Abstract
We reviewed published information on the biology of Florida lakes to determine what concentrations of total phosphorus (TP) and total nitrogen (TN) might impair their designated uses. For the designated use of swimming, lake users preferred oligotrophic to mesotrophic lakes. Eutrophic lakes in Florida generally support their designated use of the propagation and maintenance of a healthy, well-balanced population of fish and wildlife. Fish standing crops in Florida lakes increased as the concentrations of TP increased from 1 to 1000 μg/L. Florida lakes did not show the kind of changes in fish species with trophic state as might be found in northern lakes. Populations of aquatic birds and alligators also increased with increases in trophic state. Benthic macroinvertebrate indices of lake condition were not related to anthropogenic nutrient pollution when estimated by the Landscape Development Intensity index. We found no evidence that the concentrations of TP and TN in the water were responsible for excessive populations of aquatic macrophytes. A study of open-water concentrations of the cyanobacterial toxin microcystin in 187 Florida lakes found only 3 individual water samples collected from 2 lakes exceeded the World Health Organization guidance level of 20 μg/L for swimming, although high levels of microcystin can sometimes be found in some lakes in surface accumulations of cyanobacteria.
The US Environmental Protection Agency (USEPA) is asking states to replace narrative standards with numerical nutrient criteria to protect the designated uses of their waters. The designated uses for most Florida freshwater lakes (Class III) are recreation and the propagation and maintenance of a healthy, well-balanced population of fish and wildlife. The rest of the lakes (Class I) also have a designated use of potable water supplies in addition to the designated uses for Class III waters and must meet all of the criteria for Class III waters. The USEPA (2010a) promulgated numeric nutrient criteria () with the intention of protecting those uses.
Table 1 Numeric nutrient criteria for Florida lakes from the USEPA (2010a). All concentrations are annual geometric means not to be surpassed more than once in a 3-year period. Bracketed numbers reflect the range in which Florida can adjust the TN and TP criteria when data show the lake is meeting the Chl-a criteria.
The USEPA (2010b) Technical Support Document for the Florida nutrient criteria discusses the designated uses of Florida lakes as follows:
Class I waters are designated for potable water supplies while Class III waters are designated for “recreation, propagation and maintenance of a healthy, well-balanced population of fish and wildlife” (Rule 62–302.400, F.A.C.). Specifically, USEPA has derived the numeric criteria to translate the State of Florida's existing narrative water quality standard for nutrients, applicable to these waters, at Rule 62–302.530(47)(b), F.A.C.:
In no case shall nutrient concentrations of a body of water be altered so as to cause an imbalance in natural populations of aquatic flora or fauna.
The USEPA (2010b) cited 2 papers (OECD 1982, Salas and Martino Citation1991) as their basis to declare that Florida lakes with chlorophyll a (Chl-a) concentrations above 20 μg/L should be classified as eutrophic lakes, and that eutrophic lakes in Florida do not meet their designated uses. Because neither of those papers included any quantitative scientific studies relating concentrations of total phosphorus (TP), total nitrogen (TN), or Chl-a to designated uses of lakes in Florida or anywhere else, we were interested in testing the hypothesis that a Florida lake classified as eutrophic does not meet its designated uses of “recreation, propagation and maintenance of a healthy, well-balanced population of fish and wildlife,” and it has “an imbalance in natural populations of aquatic flora or fauna.”
The purpose of this study was to examine the idea that a eutrophic lake in Florida, naturally eutrophic or anthropogenically eutrophic, does not meet the criteria for Class I or Class III lakes. The question of whether the current eutrophic lakes in Florida are always the result of anthropogenic acceleration of nutrient loading is discussed in Bachmann et al. (Citation2012). Here we reviewed studies that looked at several aspects of the flora and fauna across a gradient of Florida lakes ranging from oligotrophic to eutrophic. This should give some insight into how the flora and fauna in an individual lake might change as it undergoes increases in nutrient loading.
The first objective of this study is to use available information on the biology of Florida lakes relative to their trophic states to determine what concentrations of TP, TN, and Chl-a might impair the designated uses of propagation and maintenance of a healthy, well-balanced population of fish and wildlife and the prevention of an imbalance in natural populations of aquatic flora and fauna.
The second objective is to determine if we can relate lake trophic state to the recreational use of Florida lakes. This is complicated because rather than establishing different classifications of use for different lake types, the State of Florida chose a multiple use approach and designated all its freshwater lakes for recreation and the propagation and maintenance of a healthy, well-balanced population of fish and wildlife, with some also designated for potable water supplies. This makes it difficult to create one set of criteria that will be optimal for each type of designated use in all lakes. For example, recreation on lakes includes a whole range of uses, some of which are best in biologically rich lakes (e.g., fishing and bird watching), and some are better in an unproductive oligotrophic lake (e.g., swimming and snorkeling).
The last objective is to evaluate the utility of using designated uses to set numeric nutrient criteria for Florida lakes.
General background on Florida lakes
The biology of Florida lakes is generally the same as the biology of some northern lakes, but there are important limnological differences between northern lakes and Florida lakes that must be considered before setting nutrient criteria. The trophic states of Florida lakes naturally range from oligotrophic to hypereutrophic, due in part to deposits of phosphatic materials in some soils (Canfield and Hoyer Citation1988, Griffith et al. Citation1997, Chen and Ma Citation2001, Terziotti et al. Citation2010). With the exception of some sinkhole lakes with depths of up to 30 m, most Florida lakes are shallow and well mixed (Griffith et al. Citation1997), and at least 70% of them have no surface inlet or outlet. The lakes are warm all year, and only some northern Florida lakes experience rare instances of overnight freezing of the lake surface.
The subtropical climate of Florida also influences lake biology. Several species of fish begin spawning earlier in the year, have a longer spawning season, and grow faster in Florida than they do in lakes in the northern states. There are no species of fish in Florida that require cold waters throughout the year. In a summary of differences between cold temperate versus warm temperate-subtropical-tropical lakes, Jeppesen et al. (Citation2007) note that the warm lakes have prolonged growing seasons with a greater probability of long-lasting algal blooms and dense floating plant mats. They also note smaller fish sizes, higher aggregations of fish in macrophyte beds, and more annual fish cohorts.
