Abstract
Heart failure (HF) and obesity are major public health problems. Studies have shown that obesity may increase the risk of developing new HF but after patients have developed HF, obesity may be associated with improved outcomes. This paradoxical association of obesity with HF remains poorly understood. It is believed that the obesity paradox may in part be due to the inherent limitations of body mass index (BMI) as a measure of obesity. BMI may not appropriately measure important components of body mass like body fat, fat distribution, lean body mass, and body fluid content and may not be ideal for examining the relationship of body composition with health outcomes. Differentiating between body fat and lean body mass may explain some of the paradoxical association between higher BMI and better prognosis in patients with HF. Paradoxical outcomes in HF may also be due to phenotypes of obesity. Future studies need to develop and test metrics that may better measure body composition and may serve as a better tool for the estimation of the true association of obesity and outcomes in HF and determine whether the association may vary by obesity phenotypes.
Obesity predisposes to heart failure in all age groups. But obesity in heart failure is an area of controversy, because of obesity paradox, the apparent protective effect of overweight and mild obesity on mortality after development of heart failure.
Traditional markers of obesity do not measure different components of body weight like muscle mass, fat, water, and skeletal weight. Body Mass Index in heart failure subjects does not measure accurately body fat or fluid retention. So new markers of obesity like visceral adiposity index, body composition analysis, sarcopenic status assessment may be helpful in the assessment of heart failure outcomes.
Different phenotypes of obesity may be responsible for the different morbidity, mortality as well as therapeutic outcomes in heart failure.
KEY MESSAGES
Introduction
Obesity is commonly defined as a body mass index (BMI) of 30 kg/m2. Obesity is an independent risk factor for incident heart failure (HF), by its effect on cardiovascular structure, function, and hemodynamics (Citation1,Citation2). Globally, 200 million men and 300 million women are obese (Citation3). Estimated prevalence of obesity can be up to 86% in all subjects with HF and more commonly seen in the elderly (Citation4,Citation5). Older adults are at higher risk for developing HF and are seen in 20% of octogenarians (Citation6). The relationship between obesity and cardiovascular morbidity and mortality is well known. Obesity has been associated with increased risk of cardiovascular disease like hypertension, myocardial infarction (MI), stroke, HF, and mortality (Citation7). In a study showed to prevent HF, higher levels of physical activity were needed than for other cardiovascular events like MI and stroke (Citation8). Despite advances in the diagnosis and management of HF, higher mortality rates are seen (Citation9). Fifty to seventy five percent (50–75%) of HF subjects, die within five years of diagnosis (Citation10).
In the Framingham Heart community study, investigators found increased BMI was associated with a two-fold increase in risk of HF and the risk varies with sex. With each unit increase in BMI, there was a 7% increase in risk for HF in women and 5% in men (Citation11). In another prospective cohort study on men, Kenchaiah et al. found that every 1 kg/m2 increase in BMI was associated with an 11% (95% CI 9%–13%) increase in HF risk (Citation12). Prevalence of obesity can also vary with the type of HF. A study reported the prevalence of obesity was seen in 41% of subjects with preserved LVEF and 36% with reduced LVEF (Citation13).
BMI is often used to determine obesity. Obesity is a risk factor for many cardiovascular and non-cardiovascular diseases. But once the overweight or obese patient, especially in mild or type 1 category developed the disease, studies showed that higher BMI may be protective against downstream disease outcomes including mortality, known as obesity paradox (Citation14). In a recent study of HF due to non-ischemic etiology and reduced ejection fraction, obesity was associated with a reduced risk of death (HR 0.52, 95% CI 0.28–0.99), but this protective effect disappeared after adjusting for VO2 max and BNP level (Citation15). In this study, we looked at the complex relationship between obesity and obesity paradox with HF risk as well as HF outcomes and tried to analyze the reasons for these differential HF outcomes.
Search strategy
The literature was searched using the electronic databases MEDLINE (1966–January 2016), EMBASE and SCOPUS (1965–January 2016), and DARE (1966–January 2016). The main search items were obesity, overweight, obesity paradox, BMI, obesity measures, cholesterol levels, sarcopenia, malnutrition, cardiorespiratory status, HF, mortality, morbidity, and HF outcomes. Articles not in English were excluded from this review.
