Abstract
Type 1 diabetes mellitus (T1D) is an autoimmune disease characterized by insufficient insulin production due to the destruction of insulin secreting β-cells in the Langerhans islets. A variety of factors, including chemicals, viruses, commensal bacteria and diet have been proposed to contribute to the risk of developing the disorder. In the last years, gut microbiota has been proposed as a main factor in T1D pathogenesis. Several alterations of gut microbiota composition were described both in animal model and in humans. The decrease of Firmicutes/Bacteroides ratio was the most frequent pattern described, in particular, in human studies. Furthermore, Bacteroides, Clostridium cluster XIVa, Lactobacillus, Bifidobacterium, and Prevotella relative abundances were different in healthy and affected subjects. Dysbiosis would seem to increase intestinal permeability and thus promote the development of a pro-inflammatory niche that stimulates β-cell autoimmunity in predisposed subjects. Preliminary studies on animal models were realized to investigate the role of gut microbiota modulation as therapy or prevention approach in predisposed animals: promising and stimulating results have been reported.
Dietary antigens and microbiota-derived products might act as triggers of T1D by causing a pro-inflammatory and metabolic dysfunctional environment.
Key message
Introduction
Gut microbiota comprises several microbiologic ecosystems including a variety of bacteria, archaea, microeukaryotes such as fungi and viruses. Intestinal epithelium is the natural habitat of gut microbiota, and together with the mucosal immune system makes a morpho-functional entity responsible for the homeostasis of human organism (Citation1).
Gut microbial species composition differs greatly among individuals. Each person represents a unique collection of microorganism species. Variability of gut microbiota is based on the host organism’s age, genetic, and environmental factors (Citation2,Citation3).
Recently, molecular techniques identified four major microbial phyla which represent over 90% of the gut microbiota: Firmicutes, Bacteroides, Proteobacteria, and Actinobacteria (Citation4). Most of commensal bacteria found in human fecal flora are represented by two main groups of Firmicutes, subdivided in Clostridium coccoides (Clostridium cluster XIVa) and C. leptum (Clostridium cluster IV), and the group of Cytophaga–Flavobacterium–Bacteroides (CFB) (Citation4).
In healthy individuals, gut microbiota plays a relevant role, being mainly involved in the development and growth of immune system and in the regulation of several fundamental metabolic pathways (Citation5). For instance, Clostridia cluster IV and XIVa, have an important role in immune gut homeostasis modulating the expression of Foxp3 in CD4 + T cells by the production of short-chain fatty acid (Citation6).
Quantitative and/or qualitative alterations of gut microbiota may impair homeostasis, leading to the development of a number of diseases related or not to the gastrointestinal system (Citation7). More specifically, a causal role of gut microbiota impairment on development of immunological and metabolic disorders has been investigated (Citation8–12).
Growing evidence has shown that gut microbiota is able to modulate mucosal immunity in many ways, such as development of gut-associated lymphoid tissues, promoting and enhancing adaptive immune response to pathogens (Citation13). In this review, we analyzed the role of gut microbiota in the pathogenesis of type 1 diabetes (T1D).
Type 1 diabetes mellitus and its etiopathogenesis
Diabetes mellitus (DM) comprises a heterogeneous group of metabolic diseases characterized by significant hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The vast majority of DM cases can be divided into two major types based on etiopathogenesis: type 1 diabetes mellitus (T1D) and type 2 diabetes mellitus (T2D). Moreover, about 5–10% of all patients diagnosed with T2D have markers of β cells autoimmunity, such as glutamic acid decarboxylase (GAD65Ab) and tyrosine phosphatase-2 autoantibodies (IA-2Ab) (Citation14–16). This form of autoimmune diabetes, defined by Zimmet as latent autoimmune diabetes of adults (LADA), shows clinical features somewhat intermediate between T1D and T2D (Citation17–19).
T1D is an organ-specific autoimmune disease characterized by auto-reactive T-cell mediated specific destruction of insulin-producing pancreatic β cells and accounts for about 10% of all cases of diabetes. Genetic factors play an important role in diabetic susceptibility, growing evidences has demonstrated that environmental factors can also interfere in diabetes onset and progression in the development. In T1D, there is a complex interplay between genes and the environment (Citation20). A variety of factors, including chemicals, viruses, commensal bacteria, and diet contribute to the risk of developing the disorder (Citation21,Citation22). The discordant diabetes incidence in monozygotic twins (less than 50% develop T1D) suggests that non-genetic factors might modulate genetic susceptibility. A number of genes T1D have been associated with T1D, including those located in the HLA class II locus (primarily the HLA-DRB1, -DQA1, and -DQB1 alleles), as well as the gene encoding insulin (INS), and gene encoding cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (Citation23,Citation24).
Breastfeeding
A protective role of breastfeeding on T1D is not yet fully confirmed. A recent meta-analysis reported that breastfeeding has some, although limited, protection against T1D development (Citation25). Findings obtained in animal models (Citation26), were confirmed also in humans where hydrolyzed casein reduced the positivity of one or more T1D-associated antibodies in young genetically predisposed children (Citation27). However, a recent study did not confirmed previous reports (Citation28), showing that the use of hydrolyzed casein did not reduce the incidence of diabetes-associated antibodies after 7 years. In contrast, bovine insulin appears to sensitize intestinal T lymphocytes in susceptible children, which in turn might contribute to the autoimmune destruction of pancreatic β cells (Citation29). Excessive intake of cow’s milk has been linked with an increased risk of developing T1D, especially in children with HLA-DQB1 genotypes (Citation30,Citation31). Although casein variants in vitro showed possible immunosuppressive effects (Citation32), the actual risk associated with excessive cow’s milk intake in terms of T1D development is not clear (Citation33–35).