In a review of the effects of latitude on lake properties, Lewis (Citation1996) notes that, unlike terrestrial plants that have a greater taxonomic diversity at low latitudes, the compositions of the phytoplankton communities at the genus and even species levels vary little between tropical and temperature latitudes. Lewis (Citation2000) also found that, like temperate lakes, nutrient limitation centers on deficiencies in phosphorus and nitrogen. In common with lakes in other warm climates, Florida lakes do not support large-bodied Daphnia and are dominated by small-bodied zooplankton (Chrisman and Beaver Citation1990). Some investigators attribute this size difference to intense grazing pressure by planktivorous fish (Iglesias et al. Citation2008). Mazumder and Havens (Citation1998) found that the lack of large herbivorous zooplankton in Florida lakes meant that the amount of Chl-a for a given amount of TP was greater than that found in northern temperate lakes that had large-bodied zooplankton, but the same as that for northern temperate lakes that lacked large-bodied zooplankton. Jeppesen et al. (Citation2007) and Chrisman and Beaver (1990) suggest that the lack of large zooplankton grazers means that top-down control is less important in subtropical lakes than in colder temperate lakes, so that it is difficult to apply biological restoration in warm lakes.
Methods
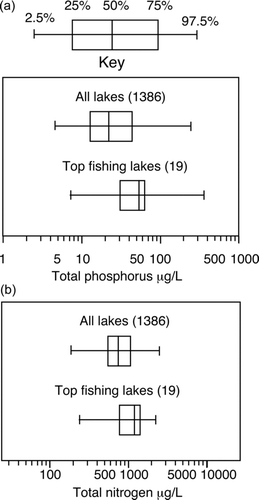
Most of our study is based on reviews of published papers on Florida lakes that focus on lake trophic states and designated uses of fish, wildlife, and recreation. While data from several lakes where changes in trophic states and biological characteristics had been documented over many years would have provided valuable information, the basic relationships can still be understood by looking at current data from studies examining a cross-section of lakes covering a broad range of trophic states. For an additional approach to determine how the trophic states of lakes are related to sport fishing, we used information on the web site of the Florida Fish and Wildlife Conservation Commission (FFWCC 2009) to identify the top fishing lakes in Florida. These lakes are based on an annual compilation by the FFWCC fisheries biologists of the top Florida fishing lakes for various popular species of sport fish. For each species of sport fish, the lakes are selected on the basis of creel surveys showing total effort and total catch, historic records of populations, and records of large fish caught. The biologists use their best professional judgment to recommend lakes where “anglers find a quality place to catch either good numbers of bass or to catch a trophy bass” (FFWCC 2009) or other species of fish of interest. We matched their list of lakes for 2009 with available data on the concentrations of TP, TN, and Chl-a in the same lakes. We then compared the distributions of those variables with the distributions of the same variables in 1386 lakes in the State of Florida. Those lakes were used in the study of Bachmann et al. (Citation2012) and represent 77% of the total surface area of all Florida lakes with surface areas of 0.1 ha or greater. We used both a t-test on logarithmically transformed concentrations of TP and TN and a nonparametric Wilcoxon/Kruskal-Wallis test using untransformed concentrations in the JMP statistical software with a 5% level for significance.
Review of relationships between lake trophic states and biota
Fish
To test the hypothesis that sport fishes in Florida lakes reach maximum biomass and optimum densities in mesotrophic–eutrophic lakes but suffer adverse effects with further enrichment (Kautz Citation1980), Canfield and Hoyer (Citation1992) initiated a fisheries and limnological study of 60 Florida lakes with trophic states ranging from oligotrophic to hypereutrophic. They sampled fish with electro-shocking and gillnets and used rotenone inside block nets to make quantitative estimates of fish population densities.
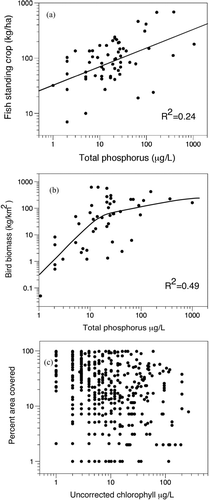
Results of the 60-lake study as reported by Bachmann et al. (Citation1996) did not show any critical level of nutrients in Florida lakes that resulted in significant changes in the composition of their fish populations. On average, the eutrophic lakes had higher standing crops of fish than did the mesotrophic and oligotrophic lakes. As expected based on historical information in Florida and elsewhere (see Bachmann et al. Citation1996), the total fish biomass per unit area correlated positively with TP (), TN, and Chl-a and inversely with Secchi disk transparency as TP ranged from 1 to >1000 μg/L. Species richness in the Florida lakes also did not change with trophic state, but increased with the surface areas of the lakes sampled (R2 = 0.70). Only 5 fish species (Lake chubsucker [Erimyzon sucetta], golden topminnow [Fundulus chrysotus], lined topminnow [Fundulus lineolatus], redfin pickerel [Esox americanus americanus], and Everglades pygmy sunfish [Elassoma evergladei]) showed decreases in frequency of occurrence with increasing lake trophic status. These 5 species are found in lakes with below average values for pH, alkalinity, and specific conductance (Hoyer and Canfield Citation1994b), which typically have low concentrations of TP and TN. Most important, the recreationally important centrarchids did not show significant reductions in their standing crops with increases in nutrients. This study did not find the kind of changes in fish species with trophic state as might be found in northern lakes, most likely because Florida lakes are warm, generally shallow, and have no winter ice cover, so oxygen replenishment at the surface can take place during the entire year. Florida lakes have no coldwater fish species like those in the family Salmonidae.
Figure 1 A. Standing crops of fish in 60 Florida lakes versus their average concentrations of total phosphorus with the linear regression line. Data are from Bachmann et al. (Citation1996). B. Observed biomass of aquatic birds on 46 Florida lakes versus their average concentrations of total phosphorus with a best-fit spline curve. Data are from Hoyer and Canfield (Citation1994a) and Hoyer et al. (Citation2006). C. Percent area covered by submersed macrophytes in 319 Florida lakes versus their average concentrations of uncorrected chlorophyll. Zero values were plotted as 1%. Data are from Bachmann et al. (Citation2002).
Another study (Schulz et al. Citation1999) investigated the potential impact of cultural eutrophication on the quality of fish populations in Florida lakes by using an index of biotic integrity (IBI). They tested 8 common fish assemblage metrics (number of fish species, number of native fish species, number of Lepomis species, number of piscivores species, number of generalist species, number of insectivore species, number of species intolerant to increased turbidity or warming and decreased dissolved oxygen concentration, and number of species tolerant to increased turbidity or warming and decreased oxygen concentration) to estimate anthropogenic impact to the 60 Florida lakes studied by Canfield and Hoyer (Citation1992) and reported by Bachmann et al. (Citation1996). They found that for 7 of the 8 metrics (number of insectivore species was the exception), the IBI metric score increased rather than decreased with increasing biological productivity. The R2 values for the 7 statistically significant IBI scores ranged from 0.06 to 0.20. Neither of these approaches supports the idea that the quantity or quality of the fish populations in eutrophic Florida lakes is impaired.
Table 2 Chemical characteristics of 19 Florida lakes selected by fisheries biologists from the Florida Fish and Wildlife Conservation Commission as the top fishing lakes in 2009. NA = data not available.
