Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) associates with cardiovascular disease independently of classic risk factors. Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) is secreted by hepatocytes and inhibits the uptake of low-density lipoproteins by targeting the receptor for degradation, and possibly lipogenesis. PCSK9 loss-of-function mutations and anti-PCKS9 drugs reduce LDL-cholesterol.
Aim: To evaluate whether hepatic fat content is associated with circulating PCSK9.
Materials and methods: In 201 consecutive patients biopsied for suspected nonalcoholic steatohepatitis, liver damage was quantified by NAFLD activity score, circulating PCSK9 by ELISA, and hepatic mRNA by qRT-PCR in a subset (n = 76).
Results: Circulating PCSK9 was associated with steatosis grade (p = 0.0011), necroinflammation (p < 0.001), ballooning (p = 0.005), and fibrosis stage (p = 0.001). At multivariate analysis, PCSK9 was associated with steatosis grade (p = 0.012), older age and lower BMI, independently of sex, hyperglycemia, and fibrosis/inflammation. Circulating PCSK9 was associated with hepatic expression of SREBP-1c (p = 0.0002) and FAS (p = 0.03). PCSK9 mRNA levels were also correlated with steatosis severity (p = 0.04) and hepatic APOB (p < 0.001), SREBP-1c (p = 0.047) and FAS expression (p = 0.001).
Conclusions: Circulating PCSK9 increases with hepatic fat accumulation and correlates with the severity of steatosis, independently of metabolic confounders and liver damage. Modulation of PCSK9 synthesis and release might be involved in NAFLD pathogenesis.
Circulating PCSK9 levels increase with hepatic fat accumulation.
Circulating PCSK9 levels are associated with increased de novo lipogenesis.
Hepatic PCSK9 expression is associated with steatosis severity and activation of lipogenesis.
Key Messages
Introduction
Fat accumulation exceeding 5% of liver weight in the absence of at risk alcohol intake defines nonalcoholic fatty liver disease (NAFLD), also known as steatosis. Lipid accumulation depends on increased flux of free fatty acids from the adipose tissue due to insulin resistance, the major risk factor for this condition (Citation1), over-stimulation of de novo lipogenesis driven by hyperinsulinemia, and a relative impairment in mitochondrial beta-oxidation (Citation2,Citation3). A relative impairment in the secretion of very low-density lipoproteins (VLDL) and free cholesterol accumulation also contribute to hepatocellular damage in the progressive inflammatory form of the disease, namely, nonalcoholic steatohepatitis (NASH) (Citation4,Citation5).
Following the epidemics of obesity, NAFLD has become the leading cause of liver damage in the Western countries (Citation6). Furthermore, NAFLD confers an increased risk of cardiovascular events independently of classic risk factors (Citation7). Increased release of inflammatory mediators and coagulation factors by the liver has been proposed to link steatosis with atherogenesis, but altered lipoproteins composition and metabolism may be involved as well.
Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) is an important player in lipoprotein metabolism (Citation8). PCSK9 is mainly expressed by hepatocytes (Citation9–11). PCSK9 undergoes an autocatalytic cleavage, and is actively secreted in the extracellular space, where it circulates mainly bound to lipoproteins. PCSK9 binds to low-density lipoprotein receptor (LDL-R), and is internalized as a PCSK9-LDL-R complex, which targets LDL-R to lysosomes for degradation. Thus, PCSK9 inhibits LDL uptake (Citation12). PCSK9 gene loss-of-function mutations are associated with reduced circulating cholesterol and protection from cardiovascular disease. Furthermore, a new class of drugs targeting PCSK9 is now available for the treatment of severe hypercholesterolemia (Citation13,Citation14).
PCSK9 release is controlled by hormonal cues, insulin and fasting (Citation10). Therefore, PCSK9 levels are correlated to insulin resistance (IR), hepatic fat and VLDL-triglycerides (Citation15). Furthermore, we and others have recently showed that PCSK9 is regulated by pro-inflammatory cytokines (Citation16–18). Finally, it has recently been reported that PCSK9 levels affect plasma lipoprotein levels also through stimulation of hepatic lipogenesis (Citation19). So far, it is still unknown whether the association between circulating PCSK9 and hepatic fat content is independent on metabolic risk factors, and on the severity of hepatic inflammation and damage.