Wheat gluten
Early weaning and introduction of cereals may be associated with an increased risk of T1D (Citation36–38). Timing seems to be important given the immune system immaturity in infant (Citation38). However, a delayed gluten exposure from 6 to 12 months does not have influence on the risk of developing islet autoimmunity at the age of 3 years (Citation39,Citation40). In contrast, studies in rodents clearly demonstrated that gluten-free diet (GFD) determines a fourfold reduction of diabetes incidence compared to a standard diet (Citation41). Marietta et al. observed that GFD was significantly protective in mice from spontaneous T1D (Citation42). More specifically, an increase of Akkermansia was detected in non-obese diabetic mice (NOD mice) on GFD. NOD mice in regular diet displayed a higher number of Barnesiella, Bifidobacterium, Tannerella, and Turcibacter. The authors suggested a pro-diabetogenic role for gluten-containing diet and a protective role of Akkermansia against T1D (Citation42). A similar Th1-based proinflammatory cytokine pattern was reported for wheat-based diet in the gut of NOD mice (Citation43) as well as in diabetes-prone rats (Citation44). Based on these results, it can be speculated that the protective effect of GFD on T1D development is modulated by diet-induced changes in the gut microbiota which in turn affects mutual immune and endocrine system interactions. Yet, the possible role of gluten in initiating the process leading to T1D in animals is not fully elucidated. Epidemiological evidence frequently shows an association between celiac disease and T1D, approximately 4% of diabetic patients have concomitant coeliac disease (Citation45). Minor evidence is available for food-related substances such as nitrite and nitrate and bisphenol and the risk of T1D (Citation46).
Microbiological agents
Since the onset of T1D follows a seasonal pattern, a variety of viruses, for example, coxsackie, rubella, and mumps, have been implicated in T1D etiology. These viruses can directly infect and disrupt β cells, but can also expose β cells to proinflammatory cytokines, through increasing expression of major histocompatibility complex class I on β cells. Viral infection might stimulate autoreactive T cells via molecular mimicry mechanisms (Citation47), although in most cases the association between viral infection (including enteroviruses) and T1D is not univocal (Citation48,Citation49).
Another putative risk factor for T1D could be the decreasing incidences of helminth infections (“hygiene hypothesis”). Helminth parasites are able to completely inhibit or significantly slow down T1D development in NOD mice (Citation50,Citation51). This protective effect might be mediated by an enhanced Th2-type response and/or the regulatory cytokines IL-10 and transforming growth factor β (Citation52,Citation53). Despite these experimental data, studies in humans on this issue are lacking.
Gut permeability
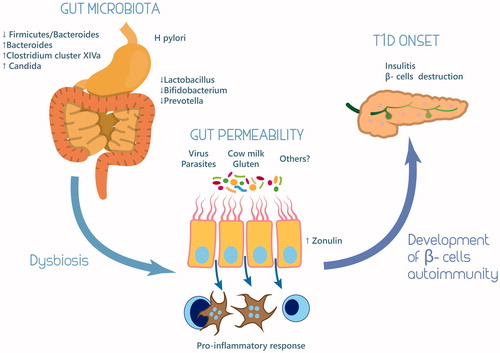
The gut barrier regulates the intestinal mucosa permeability. It is a functional unit, organized into a multilayer system, encompassing several components. From the outer layer to the inner one, the barrier consists in gut microbiota, mucus, epithelial cells, and the innate and adaptive immune cells forming the gut-associated lymphoid tissue (GALT). Disruption of the gut barrier leads to the increase of gut permeability (Citation54). The intestinal mucosa is a major site for pathogen invasion since, when undamaged, it provides the first-line of defense against antigens. Increased intestinal mucosa permeability may facilitate antigens crossing which can directly damage pancreatic β cells (Citation55). In addition, it can also allow greater exposure to the immune system of diet or pathogenic antigens, triggering inflammation and immune-mediated destruction of pancreatic β cells (Citation56). An adequate butyrate production levels are essential for gut integrity (Citation57). Moreover, the butyrate-producing bacteria have anti-inflammatory effect both in vivo and in vitro studies (Citation58). In light of the emerging evidences, the intestinal microbiota appears determinant for human health. Gut microbiota was compared between children with T1D and non-diabetic children with a case-control study design. The abundance of Bacteroides was substantially higher in cases whereas Prevotella was considerably more abundant in the controls. Lactate producers (Lactobacillus, Lactococcus, Bifidobacterium, and Streptococcus) were more common in cases in contrast to butyrate producers (Eubacterium, Fusobacterium, Anaerostipes, Roseburia, Subdoligranulum, Faecalibacterium) that were more abundant in controls (Citation59). Similar alterations in gut microbiota were reported in children with newly diagnosed T1D (Citation59,Citation60). Additional studies showed that streptococci and the phylum Bacteroidetes were more represented in diabetic children, whereas the combined abundance of Clostridium clusters IV and XIVa was higher in healthy control (Citation61). Butyrate-producing species of Clostridium reduce bacterial translocation, improve the organization of tight junctions and stimulate the synthesis of mucin, a glycoprotein maintaining the integrity of the gut epithelium, with beneficial effects against inflammation in the intestinal tissues (Citation62). These human studies support the notion that alterations in gut microbiota, in either composition and/or function, are strongly related to β cell autoimmunity and T1D development. Moreover, the available data confirmed the importance of a healthy development of microbiota in childhood. A healthy gut microbiota associated with adequately functioning of intestinal barrier during infancy will be crucial to prevent T1D development. Modification of microbiota early in life would result in aberrant microbial bowel colonization later in life.
