Abstract
Little is known about changes in carotid plaque morphology during aging and the possible impact on cardiovascular events. Only few studies addressed so far age-related modifications within atherosclerotic lesions. Therefore, in this work we endeavored to summarize the current knowledge about changing of plaque composition in elderly. The data from hitherto existing studies confirm that atherosclerotic plaques undergo distinct alternations with advanced age. However, the results are often ambiguous and the changes do not seem to be as disastrous as expected. Interestingly, none of the studies could definitely evidence increased plaque vulnerability with advanced age. Nevertheless, based on the previous work showing decrease in elastin fibers, fibroatheroma, SMCs, overall cellularity and increase in the area of lipid core, hemorrhage, and calcification, the plaque morphology appears to transform toward unstable plaques. Otherwise, even if inflammatory cells often accumulate in plaques of younger patients, their amount is reduced in the older age and so far no clear association has been observed between thin fibrous cap and aging. Thus, the accurate contribution of age-related changes in plaque morphology to cardiovascular events has yet to be elucidated.
Composition of carotid atherosclerotic lesions changes during aging. These alternations are however, just moderate and depend upon additional variables, such as life style, accompanying disease, genetics, and other factors that have yet to be determined.
Based on the current data, the age-associated plaque morphology seems to transform toward vulnerable plaques. However, the changes do not seem to be as disastrous as expected.
KEY MESSAGES
Introduction
Aging is associated with plethora of changes within human organism. These mainly irreversible processes reduce its capacity for adaption to the environment and thus increase its susceptibility to various pathological germs. In general, growing older is the greatest known risk factor for most human diseases (Citation1). Especially cardiovascular disorders are strikingly related to aging (Citation2). Although age itself is not a modifiable risk factor of cardiovascular disease, it is a reliable predictor of incoming clinical events. Aging is also associated with increase in vascular stiffness of peripheral and aortic arteries (Citation3). These changes lead inevitably to an elevated brachial and central systolic blood and pulse pressure. Hence, cardiovascular risk factors tend to cluster with hypertension (Citation4,Citation5). Furthermore, high blood pressure accelerates the pathophysiologic aging of the arteries. Thus, a vicious circle evolves that finally ends in a cardiovascular event and is frequently associated with the death of the affected person. Hypertension is also the main risk factor leading to stroke (Citation6). In an overview article, Robert L. (Citation6) considered in his work that, if no other causes of mortality, such as infection or tumour arise, the progressive changes in the cardiovascular system lead finally to death and limit the human life span to 120 years. Studies of centenarians confirmed this assumption evidencing that vascular diseases are direct or indirect reason for the mortality of these individuals. Nobody is just dying of an “old age.” The pathophysiological changes within the arterial wall in elderly are the key factors of increased arterial stiffness, its inability to regulate blood and pulse pressure, leading finally to cardiovascular events.
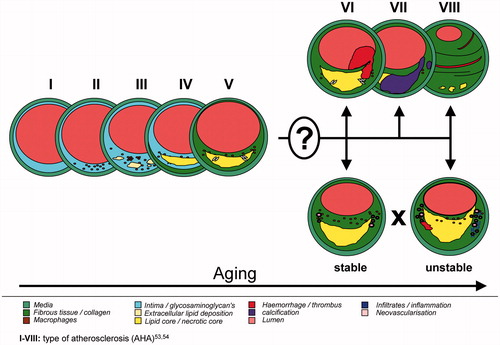
Stroke represents the second leading cause of death and is the major cause of disability around the world. Furthermore, age is the most important factor for stroke-related disease, such as brain ischemia, ischemic, or hemorrhagic stroke (Citation7). These clinical events can be partially caused also by vulnerable atherosclerotic carotid lesions (Citation8,Citation9). Extracranial carotid artery stenosis of more than 50% was detected in up to 2% of elderly men and up to 1.6% of elderly women (Citation10) (mean age 66 years) and increasing for up to 2.2% by stenosis over 60% (Citation11) (mean age 68 years). Vulnerable atherosclerotic carotid lesions, plaque rupture, and the embolized thrombotic material are thereby responsible for cerebral ischemia (Citation8,Citation9). Up to 14.3% of all ischemic strokes might be due to the carotid artery atherosclerosis (Citation12). Incidence rates of acute vascular events increase with age in all arterial territories, with 80% cerebrovascular, 73% coronary, and 8% peripheral in individuals older than 65 years (Citation13). A possible explanation might be an increase in carotid plaque instability in elderly. Redgrave and Rothwell observed different plaque morphology in younger patients with greater inflammatory cell infiltration compared to older individuals, who had in contrast larger lipid core. However, no significant differences were observed in overall plaque instability between young and old, suggesting that increased risk of stroke in the elderly patients with symptomatic carotid stenosis is due to other factors (Citation14). Nevertheless, little is still known about the exact changes in carotid plaque morphology that can be unambiguous associated with age ().
