Abstract
Tenkiller Reservoir became eutrophic between 1975 and 1986, primarily due to poultry litter practices in the watershed, resulting in rapid dissolved oxygen (DO) depletion in the meta- and hypolimnion. Depletion was driven largely by high riverine-zone algal production due to high phosphorus inflow and internal loading, and the subsequent plunging of riverine water to the meta- and hypolimnion. This process of DO depletion greatly reduced summer habitat for cool-water fish species. Optimal and suboptimal habitat for smallmouth bass (SMB) growth averaged only 10 and 25% of total reservoir volume, respectively, during mid-June to mid-September in 2005 and 2006. For walleye (WAL), acceptable (suboptimal) habitat was essentially absent (<1%) due to its lower temperature criterion. By contrast, habitat for both species was much greater in oligo-mesotrophic Broken Bow, a physically similar reference reservoir. Long-term catch rates for SMB and WAL during systematic surveys averaged 3-fold higher in Broken Bow, with greater acceptable DO–temperature habitat than in Tenkiller. Conversely, catch rates for largemouth bass, a warm-water species preferring eutrophic conditions, averaged 2-fold higher in Tenkiller than Broken Bow. These catch rate differences are consistent with the DO–temperature conditions available during the stratified period and the trophic states in these 2 reservoirs. Recovery of cool-water fish habitat in Tenkiller by ceasing poultry litter application is predicted to be slow.
[Supplemental materials are available for this article. Go to the publisher's online edition of Lake and Reservoir Management to view the supplemental file.]
Key words:
Eutrophication is well documented for lakes, and causative nutrients have usually come from point sources (Sas et al. Citation1989, Cooke et al. 2005). There are few published cases of long-term eutrophication of reservoirs and none on the importance of riverine zone internal phosphorus (P) loading. While the dissolved oxygen (DO)–temperature “squeeze” effect on fish has been well described in reservoirs, the acceptable DO and temperature habitat available to sensitive fish (and its effects) has not been quantitatively tied to eutrophication in the published literature. We herein describe the effects of diminished habitat, due largely to a long-term increase in nonpoint P leading to eutrophication, by comparing available fish habitat and catch rate in 2 physically similar reservoirs with markedly different trophic states.
The low DO effect in reservoirs that adversely squeezes cool-water fish between the too-warm epilimnion with adequate DO and the cooler meta- and hypolimnion without adequate DO is well documented for striped bass (SB; Coutant Citation1985, 1987, Zale et al. Citation1990, Young and Isely Citation2002). Adverse effects of squeeze have also been observed on growth of crappie, considered a warm-water species, (Gebhart and Summerfelt Citation1978, Hale Citation1999). Similar effects would be expected with such cool-water species as walleye (WAL), which has similar temperature requirements as SB, as well as smallmouth bass (SMB), which is slightly more temperature tolerant but also is known to prefer more oligotrophic conditions (Haines Citation1973, Buynak et al. Citation1991, Ludsin et al. Citation2001, Welch Citation2009).
Depleted DO in a large fraction of total volume during the stratified period has been described in midcontinent reservoirs (Jones et al. Citation2011). Depletion may be more pronounced in reservoirs than lakes due to higher rates of water inflow and nutrient loading, which result from higher watershed-to-reservoir area ratios, producing shorter water residence times (Thornton et al. Citation1990). That effect of greater depletion assumes that higher loading also results in higher nutrient inflow concentration, which actually determines trophic state. Also, plunging of enriched inflows, which is usually more pronounced in reservoirs due to their relatively higher inflows, contributes to DO depletion that is often more rapid in the metalimnion than hypolimnion (Thornton et al. Citation1990). Therefore, depletion of metalimnetic DO in reservoirs can result in less acceptable DO–temperature habitat than in lakes, where depletion is usually slower and confined to the hypolimnion.
Increased eutrophication of Tenkiller Reservoir in northeast Oklahoma has resulted in an early summer, rapid depletion of meta- and hypolimnetic DO, which severely restricts the acceptable DO–temperature habitat for cool-water fish. Total phosphorus (TP) loading has more than doubled since dam closure in 1953, such that average annual volume-weighted inflow TP has reached 210 μg/L and 171 μg/L during spring and summer, respectively. That increase is due largely to nonpoint sources, primarily from poultry litter disposal practices (Cooke et al. 2011).
The acceptable DO–temperature habitat available in eutrophic Tenkiller Reservoir is contrasted with that in oligo-mesotrophic Broken Bow, a reference reservoir in southeastern Oklahoma, which is physically similar but has a much lower spring–summer inflow TP of 29 μg/L (Cooke et al. 2011). This comparison illustrates that eutrophication of Tenkiller is the likely cause for the difference in DO–temperature habitat available in the 2 reservoirs. Long-term catch rates for 2 cool-water species, native and introduced SMB and introduced WAL, as well as for warm-water largemouth bass (LMB), are in turn related to DO–temperature habitat.
Site description
The dam for Tenkiller Reservoir closed in 1953 resulting in a 40 km long impoundment with a surface area of 51.6 km2 and mean and maximum depths of 15.5 and 46 m, respectively. The dam for Broken Bow Reservoir closed in 1970 forming a 34 km long water body with a surface area of 56.8 km2 and mean and maximum depths of 19.7 and 50 m, respectively. Tenkiller and Broken Bow have similar basin shapes with respect to percent volumes below 6 m (86 and 88%, respectively) and in the hypolimnion (40 and 44%, respectively). However, watershed-to-reservoir surface area ratios differ, with 80:1 for Tenkiller and 34:1 for Broken Bow. This difference produces a shorter water residence time in Tenkiller (0.7 yr) than in Broken Bow (1.7 yr). Importantly, they are both in ecoregions with streams containing low background TP concentrations of 16–20 μg/L (Omernik Citation1977, McDowell and Omernik Citation1979). Those inflows should result in 7–9 and 11–13 μg/L whole-reservoir concentrations in Broken Bow and Tenkiller, respectively, considering TP loss due to the difference in water residence times; therefore, they should be naturally oligotrophic or meso-oligotrophic.
