Abstract
Introduction: Preeclampsia is a common and partially genetic pregnancy complication characterized by hypertension and proteinuria. Association with cardiovascular disease and type 2 diabetes has been reported in 9p21 by several genome-wide association studies. It has been hypothesized that cardiometabolic diseases may share common etiology with preeclampsia.
Materials and methods: We tested association with the 9p21 region to preeclampsia in the Finnish population by genotyping 23 tagging single nucleotide polymorphisms (SNPs) in 15 extended preeclampsia families and in a nationwide cohort consisting of 281 cases and 349 matched controls. Replication was conducted in additional datasets.
Results: Four SNPs (rs7044859, rs496892, rs564398 and rs7865618) showed nominal association (p ≤ 0.024 uncorrected) with preeclampsia in the case-control cohort. To increase power, we genotyped two SNPs in additional 388 cases and 341 controls from the Finnish Genetics of Preeclampsia Consortium (FINNPEC) cohort. Partial replication was also attempted in a UK cohort (237 cases and 199 controls) and in 74 preeclamptic families from Australia/New Zealand. We were unable to replicate the initial association in the extended Finnish dataset or in the two international cohorts.
Conclusions: Our study did not find evidence for the involvement of the 9p21 region in the risk of preeclampsia.
Chromosome 9p21 is not associated with preeclampsia.
Key Message
Introduction
Preeclampsia is a multifactorial, pregnancy-specific vascular disorder characterized by hypertension and proteinuria (Citation1). Its etiology and pathophysiology remain poorly understood, but large epidemiological and family studies demonstrate a genetic contribution to preeclampsia susceptibility (Citation2–4). This genetic contribution consists of both maternal and fetal genetic factors (Citation5,Citation6).
Patients with previous preeclampsia are at elevated risk of developing cardiovascular diseases as well as impaired glucose tolerance or type 2 diabetes (T2D) in later life (Citation7–9). In line with these findings, it has been suggested that pregnancy might act as a natural stress test with the ability to unmask susceptibility to future chronic diseases such as coronary artery disease (CAD) and T2D (Citation10). The genetic risk factors predisposing to these chronic diseases and preeclampsia may thus be partially overlapping.
We have previously identified preeclampsia susceptibility regions on chromosomes 2p25 and 9p13 in a genome-wide linkage analysis studying 15 multiplex Finnish families (Citation11). Interestingly, several genome-wide association (GWA) studies have found a region on chromosome 9p21 to be associated with CAD or its main complication myocardial infarction (MI) (Citation12–14), a finding confirmed in meta-analyses and association studies of the region (Citation15,Citation16). The region is also associated with T2D, a risk factor for CAD, although different SNPs have been reported to be associated with each disease and the associated variants reside mostly in different linkage disequilibrium (LD) regions (Citation17). The SNPs showing the strongest association with CAD and T2D do not map directly within any protein-coding genes, but cyclin dependent kinase inhibitor genes CDKN2A and CDKN2B flank the associated markers. Moreover, a large non-coding RNA gene ANRIL (Antisense Non-coding RNA in the INK4 Locus) spans the associated region.
As preeclampsia has been associated with increased risk for both CAD and T2D later in life, and shares risk factors with these diseases we decided to study whether the 9p21 region confers a risk to preeclampsia. We genotyped single nucleotide polymorphisms (SNPs) previously associated with CAD and T2D as well as additional tagging SNPs in the Finnish preeclampsia families and in a nation-wide case-control sample set, and studied the placental expression of the genes in the 9p21 region for a difference between preeclamptic and normotensive pregnancies.
Materials and methods
Ethics approvals
All participants had given their written informed consent for the use of their DNA for research studies in preeclampsia. The study protocols were approved by local ethics committees at the Finnish Red Cross Blood Service, Karolinska Institutet South, the Department of Obstetrics and Gynaecology at Helsinki University Central Hospital, and Hospital District of Helsinki and Uusimaa. The South East Multi Ethics Research Committee provided ethics approval (number 00/01/027) and site-specific approvals were acquired from each participating centre of the Vitamins in Pre-eclampsia (VIP) trial consortium. For the UK Nottingham cohort, ethical approval was obtained from the hospital ethics committee. For the Australian/New Zealand (Aus/NZ) family cohort ethical approval was granted by the Research and Ethics committees of the Royal Women’s Hospital, Melbourne, Australia. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Study participants
Details of participants in each study cohort are given in Supplementary File 1.
