Abstract
Dermatitis herpetiformis (DH) is an itchy blistering skin disease with predilection sites on elbows, knees, and buttocks. Diagnosis is confirmed by showing granular immunoglobulin A deposits in perilesional skin. DH is one manifestation of coeliac disease; the skin symptoms heal with gluten free diet (GFD) and relapse on gluten challenge. Of the first-degree relatives, 5% may be affected by either condition. Tissue transglutaminase (TG2) is the autoantigen in coeliac disease and epidermal transglutaminase (TG3) in DH. Both diseases conditions exhibit TG2-specific autoantibodies in serum and small bowel mucosa; patients with DH have IgA-TG3 in the skin. There are some divergencies between these two phenotypes. One-fourth of DH patients do not have small bowel mucosal villous atrophy, but virtually all have coeliac-type inflammatory changes. The skin symptoms respond slowly to GFD. The incidence of coeliac disease is increasing, whereas the opposite is true for DH. A female predominance is evident in coeliac disease, while DH may be more common in males. Coeliac disease carries the risk of small intestinal T-cell lymphoma; in DH B-cell lymphomas at any site may prevail. Adult coeliac disease carries a slightly increased elevated mortality risk, whereas in DH, the relative mortality rate is significantly decreased.
Dermatitis herpetiformis is a cutaneous manifestation of coeliac disease; both conditions are genetically determined and gluten-dependent.
Gastrointestinal symptoms and the degree of villous atrophy are less obvious in dermatitis herpetiformis than in coeliac disease. Both show tissue transglutaminase (TG2) specific autoantibodies in serum and small bowel mucosa. In addition, TG3-targeted IgA antibodies are found in the skin of DH patients
Both conditions carry an increased elevated risk of lymphoma, in coeliac disease small intestinal T-cell lymphoma, in dermatitis herpetiformis mainly B-cell lymphoma at various sites.
Coeliac disease is currently eight times more common that DH; the incidence of DH is decreasing in contrast to that of coeliac disease, where it is increasing.
Key messages
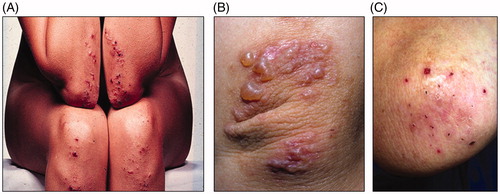
Dermatitis herpetiformis (DH) was described as a clinical entity by Louis Duhring in 1884 (Citation1). The hallmark of DH is the symmetrical distribution of small vesicles and papules on elbows, knees, and buttocks (Citation2). Lesions may also appear on the scalp, upper back, abdomen, and groin. Intense itch is very typical and due to it patients often scratch all vesicles, thus only erosions and excoriations can be seen (). DH could be distinguished from other bullous dermatoses when typical histopathological changes and granular immunoglobulin A (IgA) deposits in the skin were reported in the 1960s (Citation3). The latter finding was of great importance for accurate diagnosis and is almost pathognomic for the disorder.
Figure 1. Dermatitis herpetiformis. (A) Blisters on the elbows and knees. (B) Fresh, small blisters on the elbow. (C) Excoriated blisters and scars on the elbow, a typical clinical picture caused by scratching the lesions.
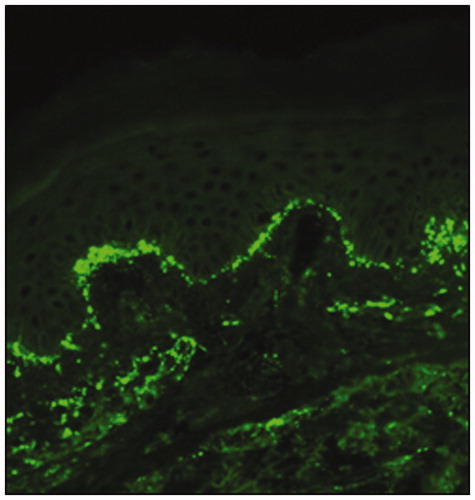
The diagnosis of DH should always be confirmed by direct immunofluorescence examination of perilesional skin showing granular IgA deposits in the papillary dermis (); false-negative results occur in about 5% of the biopsy specimens (Citation4). The disorder can thus be very accurately diagnosed – even when the cutaneous involvement is mild or barely discernible.
