Abstract
Episodic occurrences of cucumber odor caused by the alga Synura petersenii in Meander Creek Reservoir (MCR), northeastern Ohio, USA, are partly attributed to increased water transparency resulting from decreased phosphorus and suspended solids loading from the watershed. The first documented occurrence of nuisance odor levels was in 1984, 52 years after the reservoir was filled. This indicates that previous environmental factors constraining the growth of S. petersenii have been relaxed, probably from changes in the physical–chemical environment in the reservoir caused by changes in land use in the catchment. Reduction in farming since 1950, and diversion of sewage around the reservoir in 1977, reduced suspended solids and total phosphorus loading into the reservoir during the time that the cucumber odors occurred. These observations support the hypothesis that increased transparency of the reservoir, resulting from decreased sediment loading and reduced productivity, has permitted the occasional occurrence of nuisance densities of S. petersenii. Based on available data, pH, iron, and silica do not appear to be key factors regulating growth of S. petersenii in MCR. The transition to lower turbidity and total phosphorus concentrations from catchment restoration actions may increases the risk of S. petersenii blooms and cucumber odor episodes. While the overall benefits of cleaner raw water for water supply may outweigh this risk, it is desirable to understand the factors that promote nuisance growths and take actions to control them.
Objectionable odors and tastes in finished drinking water, although usually benign, are the primary criteria used by consumers to judge water safety (CitationMcQuire 1995). Aside from chlorination, most odor/taste problems are of biological origin, caused by algae, bacteria or fungi (Mallevaille and Suffet 1987, CitationSuffet et al. 1999). Most odors and tastes have more than one source; for example, musty or moldy odors are produced by many species of bacteria, cyanobacteria, fungi, and probably algae (CitationSuffet et al. 1999). However, cucumber odor, a property of the aldehyde E2,Z6 nonadienal (CitationHayes and Burch 1989), is caused by only a few species of algae. Algae implicated as possible sources of cucumber odor include Synura petersenii (CitationWee et al. 1994), Uroglenopsis (Mallevaille and Suffet 1987), Scenedesmus subspicatus (CitationCotsaris et al. 1995) Peridinium (CitationBurlingame 1992) and Uroglena (CitationPalmer 1977). Synura petersenii is the most common cause of cucumber odor in domestic water supplies (CitationWatson et al. 2001).
Nuisance levels of cucumber odor first occurred in water from Meander Creek Reservoir (MCR) in northeastern Ohio in December 1984. Subsequently, nuisance levels of the odor have occurred five times, all during winter, with the last episode in February 2009 (). During the 2002 odor episode, the causative organism was identified as Synura petersenii based on scanning electron microscope (SEM) discerned scale morphology (L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2002, unpubl.). The abundance of S. petersenii during the 2002 episode correlated with the concentration of E2,Z6 nonadienal in the reservoir water, providing substantive evidence that S. petersenii was the cause of the episodic occurrence of cucumber odor in MCR (L.A. Schroeder, S.C. Martin, Younstown State Univ., Dept. Civil Engineering, 2002, unpubl.). Synura petersenii also was identified during the 2009 episode based on colony and scale morphology with light microscopy. There is no direct evidence that the other episodes of cucumber odor are from S. petersenii; however, all episodes occurred during winter, and scales from S. petersenii were observed to a depth of about 20 cm in the sediments, corresponding to ca. 1995.
Table 1 Occurrence of cucumber odor episodes in Meander Creek Reservoir (M. Kielbasa, Mahoning Valley Sanitary District, 2003, pers. comm.).
S. petersenii is a flagellated, planktonic, colonial alga belonging to the class Synurophyceae, whose members are often abundant in the metalimnion of oligotrophic to mesotrophic lakes and reservoirs (CitationSiver 2003). S. petersenii is a strict photoautotroph (CitationHolen and Boraas 1995, CitationJansson et al. 1996) that has a wide tolerance for many environmental variables (CitationKristiansen 1975, CitationNicholls and Gerrath 1985, CitationSiver 1987, Citation1995).
Despite the wide range of conditions suitable for S. petersenii, this alga was not present in MCR at nuisance densities until 1984, suggesting that changing conditions in the reservoir released the algae from prior limiting factors. Since 1984, nonoptimum conditions have generally prevailed, keeping the population of S. petersenii below nuisance densities. Occasionally, at 3–5 yr intervals, the constraining factor or factors were relaxed and the alga attained nuisance densities, releasing precursors that form E2,Z6 nonadienal. We investigated the changes in reservoir catchment use, historical data for chemico-physical properties of MCR water, and diatom-based inferred total phosphorus (TP) levels for factors that may explain the recent and sporadic occurrence of nuisance cucumber odor in MCR.
Study site
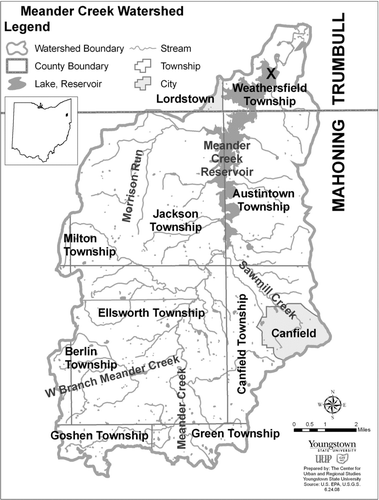
Meander Creek Reservoir, located in the Eastern-Ontario Lake Plain (EOLP) ecoregion, is the primary potable water source for 300,000 people in the Youngstown, Ohio, metropolitan area. The reservoir and the water treatment plant are managed by the Mahoning Valley Sanitary District (MVSD), a public utility. The reservoir was completed in 1931 and first filled in January 1932. An inflatable dam extension was installed in 1995, adding 41 cm to the reservoir depth. The reservoir was 11.2 km long, 1.6 km wide, and had a maximum depth 15.7 m, mean depth 5.1 m and a 221 km2 catchment area. Since 1995, the filled reservoir () had a surface area of 8.77 km2 and an impoundment capacity of 42.5 × 106 m3, with water residence time of 188 days. The reservoir was dimictic with a thermocline forming usually 3–4 m below the surface. The land immediately around the reservoir (1600 ha) was planted to red pine (Pinus resinosa). The reservoir and surrounding land is a wildlife refuge and is closed to the public.
