Abstract
Reducing the magnitude and frequency of peak algal biomass is a common goal of lake management. To better quantify such conditions in Missouri reservoirs, an upper boundary delineating maximum algal chlorophyll (Chlmax) across the range of total phosphorus (TP) was developed using summer monitoring data (n = 8839) and compared with 2 other Missouri datasets (n = 8188 and 5151). Typically, other factors constrain Chl below the maximum, and most samples contained a fraction of Chlmax. Near maximum conditions (Chlnm) were provisionally defined as 70% of Chlmax; individual reservoirs differ in their history of supporting Chlnm measurements (from 0 to 43% of samples) irrespective of nutrient status or the duration of summer monitoring. There was a rapid increase in the yield of Chlmax per unit TP across the oligo–mesotrophic range, while within the eutrophic range Chlmax varied with changes in TP in a near-unity response. This general pattern was similar for Chlnm and provides a basis for predicting how high Chl levels would change with nutrient management. Values of Chlmax in Missouri reservoirs are lower than lakes in Florida and larger than values in an international dataset, but the rate of change in Chl across the TP range is quite similar among these datasets, suggesting this pattern applies to different lake types.
Lake management efforts often focus on reducing the magnitude and frequency of peak algal biomass to prevent extreme conditions considered most objectionable (Walker Citation1985, Bachmann et al. Citation2003). In this analysis we determined both maximum chlorophyll (Chlmax) values and Chl to total phosphorus ratios (Chl:TP) in association with the upper boundary of the Chl–TP distribution in large datasets from Missouri reservoirs and compared our findings with Florida lakes (Brown et al. Citation2000) and an international data set (Pridmore and McBride Citation1984). This approach treats the cross-system pattern as the potential Chl maximum at a given TP value rather than the standard approach of accounting for variation around the center of the response, as described by best-fit regression (Jones and Knowlton Citation2005, Jones et al. Citation2008). Others have considered the Chl–TP relation from the viewpoint of the upper boundary, and our analysis contributes to this line of inquiry (Hosper Citation1980, Smith and Shapiro Citation1981, Pridmore and McBride Citation1984, White Citation1989, Kaiser et al. Citation1994, Brown et al. Citation2000, Lewis Citation2011).
Thomson et al. (Citation1996) promoted estimating the upper edge of data distributions where a variable, such as TP, acts as a limiting factor for a response variable, such as Chl, to better understand and quantify spatial structure in cross-system comparisons in ecology. Evaluating response variables relative to a potential maximum is consistent with the ecological concept of limiting factors described by the phosphorus limitation paradigm and implicit in the Chl–TP relationship (Kaiser et al. Citation1994, Smith Citation2003, Sterner Citation2008). Variation in Chl–TP is attributed to the bioavailability of nutrient pools, nitrogen supplies relative to TP, composition of the phytoplankton community, climate, hydrology, stratification patterns, grazing pressure, and light availability, as determined by color and/or mineral particulates. Regardless of other influences, most variation in Chl is related to TP in lakes.
Maximum expression of algal biomass has been addressed based on Chl–TP ratios by White (Citation1989) who considers potential phytoplankton biomass relative to the nutrient content of the sample. This approach differs from viewing algal blooms as a response to nutrient pulses from internal or external sources and does not imply that Chlmax is necessarily associated with harmful or nuisance conditions (Smayda Citation1997, Carstensen et al. Citation2007). Alternatively, high Chl events have been characterized by quantifying the frequency that observed Chl exceeds specific threshold values in individual lakes (Walmsley Citation1984, Walker Citation1985, Walker and Havens Citation1995, Bachmann et al. Citation2003).
Figure 1 Chlorophyll (Chl) and total phosphorus (TP) from Missouri reservoirs and oxbow lakes during summer (panel a, n = 8839; Jones et al. Citation2008). The upper boundary on Chl in all 3 panels was described by Equationequation 1 from the text: log10Chlmax = −0.61 + 1.62(log10TP) − 0.059(log10TP3). This upper boundary was also plotted with data from Missouri reservoirs collected by citizen volunteers (panel b, n = 8188) after TP data were increased by 2 μg/L to account for loss during storage (Obrecht et al. Citation1998) and data from Missouri reservoirs collected from reservoirs sampled daily during summer and seasons other than summer (panel c, n = 5151).