Therefore, aim of this study was to evaluate whether steatosis and liver damage are associated with circulating PCSK9 concentration in individuals at risk of NASH.
Materials and methods
Subjects
We evaluated 201 Italian patients who underwent liver biopsy for suspected NASH for whom serum samples were available. This cohort was made up of: (a) severely obese individuals (n = 76, Bariatric service cohort), who underwent routine liver biopsy during bariatric surgery between December 2013 and December 2014, (b) patients referred to the Hepatology service, who underwent liver biopsy for persistently abnormal liver enzymes or a long lasting history of steatosis associated with metabolic abnormalities (n = 125; Hepatology service cohort). Other causes of liver disease were excluded, including increased alcohol intake (>30/20 g/day for M/F) (Citation20).
For each patient demographic and anthropometric features, serum glucose and lipids, aminotransferases, PNPLA3 I148M and TM6SF2 E167K variants status, the major genetic determinants of NASH (Citation21), were available. Fifteen of 201 patients received statins as lipid lowering therapy, two received glitazones and one ursodeoxycholic acid. Thirty-nine of 201 patients received metformin as anti-diabetic treatment.
Metabolic studies were performed at the Metabolic Liver Diseases outpatient service, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico. The study protocol was approved by the Ethical Committee of the Fondazione IRCCS. Informed written consent was obtained from each patient and control subject, and the study conforms to the Ethical guidelines of the 1975 Declaration of Helsinki. Demographic and clinical features of the subjects included are shown in .
Table 1. Demographic, clinical, metabolic features of 201 patients who underwent liver biopsy for suspected NASH (Hepatology service and Bariatric Service) stratified according to steatosis severity.
Liver histology
Tissue sections were stained with hematoxylin and eosin, impregnated with silver for reticulin framework, and stained with trichrome for collagen. One expert pathologist unaware of clinical and genetic data reviewed all biopsies. The severity of steatosis (0–3), features of NASH, including necroinflammation (0–3), hepatocellular ballooning (0–2), and fibrosis stage (0–4) were assessed according to the NAFLD clinical research network scoring system (Citation22).
PCSK9 measurement
PCSK9 was measured on a serum aliquot collected after overnight fasting and stored at −80 °C, by a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). The sensitivity ranged from 0.030 to 0.219 ng/ml with a minimum detectable concentration of 0.096 ng/ml.
Hepatic gene expression evaluation
Tissue samples snap-frozen at the time of liver biopsy were available for all the severely obese patients (n = 76). Their clinical features are presented in Supplementary Table 1. Total RNA was isolated by the Trizol reagent (Lifetechnologies, Carlsbad, CA). First-strand cDNA was synthesized using 1 microg of total RNA, with the Superscript VILO cDNA synthesis kit (Lifetechnologies, Carlsbad, CA). Hepatic PCSK9, apolipoprotein-B (APOB), sterol regulatory element binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) mRNA levels were analyzed by qRT-PCR, and expression normalized for 18S. PCR cycling conditions were as follows: 94 °C for 10 min, 40 cycles at 94 °C for 15s, and 60 °C for 1 min. Ct values were used for the relative quantification of genes expression with the ΔΔCt calculation. Primer sequences are available upon request.
Statistical analysis
For descriptive statistics, results were expressed as mean ± standard deviation for normally distributed variables, median for non-normally distributed variables, which were log transformed before analysis. Ordinal regression models were fit to examine the association of steatosis grade (a semi-quantitative score) with clinical features. Variables associated with circulating PCSK9 levels were analyzed by generalized linear regression models. A p value ≤0.05 (two-tailed) was considered significant. Analyses were performed with JMP 12.0 (SAS, Cary, CA) statistical analysis software.
Results
Clinical determinants of hepatic fat content
Clinical determinants of hepatic fat content in patients stratified according to the recruitment criteria are shown in . In the Hepatology service cohort, BMI (p = 0.001), LDL cholesterol (p = 0.04), ALT (p = 0.002), impaired fasting glucose (IFG)/T2DM (p = 0.001), circulating insulin levels (p = 0.01) and PNPLA3 I148M alleles (p = 0.03) were associated with steatosis severity. Similarly, in the Bariatric service cohort, BMI (p = 0.008) and insulin levels (p = 0.02) resulted associated with steatosis grade.
