Abstract
We examined the larval remains of chironomids in 12 stocked brook trout lakes from Nova Scotia using 2 paleolimnological approaches (i.e., top–bottom approach and stratigraphic analyses). Limited monitoring data have indicated that almost all of the survey lakes contained poor coldwater-fish habitat; therefore, concerns have been raised that oxygen conditions have deteriorated and suitable habitat for brook trout may have declined. Our goal was to evaluate shifts in water quality, with a focus on understanding trends in hypolimnetic oxygen concentrations. No strong directional changes in the relative abundances of chironomids were recognized using top–bottom (12 lakes) and downcore stratigraphic (4 lakes) analyses. Chironomid assemblage compositions have not differed significantly between modern and preindustrial time periods (ANOSIM: R = −0.06, P = 0.94). Changes (if any) in oxygen conditions over the last ∼2 centuries likely were subtle and not of sufficient duration and (or) magnitude to strongly influence chironomid assemblage compositions through time. Detrended correspondence analysis of downcore assemblages indicated that taxa turnover was minimal (≤1 SD) at 3 of 4 lakes. We conclude that oxygen conditions (which our proxy data indicate were likely naturally low) have not deteriorated significantly since preindustrial times, and no overall directional trends are yet obvious in the paleolimnological record. Combining the often complimentary information gained from paleolimnological, monitoring, and modeling analyses, as we do here, can aid in the management of aquatic ecosystems and highlight the relative importance of regional trends in water quality and its influence on aquatic organisms such as recreationally significant salmonids.
The brook trout (Salvelinus fontinalis) is the most popular sport fish in Atlantic Canada and Quebec (Fisheries and Oceans Canada Citation2007). Angling-associated revenue is important to Nova Scotia, with 60,000 licensees contributing $86 million annually to the provincial gross domestic product (NSAF Citation2005). Based on sport fishing's monetary value and recreational significance, stocking of brook trout is practiced throughout Nova Scotia. However, a key factor limiting the distribution of this species is the availability of suitable summer habitat. Brook trout require coldwater (<20 C) with abundant dissolved oxygen (Power Citation1980, MacMillan et al. Citation2008). Kerr (Citation2000) suggests that brook trout generally require dissolved oxygen concentrations [DO] ≥5.0 mg/L, although optimum oxygen conditions are poorly understood (Raleigh Citation1982).
One common effect of anthropogenic development on freshwaters is greater primary productivity due to nutrient loading far above natural levels (Smith Citation2003, Smith and Schindler Citation2009). Cultural eutrophication results in increased decomposition near the sediment-water interface of deeper waters, thereby influencing [DO] during periods of thermal stratification. Fish that require high [DO] may be negatively impacted by oxygen depletion (Pollock et al. Citation2007). For example, Evans (Citation2007) demonstrated that even a minor decline of [DO], from 6 to 5 mg/L, can have considerable negative effects on lake trout (Salvelinus namaycush).
Reductions in the annual catch of brook trout by ∼60% over the last several decades in Nova Scotia have been linked to cultural eutrophication as well as other stressors such as habitat loss, introduced species, and over-exploitation by anglers; likely, declines in license sales also play a role (NSAF Citation2005, MacMillan et al. Citation2008). To understand water quality conditions, a study was commissioned by Nova Scotia Department of Agriculture and Fisheries (NSAF) and 20 brook trout lakes that were originally surveyed in July or August once during the 1970–1980s were reexamined twice in July or August 2001 (Brylinsky Citation2002). Eighteen of the 20 study lakes showed “poor” oxygen conditions (DO saturation ≤50%) for coldwater fish habitat (Brylinsky Citation2002). By comparison, the original dataset revealed poor oxygen conditions in only 11 lakes.
These “snap-shot” monitoring data must be interpreted cautiously, as acknowledged by Brylinksy (Citation2002). As such, key questions remained: Are the spot measures representative of overall limnological changes in brook trout lakes annually-stocked by the province of Nova Scotia, or are the measures an artefact of variability within a season or between years? Without long-term monitoring data, it is a challenge to track environmental changes or set mitigation goals (Quinlan et al. Citation2008, Smol Citation2010). Fortunately, lake sediments archive past environmental changes. Often, biological proxies that preserve well within the sediments can be used to reconstruct limnological change (Smol Citation2008).
The remains of midge larvae (Chironomidae and Chaoboridae) are now commonly enumerated from sediments (Walker Citation2001). Assemblages in thermally stratified lakes are often related to trophic status and (or) hypolimnetic oxygen levels (Brodersen and Quinlan Citation2006, Paterson et al. Citation2009, Quinlan and Smol Citation2002, Citation2010). The distributions of midge taxa also sort along oxygen gradients both among and within lakes (Brodersen and Lindegaard Citation1999, Brooks et al. Citation2001, Brodersen and Quinlan Citation2006). Additionally, laboratory studies provide empirical knowledge regarding the oxygen requirements of key midge taxa (Brodersen et al. Citation2004, Citation2008), and these findings compare well with the field-observed oxygen optima of similar taxa in Quinlan and Smol (Citation2001a, 2010).
Given the concerns regarding oxygen conditions in Nova Scotia brook trout lakes (Brylinsky Citation2002), a midge-based paleolimnological study was initiated to address the following questions: (1) Are chironomid assemblages, when compared between modern and preindustrial times, indicative of changes in hypolimnetic oxygen conditions, and (2) Do chironomid assemblages from dated sediment cores show directional change consistent with deteriorating water quality? Whereas recent paleolimnological studies from Nova Scotia have utilized diatom assemblages and focused on the effects of acid deposition (Ginn et al. Citation2007a, Citation2007b, Tropea et al. Citation2007), shoreline development (Thienpont et al. Citation2008), and climate (Ginn et al. Citation2007b, Citation2008), results from this invertebrate-based study offer managers a long-term perspective on oxygen conditions in Nova Scotia lakes that contain habitat perceived to be suitable for brook trout.