Recreational fishing
Freshwater recreational fishing is a billion-dollar industry nationwide, so fishing is a designated use that must be considered when establishing numeric nutrient criteria. When we compared available data on concentrations of TP, TN, Chl-a, and water color () in the top fishing lakes with similar data from 1386 other Florida lakes, we found the top fishing lakes are more eutrophic than Florida lakes as a whole (). The geometric mean TP concentration for the top fishing lakes is 49 μg/L, and for all of the 1386 lakes it is 25 μg/L. The geometric mean TN concentration for the top fishing lakes is 1066 μg/L, and for all the lakes is 764 μg/L. For both TP and TN, the differences between the top fishing lakes and all other lakes were statistically significant at the 5% level of probability for both a parametric t-test and the nonparametric Wilcoxon/Kruskal-Wallis tests. Most of the top fishing lakes have concentrations of TP, TN, or Chl-a that would place them in violation of some of the USEPA's nutrient standards. This finding alone should cause concern with the USEPA (2010a) criteria because the top fishing lakes are also excellent lakes for the propagation of fish. Thus, these lakes meet the general use standard for the propagation of fish in the Clean Water Act, but the nutrient criteria would classify them as impaired.
Figure 2 A. Distribution of total phosphorus concentrations in a sample of 1386 Florida lakes compared to the distributions in 19 of the top fishing lakes in Florida as designated by the fisheries biologists of the Florida Fish and Wildlife Conservation Commission for 2009. B. Similar plots for total nitrogen.
Aquatic birds and alligators
The lakes of Florida support a rich and diverse population of aquatic birds, which increases dramatically in the winter as migratory populations move through. Hoyer and Canfield (Citation1994a) and Hoyer et al. (Citation2006) made counts of aquatic birds on 46 Florida lakes and found the most common species observed included the great blue heron (Ardea herodias), great egret (Casmerodius albus), and anhinga (Anhinga anhinga). The species occurring with the highest densities were mallard (Anas platyrhynchos), American coot (Fulica americana), and red-winged blackbird (Agelaius phoeniceus). They also assembled trophic state data on the study lakes and found that as the concentrations of TP and Chl-a increased, both bird numbers and biomass per unit area increased. When we used linear regression with their data, the species richness increased with TP concentrations (R2 = 0.39) and lake area (R2 = 0.74). In a stepwise regression, the combination of both TP and lake area yielded an R2 of 0.79, indicating that once lake area was accounted for, TP had a small effect in increasing species richness. Bird abundance and species richness remained relatively stable as macrophyte abundance increased, but birds that use open-water habitats (e.g., double-crested cormorant [Phalacrocorax auritus]) are replaced by species that use macrophyte communities (e.g., ring-necked duck [Aythya collaris]). In other words, the more nutrients, the more productive the lake and the more attractive it is for an increasing variety of aquatic birds (). These findings are in agreement with those of Gardarsson and Einarsson (Citation1994) who found that the production of young ducks was correlated with food abundance in Lake Myvatn, Iceland, and with the study of Nummi et al. (Citation1994) who investigated a range of oligotrophic to eutrophic lakes in Finland and Sweden and found that mallard duck densities increased with food production. Other ecologists have noted that productive aquatic ecosystems are able to support a greater number and biomass of organisms and more specialized species (Hutchinson Citation1959, MacArthur Citation1970, Wright Citation1983).
Another study involved the population densities of the native American alligator (Alligator mississippiensis) in 60 Florida lakes having a wide range in trophic states (Evert Citation1999). The alligator population densities were most closely correlated with water-column TP, TN, and Chl-a concentrations and fish biomass; TP alone accounted for 55% of the variation in alligator population density. Evert noted that this finding supported the hypothesis that as nutrient levels increase among ecosystems, the abundance of the top predator increases.
Macroinvertebrates
The Florida Department of Environmental Protection (FDEP) participated in a study funded by the USEPA to examine macroinvertebrates in 310 Florida lakes as indicators of lake impairment (Gerritsen et al. Citation2000). The macroinvertebrate data were used to calculate the Lake Condition Index (LCI), which was intended to indicate lakes that were impaired due to human activities. However, subsequent reanalysis of the data by another consultant for the FDEP (Fore Citation2007) concluded that the LCI was not related to impairment due to human activities. Specifically, there was no correlation between indices of macroinvertebrate species composition and 2 independent measures of human disturbance. One of these is the Landscape Development Intensity index (LDI) developed by Brown and Vivas (Citation2005) and Lane and Brown (Citation2006), a GIS-based index of the intensity of land uses around lakes. The other is the Habitat Index based on Secchi depth and field estimates of vegetation quality, stormwater inputs, bottom substrate quality, lakeside adverse human alterations, upland buffer zone, and adverse watershed land uses. Fore (Citation2007) did find a correlation between the macroinvertebrate community compositions and phosphorus, nitrogen, and water clarity but noted that this correlation could be due to either anthropogenic or natural sources. Thus, Fore (Citation2007) concluded that macroinvertebrate indicators might not be reliable for assessing and reporting the biological condition of Florida lakes.
Aquatic macrophytes
As noted previously, the USEPA (2010a, page 75780) stated that lakes with Chl-a concentrations >20 μg/L are more likely to be eutrophic with reduced water clarity that would negatively affect native submerged macrophytes and the fauna that depend upon them. We with Bachmann et al. (Citation2002) tested this hypothesis about aquatic macrophytes with a large-scale study of aquatic macrophytes in 319 mostly shallow, polymictic, Florida lakes. Their aim was to look for relationships between trophic state indicators and the biomasses of plankton algae, algal periphyton, and macrophytes. The lakes ranged from oligotrophic to hypereutrophic with total algal chlorophylls ranging from 1 to 241 μg/L. There were strong positive correlations between planktonic chlorophylls and TP and TN, but there were weak inverse relationships between the densities of periphyton and the trophic state indicators TP, TN, and Chl-a. Periphyton biomass was positively correlated with Secchi depth.
There was no predictable relationship between the abundance of emergent, floating-leaved, and submersed aquatic vegetation and the examined trophic state indicators. For example, the percent of the lake area covered by submerged macrophytes at different concentrations of chlorophyll () was so variable that, contrary to the assumption of the USEPA (2010b), the biomasses of submersed macrophytes were no lower in eutrophic lakes than mesotrophic lakes, except for the most eutrophic lakes with TP concentrations >100 μg/L. The phosphorus–chlorophyll and phosphorus–Secchi depth relationships in this dataset of Florida lakes were not influenced by the amounts of aquatic vegetation present, indicating that the role of macrophytes in clearing lakes may be primarily to reduce nutrient concentrations for a given level of loading. Rather than nutrient concentrations controlling macrophyte abundance, it seems that macrophytes reduced nutrient concentrations in the water (Bachmann et al. Citation2002).