The stroke mortality has rapidly decreased over the last decades (Citation15). Currently, it is suggested that the reduction of stroke incidence is because of better and faster recognition, improved treatment options, newer medications, and secondary prevention in addition to changes in people lifestyles. Furthermore, the recent downward trends in stroke death and disability rates are indications that stroke does not need to be an inevitable consequence of aging (Citation16). Data from longitudinal studies have shown that the most powerful lifestyle modifications to lower the risk of stroke are reduction of blood pressure, cessation of smoking, regular physical activity, and maintenance of a healthy diet (Citation17). On the other hand, stroke incidence forecasts (Citation15) reveal that the number of stroke will notwithstanding increase in the future, with 34% of the stroke events of the age above 85 and 66% over 75 years. Several other reports indicate that more than 80% of ischemic stroke occurs in individuals aged over 65 years (Citation18). Of these cases, nearly 25% will occur in patients >85 years old. Several age-related changes in the brain have been identified to be associated with the increase of ischemic stroke in elderly (Citation19). Furthermore, the mechanism of ischemic injury seems to be different between young and old patients. Sex-related differences were observed in the elderly as well. The incidence of ischemic stroke in patients <80 years was higher in men than in women. In contrast, in very old patients (>80 years), women seem to be at a higher risk of stroke (Citation19). In addition, hypertension appears to be the main risk factor for all stroke subtypes in patients <80 years but is e.g., less important for individuals >80 years. Hyperlipidemia is another risk factor for stroke in the elderly but not in the very old (Citation19). Finally, the metabolic syndrome is a strong independent risk factor for atherothrombotic stroke especially in very old individuals. In line with the changes in plaque morphology with age, van Lammeren et al. analyzed histopathologic features of carotid atherosclerotic plaques of 1583 patients (Citation20). Large atheroma, plaque thrombosis, macrophages, and calcifications were less frequently observed per 2-year increase in time. These changes were found in patients with stroke, transient ischemic attack (TIA), ocular symptoms, and asymptomatic patients. The authors conclude that over the past decade, a time-dependent change in plaque composition occurs, characterized by a decrease in plaque instability. These circumstances appear to be associated with the improvements in risk factor management to prevent stroke.
In the treatment of carotid artery stenosis and in addition to best medical therapy (BMT), carotid endarterectomy (CEA) is a good established surgical procedure. Over decades, several large well-structured trials have been conducted in order to identify the patient groups that benefit from surgery (Citation8,Citation21). However, new options for intervention, improvements in medications and a changing lifestyle force to reconsider the current approaches. The most important guidelines used in the treatment of carotid artery stenosis are based on the results of The North American Symptomatic Carotid Endarterectomy Trial (NASCET) (Citation22) and the European Carotid Surgery Trial (ECST) (Citation23). Rothwell et al. demonstrated in a post-hoc analysis of the ECST and NASCET data the importance of timing of the surgical intervention (Citation24). The number needed to treat to prevent one neurological event (NNT) was five for that randomized within 2 weeks after their ischemic event and 125 for that randomized first after more than 12 weeks. Accordingly, the recommendations are that symptomatic carotid stenosis should be treated within 14 d of the index event. Nevertheless, the ideal timing is still subject of controversies. According to a recent systematic review, the stroke risk is as high as 6.4% within the first 2–3 d, 19.5% within 7 d, and 26.1% within 14 d after an incidence of ischemic stroke (Citation25). Furthermore, a Swedish register study showed that patients treated within 2 d following the index event had a significantly increased perioperative mortality and stroke risk (Citation26). Consequently, the optimal time interval following the incidence of stroke is still not clear. With regard to the intraoperative management of CEA, little has changed so far. The focus was set on the local anesthetic and improved neuromonitoring to prevent unnecessary shunting and avoid hyperfusion (Citation21). Regarding the operative techniques in common practice, eversion endarterectomy appears to have the lowest stroke risk (Citation27). In summary, CEA, timely performed within 14 d after NNT and good monitoring seem to be an essential part of stroke prevention strategies and significantly contribute to the reduction of stroke incidence in elderly population.
Carotid artery stenting (CAS) is a promising alternative to the established CEA described above. However, uncertainty exists about which of the technique, CEA or CAS, is more suitable, especially in elderly patients. Antoniou et al. (Citation28) performed shortly a detailed meta-analysis of all this studies comparing CEA and CAS including over 500,000 individuals. The authors compared these both interventions between young and old with 80 years as a cut-off for the study groups. Using CEA, no significant differences were observed for incidence of stroke, TIA or both between the old and young. In contrast, CAS caused in elderly population significant more stroke or TIA rates. Interestingly, following CEA, overall mortality was significantly increased in older patients, not however, applying CAS. It is however, to mention, that the mortality rate was quite low, 0.4% vs. 0.5% for CEA, 0.6% vs. 0.7% for CAS (young vs. old). Thus, CEA is associated with better neurologic outcome compared to CAS. The reasons for these discrepancies seem to be the unfavorable anatomy in elderly patients, such as calcified supra-aortic branches and aortic arch, elongation, distortion, or stenosis (Citation28,Citation29). The wire manipulation and navigation through the impaired aortic arch in elderly may result in increased risk of endothelial trauma or thromboembolic events. Furthermore, aged patients are likely to have compromised cerebrovascular reserve, being less tolerant for cerebral embolization (Citation28,Citation30). Interestingly, log odds ratio for stroke and mortality after CAS significantly decreases in new studies compared to older ones, which is not the case for CEA. This finding reflects the improvement of stenting technique and devises in recent years (Citation28). Another study from Bazan et al. (Citation30) and Giannopoulos et al. (Citation31), who focused on the large randomized studies (ACST, ACAS, NASCET, ECST, CREST) concluded that CAS has a significantly higher incidence of stroke, MI or death in patients >75 years. Surprisingly, CAS was most beneficial in the youngest patients (<65 years). Finally, Howard et al. (Citation32) performed recently a meta-analysis from four randomized controlled trials within the Carotid Stenosis Trialists’ Collaboration (CSTC) for symptomatic patients focusing on the outcome of CAS and CEA in elderly patients. Also in this work, CEA was superior to CAS in patients older than 70 years, especially regarding NNTs.