The role of increased gut permeability in the pathogenesis of T1D was studied by several authors in animal models. In spontaneously diabetic rat, it was observed that a “leaky gut” was an early lesion (Citation63). For example, in bio-breeding diabetes prone (BB-DP) rats, compared to bio-breeding diabetes-resistant (BB-DR) rats, the enteropathy represented by an increase in crypt length and in the proliferative activity of crypt epithelial cells, precedes T1D onset (Citation64). Lee et al. demonstrated that loss of intestinal barrier integrity caused by enteric bacterial pathogen, such as Citrobacter rodentium, accelerates insulitis in NOD mice (Citation65).
Neu et al. realized a BB rat model to explore the mechanism of gut permeability in BB prone rat (Citation66). They found a percentage of goblet cells and mucosal crypt depth significantly greater in BB-DP than BB-DR rats. Notably, intestinal permeability decreased in all rat strains according to age. This observation demonstrates that in a genetically susceptible rodent model of diabetes, early increase of the intestinal permeability might allow unregulated passage of environmental antigens that could potentially trigger the autoimmune response leading to T1D (Citation66). Zonulin up-regulation is coincident with an increased permeability, and is followed by the production of autoantibodies against pancreatic β cells, which preceded the onset of clinically evident T1D. In BB-DP rats, inhibition of zonulin receptor reduced the cumulative incidence of T1D by 70%, despite the persistence of intraluminal zonulin up-regulation (Citation67). Experimental model demonstrated that hydrolyzed casein diet could contribute to the prevention of T1D modulating gut permeability. Modulation of tight junctions, ileal cytokines and zonulin production might be important mechanisms for this effect (Citation68). Preclinical data, on animal models, were confirmed in human studies. Subclinical enteropathy is already detectable before clinical onset of T1D (Citation69). In these subjects, an alteration of intestinal barrier function, associated with mucosal ultra-structural lesions, were detected. Small bowel samples did not show signs of atrophy or inflammation at the light microscopy level, whereas transmission electron microscopy analysis showed remarkable ultra-structural alterations, in particular height and thickness of microvilli, space between microvilli and thickness of tight junctions (Citation70). Moreover, an increased gut permeability was observed in T1D subjects with the HLA-DQB1*02 allele, suggesting that these patients may be more prone to develop abnormal immune responses to food antigens (Citation71). Recently, it has been investigated the role of zonulin in increased gut permeability in T1D. Zonulin up-regulation seems to precede the onset of the disease, providing a possible link between increased intestinal permeability, environmental exposure to non-self-antigens, and the development of autoimmunity in genetically susceptible subjects (Citation72).
Gut microbiota and its association with type 1 diabetes
Gut microbiota have been proposed as a main actor in the pathogenesis of T1D (Citation73–76). An increasing number of models for T1D have considered the potential role of microbes, with particular emphasis of those harbored in the gut, as an important factor in the pathogenesis of T1D.
The role of the intestinal microbiota as an integral determinant of human health has become increasingly evident in the past decade (Citation77,Citation78). The gut microbiota interacts with the adjacent mucosal environment directly, impacts on the intestinal permeability, and influences local and systemic inflammatory activity (Citation75). The intestinal microbiota thrives mainly on diet-derived nutrients, while the host benefits from its metabolites, which in turn modulate host mucosal immune response throughout different mechanisms, including stimulation of regulatory T cells (Citation75,Citation79). Moreover, specific combinations of microbial groups can induce specific immune responses (Citation80). It is clear that the human microbiota hold a pivotal role in health and in disease. Gastrointestinal microbes introduced through dietary exposure may act as direct modulators of the immune system through alteration of the gut microbiota. Several T1D animal model of have been studied to explore the relationship between the gut microbiota and T1D.
Animal models
Most of evidences have been indirectly and largely derived from two rodent models: the NOD mouse and the BB-DP rat (Citation81,Citation82). In both the models, microorganism was a necessary condition for T1D development. Hara et al. used the LEW1.WR1 rat model of Kilham rat virus (KRV)-induced T1D to explore the association between T1D and gut dysbiosis. They demonstrated that KRV infection resulted in a transient increase in the abundance of Bifidobacterium spp. and Clostridium spp. in fecal samples of infected versus uninfected rat (Citation83). An increased abundance of Lactobacillus and Bifidobacterium was detected in the BB-DR samples, while Bacteroidetes, Eubacterium, and Ruminococcus were more abundant in BB-DP rats (Citation84). Diet components such as acid liquid, fiber, and casein were investigated. Consuming acid water lead to a decrease in Firmicutes and an increase in Bacteroidetes and was associated to an increased onset of diabetes in NOD mice (Citation85). A recent study in NOD mice suggests that instead of gluten and milk proteins, the fraction of fibers, especially fermentable fibers, might be the diabetogenic component of normal laboratory chow (Citation86). A potential link to gut microbiota was also observed, as inclusion of such fibers to otherwise diabetes-retarding diet appeared to promote outgrowth of Bacteroidetes in young NOD mice.