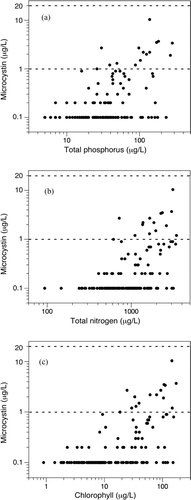
Figure 3 Average annual concentrations of microcystins in 187 Florida lakes versus their average concentrations of (A) total phosphorus, (B) total nitrogen, and (C) uncorrected chlorophyll. The dashed lines represent the WHO guidance values for drinking water (1 μg/L) and contact recreation (20 μg/L). Lakes with undetectable microcystin concentrations (0.1 μg/L) were plotted as 0.1 μg/L. Data are from Bigham et al. (Citation2009).
Plankton algae
Recreational uses
Numerous studies on Florida lakes have shown a correlation between the nutrients TP and TN and algal populations as measured by Chl-a (Canfield Citation1983, Bachmann et al. Citation2003, Brown et al. Citation2000); however, these studies do not directly provide a concentration threshold for TP, TN, or Chl-a that would cause impairment of recreational uses.
Heiskary and Walker (Citation1988) reported on the use of surveys of lake users participating in citizen monitoring programs in Minnesota to determine relationships between water quality measurements and the perceptions of lake users on physical appearance and recreational suitability. One of the questions sought opinions on how suitable a lake was for recreation and aesthetic enjoyment on the day of sampling. The answers were compared with the measured concentrations of TP, Chl-a, and Secchi depth on that day. The interquartile range (25th–75th percentile) of Chl-a concentrations for respondents who checked “excellent for swimming” were 5–14 μg/L. Lakes where the users checked “swimming and aesthetic enjoyment slightly impaired because of algal levels” had an interquartile range of Chl-a concentrations of 14–54 μg/L. In a similar survey in Florida with the same questions, Hoyer et al. (Citation2004) found the interquartile range of Chl-a concentrations for respondents who checked “excellent for swimming” was 3–13 μg/L; however, the response of “swimming and aesthetic enjoyment slightly impaired because of algal levels” had a smaller interquartile range of 5–18 μg/L than the range found for the Minnesota survey. Both studies showed that for aesthetic purposes and swimming lake users preferred lakes with greater Secchi depths and lower rather than higher concentration of TP and Chl-a; however, there was a great deal of variation in the responses.
Both Heiskary and Walker (1988) and Smeltzer and Heiskary (Citation1990) found regional differences both within and among states in the user perceptions of what levels of Chl-a constituted recreational impairment. Users in regions with very low concentrations of Chl-a in their lakes had a lower tolerance for lake algae than did lake users in regions having lakes with higher concentrations of Chl-a. In a less comprehensive study, Hoyer et al. (Citation2004) found the same trend in Florida lakes. Smeltzer and Heiskary (Citation1990) used those differences in a 3-step process to set TP criteria for each ecoregion in Minnesota. The methodology took into account the TP impacts on lake conditions, the TP impacts on lake users, and the attainability of achieving desired TP concentrations in each of the ecoregions for their most sensitive uses. Their regional criteria for TP ranged from 15 to 90 μg/L. Both of these studies would support the idea of protecting oligotrophic lakes from increases in trophic state to meet user desires for recreational swimming.
Cyanobacteria and microcystin
A problem with plankton algae cited by the USEPA (2010a) concerns the presence of high populations of cyanobacteria in eutrophic or hypereutrophic lakes. Under certain conditions, some of the species of this group have buoyant cells that float to the surface and can form scums that detract from the beauty of a lake. These scums can be concentrated by the wind and accumulate along the shore and on beaches.
Canfield et al. (Citation1989) and Duarte et al. (Citation1992) have studied the factors influencing the abundance of cyanobacteria in Florida lakes. They found that, while cyanobacteria biomass in Florida lakes had a weak negative relationship with water transparency (R2 = 0.12) and positive relationships with TP (R2 = 0.11) and TN (R2 = 0.22), it was positively correlated to total algal biomass (R2 = 0.81). While the biomasses of cyanobacteria are consistently dominant in hypereutrophic lakes when total algal biomass exceeds 100 mg/L, they could also dominate in lakes of a lesser trophic state as well.
In addition to the surface scums, some cyanobacterial species under certain circumstances can produce toxins such as microcystins that may be harmful to wildlife or humans at high enough concentrations (USEPA 2010b). The USEPA has been studying the algal toxin problem for some time; however, they have not yet established numeric criteria for algal toxins in the waters of the United States. The World Health Organization (WHO; Chorus and Bartram 1999) suggests a drinking water guidance value of 1μg/L for microcystin, a recreational guidance value of 20 μg/L for activities in direct contact with water (e.g., swimming), and 100 μg/L for activities having indirect contact with water (e.g., boating).
In Florida, Williams et al. (Citation2007) reported measurements of microcystins from 90 open-water samples taken from 6 lakes in the Harris Chain of Lakes and 67 samples from Lake Okeechobee and found that the median concentrations for each lake did not exceed the drinking water guidance value of 1μg/L. However, on 12 occasions they took water samples from surface scums in the Harris Chain of Lakes where floating cyanobacterial cells had accumulated, and the median concentration of microcystins was 550 μg/L.
Bigham et al. (Citation2009) developed a dataset to relate microcystin concentrations to lake trophic state as estimated by TP, TN, and Chl-a. They reported on a survey of microcystin concentrations in 187 Florida lakes sampled 6 times a year for 1 year. They sampled at open-water locations that are routinely used in the LAKEWATCH sampling program in the open waters of these lakes and they intended to sample in concentrated patches of floating cyanobacteria when they were observed; however, none were observed during that study. The lakes had a statewide distribution and a wide range in trophic states.
Table 3 Percent of water samples from 187 Florida lakes where the microcystin concentrations exceeded 1 μg/L for different ranges of total phosphorus (TP), total nitrogen (TN), and uncorrected chlorophyll a (Chl-a). All concentrations are in μg/L. Data are from Bigham et al. (Citation2009).
The average annual microcystin concentrations in these 182 studied lakes ranged from undetectable (<0.1 μg/L) to 12 μg/L. Only 29% of the lakes had detectable microcystin (>0.1 μg/L), and just 13 of the 187 lakes had annual lake averages that exceeded the WHO (2003) drinking water guidance value of 1 μg/L. None of these lakes had average microcystin concentrations that exceeded the WHO (2003) recreational guidance value of 20 μg/L (). The microcystin concentrations in individual water samples (N = 862) from all lakes ranged from undetectable to 32 μg/L. Only 7% of all the individual samples exceeded the WHO (2003) drinking water guidance value of 1 μg/L. Only 3 individual water samples collected from 2 lakes (0.3%) exceeded the WHO (2003) recreational guidance value of 20 μg/L.
