , E. A. and Purdum , E. D. 64 – 81 . Tallahassee : Florida State University, Institute of Science and Public Affairs .
- Oliver , R. L. and Ganf , G. G. 2000 . “ Freshwater blooms ” . In The ecology of cyanobacteria their diversity in time and space , Edited by: Whitton , B. A. and Potts , M. 149 – 194 . Boston : Kluwer Academic Publishers .
- Paerl , H. W. , Fulton , R. S. III , Moisander , P. H. and Dyble , J. 2001 . Harmful freshwater algal blooms, with an emphasis on cyanobacteria . Sci. World. , 1 : 76 – 113 .
- Paerl , H. W. , Joyner , J. J. and Piehler , M. F. 2005 . Sources, patterns, and degradability of organic matter and oxygen deficits in the Lower St. Johns River Final report to the St. Johns River Water Management District
- Pearsall , W. H. 1932 . Phytoplankton in the English lakes: II. The composition of the phytoplankton in relation to dissolved substances . J. Ecol. , 20 : 241 – 262 .
- Phlips , E. and Cichra , M. Sr. 1999 . Lake Washington phytoplankton community composition October 1997 through December 1998. Final report to the St. Johns River Water Management District, Palatka FL
- Phlips , E. , Badylak , S. and Grosskopf , T. 2002 . Factors affecting the abundance of phytoplankton in a restricted subtropical lagoon, the Indian River Lagoon, Florida, USA . Estuarine Coast. and Shelf Sci. , 55 : 385 – 402 .
- Proulx Pick , M. F. R. , Mazumder , A. , Hamilton , P. B. and Lean , D. 1996 . Experimental evidence for interactive impacts of human activities on lake algal species richness . Oikos. , 76 : 191 – 195 .
- Reynolds , C. S. 1998 . What factors influence the species composition of phytoplankton in lakes of different trophic status? . Hydrobiologia , 369/370 : 11 – 26 .
- Robarts , R. D. and Zohary , T. 1987 . Temperature effects on photosynthetic capacity, respiration, and growth rate of bloom-forming cyanobacteria . N. Z. J. Mar. Freshw. Res. , 21 : 391 – 399 .
- Ryder , R. A. and Pesendorfer , J. 1989 . Large rivers are more than flowing lakes: a comparative review Edited by: Dodge , D. P. 65 – 85 . Proceedings of the International Large River Symposium. Can. Spec. Publ. Fish. Aquat. Sci. 106
- Schelske , C. L. , Donar , C. M. and Stoermer , E. F. 1996 . A test of paleolimnologic proxies for the plamktonic/benthic ratio of microfossil diatoms in Lake Apopka , Koeltz Scientific Books . 14th Diatom Symposium
- Smith , V. H. 1983 . Low nitrogen to phosphorus ratios favor dominance by cyanobacteria in lake phytoplankton . Science , 221 : 669 – 671 .
- Stoermer , E. F. and Julius , M. L. 2003 . “ Chapter 15-Centric Diatoms ” . In Freshwater algae of North America ecology and classification , Edited by: Wehr , J. D. and Sheath , R. G. New York : Academic Press .
- USEPA . 1983 . Methods for the Chemical Analysis of Water and Wastes , Cincinnati , OH : U. S. Environmental Protection Agency. Environ. Monit. Support Lab. .
- Utermöhl , H. and White , W. A. 1958 . Some geomorphic features of central peninsular Florida , Florida Geological Survey . Geological Bulletin no. 41
- Whitmore , T. J. 1989 . Florida diatom assemblages as indicators of trophic state and pH . Limnol. Oceanogr. , 34 : 882 – 895 .
- Yount , J. R. and Belanger , T. V. 1988 . Population trends in the phytoplankton and zooplankton of the Upper and Middle St. Johns River, Florida, 1893-1984 . Florida Scientist. , 51 : 7685