After adjustment for BMI, circulating PCSK9 concentration was associated with steatosis grade in the Hepatology service cohort (p = 0.03) and nearly associated with steatosis in the Bariatric service cohort (p = 0.07).
PCSK9 is associated with histological features of NAFLD
At univariate analysis, circulating PCSK9 concentration was associated with age (estimate 0.015 ± 0.004 log ng/mL; p < 0.001) and inversely correlated with BMI (−0.025 ± 0.006; p < 0.001). There was no significant association between circulating PCSK9 and total cholesterol, HDL and LDL cholesterol, triglycerides, and ALT levels ().
Table 2. Variables associated with circulating PCSK9 levels (log ng/mL) in 201 Italian subjects who underwent liver biopsy for suspected NASH.
The association between circulating PCSK9 levels and histological damage is shown in . Circulating PCSK9 levels were associated with both steatosis grade (panel A; estimate +0.18 ± 0.06 log ng/mL; p = 0.001), and all the parameters reflecting hepatic damage, namely, necroinflammation (panel B; +0.27 ± 0.07; p < 0.001), hepatocellular ballooning (panel C; +0.28 ± 0.09; p = 0.005), and with fibrosis stage (panel D; +0.16 ± 0.05; p = 0.001).
Figure 1. Serum PCSK9 and histological liver damage. Correlation between histological steatosis severity (A), necroinflammation (B), ballooning (C), fibrosis (D) and circulating PCSK9 concentration.
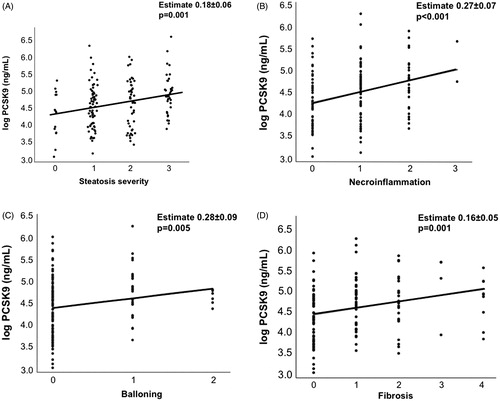
At multivariate analysis, circulating PCSK9 concentration was associated with steatosis grade (p = 0.012), older age (p = 0.038), and lower BMI (p = 0.003), independently of sex, presence of IFG/T2DM, and fibrosis severity (). Conditioning the aforementioned model for lobular necroinflammation or hepatocellular ballooning in alternative to fibrosis stage did not abolish the independent association between steatosis severity and circulating PCSK9 (adjusted p = 0.02), whereas liver damage was again not independently associated with PCSK9 (p > 0.05).
At univariate analysis, statin use was not significantly associated with circulating PCSK9 levels (estimate 0.11 ± 0.24, p = 0.65) suggesting that increased PCSK9 levels in NAFLD patients are not explained by pharmacological treatment ().
Finally, PNPLA3 I148M and TM6SF2 E167 variants were not significantly associated with circulating PCSK9 ().
PCSK9 serum levels and gene expression
Circulating PCSK9 levels were associated with hepatic SREBP-1c (estimate +0.24 ± 0.06 log ng/mL; p = 0.0002) and FAS mRNA levels (estimate +0.12 ± 0.06 log ng/mL; p = 0.03) ().
Figure 2. Serum and hepatic PCSK9 levels correlate with lipogenic genes. Correlation between circulating and hepatic PCSK9 expression with hepatic SREBP-1c and FAS mRNA levels.