These data provide some guidance for drinking water lakes (Class I) using the WHO (2003) drinking water guidance value of 1 μg/L. Annual average microcystin concentrations start to exceed 1 μg/L at average values of TP of about 25 μg/L, TN of about 700 μg/L, and uncorrected chlorophyll of about 25 μg/L. The frequencies that individual samples exceed 1μg/L of microcystin () show similar thresholds for TP, TN, and Chl-a. For lakes with contact recreation (Class III), even the most eutrophic lakes in the sample did not have average microcystin concentrations that exceeded the WHO guidance level of 20 μg/L. The probability of an open-water sample exceeding the drinking water guidance value begins at a chlorophyll concentration of about 10 μg/L, while the 3 samples that exceeded the 20 μg/L recreational guideline had uncorrected chlorophyll concentrations of 130 μg/L or more.
The results of these studies would indicate that the risks of microcystins for recreational uses in the open waters of Florida lakes are low, even in eutrophic lakes. The problems would seem to be with the concentrated patches of floating cyanobacteria. When Williams et al. (Citation2007) sampled such patches, they found that the concentrations of microcystin could be high enough to be of concern if swimmers ingested them.
There is no easy way to predict where or when cyanobacteria patches may form on a lake. The use of numeric nutrient criteria to prevent them from forming might require unattainable concentrations of TP and TN. Management practices such as closing beaches and notifying swimmers to avoid patches with algal scums might be the most effective management solution to this problem.
Discussion
Biology
Our goal was to determine how the concentrations of TP and TN and the resultant phytoplankton biomasses as measured by Chl-a are related to the designated uses of Florida lakes. In particular, we wanted to test the assumption that all eutrophic lakes in Florida, regardless of whether they are naturally eutrophic or culturally eutrophic, do not meet their designated uses. When setting criteria for potentially toxic substances in lake and river waters, a series of standard protocols can be followed. Laboratory bioassays such as those outlined in APHA (1989) are often used where several different aquatic organisms are exposed to a range of concentrations of the toxin to find the harmful or lethal concentration. This information would form the basis for a criterion for that substance in natural waters that would not be harmful to the biota. Because the plant nutrients phosphorus and nitrogen are not toxic at the levels found in Florida lakes, but rather are essential for the support of living organisms, a different approach has to be used to set critical levels.
Our approach has been to examine studies of the biological communities among Florida lakes that have a range of trophic states from oligotrophic to hypereutrophic as expressed by TP, TN, and Chl-a concentrations. Because we were looking empirically at the response of organisms to nutrients, it was not necessary to know the extent to which the TP and TN were derived from natural sources or from anthropogenic loading. We were looking for changes in the biological characteristics across the spatial gradients in nutrient concentrations that might bear on the designated uses of individual Florida lakes.
We did not find any biological threshold that can be used to establish numeric nutrient criteria in Class III Florida lakes that have multiple designated uses of recreation and the propagation and maintenance of a healthy, well-balanced population of fish and wildlife. We found that in Florida lakes, fish standing crops increased with the concentrations of TP, TN, and Chl-a, with seemingly no absolute upper limit within the concentration ranges currently found in Florida lakes. Wagner and Oglesby (Citation1984) also published a similar graph showing fish yields increasing linearly with Chl-a in a group of lakes with a maximum Chl-a concentration of 200 μg/L. The important sport fish did not decrease in abundance as lakes became more eutrophic, and there was no change in common indices of biological integrity for fish related to human impacts (Schulz et al. Citation1999). The top fishing lakes in Florida, as determined by the FFWCC biologists, have higher nutrient concentrations than average Florida lakes, and many of the top fishing lakes would be classified as impaired under the USEPA (2010a) nutrient criteria for TP, TN, or Chl-a.
Florida is different from some northern states like Minnesota (Heiskary and Wilson Citation2008) and elsewhere where coldwater fish like trout and whitefish need a cold, oxygenated hypolimnion to survive the summer. In colder waters, nutrient criteria are set to limit primary production in the epilimnion to reduce the contribution of dead organic material to the hypolimnion, which might deplete the dissolved oxygen during stratification. Also, in northern lakes where warm-water fish are dominant, numeric standards are established to limit biological productivity to prevent the loss of oxygen in the winter when ice cover prevents replenishment of oxygen from the atmosphere. This is not a problem with Florida lakes that do not have a winter ice cover.
With regard to maintaining a favorable environment for wildlife, we found the abundance and diversity of aquatic birds also increase with nutrient concentrations, as did the abundance of alligators. We also noted that benthic macroinvertebrate indices of lake condition by themselves could not be related to anthropogenic nutrient pollution. We could find no evidence that plant nutrients in the water are responsible for excessive populations of aquatic macrophytes. We also found that, contrary to the assumption of the USEPA (2010b), the biomasses of submersed macrophytes were no lower in eutrophic lakes than mesotrophic lakes, except for the most eutrophic lakes with TP concentrations >100 μg/L.
When the macroinvertebrate communities were studied to develop a biological assessment index that could be used to identify Florida lakes impaired by anthropogenic nutrient enrichment, Fore (Citation2007) did find changes in some of the macroinvertebrate community metrics that could be related to the concentrations of trophic state indicators such as TP, total Kjeldahl nitrogen, Chl-a, and Secchi disk transparency. This finding was expected because limnologists have long known that the benthic macroinvertebrate communities of lakes reflect their trophic state (Wetzel Citation2001), so the macroinvertebrate communities of eutrophic lakes are naturally different from those of oligotrophic and mesotrophic lakes. However, Fore (Citation2007) found no correlation between 2 measures of anthropogenic activity around the study lakes and either the macroinvertebrate communities or the nutrient concentrations and clarity of the water. She concluded that the index could not be used to identify anthropogenically-impacted lakes because it was not possible to distinguish between anthropogenic and natural sources of phosphorus and nitrogen. This same problem will probably be true for the use of any kind of biological index to determine if a lake has been impacted by anthropogenic sources of nutrients because there is no evidence that the biological communities of lakes naturally rich in TP and TN are any different from those in lakes with the same nutrient status due to artificial enrichment.
The biological indices do not stand alone but can be useful if they are measured over time and show a significant change that can be related to artificial eutrophication. Many Florida lakes, such as Lake Wauberg, have had naturally high concentrations of TP and TN since before significant European settlement in the surrounding region (Riedinger-Whitmore et al. Citation2005), while other lakes, such as Lake Jessup (Cable et al. Citation1997), at one time had high levels of TP and TN in part due to the inflow of effluents from 7 different sewage treatment plants. In both cases, the algal populations are high, one because of natural factors and the other because of pollution. This means that we cannot use a current concentration of Chl-a or a macroinvertebrate index alone as an absolute indicator of impairment of designated uses due to man-caused loadings of TP and/or TN.