Interestingly, early treatment with vancomycin reduces the risk of diabetes development in NOD mouse. Culture independent method revealed a relative increase in the abundance of a single species, Akkermansia muciniphila, while a depletion of many major genera of Gram-positive and Gram-negative microbes was observed (Citation87). Similarly to Akkermansia muciniphila, a protective role against T1D was reported for segmented filamentous bacteria (SFB) (Citation88). Also Lactobacillus johnsonii, isolated from BB-DR can reduce T1D development in BB-DP, while Lactobacillus reuteri failed in this scope (Citation89).
Diabetes resistance in Lactobacillus johnsonii N6.2-fed BB-DP rodents was correlated to a Th17 cell bias among the mesenteric lymph nodes (Citation82). Recently, Krych et al. confirmed the hypothesis of a diabetogenic microbiota. They showed that members of Firmicutes, as Lachnospiraceae, Ruminococcus, and Oscillospira promote T1D, while Bacteroidetes as Prevotella, and an unknown Bacteriodales act in favor of diabetes protection (Citation90). This finding on Bacteroidetes seems to contradict the data previously described. The differences between the results shown by some experimental models could be caused by the design of the experimental model, by environmental factors and further by the conservation and processing of the biological sample. Finally, regionally distinct alterations in gut microbiota were observed in rats with Streptozotocin-induced diabetes. Although the duodenum was not interested, distinct patterns of microbiota were detected in the ileum and colon. In particular, an increase in Proteabacteria and Klebsiella (Citation91) was observed, in diabetic rats.
The data would suggest that modification of the innate immune response and/or the presence of specific components of the gut microbiota may prevent the activation of T cells. However, the pathways how the gut microbiota interact and modulate host immunity and β cell autoimmunity changes are not clear. MyD88 knockout mice are protected from developing T1D, showing lower expression of tumor necrosis factor-alpha (TNF-α) and increased the counts of Lactobacilli, Rikenellaceae, and Porphyromonadaceae (Citation92). Moreover, in these mice, gut microbiota destruction by broad-spectrum antibiotics was associated with an increased incidence of T1D (Citation92). Studies in T1D animal models have shown that pro-inflammatory cytokines induce changes in β cells, whereas β cells apoptosis was induced by molecules of the TNF family (Citation93). For example, IL-17 is likely diabetes-promoting cytokine, and IL-17-deficient NOD mice are protected from diabetes development (Citation94). It is known that segmented filamentous bacteria (SFB) are associated with the differentiation of Th17 cells. This process is mediated by CD4 T cells in the gut where CD4 cells differentiated into either Th1 or Th17 cells, depending on the antigen delivered from gut bacteria (Citation95). Thus, commensal bacteria-derived peptides can activate antigen-specific T cells contributing to autoimmune diseases. The Bacteroides fragilis, a member of the Bacteroidetes phyla is one more example of a defined bacterial strain able to regulate the immune system (Citation80). Bacteroides fragilis reduce intestinal inflammation while SFB are able to activate IL-17 producing CD4 helper cells which in turn stimulate autoimmune responses and the production of inflammatory cytokines (Citation88,Citation96). The immune regulatory and disease protective effects of B. fragilis are abolished if the polysaccharide A gene is deleted (Citation97). It has been proposed that the maintenance of normal gut microbiota is promoted by the modulatory role of T cells secreting IL-10 and transforming growth factor β (Citation97), in a complex gut flora inclusive of symbiotic, potentially pathogenic commensal and pathogenic microorganisms working together for the maintenance of self-tolerance.
Human studies
Results obtained in animal models were translated to human studies confirming a relationship between gut microbiota and T1D. In T1D patients, compared to healthy controls, it is observed a decrease in Firmicutes/Bacteroides (F/B) ratio. Murri et al., described that intestinal dysbiosis can trigger T1D. Interestingly, they found a significant increase in the number of Clostridium, Bacteroides and Veillonella and a significant decrease in the number of Lactobacillus, Bifidobacterium, Blautiacoccoides/Eubacteriumrectale group, and Prevotella in children with diabetes. Furthermore, a significant difference in the number of Bifidobacterium, Lactobacillus, and Clostridium and in the F/B ratio, was observed between diabetic and not diabetic subjects, maybe related to the glycemic level (Citation98). A decrease in F/B ratio was confirmed by other studies (Citation99–101), but conflicting data have been published too. For instance, Mejia-Leon et al., reported an increase in genus Bacteroides, but unaltered F/B ratio, in the newly diagnosed T1D cases and a decrease in Prevotella, Acidaminococcus, and Megamonas. Notable, these authors showed that glycemic control in diabetic subjects normalized the gut microbiota profile (Citation60).