SNP selection and genotyping
Based on the HapMap data and the GWA study results on the chromosome 9p21 region, we selected 12 SNPs previously associated with CAD or T2D and 14 additional tagging SNPs using Tagger (Citation18) (http://www.broad.mit.edu/mpg/tagger/server.html) to cover the majority of common variation within the region. We excluded SNPs with minor allele frequency (MAF) < 5% as we would have been underpowered to detect association with low-frequency variants. Genotyping of the original Finnish case-control dataset and families was performed using Sequenom iPLEX platform as described previously (Citation19). Two markers (rs10757278 and rs10965241) could not be multiplexed in assay design and were excluded. One marker (rs12379111) failed assay validation and was also excluded. In total, 23 markers ( and Supplementary Table 1 in Supplementary File 1) were successfully genotyped in the original Finnish case-control and family datasets. Genotypes were verified independently by two investigators and the Hardy–Weinberg equilibrium (HWE) was assessed for each marker as a quality control procedure.
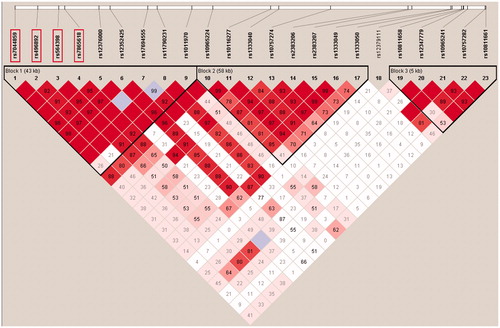
Figure 1. Linkage disequilibrium structure in 9p21. This Haploview image shows three distinct haploblocks formed by 23 SNPs genotyped in the original Finnish case-control cohort. The replicated SNPs (boxed) are all located in Haploblock 1, which spans the region of three genes that associate with coronary artery disease and type 2 diabetes.
We aimed to genotype the same 23 markers in the UK cohorts using the Sequenom iPLEX platform, but markers rs1333049 and rs12379111 failed assay validation and were excluded. Two markers showing association in the Finnish datasets (rs496892 and rs7044859) were genotyped in the Aus/NZ cohort consisting of 74 families. In the Aus/NZ cohort, rs496892 was genotyped using the Illumina GoldenGate® SNP Genotyping Assay (Illumina, San Diego, CA). The SNP was incorporated into a 384-plex Illumina GoldenGate™ assay and genotyped following the manufacturer’s instructions. Genotypes were detected on BeadXPress Reader using VeraScan software v.1.1 and called using Illumina BeadStudio software v.3.2.32, Genotyping Module. Genotyping of rs7044859 in the Aus/NZ cohort was performed using a custom TaqMan genotyping assay (Applied Biosystems, Foster City, CA). Five microlitre PCR reactions contained 50 ng of genomic DNA, 2.5 μl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems), 0.125 μl of 40× SNP genotyping assay mix and 1.374 μl of H2O. The PCR amplification conditions were 95 °C for 10 min followed by 40 cycles of 92 °C for 15 s and 60 °C for 1 min. Thermal cycling was performed on an Applied Biosystem 7900HT Fast Real-Time PCR System and genotype data were obtained using the ABI Sequence Detection System (SDS) software v.2.2.2. The two SNPs showing the strongest association in the original Finnish sample set (rs7044859 and rs7865618) were genotyped in the Finnish Genetics of Preeclampsia Consortium (FINNPEC) replication cohort by Taqman genotyping assays with 7500 Fast Real-Time qPCR machine (Applied Biosystems) according to the manufacturer’s instructions.