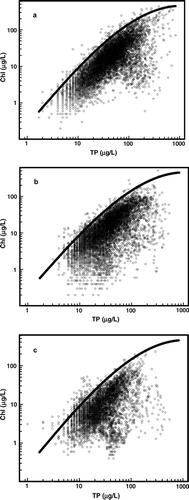
Using individual Chl–TP pairs from Missouri reservoirs during summer, we fitted a reference line to delineate the upper boundary of Chl (uncorrected for pheophytin, n = 8839, 0.2–447 μg/L, median 13.5 μg/L) across the range of TP (2–831 μg/L, median 36 μg/L; ). The data were binned based on the distribution of log10TP values (n = 38 bins, each with <6% of the total observations). Within each bin, Chl–TP pairs were ranked to identify Chlmax within the given nutrient range; obvious outliers were excluded. A line was fitted to the Chlmax values using stepwise regression with log10TP and log10TP3 (R2 = 0.98, p < 0.01) to describe the upper edge of the distribution of the data; log10TP2 and higher-order terms for log10TP were not significant:
Most samples contained a fraction of Chlmax; the median ratio of Chlobs:Chlmax was 0.31 (, interquartile range 0.22–0.44, mean 0.35). This ratio was >0.8–1 in only 2.4% of the observations, and an additional 2.7% of the values had ratios >0.7<0.8. This distribution suggests values near Chlmax are infrequent in routine summer monitoring data. Noteworthy, Chlmax values and Chlmax:TP ratios are at least 3 times larger than the conventional limits used to categorize reservoir trophic state in Missouri reservoirs (Jones et al. Citation2008; ). This comparison further illustrates that Chlmax values represent extreme conditions associated with a given nutrient value.
Table 1 Trophic state criteria for Missouri reservoirs (Jones et al. Citation2008) with the corresponding Chl:TP ratios for conditions at the upper boundary. Maximum and near-maximum chlorophyll (see text) and corresponding Chl:TP ratios are shown for the upper TP value in each trophic state category.
Figure 2 Plot of maximum chlorophyll (Chlmax) against total phosphorus (TP) calculated using a nonlinear (upper line, panel a) equation for Florida lakes by Brown et al. (Citation2000) and Equationequation 1 from the text for Missouri reservoirs and for international lakes (Pridmore and McBride Citation1984). Data from panel a were replotted in panel b to show the rate of change in Chlmax [ = (Chlmax)TP – (Chlmax)TP-1] / (Chlmax)TP] across much of the observed TP range in the dataset.
![Figure 2 Plot of maximum chlorophyll (Chlmax) against total phosphorus (TP) calculated using a nonlinear (upper line, panel a) equation for Florida lakes by Brown et al. (Citation2000) and Equationequation 1 from the text for Missouri reservoirs and for international lakes (Pridmore and McBride Citation1984). Data from panel a were replotted in panel b to show the rate of change in Chlmax [ = (Chlmax)TP – (Chlmax)TP-1] / (Chlmax)TP] across much of the observed TP range in the dataset.](/cms/asset/841df960-b5fd-49c2-b726-95ec468f0ddb/ulrm_a_627625_o_f0002g.gif)
The empirical Chlmax described by Equationequation 1 also applies to other datasets from Missouri reservoirs. It envelops the upper boundary of data collected by citizen volunteers (n = 8188, TP 3–539 μg/L, median 31 μg/L; ); some 1.6% of Chlobs were larger than Chlmax and most come from Table Rock Lake, an impoundment with low mineral turbidity with large Chl:TP ratios (Thorpe and Obrecht Citation2008). The Chlmax boundary (Equationequation 1) also envelops an aggregated dataset that includes daily collections from several reservoirs during summer and numerous nonsummer samples (n = 5151, TP 2–543 μg/L, median 25 μg/L; ). Some 2.6% of Chlobs was larger than Chlmax; most were from Table Rock Lake or collected during fall destratification, a period of high Chl:TP ratios (Jones and Knowlton Citation2005). The median ratio Chlobs:Chlmax of 0.27 was, however, somewhat lower than the other datasets (), in part because this dataset includes midwinter collections when low Chl:TP ratios are common (Jones and Knowlton Citation2005). Together, these comparisons suggest the equation for Chlmax broadly applies to Missouri reservoirs.