Consequently, age should be considered when planning carotid intervention, taken into account that CEA is not associated with deteriorated neurological outcome in elderly, however, with increased overall mortality. CAS in contrast has an increased risk of cerebrovascular events in older patients but mortality does not change.
In this review, we summarize the current knowledge of the changes in carotid plaque morphology during aging. First, we sought to address different non-invasive approaches that can be principally used to monitor plaque morphology and age-related alternations in atherosclerotic lesions, considering especially carotid intima-media thickness (CIMT) and high-resolution magnetic resonance imaging (MRI). Second, we recapitulate what has been actually done on this issue using these techniques, especially with focus on changes in plaque composition during aging. Third, we particularly address studies with histological characterization of lesion morphology and their changes in elderly focusing on the individual components within the atherosclerotic plaques.
CIMT, plaque morphology, and aging
The most widely used predictor of cardiovascular events during aging is the measurement of CIMT by B-mode ultrasound (Citation34). A number of studies have already shown clear association between CIMT and dyslipidemia or high blood pressure (Citation33–37). Furthermore, de Freitas et al. showed that aging is an independent factor positively related to CIMT (Citation38). The accuracy of CIMT is however, impaired by the fact that intima-media thickness changes with age independent of the absence of atherosclerosis (Citation33). Therefore, measurement of CIMT serves more as an indicator for overall risk of cardiovascular disease rather than an accurate measure of plaque progression or vulnerability (Citation39). Pathological thickening of the intima is generally the first manifestation of atherosclerosis, accompanying by lipid deposition, loss of smooth muscle cells (SMCs), and macrophage infiltration (Citation40).
The CIMT as a predictor of cardiovascular events by ultrasound has several drawbacks. First, the B-mode ultrasound is performed mostly by measuring common carotid artery rather than bifurcation or the internal carotid artery, which does not necessarily need to correspond with the atherosclerotic plaque formation in the internal carotid vessel (Citation39). Second, there is no evidence that CIMT is truly associated with increased vascular risk. Den Ruijter et al. analyzed data from 45,828 individuals (Framingham study) and found that the addition of CIMT measurement to the Framingham Risk Score can only marginally improved the 10-year risk prediction of myocardial infarction or stroke and is therefore unlikely to be of clinical importance (Citation41). Third, CIMT is unable to recognize lesions with necrotic core or thin cap fibroatheroma, which has already been described as important criteria of carotid plaque vulnerability (Citation39,Citation40). It is therefore important to evaluate other features of atherosclerotic lesions rather than CIMT, such as e.g., plaque echolucency or surface irregularity, which has already been associated with a higher risk of ischemic stroke (Citation42,Citation43). In addition, 3-dimensional (3D) ultrasound allows more accurate quantification of carotid plaque morphology compared to B-mode ultrasound (Citation42). The determination of 3D plaque volume was already shown to have high negative predictive value, excluding patients with low risk by clinical screening (Citation44). Recent studies have also shown that 3D ultrasound is able to characterize plaque morphology. A specific scoring system was developed that incorporates degree of stenosis, irregularity of plaque surface, echolucency, and plaque texture (Citation45). Another ultrasound-based score including clinical features was developed by Nicolaides et al. (Citation46). However, first the reproducibility of the individual plaque features ascertained by 3D ultrasound needs to be clearly defined in large multicentric studies and validated by true plaque morphology from pathological tissue samples prior to clinical use.
In summary, ultrasound is a helpful tool to evaluate plaque stenosis and some of its characteristics. At the moment, however, the current methodologies are not sensitive enough to recognize vulnerable plaques or to significantly improve risk stratification of stroke patients. So far, with the exception of CIMT being an independent factor associated with age, no other data regarding changes in atherosclerotic plaque morphology in elderly assessed by ultrasound are available.
MRI, plaque morphology, and aging
Despite ongoing advances in imaging techniques and understanding the pathogenesis of atherosclerotic lesion, adequate imaging tools for early detection of patients at increased risk of cardiovascular disease, especially stroke, are still missing. Several non-invasive methods are available to monitor atherosclerotic plaques, such as computed tomography angiography (CTA), high-resolution MRI, multidetector CT angiography (MDCTA), or positron emission tomography using fluorodeoxyglucose (PET-FDG) (Citation47,Citation48,Citation50–57).