Statistical analysis
Haploview 4.0 software (Citation20) was used to assess LD between markers and to run haplotype association tests in the case-control cohorts. Association tests for single markers were performed in PLINK v1.07 (Citation21). Chi-square was used as the test statistic and the overall significance for multiple testing was corrected using max(T) permutation (mperm 10,000) in PLINK for single markers and 10,000 permutations in Haploview for haplotypes. PDTPHASE v.2.4 from the software package UNPHASED (Citation22) was used to calculate single-marker and haplotype associations in the family cohorts. The analyses in the family cohorts were conducted both with strict (cases with gestational hypertension (GH) excluded) and general (cases with GH included) diagnostic criteria, but only the results obtained with strict criteria are reported.
The analysis was restricted to markers with at least 5% MAF. Genetic Power Calculator (Citation23) was used to evaluate the ability to detect association with the marker rs1333049, which was originally described as the most significantly associated CAD marker (Citation12). The assumptions for the power calculation were preeclampsia prevalence of 3%, risk allele frequency of 0.456 as observed in HapMap CEU data and D′=1. Under these parameters, the study had 80% power to detect genotypic relative risk of 1.40 for Aa and 1.86 for AA in the original Finnish case-control cohort at α = 0.05. In the replication phase, our combined case-control cohort of 669 cases and 690 controls had 80% power to detect genotypic relative risk of 1.25 for the heterozygous and 1.52 for the homozygous risk genotype of rs7044859 at α = 0.05 when assuming preeclampsia prevalence of 3%, D′=1, and risk allele frequency of 0.45 observed in the controls of the original case-control cohort.
Transcript expression
In a parallel study line to genotyping, we analyzed the transcription levels of the 9p21-located genes ANRIL, CDKN2A and CDKN2B in the placental samples from eight preeclamptic women and six healthy controls. After isolation using TRIzol kit (Invitrogen, Carlsbad, CA) and purification with RNAeasy total RNA isolation kit (Qiagen, Hilden, Germany), 6 μg of total RNA obtained from the placental samples was reverse transcribed, amplified, labelled according to the alternative protocol for one-cycle cDNA synthesis and hybridized on Affymetrix Human Genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA). The arrays were stained and scanned according to Affymetrix protocols.
Transcript levels were determined from data images with Affymetrix GeneChip® DNA analysis software (GDAS). Arrays were background corrected and normalized using GC Robust Multi-array Average (GCRMA) adjustment, and differential expression was assessed using linear models from Limma. Both software packages are available from the open-source Bioconductor project (Citation24), and were used under the R environment for statistical computing.
The placental expression levels of ANRIL, CDKN2A and CDKN2B were also studied using RNA sequencing as described previously (Citation25).
Results
Association analysis
In total, 23 markers were successfully genotyped in the original Finnish case-control and family cohorts, with an average Sequenom genotype call rate of 99% across all samples. The allele frequencies observed in the Finnish controls were in concordance with allele frequencies in the 1000 Genomes Project European/CEU/Finnish samples and the HapMap Project CEPH reference samples.
In the original Finnish case-control cohort, four SNPs (rs7044859, rs496892, rs564398 and rs7865618) showed nominal association with preeclampsia (), although the association did not remain statistically significant after permutation correction for multiple testing. Rs7044859 had a predisposing alternative allele with OR 1.36 (CI 95% 1.09–1.71) and for the other three, the alternative allele was protective. These SNPs are located in the same haplotype block within a 12 kbp interval (). In the haplotype analysis for the original Finnish case-control cohort, the 23 genotyped markers formed five haplotype blocks, but no haplotype association with preeclampsia was observed (data not shown). Stringent preeclampsia phenotype was tested by removing possibly confounding diagnosis of twin pregnancies or eclamptic/superimposed preeclampsia patients from the analysis. In these analyses, association was weakened probably due to the loss of power in the smaller but more defined phenotype (data not shown).
Table 1. The results of four associating SNPs from the original Finnish case-control cohort as well as from the replication case-control cohorts in Finland and UK and from the family replication cohorts in Finland and Australia/New Zealand.
The two SNPs with the strongest association (rs7044859 and rs7865618) were genotyped in the FINNPEC case-control replication dataset with success rate >96%. The SNPs were not, however, associated with preeclampsia in this cohort in an independent analysis (data not shown). Furthermore, when the two Finnish case-control datasets were pooled creating a larger dataset consisting of 690 cases and 669 controls, the original association failed to replicate ().