The Chlmax response for Florida lakes by Brown et al. (Citation2000) is about twice the value for the Missouri Chl–TP pattern (). This discrepancy likely reflects differences in climate and lake type between the 2 regions; Brown et al. (Citation2000) previously concluded some Florida lakes have larger Chl:TP ratios than northern lakes. Suppression of Chl yields by mineral turbidity could also reduce Chlmax in some Missouri reservoirs (Jones and Knowlton Citation2005). In contrast, values of Chlmax estimated for an international suite of lakes (Pridmore and McBride Citation1984) averaged about 60% of Chlmax in Missouri (). Longer collection records for the Florida and Missouri datasets would increase the likelihood of sampling high Chl events, thereby contributing to larger Chlmax values (Brown et al. Citation2000, Jones et al. Citation2008). Regardless, these comparisons suggest regional differences in Chlmax.
As a preliminary approach to identify near-maximum algal biomass in Missouri reservoirs and to broaden the scope of the comparative analysis beyond Chlmax, we calculated the upper 95% confidence limit on mean Chl within each of the log10TP bins used to generate Equationequation 1 (mean + 1.64*Standard Deviation). The cross-system pattern matched 70% of Chlmax and serves as a provisional limit for identifying near-maximum Chl (Chlnm) in these reservoirs. These data include samples within approximately 5% of Chlmax and those located above the upper boundary (). Values of Chlnm and Chlobs:TP ratios are more than double the conventional limits used to categorize reservoir trophic state boundaries ().
Phytoplankton taxonomic composition varies with lake trophic state, and the cellular Chl content differs within and among species (Watson et al. Citation1992, Citation1997); both factors may influence Chlnm in Missouri reservoirs. Taxonomic data from July 2003 (63 reservoirs; Jones et al. Citation2008) showed that 6% of the samples exceeded Chlnm criteria, as did 1 of 15 reservoirs in August 2004; these samples were from eutrophic reservoirs dominated by either Anabaena or Aphanizomenon (87–98% of total biovolume). These limited data indicate Chlnm can be exceeded when the phytoplankton community is dominated by large cyanobacteria. Additional taxonomic information is needed to determine the algal community dominating other Chlnm events, particularly in oligotrophic and mesotrophic reservoirs.
Table 2 Trophic state criteria for Missouri reservoirs based on TP (Jones et al. Citation2008) with the mean ratio of observed average chlorophyll:near-maximum chlorophyll (Chlobs:Chlnm) in Chlobs samples of ≥10, ≥20, ≥30, ≥40, and ≥50 μg/L from intensively sampled reservoirs in the dataset (n = 113). The eutrophic category was divided at 50 μg TP/L to better illustrate the cross-system pattern in Chlobs:Chlnm.
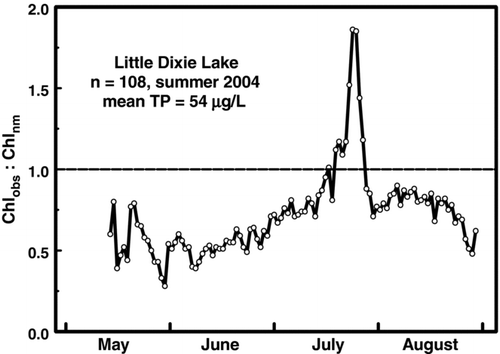
Conditions that favor Chlnm in individual reservoirs may be short-lived. Daily collections from Little Dixie Lake during summer 2004 (n = 108; ) show 9% of samples exceeded the Chlnm threshold during a single event in late July. This ephemeral peak was consistent with a bloom event wherein Chl deviates from the normal seasonal cycle for a short period of time (Hutchinson Citation1967, Carstensen et al. Citation2007). These events would not always be captured in routine summer monitoring (Knowlton and Jones Citation2000, Jones et al. Citation2008) and suggest that Chlmax and Chlnm are best assessed using large datasets.