CTA can achieve an excellent spatial resolution and detect degree of stenosis, luminal narrowing, or some of the plaque components. This technique is also suitable for longitudinal follow-up studies. The disadvantages of CTA in comparison to ultrasound are the high cost and in contrast to other imaging techniques the fact that the patients are submitted to radiation. Brenner and Hall reported, e.g., that 0.4% of current cancers in the USA were due to CT scans and that this incidence may increase in the future for up to 2% (Citation49). Another modality is a MDCTA. Homburg et al. was able to detect different plaque components in symptomatic carotid lesions, such as lipid core, fibrosis, calcification, and intraplaque hemorrhage (Citation50). Furthermore, various plaque features, such as, e.g., plaque volume, degree of stenosis, and large lipid core were associated with lesion ulceration. Thus, MDCTA might reliably identify rupture prone plaques and improve risk stratification in patients with ischemic stroke. However, no data about using this imaging modality to analyze plaque morphology as a function of aging are available. PET-FDG, which is also often used to image vessels, possesses only low spatial resolution of 4–10 mm, which is not sufficient to recognize reliable components of atherosclerotic plaques (Citation48).
The most promising non-invasive technique seems to be at the moment high-resolution MRI. MRI allows reliable characterization of various features of atherosclerotic lesions (Citation51–57). Den Hartog et al. provide a nice overview of the literature about non-invasive carotid plaque characterization using MRI (Citation53). The other advantage of MRI is the possibility to monitor atherosclerotic lesions over time. However, no standardized protocols are so far established. Using combined analysis of various tissue signals, relaxation times, and contrast weightings (TOF, T1, T2, PDW), plaque size, plaque thickness, fibrous cap, calcification, intraplaque hemorrhage, or thrombus, and lipid-rich necrotic cores can be reliably identified with very good sensitivity and specificity (Citation47,Citation52–56). Cai et al. demonstrated in this content that high-resolution multi-contrast MRI is able to classify different atherosclerotic lesions in the human carotid artery in the same way as recommended by American Heart Association (Citation57–59).
Albeit several hundred publications have already been dedicated to determine the morphology of atherosclerotic lesions in carotid or coronary arteries by MRI, very view studies focused so far on the changes of plaque morphology with age. Cheung et al. published in 2011 that complicated carotid atheroma among symptomatic patients is associated with increasing age and male sex (Citation60). Complicated lesions were considered those with intraplaque hemorrhage. Bouwhuijsen et al. confirmed in 2012 that intraplaque hemorrhage occurs more frequently at older age (Citation61). Another feature of atherosclerotic plaques changing with aging using MRI was found to be carotid wall thickening in elderly subjects suffering from chronic obstructive pulmonary disease (Citation62). These results were in line with the findings measuring CIMT by ultrasound (Citation38). In another study, MRI demonstrated distensibility of the carotid artery due to aging (Citation63). Finally, Zhao et al. performed an extended characterization of carotid arteries by MRI to assess plaque burden and tissue contents in accordance with the MRI-modified American Heart Association lesion types (Citation64). The authors observed that the type VI lesions were significantly associated with older age. Atherosclerotic lesion type VI is characterized by thrombotic deposits, intraplaque hemorrhage and markedly contribute to plaque vulnerability.
In summary, MRI is a promising non-invasive technique to determine the individual components of atherosclerotic lesions. With regard to the changes of plaque morphology with age, longitudinal prospective studies, which would follow the development of atherosclerotic plaques over time, and thus allow better understanding of the critical changes within atherosclerotic plaques that lead to rupture and consequent cardiovascular events, are however still missing.
Histological studies of plaque morphology and aging
Surprisingly, only couple of researchers performed direct histological studies on carotid atherosclerotic tissues samples considering plaque morphology as a function of age (Citation14,Citation65–69). In addition, the authors have reported inconsistent results (). Particularly the patient population was not uniform and the distribution of the age-related groups was different. The first histological characterization of carotid atherosclerotic plaques related to age was described by Spagnoli et al. (Citation65). The authors analyzed 180 individuals with neurological symptoms and observed that the presence of particular plaque components are non-random but significantly correlate with specific risk factors. For example, fibrous plaque was significantly associated with aging and diabetes, the granulomatous plaques, rich in giant multinuclear and mononuclear cells were correlated with female sex and hypertension, the xanthomatous plaques, rich in foam cells, and proteoglycans, correlated with hypercholesterolemia. Discriminant analysis of age-related groups (<50, 50–70, >70) showed that with increased age the plaques tended to have a greater extension and larger amount of connective tissue associated with neovascularization.
Table 1. Summary of the most important features from studies addressing plaque morphology and aging.
Another study from Grufman et al. (Citation66) analyzed 200 plaques from patients undergoing CEA comprising both symptomatic (n = 105) and asymptomatic (n = 95) individuals. The study participants were divided into two age-related groups with cut-off by 70 years (median). Carotid plaques from elderly patients contained lower amount of elastic fibers. Interestingly, inflammatory markers interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), soluble CD40 ligand (sCD40L), and fractalkine were significantly decreased in atherosclerotic lesions of these older individuals. In addition, the content of macrophages and lipids was significant higher in plaques from symptomatic patients in the elderly group, but not in younger patients. The authors concluded that increased plaque vulnerability in the symptomatic elderly patients was associated with the accumulation of lipid components and impaired tissue repair, rather than with inflammatory processes in the diseases carotid arterial wall.