Cool-water fish DO-temperature criteria
Low DO is the principal environmental factor that adversely affects reservoir fish species that are intolerant of eutrophication. The effect results from decreased meta- and hypolimnetic DO, along with increasing epilimnetic temperature, which effectively squeezes the habitat volume available for growth, and ultimately survival, of cool-water sport fish. The squeeze effect has been observed for striped bass and crappie, as indicated earlier.
Native sport fish in Tenkiller that are most likely intolerant to this effect of eutrophication are primarily SMB, although temperature–DO criteria are similar for spotted bass and channel catfish (USFWS 1982a, 1983, 1984a). For SMB, a cool-water species emphasized here, the maximum temperature criterion for optimal growth is 27 C, while 29 C is generally recognized as the maximum of its preferred range (Fry Citation1947, Horning and Pearson Citation1973, MacClean et al. Citation1981, USFWS 1983). Fish are known to prefer temperatures that are optimal for growth and activity and avoid higher temperatures (Beitinger and Fitzpatrick Citation1979, Headrick and Carline Citation1993). Even lower temperature criteria were recommended for successful introduction of SMB to Kentucky reservoirs: 21–23 C as acceptable and 20–21 C as ideal (Buynak et al. Citation1991). For LMB, the maximum temperature for optimal growth is 30 C (USFWS 1982b).
Striped bass and WAL are also cool-water species that are intolerant of DO–temperature effects of eutrophication (Leach et al. Citation1977, Coutant Citation1985, 1987). Walleye were introduced in large numbers in Tenkiller and Broken Bow reservoirs, as were SB in Tenkiller. Striped bass and WAL avoid temperatures above 24 C, preferring 21–22 C (USFWS 1984b, 1984c). The maximum temperature acceptable for WAL is 24 C; above that growth stops (Kitchell and Stewart Citation1977, Hurley Citation1986a). Therefore, epilimnetic temperature may restrict SB and WAL even more than SMB.
Minimum DO criteria for optimal and suboptimal growth, respectively, for SMB, WAL, and SB are 6 and 5 mg/L (USFWS 1983, 1984b, 1984c). While 5 mg/L is considered suboptimal for these species, it will allow some growth, and survival is possible down to 2–3 mg/L (Welch and Jacoby Citation2004). Even LMB, which are considered more tolerant of eutrophication than SMB, showed decreased food consumption and growth at DOs less than saturation (9.2 mg/L at 20 C; Stewart et al. Citation1967). Similar to the response to temperature, warm- and cool-water species alike avoided low DO and selected available cool waters nearer 5 mg/L (Headreck and Carline 1993, Burleson et al. Citation2001).
Methods and materials
Tenkiller Reservoir was sampled at 4 sites established with respect to functional zones (Thornton et al. Citation1990); riverine (LK04), transition (LK03); and lacustrine (LK01 and LK02; see Cooke et al. Citation2011 for map and locations). The lacustrine zone is largest, representing about 65% of the reservoir area, while the transition and riverine are 22 and 13%, respectively. Volumes are about 70% for lacustrine and 22 and 8% for transition and riverine zones, respectively.
Sampling was conducted by personnel from Camp Dresser and McKee (CDM) twice monthly from May to November in 2005, March to September in 2006, and monthly during June to September in 2007 at each of the 4 sites. A multiparameter Hydro-Lab with a datasonde unit was used to determine DO, temperature, and specific conductance at 1 m intervals with depth at the cross-sectional midpoint at the 4 sites. Maximum water column depths sampled at the 4 sites, LK01 to LK04, were usually 26, 24, 7, and 6 m. Air calibration for DO was routinely checked with Winkler titrations. These data were used to determine available DO–temperature habitat. In addition, 5 profiles of DO–temperature, distributed over the reservoir cross-section, were determined at LK01 on 10 August 2005 to evaluate the representativeness of midreservoir DO–temperature profiles.
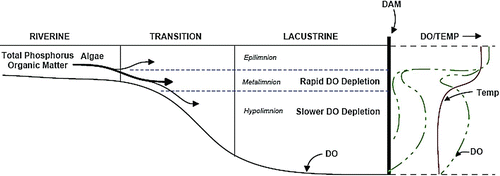
Figure 1 Longitudinal cross-section schematic of the zones and directional pattern of inflow and DO depletion in Tenkiller Reservoir. Riverine and transition zones actually represent 13 and 22% of reservoir area but are exaggerated to indicate their importance to inflow direction. High density inflows preferentially enter the metalimnion in early summer, depleting DO faster in the metalimnion, indicated by DO profiles progressing from right to left with time (color figure available online).
Reservoir volumes available for SMB and WAL were calculated separately for each delineated sampling section (LK01 to LK04; Cooke et al. 2011) of Tenkiller and then summed. Calculations were based on volume–depth relationships computed for 1 m intervals and twice monthly DO– temperature profiles with recorded values at 1 m intervals. Volumes were adjusted for each sample time because reservoir elevation varied.
DO–temperature profile data from 3 sites (BB01, 03, 06) in Broken Bow Reservoir were collected by the Oklahoma Water Resources Board (OWRB) at various times during the stratified period from 1997 to 2006 (see , Cooke et al. Citation2011). September data were missing, so to determine available habitat volume, as was done for Tenkiller, a DO–temperature profile was interpolated to lie equidistant between those for August and October.
Fish-catch data were obtained from the Oklahoma Department of Wildlife Conservation (ODWC), which used standardized sampling procedures to compare the quality of fisheries among reservoirs. Electrofishing surveys were conducted systematically for SMB and LMB during the spring at regular prime habitat sites to maximize catch. Gill nets were used at regular sites for WAL. Net size was 200 ft long by 6 ft deep with mesh size ranging from 0.5 to 3 inches. Catch per unit effort rates (c/f) were reported in no./hr. Catch rate differences between Tenkiller and Broken Bow Reservoirs were analyzed by 2 sample t-test for WAL and LMB and the Mann-Whitney nonparametric test for SMB data, which were not normally distributed.