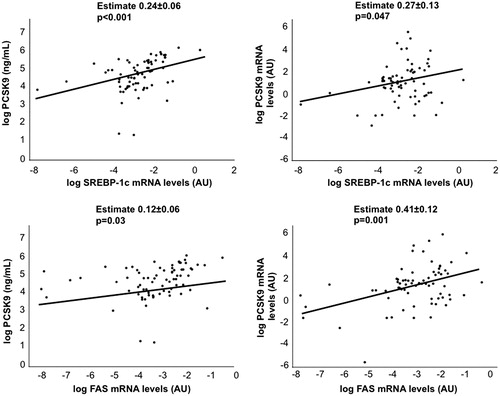
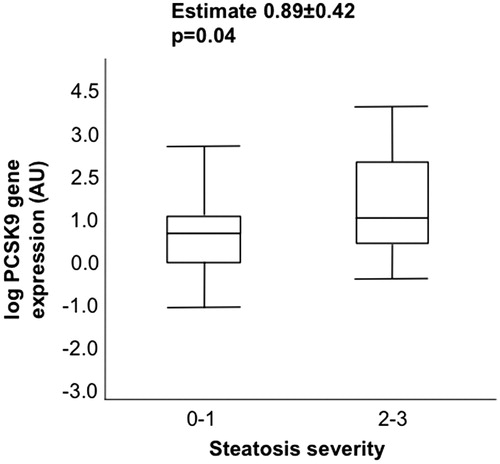
Hepatic PCSK9 mRNA levels were associated with steatosis severity (estimate +0.89 ± 0.42; p = 0.04) (). Moreover, we found a significant correlation between hepatic PCSK9, APOB, SREBP-1c and FAS expression (estimate + 0.51 ± 0.05, p < 0.001; +0.27 ± 0.13, p = 0.047; +0.41 ± 0.12, p = 0.001, respectively). Hepatic APOB, SREBP-1c and FAS mRNA levels were also associated with steatosis severity (estimate + 0.54 ± 0.24, p = 0.02; + 039 ± 0.13, p = 0.003; +0.54 ± 0.15, p < 0.001, respectively).
Figure 3. Hepatic PCSK9 expression and steatosis severity. Correlation between histological steatosis severity and hepatic PCSK9 mRNA levels.
The independent predictors of PCSK9 mRNA levels are shown in Supplementary Table 2.
Discussion
In this study, we examined whether histological steatosis and liver damage are associated with circulating PCSK9 in individuals at risk of NASH. We observed that circulating PCSK9 was correlated with the severity of hepatic steatosis, independently of metabolic confounders and liver damage, including necroinflammation and fibrosis severity.
Evidence is accumulating that NAFLD is a cardiovascular disease determinant independently of classic risk factors (Citation23). However, the specific molecular mechanisms underpinning this association remain to be elucidated. Recently, PCSK9 was identified as a major player in lipoprotein metabolism and PCSK9 expression has been shown to modulate serum lipids and the susceptibility to cardiovascular disease (Citation13,Citation14). PCSK9 is mainly secreted by the liver, and the circulating form is involved in regulating lipoprotein metabolism by inhibiting the uptake of LDL and other lipoproteins (Citation14). Therefore, the altered secretion of PCSK9 related to hepatocellular fat accumulation could be involved in the pathogenesis of cardiovascular disease in patients with NAFLD.
We observed a robust and, for the first time reported, independent association between liver fat and circulating PCSK9, which was evident both in patients referred for suspected liver disease and in those undergoing bariatric surgery, which was not accounted for lipid lowering therapies. Most importantly, the link between steatosis severity and PCSK9 was independent from necroinflammation and hepatocellular ballooning. This suggests that PCKS9 is not induced by inflammatory cytokines, but is directly linked to hepatocellular triglycerides content and stimulation of hepatic lipogenesis.
Indeed, in a subgroup of severely obese individuals, we found that circulating PCSK9 and its hepatic expression correlate with SREBP-1c and FAS mRNA levels, suggesting a correlation between PCSK9 and increased de novo lipogenesis. Of note, it has recently been reported that PCSK9 levels affect plasma lipid and lipoprotein levels not only by reducing lipoproteins clearance but also through induction of hepatic lipid synthesis, which was triggered by high-fat diet (Citation19).
In keeping, in this subgroup of obese individuals PCSK9 mRNA levels also correlated with the histological severity of steatosis. It could therefore be speculated that both hepatic fat accumulation and PCSK9 transcription are induced by hyperinsulinemia and activation of SREBP-1c, the master regulator of lipogenesis (Citation10,Citation24). However, in line with previous data (Citation25,Citation26), we could not detect a direct relationship between PCSK9 mRNA levels and secreted protein. Future studies are thus required to better address the mechanism of this association.