When we reviewed the studies on open-water concentrations of the cyanobacterial toxin microcystin in 187 Florida lakes, we found that none of the lakes had annual average concentrations that exceeded the WHO (2003) guidance level of 20 μg/L for recreational activities. Only 3 individual water samples of 862 samples collected from 2 of 187 lakes exceeded the WHO recreational guidance value. We did note a study (Williams et al. Citation2007) that sampled floating accumulations of cyanobacteria and found that the concentrations of microcystin could be high enough to be of concern if ingested by swimmers. The USEPA chlorophyll criterion of 20 μg/L would not necessarily prevent the formation of such patches in a lake because cyanobacteria are also found in mesotrophic and oligotrophic lakes and would be subject to concentration by wind-driven currents. It is common practice to close beaches when such patches of algae are found in swimming areas.
Only 16 Florida lakes are classified for drinking water supplies (Class I), and we could not find literature reporting on their suitability as raw water supplies. For this reason we have directed our discussions to the designated uses of the Class III lakes because those uses apply to all 7700 lakes, in Florida, including the Class I lakes. We have presented available information on the trophic states of some lakes where microcystin concentrations exceeded the WHO suggested guidance concentration of 1 μg/L. We noted that annual average microcystin concentrations start to exceed this level at average TP values of about 25 μg/L, TN of about 700 μg/L, and uncorrected chlorophyll of about 25 μg/L. These numbers might provide some guidance for establishing numeric nutrient criteria for Class I lakes in Florida; however, we do not have sufficient information to recommend criteria that would be protective of public health when these waters are used as a raw water supply.
Designated uses
A lake cannot be all things to all people, and the natural diversity of lakes in Florida (Griffith et al. Citation1997, Bachmann et al. Citation2012) will make individual lakes suited for different uses. For example, the natural oligotrophic lakes will have aesthetic values for their great water transparency and would also be excellent sources for potable water supplies because little treatment would be needed. In comparison, the more productive eutrophic lakes make excellent lakes for the propagation of fish and wildlife and are economically valuable for that reason. Wagner and Oglesby (Citation1984) also noted the same incompatibility of fishery optimization and other management objectives such as water supply, contact recreation, and aesthetics. Different kinds of lakes serve different kinds of recreational activities, including but not limited to, swimming, water skiing, boating, air boating, bird watching, hunting, and fishing. The result is a diversity of uses that fit the diversity of Florida lakes.
Rather than establishing different classifications of use for different lake types, the State of Florida chose a multiple use approach and designated all its freshwater lakes for recreation and the propagation and maintenance of a healthy, well-balanced population of fish and wildlife, with some also designated for potable water supplies. This makes it impossible to create one set of criteria that will be optimal for each designated use in all lakes.
USEPA approach
The USEPA (2010b) used a different approach to determine what concentrations of TP, TN, or Chl-a would place Florida lakes in an impaired category that would not meet their designated uses. As outlined in the technical document, the USEPA (2010b) started with the assumption that eutrophic lakes did not meet the designated uses for Florida lakes and then determined what concentrations of TP, TN, or Chl-a would place Florida lakes in a eutrophic category. The USEPA (2010b) did not present any data or studies on Florida lakes, as we did, that would show why eutrophic lakes would not be suitable for any or all of the designated uses for Class I or Class III lakes.
The decision that eutrophic lakes did not meet their designated uses was based on Table 2.3 from the USEPA (2010b) technical document, derived from 2 publications (OECD 1982, Salas and Martino Citation1991) listing trophic categories and use impairment. The Organisation for Economic Co-operation and Development study (OECD 1982) did not use a quantitative procedure to relate how well a eutrophic lake would meet various designated uses, and the OECD (1982, p 89–92) discussion of water quality and lake uses did not categorically state that all eutrophic lakes were of poor water quality. It pointed out that when Vollenweider (Citation1968) designated the TP loading that would result in a eutrophic lake as “excessive,” he was basing this boundary on oligotrophic lakes that received accelerated loading that resulted in increased production with attendant problems. In other words, if an oligotrophic lake received an artificial loading of TP and/or TN that transformed it into a eutrophic lake, then there would be use impairment. The OECD (1982) report went on to point out that it would not be excessive loading if it was a natural loading that produced a eutrophic lake, and in areas with high soil fertility a moderately eutrophic condition would be quite acceptable for many intended uses. This statement is particularly important in Florida where there are many naturally eutrophic lakes due to deposits of phosphatic rocks in various parts of the state.
The OECD (1982) discussion also pointed out that water quality objectives relate to the intended water use, so the adjectives “good” or “poor” quality water are meaningless without reference to the intended use. The text stated that, considering the various trophic classes for the purpose of multiple use (disregarding eutrophic waters used for fish and wildlife production), oligotrophic lakes would create no problems, mesotrophic lakes would create some problems, and eutrophic lakes would pose many problems for various uses. In our opinion this is not a blanket justification to declare all eutrophic lakes in Florida as impaired because OECD (1982) excluded fish and wildlife production from their analysis of water quality and intended lake uses. Fish and wildlife propagation are highly valued designated uses for Class III lakes in Florida and are specifically mentioned in the laws governing designated uses.
Just as the OECD (1982) report provided no quantitative basis to support the contention that eutrophic Florida lakes do not meet their designated uses, the same is true for the report of Salas and Martino (Citation1991). They did not conduct any studies related to trophic state and use impairment, but rather summarized the subjective opinions of unnamed investigators who reported on a small sample of warm-water tropical lakes. Most of the lake examples were artificial reservoirs in Brazil and Argentina, with only one Texas lake representing the United State and no lakes located in Florida.
In summary, the USEPA (2010b) presented no quantitative studies demonstrating that eutrophic lakes in Florida do not meet their designated uses. Our approach using data from Florida lakes indicates that trophic state by itself, without considering changes over time, should not be used to determine whether lakes meet their designated uses.
Need for an alternative approach
The problem with the USEPA approach lies in the word “altered” in the Florida narrative standard for lakes: “In no case shall nutrient concentrations of a body of water be altered so as to cause an imbalance in natural populations of aquatic flora or fauna.” This is illustrated by the example of 2 Florida lakes selected by the Florida Department of Environmental Protection as Benchmark lakes (USEPA 2010b). The Benchmark lakes were selected on the basis of no or minimal anthropogenic disturbance in their watersheds and as such should be close representations of Florida lakes prior to European settlement. Lake Annie in Highlands County is oligotrophic (TP = 7 μg/L, TN = 394 μg/L, and Chl-a = 4 μg/L) and is located in the Southern Lake Wales Lake Region (Griffith et al. Citation1997), where oligotrophic to mesotrophic lakes are common. Lake Wauberg in Alachua County is eutrophic (TP = 127 μg/L, TN = 1952 μg/L and Chl-a = 75 μg/L) and is in the Central Valley Lake Region (Griffith et al. Citation1997), where eutrophic lakes are common, due in part to extensive deposits of phosphatic rocks. Analyses of core samples from Lake Wauberg show it was highly eutrophic at least back to 1884 (Riedinger-Whitmore et al. Citation2005).