Materials and methods
Study area
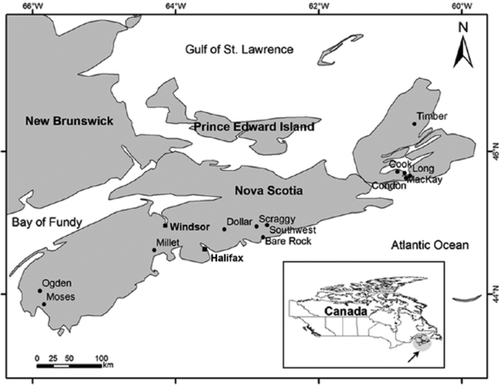
The lakes used in this study are located in the province of Nova Scotia, Canada, along a southwest to northeast gradient (). Nova Scotia is the second most densely populated province in Canada and contains ∼9400 lakes >1 ha in surface area (Davis and Browne Citation1996). The geology of the province is divided across a northwest transect from Halifax to the Windsor area of the Minas Basin into distinctive regions of northern and southern geology, which ultimately influences regional lake water chemistry. The northern portion of the province is composed mainly of Carboniferous-aged sedimentary rocks, whereas the southern region is composed of Triassic-aged volcanics and Devonian-aged slates, limestone, and granite (Davis and Browne Citation1996). Much of the province is highly susceptible to acid deposition due to poor buffering from noncarbonate bedrock (Davis and Browne Citation1996). Regions of Nova Scotia record some of the lowest surface water pH values on the continent (Morrison Citation2004). However, many Nova Scotian lakes were historically acidic because of natural processes associated with high dissolved organic carbon (DOC) and the deposition of sulphate from natural marine sources (Jeffries Citation1997).
We selected 12 lakes for study to capture a region-wide assessment of lake responses to potential water quality changes based on results from the Nova Scotia Lake Hypolimnion Project (Brylinsky Citation2002). Generally, lakes were slightly acidic to circumneutral (pH: 5.1–7.9), relatively shallow (maximum depth: 6–27 m), mesotrophic (total phosphorus (TP): 7–30 μg/L), and showed a hypoxic to well-oxygenated hypolimnion ([DO]: 2.0–9.1 mg/L). All study lakes exhibited thermal stratification except Ogden Lake, the shallowest study site (). Of the lakes in common to this study and the Hypolimnion Project, Brylinsky (Citation2002) reported poor habitat status for brook trout during both July and August 2001 sampling periods at 7 lakes, whereas “good” habitat status was observed only at Millet Lake. Catchments ranged from pristine with no surrounding development to low-to-moderate shoreline development.
Table 1 Summary of physical and water quality measurements for the 12 study lakes. All lakes were sampled in Jul 2003, except Timber and Millet (Jul 2004). [DO] = dissolved oxygen concentration.
Field and laboratory methods
During field seasons in July 2003 and 2004, sediment cores were collected from the deepest basin in each of the study lakes using a standard gravity corer (Glew Citation1989). Cores ranged in length between 30 and 40 cm and were sectioned in the field at 0.5 cm intervals using a vertical extruder (Glew Citation1988). The sectioned intervals were then placed into labeled, sealable bags for transport and cold storage at 4 C until analyzed. Using standard protocols, [DO], Secchi depth, pH, and water temperature measurements were collected by NSAF concurrent with sediment coring ().
Four of the 12 lakes were selected for detailed paleolimnological analyses; therefore, cores required age estimation of sediment intervals. Select sediment intervals from each core were analyzed for 210Pb, 137Cs, and 214Bi activities using gamma spectrometry. Chronologies were determined by 210Pb activities and cumulative dry mass using the constant rate of supply model (Appleby Citation2001).
Samples of 0.28–0.78 g of freeze-dried sediment were prepared for chironomid analyses following standard procedures outlined by Walker (Citation2001). Briefly, sediments were deflocculated in 5% potassium hydroxide (KOH) at 70 C for ∼30 min, then rinsed with deionized water onto a 100 μm sieve. Sediments remaining on the sieve were then concentrated into a beaker with deionized water, and aliquots were poured into a Bogorov tray. Chironomid head capsules and Chaoborus mandibles were manually retrieved from the Bogorov tray using a dissecting microscope at ∼25× magnification and forceps. A sample was considered complete when all chironomid head capsules and chaoborid mandibles were retrieved. Remains were then placed onto coverslips and mounted permanently onto slides.
Midges were examined using bright-field microscopy at 200–400× magnification. Chironomids were identified primarily using Wiederholm (Citation1983) and Walker (Citation1988). A minimum count of 50 chironomids was targeted as a reliable estimate of the assemblage (Heiri and Lotter Citation2001, Larocque Citation2001, Quinlan and Smol Citation2001b). Of 56 total samples, only 2 samples (i.e., 0–0.5 cm from Long and Ogden lakes) did not meet this target (mean = 133 individuals, min = 29, max = 310). Taxa were expressed as percent relative abundances of the total chironomid count (i.e., 2 mentum halves equal 1 individual).
Numerical analyses of chironomid assemblages
To examine assemblage responses to recent limnological change in our 12 study lakes, we used a top–bottom paleolimnological approach (Smol Citation2008). Our approach compares assemblages from lake surface sediments, which represent modern conditions (lakes were cored in Jul 2003 and 2004), to assemblages from older lake sediments, which likely represent preindustrial conditions (∼1850). Radiometric dating of sediments from Nova Scotia lakes varying in size and basin morphometry have demonstrated that a sediment interval beyond ∼15 cm depth is generally representative of the preindustrial period (Ginn et al. Citation2007a, Citation2007b, Citation2008, Korosi and Smol Citation2011).