Figure 3 Ratio of observed chlorphyll to near-maximum chlorophyll (Chlobs:Chlnm) in daily collections from Little Dixie Lake during summer 2004. Values above the horizontal line indicate samples where Chlobs exceeded Chlnm.
Among the most intensively sampled reservoirs in our dataset (n = 113, 33–151 summer samples, median 53), two-thirds of samples exceeding Chlnm were collected during July and August, consistent with an earlier finding that Chl increases in late summer (Jones and Knowlton Citation2005). Individual reservoirs in this group differ in their history to support Chlnm; 23% never expressed Chlnm, 37% supported Chlnm in 0.1–5% of samples, and 40% supported Chlnm in ≥5–43% of samples. Interestingly, neither mean TP (6–180 μg/L, median = 39 μg/L) nor the number of samples collected from an individual reservoir showed a significant correlation with Chlnm (p > 0.05). These outcomes suggest frequency of Chlnm is not a simple function of nutrient status or the duration of monitoring as represented in our summer inventory.
Lake managers have addressed undesirable algal abundance as the frequency that Chlobs exceeds nuisance levels (Walmsley Citation1984, Walker Citation1985, Walker and Havens Citation1995, Bachmann et al. Citation2003). The frequency of high Chl levels is known to increase with trophic state, with large values common in enriched lakes. We followed this convention and calculated the frequency of Chl values of ≥10, ≥20, ≥30, ≥40, and ≥50 μg/L from intensively sampled reservoirs in the dataset (n = 113; ) and found similarities with lakes in other regions and previous findings for Missouri reservoirs (Jones et al. Citation2008). In general, the frequency of Chl ≥10 μg/L increased sharply among reservoirs with mean TP ≥20 μg/L but was uncommon in reservoirs with lower mean TP ().
Figure 4 The proportion of observed chlorophyll (Chlobs) values that exceed 10, 20, 30, 40 and 50 μg/L (panels a–e, respectively) plotted against the mean log10TP value from intensively sampled reservoirs in the dataset (n = 113). The mean ratio of Chlobs to near-maximum chlorophyll (Chlnm) was calculated for each reservoir using Chl values that exceeded the cutpoint for the respective panels. Mean Chlobs:Chlnm ratios were divided into 4 categories and are represented in the panels by unique symbols to show the cross-system pattern.
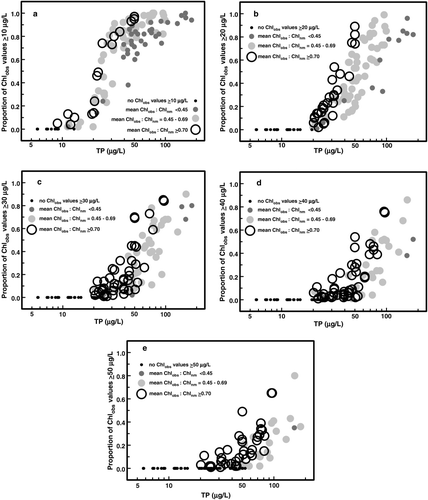
Another feature of this analysis is that within each trophic state category an increase in Chlobs represents a progressively larger ratio of Chlobs:Chlnm and therefore is less frequently observed in the data distribution (; ). For example, among mesotrophic reservoirs, a Chl value of ≥10 μg/L equates to nearly two-thirds of Chlnm, while 40 μg/L Chl closely matches Chlnm, and 50 μg/L Chl exceeds the Chlnm criteria (). This general pattern holds for Chl values across all trophic states (). Conversely, for any given Chl value, the ratio of Chlobs:Chlnm declines with trophic state (). To illustrate, ≥10 μg/L Chl closely matches Chlnm in oligotrophic reservoirs, and equates to nearly two-thirds, half, and one-third of Chlnm in mesotrophic, eutrophic, and hypereutrophic systems, respectively (). The magnitude of these high Chl events in individual reservoirs () is masked by aggregation in the presentation of the Chl–TP relationship as seasonal or long-term mean values (Jones et al. Citation1998, Jones and Knowlton Citation2005). These extreme values are the basis for estimating Chlmax in summer monitoring data ().