Methods
Sediment cores
Two sediment cores were collected during June 2005 from the deepest part of the reservoir () using a 5-cm dia piston corer. One core was analyzed for sediment particle size distribution and the other was sectioned for analysis of diatom remains, wet sediment specific gravity, dry matter content, and loss on ignition (LOI). Particle size of the sieved material was determined using a hygrometer method (CitationDas 2001). Specific gravity was determined as the mass of a measured volume of sediment compacted by centrifugation in a calibrated centrifuge tube. Dry matter was determined as the mass remaining after drying at 105 C for 24 hr. Loss on ignition was determined as mass loss after heating to 550 C for 4 hr.
The 144-cm long diatom core was uniformly divided into 1.12 cm sections. Sediment chronology was determined by Daniel Engstrom (St. Croix Watershed Research Station, Science Museum of Minnesota, Marine on St. Croix, MN) from 137Cs activity on core sections that bracketed the maximum 137Cs activity and extended to a depth of no activity. Diatom sediment subsamples were taken from each section with a #2 cork borer (0.1–0.2 g wet weight), cleaned by concentrated nitric acid, washed with DI water, settled onto 22 × 22 mm glass cover slips and mounted in ZERAX®. Diatom valves were counted with a 100× objective using an Olympus AO 70A microscope. Consecutive 100-μm square quadrats were scored along a transect of the slide until at least 400 valves were counted. Diatom identification was based primarily on the works of CitationKrammer and Lange-Bertalot (1986–Citation1991) and CitationPatrick and Reimer (1966, Citation1975). Diatom nomenclature primarily follows CitationStoermer et al. (1999) then CitationKrammer and Lange-Bertalot (1986–Citation1991, (see Supplement for additional details).
Determination of inferred relative total phosphorus
Diatom inferred relative total phosphorus (IRTP) was determined using the weighted averaging method (CitationMcCune and Medford 1999). No regional training set includes reservoirs and/or lakes in the EOLP ecoregion of Ohio; therefore, a surrogate training set was constructed from appropriate published training sets. Five training sets were use: (1) Reavie set, 64 alkaline lakes in southeastern Ontario (CitationReavie and Smol 2001); (2) Bennion set, 32 eutrophic ponds in southeast England (CitationBennion 1994); (3) Fritz set, 42 lakes in northern and western Michigan (CitationFritz et al. 1993); (4) Dixit set, 257 lakes and reservoirs in northeastern USA (CitationDixit et al. 2006); and (5) Ramstack set, 55 lakes in Minnesota (CitationRamstack et al. 2003). Because of the high variability in the optimum TP among training sets, the data were normalized to the maximum value in each training set. The surrogate training set was then calculated as the mean of the normalized TP optima for each taxon. Therefore, the normalized optimum TP range for each training set and also the surrogate set was between 0 and 1. The resulting inferred TP is a relative value and indicative of magnitude of changes in TP with time but not absolute TP concentrations (see Supplement for additional details).
Estimation of total phosphorus (TP) and suspended solids (SS) loading
Total phosphorus and SS loadings into MCR were estimated from land use and mean export coefficients derived from published data (see Supplement for details).
Results
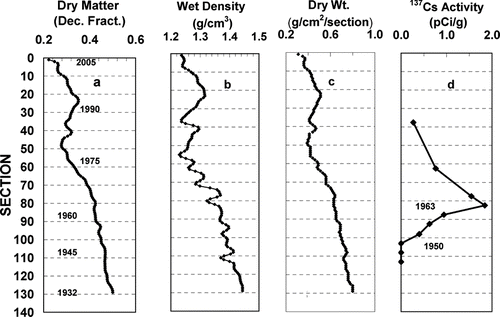
The core section thickness (1.12 cm) is less than the annual sediment deposition rate of 3.2 cm/yr for upper sections and 1.6 cm/yr for the deepest part of the core. Thus, seasonal differences in deposition rates may increase the variability in data derived from core section analysis: LOI, density, dry matter and diatoms abundance. To compensate for the seasonal source of variability in the core sections, the data were smoothed using the T3524 function in SPSS® (CitationNorusis 1993).
Sediment dry matter and density
The sediment dry matter content increased with sediment depth from 22% near the surface to a maximum of 50% in the deepest sediment, (). Sediment wet density follows a pattern similar to dry matter content (). The upper sediment has relatively low density (mean = 1.3 ± 0.03 g/cc, sections 1–57) with peaks at sections 22 and 40 corresponding to maxima in the dry matter content ( and ). The density then increases with sediment depth through the remainder of the core to a maximum of 1.45 g/cc in the deepest layers (). The dry matter content of each core section increased from 0.25 g/cm2/section at the surface to 0.92 g/cm2/section at the bottom of the core ().
Chronology
Four time markers establish the chronology of core deposition. The top of the core was deposited in 2005 and the bottom in 1932. Maximum 137Cs activity occurred in section 83, representing 1963, and last detectable activity in section 98, representing ca. 1950 (). Aerial annual deposition rates (AAD) were calculated for three of the core regions delimited by the 137Cs chronology: sections 1–83, 84–102 and 103–139 (). The AAD increased with core depth from 0.93 g/cm2/yr for the upper region (2005–1965) to 0.98 g/cm2/yr for the mid region (1964–1950) and 1.2 g/cm2/yr in the deepest region (1951–1932). The time span represented by each core section was estimated from the linear regression of AAD on core section and the measured dry weight content of the section (see Supplement for additional details). Although the change in deposition rate is likely nonlinear, the estimates of sediment age based on AAD and measured dry matter content of each section are likely more accurate than those based on a constant deposition rate.
Table 2 Mean dry matter deposited during time intervals delineated by the 137Cs chronology (n = core section number from top of core).
Loss of weight on ignition (LOI)
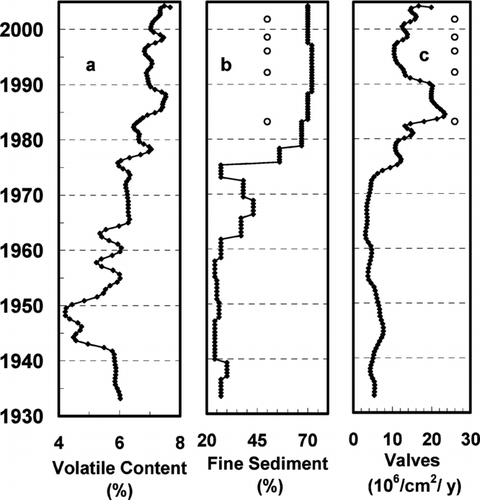
Organic content of the sediments (LOI) decreased steadily with sediment age, from 7.5% of dry mass in 2005 to 5.5% in 1955, then formed a minimum between 1950 and 1942 and increased to 6% from 1946 through 1932 (). There was no evidence of organic matter from terrestrial sources in the core (e.g., tree leaves and twigs); thus, the organic content of the sediment is attributed predominantly to reservoir productivity. The pattern of change in organic matter depends on both the productivity of the reservoir and the rate of dilution by inorganic material from the catchment. The pattern is consistent with the decreased rate of inorganic deposition with time (see suspended solids export, below); the lowest organic content occurred in the deepest sediment, which also had the greatest rate of inorganic deposition.