Taxa that were not present in at least 2 samples with a relative abundance of ≥2% were removed prior to numerical analyses. Assemblage similarity between the top (0–0.50 cm) and bottom (depths were between 28.5 and 41.5 cm) sediment samples from each lake were analyzed using the Bray-Curtis dissimilarity coefficient (Bray and Curtis Citation1957). Analysis of similarities (ANOSIM) was then completed to test for significant (P < 0.05) differences in chironomid assemblage composition between modern and preindustrial periods, using R v 2.11.1 (R Development Core Team Citation2010) and the vegan package (Oksanen et al. Citation2010). ANOSIM is a nonparametric analysis that compares within and across group-rank dissimilarities between sample pairs and the original rank dissimilarities to determine statistical significance between predefined groups (Clarke Citation1993). To further assess assemblage change between modern and preindustrial periods, we also examined differences between taxa turnover and Bray-Curtis dissimilarities across time periods in relation to measured hypolimnetic oxygen concentrations. Detrended correspondence analysis (DCA) of assemblages from each time period was used as a measure of the magnitude of assemblage change (Birks Citation1998). Rare species were not downweighted.
Downcore assemblage changes from 4 study lakes (Ogden, Millet, Timber, and Scraggy) were also examined. These 4 lakes were selected because of their priority to NSAF (Crandlemere T and MacMillan J, NSAF, Citation2004, pers. comm.). Midge-based zones were established by cluster analysis with constrained incremental sum of squares (CONISS) in R v 2.11.1 (R Development Core Team Citation2010) using the rioja package (Juggins Citation2009). Prior to zoning, chironomid data were square-root transformed to equalize taxa variances and those taxa that were not present in at least 2 samples with a relative abundance of ≥2% were removed. The number of zones for each record was determined using a broken-stick model and examining variance reduction as a percentage of the total variance (Jackson Citation1993, Bennett Citation1996). As a measure of taxa turnover downcore, DCAs were performed and the gradient lengths of axis 1 sample scores were examined. Gradient lengths ≤1 SD (standard deviation units) were interpreted as reflecting minimal ecological change (e.g., Smol et al. Citation2005) given the time periods captured by each dated core.
Results
Top–bottom analysis
In general, chironomid assemblages of the 12 brook trout lakes chosen for the regional paleolimnological analysis were composed of many of the same taxa, and assemblages varied little within lakes (). Assemblages in the modern and preindustrial sediments were not significantly different from one another (ANOSIM: R = −0.06, P = 0.94, n = 999). Lakes that experienced the least amount of change (assessed by Bray-Curtis dissimilarity) in chironomid assemblages include Condon, Moses, Bare Rock, and Southwest. Those lakes that showed the largest change in assemblages include MacKay and Ogden. There was no significant relationship (P = 0.66) with respect to the measured hypolimnetic oxygen values and lakes that showed the least or largest change in assemblage dissimilarity (). Similarly, differences in DCA axis 1 sample scores showed little change through time, except at MacKay and Condon lakes (). Gradient lengths of the modern and preindustrial assemblages were relatively short and comparable at 1.7 and 1.5 SD, respectively.
Figure 2 Modern (solid bars) and preindustrial (open bars) relative abundances of common chironomid taxa from 12 Nova Scotia brook trout lakes. Lakes are ordered from highest (Dollar, 9.1 mg/L) to lowest (Cook, 2.0 mg/L) hypolimnetic dissolved oxygen concentrations. Oxygen optima from Quinlan and Smol (Citation2001a) are noted in brackets for taxa common to both datasets.
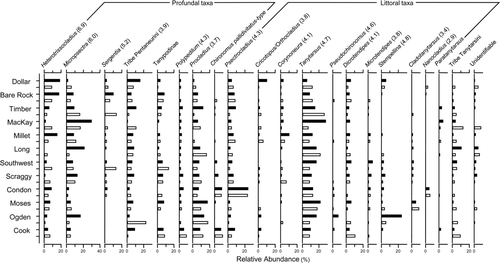
Figure 3 Differences in detrended correspondence analysis (DCA) axis 1 sample scores of modern and preindustrial assemblages and the Bray-Curtis dissimilarity of modern and preindustrial assemblages compared across measured hypolimnetic dissolved oxygen concentrations.
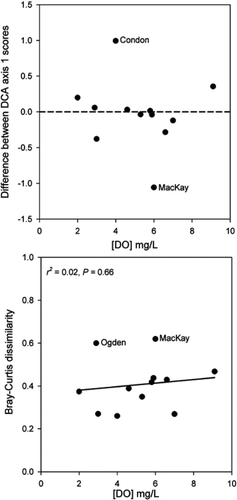
Members of the subfamily Tanypodinae, Polypedilum, Psectrocladius, Tanytarsus, and Dicrotendipes showed relative stability through time and were often moderately abundant at ∼10–20% (). Heterotrissocladius, Micropsectra, and Sergentia were also abundant, and at several lakes (e.g., Dollar, Ogden, and MacKay) typically showed modest abundance changes when modern and preindustrial assemblages were compared. Stempellina increased by ∼20% within the modern sediments of only Ogden Lake, although the individual count from the modern time period of this lake was less than our target of 50 individuals.
Downcore assemblage changes
Zonation and DCA
Cluster analyses with CONISS and comparisons of variance reduction using broken-stick models (not shown) did not identify major chironomid assemblage zones within any of the 4 sediment records. Overall, this suggests that assemblage changes have been minor in our 4 downcore records. DCAs further support interpretations of low taxa turnover through time. Gradient lengths were ≤1 SD at Scraggy, Timber, and Millet lakes. At Ogden Lake, taxa turnover was moderate at 1.5 SD and driven by recent shifts in Micropsectra, members of Tanypodinae, and Stempellina.