In the Finnish family dataset, rs7044859 was nominally associated with preeclampsia (p = 0.048 uncorrected) () when the cases with GH were excluded from the analysis.
We investigated our original findings in two separate preeclampsia cohorts recruited in the United Kingdom (UK) and in Australia/New Zealand (Aus/NZ). The average call rate for the 21 SNPs genotyped in the UK sample set was 99%. In the Aus/NZ families, the top two markers rs7044859 and rs496892 were genotyped with a success rate of >95%. Both in the UK controls and in the Aus/NZ families, the allele frequencies for the genotyped markers were similar to those observed in the Finnish controls. We did not observe any single-marker or haplotype association in the UK or Aus/NZ datasets and were thus unable to replicate our initial findings in these datasets ().
Expression analysis of CDKN2A, CDKN2B and ANRIL transcripts
We examined the expression of the 9p21-located genes CDKN2A, CDKN2B and ANRIL in the RNA samples obtained from placentas from eight preeclamptic and six normotensive pregnancies using Affymetrix array. None of the four gene-specific probe sets present on the array (one for ANRIL, two for CDKN2A and one for CDKN2B) showed statistically significant difference in the expression levels between the cases and controls, even when the two groups were subdivided according to the delivery method (vaginal or Caesarean section; data not shown).
In addition to the expression array, we measured placental expression of CDKN2A, CDKN2B and ANRIL with RNA sequencing. For ANRIL there were not enough alignments for testing expression difference between the cases and controls, and no expression differences were seen for the other two genes either (data not shown).
Discussion
The existence of common risk factors for preeclampsia, CAD and T2D (Citation26,Citation27) and elevated risk of developing cardiovascular disease and T2D for women with previous preeclampsia (Citation7–9,Citation28) support the theory of pregnancy as a stress test with the ability to reveal susceptibility for future chronic diseases (Citation10). Knowing that CAD and T2D also share common metabolic characteristics with preeclampsia, we set out to look for preeclampsia association in 9p21 where several genes of interest in CAD and T2D are located. In the original Finnish case-control cohort, we observed a nominal association for the SNPs rs7044859 and rs496892, but this association was not replicated when sample size was increased by combining the additional FINNPEC case-control samples with the original sample set. In the small Finnish family cohort, none of the studied SNPs reached a statistical significance that would have survived a correction for multiple testing. In line with these results, the initial association was not replicated in any of the SNPs in the UK case-control sample set or in the multiplex Aus/NZ families.
The present study contains limitations that should be considered in further studies. First, as the sample sizes of the replication cohorts used in the study are modest, this study, as well as many previously published studies investigating the genetic basis of preeclampsia, was underpowered to detect association of variants with relatively low genetic risk. Given the effect size of rs7044859 in the original Finnish case-control cohort and its observed allele frequency in the UK samples, a power calculation indicated that with our sample size we had only 35% power to detect association of this particular marker with preeclampsia in the UK cohort. Second, our study did not assess the role of low-frequency variants in the risk of preeclampsia. We cannot therefore exclude the possibility that low-frequency variants or SNPs located in other LD blocks in this region could be associated with preeclampsia. Third, in the expression study, we did not have a chance to examine gene expression in early pregnancy placental samples, when the disease process has its origins, or to measure expression of proteins of interest in serum samples.
The association of the 9p21 locus with CAD and T2D makes this region a meaningful target in the search for preeclampsia susceptibility genes. We observed an initial association between markers on chromosome 9p21 and preeclampsia in a Finnish population, but the result was not confirmed in the expanded Finnish case-control dataset or in the additional replication cohorts. In conclusion, we did not find evidence for the involvement of 9p21 in the risk of preeclampsia.
Funding information
This study was supported by Academy of Finland; Finnish Medical Foundation, Jane and Aatos Erkko Foundation; Sigrid Jusélius Foundation, Päivikki and Sakari Sohlberg Foundation; Research Funds of the University of Helsinki; Government Special state subsidy for Health Sciences (EVO funding) at Helsinki and Uusimaa Hospital District.