Frequently, in human studies it has been reported an increase in Bacteroides genus, in particular B. dorei and vulgatus. Bacteroides dorei abundance raised the appearance of autoantibodies before eight months (Citation101). Additional modifications such as abundance of several lactate and butyrate-producing bacteria, inversely related to the number of β cell autoantibodies in children were described. The Bacteroides genus and Clostridium perfringens, which are known to be associated with increased gut permeability and inflammation, were positively associated with β cell autoimmunity. Bacteroides adolescentis and Roseburia faecis, a member from Clostridium cluster XIVa, which produces butyrate using acetate and Faecalibacterium prausnitzii, a member from Clostridium cluster IV (an acetate utilizing butyrate-producing bacterium with anti-inflammatory properties), correlated positively with antibody titers against β cell, while Eubacterium hallii, was inversely related (Citation100). Kostic et al. described a decrease in microbiota diversity that occurs after antibodies appearance but before the onset of T1D, and an increase in Blautia, Rikenallacea, Ruminococcus gnavus, and Streptococcus infantarius (Citation102).
A relative reduction in Lactobacillus spp. and Staphylococcus spp. in the T1D new-onset seropositive and seronegative groups versus the unrelated healthy control group was reported. Furthermore, it has been reported a correlation between the number of antibodies and type of dysbiosis, such as reduction of Butyricimonas and Coprococcus and increase in Akkermansia in subjects with multiple-type autoantibodies compared with one autoantibody (Citation103).
Gut microbiota seems to influence also the age of detectable antibodies. Dorea and Barnesiella (at 6 months of age), Candidatus Nardonella (at 1 year of age) Erwinia and Enterobacter (at 2 year of age) abundances differed between children positive and negative for anti-islet cell autoantibodies. These include Veillonella abundance, which was lower in children in whom anti-islet cell autoantibodies developed (average 3%) than in children who remained autoantibody-negative, and Enterococcus abundance, which was higher in children in whom anti-islet cell autoantibodies developed (average 3%) than in children who remained autoantibody-negative (Citation104).
The significant role of the age was confirmed in a study on European children. Abundance of Bacteroidetes and Streptococcus spp. was positively associated with diabetes in subjects younger than 2.9 years. In the group of children older than 2.9 years, the largest difference between the control and the diabetic children was found within Clostridium cluster XIVa (Citation61).
Not only bacteria influenced the development of T1D, Candida albicans colonization was identified in a higher number of T1D subjects than controls. Additionally, colonization by unclassified Candida spp. other than C. albicans was investigated, but not significant difference was identified (Citation105).
Finally, the association between Helicobacter pylori and T1D has been investigated. Some researchers suggest a protective role of H. pylori based on epidemiologic data on the prevalence of H. pylori in schoolchildren. Interestingly, it has been reported that 73% of the schoolchildren in Russian Karelia were seropositive for H. pylori compared to 5% among Finnish peers. These data are in contrast to the difference observed in the incidence rate of T1D, which is around six times higher in Finland (Citation106). On the other hand, a recent study report a correlation among H. pylori infection and the risk of T1D compared to T2D was investigated. Helicobacter pylori seroprevalence was comparable in T1D and T2D, however, anti-CagA IgG were significantly higher in autoimmune diabetes. This finding suggest that more virulent H. pylori strains might be a trigger for immune diabetes (Citation107).
It was also suggested that in genetically susceptible individuals emergence and progression of T1D might be associated with dysbiosis within the gut microbiota and interruption of microbial colonization early in life. However, it needs to be emphasized that these observations are no more than correlation, and that it is not clear whether they are pre-existent or induced by the disease, or whether a particular gut microbial community is linked to cause or effect in disease pathogenesis.
Gut microbiota modulation and T1D
Does manipulation of gut microbiota have a role in modulating the onset of T1D in predisposed subjects? To date, this hypothesis was tested only on animal models.
In an experimental approach, gut microbiota from MyD88-deficient non-obese diabetic (MyD88-/-NOD) mice, which were protected from T1D development, was transplanted in NOD mice. A reduced onset of insulitis and a significantly delayed diabetes was observed (Citation108). Specific probiotic strains were tested in rodent model. Strains of L. lactis, genetically modified to secrete the whole pro-insulin autoantigen along with the immunomodulatory cytokine IL-10 were able to revert diabetes in NOD mice and increased the frequencies of local T regs (Citation109). These data were confirmed by a more recent study. Administration of L. lactis that express GAD65 and IL-10, in combination with short-course low-dose anti-CD3, stabilized insulitis, preserved functional β cell mass, and restored glycemia to normal levels in NOD mice with recent-onset diabetes (Citation110). It has been also demonstrated that gender could play a pivotal role. Indeed, NOD T1D female mice were significantly more susceptible to disease than males. Transfer of male microbiota to female NOD prior to disease onset protected against pancreatic islet inflammation, autoantibody production, and the development of diabetes (Citation111).