Ogden Lake
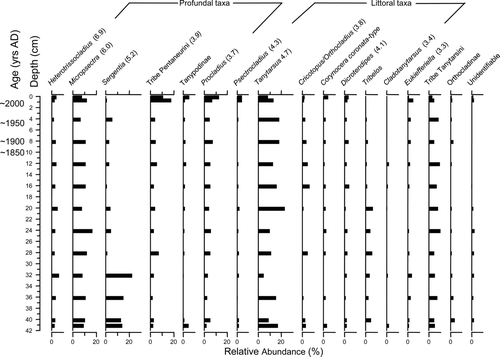
Despite Ogden Lake's low hypolimnetic oxygen levels at 2.9 mg/L, the main chironomid assemblage trend was a shift from taxa indicative of low-to-moderate oxygen levels (e.g., Tanypodinae and Polypedilum) to taxa often associated with higher oxygen levels (e.g., Heterotrissocladius and Micropsectra; ). Combined, Heterotrissocladius and Micropsectra showed an increase from ∼5% relative abundance in the preindustrial intervals to ∼20% relative abundance in the modern sediments. Notably, the littoral genus Stempellina was the dominant taxon in the modern sediments (∼25%); however, its relative abundance was <10% in all previous sediment intervals.
Millet Lake
Preindustrial age (∼1850) sediments from Millet Lake were reached at approximately twice the depth as the other 3 dated sediment records (). Despite the greater sedimentation rate, relative abundances of chironomid taxa from Millet Lake were remarkably stable throughout the core (). There was no major directional change in the assemblages. The relative abundances of several common profundal and littoral taxa were comparable through time at between ∼5 and 10%. Heterotrissocladius, Micropsectra, Sergentia, and Tribe Pentaneurini were the most abundant profundal taxa. The most abundant littoral taxa also showed very little change through time (e.g., Corynoneura coronata-type and Tanytarsus).
Figure 4 Relative abundances of common chironomid taxa from the Ogden Lake (southwestern Nova Scotia) sediment core collected in Jul 2003. Hypolimnetic dissolved oxygen concentration was 2.9 mg/L and downcore gradient length was 1.5 SD. Oxygen optima from Quinlan and Smol (2001a) are noted in brackets for taxa common to both datasets.
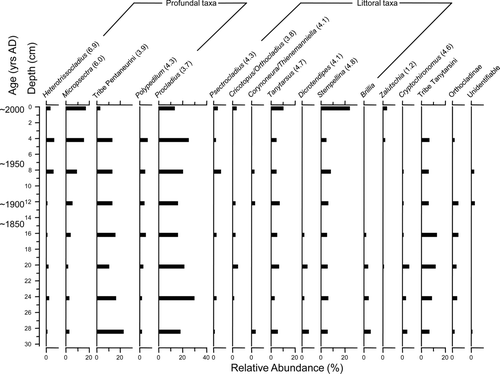
Figure 5 Relative abundances of common chironomid taxa from the Millet Lake (central Nova Scotia) sediment core collected in Jul 2004. Hypolimnetic dissolved oxygen concentration was 5.9 mg/L and downcore gradient length was 0.85 SD. Oxygen optima from Quinlan and Smol (2001a) are noted in brackets for taxa common to both datasets.
Scraggy Lake
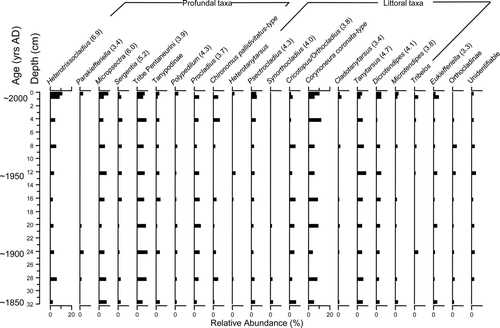
There were no major changes in the relative abundances of chironomid taxa within the Scraggy Lake sediment core (). Tribe Pentaneurini, Procladius, and Tanytarsus were generally the most abundant taxa. Heterotrissocladius and Micropsectra were also observed throughout the record with relative abundances of typically <5%. Protanypus occurred throughout the core with relative abundances of <5%. Mesocricotopus was only found in preindustrial sediments, although at low abundance.
Figure 6 Relative abundances of common chironomid taxa from the Scraggy Lake (central Nova Scotia) sediment core collected in Jul 2003. Hypolimnetic dissolved oxygen concentration was 4.6 mg/L and downcore gradient length was 1.0 SD. Oxygen optima from Quinlan and Smol (2001a) are noted in brackets for taxa common to both datasets.
Timber Lake
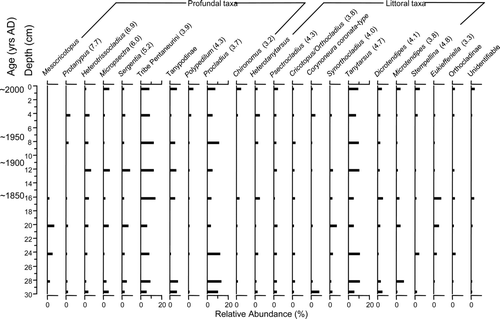
Chironomid assemblages from Timber Lake were relatively stable throughout the core, despite the likelihood of sediments from this 42 cm core being much older than ∼1850 (). Micropsectra and Tanytarsus were the dominant profundal and littoral taxa, respectively. Heterotrissocladius abundances varied little throughout the record. Overall, members of the subfamily Tanypodinae increased in abundance toward modern times. The most apparent change in assemblage composition occurred prior to ∼1850, although the age of this period remains undetermined. It is characterized by a decrease of Sergentia from ∼20% abundance between 42 and 32 cm to <5% relative abundance between 30 cm and modern times. Also of note, Chaoborus mandibles were present throughout the core, and C. americanus, an indicator of fishless lakes, was observed from all core intervals examined between 4 and 41.5 cm (not shown). Timber Lake was the only study lake where chaoborid mandibles occurred and was presumably fishless prior to stocking after the ∼1950s.