Results
DO depletion and inflow direction
The high TP internal loading rate in the shallow, unstratified riverine zone (LK04) during 2005 and 2006 was fully available to algae and was at least half the cause of the high summer average chlorophyll (chl) concentration (26.5 μg/L, 11 summers). Net internal loading was indicated by the near doubling of riverine zone TP over inflow TP concentration (Cooke et al. 2011). Furthermore, the plunging of riverine zone water, containing the large mass of algae, into the meta- and hypolimnion was instrumental in driving the extensive rate of DO depletion in those layers (). Much of the denser riverine water entered the metalimnion as an interflow in late spring and early summer, causing more rapid DO depletion there than in the hypolimnion (). Denser riverine water entered the hypolimnion as flow decreased in late summer. Metalimnetic DO was essentially depleted between 8 and 12 m by the end of June 2005, while the hypolimnion was nearly depleted by the end of July, indicating a greater depletion rate in the metalimnion (). The pattern was similar in 2006, except that near depletion by the end of June was between 10 and 13 m instead of 8 and 12 m, a difference attributed to higher river flow in 2005.
The rapid metalimnetic DO depletion was largely caused by transport of respiring and decomposing algae from the riverine zone rather than from inflowing organic matter from the river or from settling algae from the lacustrine epilimnion. The depth interval of DO depletion was sufficiently below the lighted zone, so there was no contribution of DO from photosynthesis at 2–4 m (supersaturated DO; ). Settling of algae from the lacustrine epilimnion was probably less important than transport from the riverine zone because lacustrine chl averaged 9 μg/L and riverine 28 μg/L during summer 2005 and 2006 (Cooke et al. 2011). River inflow also contributed some organic matter, but total organic carbon averaged higher in the riverine zone (2.5–3.0 μg/L) than in the river (1.75 mg/L) during spring–summer 2005 and 2006.
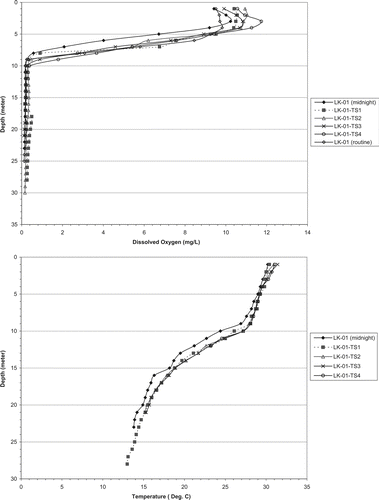
Figure 2 DO and temperature profiles at lacustrine station LK01 in Tenkiller Reservoir during spring–summer 2005. See map in Cooke et al. 2001, for location.
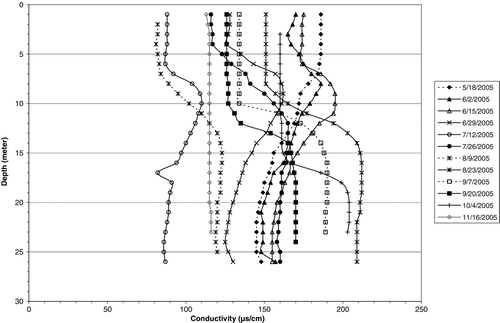
Inflow was directed primarily into the lacustrine metalimnion during late spring–early summer. Specific conductance, used as a tracer, showed a “bulge” of slightly higher values between 6 and 12 m during June 2005 (). That conductance bulge averaged 180 μS/cm, while higher values occurred toward the bottom of the transition zone, and river values were still higher (). Interflow conductance decreased with dilution through the reservoir. The pathway of higher density water was probably deeper than sampled profiles because maximum depth in the cross-section of LK03 ranged from 14 to 17 m during the period versus the sampling site depth of 6–7 m.
The pattern was similar in 2006, but conductance was much higher due to about one-third as much inflow during the spring (). Nevertheless, there was a consistent gradient down reservoir as inflow plunged into the lacustrine metalimnion, although to a slightly greater depth interval (8–14 m) than in 2005 (). Riverine zone water was directed to the lacustrine hypolimnion in late summer, as indicated by the highest conductance occurring in August at 16–19 m in 2005 and 12–18 m in 2006 (; ).
Table 1 Mean conductance, in μS/cm at 25 C, in the river, riverine (LK04), transition LK03, and lacustrine (LK02, 01) zones during 2005–2006 for the depth intervals indicated. Sample size (LK03) indicates the number of dates for which individual means were computed from the consecutive 1 m observations over the depth intervals indicated.
Figure 3 Profiles of specific conductance at lacustrine station Lk01 in Tenkiller Reservoir during spring–summer 2005.
The 5 cross-sectional profiles at LK01 on 10 August 2005 showed that temperature and DO profiles were horizontally consistent across the reservoir (). Temperature ranged only 1–2 C among profiles throughout the water column, and most of that variation was in the metalimnion. The 1–2 C represents a depth interval difference of <1 m. Epilimnetic depth was consistently at 8 m. The range in DO was about 2 mg/L in the epilimnion, and the depth below which DO was minimum (<0.5 mg/L) ranged from 8 to 10 m. The average depth interval represented by a given DO was <1 m through the metalimnion, similar to temperature. Thus, profiles at the regular sampling stations were considered to represent their respective whole-zone volumes with a relatively small error (<2%) considering the large range in calculated available DO–temperature habitat through the season. This means that stress from low DO would force cool-water species into the epilimnion where temperatures were near or exceeded 30 C during July and August throughout the reservoir ().
Available DO-temperature habitat
Available habitat for cool-water fish in Tenkiller is shown more clearly by volume than by inspecting DO–temperature profiles (). For SMB, there was little or no volume with optimal (>6 mg/L, < 27 C) habitat available during most of July–August and no suboptimal (>5 mg/L, <29 C) habitat for one-half to one month. From mid-June to mid-September, optimal habitat averaged only 10% of reservoir volume and suboptimal only 25% in 2005 and 2006. The less frequent data in 2007 did not allow a comparable volume estimate.
Even suboptimal habitat was essentially absent during most of the stratified period for WAL and SB (). The average volume meeting those DO–temperature criteria for mid-June to mid-September in 2005 and 2006 was <1% of the total. For these species, lethal temperatures of 29 and 28 C were exceeded in surface waters (30–31 C; and 4), so stresses on their growth and survival were even more severe than for SMB.