The ratio of Chlmax:TP forms a dome-shaped distribution across the range of Chlobs:TP values in the dataset when both are plotted against trophic state (as log10TP; ). This pattern clearly shows a rapid increase in the yield of Chlmax per unit TP across the oligo- and mesotrophic ranges, followed by high ratios throughout the eutrophic range and subsequent decline among the most fertile samples. Overall, ratios of Chlmax:TP increase sharply from ∼0.6 at 5 μg TP/L to unity at 13 μg TP/L, and the ratio increases to 1.25 at the upper boundary of the mesotrophic conditions (25 μg TP/L). Across this range, the increase in Chlmax, from 3.2 to 31.2 μg/L, was double the 5-fold increase in TP. Between 25 and 30 μg TP/L, the increase in Chlmax was just slightly larger than the proportional increase in TP. Near the center of the data distribution, Chlmax:TP forms a broad dome. Ratios were 1.4 at 44 and 125 μg TP/L with a peak ratio of 1.46 at 74 μg TP/L. As a consequence, Chlmax closely tracks changes in TP in a near-unity response within the eutrophic range. For example, halving TP from 100 to 50 μg TP/L corresponds with halving Chlmax (from 144 to 71 μg/L). The decline in Chlmax:TP among the most fertile samples (>125 μg TP/L, 8% of the total; ) is largely a function of light limitation and available supplies of dissolved P in turbid samples (Knowlton and Jones Citation2000, Jones and Knowlton Citation2005, Jones et al. Citation2008).
Figure 5 Chlorophyll (Chlobs) and total phosphorus (TP) data from were replotted in panel a as the Chlobs:TP ratio against log10TP with trophic state boundaries for TP (Jones et al. Citation2008) shown on the x-axis. The ratio of maximum chlorophyll (Chlmax) to TP (as calculated from Equationequation 1 divided by the observed TP) forms a dome across the data distribution. Samples with the most extreme Chlobs:TP ratios were not included. In panel b, Chlobs:TP ratios for the 90th, 70th, and 50th percentile values in the TP bins used to generate Equationequation 1 (see text) were plotted against the corresponding median TP value for each bin. In panel c, Chlobs:TP ratios for the 30th, 25th, and 20th percentile values in the TP bins were plotted against the corresponding median TP value.
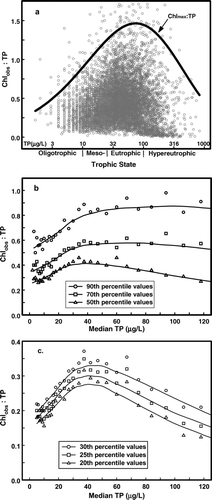
The pattern seen in Chlmax:TP ratios also holds for the 90th, 70th, and 50th percentile values in the TP bins used to generate Equationequation 1 (, values >125 μg TP/L not shown). Within each category, Chl:TP shows a statistically significant increase with TP across oligotrophic to near-eutrophic range (r ≥ 0.93, n = 17, TP = 5–38 μg/L), with a nonsignificant, near-flat response between 40 and 125 μg TP/L. Collectively, these patterns illustrate how high Chl levels in Missouri reservoirs would respond to changes in TP ( and b).
Among samples in the 30th, 25th, and 20th percentiles in the various TP bins (), the initial significant increase in Chlobs:TP with TP (r ≥ 0.93, n = 17, TP = 5–38 μg/L) was followed by a significant decline in Chlobs:TP (r ≥ −0.94, n = 10). This pattern indicates the yield of Chl per unit of TP is not asymptotic across all samples within the eutrophic range. For example, halving TP from 80 to 40 μg TP/L only results in a 20–35% reduction in Chl within the 20th to 30th percentiles within the cross-system pattern (). This analysis suggests the response to phosphorus reduction would differ between the upper and lower half of the Chl–TP data distribution ().