Tea Kaartokallio was supported by Doctoral Programme in Biomedicine (DPBM), Doctoral Programme in Clinical Research (KLTO), Research Foundation of the University of Helsinki and Biomedicum Helsinki Foundation.
Ayat Sayed was supported by a grant from the Egyptian Ministry of Higher Education.
Elina Salmela was supported by Antti and Jenny Wihuri Foundation.
Tuuli Lappalainen was supported by Emil Aaltonen Foundation.
The Australian and New Zealand component of the study were funded by grants from the Australian National Health and Medical Research Council (# 1053152) and the National Institutes of Health of the USA (# HD049847).
Funding for the UK VIP (vitamins in pre-eclampsia) trial was provided by the Wellcome Trust.
Acknowledgements
We sincerely thank all the participants and the support of all the clinicians, research midwives, researchers and technicians. We would like to express our thanks to Ms. Leena Järvinen for technical assistance with the Finnish family cohort.
Disclosure statement
The authors report no conflicts of interest. The funders did not participate in study design, analysis or reporting of the results.
References
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4.
- Baxter R, Chang C, Chelliah Y, Blandin S, Levashina E, Deisenhofer J. Structural basis for conserved complement factor-like function in the antimalarial protein TEP1. PNAS. 2007;104:11615–20.
- Arngrimsson R, Björnsson S, Geirsson RT, Björnsson H, Walker JJ, Snaedal G. Genetic and familial predisposition to eclampsia and pre-eclampsia in a defined population. Br J Obstet Gynaecol. 1990;97:762–9.
- Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–72.
- Lie RT, Rasmussen S, Brunborg H, Gjessing HK, Lie-Nielsen E, Irgens LM. Fetal and maternal contributions to risk of pre-eclampsia: population based study. BMJ. 1998;316:1343–7.
- Skjaerven R, Vatten LJ, Wilcox AJ, Ronning T, Irgens LM, Lie RT. Recurrence of pre-eclampsia across generations: exploring fetal and maternal genetic components in a population based cohort. BMJ. 2005;331:877.
- Haukkamaa L, Salminen M, Laivuori H, Leinonen H, Hiilesmaa V, Kaaja R. Risk for subsequent coronary artery disease after preeclampsia. Am J Cardiol. 2004;93:805–8.
- Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–6.
- Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–51.
- Roberts JM, Hubel CA. Pregnancy: a screening test for later life cardiovascular disease. Womens Health Issues. 2010;20:304–7.
- Laivuori H, Lahermo P, Ollikainen V, Widen E, Haiva-Mallinen L, Sundstrom H, et al. Susceptibility loci for preeclampsia on chromosomes 2p25 and 9p13 in Finnish families. Am J Hum Genet. 2003;72:168–77.
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78.
- McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–91.
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53.
- Schunkert H, Gotz A, Braund P, McGinnis R, Tregouet DA, Mangino M, et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008;117:1675–84.
- Palomaki GE, Melillo S, Bradley LA. Association between 9p21 genomic markers and heart disease: a meta-analysis. JAMA. 2010;303:648–56.
- Cugino D, Gianfagna F, Santimone I, de Gaetano G, Donati MB, Iacoviello L, et al. Type 2 diabetes and polymorphisms on chromosome 9p21: a meta-analysis. Nutr Metab Cardiovasc Dis. 2012;22:619–25.
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–23.
- Ylisaukko-Oja T, Peyrard-Janvid M, Lindgren CM, Rehnstrom K, Vanhala R, Peltonen L, et al. Family-based association study of DYX1C1 variants in autism. Eur J Hum Genet. 2005;13:127–30.
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–21.
- Purcell S, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–50.
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80.
- Kaartokallio T, Cervera A, Kyllonen A, Laivuori K, FINNPEC Core Investigator Group. Gene expression profiling of pre-eclamptic placentae by RNA sequencing. Sci Rep. 2015;5:14107.
- Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension. 2001;37:232–9.
- Ness RB, Hubel CA. Risk for coronary artery disease and morbid preeclampsia: a commentary. Ann Epidemiol. 2005;15:726–33.
- Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77:154–8.