Figure 7 Relative abundances of common chironomid taxa from the Timber Lake (northeastern Nova Scotia) sediment core collected in Jul 2004. Hypolimnetic dissolved oxygen concentration was 6.6 mg/L and downcore gradient length was 0.89 SD. Oxygen optima from Quinlan and Smol (2001a) are noted in brackets for taxa common to both datasets.
Discussion
One of the most critical biological impacts of land-use changes and cultural eutrophication is an overall reduction in hypolimnetic [DO] in thermally stratified lakes. Our analyses of modern and preindustrial chironomid assemblages from 12 lakes demonstrates that assemblage compositions have not significantly differed between the time periods examined (ANOSIM: R = −0.06, P = 0.94), and there is no significant relationship between measured oxygen conditions and the dissimilarity coefficient derived from comparisons of modern and preindustrial assemblages at each lake. Additionally, the 4 lakes analyzed in greater temporal detail show that assemblages have been remarkably stable since preindustrial times, with 3 of 4 lakes recording gradient lengths ≤1 SD. Long-term changes (if any) in hypolimnetic oxygen conditions likely have not been of sufficient magnitude or duration to cause major chironomid assemblage shifts (i.e., increased abundances of taxa associated with eutrophic or low oxygen conditions).
The stable abundances, and at some lakes (e.g., Dollar, Bare Rock, MacKay, Long) even increased abundances, of taxa indicative of at least a moderately oxygenated hypolimnion (Heterotrissocladius, Sergentia, Micropsectra), also suggest that long-term oxygen conditions have not deteriorated. Additionally, assemblages from the lakes with the lowest modern hypolimnetic oxygen measures (<5.0 mg/L) are remarkably stable, notwithstanding an increase in Stempellina at Ogden. Procladius, an indicator of poor oxygen conditions (Quinlan et al. Citation1998, Little et al. Citation2000, Quinlan and Smol Citation2001a), was ubiquitous in all study lakes. This taxon shows greater abundances at the lower end of our oxygen gradient and increases of ∼10% within the modern sediments of Timber and Moses lakes, which may suggest some decline in hypolimnetic oxygen conditions since preindustrial times. Moses Lake has always recorded poor oxygen conditions at [DO] of 1.5–3.1 mg/L (Brylinsky Citation2002). Despite regional stressors affecting water quality in 54 south-central Ontario lakes, Quinlan and Smol (Citation2002) also concluded that in general, modern hypolimnetic oxygen conditions were similar to those present during preindustrial times prior to large-scale land clearance and logging, as inferred by fossil chironomids after application of a quantitative inference model.
The paleolimnological approach we utilized may provide a more realistic picture of long-term limnological change compared to infrequent spot measures of water quality. In many instances, fossil chironomid assemblages reflect overall oxygen conditions both in time and space due to life cycles of seasons to 2 yr in temperate lakes (Oliver Citation1971, Pinder Citation1986) and the focusing of fossil remains from various habitats within a lake basin. However, limnological monitoring conducted by Brylinsky (Citation2002) and NSAF clearly show late-summer periods of low [DO], although these periods may be of short duration or occur infrequently across several years. The discrepancy between our paleolimnological data and water quality measures may be explained by Quinlan and Smol's (2001a) observation of taxa representative of a well-oxygenated hypolimnion in lakes that experience late-summer oxygen depletion. They postulated that, if the seasonal warming of lake water is gradual, stenoxic taxa may begin their lifecycle and deposit head capsules during instar development prior to unfavorable conditions developing in the hypolimnion. Thus, stenoxic taxa could therefore be present at low-to-moderate abundances, despite the lack of suitable habitat throughout their entire lifecycle in lakes that experience near anoxic late-summer oxygen conditions. Also, caution is warranted because taxa associated with a moderate-to-well oxygenated hypolimnion may occur at low abundances in lakes where late-summer oxygen conditions are much lower than the taxon's optimum (Quinlan and Smol Citation2001a, Brodersen and Quinlan Citation2006). We stress that it is likely the duration and absolute oxygen conditions combined that influence chironomid assemblage composition (Heinis and Davids Citation1993, Paterson et al. Citation2009).
Despite differences in geography, land-use patterns, lake morphometry, and water quality, numerous studies have demonstrated that chironomid assemblages reflecting mesotrophic to oligotrophic conditions are represented by taxa such as Heterotrissocladius, Micropsectra, Protanypus, and Parakiefferiella nigra-type (Clerk et al. Citation2000, Quinlan and Smol Citation2001a, Brodersen et al. Citation2001, 2004), whereas eutrophic lakes are often dominated by Chironomus and Procladius, among several other littoral taxa (Quinlan et al. Citation1998, Little et al. Citation2000, Brodersen and Quinlan Citation2006). In thermally stratified lakes, the succession of assemblages composed largely of Heterotrissocladius, Micropsectra, Stictochironomus, and (or) Sergentia to assemblages dominated by Chironomus and Procladius typify a shift in water quality to a eutrophic state and indicate declines in overall oxygen conditions (Brodersen et al. Citation2001, Reavie et al. Citation2006, Brodersen and Quinlan Citation2006). Based on the moderately stable abundances of Heterotrissocladius and Micropsectra and the high similarity between assemblages from modern and preindustrial periods, long-term water quality conditions within many of our study lakes have been and continue to be relatively stable.
The lack of directional change in the top–bottom analysis is also supported by only subtle changes in relative abundances observed in the sediment cores studied at greater temporal detail from Millet, Scraggy, and Timber lakes. Additionally, taxa turnover was low at these 3 lakes, and our clustering methods did not recognize any major zone delineations within the downcore assemblages, further suggesting no strong directional changes. Some of the subtle changes in the relative abundances of common taxa can likely be attributed to natural habitat variability and (or) the coarseness of our sample intervals. At Ogden, the gradient length was still relatively low (1.5 SD), but may suggest some recent environmental changes. Despite this, we rule out oxygen declines as a driver of these changes given that while Stempellina may indicate hypoxic conditions, increases in stenoxic taxa with well-established preferences for higher oxygen concentrations (e.g., Heterotrissocladius and Micropsectra) occur from ∼1950 to the present.