Obesity paradox in HF and association with morbidity and mortality
Obesity paradox in HF is a term used to describe better prognosis with HF in overweight and mildly obese patients (Citation16). Obesity and hypercholesterolemia can be associated with increased survival in HF patients. Studies had shown that high cholesterol did not predict worse outcomes in subjects with chronic HF, but rather were associated with improved outcomes. This was described as reverse epidemiology in patients with HF (Citation17). Lower total cholesterol level predicts increased mortality risk in the hospital in acutely decompensated subjects with chronic HF (Citation18). Similarly another study in advanced HF subjects found that low serum cholesterol was a risk factor for increase in mortality, whereas overweight status was not found to be a risk factor. In this study, after adjusting for HF prognostic factors and lipid lowering medications, total cholesterol in the lowest quintile (<129 mg/dl) was associated with more than two times higher risk of death (Citation19). Similar findings regarding the association of low serum cholesterol and mortality had been shown in HF subjects with CAD and not seen in subjects without CAD (Citation20). However, in idiopathic dilated cardiomyopathy subjects, low cholesterol levels did not independently predict adverse prognosis (Citation21). In a study on male veterans with HF, surviving subjects had a higher prevalence of hyperlipidemia than deceased subjects (Citation22). In a recent study on systolic HF subjects with LVEF <40%, overweight females has a significant survival benefit (HR 0.84, 95% CI 0.77–0.93, p = 0.0005), whereas overweight and obese males had higher adjusted mortality than normal-weight males (p for interaction <0.0001) (Citation23). Subjects with identical BMI may have varying body composition and misclassification was shown in patients with chronic HF (Citation24). In obesity paradox studies, less than 10% used a direct measure of body composition (Citation25). Because of the association of obesity and high cholesterol with improved survival in established HF subjects, the role of cholesterol lowering therapy in chronic HF remains controversial ().
Table 1. Selected studies on obesity and obesity paradox on heart failure and its outcomes.
Mechanisms of obesity and obesity paradox in increasing the risk and outcomes of HF
Mechanisms of obesity on risk and outcomes of HF
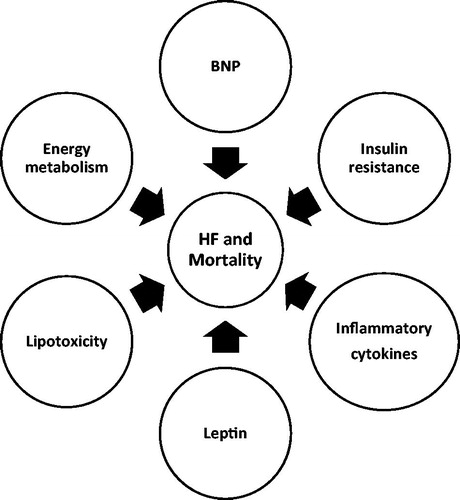
Obesity causes excess epicardial adipose tissue deposition, hypertrophy of different chambers of heart, impaired LV diastolic filling/relaxation, increased cardiac output with increased left ventricular stroke volume, increased myocardial oxygen consumption and increased pulmonary artery, and capillary wedge pressure, which can cause HF and also lead to downstream outcomes after development of HF (Citation34) (). With obesity, insulin resistance and hyperinsulinism were seen and associated with abnormal left ventricular energy metabolism (Citation35). Obesity leads to metabolic abnormalities that can increase the levels of inflammatory cytokines (interleukins, tumor necrosis factor-alpha, CRP), which can lead to cardiac fibrosis and myocardial stiffening (Citation36). Increased abnormal adipose tissue leads to lipotoxicity and apoptosis of cardiac myocytes, leading to myocardial dysfunction (Citation37).