Overall, this analysis proposes Chlmax and Chlnm metrics for Missouri reservoirs that characterize peak algal biomass and serves as a basis to quantify controlling factors, assess seasonal patterns, and compare with lakes in other regions. The upper boundary on the cross-system Chl–TP pattern () represents the general distribution in which other factors constrain responses below the maximum. Conditions of Chlmax and Chlnm represent near-potential algal biomass for a given TP concentration (; ) and were generally rare in summer monitoring data (). An important outcome is that history and frequency of high Chl events differ among individual reservoirs and suggest system-specific constraint of Chl by biotic and abiotic factors. A detailed analysis of factors that determine the degree to which Chlobs is less than Chlmax or Chlnm in individual reservoirs is beyond the scope of this note but remains a research question.
The distribution of Chlmax:TP ratios across the TP range has management implications. The sharp increase across the least fertile samples and the near-asymptote across the eutrophic range () provide a framework for interpreting how phosphorus control will reduce Chl. Interestingly, when expressing this relationship as the rate of change in Chl per unit TP, the pattern is quite similar for Missouri reservoirs, Florida lakes, and a selection of international lakes (), despite large differences in actual Chlmax values among the datasets (). This pattern also holds for average Chl values predicted using the least squares regression based on long-term reservoir means (Jones et al. Citation2008, data not shown). The clear inference is that the rate of change in Chl across observed TP values applies to a broad range of lake types and is consistent with the early finding that the slope coefficient of the Chl–TP relationship differs with TP and is nonlinear (Straskraba Citation1980, Watson et al. Citation1992, Brown et al. Citation2000). The transition between rapid change in Chlmax:TP and gradually declining rate of change is near the conventional boundary between meostrophic and eutrophic conditions (30 μg TP/L; Nürnberg Citation1996; ).
Control of algal biomass and associated nuisance conditions is an objective of most lake management efforts (Bachmann et al. Citation2003), and this analysis furthers our understanding of these issues in Missouri reservoirs.
Acknowledgments
Funding was provided by the Missouri Department of Natural Resources and Missouri Agricultural Experiment Station. We thank Jennifer Graham, Joshua Millspaugh, and Robert Gitzen for advice. Roger Bachmann, Gertrud Nürnberg, and 2 anonymous reviewers provided most helpful suggestions.
References
- Bachmann , R W , Hoyer , M V and Canfield , D E Jr . 2003 . Predicting the frequencies of high chlorophyll levels in Florida lakes from average chlorophyll or nutrient data . Lake Reserv Manage. , 19 : 229 – 241 .
- Brown , C D , Hoyer , M V , Bachmann , R W and Canfield , D E Jr . 2000 . Nutrient-chlorophyll relationships: an evaluation of empirical nutrient-chlorophyll models using Florida and north-temperate lake data . Can J Fish Aquat Sci. , 57 : 1574 – 1583 .
- Carstensen , J , Henriksen , P and Heiskanen , A-S . 2007 . Summer algal blooms in shallow estuaries: definition, mechanisms, and link to eutrophication . Limnol Oceanogr. , 52 : 370 – 384 .
- Hosper , SH. 1980 . Development and partial application of limiting values for the phosphate concentration in surface waters in the Netherlands . Hydrobiol Bull. , 14 : 64 – 72 .
- Hutchinson , GE. 1967 . A treatise on limnology. II Introduction to lake biology and the limnoplankton , New York (NY) : John Wiley & Sons .
- Jones , J R , Knowlton , M F and Kaiser , M S . 1998 . Effects of aggregation on chlorophyll-phosphorus relations in Missouri reservoirs . Lake Reserv Manage. , 14 : 1 – 9 .