At Ogden, Millet, Scraggy, and Timber lakes, taxa representing a well-oxygenated hypolimnion (Heterotrissocladius and Micropsectra) were observed throughout the cores and even showed moderate increases at Ogden Lake, our shallowest study site with the second lowest hypolimnetic oxygen concentration (2.9 mg/L). At Timber Lake, Sergentia, an indicator of a moderate-to-well oxygenated hypolimnion (Little et al. Citation2000, Quinlan and Smol Citation2001a), showed large-scale changes with declines from ∼20 to 5%. However, these changes occurred between about 40 and 30 cm, much earlier than 1850, which we estimate at 10 cm. At Timber Lake, there is also some indication of recent increases in Tribe Pentaneurini and Procladius, an indicator of lower hypolimnetic oxygen. This may suggest some deterioration in oxygen conditions, although both Heterotrissocladius and Micropsectra abundances remain stable; therefore, we believe these changes (if any) may be only minor and may reflect changes in available habitat or possibly shifts in predation as evidenced by the extirpation of Chaoborus americanus post stocking. To summarize, 3 of the 4 study lakes examined at greater temporal detail show no substantial directional changes, and long-term oxygen conditions at these study sites remain comparatively stable.
Smol (Citation2008) outlines 4 main data sources and (or) approaches managers can choose from to assess long-term environmental change: modeling, monitoring, paleoenvironmental reconstructions, and comparisons of impacted and control sites. Each approach compliments one another and, in combination, they may provide substantial insights to the complex nature of ecosystems. In the case of these 20 Nova Scotia brook trout lakes, there exists at least some data generated by the following 3 approaches for most of these lakes: water quality monitoring (Brylinksy Citation2002), paleolimnological assessment of chironomid assemblages (presented here), and modeling of water quality (Soliman et al. Citation2009). Soliman et al. (Citation2009) applied the Ontario Lakeshore Capacity model to 10 Nova Scotia brook trout lakes and concluded that both current measurements and hindcasted predevelopment water quality variables (i.e., hindcasted hypolimnetic oxygen concentration ranged between 0.3 and 4.9 mg/L) clearly indicated poor habitat for coldwater fish at all 10 study lakes. Collectively, these 3 diverse approaches question the sustainability of the near-annual stocking of brook trout in potentially marginal coldwater habitats given the threat of negative impacts from climate change scenarios on lakes (Schindler Citation2001, Smol Citation2010). Efforts to enhance angling opportunities in Nova Scotia for brook trout should focus on well-studied lakes that currently provide better-than-marginal summertime habitat.
Conclusions
Changes in chironomid assemblage composition between modern and preindustrial time periods have been minimal in 12 Nova Scotia brook trout lakes. Sediment cores examined at greater temporal detail from 4 of the study lakes further support the findings from the top–bottom analysis and show no substantial directional changes in chironomid assemblages related to declines in oxygen conditions. Collectively, our findings indicate that chironomid-inferred hypolimnetic oxygen conditions have remained remarkably stable over approximately the last 2 centuries. Therefore, the results of water quality measures reported in Brylinsky (Citation2002) and compared to earlier surveys conducted during the 1970–1980s, are not believed to be representative of any long-term directional trends in the hypolimnetic oxygen conditions of Nova Scotia lakes assessed for suitable brook trout habitat. Combining the knowledge gained by paleolimnological study, in addition to limnological monitoring and modeling, provides greater insight toward the management of freshwaters and the long-term changes these aquatic ecosystems may experience.
Acknowledgments
This study would not have been possible without the assistance of J. MacMillan and T. Crandlemere of NSAF. We also thank J. Sweetman, B. Ginn, and S. Pla of PEARL for assistance in this project, M. Brylinsky for interpretations from his earlier work on these lakes, and the constructive comments from 3 anonymous reviewers. This study was funded by a NSERC Strategic Grant to J.P. Smol, B.F. Cumming, and P. Dillon.
Authors contributed equally and listed alphabetically
References
- Appleby , PG. 2001 . “ Chronostratigraphic techniques in recent sediments ” . In Tracking environmental change using lake sediments. Volume 1: Basin analysis, coring and chronological techniques , Edited by: Last , W M and Smol , J P . 171 – 203 . Dordrecht (NL) : Kluwer Academic Publishers .
- Bennett , KD. 1996 . Determination of the number of zones in a biostratigraphical sequence . New Phytol. , 132 : 155 – 170 .
- Birks , HJB. 1998, 20 . Numerical tools in palaeolimnology - Progress, potentialities, and problems . J Paleolimnol , : 307 – 332 .
- Bray , J R and Curtis , J T . 1957 . An ordination of the upland forest communities of southern Wisconsin . Ecol Monogr. , 27 : 326 – 349 .
- Brodersen , K P and Lindegaard , C . 1999 . Classification, assessment and trophic reconstruction of Danish lakes using chironomids . Freshwater Biol. , 42 : 143 – 157 .
- Brodersen , K P , Odgaard , B , Vestergaard , O and Anderson , N J . 2001 . Chironomid stratigraphy in the shallow and eutrophic Lake Søbygaard, Denmark: Chironomid-macrophyte co-occurrence . Freshwater Biol. , 46 : 253 – 267 .
- Brodersen , K P , Pedersen , O , Lindegaard , C and Hamburger , K . 2004 . Chironomids (Diptera) and oxy-regulatory capacity: An experimental approach to paleolimnological interpretation . Limnol Oceanogr. , 49 : 1549 – 1559 .