Mechanisms of obesity paradox on risk and outcomes of HF
The underlying mechanism of obesity paradox is not clear. Possible mechanisms include: 1. Insulin resistance/insulin hypersensitivity, 2. Higher lean body mass may be protective by role of myocytes on vasculature and by favorable cytokines or myokines. 3. The sex difference on survival could be due to differences in adipokine hormone (adiponectin, resistin, leptin, tumor necrosis factor-alpha) which signal between adipose tissue and myocardium. 4. Female HF hearts may have greater myocardial fatty acid uptake and lesser myocardial glucose utilization (Citation38). Fat tissue has been shown to produce tumor necrosis factor alpha receptors which may be protective. High circulating lipoprotein levels in obese subjects may bind and detoxify lipopolysaccharides that may play a role in the release of inflammatory cytokines (Citation39,Citation40). Several other mechanisms may explain the protective effect of higher BMI on mortality (Citation41). NT-pro BNP levels were lower in overweight and obese subjects which predict lower mortality (Citation42). Experimental study suggested that leptin produced by fat tissue may have a protective effect in HF (Citation43). In obesity, lower levels of adiponectin were seen and it was associated with lower mortality in HF (Citation44).
Traditional and new markers for measuring obesity
Traditional measures of obesity
Traditional anthropometric measures include body weight, BMI, waist circumference, and hip–waist ratio (). BMI measures weight related to height and does not measure fat. Obesity is a condition, where there is abnormal accumulation of body fat (Citation45).
Table 2. Traditional and new markers of obesity.
Limitations of BMI as a marker of obesity in HF risk and outcomes
BMI can classify people as overweight or obese, when they do not have an excess of fat. It can misclassify when subjects have changes in body composition with loss of muscle and increase in fat, commonly seen in aging, also known as sarcopenic obesity. BMI may mislead in these subjects because of low lean body mass and high percentage of fat (Citation46–48). The waist circumference was shown to be even better than the BMI as a measure of obesity, because of the age-dependent decrease in height in older adults (Citation49,Citation50). In a study by De Schutter et al. on 47,866 with preserved left ventricular ejection fraction over 3.1 years found that in subjects with higher lean body mass, skeletal muscle was associated with 29% lower mortality compared with subjects who had higher body fat (Citation51).
Central obesity is measured by waist circumference and waist to hip ratio. Obesity is defined as a waist circumference more than or equal to 102 cm for men and 88 cm for women or waist to hip ratio of >0.9 or >0.8 in women. In a study by Clark et al., on advanced systolic HF subjects, high waist circumference, as well as combination of high BMI and waist circumference was independently associated with better survival (Citation31). Even though fat distribution varies by sex, this study showed that high BMI and waist circumference were associated with improved outcomes like death, urgent status heart transplant, and ventricular assist device placement in both sexes (Citation52).
New measures
HF is a chronic disease associated with muscle wasting and water retention. Some HF subjects who are considered to be normal weight may actually be underweight. So new measures of obesity including lean body weight calculations were used in recent studies. Body fat % calculations are calculated by calculating the quotient total body fat by weight multiplied by 100. Total body fat is calculated as the difference between weight (kg) and fat free (lean) mass. According to Baumgartner’s criteria, obesity was defined as % of body fat >27 in men and >38 in women (Citation53). Another measure used was the visceral adiposity index (Citation54). Body composition analysis was assessed by using bioelectrical impedance (Citation24). Sarcopenia was defined as the height-adjusted appendicular skeletal muscle mass, assessed by dual energy X-ray absorptiometry (DEXA) with two standard deviations below the reference for healthy younger persons (Citation55). Janssen et al. proposed sex specific cutoffs for sarcopenia (men, normal: >10.76 kg/m2, class I sarcopenia: 8.51–10.75 kg/m2, class 2 sarcopenia: <8.50kg/m2; females, normal >6.76 kg/m2, class I sarcopenia: 5.76–6.75 kg/m2, class II sarcopenia: <5.75 kg/m2) (Citation56). The definition of sarcopenia has been changed by the European working group on sarcopenia in older people, which includes low muscle function (strength or performance) in addition to low muscle mass (Citation57). Subjects fulfilling criteria for sarcopenia and obesity were classified as sarcopenic obesity.
Types (phenotypes) of obesity and HF outcomes
There are different types of obesity which may be responsible for the paradox seen in obesity as well as different HF outcomes including survival ().
Table 3. Types (phenotypes) of obesity.