Sediment particle size
Coarse silt and sand (particle size 8–74 μm) dominated the sediment from 1932 to ca. 1957. Between 1957 and ca. 1980 sediment particle size changed to predominantly fine silt and clay (size < 8 μm) and remained clay–silt through 2005 (). All odor episodes occurred after the transition from coarse to fine sediment size ().
Diatom abundance and composition
Diatom valve production remained relatively low from 1932 to ca. 1973 at about 6 × 106 valves/cm2/yr, then increased to a maximum of about 23 × 106 valves/cm2/yr by 1987 and remained relatively high to the core top (2005) during the period when odor episodes occurred ().
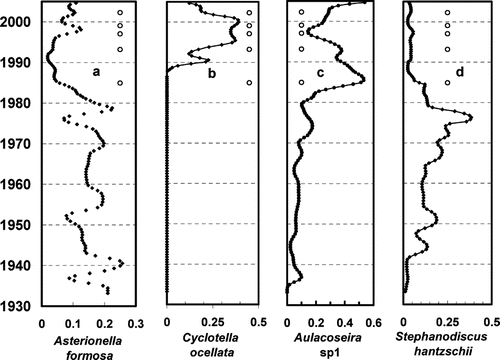
The relative abundance (RA, the ratio of number of valves for a taxon to the total valves counted) of the diatom assemblage changed from predominantly Asterionella formosa, (RA = 0.15) and Stephanodiscus hantzschii (RA = 0.4; , ) prior to 1985 to predominantly Cyclotella ocellata (RA = 0.4) and Aulacoseira sp1 (RA = 0.2) ( and ) after 1985. The decrease in A. formosa, Stephanodiscus hantzschii ( and ) and increases in C. ocellata and A. sp1 ( and ) roughly correspond to the onset of cucumber odor in MCR. Stephanodiscus hantzschii has a high, and A. formosa moderately high, relative TP optima (0.62 and 0.35, respectively). Cyclotella ocellata and A. sp1 (A. cf alpigena) have relatively low optimal TP requirements (0.23 and 0.21, respectively), which is consistent with increased RA as TP decreases. The TP optimum for A. sp1 is not available, but a similar species, Aulacoseira alpigena, has an optimum relative TP value of 0.2l. Changes in relative abundance of diatom taxa with sediment depth imply changes in water chemical and/or physical characteristics. Patterns of changes in diatom community composition and abundance correspond with the occurrence of odor episodes in MCR. Both patterns are consistent with decreased nutrient (TP) levels during the period that odor episodes were observed.
Water chemistry
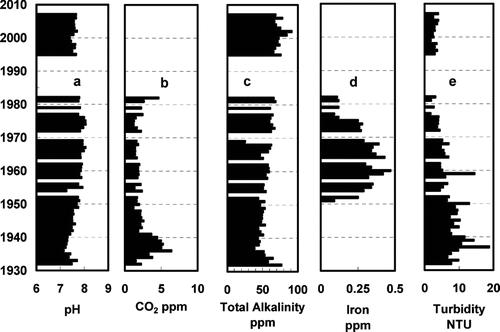
The MVSD has maintained water chemistry records from the beginning of water treatment plant operations in 1932. Although some of the records, particularly between 1984 and 1995, are missing, the available data are useful in assessing the trends in reservoir conditions. The pH of MCR has remained relatively constant (geometric mean 7.67 ± 0.23; ). The pH decreased during the first 5 years after construction to the lowest value (7.1) then increased steadily to a maximum of 8.05 ca. 1972, then decreased to 7.7 at present (). Free carbon dioxide was not monitored after 1983. The highest CO2 values of 5 ppm were recorded shortly after the reservoir was filled and declined to 2 ppm by 1980 (). Total alkalinity trended higher from 45 ppm shortly after reservoir filling to 65 ppm (CaCO3 equivalents) by 1995 (). Total iron was recorded between 1950 and 1982. Iron content was relatively high, 40 ppb, until 1949 then decreased to 20 ppb by 1983 (). Turbidity was high following reservoir filling, > 10 NTU, then declined to 4 NTU by 1980 and remained relatively low for the remainder of the study period ().
Figure 5 Meander Creek Reservoir water analysis data: pH, free carbon dioxide, total alkalinity, iron, turbidity and turbidity. Data are from Mahoning Valley Sanitary District, blank space = no available data.
There are no early direct measures of transparency in MCR; however, reservoir turbidity (tb) is correlated with Secchi disk transparency (sd in m; Equation Equation1). The regression equation for transparency as a function of turbidity in MCR, calculated from measurements during three winter seasons, 2000–2003 (L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2003, unpubl), is:
Water chemistry including TP, silica, pH, conductivity, turbidity, suspended solids (SS) and transparency were monitored during the winter seasons 2000–2003 (, data from L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2003, unpubl.).
Table 3 Summary of chemical/physical data for Meander Creek Reservoir for the winter seasons of 2000–2003.
Land use patterns
Three major changes in the 221 km2 MCR catchment have occurred since reservoir construction: (1) total farmland decreased but the proportion planted to soybeans increased; (2) the human population increased with the concomitant increase in residential area; and (3) the sewage effluent from the City of Canfield was diverted around the reservoir in 1977. Land use in the catchment in 1932 when MCR was completed was: water 3.5%; residential 1.4%; woods and shrub 33%; and farmland 62%. By 2005 land use was: water 4%; residential 16%; shrub and woods 53%; and farmland 27% (NASS 2006 and extrapolated from Christou 2002). There is little industry, commercial or dense urban development in the catchment.