In particular, two groups have been addressing the subject of changes in plaque morphology with age, sex, or neurological symptoms, namely of Pasterkamp and Rothwell. Recently, they joined their large atherosclerotic biobanks and analyzed the composition of 1640 carotid plaques from symptomatic patients focusing on prediction of individual risk of stroke (Citation70). They evaluated, whether specific changes of various carotid plaque components might predict the risk of future ipsilateral ischemic stroke. The predicted 1- and 5-year stroke risk was related particularly to plaque thrombus, decrease of fibrous content, increased number of macrophages, and high microvessel density. Interestingly, no association was found for cap thickness, extent of calcification, intraplaque hemorrhage, or lymphocyte infiltration. However, the risk for ischemic ipsilateral stroke was calculated using a carotid stenosis risk prediction model (www.stroke.ox.ac.uk/model/form1.html), which does not necessarily correlate with the true clinical outcome for each individual patient. Furthermore, some of their results were discrepant to other former published data, which demonstrated, e.g., that intraplaque hemorrhage was significantly associated with the degree of stenosis and cerebrovascular events (Citation71). The authors suggest that as soon as important risk factors for ipsilateral stroke are taken into account, intraplaque hemorrhage is probably less predicting the potential risk of stroke. Interestingly, the authors found stronger association between plaque characteristics and stroke risk, if the plaques originated from patients, removed between 14 and 30 d after cerebrovascular symptoms. Consequently and in line with the current guidelines, patients with symptomatic carotid stenosis should undergo surgical intervention within 14 d following the cerebrovascular event (Citation72).
Another study from Oostrom and Pasterkamp analyzed age-related changes in carotid plaque composition from 383 patients undergoing CEA including both symptomatic and asymptomatic patients (Citation69). The selection of different age groups was performed by categorical analysis using quartiles with following cut off values: 39–61, 62–67, 68–72, and 73–89 years. The authors detected more fibrous and less atheromatous plaques within the youngest age quartile. No further changes were observed between the other quartiles. Interestingly, no significant differences were found for collagen. In contrast, continuous decrease in SMCs was detected until the third quartile (<73 years). Quantitative analysis of CD68 showed significant reduction of macrophages in the youngest quartile and highest number of these inflammatory cells in the second quartile. In summary, the authors conclude that carotid atherosclerotic plaque morphology does change with age, including increase in the extent of atheroma, less SMCs and higher amount of macrophages in the middle-aged patients. These age-related changes of plaque composition appear predominantly in the youngest without any further deterioration in the older age.
In a similar way as described above, Redgrave and Rothwell evaluated carotid plaques from 526 patients intended for CEA by means of histology (Citation14). In contrast to the previous studies (Citation65,Citation69), only symptomatic patients were included and adjustment for sex, smoking, and hypertension was performed. Using Chi-squared test, significant differences between the age groups (<55, 55–64, 65–74, ≥75 years) were observed for large lipid core, fibroatheroma, calcification, and inflammatory infiltration. However, it is to note that the increase was observed only within the first three groups for up to 74 years. The oldest patients in the fourth group showed in contrast a significant decrease in the quantity of all the mentioned plaque components. Subsequent analysis revealed no association between age and fibrous cap, representing normally the plaque instability. At the other side, lymphocyte infiltration was associated with age (again up to 74 years), not however, the content of macrophages. Aging was also related to the increase in plaque calcification. In conclusion, the authors considered that even if the atherosclerotic lesions change during aging, they do not become significantly more unstable, suggesting that plaque vulnerability does not markedly contribute to the stroke in elderly.
In 2011, van Lammeren and Pasterkamp performed a study on carotid atherosclerotic plaques harvested from 1385 consecutive patients following carotid artery endarterectomy, evaluating the hypothesis of plaque vulnerability as a possible explanation for the increased risk of stroke in elderly (Citation67). The patients were divided into four age groups: <60, 60–69, 70–79, and ≥80 years. As already discovered in the former studies, no significant changes between the groups were observed for macrophages. In line with the previous studies, especially the decrease in SMCs, increased extent of lipid core, and calcification seem to be relevant features of plaque morphology being on the rise during aging. In contrast to Redgrave and Rothwell (Citation14), the authors concluded that plaque stability decreases with age, mainly due to the lower content of SMCs and enlargement of the lipid core. These results were confirmed by other work of van Lammeren (Citation73), where the authors compared the plaque composition between truly asymptomatic patients and patients with index events more than six months prior to surgical intervention. The second group showed significant less SMCs and increased intraplaque hemorrhage, features that correspond with increased lesion vulnerability. Interestingly, the plaques of truly asymptomatic patients were significantly more calcified, which would suppose that under certain conditions calcium deposits might contribute to plaque stabilization.