- Brodersen , K P , Pedersen , O , Walker , I R and Jensen , M . 2008 . Respiration of midges (Diptera; Chironomidae) in British Columbian lakes: oxy-regulation, temperature and their role as palaeo-indicators . Freshwater Biol. , 53 : 593 – 602 .
- Brodersen , K P and Quinlan , R . 2006 . Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen . Quat Sci Rev. , 25 : 1995 – 2012 .
- Brooks , S J , Bennion , H and Birks , H JB . 2001 . Tracing lake trophic history with a chironomid-total phosphorus inference model . Freshwater Biol. , 46 : 513 – 533 .
- Brylinsky , M. 2002 . “ Nova Scotia Hypolimnion Project ” . Wolfville (NS) : Publication No. 67 of the Acadia Centre for Estuarine Research .
- Clarke , KR. 1993 . Non-parametric multivariate analyses of changes in community structure . Aust J Ecol. , 18 : 117 – 143 .
- Clerk , S , Hall , R , Quinlan , R and Smol , J P . 2000 . Quantitative inferences of past hypolimnetic anoxia and nutrient levels from a Canadian Precambrian Shield lake . J Paleolimnol. , 23 : 319 – 336 .
- Davis , D S and Browne , R . “ 1996. The natural history of Nova Scotia ” . In Theme regions , Vol. 2 , Halifax (NS) : Nimbus Publishing .
- Evans , DO. 2007 . Effects of hypoxia on scope-for-activity and power capacity of lake trout (Salvelinus namaycush) . Can J Fish Aquat Sci. , 64 : 345 – 361 .
- Fisheries and Oceans Canada . 2007 . “ Survey of recreational fishing in Canada 2005 ” . Ottawa (ON) : Catalogue No. Fs23-522/2005E .
- Ginn , B K , Cumming , B F and Smol , J P . 2007a . Assessing pH changes since pre-industrial times in 51 low-alkalinity lakes in Nova Scotia, Canada . Can J Fish Aquat Sci. , 64 : 1043 – 1054 .
- Ginn , B K , Cumming , B F and Smol , J P . 2007b . Long-term acidification trends in high- and low-sulphate deposition regions from Nova Scotia, Canada. Hydrobiologia . 586 : 261 – 275 .
- Ginn , B K , Grace , L , Cumming , B F and Smol , J P . 2008 . Tracking anthropogenic- and climatic-related environmental changes in the remaining habitat lakes of the endangered Atlantic whitefish (Coregonus huntsmani) using paleolimnological techniques . Aquat Conserv. , 18 : 1217 – 1286 .
- Glew , JR. 1988 . A portable extruding device for close interval sectioning of unconsolidated core samples . J Paleolimnol , 1 : 235 – 239 .
- Glew , JR. 1989 . A new trigger mechanism for sediment samples . J Paleolimnol. , 2 : 241 – 243 .
- Heinis , F and Davids , C . 1993 . Factors governing the spatial and temporal distribution of chironomid larvae in the Maarsseveen Lakes with special emphasis on the role of oxygen conditions . Neth J Aquat Ecol. , 27 : 21 – 34 .
- Heiri , O and Lotter , A F . 2001 . Effect of low count sums on quantitative environmental reconstructions: an example using subfossil chironomids . J Paleolimnol. , 26 : 343 – 350 .
- Jackson , DA. 1993 . Stopping rules in principal components analysis – a comparison of heuristic and statistical approaches . Ecology. , 74 : 2204 – 2214 .
- Jeffries , DS. 1997 . “ Canadian acid rain assessment Vol. 3: the effects on Canada's lakes rivers, and wetlands ” . Ottawa (ON) : Environment Canada .
- Juggins , S . 2009 . rioja: Analysis of Quaternary Science Data , R package version 0.5-6 . http://CRAN.R-project.org/package=rioja
- Kerr , SJ. 2000 . “ Brook trout stocking: An annotated bibliography and literature review with an emphasis on Ontario waters ” . Peterborough (ON) : Fish and Wildlife Branch, Ontario Ministry of Natural Resources .
- Korosi , J B and Smol , J P . 2011 . A comparison of present-day and pre-industrial cladoceran assemblages from softwater Nova Scotia (Canada) lakes with different regional acidification histories . J Paleolimnol , DOI 10.1007/s10933-011-9547-4
- Larocque , I. 2001 . How many chironomid head capsules are enough? A statistical approach to determine sample size for palaeoclimatic reconstructions . Palaeogeogr Palaeoclimatol Palaeoecol. , 172 : 133 – 142 .
- Little , J L , Hall , R I , Quinlan , R and Smol , J P . 2000 . Past trophic status and hypolimnetic anoxia during eutrophication and remediation of Gravenhurst Bay, Ontario: comparison of diatoms, chironomids, and historical records . Can J Fish Aquat Sci. , 57 : 333 – 341 .
- MacMillan , J L , Caissie , D , Marshall , T J and Hinks , L . 2008 . “ Population indices of brook trout (Salvelinus fontinalis), Atlantic salmon (Salmo salar), and salmonid competitors in relation to summer water temperature and habitat parameters in 100 streams in Nova Scotia. Moncton (NB): Department of Fisheries and Oceans Gulf Region ” . Oceans and Science Branch .
- Morrison , HA. 2004 . “ Canadian acid deposition science assessment ” . Ottawa (ON) : Environment Canada .
- [NSAF] Nova Scotia Department of Agriculture and Fisheries . 2005 . “ Nova Scotia Trout Management Plan ” . Pictou (NS) : Inland Fisheries Division .
- Oksanen , J , Blanchet , F G , Kindt , R , Legendre , P , O’Hara , R B , Simpson , G L , Solymos , P , Stevens , M HH and Wagner , H . 2010 . “ vegan: Community Ecology Package ” . R package version 1.17-4. http://CRAN.R-project.org/package=vegan
- Oliver , DR. 1971 . Life history of the Chironomidae . Ann Rev Entomol. , 16 : 211 – 230 .