Because these lakes are so different in their trophic states, we would expect a detailed examination of the species compositions of the flora and fauna of the 2 lakes to show some differences, and that their suitability for different kinds of designated uses such as recreation (e.g., swimming, bird watching, and boating) and the propagation of fish and wildlife would vary as well. Because apparently neither lake has been subject to anthropogenic pollution that could alter their nutrient status, neither meet the Florida definition of being impaired; yet the USEPA approach based on current trophic state would place Lake Wauberg on the impaired list without evidence that it has received pollution. Our data indicate that many naturally eutrophic lakes in Florida will be wrongly declared as impaired (Bachman et al. 2012).
The logical conclusion is that in Florida we should not use trophic state measurements at just one point in time to determine if a lake has been impaired. We need to show that the nutrient concentrations have been altered from a previous state due to anthropogenic activities. If long-term data are not available to show a change, paleolimnological analyses on long sediment cores might be used to infer past conditions. An additional approach would be to group similar types of lakes based on the USEPA lake regions (Griffith et al. Citation1997). This type of regionalization approach has worked well in Minnesota (Heiskary and Wilson Citation2008) where lakes also have a broad range of trophic states that can be related to geographic regions. One could then establish the expected trophic character of the lakes in that region and set criteria that would maintain them in their expected state.
References
- [APHA] American Public Health Association . 1989 . Standard methods for the examination of water and wastewater. 17th ed , New York : American Public Health Association, Inc .
- Bachmann , R W , Bigham , D L , Hoyer , M V and Canfield , D E Jr . 2012 . Factors determining the distributions of total phosphorus, total nitrogen and chlorophyll a in Florida lakes . Lake Reserv Manage. 28(1):10–26 ,
- Bachmann , R W , Horsburgh , C A , Hoyer , M V , Mataraza , L K and Canfield , D E Jr . 2002 . Relations between trophic state indicators and plant biomass in Florida lakes . Hydrobiologia. , 470 : 219 – 234 .
- Bachmann , R W , Hoyer , M V and Canfield , D E Jr . 2003 . Predicting the frequencies of high chlorophyll levels in Florida lakes from average chlorophyll or nutrient data . Lake Reserv Manage. , 19 : 229 – 241 .
- Bachmann , R W , Jones , B L , Fox , D D , Hoyer , M V , Bull , L A and Canfield , D E Jr . 1996 . Relations between trophic state indicators and fish in Florida (USA) lakes . Can J Fish Aquat Sci. , 53 : 842 – 855 .
- Bigham , D L , Hoyer , M V and Canfield , D E Jr . 2009 . Survey of toxic algal (microcystin) distribution in Florida lakes . Lake Reserv Manage. , 25 : 264 – 275 .
- Brown , C D , Hoyer , M V , Bachmann , R W and Canfield , D E Jr . 2000 . Nutrient-chlorophyll relationships: an evaluation of empirical nutrient-chlorophyll models using Florida and north-temperate lake data . Can J Fish Aquat Sci. , 57 : 1574 – 1583 .
- Brown , M T and Vivas , M B . 2005 . Landscape development index . Environ Monit Assess. , 101 : 289 – 309 .
- Cable , J E , Schelske , C L , Hansen , P S , Kenney , W F and Whitmore , T J . 1997 . Sediment and nutrient deposition in Lake Jessup, Florida (USA) , Palatka (FL) : Final Report to the St. Johns River Water Management District; SJ98–SP18 .
- Canfield , D E Jr. 1983 . Sensitivity of Florida lakes to acidic precipitation . Water Resour Res. , 19 : 833 – 839 .
- Canfield , D E Jr. and Hoyer , M V . 1988 . Regional geology and the chemical and trophic state characteristics of Florida lakes . Lake Reserv Manage. , 4 : 21 – 31 .
- Canfield , D E Jr and Hoyer , M V . 1992 . Aquatic macrophytes and their relation to the limnology of Florida lakes , Tallahassee (FL) : Florida Department of Natural Resource; Final Report submitted to the Bureau of Aquatic Plant Management .
- Canfield , D E Jr. , Phlips , E and Duarte , C M . 1989 . Factors influencing the abundance of blue-green algae in Florida lakes . Can J Fish Aquat Sci. , 46 : 1232 – 1237 .
- Chen , M and Ma , L Q . 2001 . Taxonomic and geographic distribution of total phosphorus in Florida surface soils . Soil Sci Soc Am J. , 65 : 1539 – 1547 .
- Chorus , I and Bartram , J , eds. 1999 . Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management , London and New York : E & FN Spon Publishers (published on behalf of the World Health Organization) .
- Chrisman , T L and Beaver , J R . 1990 . Applicability of planktonic biomanipulation for managing eutrophication in the subtropics . Hydrobiologia. , 200 : 177 – 185 .
- Duarte , C M , Agusti , S and Canfield , D E Jr . 1992 . Patterns in phytoplankton community structure in Florida lakes . Limnol Oceanogr. , 37 : 155 – 161 .
- Evert , JD. 1999 . Relationships of alligator (Alligator mississippiensis) population density to environmental factors in Florida lakes [master's thesis] , [Gainesville (FL):] : University of Florida .
- [FFWCC] Florida Fish and Wildlife Conservation Commission . 2009 . Florida's top fishing lakes as compiled by the Florida Fish and Game Commission [cited 29 Jun 2009]. Available from http://myfwc.com/
- Fore , LS. 2007 . Evaluation of benthic macroinvertebrate assemblages as indicators of lake condition , Florida : Prepared for the Florida Department of Environmental Protection, Tallahassee . [cited 25 May 2011]. Available from http://www.dep.state.fl.us/labs/docs/lake_macro_testing.pdf
- Gardarsson , A and Einarsson , A . 1994 . Response of breeding duck populations to changes in food supply . Hydrobiologia. , 279/280 : 15 – 27 .
- Gerritsen , J B , Jessup , B K , Leppo , E and White , J . 2000 . Development of lake condition indexes (LCI) for Florida , Florida : Prepared for the Florida Department of Environmental Protection, Tallahassee . [cited 25 May 2011]. Available from http://publicfiles.dep.state.fl.us/dear/sas/sopdoc/lci_final.pdf
- Griffith , G E , Canfield , D E Jr. , Horsburgh , C A and Omernik , J M . 1997 . Lake Regions of Florida , Corvallis (OR) : US Environmental Protection Agency; National Health and Environmental Effects Research Laboratory . EPA/R-97/127; [cited 5 July 2010] Available from http://www.epa.gov/wed/pages/ecoregions/fl_eco.htm.