Surprisingly, in the present cohort we found an inverse correlation between circulating PCSK9 and BMI, which was maintained by analyzing separately patients referred for liver disease and severe obesity. Therefore, the association between PCSK9 and steatosis severity seems to be independent of fat mass. It could be speculated that previous studies detected a positive association between BMI and PCSK9 levels because they were conducted in the general population, where overweight/obesity is strongly linked with increased hepatic fat content. In contrast, in a selected population with high hepatic fat content, such as that described in the present study, where most of the patients had NAFLD and overweight/obesity, we could better dissociate the impact of this two factors on PCSK9 levels (Citation27). Overall, our findings seem to suggest that increased hepatic fat and not adipose tissue mass are responsible for increased PCSK9 levels in obese people.
Another aspect that should be taken into account is that usually women have higher circulating PCKS9 levels than men. In our study, we found a negative association of PCSK9 with female gender, even if the association was lost after adjustment for confounders at multivariate analysis. Remarkably, almost half of females included in our study were older than 50 years, so likely in post-menopausal state, and it has been reported that PCSK9 levels are higher in postmenopausal than in premenopausal females (Citation28,Citation29). However, not all the studies are consistent with the evidence that estrogens induce PCSK9 levels, and it could be hypothesized that induction of PCSK9 during NAFLD may overcome its regulation by sexual hormones.
Therefore, induction of PCKS9 may be involved in the pathogenesis of NAFLD modulating de novo lipogenesis. Given that (a) neutralization of circulating PCSK9 and that loss-of-function PCSK9 mutations reduce cardiovascular mortality (Citation30,Citation31), and that (b) circulating PCSK9 is associated with vascular damage and cardiovascular events independently of serum lipids (Citation25,Citation26), these data also suggest that increased PCSK9 secretion could contribute to the pathogenesis of vascular disease in patients with NAFLD.
However, unlike previous published studies, we did not find a significant association between PCSK9 and LDL cholesterol. However, the lack of association between PCSK9 and LDL cholesterol is in line with recent studies conducted in other cohorts of patients (Citation17,Citation25,Citation32). It is important to point out that the association between plasma PCSK9 and LDL cholesterol levels, although reproducible, is relatively weak. Indeed, in the Dallas Heart Study variations in fasting plasma PCSK9 only accounted for approximately 7% of the variations in LDL cholesterol (Citation18). Thus, it seems conceivable to hypothesize that, in our and other studies, the sample size was not sufficient to demonstrate the association between circulating PCSK9 and the LDL cholesterol levels.
Furthermore, it has recently been demonstrated that PCSK9 promotes atherogenesis both indirectly, by raising plasma lipids, and directly by modulating the entry of inflammatory monocytes into the artery wall (Citation19).
However, limitations of the study include the lack of assessment of the relationship between circulating PCSK9 and lipoproteins composition, and the impact on the severity of atherosclerosis. Larger prospective studies are thereby warranted to address the impact of the present findings.
In conclusion, circulating PCSK9 concentration increases with the severity of hepatic fat accumulation in patients at risk of NASH. Modulation of PCSK9 synthesis and release might be involved in the pathogenesis of NAFLD, with potential therapeutic implications.
Funding information
This work was supported by the Ricerca Corrente Fondazione Ca’ Granda IRCCS Milan (Public research institution internal grant), Associazione Malattie Metaboliche del Fegato ONLUS (non-profit organization for the study and care of metabolic liver diseases), grant 2012-0549 from Fondazione Cariplo (NF) and Piano di Sostegno per la Ricerca, Università degli Studi di Milano, 2015-2017 - Linea 2 (Azione A) (MR).