- Paterson , A M , Quinlan , R , Clark , B J and Smol , J P . 2009 . Assessing hypolimnetic oxygen concentrations in Canadian Shield lakes: deriving management benchmarks using two methods . Lake Reserv Manage. , 25 : 313 – 322 .
- Pinder , L CV . 1986 . Biology of freshwater Chironomidae . Ann Rev Entomol. , 31 : 1 – 23 .
- Pollock , M S , Clarke , L MJ and Dubé , M G . 2007 . The effects of hypoxia on fishes: from ecological relevance to physiological effects . Environ Rev. , 15 : 1 – 14 .
- Power , G. 1980 . “ The brook charr, Salvelinus fontinalis ” . In Charrs: salmonid fishes of the genus Salvelinus , Edited by: Balon , E K . 141 – 203 . Hague (NL) : Dr W Junk Publishers .
- Quinlan , R , Hall , R I , Paterson , A M , Cumming , B F and Smol , J P . 2008 . Long-term assessments of ecological effects of anthropogenic stressors on aquatic ecosystems from paleoecological analyses: challenges to traditional perspectives of lake management . Can J Fish Aquat Sci , 65 : 933 – 944 .
- Quinlan , R , Smol , J P and Hall , R I . 1998 . Quantitative inferences of past hypolimnetic anoxia in south-central Ontario lakes using fossil midges (Diptera: Chironomidae) . Can J Fish Aquat Sci. , 55 : 587 – 596 .
- Quinlan , R and Smol , J P . 2001a . Chironomid-based inference models for estimating end-of-summer hypolimnetic oxygen from south-central Ontario shield lakes . Freshwater Biol. , 46 : 1529 – 1551 .
- Quinlan , R and Smol , J P . 2001b . Setting minimum head capsule abundance and taxa deletion criteria in chironomid-based inference models . J Paleolimnol. , 26 : 327 – 342 .
- Quinlan , R and Smol , J P . 2002 . Regional assessment of long-term hypolimnetic oxygen changes in Ontario (Canada) shield lakes using subfossil chironomids . J Paleolimnol. , 27 : 249 – 260 .
- Quinlan , R and Smol , J P . 2010 . The use of Chaoborus subfossil mandibles in the development of paleoecological inference models of hypolimnetic oxygen . J Paleolimnol. , 44 : 43 – 50 .
- R Development Core Team . 2010 . “ R: a language and environment for statistical computing ” . Vienna : R Foundation for Statistical Computing . http://www.R-project.org
- Raleigh , RF. 1982 . “ Habitat suitability index models: Brook trout ” . US Dept Int . Fish Wildl Servo FWS/OBS-82/10.24
- Reavie , E D , Neill , K E , Little , J L and Smol , J P . 2006 . Cultural eutrophication trends in three southeastern Ontario lakes: a paleolimnological perspective . Lake Reserv Manage. , 22 : 44 – 58 .
- Schindler , DW. 2001 . The cumulative effects of climate warming and other human stressors on Canadian freshwaters in the new millennium . Can J Fish Aquat Sci , 58 : 18–29.
- Smith , VH. 2003 . Eutrophication of freshwater and marine ecosystems: a global problem . Environ Sci Pollut Res Int. , 10 : 126 – 139 .
- Smith , V H and Schindler , D W . 2009 . Eutrophication science: Where do we go from here? . Trends Ecol Evol. , 24 : 201 – 207 .
- Smol , JP. 2008 . “ Pollution of lakes and rivers: a paleoenvironmental perspective ” . Oxford (UK) : Blackwell Publi- shing .
- Smol , JP. 2010 . The power of the past: using sediments to track the effects of multiple stressors on lake ecosystems . Freshwater Biol. , 55 ( Suppl. 1 ) : 43 – 59 .
- Smol , J P , Wolfe , A P , Birks , H JB , Douglas , M SV , Jones , V J , Korhola , A , Pienitz , R , Rühland , K , Sorvari , S Antoniades , D . 2005 . Climate-driven regime shifts in the biological communities of arctic lakes . Proc Nat Acad Sci. , 102 : 4397 – 4402 .
- Soliman , C , Dillon , P J , Aherne , J and Lasenby , D . 2009 . Predicting oxygen profiles in Nova Scotia brook trout lakes using the Lakeshore Capacity Model: considerations for brook trout habitat, remediation and future development . Verh Int Ver Theor Angew Limnol , 30 : 1137–1140.
- Thienpont , J R , Ginn , B K , Cumming , B F and Smol , J P . 2008 . An assessment of environmental changes in three lakes from King's County (Nova Scotia, Canada) using diatom-based paleolimnological techniques . Water Qual Res J Can , 43 : 85–98.
- Tropea , A E , Ginn , B K , Cumming , B F and Smol , J P . 2007, 23 . Environmental changes in the Halifax municipal water supply (Pockwock Lake) related to acidic deposition . Lake Reserv Manage , : 279 – 286 .
- Walker , IR. 1988 . “ Late-Quaternary Palaeoecology of Chironomidae (Insecta: Diptera) in Lake Sediments from British Columbia. [dissertation] ” . [Burnaby (BC)] : Simon Fraser University .
- Walker , IR. 2001 . “ Midges: Chironomidae and related Diptera ” . In Tracking environmental change using lake sediments. Vol 4: Zoological indicators , Edited by: Smol , J P , Birks , H JB and Last , W M . 43 – 66 . Dordrecht (NL) : Kluwer Academic Publishers .
- Wiederholm , T. 1983 . Chironomidae of the Holarctic region. Keys and diagnoses, Part 1-Larvae . Entomologica Scandinavica. ,