Non modified probiotic strains were also investigated. Early oral administration of VSL#3, a probiotic mixture containing S. thermophiles, Bifidobacterium spp and Lactobacillus spp was shown to prevent diabetes development in NOD mice. Reduction of insulitis and a decreased rate of β cell destruction in protected mice were reported (Citation112). Dolpady et al. confirmed these results. They showed that treatment with oral probiotic VSL#3, alone or in combination with retinoic acid, protect NOD mice from T1D. They demonstrated that this approach acts by affecting inflammasome at the intestinal level. In particular, it inhibits IL-1β expression while enhancing the release of protolerogenic components of the inflammasome, such as indoleamine 2,3-dioxygenase and IL-33 (Citation113). Finally, also Lactobacillus spp. was investigated in the prevention of T1D. Lactobacillus johnsonii or L. reuteri isolated from diabetes-resistant rat was administered to BB-DP rats. Lactobacillus johnsonii, but not L. reuteri, post-weaned the onset of T1D. Moreover, the administration of L. johnsonii also resulted in higher levels of the tight junction protein claudin (Citation89), as shown in .
Table 1. Role of probiotics in type 1 diabetes: results from animal studies.
Discussion
T1D most likely results from a combination of genetic susceptibility and exposure to environmental trigger(s). The main mechanism is clearly an autoimmune process, which is also evident at the time of clinical diagnosis. The drivers of T1D development have not been identified, but a variety of candidates have been proposed, including exposure to certain viruses and microbes, or dietary habits (i.e., breast feeding vs. infant formula, highly hydrolyzed infant formula, exposure to gluten, vitamin D deficiency etc.). Most of the effects of these varaibles are certainly mediated via the gut and the intestinal microbiota. Both animal models and human studies have suggested that the gut microbiota may be considered a super-organ, which has coevolved with the host, showing a strong association in health an disease. Moreover, the composition of the gut microbiota can be modulated by diet and environmental factors. This modulation can induce the proper maturation of the immune system or result in gut dysbiosis and altered immune response. There is clear and increasing evidence that changes in the microbiota are associated with some autoimmune diseases, either involving the gut mucosa as exemplified by coeliac disease or other distant sites such as joints in rheumatoid arthritis and the pancreas in T1D (Citation75). Thus, dietary antigens and microbiota-derived products might act as triggers of T1D by causing a pro-inflammatory and metabolic dysfunctional environment. The importance of intestinal microbiota in T1D etiology is further suggested by the differences in gut microbiota composition observed between individuals with autoimmune T1D and healthy controls that may influence the etiology and the progression of the disease. In particular, it has been shown that F/B ratio is reduced in T1D patients, furthermore, Bacteroides and Clostridium cluster XIVa are more abudant in contrast to Lactobacilli, Bifidobacteria, and Prevotella which are less represented in gut microbiota of T1D subjects (). Gut dysbiosis can lead to the increase in gut permeability, in this way several microbial and nutraceutical antigen can stimulate a local and systemic proinflammatory response. So, an adequately functioning intestinal barrier during infancy seems to prevent T1D development. Further, some found that manipulation of intestinal microbiota can be used to prevent or reduce T1D.
Overall, data on animal models are promising but additional studies will be required to translate into effective human treatments. Findings on humans are scanty, not univocal, and often based on a small sample size. Most of the observations are derived from cross-sectional studies and report associations, since the studies do not allow determining the cause and effect relationships. The variable results reported in the literature could be due to methodological issues and in particular to many confounding factors such as stool collection procedure, diet, culture, water supply, climate, pollution levels, and medical practices.
Despite the available evidences obtained in humans and experimental animals, it is presently unknown whether the observed correlations have any causative role or result from disease development itself. The question “cause or effect” still remains unanswered, and additional longitudinal studies are needed to change this interesting and fascinating hypothesis to an undeniable support for the multifactorial pathogenesis of T1D. In addition, the role of other components of the gut barrier in T1D requires more investigations, as in animal models as in humans. Once a specific knowledge of how interactions betweeen host and gut micriobiota is gained, it could predispose to autoimmune diabetes, microbiota-based therapies can be developed to prevent/cure T1D.
Acknowledgements
The authors are grateful to Salvatore Satta for having contributed to the realization of the figure.
Disclosure statement
The authors report no declarations of interest.
Funding
This work was done by receiving grant from Fondazione Banco di Sardegna (Studio di identificazione dei fattori che influenzano la durata e la qualità della vita umana in una popolazione scelta di individui longevi della Sardegna), Prot. U999.2014/AI.881.MGB, scientific coordinator Prof. Giuseppe Delitala.
References
- Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146:1449–58.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8.
- Marchesi J, Shanahan F. The normal intestinal microbiota. Curr Opin Infect Dis. 2007;20:508–13.
- Cani PD. Metabolism in 2013: the gut microbiota manages host metabolism. Nat Rev Endocrinol. 2014;10:74–6.
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904.
- Longman RS, Yang Y, Diehl GE, Kim SV, Littman DR. Microbiota: host interactions in mucosal homeostasis and systemic autoimmunity. Cold Spring Harb Symp Quant Biol. 2013;78:193–201.
- Ianiro G, Bibbo S, Gasbarrini A, Cammarota G. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets. 2014;15:762–70.
- Cammarota G, Ianiro G, Cianci R, Bibbo S, Gasbarrini A, Curro D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol Ther. 2015;149:191–212.
- Bibbo S, Lopetuso LR, Ianiro G, Di Rienzo T, Gasbarrini A, Cammarota G. Role of microbiota and innate immunity in recurrent Clostridium difficile infection. J Immun Res. 2014:462740.
- Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146:1525–33.
- Kamada N, Nunez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146:1477–88.
- Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M, et al. Variation in microbiome lps immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–53.
- Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol.2013;13:321–35.