Obesity based on BMI
According to the World Health Organization (WHO) classification, subjects are considered overweight, when they have a BMI of 25.0–29.9 kg/m2, class I obesity a BMI of 30.0–34.9 kg/m2, class II obesity a BMI of 35.0–39.9 kg/m2, and class III or morbid obesity for BMI ≥40 kg/m2 (Citation51). In a meta-analysis done by Orepoulos et al. on BMI and mortality in HF subjects over a period of 2.7 years, BMI ≥ 30 kg/m2 was associated with lower all-cause (RR 0.67, 95% CI 0.62–0.73) and lower cardiovascular mortality (RR 0.60, 95% CI 0.62–0.73) (Citation58). Sharma et al. in their meta-analysis showed decreased risk of CV mortality and hospitalization in overweight subjects, whereas underweight (<20 kg/m2) was associated with increased risk of CV mortality, total mortality, and hospitalization (Citation59). A recent meta-analysis looked at the dose–response relationship of BMI, as well as compared the relationship with abdominal obesity using waist circumference and waist to hip ratio. For every 5 unit increase in BMI, HF incidence increased by 41% (RR 1.41, 95% CI 1.37–1.47), relative risk for a 10 cm increase in waist circumference increased HF risk by 29% (1.29, 95% CI 1.27–1.37) and for an increase 0.1 unit in waist to hip ratio (1.29, 95% CI 1.13–1.47). No increase in mortality after HF was seen in this study (Citation28) (). Body weight and BMI do not analyze the body composition (i.e. different proportion of lean vs. adipose tissue). Oreopoulos et al. showed BMI misclassified body composition in 41% of subjects with chronic HF (Citation24). Such misclassification may explain the controversial results in obesity studies using BMI and the obesity paradox seen in HF. Because of the phenotypes of obesity, assuming homogeneity of risk with obesity groups based on BMI may not be correct. Failing to account for the positive confounding effect of abdominal circumference and the negative or suppression confounding effect of hip circumference in obesity related studies may also lead to biased estimates. So the non-linear or U shaped association of obesity to mortality could also be due to confounding bias of the hip and waist circumference (Citation16,Citation60). A recent prospective study on different types of HF showed adding mid-upper arm circumference to BMI (0.70 vs. 0.63, p = 0.012), but not waist circumference to BMI (0.64 vs. 0.63, p = 0.763) plays a complementary role in predicting all-cause mortality in patients with HF (Citation61). Khalid et al. in Atherosclerosis Risk in Communities Study, used BMI measured ≥6 months before incident HF (pre-morbid BMI) and found significant component of the obesity paradox on mortality in established HF could be due to pre-morbid obesity (Citation33).
Sarcopenic obesity
With aging there is also progressive loss of skeletal muscle, with an increase in fat tissue known as sarcopenic obesity. Subjects meeting criteria of sarcopenia and obesity are known as sarcopenic obesity and are a new category of obesity. Baumgartner criteria for sarcopenic obesity include: (Citation1) an appendicular skeletal muscle index (legs and arms muscle mass/height in m2) less than two standard deviations in comparison to young adult reference group aged between 20 and 30 years old and (Citation2) a percentage of body fat above the 60th percentile for the same gender and age (Citation30). In NHANES III study on older adults in the general population found sarcopenic obesity prevalence was 18% in women and 42% in men and also found that sarcopenic obesity is associated with a higher mortality risk when compared to generalized obesity (HR 1.29, 95% CI 1.03–1.60) (Citation62). Muscle wasting is commonly seen with HF. A study by Kitzman et al. showed significant skeletal muscle abnormalities in elderly HF with preserved ejection fraction (HFpEF) subjects (Citation63). In the prospective Cardiovascular Health Study, sarcopenic obesity was associated with a 42% increase in risk of HF over eight years of follow-up (Citation64). In a study on men with HF, overweight and obese subjects had a trend towards lower risk of mortality and it got attenuated when adjusted for muscle mass and BNP (p = 0.05) (Citation65). Literature had shown that therapeutic approaches using ghrelin and selective androgen receptor modulators (SARMs) are under study for the management of sarcopenia (Citation66).