Our own data on 763 atherosclerotic plaques from CEA patients (Citation68) confirmed more or less the previous results of Rothwell and Pasterkamp. We have included both symptomatic and asymptomatic individuals. In contrast to the previous studies, we used a complex multinomial statistical regression model, adjusted for common risk factors of atherosclerotic burden, such as sex, neurological symptoms, arterial hypertension, diabetes mellitus, hyperlipidemia, smoking, history of coronary heart and peripheral artery disease, as well as medication. Furthermore, we tried to associate the clinically relevant atherosclerotic plaque types according to AHA with age, neurological symptoms, and sex. With regard to carotid lesion morphology, type VII vs. type V, and complex plaques (VI/VII) vs. type V were significantly associated with age. Especially the complex lesion can be considered as vulnerable. Consequently, more complicated and unstable plaques seem to correlate with aging. Furthermore, higher age was significantly related to increased calcification. The other features of atherosclerotic plaques, such as cellularity, collagen, elastin, inflammatory infiltration, and neovascularisation did not show any statistically significant differences, even if there were some promising tendencies. Particularly inflammation and overall cellularity appear to be decreased in elderly, which was in concordance with some of the previous studies (Citation14,Citation67,Citation69).
Summary
Currently, little is known about the real changes of carotid plaque morphology in elderly and its possible impact on cardiovascular events, especially the risk of stroke. The most widely described atherosclerotic feature during aging is the pathological thickening of the intima compared to media, called CIMT. However, no clear consensus exists on which CIMT is evidently associated with cardiovascular risk. Another technique, the 3D ultrasound would allow for more accurate quantification of plaque morphology. However, data using such an approach, addressing plaque composition and aging, are still missing. The high-resolution MRI is a powerful and promising non-invasive technique that has sufficient spatial resolution to distinguish between various components of atherosclerotic lesions. Interestingly, very view studies focused on the changes of plaque morphology with age thus far. Up to now, MRI technique associated intraplaque hemorrhage and calcification unambiguously with age. Plethora of studies has also been addressed to evaluate individual components of atherosclerotic lesions in coronary and carotid arteries by means of histology and immunohistochemistry. Nevertheless, very few associated the ascertained plaque features with aging (Citation14,Citation65,Citation66–69). The hitherto existing results confirm that lesion morphology markedly changes with age; the data are however, ambiguous. First, the aged groups of the mentioned studies are very different. While Spagnoli distinguished between <50, 50–70, and >70 years (Citation65), Oostrom used quartiles of 39–61, 62–67, 68–72, and 73–89 years (Citation69), Redgrave built another four aged groups with <55, 55–64, 65–74, and <75 years (Citation14), and van Lammeren (Citation67) decided for decades (<60, 60–69, 70–79, ≥80). Furthermore, Grufman (Citation66) compared only two groups with a cut-off by 70 years and our own group used a multimodal regression model with a 10-year gradient (Citation68). Second, depending upon the methodology and histological examinations of the individual studies, different features of atherosclerotic lesions have been evaluated. Third, some researchers analyzed plaques from both symptomatic and asymptomatic carotid patients, others focused only on individuals with neurological symptoms. Thus, a clear consensus is difficult to reach. Fourth, due to the quite large number of tissue samples (between 180 and 1385), semi-quantitative score-based analyses were frequently performed to evaluate the individual components within the plaques. Hence, it is difficult to compare these studies among themselves. Surprisingly, however, one conclusion can be drawn, which was common in all studies, namely that the age-associated changes in the plaque morphology were lesser than expected. None of these studies could definitely evidence that atherosclerotic plaques are growing more unstable during aging. Even if, e.g., van Lammeren and van Oostrom observed significant reduction of SMCs and increase in the extent of lipid/necrotic core in elderly (Citation14,Citation65,Citation67,Citation69), inflammation increased only partially and dropped even down above 75 years. Interestingly, not all studies observed significant decrease in the content of collagen. In addition, the results from Redgrave et al. could not associate age with thin fibrous cap (Citation14,Citation70), an established marker of lesion vulnerability. Finally, all the age-related alternations in plaque morphology were more or less only “moderate.”
Conclusion
Inferential, our present review article highlights the current state of art regarding changes in atherosclerotic plaque morphology during aging. Following conclusions can be made so far: (i) Carotid atherosclerotic lesions do change during aging. However, these changes are just moderate and depend probably upon various additional variables, such as patient life style, accompanying disease, genetics, and other factors that have yet to be determined. (ii) Based on the current data, following changes in plaque composition can be observed in elderly: decrease in elastin fibers, SMCs, and overall cellularity and increase in the extent of lipid core, hemorrhage, and calcification. Consequently, the age-associated plaque morphology seems to change toward more vulnerable plaques. Large prospective and longitudinal studies using, e.g., non-invasive MRI technique or 3D ultrasound are however, necessary to allow for more accurate argumentation about the impact of the individual components of atherosclerotic lesions on cardiovascular events during aging.
Disclosure statement
The authors declare no conflicts of interest.
References
- Dillin A, Gottschling DE, Nystrom T. The good and the bad of being connected: the integrons of aging. Curr Opin Cell Biol. 2014;26:107–12.
- Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–9.
- Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–6.
- Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S.