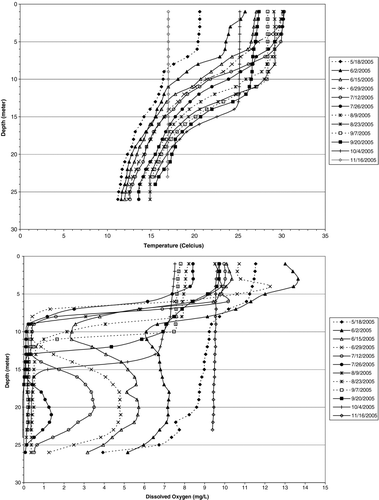
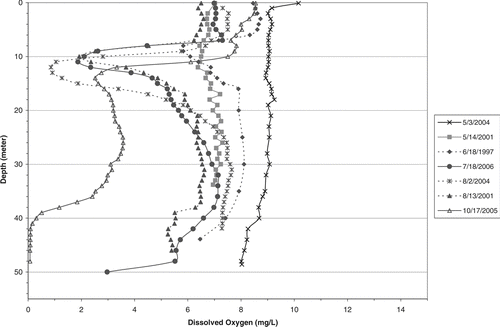
In contrast to Tenkiller, DO concentrations in Broken Bow remained above 4 mg/L in the hypolimnion for most of the summer, offering a refuge for cool-water species below about 15 m (). However, DOs were low, between 10 and 15 m, showing a similar pattern of metalimnetic DO depletion as in Tenkiller. While these DO profiles were from different years, all months were represented except September, and DO was <2 mg/L in mid-October, in contrast to Tenkiller (). Comparing profiles for July and August, DO below 8 m in Tenkiller averaged <1 mg/L, but >4.5 mg/L in Broken Bow.
Figure 5 Habitat volume available for 3 DO–temperature criteria in Tenkiller during 2005, 2006, and 2007 (data limited in 2007). (A) Optimal (>6 mg/L, <27 C) and (B) suboptimal (>5 mg/L, <29 C) for SMB and (C) suboptimal (>5 mg/L, <24 C) for WAL and SB.
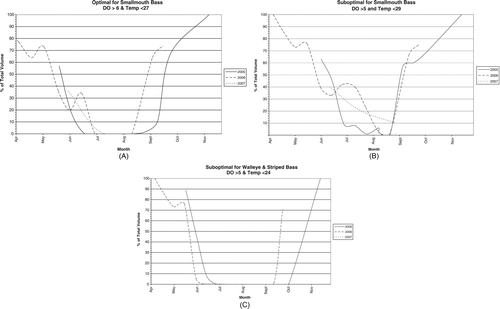
Figure 6 DO profiles at lacustrine station BBL01 in Broken Bow Reservoir for the indicated years with data from OWRB. See map in Cooke et al. 2011 for station location.
This contrast in DO between the 2 reservoirs is illustrated by the calculated average habitat volume available in Broken Bow. Volumes meeting optimal and suboptimal criteria for SMB were 14 and 49%, respectively, and 32% met suboptimal criteria for WAL. From the standpoint of DO and temperature, Broken Bow offered a larger suitable habitat volume than Tenkiller, consistent with their oligo–meso- and meso-eutrophic states, respectively.
Long-term fish catch data
Smallmouth bass known as Neosho and Quachita, were native to northeastern Oklahoma tributaries of Tenkiller and Broken Bow reservoirs, respectively, prior to dam closures (Moore and Paden Citation1950, Hall Citation1952, 1953, Hubbs and Lagler Citation2004, Miller and Robinson Citation2004) and continued to populate the reservoirs following dam closures, prior to stocking (Eley et al. Citation1971). A creel census in 1974–1975 (prior to stocking) reported 2930 SMB caught in Tenkiller and 1489 in Broken Bow (Summers Citation1978). Although catch rates were low (0.006 and 0.008/hr, respectively), they were similar for SMB in 4 other Oklahoma reservoirs. More than 100,000 LMB were caught in each reservoir in 1974–1975 at respective rates of 0.24 and 0.61/hr (Summers Citation1978).
Table 2 Stocking history in thousands for Tenkiller and Broken Bow Reservoirs (P. Balkenbusch, ODWC, 2008, pers. comm.).
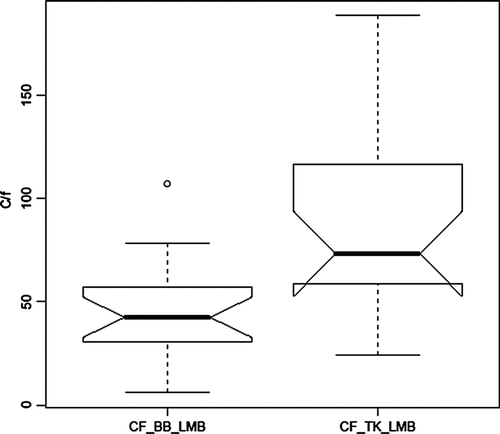
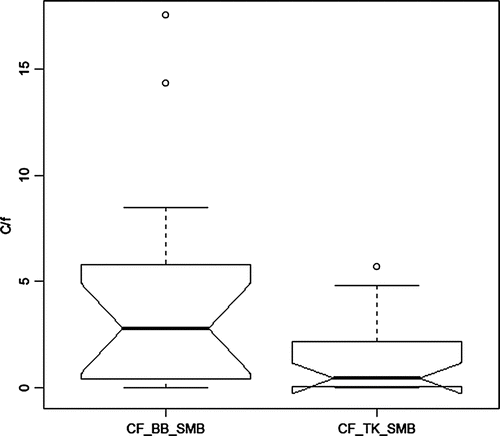
Considerable effort was expended by ODWC to enhance SMB fisheries by stocking “lake-strain” fry from Tennessee into Tenkiller in the early 1990s and from National hatcheries into Broken Bow during the mid-1970s to mid-1980s (). Despite this effort, catch rates in Tenkiller by systematic electrofishing surveys remained low, averaging 1.4/hr, well below the quality fishery level established by ODWC of 10/hr (). Catch rates in Broken Bow have averaged 3-fold higher at 4.4/hr and have approached or exceeded the quality rate in a couple years (). The catch rate difference between the 2 reservoirs is significant (p = 0.04).
Figure 7 Box plots showing median, quartiles, minimum and maximum, and outliers (circle) for catch rates per hour (c/f) of SMB by electrofishing in Broken Bow (BB) and Tenkiller (TK) Reservoirs during 1977–2006; N = 16 (BB) and 22 (TK).