Residential:
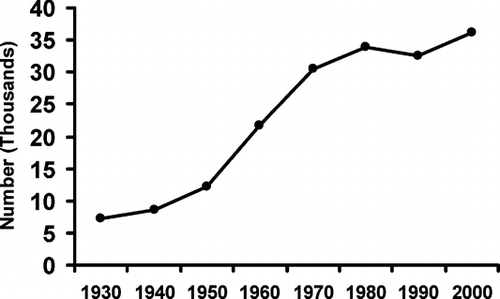
Population changes in the catchment were estimated from the U.S. 10-year township census data weighted for the proportion of each township included in the catchment (). The catchment population grew exponentially, with a doubling time of about 18 yr between 1930 and 1970, then more slowly during the 1970s, and even declining during the 1980s. The population growth resumed during the 1990s (). Residential area of the catchment increased from 10.3 km2 in 1965 to 30.0 km2 in 1994 (measurements from aerial photographs, CitationChristou 2002). Extrapolating from these data, the residential area increased from 3.7 km2 in 1950 to 14 km2 by 1970 and 45 km2 in 2005 (). Most of the residential area in the catchment is in and around the City of Canfield (). Prior to 1977, all sewage from the city was treated at the Canfield waste water treatment plant (WWTP) and the effluent discharged into Sawmill Creek, a tributary that flows into the southern end of MCR (). The Canfield WWTP was closed in 1977, and the raw sewage from Canfield was diverted around MCR to a county WWTP downstream.
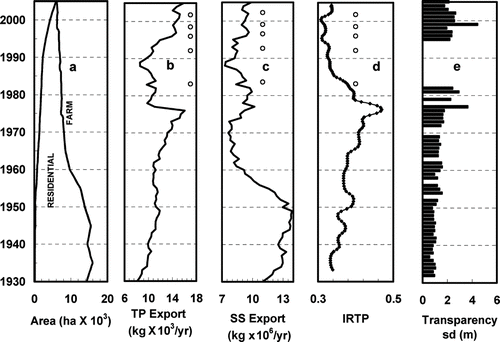
Figure 7 Factors affecting the growth of Synura petersenii in Meander Creek Reservoir (MCR): (a) change in farmland and residential area on MCR catchment; (b) estimated total phosphorus export from MCR catchment; (c) estimated suspended solids export from MCR catchment; (d) relative inferred total phosphorus (IRTP) and (e) Secchi disk transparency (sd) calculated from measured turbidity. Odor episodes are marked by an “o.”
Agriculture:
Farmland area (NASS 2006) of the catchment steadily decreased after ca. 1945 (). The most rapid change in farmland occurred between 1945 and 1965 when farmland decreased by nearly 50% (). Changes in catchment land use are reflected in the loading of nutrients and suspended materials entering the reservoir.
The SS loading is dominated by agricultural land use (). Suspended solids export was high until 1950 then decreased rapidly, in concert with the decrease in farmland, to relatively low values by 1970. The decline in farmland corresponds to the change in particle size of the sediment from predominately coarse silt to fine silt and clay (). This is in contrast to the TP export, where the rapid decrease occurred after 1977 in response to completion of the sewage bypass. After 1990, the TP export from increasing residential area became substantial. All of the recorded incidents of cucumber odor occurred after the closing of the WWTP, and the marked decrease in TP, and during a period with relatively low SS loading (). The pattern of change in SS export () with time corresponds to changes in measured turbidity in the reservoir. Turbidity was high during the period of high farming activity (1932–1950) then decreased through about 1980, remaining low until 2005 ().
Inferred relative total phosphorus (IRTP)
Inferred relative total phosphorus increased steadily from 0.34 after reservoir filling in 1932 to a maximum of 0.47 in 1977 then decreased to a minimum of 0.32 ca. 1995 (). The decrease in IRTP beginning in 1977 corresponds to completion of the sewage bypass by the City of Canfield. At the time of the first odor episode in 1984, IRTP was 0.37; during the subsequent episodes between 1995 and 2002, the IRTP was at a minimum of about 0.32 (). Changes in IRTP reflect the changes in TP export, especially from 1932 to about 1990. After ca. 1990, increases in TP export do not correspond to changes in IRTP ( and ), possibly due to over-estimation of phosphorus export from the developing urban areas. The mean TP measured during winter seasons 2000–2003 was 27 ppb, N = 47 (). If TP remains proportional to IRTP then the IRTP cardinal points correspond to TP of: 29 ppb following reservoir filling, 1932; 40 ppb at maximum TP, 1977; 31 ppb for first odor episode, 1984; and about 27 ppb during the remaining odor episodes.
The decrease in SS () and TP ( and ) is manifested in increased transparency (). Transparency, calculated from turbidity data (), increased slowly from 1932 to ca. 1977 then increased rapidly and remained relatively high until 2005 (). The change in transparency is consistent with changes in TP and SS export and with the changes in IRTP. All odor episodes occurred during the period of relatively high transparency.
Discussion
Six episodes of cucumber odor in water from MCR have occurred between December 1984 and 17 February 2009. The synurophyte, S. petersenii has been identified as causing the cucumber odor of the 2002 and the 2009 episodes and presumably is the causative agent for the previous episodes (L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2002, unpubl.). The relatively recent (1984) first odor episode implies that conditions in the reservoir changed in a manner that released S. petersenii from prior limiting factors. The sporadic nature of the nuisance odor implies that constraining factors generally prevail but occasionally are relaxed, permitting the alga to attain nuisance densities. Thus, an explanation for the occurrence of cucumber odor in MCR must include a general change in one or more factors that permit the growth of S. petersenii to nuisance densities and recognize that these factors are only occasionally effective.
Several environmental factors have been implicated as affecting, or at least corresponding with, growth of S. petersenii populations, including: pH (CitationSiver and Hammer 1989); phosphorus (CitationSiver and Hammer 1989, CitationRashash et al. 1995); conductivity (CitationDixit et al. 2000); light (CitationRashash et al. 1995, CitationPaterson et al. 2004); silica (CitationSandgren et al. 1996); free carbon dioxide (CitationSaxby-Rouen et al. 1998); and iron (CitationGuseva 1935, cited in CitationNicholls 1995).
Scaled chrysophytes in general and the synurophyte S. petersenii in particular have recently increased in abundance in many boreal lakes in North America (CitationPaterson et al. 2004). The recent increases were attributed to “broadly applicable anthropogenic effects” but not to changes in pH (acid precipitation), TP, or catchment disturbances (CitationPaterson et al. 2004). They proposed that climatic changes or synergism between climatic changes and acidification cause an increase in lake transparency resulting in increased productivity in deeper layers of the lakes. Because many colonial scaled chrysophytes and synurophytes, including S. petersenii, are most prevalent in the metalimnion (CitationPaterson et al. 2004 and references therein), they would benefit from greater lake transparency.
The silica requirement for S. petersenii is low, less than for most diatoms (CitationKlaveness and Guillard 1975, CitationSandgren et al. 1996). Growth rate of S. petersenii was reduced in media containing < 1.0 μM silica (CitationKlaveness and Guilliard 1975). The mean silica concentrations of MCR measured during winter 2002 (2.4 ppm = 40 μm/l; ) is 40 times the threshold for reduced growth of S. petersenii (CitationKlaveness and Guilliard 1975); therefore, silica is not likely a limiting factor for the growth of S. petersenii in MCR.