- Nilsson PM, Khalili P, Franklin SS. Blood pressure and pulse wave velocity as metrics for evaluating pathologic ageing of the cardiovascular system. Blood Press. 2014;23:17–30.
- Robert L. Aging of the vascular-wall and atherosclerosis. Exp Gerontol. 1999;34:491–501.
- Johansson BB. Hypertension mechanisms causing stroke. Clin Exp Pharmacol Physiol. 1999;26:563–5.
- Eckstein HH. Evidence-based management of carotid stenosis: recommendations from international guidelines. J Cardiovasc Surg (Torino). 2012;53:3–13.
- Pelisek J, Eckstein HH, Zernecke A. Pathophysiological mechanisms of carotid plaque vulnerability: impact on ischemic stroke. Arch Immunol Ther Exp (Warsz). 2012;60:431–42.
- Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–50.
- Ratchford EV, Jin Z, Di Tullio MR, Salameh MJ, Homma S, Gan R, et al. Carotid bruit for detection of hemodynamically significant carotid stenosis: the Northern Manhattan Study. Neurol Res. 2009;31:748–52.
- Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–73.
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1773–83.
- Redgrave JN, Lovett JK, Rothwell PM. Histological features of symptomatic carotid plaques in relation to age and smoking: the oxford plaque study. Stroke. 2010;41:2288–94.
- Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20.
- Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004;363:1925–33.
- Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010;58:S325–S8.
- Orzuza G, Zurrú MC. Epidemiological aspects of stroke in very old patients. Cardiovasc Hematol Disord Drug Targets. 2011;11:2–5.
- Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM. Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol. 2010;6:256–65.
- van Lammeren GW, den Ruijter HM, Vrijenhoek JE, van der Laan SW, Velema E, de Vries JP, et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation. 2014;129:2269–76.
- Ritter JC1, Tyrrell MR. Carotid endarterectomy: where do we stand at present? Curr Opin Cardiol. 2013;28:619–24.
- Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–8.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379–87.
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR. Carotid Endarterectomy Trialists' Collaboration, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–16.
- Tsantilas P, Kuehnl A, Kallmayer MA, Knappich C, Schmid S, Kuetchou A, et al. Stroke risk in the early period after carotid related symptoms: a systematic review. J Cardiovasc Surg (Torino). 2015;56:845–52.
- Stromberg S, Gelin J, Osterberg T, Bergstrom GM, Karlstrom L, Osterberg K. Very urgent carotid endarterectomy confers increased procedural risk. Stroke. 2012;43:1331–5.
- Demirel S, Attigah N, Bruijnen H, Ringleb P, Eckstein HH, Fraedrich G, et al. Multicenter experience on eversion versus conventional carotid endarterectomy in symptomatic carotid artery stenosis: observations from the Stent-Protected Angioplasty Versus Carotid Endarterectomy (SPACE-1) trial. Stroke. 2012;43:1865–71.
- Antoniou GA, Georgiadis GS, Georgakarakos EI, Antoniou SA, Bessias N, Smyth JV, et al. Meta-analysis and meta-regression analysis of outcomes of carotid endarterectomy and stenting in the elderly. JAMA Surg. 2013;148:1140–52.
- Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007;45:875–80.
- Bazan HA, Smith TA, Donovan MJ, Sternbergh WC. 3rd. Future management of carotid stenosis: role of urgent carotid interventions in the acutely symptomatic carotid patient and best medical therapy for asymptomatic carotid disease. Ochsner J. 2014;14:608–15.
- Giannopoulos S, Katsanos AH, Vasdekis SN, Boviatsis E, Voumvourakis KI, Tsivgoulis G. Age and gender disparities in the risk of carotid revascularization procedures. Neurol Sci. 2013;34:1711–17.
- Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G. Carotid Stenting Trialists' Collaboration, et al. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: a meta-analysis of pooled patient data from four randomised trials. Lancet. 2016;387:1305–11.
- Spence JD. Technology Insight: ultrasound measurement of carotid plaque–patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol. 2006;2:611–19.
- Cutler JA. High blood pressure and end-organ damage. J Hypertens Suppl. 1996;14:S3–S6.
- Fabris F, Zanocchi M, Bo M, Fonte G, Poli L, Bergoglio I, et al. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke. 1994;25:1133–40.
- LaRosa JC. Dyslipidemia and coronary artery disease in the elderly. Clin Geriatr Med. 1996;12:33–40.
- Milio G, Corrado E, Sorrentino D, Muratori I, La Carrubba S, Mazzola G, et al. Asymptomatic carotid lesions and aging: role of hypertension and other traditional and emerging risk factors. Arch Med Res. 2006;37:342–7.
- de Freitas EV, Brandao AA, Pozzan R, Magalhies ME, Castier M, Brandao AP. Study of the intima-media thickening in carotid arteries of healthy elderly with high blood pressure and elderly with high blood pressure and dyslipidemia. Clin Interv Aging. 2008;3:525–34.
- Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–81.
- Kolodgie FD, Burke AP, Nakazawa G, Virmani R. Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscler Thromb Vasc Biol. 2007;27:986–9.
- Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308: 796–803.
- Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–38.