| Abbreviations | ||
| NAFLD | = | nonalcoholic fatty liver disease |
| PCSK9 | = | Proprotein Convertase Subtilisin/Kexin type 9 |
| NASH | = | nonalcoholic steatohepatitis |
| BMI | = | body mass index |
| VLDL | = | very low-density lipoproteins |
| LDL | = | low-density lipoprotein |
| LDL-R | = | low density lipoprotein receptor |
| IR | = | insulin resistance |
| PNPLA3 | = | Patatin-like phospholipase domain-containing protein 3 |
| ApoB | = | apolipoprotein B |
| IFG | = | impaired fasting glucose |
| T2DM | = | type 2 diabetes mellitus |
| ALT | = | alanine aminotransferase |
| GGT | = | gamma-glutamyltransferases |
| ELISA | = | enzyme-linked immunosorbent assay |
| SREBP | = | Sterol-regulatory element binding protein |
REVISED_SUPPPLEMENTAL_TABLES.doc
Download MS Word (47 KB)Acknowledgements
We thank all the members of the Metabolic Liver Disease Center for helpful comments and discussion.
Disclosure statement
The authors report no conflicts of interest.
References
- Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5.
- Rametta R, Mozzi E, Dongiovanni P, Motta BM, Milano M, Roviaro G, et al. Increased insulin receptor substrate 2 expression is associated with steatohepatitis and altered lipid metabolism in obese subjects. Int J Obes (2005). 2013;37:986–92.
- Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–42.
- Musso G, Gambino R, Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res. 2013;52:175–91.
- Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–14.
- Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608.
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50.
- Ferri N, Corsini A, Macchi C, Magni P, Ruscica M. Proprotein convertase subtilisin kexin type 9 and high-density lipoprotein metabolism: experimental animal models and clinical evidence. Transl Res. 2015. doi:10.1016/j.trsl.2015.10.004.
- Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–33.
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–83.
- Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–6.
- Guo YL, Zhang W, Li JJ. PCSK9 and lipid lowering drugs. Clin Chim Acta. 2014;437:66–71.
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–5.
- Tibolla G, Norata GD, Artali R, Meneghetti F, Catapano AL. Proprotein convertase subtilisin/kexin type 9 (PCSK9): from structure-function relation to therapeutic inhibition. Nutr Metab Cardiovasc Dis. 2011;21:835–43.
- Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205–15.
- Ruscica M, Ricci C, Macchi C, Magni P, Cristofani R, Liu J, et al. Suppressor of Cytokine signaling-3 (SOCS-3) induces proprotein convertase subtilisin kexin type 9 (PCSK9) expression in hepatic HepG2 cell line. J Biol Chem. 2016;291:3508–19.
- Cariou B, Langhi C, Le Bras M, Bortolotti M, Le KA, Theytaz F, et al. Plasma PCSK9 concentrations during an oral fat load and after short term high-fat, high-fat high-protein and high-fructose diets. Nutr Metab (Lond). 2013;10:4.
- Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–43.
- Tavori H, Giunzioni I, Predazzi IM, Plubell D, Miles J, DeVay RM, et al. Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE - and LDLR-mediated mechanisms. Cardiovasc Res. 2016;110:268–78.
- Dongiovanni P, Petta S, Mannisto V, Margherita Mancina R, Pipitone R, Karja V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–12.
- Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19:6969–78.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
- Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104.
- Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:6211–18.
- Werner C, Hoffmann MM, Winkler K, Bohm M, Laufs U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. 2014;62:94–102.
- Lee CJ, Lee YH, Park SW, Kim KJ, Park S, Youn JC, et al. Association of serum proprotein convertase subtilisin/kexin type 9 with carotid intima media thickness in hypertensive subjects. Metabolism. 2013;62:845–50.
- Lakoski SG, Xu F, Vega GL, Grundy SM, Chandalia M, Lam C, et al. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J Clin Endocrinol Metabol. 2010;95:800–9.
- Ghosh M, Galman C, Rudling M, Angelin B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res. 2015;56:463–9.
- Guo W, Fu J, Chen X, Gao B, Fu Z, Fan H, et al. The effects of estrogen on serum level and hepatocyte expression of PCSK9. Metabolism. 2015;64:554–60.
- Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:40–51.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
- Nekaies Y, Baudin B, Kelbousi S, Sakly M, Attia N. Plasma proprotein convertase subtilisin/kexin type 9 is associated with Lp(a) in type 2 diabetic patients. J Diabetes Complications. 2015;29:1165–70.