- Heiskary , S A and Walker , W W . 1988 . Developing phosphorus criteria for Minnesota lakes . Lake Reserv Manage. , 4 : 1 – 9 .
- Heiskary , S A and Wilson , C B . 2008 . Minnesota's approach to lake nutrient criteria development . Lake Reserv Manage. , 24 : 282 – 297 .
- Hoyer , M V , Brown , C D and Canfield , D E Jr . 2004 . Relations between water chemistry and water quality as defined by lake users in Florida . Lake Reserv Manage. , 20 : 240 – 248 .
- Hoyer , M V and Canfield , D E Jr . 1994a . Bird abundance and species richness on Florida lakes: Influence of trophic status, lake morphology, and aquatic macrophytes . Hydrobiologia. , 297/280 : 107 – 119 .
- Hoyer , M V and Canfield , D E Jr . 1994b . Handbook of common freshwater fish in Florida lakes , Gainesville (FL) : University of Florida; Institute of Food and Agricultural Sciences; Spec Pub. 160 .
- Hoyer , M V , Notestein , S K , Frazer , T K and Canfield , D E Jr . 2006 . Bird density, biomass, and species richness on five Florida coastal rivers, with comparisons to Florida lakes . Hydrobiologia. , 567 : 5 – 18 .
- Hutchinson , GE. 1959 . Homage to Santa Rosalia, or ‘Why are there so many kinds of animals?’ . Am Natl. , 93 : 137 – 145 .
- Iglesias , C , Mazzeo , N , Goyenola , G , Fosalba , C , De Mello , F T , Garcia , S and Jeppesen , E . 2008 . Field and experimental evidence of the effect of Jenynsia multidentata, a small omnivorous-planktivorous fish, on the size distribution of zooplankton in subtropical lakes . Freshwater Biol. , 53 : 1797 – 1807 .
- Jeppesen , E , Meerhoff , M , Jacobsen , B A , Hansen , R S , Sondergaard , M , Jensen , J P , Lauridsen , T L , Mazzeo , N and Branco , C WC . 2007 . Restoration of shallow lakes by nutrient control and biomanipulation-the successful strategy varies with lake size and climate . Hydrobiologia. , 581 : 269 – 285 .
- Kautz , RS. 1980 . Effects of eutrophication on the fish communities of Florida lakes . Proceedings of the Annual Conference of the SE Association of Fish and Wildlife Agencies , 34 : 67 – 80 .
- Lane , C R and Brown , M T . 2006 . Energy-based land use predictors of proximal factors and benthic diatom composition in Florida freshwater marshes . Environ Monit Assess. , 117 : 433 – 450 .
- Lewis , W M Jr . 1996 . “ Tropical lakes: how latitude makes a difference ” . In Perspectives in tropical limnology , Edited by: Schiemer , F and Boland , K T . 43 – 64 . Amsterdam : SPB Academic Publ. .
- Lewis , W M Jr . 2000 . Basis for the protection and management of tropical lakes . Lake Reserve Manage. , 5 : 35 – 48 .
- MacArthur , R. 1970 . Species packing and competitive equilibrium for many species . Theor Popul Biol. , 1 : 1 – 11 .
- Mazumder , A and Havens , K E . 1998 . Nutrient-chlorophyll-Secchi relationships under contrasting grazer communities of temperate versus subtropical lakes . Can J Fish Aquat Sci. , 55 : 1652 – 1662 .
- Nummi , P , Poysa , H , Elmberg , J and Sjoberg , K . 1994 . Habitat distribution of the mallard in relation to vegetation structure, food, and population density . Hydrobiologia. , 279/280 : 247 – 252 .
- [OECD] Organisation for Economic Development and Co-Operation . 1982 . Eutrophication of waters. Monitoring, assessment and control , Paris. : OECD .
- Riedinger-Whitmore , M , Whitmore , T , Smoak , J , Brenner , M , Moore , A , Curtis , J and Schelske , C . 2005 . Cyanobacterial proliferation is a recent response to eutrophication in many Florida lakes: A paleolimnological assessment . Lake Reserv Manage. , 21 : 423 – 435 .
- Salas , H J and Martino , P . 1991 . A simplified phosphorus trophic state model for warm-water tropical lakes . Water Res. , 25 : 341 – 350 .
- Schulz , E J , Hoyer , M V and Canfield , D E Jr . 1999 . An index of Biotic Integrity: A test with limnological and fish data from sixty Florida lakes . Trans Am Fish Soc. , 128 : 564 – 577 .
- Smeltzer , E and Heiskary , S A . 1990 . Analysis and applications of lake user survey data . Lake Reserv Manage. , 6 : 109 – 118 .
- Terziotti , S , Hoos , A B , Harned , D A and Garcia , A M . 2010 . Mapping watershed potential to contribute phosphorus from geologic materials to receiving streams, Southeastern United States , Scientific Investigations Map 3102; [cited 25 May 2011] . Available from http://pubs.usgs.gov/sim/3102/
- [USEPA] United States Environmental Protection Agency . 2010a . Water quality standards for the state of Florida's lakes and flowing waters . Federal Register , 75 : 131 (26 January 2010) 4173–4226
- [USEPA] United States Environmental Protection Agency . 2010b . Technical support document for the U. S , EPA's final rule for numeric criteria for nitrogen/phosphorus pollution in Florida's inland surface fresh waters; [cited 6 Mar 2011] . Available from http://water.epa.gov/lawsregs/rulesregs/upload/floridatsd1.pdf
- Vollenweider , RA. 1968 . Scientific fundamentals of the eutrophication of lakes and flowing waters with particular reference to nitrogen and phosphorus as factors in eutrophication , Paris (France) : Organization for Economic Co-Operation and Development; Tech Report No. DAS/CSI/68.27 .
- Wagner , K J and Oglesby , R T . 1984 . Incompatibility of common lake management objectives . Lake Reserv Manage. , 1 : 97 – 100 .
- Wetzel , RG. 2001 . Limnology: Lake and river ecosystems. 3rd ed , New York (NY) : Academic Press .
- Williams , C D , Aubel , M T , Chapman , A D and D’Aiuto . 2007 . Identification of cyanobacterial toxins in Florida's freshwater systems . Lake Reserv Manage. , 23 : 144 – 152 .
- [WHO] World Health Organization . 2003 . Guidelines for Safe Recreational Water Environments, Volume 1: Coastal and Fresh Waters Geneva [cited 27 Aug 2010]. Available from http://www.who.int/water_sanitation_health/bathing/srwe1/en/
- Wright , DH. 1983 . Species-energy theory: an extension of species-area theory . Oikos. , 41 : 496