- Mathiesen EB, Bonaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromsø study. Circulation. 2001;103:2171–5.
- Johri AM, Chitty DW, Hua L, Marincheva G, Picard MH. Assessment of image quality in real time three-dimensional dobutamine stress echocardiography: an integrated 2D/3D approach. Echocardiography. 2015;32:496–507.
- Prati P, Tosetto A, Casaroli M, Bignamini A, Canciani L, Bornstein N, et al. Carotid plaque morphology improves stroke risk prediction: usefulness of a new ultrasonographic score. Cerebrovasc Dis. 2011;31:300–4.
- Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, et al. Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486–96.
- Corti R, Fuster V. Imaging of atherosclerosis: magnetic resonance imaging. Eur Heart J. 2011;32:1709–19b.
- Matter CM, Stuber M, Nahrendorf M. Imaging of the unstable plaque: how far have we got? Eur Heart J. 2009;30:2566–74.
- Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84.
- Homburg PJ, Rozie S, van Gils MJ, van den Bouwhuijsen QJ, Niessen WJ, Dippel DW, et al. Association between carotid artery plaque ulceration and plaque composition evaluated with multidetector CT angiography. Stroke. 2011;42:367–72.
- den Hartog AG, Bovens SM, Koning W, Hendrikse J, Luijten PR, Moll FL, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg. 2013;45:7–21.
- Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–25.
- Fayad ZA, Fallon JT, Shinnar M, Wehrli S, Dansky HM, Poon M, et al. Noninvasive In vivo high-resolution magnetic resonance imaging of atherosclerotic lesions in genetically engineered mice. Circulation. 1998;98:1541–7.
- Helft G, Worthley SG, Fuster V, Zaman AG, Schechter C, Osende JI, et al. Atherosclerotic aortic component quantification by noninvasive magnetic resonance imaging: an in vivo study in rabbits. J Am Coll Cardiol. 2001;37:1149–54.
- Toussaint JF, Southern JF, Fuster V, Kantor HL. T2-weighted contrast for NMR characterization of human atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1533–42.
- Yuan C, Beach KW, Smith LH Jr., Hatsukami TS. Measurement of atherosclerotic carotid plaque size in vivo using high resolution magnetic resonance imaging. Circulation. 1998;98:2666–71.
- Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–73.
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74.
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr., Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–78.
- Cheung HM, Moody AR, Singh N, Bitar R, Zhan J, Leung G. Late stage complicated atheroma in low-grade stenotic carotid disease: MR imaging depiction-prevalence and risk factors. Radiology. 2011;260:841–7.
- van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Eur Heart J. 2012;33:221–9.
- Lahousse L, van den Bouwhuijsen QJ, Loth DW, Joos GF, Hofman A, Witteman JC, et al. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam Study. Am J Respir Crit Care Med. 2013;187:58–64.
- Canton G, Hippe DS, Sun J, Underhill HR, Kerwin WS, Tang D, et al. Characterization of distensibility, plaque burden, and composition of the atherosclerotic carotid artery using magnetic resonance imaging. Med Phys. 2012;39:6247–53.
- Zhao XQ, Hatsukami TS, Hippe DS, Sun J, Balu N, Isquith DA, et al. Clinical factors associated with high-risk carotid plaque features as assessed by magnetic resonance imaging in patients with established vascular disease (from the AIM-HIGH Study). Am J Cardiol. 2014;114:1412–19.
- Spagnoli LG, Mauriello A, Palmieri G, Santeusanio G, Amante A, Taurino M. Relationships between risk factors and morphological patterns of human carotid atherosclerotic plaques. A multivariate discriminant analysis. Atherosclerosis. 1994;108:39–60.
- Grufman H, Schiopu A, Edsfeldt A, Bjorkbacka H, Nitulescu M, Nilsson M, et al. Evidence for altered inflammatory and repair responses in symptomatic carotid plaques from elderly patients. Atherosclerosis. 2014;237:177–82.
- van Lammeren GW, Reichmann BL, Moll FL, Bots ML, de Kleijn DP, de Vries JP, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke. 2011;42:2550–5.
- Wendorff C, Wendorff H, Pelisek J, Tsantilas P, Zimmermann A, Zernecke A, et al. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke. 2015;46:3213–19.
- van Oostrom O, Velema E, Schoneveld AH, de Vries JP, de Bruin P, Seldenrijk CA, et al. Age-related changes in plaque composition: a study in patients suffering from carotid artery stenosis. Cardiovasc Pathol. 2005;14:126–34.
- Howard DP, van Lammeren GW, Rothwell PM, Redgrave JN, Moll FL, de Vries JP, et al. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46:182–9.
- Turc G, Oppenheim C, Naggara O, Eker OF, Calvet D, Lacour JC, et al. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: the HIRISC study. Arterioscler Thromb Vasc Biol. 2012;32:492–9.
- Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke. 2015;46:3288–301.
- van Lammeren GW, den Hartog AG, Pasterkamp G, Vink A, de Vries JP, Moll FL, et al. Asymptomatic carotid artery stenosis: identification of subgroups with different underlying plaque characteristics. Eur J Vasc Endovasc Surg. 2012;43:632–6.