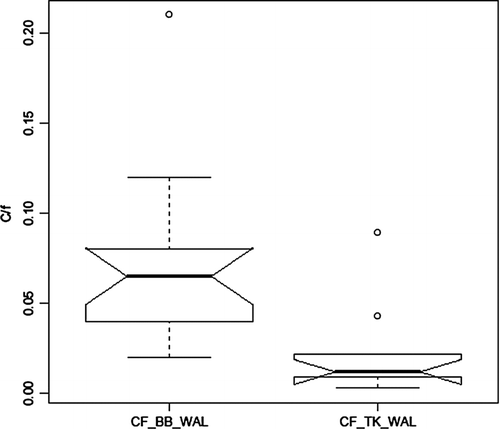
Efforts to establish WAL fisheries in the 2 reservoirs involved stocking millions over many years, with more in Tenkiller than Broken Bow (). Despite the larger and more widespread stocking, WAL catch rate by gill net has remained very low (0.02/hr), and they are considered uncommon in Tenkiller. In contrast, WAL catch in Broken Bow has averaged 0.07/hr, consistently near and sometimes above the quality fishery level of 0.1/hr and 3.5 times greater than in Tenkiller (). The difference in catch rate is highly significant (p = 0.005).
Figure 8 Catch rates per hour (c/f) of WAL by gillnet in Broken Bow (BB) and Tenkiller (TK) Reservoirs during 1981–2006; N = 16 (BB) and 9 (TK).
SB were stocked in Tenkiller in the 1970s and 1980s (), but this effort failed to establish a fishery, most likely due to the inadequate DO–temperature habitat. Striped bass were not stocked in Broken Bow.
Results were opposite for LMB, a warm-water species that, in contrast to SMB, thrives in eutrophic waters (Greene and Maceina Citation2000, Maceina and Bayne Citation2001). Largemouth bass were stocked in both Tenkiller and Broken Bow over many years (). As expected, given the trophic preference of LMB, electrofishing catch rate in Tenkiller has averaged 88/hr, double that in Broken Bow (44/hr) and more than double the ODWC quality fishery level (40/hr; ). The higher catch rate in Tenkiller is despite much greater stocking in Broken Bow (), and the difference in catch rate is highly significant (p = 0.001).
Discussion
DO depletion and inflow direction
Metalimnetic DO depletion, driven by density interflows, is common in reservoirs (Thornton et al. Citation1990) and contributes to higher aerial hypolimnetic oxygen deficits (AHOD) than in lakes (Cooke et al. 2011). Despite greater watershed area and thus greater TP loading in reservoirs, AHOD is directly related to TP concentration, as it is in lakes (Nürnberg Citation1996). This is evidenced by the more than 2-fold higher AHOD in Tenkiller than in Broken Bow and their corresponding differences in inflow TP concentration and trophic state. Moreover, algal production in the riverine and transition zones of Tenkiller was partly caused by net internal P loading, a process heretofore largely ignored in reservoir literature. While organic matter in the river inflow and lacustrine autochthonous production may have contributed to DO depletion, the high riverine chl and higher reservoir-than-inflow organic carbon concentrations indicate that the down-reservoir DO depletion was largely caused by riverine and transition zone production.
Eutrophication effect on cool water fish
While production and biomass of fish generally increase with eutrophication (Leach et al. Citation1977, Oglesby Citation1977, Jones and Hoyer Citation1982, Downing et al. Citation1990) the structure of fish communities changes. Smallmouth bass is an important cool-water sport fish considered intolerant of eutrophication, due largely to decreased DO and transparency (Haines Citation1973, Buynak et al. Citation1991). Their responses have been likened to SB and trout when deprived of suitably oxygenated water at preferred temperatures (Buynak et al. Citation1991).
As evidence of SMB intolerance of eutrophic waters, their populations have increased in response to decreased phosphorus and subsequent oligotrophication in Lake Erie (Ludsin et al. Citation2001); Bay of Quinte, Ontario (Hurley Citation1986b); and Moses Lake, Washington (Welch Citation2009). They are known to prefer hard, rocky, gravelly substrata and drop off areas in clear cool lakes (Pflug and Pauley Citation1984, Hubbs and Lagler Citation2004), and the oligotrophic areas of reservoirs (Buynak et al. Citation1991; J. Lott, SD Game, Fish and Parks, 2009, pers. comm.). Even in a lake and reservoir where temperatures in surface waters were cool and not stressful, SMB sought out deeper strata during summer stratification (Pflug and Pauley Citation1984, Lott Citation2000).
The lower catch rate of SMB in Tenkiller than in Broken Bow is therefore expected based on the difference in reservoir trophic state. The persistence of no or very low DO in the cooler meta- and hypolimnion during most of the summer greatly compromised the available habitat acceptable to SMB. Optimal (<27 C and >6 mg/L DO) conditions for SMB were largely absent during the summer in meso-eutrophic Tenkiller. Even suboptimal conditions (<29 C and >5 mg/L DO) were highly restrictive, forcing fish to retreat to epilimnetic water with adequate DO but higher than preferred temperature. While the lethal temperature (32 C) was not exceeded, little or no growth would be expected at above-preferred temperatures (Beitinger and Fitzpatrick Citation1979, Hale Citation1999). Even if a small portion of reservoir volume were suitable, crowding and food limitation could result. In contrast, DO–temperature conditions in oligo-mesotrophic Broken Bow were much less restrictive.
The greater and earlier stocking of SMB in Broken Bow than in Tenkiller () does not explain the higher catch rates in Broken Bow. More WAL were stocked in Tenkiller and more LMB in Broken Bow, yet catch rates for WAL were greater in Broken Bow and rates for LMB were greater in Tenkiller. Further, native SMB were established in both reservoirs before any stocking, and DNA evidence in 2003 showed that the native strain still dominated (59%) in Broken Bow while fish in Tenkiller were 85–90% nonnative (G. Gilliland, ODWC, 2003, pers. comm.). Therefore, habitat best explains the greater catch rate and persistence of native fish in Broken Bow.