The use of cytochrome c as an electron donor by S. petersenii results in relatively high requirements for iron (CitationRaven 1995). Synura petersenii growth was enhanced by the addition of iron to Hall Lake water (CitationMunch 1972 cited in Sandgren 1988). CitationGuseva (1935 cited in CitationNicholls 1995) found that iron was limiting to growth of S. petersenii below about 1.2–1.4 ppm. The measured iron content in MCR (mean = 0.3 ppm between 1951 and 1983; data from MVSD) is considerably less than the reported limiting concentration (CitationGuseva 1935 cited in CitationNicholls 1995); however, the iron content of MCR was decreasing rather than increasing prior to the first occurrence of cucumber odor (). The trend in iron concentration is consistent with expectations from changes in watershed use (i.e., decreased SS and TP loading). Thus the episodic increases in S. petersenii are not likely due to relief from iron limitation.
Synura petersenii has been described as pH indifferent (CitationKristiansen 1975, CitationTakahashi 1978) and is found over a wide range of pH conditions (CitationRoijackers and Kessels 1986). The optimum pH reported for S. petersenii (i.e., the mean pH weighted for abundance) ranges from 6.4 for 105 Sudbury Ontario lakes (CitationDixit et al. 2002) to 7.2 for 146 northeastern USA lakes (CitationDixit et al. 1999). Synura petersenii abundance and distribution was not correlated with pH among 30 Sudbury Ontario lakes (CitationDixit et al. 1988), and pH was not implicated in the recent increase in S. petersenii abundance in boreal lakes of Canada (CitationPaterson et al. 2004). Changes in abundance of S. petersenii in five Connecticut lakes were not attributed to pH, but to changes in land use that affected conductivity and alkalinity (CitationMarsicano and Siver 1993). Laboratory cultures of S. petersenii showed no significant difference in growth rates over a pH range of 5.5 to 8.5 (CitationWee et al. 1991); however, pH and conductance were the dominant variables for axis 1 in a Principal Component Analysis (PCA) of environmental factors affecting the distribution of S. petersenii in northeastern U.S. lakes (CitationSiver and Hammer 1989). Although the decrease in pH in MCR between 1972 and 2005 includes the period when cucumber odor episodes occurred and is consistent with more favorable pH conditions for the growth of S. petersenii, it is unlikely the causative factor for the episodic occurrence of cucumber odor in MCR water. The pH of MCR (7.8; ) remains well within the range for occurrence of S. petersenii, and prior to the odor episodes there were periods when the pH was considerably lower (pH = 7.2, 1933–1940; ) with no recorded reports of cucumber odor episodes.
The inability of S. petersenii to utilize bicarbonate as a carbon source for photosynthesis (CitationSaxby-Rouen et al. 1998) limits their distribution to lakes, or regions within lakes, with sufficient concentrations of free CO2 to support growth. Because free CO2 is inversely related to both alkalinity and pH, as described by the Henderson-Hasselbalch equation (see CitationHutchinson 1957), S. petersenii is indirectly sensitive to alkalinity and pH. This is consistent with the optimum pH for S. petersenii of 6.7 and the observation that all 11 incidents of cucumber odor problems in Ontario, noted by CitationNicholls and Gerrath (1985), were in Canadian Shield lakes of low alkalinity (< 45 mg CaCO3 /L).
In soft water lakes with low alkalinity, pH must be low enough to provide sufficient free CO2 for S. petersenii growth; however, in lakes with moderately high hardness and bicarbonate alkalinity, sufficient free CO2 can be available even at relatively high pH. The geometric mean pH in MCR is 7.8, but, because of the high total inorganic carbon, the free CO2 is relatively high at about 2 ppm. Free CO2 in MCR does not vary in a manner consistent with the recent sporadic winter occurrence of cucumber odor; therefore, we do not expect that free CO2 is limiting S. petersenii growth in MCR.
Cucumber odor episodes in MCR (1984–1999) occurred during a period of inferred TP minima, implicating TP as a factor related to the abundance of S. petersenii. Synura petersenii were more prevalent in Experimental Lake 227 prior to experimental nutrient enrichment with nitrogen and phosphorus (CitationZeeb et al. 1994). Synura petersenii occurred in substantial numbers after TP was lowered by 90% by dredging and other restoration procedures in Lake Trummen, Sweden (CitationCronberg 1982). Recent increases in S. petersenii in two oligotrophic lakes, Russell and Willard Ponds, NH, were attributed to watershed disturbances but could not be directly linked to changes in pH, chloride or phosphorus (CitationDixit et al. 2001).
By contrast, anthropogenic effects including eutrophication have been proffered as a cause for recent increases in S. petersenii in Joe's Pond, VT (TP = 7.9 ppb) and Kenoza Lake, MA (TP = 13 ppb), while an increase in S. petersenii in the third lake, French Pond, NH (TP = 14 ppb) was attributed to increases in electrolytes and TP (CitationDixit et al. 2000). CitationMarsicano and Siver (1993) attributed recent increases in S. petersenii in sediments of lakes from northeastern U.S. to increased TP. Principle Component Analysis of the abundance of S. petersenii and environmental factors in 62 lakes (TP = < 10 to > 40 ppb) from New York and New Hampshire revealed a high correspondence of TP (second PC axis) to the abundance of S. petersenii (CitationSiver and Hammer 1989).
The inconsistent response of S. petersenii populations to changes in nutrient levels in fresh water is also characteristic of other chrysophytes populations (CitationNicholls 1995). Recent widespread increase in S. petersenii in oligotrophic and mesotrophic boreal lakes of North America could not be attributed to TP enrichment (CitationPaterson et al. 2004). The requirement for phosphorus, as determined in laboratory cultures, is lower than for most other algae (CitationSandgren 1988, CitationSandgren et al. 1996). The TP in MCR, measured during winter seasons 2000–2003, was 27 ppb (), which is considerably greater than the weighted average TP of 17 ppb from lakes in northeastern U.S. where S. petersenii were present (CitationSiver and Hammer 1989).
There is no evidence for inhibition of S. petersenii by high concentrations of TP (CitationRashash et al. 1995). High TP concentrations may indirectly suppress S. petersenii populations by stimulating growth of competing algae that limit light availability in the deeper water where S. petersenii and other colonial scaled synurophytes and chrysophytes are commonly found (CitationFee et al. 1977, CitationSandgren 1988). The recent occurrence of S. petersenii in MCR is consistent with this hypothesis.