Metabolically normal/abnormal obesity and metabolically obese normal-weight persons
Metabolically Healthy Obese (MHO) phenotype has been defined as a subgroup of obese individuals who do not have insulin resistance, lipid disorders, or hypertension (Citation67,Citation68). Prevalence of MHO ranges from 10 to 30% among obese adults (Citation69,Citation70). In a prospective study by Voulgan et al. comparing MHO with normal-weight individuals with metabolic syndrome over a period of six years, showed MHO individuals had a decreased risk of HF (HR 1.12, 95% CI 0.35–1.33), whereas in normal-weight individuals with metabolic syndrome the risk of HF was higher (RR 2.5, 95% CI 1.68–3.40) (Citation71). Epidemiological studies suggested that MHO carries a lower risk of CV disease and mortality when compared to normal weight or obese but metabolically unhealthy subjects (Citation26). MHO individuals had been shown to have increased subcutaneous relative to visceral fat, lower fat in skeletal muscle and liver, better insulin sensitivity, and inflammatory status (Citation72,Citation73). In a small study on middle aged subjects, MHO subjects had normal myocardial performance, and significant subclinical systolic and diastolic abnormalities were seen in metabolically unhealthy subjects (p = 0.001) (Citation74). In a recent Brazilian population study in middle age individuals, both MHO and metabolically unhealthy normal-weight individuals were associated with elevated high sensitivity C-reactive protein and hepatic steatosis (Citation75). However a Norwegian population based prospective cohort study (HUNT study) found that even in MHO individuals, metabolically healthy severe obese (BMI ≥35 kg/m2) and also having long lasting obesity (>30 years) were associated with increased risk for HF (HR 1.7, 95% CI 1.3–2.3) (Citation76). This study points out that even among MHO individuals, certain subgroups (severe obesity and long-lasting obesity) may have higher risk for CV outcomes.
Obesity with high or low cardiorespiratory fitness
Cardiorespiratory fitness (CRF) with obesity also influences the HF outcomes. It modifies the association between obesity and mortality in HF subjects. CRF measured as peak oxygen uptake (VO2) or minute ventilation (VE/carbon dioxide production (VCO2) has been identified as an important predictor of survival in HF (Citation27). A study in the community-dwelling older adults showed high level of physical activity was associated with lower risk of incident HF (Citation8). Physical activity is the strongest modifiable determinant of CRF and independently associated with HF (Citation77–80). Barry et al. in their meta-analysis of cohort studies showed mortality risk was not significantly different between overweight and obese-fit individuals from normal-weight fit individuals. This study suggested that higher levels of CRF attenuated the long-term mortality effect associated with obesity (Citation81). CRF modifies the association between obesity and mortality both in non-HF as well as in HF subjects. McAuley et al., after analyzing few observational studies showed high CRF had better survival compared to subjects with low CRF in the same BMI category (Citation68). In the Aerobics Center Longitudinal Study (ACLS) on adult men and women in the general population, MHO individuals was shown to have a higher fitness level and a lower risk of CVD mortality and all-cause mortality, when compared to metabolically abnormal individuals (Citation82). In the same ACLS study, Lee et al. showed in males maintaining or improving CRF over a period of 6.3 years was associated with reduced CV mortality, regardless of BMI change (HR 0.56, 95% CI 0.37–0.85) (Citation83). Lavie et al. found in systolic HF subjects that BMI was a significant predictor of age and sex adjusted survival in the low CRF category, but not in the high-CRF group. This study demonstrated that CRF level may actually mitigate the impact of the obesity paradox in HF (Citation84). Clarke et al. in their study showed in advanced systolic HF subjects obesity paradox was only seen in subjects with lower cardiorespiratory status (low PKVO2 group, p < 0.001vs high PKVO2, p = 0.1) (Citation29). It looks that the modifying effects of CRF on survival in relation to the obesity paradox is seen both in patients with and without HF.