Little or no habitat was available in Tenkiller to allow minimum growth of WAL during the summer period, and they were even more restricted in recent years than were SMB. In contrast, much more favorable habitat was available in Broken Bow. The much greater catch rate in Broken Bow than in Tenkiller is expected based on the reservoir's respective differences in trophic state and related DO–temperature conditions.
Acceptable DO–temperature conditions for WAL were considered present in Tenkiller in 1960 (Summers Citation1961). DO data show an AHOD rate for Tenkiller in 1960 quite similar to that in Broken Bow in recent years (Cooke et al. 2011). The acceptable summer DO–temperature regime observed in 1960 apparently prompted the large stocking effort through the 1990s to establish a fishery in Tenkiller. Summers (Citation1961) concluded that “The limnological investigations did not indicate any chemical or physical limitations that would be undesirable to the introduction of walleye in 1961. On the contrary, the reservoir has a very high productive potential and the introduction of this species should prove successful.”
The WAL population in Broken Bow is considered self-sustaining, while stocking to establish a fishery in Tenkiller has ceased (L. Bolton, Chief of Fisheries, ODWC, 2008, pers. comm.). The success of WAL in Broken Bow but not in Tenkiller is consistent with the contrast between the 2 reservoirs in trophic state and the acceptable DO– temperature habitat available during summer for this cool-water species.
Both SMB and WAL have declined with eutrophication elsewhere and have recovered with oligotrophication from reduced P loading (Hurley, Citation1986b, Ludsin et al. Citation2001, Culver et al. Citation2009, Welch Citation2009). Part of the adverse effect of eutrophication on WAL and SMB may be due to increased sedimentation and decreased transparency. Sedimentation on rocky, gravelly spawning areas adversely affected WAL in several lakes (Leach et al. Citation1977), and decreased turbidity and sedimentation were important to SMB recovery in Lake Erie (Ludsin et al. Citation2001). Although transparency averaged less in Tenkiller (2.2 m) than in Broken Bow (3 m) during summer, those values were within the mesotrophic range (2–4 m) found favorable to WAL (Schupp and Wilson Citation1993, Hiskary and Wilson 2008). While the extent of sedimentation on rocky, gravely lacustrine substrata in these reservoirs is unknown, the transparency differences that could affect sight feeding by WAL and SMB is likely unimportant, because the fish were excluded (due to DO) from depths below 8–10 m, during most of the summer stratified period in Tenkiller when light reduction at depth would have had any effect. At those transparencies, depths to 1% light averaged 7.3 m in Broken Bow and 5.4 m in Tenkiller, assuming transparency depths were 15% of surface intensity.
The little or no favorable DO–temperature habitat during summer in Tenkiller, a primary cause for the poor success of WAL, is also consistent with the failure of SB, which has the same requirements as WAL. Poor growth and survival of SB in stratified reservoirs, with limiting DO–temperature habitat, is well documented (Coutant Citation1985, 1987, Matthews et al. Citation1989, Zale et al. Citation1990, Young and Isely Citation2002).
The much higher catch rates for LMB in Tenkiller than in Broken Bow further support the hypothesis that eutrophication is the cause of the SMB and WAL response in the 2 reservoirs. Largemouth bass is a warm-water species that tolerates higher epilimnetic temperatures during summer (USFWS 1982b) and thus would not suffer from a DO–temperature squeeze. They are said to prefer “weedy or brushy mud bottom lakes and ponds” (Hubbs and Lagler Citation2004). They were observed to thrive in mildly eutrophic waters (Greene and Maceina Citation2000, Maceina and Bayne Citation2001) and select shallow water (<1–3 m) with vegetation or large woody debris, as shown by radio tracking (Sammons and Maceina Citation2005). That pattern of habitat selection is opposite to that of SMB, which seek deeper, cooler, nonweedy depths (Buynak et al. Citation1991; J. Lott, South Dakota Game Fish & Parks, 2000, pers. comm.). That probably accounts in large part for the respective responses of these cool- and warm-water species to the eutrophication of Tenkiller Reservoir.
The increasing eutrophication of Tenkiller, due primarily to poultry litter practices, has greatly reduced the acceptable DO–temperature habitat for cool-water fish. That conclusion is supported by the contrast with DO–temperature conditions in oligo-mesotrophic Broken Bow Reservoir, the catch-rate differences between the 2 reservoirs for cool-water and warm-water fish, and the response of these fishes to eutrophication and oligotrophication in other water bodies. These results have tied long-term eutrophication to habitat loss and low catch rate for fish sensitive to eutrophication.
Table 3 Mean outlet DO concentration from mid-May to mid-October and mean available DO–temperature habitat available for SMB and WAL (optimal = O and suboptimal = SO) during mid-June to mid-September following cessation of litter application resulting in a 40% reduction in external and 55% reduction in internal TP loading. Results predicted using CE-QUAL-W2 with 200 cross sectional segments (Wells et al. 2008; Appendix A).
To markedly increase the available DO–temperature habitat for cool-water fish will probably require hypolimnetic or possibly layer aeration as well as cessation of litter application to the watershed. A realistic goal should be to restore DO in Tenkiller to the 1960 level, similar to the current level in Broken Bow. AHODs in Tenkiller then and Broken Bow now are 0.6 and 0.5 g/m2 per day, respectively (Cooke et al. 2011). Cessation alone is not expected to increase hypolimnetic DOs in Tenkiller to levels from 1960 and currently in Broken Bow for more than 50 years according to model predictions (). That prediction is indicated by mean May–October outlet (at 37 m) DO increasing from 1.4 mg/L for the current 10-year base case TP loading to only 2.9 mg/L the last 10 years of a postcessation, 50-year period. Natural outlet DO, given a background 20 μg/L TP inflow concentration, would be 6.1 mg/L (). Cessation would provide only a slight increase in available habitat for cool-water fish from the base case prediction and that currently observed during June–September (). Habitat would remain well below natural levels and those currently existing in Broken Bow. The slow recovery is due to the expected slow decrease in TP loading, which in turn is due to continued leaching from supersaturated soil in the watershed and internal loading in the reservoir, despite a projected 40% and 55% reduction in external and internal loading, respectively.