- Irvine WJ, McCallum CJ, Gray RS, Duncan LJ. Clinical and pathogenic significance of pancreatic-islet-cell antibodies in diabetics treated with oral hypoglycaemic agents. Lancet. 1977;1:1025–7.
- Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care. 2001;24:1460–7.
- Zimmet PZ. The pathogenesis and prevention of diabetes in adults. Genes, autoimmunity, and demography. Diabetes Care. 1995;18:1050–64.
- Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288–93.
- Delitala AP, Fanciulli G, Zoledziewska M, Pitzalis M, Pusceddu P, Frongia P, et al. Allelic variant in CTLA4 is associated with thyroid failure and faster β-cell exhaustion in latent autoimmune diabetes in adults . J Diabetes 2015;7:68–73.
- Pes GM, Delitala AP, Delitala G, Errigo A, Costantino S, Fanciulli G. Phenotypic heterogeneity of latent autoimmune diabetes in adults identified by body composition analysis. Diabetol Metab Syndr. 2014;6:128.
- Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–67.
- Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304:1020–2.
- Dahlquist GG. Viruses and other perinatal exposures as initiating events for beta-cell destruction. Ann Med. 1997;29:413–17.
- Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57:176–85.
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature Genet. 2009;41:703–7.
- Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, et al. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35:2215–25.
- Emani R, Asghar MN, Toivonen R, Lauren L, Soderstrom M, Toivola DM, et al. Casein hydrolysate diet controls intestinal T cell activation, free radical production and microbial colonisation in NOD mice. Diabetologia. 2013;56:1781–91.
- Knip M, Virtanen SM, Seppa K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–8.
- Knip M, Akerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, et al. Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA.2014;311:2279–87.
- Vahasalo P, Petays T, Knip M, Miettinen A, Saukkonen T, Karjalainen J, et al. Relation between antibodies to islet cell antigens, other autoantigens and cow's milk proteins in diabetic children and unaffected siblings at the clinical manifestation of IDDM. The Childhood Diabetes in Finland Study Group. Autoimmunity. 1996;23:165–74.
- Saukkonen T, Virtanen SM, Karppinen M, Reijonen H, Ilonen J, Rasanen L, et al. Significance of cow's milk protein antibodies as risk factor for childhood IDDM: interactions with dietary cow's milk intake and HLA-DQB1 genotype. Childhood Diabetes in Finland Study Group. Diabetologia. 1998;41:72–8.
- Martin JM, Trink B, Daneman D, Dosch HM, Robinson B. Milk proteins in the etiology of insulin-dependent diabetes mellitus (IDDM). Ann Med. 1991;23:447–52.
- Merriman TR. Type 1 diabetes, the A1 milk hypothesis and vitamin D deficiency. Diabetes Res Clin Prac. 2009;83:149–56.
- Elliott RB, Reddy SN, Bibby NJ, Kida K. Dietary prevention of diabetes in the non-obese diabetic mouse. Diabetologia. 1988;31:62–4.
- Muntoni S, Cocco P, Aru G, Cucca F. Nutritional factors and worldwide incidence of childhood type 1 diabetes. Am J Clin Nutr. 2000;71:1525–9.
- Muntoni S, Muntoni S. Epidemiological association between some dietary habits and the increasing incidence of type 1 diabetes worldwide. Ann Nutr Metab. 2006;50:11–9.
- Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. Europ J Nutr. 2009;48:243–9.
- Ziegler AG, Schmid S, Huber D, Hummel M. Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–8.
- Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–20.
- Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–5.
- Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr. 2011;94:1821S–3S.
- Funda DP, Kaas A, Bock T, Tlaskalova-Hogenova H, Buschard K. Gluten-free diet prevents diabetes in NOD mice. Diabetes/Metab Res Rev. 1999;15:323–7.
- Marietta EV, Gomez AM, Yeoman C, Tilahun AY, Clark CR, Luckey DH, et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS One. 2013;8:e78687.
- Flohe SB, Wasmuth HE, Kerad JB, Beales PE, Pozzilli P, Elliott RB, et al. A wheat-based, diabetes-promoting diet induces a Th1-type cytokine bias in the gut of NOD mice. Cytokine. 2003;21:149–54.
- Patrick C, Wang GS, Lefebvre DE, Crookshank JA, Sonier B, Eberhard C, et al. Promotion of autoimmune diabetes by cereal diet in the presence or absence of microbes associated with gut immune activation, regulatory imbalance, and altered cathelicidin antimicrobial Peptide. Diabetes. 2013;62:2036–47.
- Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev. 2016;15:644–648.
- Virtanen SM, Jaakkola L, Rasanen L, Ylonen K, Aro A, Lounamaa R, et al. Nitrate and nitrite intake and the risk for type 1 diabetes in Finnish children. Childhood Diabetes in Finland Study Group. Diabetic Med. 1994;11:656–62.
- von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol. 2003;1:151–7.
- Coppieters KT, Wiberg A, von Herrath MG. Viral infections and molecular mimicry in type 1 diabetes. APMIS. 2012;120:941–9.
- Kondrashova A, Hyoty H. Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int Rev Immunol. 2014;33:284–95.
- Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45:588–94.
- Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407.
- Zaccone P, Cooke A. Vaccine against autoimmune disease: can helminths or their products provide a therapy? Curr Opin Immunol. 2013;25:418–23.