Obesity with malnutrition
A study by Colin-Ramprez et al. showed low BMI in HF subjects independently did not predict all-cause (RR 2.03, 95% CI 0.59–7.04) and cardiovascular mortality (RR 2.10. 95% CI 0.37–12.11) among chronic HF subjects when adjusted for nutritional, body composition, and clinical status parameters (Citation32). This study indicates condition like malnutrition may play a significant role in HF outcomes other than BMI. Malnutrition is seen in HF subjects even with normal-weight, overweight, and obese groups. So malnutrition has to be assessed in obese subjects using markers of malnutrition like albumin, prealbumin, total lymphocyte count, cholesterol levels, tricipital fold thickness, and arm circumference. The PLICA study in HF subjects had shown, BMI masked true nutritional status which is an independent prognostic factor above and beyond BMI. The authors of this study stressed the need for nutritional assessment using measures of composition in subjects with obesity paradox (Citation85). In a recent small study, lean HF subjects were found to have greater number of micronutrient deficiencies when compared to overweight and obese subjects (p < 0.01) (Citation86). In subjects with high BMI, malnutrition may be seen infrequently which may partly help to explain the obesity paradox seen in some obese subjects. A cross-sectional study by Casas-Vara et al. in elderly hospitalized HF subjects showed improved survival in subjects with better nutritional status (Citation87). A cohort study done on hospitalized advanced HF subjects showed a simple validated tool, Nutritional Risk Index (NRI) in predicting six-month mortality (38% in subjects with NRI less than 100 and 14% in those with NRI more than 100) (p = 0.04) (Citation88).
Conclusions
Obesity predisposes to HF in all age groups. Once the subject develops HF there is evidence pointing out that certain group of patients who are overweight or mildly obese may have a short-term survival benefit than moderate to severe obese and cachectic subjects, which is known as obesity paradox. Differential outcomes of obesity including obesity paradox on HF could be due to limitations of the traditional marker of BMI, which has been used in most studies of obesity and obesity paradox, and may misclassify obesity. BMI is correlated to some extent to lean body mass, but does not measure accurately body fat or fluid retention in HF subjects. So studies with new markers for measurement of obesity may give more information or insight into this issue. Even though techniques to analyze body composition is evolving, it looks that excess adipose tissue alone may have no protective effect in the presence of low muscle mass. Different phenotypes of obesity may also be responsible for the different morbidity, mortality as well as therapeutic outcomes in HF. Prognostic and therapeutic goals in HF should be based on a combination of weight, body composition biomarkers and measures of metabolic and CRF. So, future studies should focus as well as on the different phenotypes of obesity on HF.
Disclosure statement
The authors report no conflict of interest.
References
- Baena-Diez JM, Bynan AO, Grau M, Gornez-Fernandez C, Vidal-Solsona M, Ledesma-Ulloa G, et al. Obesity is an independent risk factor for heart failure: Zona Franca Cohort study. Clin Cardiol. 2010;33:760–4.
- Nicklas BJ, Lesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–20.
- World Health Organization. Obesity and overweight; 2013. Available from: http://www.who.int.login.ezproxy.library.ualberta.ca/mediacentre/factsheets/fs311/en/
- Alpert MA, Lavie CJ, Agarwal H, Aggarwal K, Kumar SA. Obesity and heart failure. Epidemiology, pathophysiology, clinical manifestations and management. Trans Res. 2014;164:345–56.
- Lavie CJ, Alpert Ma Arena R, Mehra MR, Milani RV Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102.
- Alagiakrishnan K, Banach M, Jones LG, Ahmed A, Aronow WS. Medication management of chronic heart failure in older adults. Drugs Aging. 2013;30:765–82.
- Metra M, Dei Cas L, Massie BM. Treatment of heart failure in the elderly: never say it's too late. Eur Heart J. 2009;30:391–3.
- Patel K, Sui X, Zhang Y, Fonarow CC, Aban IB, Brown CJ, et al. Prevention of heart failure in older adults may require higher levels of physical activity than needed for other cardiovascular events. Int J Cardiol. 2013;168:1905–9.
- Alagiakrishnan K, Banach M, Jones LG, Datta S, Ahmed A, Aronow WS. Update on diastolic heart failure or heart failure with preserved ejection fraction in the older adults. Ann Med. 2013;45:37–50.
- Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Midland Research practices Consortium (MidReC). Prognosis of all-cause heart failure and borderline left ventricular systolic function: 5-year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J. 2007;28:1128–34.
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13.
- Kenchaiah S, Sesso HD, Gaziono JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52.
- Owan TE, Hudge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9.
- Flegal KN, Kalantar-Zadeh K. Overweight, mortality and survival. Obesity (Silver Spring). 2013;21:1744–5.
- Pozzo J, Fourner P, Lairez O, Velvueren PL, Delmas C, Elbaz M, et al. Obesity paradox: origin and best way to assess severity in patients with systolic HF. Obesity (Silver Spring). 2015;23:2002–8.
- Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2013;56:409–14.
- Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–44.
- Horwich TB, Hernandez AF, Dai D, Yancy CW, Fonarow GC. Cholesterol levels and in-hospital mortality in patients with acute decompensated heart failure. Am Heart J. 2008;156:1170–6.
- Horwich TB, Hamilton MA, MacLellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–24.
- Sakatani T, Shirayama T, Suzaki Y, Yamamoto T, Mani H, Kawasaki T, et al. The association between cholesterol and mortality in heart failure. Comparison between patients with and without coronary artery disease. Int Heart J. 2005;46:619–29.
- Christ M, Klima T, Grimm W, Mueller HH, Maisch B. Prognostic significance of serum cholesterol levels in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2006;27:691–9.
- Lissin LW, Gauri AJ, Froelicher VF, Ghayourmi A, Myers J, Glacommini J. The prognostic value of body mass index and standard exercise testing in male veterans with congestive heart failure. J Card Fail. 2002;8:206–15.
- Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The Heart Failure overweight/obesity survival Paradox: the missing sex link. JACC Heart Fail. 2015;3:917–26.
- Oreopoulos A, Ezekowitz JA, Mc Alister FA, Kalantar-Zadeh K, Fonarow GC, Norris CM, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85:609–17.
- Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care. 2015;18:535–51.
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hanners L, Macinnis RJ, et al. Body mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211.
- Pandey A, Berry JD, Lavie CJ. Cardiometabolic disease leading to heart failure: better fat and fit than lean and lazy. Curr Heart Fail Rep. 2015;12:302–8.
- Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, et al. Body mass index, abdominal fitness and heart failure incidence and mortality. A systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133:639–49.
- Colin-Ramirez E, Orea-Tejeda A, Castillo-Martinez L, Montano- Hernandez P, Sanchez -Ramirez A, Pineda- Juarez JA, et al. Malnutrition syndrome, but not body mass index is associated with worse prognosis in heart failure patients. Clin Nutr. 2011;30:753–8.
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallager D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004.
- Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–80.
- Gastelurritia P, Lupon J, de Antonio M, Zamora E, Domingo M, Urrulia A, et al. Body mass index, body fat and nutritional status of patients with heart failure: the PLICA study. Clin Nutr. 2015;34:1233–8.
- Khalid U, Ather S, Bavishi C, Chan W, Loehr LR, Wruck LM, et al. Pre-morbid body mass index and mortality after incident heart failure. The ARIC study. J Am Coll Cardiol. 2014;64:2743–9.
- Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419.
- Perseghin G, Ntali G, De Cobelli F, Lattuada G, Esposito A, Belloni E, et al. Abnormal left ventricular energy metabolism in obese men with preserved systolic and diastolic functions is associated with insulin resistance. Diabetes Care. 2007;30:1520–6.
- Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, et al. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One. 2013;8:e68893.
- Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–67.
- Creopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25:643–59.
- Lavie CJ, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the “obesity paradox”. Mayo Clin Proc. 2010;85:605e8.
- Arena R, Lavie CJ. The obesity paradox and outcome in heart failure: is excess bodyweight truly protective? Future Cardiol. 2010;6:1–6.
- Dorner TE, Rieder A. Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol. 2012;155:56–65.
- Clerico A, Gionnoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev. 2012;17:81–96.
- McGaffin KR, Moravec CS, Mctiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circ Heart Fail. 2009;2:676–83.
- Kistorp C, Faber J, Galatus S, Gustafsson F, Frystyk J, Flyybjerg A, et al. Plasma adiponectin, body mass index and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62.
- Sethi JK. Activatin' human adipose progenitors in obesity. Diabetes. 2010;59:2354–7.
- Beaufrere B, Morio B. Fat and protein distribution with aging. Metabolic considerations. Eur J Clin Nutr. 2000;54:548–53.
- Horani MH, Mooradian AD. Management of obesity in the elderly: special considerations. Treat Endocrinol. 2002;1:387–98.
- Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8.
- Tucato E, Bosello O, Di Francesco V, Harris TB, Zoico E, Bissoli L, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord. 2000;24:1005–10.
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist–hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–5.
- De schutter A, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: untangling the obesity paradox. Mayo Clin Proc. 2014;89:1072–9.
- Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82.
- Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnsol CL, Heymsfield SB, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–609.
- Bozorgmanesh M, Hadaegh F, Khalili D, Azizi F. Prognostic significance of the complex “Visceral Adiposity Index” US simple anthropometric measures: Tehran lipid and glucose study. Cardiovasc Diabetol. 2012;11:20.
- Baumgartner RM, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63.
- Janssen I. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
- World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization, 2000. Technical report 894.
- Sharma A, Lavie CJ, Borer JS, Vallakali A, Goel S, Lopez-Jinemez F, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;15:1428–34.
- Lavie CJ, Sharma A, Alpert Ma De Schutter A, Lopez-Jimenez F, Milani RV Ventura HO. Update on obesity and obesity paradox in heart Failure. Prog Cardiovasc Dis. 2015;58:393–400.
- Kamiya K, Masuda T, Matsue Y, Inomata T, Hamazaki N, Matsuzawa R, et al. Complementary role of arm circumference to body mass index in risk stratification in heart failure. JACC Heart Fail. 2016;4:265–73.
- Batsis JA, Mackenzie TA, Barre LK, Lopez J, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–7.
- Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70.
- Stephen WC, Janssen I. Sarcopenic obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. 2009;13:460–6.
- Wannamethee SG, Shaper AG, Whineup PH, Lennon L, Papacosta O, Sattar N. The obesity paradox in men with coronary heart disease and heart failure: the role of muscle mass and leptin. Int J Cardiol. 2014;171:49–55.
- Anker MS, von Haehling S, Springer J, Banach M, Anker SD. Highlights of mechanistic and therapeutic cachexia and sarcopenia research 2010 to 2012 and their relevance for cardiology. Arch Med Sci. 2013;9:166–71.
- Bluher M. The distinction of metabolically healthy from unhealthy obese individuals. Curr Opin Lipidol. 2010;21:38–43.
- Ortega FB, Lee D, Katzmarezyk PT, Ruiz JR, Sui X, Church TS, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–97.
- Karelis AD. To be obese – does it matter if you are metabolically healthy? Nat Rev Endocrinol. 2011;7:699–700.
- Waldman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–24.
- Voulgari C, Tentolouris N, Dilaveris P, Tousolis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–50.
- Stefan N, Harring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62.
- Arsenault BJ, Beaumont EP, Despres JP, Larose E. Mapping body fat distribution: a key step towards the identification of the vulnerable patient? Ann Med. 2012;44:758–72.
- Dobson R, Burgess MI, Sprung VS, Irwin A, Hamer M, Jones I, et al. Metabolically healthy and unhealthy obesity. Differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes. 2010;40:153–61.
- Shaharyar S, Roberson LL, Jamal O, Younus A, Blaha MJ, Ali SS, et al. obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are sensitivity associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy Brazilian population. J Obes. 2015;2015:178526.
- Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (NORD-Trondelag Health Study) Norway. J Am Coll Cardiol. 2014;63:1071–8.
- Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far A, Ayers C, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–34.
- Cornwell WNI, Pandey A, Wilis B, leonard D, Gao A, Defina L, et al. Combined association of midlife obesity and fitness with long-term risk of heart failure – the cooper center longitudinal study. Circulation. 2013;128:A 15669.
- Pandey A, Patel M, Gao A, Willis BL, Das LR, Leonard D, et al. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and non-cardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J. 2015;169:290–7.
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56:382–90.
- McAuley PA, Bevers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56:434–40.
- Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011;124:2483–90.
- Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–8.
- Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. Am J Cardiol. 2015;115:209–13.
- Shetty PM, Hauptman PJ, Landfried LK, Patel K, Weiss EP. Micronutrient deficiencies in patients with heart failure: relationships with body mass index and age. J Card Fail. 2015;21:968–72.
- Casas-Vara A, Santolaria F, Fernandez-Bereciartua A, Gonzalez-Reimers E, Garcia-Ochoa A, Martinez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition. 2012;28:616–22.
- Adejumo OL, Koeling TM, Hummel SL. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant. 2015;34:1385–9.
- Orepoulos A, Padwal R, Kalantar-Zadeh K, Fonarow CC, Norris CM, Mc Alister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22.