Acknowledgments
The field and technical assistance supplied by CDM personnel, especially Brian Bennett, Ron French, Roger Olsen, and Drew Santini is greatly appreciated. Special thanks to Malena Foster for data acquisition and illustrations and Donie Jordan and Leah Serra for report preparation, all with CDM. Kari Kimura with Tetra Tech also helped with illustrations. Fishery data and advice provided by ODWC, especially Paul Balkenbusch, Larry Bolton, and Josh Johnson were critical to the study. Such standardized, regular monitoring of fish stocks is not only invaluable to fisheries management but often is useful for other environmental purposes, as demonstrated here. Many thanks to Todd King (CDM) and Dr. James Loftis (Colorado State University) for statistical analysis. The helpful advice and understanding of processes in the Illinois River Watershed contributed by Bernard Engel, Berton Fisher, and Scott Wells are also recognized.
References
- Beitinger , T L and Fitzpatrick , L C . 1979 . Physiological and ecological correlates of preferred temperature in fish . Am Zool. , 19 : 319 – 329 .
- Burleson , M L , Wilhelm , D R and Smatresk , N J . 2001 . The influence of fish size on the avoidance of hypoxia and oxygen selection by largemouth bass . J Fish Biol. , 59 : 1336 – 1349 .
- Buynak , G L , Kornman , L E , Surmont , A and Mitchell , B . 1991 . Evaluation of a smallmouth bass stocking program in a Kentucky Reservoir . Am J Fish Manage. , 11 : 293 – 297 .
- Cooke , G D , Welch , E B and Jones , J R . 2011 . Eutrophication of Tenkiller Reservoir, Oklahoma, from nonpoint agricultural runoff . Lake Reserv Manage. , 27 : 256 – 270 .
- Cooke , D C , Welch , E B , Peterson , S A and Nichols , S A . 2005 . Restoration and management of lakes and reservoirs . Boca Raton (FL): CRC Press. 3rd ed.
- Coutant , CC. 1985 . Striped bass, temperature, and dissolved oxygen: a speculative hypothesis for environmental risk . T Am Fish Soc. , 114 : 31 – 61 .
- Coutant , CC. 1987 . Poor reproductive success of striped bass from a reservoir with reduced summer habitat . T Am Fish Soc. , 116 : 154 – 160 .
- Culver , D A , Conroy , J D , Tyson , J T , Crane , V C and Zhang , H . 2009 . “Optimal” P loading in large lakes affects fish communities: Do you prefer walleye or yellow perch? . Verh Internat Verein Limnol. , 30 : 1070 – 1072 .
- Downing , J A , Plante , C and LaLonde , S . 1990 . Fish production correlated with primary productivity, not morphoedaphic index . Can J Fish Aquat Sci. , 47 : 1929 – 1936 .
- Eley , R , Randolf , J and Carroll , J . 1981 . A comparison of pre- and post- impoundment fish populations in the Mountain Fork River in southeastern Oklahoma . Proc OK Acad Sci. , 61 : 7 – 14 .
- Fry , FEJ. 1947 . Effects of the environment on animal activity. University Toronto Studies Biol Series No. 55 . Pub Ontario Fish Res Lab. No. , 68 : 1 – 62 .
- Gebhart , G E and Summerfelt , R C . 1978 . Seasonal growth rate of fishes in relation to conditions of lake stratification . Proc Okla Acad Sci. , 58 : 6 – 10 .
- Greene , J C and Maceina , M J . 2000 . Influence of trophic state on spotted bass and largemouth bass spawning time and age-0 population characteristics in Alabama reservoirs . N Am J Fish Manage. , 20 : 100 – 108 .
- Haines , TA. 1973 . Effects of nutrient enrichment and a rough-fish population (carp) on a game-fish population (smallmouth bass) . T Am Fish Soc. , 102 : 346 – 354 .
- Hale , RS. 1999 . Growth of white crappie in response to temperature and dissolved oxygen conditions in a Kentucky reservoir . N Am J Fish Manage. , 19 : 591 – 598 .
- Hall , GE. 1952 . Observations on the fishes of the Fort Gibson and Tenkiller Ferry Reservoir areas, 1952 . Proc Okla Dept Wildl Conserv. , : 52
- Hall , GE. 1953 . Preliminary observations on the presence of stream-inhabiting fishes in Tenkiller Reservoir, a new Oklahoma impoundment . Proc Okla Acad Sci. , 46 : 55 – 62 .
- Headrick , H R and Carline , R F . 1993 . Restricted summer habitat and growth of northern pike in two southern Ohio impoundments . T Am Fish Soc. , 122 : 228 – 236 .
- Heiskary , S A and Wilson , C B . 2008 . Minnesota's approach to lake nutrient criteria development . Lake Reserv Manage. , 24 : 282 – 297 .
- Horning , W B and Pearson , R E . 1973 . Growth temperature requirements and lower lethal temperatures for juvenile smallmouth bass (Micropterus dolomieu) . J Fish Res Board Can. , 30 : 1226 – 1230 .
- Hubbs , C L and Lagler , K F . 2004 . Fishes of the Great Lakes Region. Revised ed. , Ann Arbor , MI : Univ of Michigan Press .
- Hurley , DA. 1986a . “ Growth, diet, and food consumption of walleye (Stizostedion vitreum vitreum): An application of bioenergetics modeling to the Bay of Quinte, Lake Ontario, population ” . In Project Quinte: point source phosphorus control and ecosystem response in the Bay of Quinte , Edited by: Minn , C K , Hurley , D A and Nicnolls , K H . Vol. 86 , 224 – 236 . Lake Ontario : Can Spec Publ Fish Aquat Sci. .
- Hurley , DA. 1986b . “ Fish populations of the Bay of Quite, Lake Onterio, before and after phosphorus control ” . In Project Quinte: point-source phosphorus control and ecosystem response in the Bay of Quinte , Edited by: Minn , C K , Hurley , D A and Nicnolls , K H . Vol. 86 , 210 – 214 . L. Ontario : Can Spec Publ Fish Aquat Sci. .
- Jones , J R and Hoyer , M V . 1982 . Sportfish harvest predicted by summer chlorophyll-a concentration in midwestern lakes and reservoirs . T Am Fish Soc. , 111 : 176 – 179 .