- Jones , J R and Knowlton , M F . 2005 . Chlorophyll response to nutrients and non-algal seston in Missouri reservoirs and oxbow lakes . Lake Reserv Manage. , 21 : 361 – 370 .
- Jones , J R , Obrecht , D V , Perkins , B D , Knowlton , M F , Thorpe , A P , Watanabe , S and Bacon , R R . 2008 . Nutrients, seston and transparency of Missouri reservoirs and oxbow lakes: An analysis of regional limnology . Lake Reserv Manage. , 24 : 155 – 180 .
- Kaiser , M S , Speckman , P L and Jones , J R . 1994 . Statistical models for limiting nutrient relations in inland waters . J Am Statist Assoc. , 89 : 410 – 423 .
- Knowlton , M F and Jones , J R . 2000 . Non-algal seston, light, nutrients and chlorophyll in Missouri reservoirs . Lake Reserv Manage. , 16 : 322 – 332 .
- Lewis , W M Jr . 2011 . Global primary production of lakes: 19th Baldi Memorial Lecture . Inland Waters. , 1 : 1 – 28 .
- Nürnberg , GK. 1996 . Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish . Lake Reserv Manage. , 12 : 432 – 447 .
- Obrecht , D V , Milanick , M , Perkins , B D , Ready , D and Jones , J R . 1998 . Evaluation of data generated from lake samples collected by volunteers . Lake Reserv Manage. , 14 : 21 – 27 .
- Pridmore , R D and McBride , G B . 1984 . Prediction of chlorophyll-a concentrations in impoundments of short hydraulic retention time . J Environ Manage. , 19 : 343 – 350 .
- Smith , VH. 2003 . Eutrophication of freshwater and coastal marine ecosystems: a global problem . Environ Sci Pollut Res. , 10 : 126 – 139 .
- Smith , V H and Shapiro , J . 1981 . Chlorophyll-phosphorus relations in individual lakes. Their importance to lake restoration strategies . Environ Sci Tech. , 15 : 444 – 451 .
- Smayda , TJ. 1997 . What is a bloom? A commentary . Limnol Oceanogr. , 42 : 1132 – 1136 .
- Sterner , RW. 2008 . On the phosphorus limitation paradigm for lakes . Internat Rev Hydrobiol. , 93 : 433 – 445 .
- Straskraba , M. 1980 . “ Effects of physical variables on production ” . In The functioning of freshwater ecosystems. IBP 22 , Edited by: LeCren , E D and Lowe-McConnel , R H . Cambridge (UK) : Cambridge University Press. p. 13–84 .
- Thomson , J D , Weiblen , G , Thomson , B A , Alfaro , S and Legender , P . 1996 . Untangling multiple factors in spatial distributions: lilies, gophers and rocks . Ecology , 77 : 1698 – 1715 .
- Thorpe , A P and Obrecht , D V . 2008 . The Lakes of Missouri Volunteer Program 2008 data report , Columbia : University of Missouri .
- Walker , WW Jr. 1985 . “ Statistical bases for mean chlorophyll a criteria ” . In Lake and Reservoir Management – Practical Applications , 57 – 62 . McAffee (NJ) : Proc. 4th Annual Conference, North American Lake Management Society .
- Walker , W W Jr. and Havens , K E . 1995 . Relating algal bloom frequencies to phosphorus concentrations in Lake Okeechobee . Lake Reserv Manage. , 11 : 77 – 83 .
- Walmsley , RD. 1984 . A chlorophyll a trophic status classification system for South African reservoirs . J Environ Qual , 13 : 97 – 104 .
- Watson , S , McCauley , E and Downing , J A . 1992 . Sigmoid relationships between phosphorus, algal biomass, and algal community structure . Can J Fish Aquat Sci. , 49 : 2605 – 2610 .
- Watson , S , McCauley , E and Downing , J A . 1997 . Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status . Limnol Oceanogr , 42 : 487 – 495 .
- White , E. 1989 . Utility of relationships between lake phosphorus and chlorophyll a as predictive tools in eutrophication control studies . N Z J Mar Freshw Res. , 23 : 35 – 41 .