As farming activities on MCR catchment declined (), the SS load to the reservoir decreased (). The decrease in farming along with completion of a sewage effluent bypass in 1977 reduced nutrient loading to the reservoir (), confirmed by the pattern of IRTP (). The reduced TP and SS increased reservoir transparency during the time that odor episodes were observed (), consistent with the hypothesis that S. petersenii were consistently light limited prior to ca. 1980 and periodically light limited after ca. 1980. Superimposed on the increased light penetration are physical vagaries affecting light penetration on a shorter time scale that may be caused by snow covered ice, irregular runoff events that temporarily increase turbidity or prolonged cloudy periods. These short-term vagaries in light penetration provide a mechanism for the sporadic occurrence of nuisance levels of S. petersenii in MCR.
The onset and duration of ice cover, and depth and character of snow cover in particular, may affect the sporadic growth of nuisance levels of S. petersenii. The onset and duration of ice cover on MCR is quite variable, ranging from early December to mid-January with duration of a few weeks to several months (L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2000, unpubl.). Ice cover eliminates wind mixing, resulting in reduced SS with a concomitant increase in transparency. Snow cover on the ice reduces light penetration, perhaps favoring growth of low-light tolerant species (e.g., S. petersenii). When ice melt occurs after prolonged ice cover, especially when not accompanied by a major runoff, the nascent S. petersenii populations may be released to produce nuisance density populations. This is consistent with the correspondence between January snow melt and odor episodes in MCR (L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2000, unpubl.).
The phenomenon of nuisance growth of S. petersenii triggered by increasing reservoir transparency would most likely occur in dimictic, meso- to eutrophic reservoirs. As transparency increases, S. petersenii are released from the limitation of low light intensity and may form nuisance level populations, if other conditions are favorable. In reservoirs with moderate bicarbonate, such as MCR, subsequent phytoplankton growth in the epilimnion would reduce free CO2 (CitationWetzel 1983), probably limiting the duration of light-induced S. petersenii growth. In oligotrophic reservoirs with greater free CO2 and high transparency (e.g., Cannon Reservoir, on a branch of the Delaware River in New York), the duration of S. petersenii problems may persist for longer periods (CitationBurlingame et al. 1992).
Synura petersenii in MCR present a paradox where improving water quality, as assessed by increased water transparency, was accompanied by instances of excessive growth of a nuisance alga, S. petersenii. This is, of course, not a valid argument for reducing watershed management efforts. Water treatment plant managers reported that only 7% of taste/odor problems were attributed to S. petersenii, while over 56% were caused by cyanobacteria (CitationKnappe et al. 2004). Cyanobacteria and other problem algae are most common in eutrophic conditions and could be alleviated by catchment and reservoir management that reduces nutrient loading. The increased cost of water treatment to remove excess algae and odors formed in eutrophic reservoirs likely exceeds the additional cost of removing the occasional cucumber odor that may result from S. petersenii growth in reservoirs of improving water quality (i.e., increased transparency).
The threshold concentration for detection of cucumber odor is low, from 2 ng/L (CitationBurlingame et al. 1992) to 60 ng/L of E2,Z6 nonadienal (CitationYoung et al. 1996), corresponding to a S. petersenii cell density of about 20 cells/ml (CitationRashash et al. 1995, CitationWatson et al. 2001). Peak density of S. petersenii observed in MCR was 300 colonies/ml (21,000 cells/ml). The corresponding concentration of E2,Z6 nonadienal was 64 ng/L in suspended particulate matter on 22 January 2002 (L.A. Schroeder, S.C. Martin, Youngstown State Univ., Dept. Civil Engineering, 2002, unpubl.). The aldehyde E2,Z6 nonadienal is found only at very low concentrations in living cells (CitationWee et al. 1994, CitationRashash et al. 1995); cucumber odor forms primarily after cell death and lysis.
Cucumber odor can be readily removed from water supplies by activated carbon adsorption (CitationBurlingame et al. 1992) or chlorine (CitationBurlingame et al. 1992, CitationWatson et al. 2001). In situ treatment with algaecides is more problematic. During summer thermal stratification, S. petersenii are most often found below the thermocline and therefore are less susceptible to surface application of algaecides (CitationSung 1990). During thermal turnover when algae are vulnerable, the entire reservoir water column must be treated, requiring larger dosages of algaecides (CitationSung 1990). Often S. petersenii produce odor problems during winter and early spring when the reservoir is wholly or partially ice covered, making application of algaecides difficult. If algaecide treatment is successful, the death of S. petersenii can be expected to result in a temporary increase in cucumber odor.
Acknowledgments
Dr. Eduardo A. Morales, Curador, Herbario Criptogámico, Universidad Católica Boliviana San Pablo, Cochabamba, Bolivia and research scientist at the Philadelphia Academy of Sciences, Philadelphia, PA, helped with identification of diatoms. Dr. Gary Walker, Department of Biological Sciences, Youngstown State University (YSU), gave valuable advice on microscopy and microphotography and permitted use of his dark room and dark room facilities. Carl Leet III, YSU Media Center, provided resources and help digitizing film negatives and advice on microphotography. John Bralich, Center for Urban and Regional Studies, YSU, provided the census data and the map of MCR catchment. Ellen Wakefield-Banks at the YSU library was exceptionally helpful in tracking literature sources. Ed McCormack, Joe Paris and Martin Kielbasa of the MVSD provided access to MVSD data and logistical support for field work. We are especially appreciative to the editor of LRM and to the several reviewers for their insightful and constructive suggestions. Funding was provided by the MVSD and YSU Research Council, grant number 2004–2005 #1.
References
- Bennion , H. 1994 . A diatom-phosphorus transfer function for shallow, eutrophic ponds in southwest England . Hydrobiologia , 275/276 : 391 – 410 .
- Burlingame , G. A. , Muldowney , J. J. and Maddrey , R. E. 1992 . Cucumber flavor in Philadelphia's drinking water . J. Am. Water Res. Assn. , : 92 – 97 . August
- Christou , C. 2002 . Impact of historic trends in nutrient loading on the trophic status of Meander Creek Reservoir , MS Thesis Youngstown , OH : Youngstown State University .
- Cotsaris , E. , Bruchet , A. , Maillevialle , J. and Bursill , D. B. 1995 . The identification of odorous metabolites produced from algal monocultures . Water Sci. Tech. , 31 ( 11 ) : 251 – 258 .
- Cronberg , G. 1982 . Changes in phytoplankton of Lake Trummen induced by restoration . Hydrobiologia , 86 : 185 – 193 .