- Hubner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-beta. J Immunol. 2012;188:559–68.
- Viggiano D, Ianiro G, Vanella G, Bibbo S, Bruno G, Simeone G, et al. Gut barrier in health and disease: focus on childhood. Europ Rev Med Pharmacol Sci. 2015;19:1077–85.
- Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes-a solid or leaky concept? Pediatr Diabetes. 2015;16:485–92.
- Gomes AC, Bueno AA, de Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13:60.
- Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–84.
- Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101–11.
- Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792.
- Mejia-Leon ME, Petrosino JF, Ajami NJ, Dominguez-Bello MG, de la Barca AM. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep. 2014;4:3814.
- de Goffau MC, Fuentes S, van den Bogert B, Honkanen H, de Vos WM, Welling GW, et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57:1569–77.
- Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–61.
- Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–7.
- Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AM. Enteropathy precedes type 1 diabetes in the BB rat. Gut. 2004;53:1437–44.
- Lee AS, Gibson DL, Zhang Y, Sham HP, Vallance BA, Dutz JP. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010;53:741–8.
- Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2005;40:589–95.
- Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Nat Acad Sci USA. 2005;102:2916–21.
- Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010;53:2621–8.
- Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–7.
- Secondulfo M, Iafusco D, Carratu R, deMagistris L, Sapone A, Generoso M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis.2004;36:35–45.
- Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. 2002;35:365–8.
- Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9.
- Mejia-Leon ME, Barca AM. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients. 2015;7:9171–84.
- Gulden E, Wong FS, Wen L. The gut microbiota and Type 1 Diabetes. Clin Immunol.2015;159:143–53.
- McLean MH, Dieguez D, Jr., Miller LM, Young HA. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut. 2015;64:332–41.
- Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12:154–67.
- Borody TJ, Campbell J. Fecal microbiota transplantation: techniques, applications, and issues. Gastroenterol Clin North Am. 2012;41:781–803.
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30.
- Shapiro H, Thaiss CA, Levy M, Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol. 2014;30:54–62.
- Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev. 2012;245:13–26.
- Hoorfar J, Buschard K, Dagnaes-Hansen F. Prophylactic nutritional modification of the incidence of diabetes in autoimmune non-obese diabetic (NOD) mice. Brit J Nutr. 1993;69:597–607.
- Lau K, Benitez P, Ardissone A, Wilson TD, Collins EL, Lorca G, et al. Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. J Immunol. 2011;186:3538–46.
- Hara N, Alkanani AK, Ir D, Robertson CE, Wagner BD, Frank DN, et al. Prevention of virus-induced type 1 diabetes with antibiotic therapy. J Immunol. 2012;189:3805–14.
- Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. The ISME J. 2009;3:536–48.
- Wolf KJ, Daft JG, Tanner SM, Hartmann R, Khafipour E, Lorenz RG. Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J Histochem Cytochem.2014;62:237–50.
- Toivonen RK, Emani R, Munukka E, Rintala A, Laiho A, Pietila S, et al. Fermentable fibres condition colon microbiota and promote diabetogenesis in NOD mice. Diabetologia. 2014;57:2183–92.
- Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sorensen SJ, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–94.
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Nat Acad Sci USA. 2011;108:11548–53.
- Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One. 2010;5:e10507.
- Krych L, Nielsen DS, Hansen AK, Hansen CH. Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-γ level in NOD mice . Gut Microbes. 2015;6:101–9.
- Wirth R, Bodi N, Maroti G, Bagyanszki M, Talapka P, Fekete E, et al. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS One. 2014;9:e110440.
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13.
- Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. Beta cell apoptosis in diabetes. Apoptosis. 2009;14:1389–404.
- Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16:55–65.
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–6.
- Geuking MB, McCoy KD, Macpherson AJ. The continuum of intestinal CD4+ T cell adaptations in host-microbial mutualism. Gut Microbes. 2011;2:353–7.
- Ochoa-Reparaz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103–8.
- Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Medicine. 2013;11:46.
- Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91.
- de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T, et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. 2013;62:1238–44.
- Davis-Richardson AG, Ardissone AN, Dias R, Simell V, Leonard MT, Kemppainen KM, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol. 2014;5:678.
- Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–73.
- Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD, et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes. 2015;64:3510–20.
- Endesfelder D, zu Castell W, Ardissone A, Davis-Richardson AG, Achenbach P, Hagen M, et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes. 2014;63:2006–14.
- Soyucen E, Gulcan A, Aktuglu-Zeybek AC, Onal H, Kiykim E, Aydin A. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int. : 2014;56:336–43.
- Kondrashova A, Seiskari T, Ilonen J, Knip M, Hyoty H. The ‘Hygiene hypothesis’ and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. APMIS. 2013;121:478–93.
- Delitala AP, Pes GM, Malaty HM, Pisanu G, Delitala G, Dore MP. Implication of cytotoxic helicobacter pylori infection in autoimmune diabetes. J Diabetes Res. 2016:7347065.
- Peng J, Narasimhan S, Marchesi JR, Benson A, Wong FS, Wen L. Long term effect of gut microbiota transfer on diabetes development. J Autoimmun. 2014;53:85–94.
- Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. 2012;122:1717–25.
- Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes. 2014;63:2876–87.
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8.
- Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–75.
- Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D, et al. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res. 2016;2016:7569431.