- Jones , J R , Knowlton , M F , Obrecht , D V and Graham , J L . 2011 . Temperature and oxygen in Missouri reservoirs . Lake Reserv Manage. , 27 : 173 – 182 .
- Kitchell , J F and Stewart , D J . 1977 . Applications of a bioenergetics model to yellow perch (Perca flavescens) and walleye (Stizostidion vitreum) . Can J Fish Aquat Sci. , 34 : 1922 – 1935 .
- Leach , J H , Johnson , M G , Kelso , J RM , Hartmann , J , Numann , W and Entz , B . 1977 . Responses of percid fishes and their habitats to eutrophication . J Fish Res Board Can , 34 : 1964 – 1971 .
- Lott , J. 2000 . Smallmouth bass movement, habitat use and electrofishing susceptibility in lower Lake Oache, SD . South Dakota Dept Game Fish and Parks , Annual Rept 00–15
- Ludsin , S A , Kershner , M W , Blochsom , K A , Knight , R L and Stein , R A . 2001 . Life after death in Lake Erie: Nutrient controls drive fish species richness, rehabilitation . Ecol Appl. , 11 : 731 – 746 .
- MacClean , J A , Shuter , B J , Regier , H A and MacLeod , J C . 1981 . Temperature and year-class strength of smallmouth bass . Cons Int Explor Mer. , 178 : 30 – 40 .
- Maceina , M J and Bayne , D R . 2001 . Changes in the black bass community and fishery with oligotrophication in West Point Reservoir, Georgia . N Am J Fish Manage. , 21 : 745 – 755 .
- Matthews , W J , Hill , L G , Edds , D R and Gelwick , F P . 1989 . Influence of water quality and season on habitat use by striped bass in a large southwestern reservoir . T Am Fish Soc. , 118 : 243 – 250 .
- McDowell , T R and Omernik , J M . 1979 . Nonpoint source - stream nutrient level relationships: A nation-wide survey . : 31 EPA - 600/3–79-103
- Miller , R J and Robinson , H W . 2004 . Fishes of Oklahoma , Norman , OK : Univ. of Oklahoma Press .
- Moore , G A and Paden , J M . 1950 . The fishes of the Illinois River in Oklahoma and Arkansas . Am Midl Nat. , 44 : 76 – 95 .
- Nürnberg , GK. 1996 . Trophic state of clear and colored, soft-and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish . Lake Reserv Manage. , 12 : 432 – 447 .
- Oglesby , RT. 1977 . Relationship of fish yield to phytoplankton standing crop, production and morphoedaphic factors . J Fish Res Board Can , 34 : 2271 – 2279 .
- Omernik , JM. 1977 . Nonpoint source-stream nutrient level relationships . EPA-660/3-77-105
- Pflug , D E and Pauley , G B . 1984 . Biology of smallmouth bass (Micropterus dolomieu) in Lake Sammamish, Washington . Northwest Sci. , 58 : 118 – 129 .
- Sammons , S M and Maceina , M J . 2005 . Activity patterns of largemouth bass in a subtropical US reservoir . Fish Manage Ecol. , 12 : 331 – 339 .
- Schupp , O and Wilson , B . 1993 . Developing lake goals for water quality and fisheries . Lakeline. , 13 : 18 – 21 .
- Sas , H , Ahlgren , I , Bernhardt , B , Boström , B , Clasen , J , Forsberg , C , Imboden , D , Kamp-Nielson , L , Mur , L de Oude , N . 1989 . Lake restoration by reduction of nutrient loading: expectations, experiences, extrapolation , St. Augustine , , Germany : Richarz .
- Stewart , N E , Shumway , D L and Douderoff , P . 1967 . Influence of oxygen concentration of the growth of juvenile largemouth bass . J Fish Res Board Can , 24 : 475 – 494 .
- Summers , PB. 1961 . Observations on the limnological dynamics of Tenkiller Ferry Reservoir . Report Okla Dept Wildl Conserv ,
- Summers , GL. 1978 . Sportfishing statistics of Oklahoma Reservoirs . Oklahoma Fish Res Lab and University of Oklahoma , Bull No 14
- Thornton , K , Payne , F E and Kimmel , B L . 1990 . “ Reservoir limnology: ecological perspectives ” . New York , NY : John Wiley & Sons .
- [USFWS] US Fish and Wildlife Service . 1982a . Habitat suitability index models: channel catfish . Biological Services Program and Division of Ecological Services , FWS/OBS-82/10.2
- [USFWS] US Fish and Wildlife Service . 1982b . Habitat suitability mndex models: largemouth bass . Biological Services Program and Division of Ecological Services , FWS/Obs-82/10.16
- [USFWS] US Fish and Wildlife Service . 1983 . Habitat suitability index models: smallmouth bass . Biological Services Program and Division of Ecological Services , FWS/OBS-82/10.36
- [USFWS] US Fish and Wildlife Service . 1984a . Habitat suitability index models and instream flow suitability curves: spotted bass . Biological Services Program and Division of Ecological Services , FWS/OBS-82/10.72
- [USFWS] US Fish and Wildlife Service . 1984b . Habitat suitability index models and instream flow suitability curves: inland stocks of striped bass . Biological Services Program and Division of Ecological Services , FWS/OBS-82/10.85
- [USFWS] US Fish and Wildlife Service . 1984c . Habitat suitability information: walleye . Biological Services Program and Division of Ecological Services , FWS/OBS-82/10.56
- Welch , EB. 2009 . Phosphorus reduction by dilution and shift in fish species in Moses Lake , Vol. 25 , 276 – 283 . WA : Lake Reserv Manage .
- Welch , E B and Jacoby , J M . 2004 . Pollutant effects in freshwater. Appl Limnol , 3rd ed. , Boca Raton , FL : Taylor and Francis .
- Young , S P and Isely , J F . 2002 . Striped bass annual site fidelity and habitat utilization in J. Strom Thurmond Reservoir, South Carolina, Georgia . T Am Fish Soc. , 131 : 828 – 837 .
- Zale , A V , Wiechman , J D 1990 , Lochmiller , R L and Burroughs , J . 1990 . Limnological conditions associated with summer mortality of striped bass in Keystone Reservoir, Oklahoma . T Am Fish Soc. , 119 : 72 – 76 .