- Das , B. 2001 . Soil Mechanics Laboratory Manual , San Jose , CA : Engineering Press .
- Dixit , S. S. , Connor , J. N. and Landry , S. C. 2001 . Paleolimnological study of Willard and Russell Ponds in New Hampshire . Lake Reserv. Manage. , 17 ( 3 ) : 197 – 216 .
- Dixit , S. S. , Dixit , A. S. and Evans , R. D. 1988 . Scaled chrysophytes (Chrysophyceae) as indicators of pH in Sudbury, Ontario, Lakes . Can. J. Fish. Aquat. Sci. , 45 : 1411 – 1421 .
- Dixit , S. S. , Dixit , A. S. and Smol , J. P. 1999 . Lake sediment chrysophyte scales from the northeastern USA and their relationship to environmental variables . J. Phycol. , 35 : 903 – 918 .
- Dixit , S. S. , Dixit , A. S. and Smol , J. P. 2002 . Relationship between Chrysophyte assemblages and environmental variables in seventy two Sudbury lakes as examined by canonical correpondence analysis (CCA) . Can. J. Fish. Aquat. Sci. , 46 : 1667 – 1676 .
- Dixit , S. S. , Dixit , A. S. , Smol , J. P. , Hughes , R. M. and Paulsen , S. G. 2000 . Water quality changes from human activities in their Northeastern USA lakes . Lake Reserv. Manage. , 16 ( 4 ) : 305 – 321 .
- Dixit , S. S. , Smol , J. P. , Charles , D. F. , Hughes , R. M. , Paulsen , S. G. and Collins , G. B. 2006 . Excerpts from: 1999. Assessing water quality changes in lakes of the northeastern United States using sediment diatoms . Can. J. Fish. Aquat. Sci. , 56 : 131 – 152 . http://lakes.chebucto.org/PALEO/ne_usa-257.html
- Fee , E. J. , Shearer , J. A. and DeClercq , D. R. 1977 . In vivo chlorophyll profiles in lakes from the Experimental Lakes Area, Northwestern Ontario , Winnipeg , , Canada : Department of Environment, Freshwater Institute . Technical Report 703
- Fritz , S. C. , Kingston , J. C. and Engstrom , D. R. 1993 . Quantitative trophic reconstruction from sedimentary diatom: Assemblages. A cautionary tale . Freshwater Biol. , 30 : 1 – 23 .
- Guseva , K. A. 1935 . The conditions of mass development and the physiology of nutrition of Synura . Mikrobiologia , 4 : 24 – 32 .
- Hayes , K. P. and Burch , M. D. 1989 . Odorous compounds associated with algal blooms in South Australian waters . Water Res. , 23 ( 1 ) : 115 – 121 .
- Holen , D. A. and Boraas , M. E. 1995 . “ Mixotrophy in Chrysophytes ” . In Chrysophyte algae: ecology, phylogeny and development , Edited by: Sandgren , C. D. , Smol , J. P. and Kristiansen , J. 119 – 140 . UK : Cambridge University Press .
- Hutchinson , G. E. 1957 . A Treatise on Limnology. Volume I , New York , NY : John Wiley and Sons .
- Jansson , M. P. , Blomqvist , P. , Johnson , A. and Bergstrijm , A.-K. 1996 . Nutrient limitations of bacterioplankton, autotrophic and mixotrophic phytoplankton, and heterotrophic nanoflagelates in Lake Ortrasket . Limnol. Oceanogr. , 41 : 1552 – 1559 .
- Klaveness , D. and Guillard , R. R. L. 1975 . The requirement for silica in Synura petersenii . J. Phycol. , 11 : 349 – 355 .
- Knappe , D. R. U. , Belk , R. C. and Briley , D. S. 2004 . Algae detection and removal strategies for drinking water treatment plants , Denver , CO : Am. Water Works Assn. .
- Krammer , K. and Lange-Bertalot , H. 1986 . “ Bacillariophyceae. 1. Teil: Naviculaceae ” . In Süsswasserflora von Mitteleuropa , Edited by: Ettl , H. , Gerloff , J. , Heynig , H. and Mollenhauer , D. Jena , , Germany : Fisher Verlag . 2(1) (in German)
- Krammer , K. and Lange-Bertalot , H. 1988 . “ Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae ” . In Süsswasserflora von Mitteleuropa , Edited by: Ettl , H. , Gerloff , J. , Heynig , H. and Mollenhauer , D. Stuttgart , , Germany : Fisher Verlag . 2(2). Gustav Fisher Verlag, Stuttgart, Germany (in German)
- Krammer , K. and Lange-Bertalot , H. 1991 . “ Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae ” . In Süsswasserflora von Mitteleuropa , Edited by: H , Ettl , Gerloff , J , Heynig , H and Mollenhauer , D . Jena , , Germany : Fisher Verlag . 2(3) (in German)
- Krammer , K. and Lange-Bertalot , H. 1991 . “ Bacillariophyceae. 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema ” . In Süsswasserflora von Mitteleuropa , Edited by: Ettl , H. , Gerloff , J. , Heynig , H. and Mollenhauer , D. Jena , , Germany : Fisher Verlag . 2(4) (in German)
- Kristiansen , J. 1975 . On the occurrence of the species Syunura (Chrysophyceae) . Verh. Int. Ver. Limnol. , 19 : 2709 – 2715 .
- Mallevialle , J. and Suffet , I. H. , eds. 1987 . Identification and treatement of tastes and odors in drinking water , Denver , CO : J. Am. Water Works Assn. .
- Marsicano , L. J. and Siver , P. A. 1993 . A paleolimnological assessment of lake acidification in five Connecticut lakes . J. Paleolim. , 9 : 209 – 221 .
- McCune , B. and Mefford , M. J. 1999 . PC-ORD. Multivariate analysis of ecological data, Version 4. MJM Software Design Gleneden Beach , OR
- McQuire , J. M. 1995 . Off-flavor as the consumer's measure of drinking water safety . Water Sci. Tech. , 31 ( 11 ) : 1 – 8 .
- Munch , C. S. 1972 . An ecological study of the planktonic Chrysophytes in Hall Lake, Washington , PhD dissertation Seattle , WA : University of Washington .
- Nicholls , K. H. 1995 . “ Chyrsophyte blooms in the plankton and neuston of marine and freshwater systems ” . In Chrysophyte Algae , Edited by: Sandgren , C. D. , Smol , J. P. and Kristiansen , J. 181 – 213 . Cambridge , , UK : Cambridge University Press .
- Nicholls , K. H. and Gerrath , J. F. 1985 . The taxonomy of Synura in Ontario with special reference to taste and odor in water supplies . Can. J. Bot. , 63 : 1482 – 1493 .
- Norusis , M. J. 1993 . SPSS® for Windows™ Release 6.0 Chicago , IL
- Palmer , C. M. 1977 . Algae and water pollution , Cincinnati , OH : USEPA . EPA-600/9-77-036
- Paterson , A. M. , Cumming , B. F. , Smol , J. P. and Hall , R. I. 2004 . Marked recent increase of scaled chrysophytes in boreal lakes: implications for management of taste and odour events . Freshwater Biol. , 49 : 199 – 207 .
- Patrick , R. and Reimer , C. W. 1966 . The Diatoms of the United States Vol. 1. Monogr. Acad. Nat. Sci. Phila. 13
- Patrick , R. and Reimer , C. W. 1975 . The Diatoms of the United States Vol. 2, Part 1. Monogr. Acad. Nat. Sci. Phila. 13
- Ramstack , J. M. , Fritz , S. C. , Engstrom , D. R. and Heiskary , S. A. 2003 . The application of a diatom-based transfer function to evaluate regional water-quality trends in Minnesota since 1970 . J. Paleolimn. , 29 : 79 – 94 .
- Rashash , D. M. , Dietrich , C. A. M. , Hoehn , R. C. and Parker , B. C. 1995 . The influence of growth conditions on odor-compound production by two crysophytes and two cyanobacteria . Water Sci. Tech. , 31 ( 11 ) : 165 – 172 .
- Raven , J. A. 1995 . “ Comparative aspects of Chrysophyte nutrition with emphasis on phosphorus and carbon ” . In Chrysophyte algae ecology, phylogeny and development , Edited by: Sandgren , C. D. , Smol , J. P. and Kristiansen , J. 95 – 118 . Cambridge , , UK : Cambridge University Press .
- Reavie , E. D. and Smol , J. P. 2001 . Diatom-environmental relationships in 64 alkaline southeastern Ontario (Canada) lakes: a diatom based model for water quality reconstruction . J. Paleolimn. , 25 : 25 – 42 .
- Roijackers , R. M. and Kessels , H. 1986 . Ecological characteristics of scale- bearing Chrysophyceae from The Netherlands . Nord. J. Bot. , 6 ( 3 ) : 373 – 385 .
- Saxby-Rouen , K. J. , Leadbeater , B. S. C. and Reynolds , C. S. 1998 . The relationship between the growth of Synura petersenii (Syanurophyceae) and the components of the dissolved inorganic carbon system . Phycologia , 37 : 467 – 477 .
- Sandgren , C. D. 1988 . “ The ecology of chrysophyte flagellates: their growth and perennation strategies as freshwater plankton ” . In Growth and reproductive strategies of freshwater phytoplankton , Edited by: Sandgren , C. D. 9 – 104 . Cambridge , , UK : Cambridge University Press .
- Sandgren , C. D. , Hall , S. A. and Barlow , S. B. 1996 . Siliceous scale production in chrysophyte and synurophyte algae, I. Effects of silica limited growth on cell silica content, scale morphology, and construction of the scale layer of Synura petersenii . J. Phycol. , 32 : 675 – 692 .
- Siver , P. A. 1987 . The distribution and variation of Synura species (Chrysophyceae) in Connecticut, USA . Nord. J. Bot. , 66 : 1391 – 1403 .
- Siver , P. A. 1995 . “ The distribution of chrysophytes along environmental gradients: their use as biological indicators ” . In Chrysophyte Algae: Ecology, Physiology and Development , Edited by: Sandgren , C. D. , Smol , J. P. and Kristiansen , J. 232 – 268 . Cambridge , , UK : Cambridge University Press .
- Siver , P. A. 2003 . “ Synurophyte algae ” . In Freshwater algae of North America ecology and classification , Edited by: Wehr , J. D. and Sheath , R. G. 523 – 557 . Academic Press .
- Siver , P. A. and Hammer , J. S. 1989 . Multivariate statistical analysis of the factors controlling the distribution of scaled chrysophytes . Limnol. Oceanogr. , 34 : 368 – 381 .
- Stoermer , E. F. , Kreis , R. G. Jr. and Andressen , N. A. 1999 . Checklist of diatoms from the Laurentian Great Lakes. II . J. Great Lakes Res. , 25 ( 3 ) : 515 – 566 .
- Suffet , I. H. , Khiari , D. and Bruchet , A. 1999 . The drinking water taste and odor wheel for the millennium: beyond geosmin and 2-methylisoborneol . Water Sci. Tech. , 40 ( 6 ) : 1 – 13 .
- Sung , W. 1990 . Taste and odor episodes at Wachusett Reservoir . J. New England Water Assn. , 105 ( 3 ) : 165 – 178 .
- Takahashi , E. 1978 . Electron microscopical studies of the Synuraceae (Chrysophyceae) in Japan: taxonomy and ecology , Tokyo : Tokai University Press .
- [NASS] United States Department of Agriculture National Agricultural Statistics Service . 2006 . http://www.nass.usda.gov/ Accessed 2006
- Watson , S. B. , Satchwill , T. , Dixon , E. and McCauley , E. 2001 . Under-ice blooms and source-water odour in a nutrient-poor reservoir: biological, ecological and applied perspectives . Freshwater Biol. , 46 : 1553 – 1567 .
- Wee , J. S. , Harris , S. , Smith , J. , Dionigi , C. and Millie , D. F. 1994 . Production of the taste/odor compound trans-2, cis-6-nonadienal within the Synurophyceae . J. Appl. Phycol. , 6 : 365 – 369 .
- Wee , J. S. , Millie , D. F. and Walton , S. P. 1991 . A statistical comparison of growth among clones of Synura petersenii (Synurophyceae) . J. Phycol. , 27 : 570 – 575 .
- Wetzel , R. G. 1983 . Limnology , Philadelphia , PA : Saunders College Publishing .
- Young , W. F. , Horth , H. , Crane , R. , Ogden , T. and Arnott , M. 1996 . Taste and odor threshold concentrations of potential potable water contaminants . Water Res. , 30 : 331 – 340 .
- Zeeb , B. A. , Christie , C. E. , Smol , J. P. , Findlay , D. L. , Kling , H. J. and Birks , H. J. B. 1994 . Responses of diatoms and chrysophyte assemblages in Lake 227 sediments to experimental eutrophication . Can. J. Fish. Aquat. Sci. , 51 : 2300 – 2311 .