Dermatitis herpetiformis: links to coeliac disease
Samuel Gee described the symptoms of coeliac disease in 1888 (Citation5), 4 years after the discovery of DH. Small bowel inflammation, villous atrophy, and crypt hyperplasia are typical and well-known manifestations of coeliac disease, and biopsy has been the diagnostic gold standard up to the present day. The treatment of coeliac disease is gluten-free diet (GFD), where patients are not allowed to ingest products containing wheat, rye, or barley; this diet will result in mucosal recovery, provided that the diet is strict enough. Small bowel biopsies taken from patients with DH in the 1960s showed villous atrophy identical to that in coeliac disease (Citation6,Citation7); however, one-fourth had normal small bowel villous architecture. This caused much confusion as to whether DH is a specific cutaneous manifestation of coeliac disease, or only a disease associated with coeliac disease, like some autoimmune disorders. This issue began to be resolved in the 1970s, when it was understood that the skin symptoms in DH respond, albeit slowly, to strict GFD, even in patients with morphologically normal villous mucosa (Citation8,Citation9), and the symptoms recur on gluten challenge (Citation10). Both DH and coeliac disease have circulating antibodies against tissue transglutaminase (TGab), and TGab test is also highly specific in DH (Citation11).
There is more evidence that DH is one form of coeliac disease and not only a gluten-related disorder. Even though some patients with DH have seemingly normal small bowel mucosa (Citation7,Citation9), they do in fact have coeliac-type minor enteropathy. Increased density of small bowel mucosal intraepithelial lymphocytes (IELs), especially gamma/delta IELs is fairly characteristic of coeliac disease (Citation12,Citation13). An increased number of these IELs are also present in DH, regardless of the mucosal lesion (Citation14,Citation15). Some individuals have no villous atrophy, but have minor mucosal changes and circulating IgA antibodies either to endomysium or TG2, and will later develop overt coeliac disease. They are at this point called early developing, latent or potential coeliac disease patients, and they also benefit from GFD treatment (Citation16–18). Such minor mucosal morphology has long been recognized in DH (Citation7,Citation9), which is indeed representative for the currently well-acknowledged form of incipient coeliac disease (Citation16,Citation17).
Genetic and family studies bind dermatitis herpetiformis and coeliac disease convincingly together
The genetic association among coeliac disease, DH and HLA class II genes was documented 40 years ago (Citation19,Citation20). Almost every patient with coeliac disease or DH has the alleles contributing to HLA DQ2 or HLA DQ8 haplotype (Citation21,Citation22). Significantly, these haplotypes are present in about 30% of the population, which means that their occurrence is not of diagnostic value, but their absence may be useful in eliminating the possibility of coeliac disease (Citation23).
Due to their genetic susceptibility, first-degree relatives are at increased risk of developing coeliac disease or DH. A recent meta-analysis documented that the pooled prevalence of coeliac disease among first-degree relatives was 7.5% (Citation24). Only a few family studies have been conducted on DH. Meyer and Zone (Citation25) found that among 92 patients with DH, six (6.5%) had a first-degree relative afflicted with the disease. DH family studies from Finland showed that first-degree relatives may also be affected with coeliac disease (Citation26). As many as 18% of patients with DH and 19% of those with coeliac disease were found to have affected relatives, and irrespective of the phenotype of the index case, the disorder in the relative could be either DH or coeliac disease (Citation27).
A high disease concordance (71% pairwise) of coeliac disease was demonstrated in 23 Italian monozygotic twins (Citation28). We reported on six monozygotic twins with DH: three were DH-DH pairs, two DH-coeliac disease pairs, and one pair was discordant (Citation29). The fact that genetically identical individuals can have differentiated phenotypes suggests that some environmental factors determine the development of the cutaneous phenotype. Prolonged gluten ingestion may be one factor, because there are cases where the phenotype has changed from classic coeliac disease to DH in patients not complying with strict GFD treatment (Citation30). Nevertheless, DH was proven a gluten sensitive disorder and in Finnish and Swedish it is now literally referred to as “coeliac disease of the skin” (Citation31).
The dietary response to gluten-free diet takes longer in dermatitis herpetiformis than in coeliac disease
Dietary adherence to the GFD is essential in coeliac disease to achieve small bowel mucosal healing and alleviation of gastrointestinal symptoms. The mucosal recovery often takes 1 year or even longer, but abdominal symptoms usually abate in a few weeks. By contrast, the itchy skin lesions in DH may take months or years to recover on GFD (Citation8,Citation9). Therefore, most patients with DH initially receive additional treatment with dapsone, which usually clears the skin lesions within 2–3 d but has no effect on the possible gastrointestinal symptoms or small bowel mucosal damage. Dapsone is slowly tapered off as the skin symptoms heal; in the initial study, the mean time for discontinuing the use of the drug was 29 months (Citation8), and after this a strict GFD was the only treatment needed. The itchy papulovesicles recur rapidly after dietary transgressions, which help to maintain dietary compliance; symptoms in coeliac disease may appear much later. In our recent study, 98% of the patients with DH adhered to GFD, 72% of them strictly (Citation32). The adherence to GFD in patients with coeliac disease has been decidedly variable in different countries, with 40–96% maintaining a strict diet, but in Finland it is as good as in DH (Citation33). Nevertheless, there is a demand for guidelines on the follow-up of dietary treatment in coeliac disease or DH, since in many places this seems at present inadequate (Citation34,Citation35).
Refractory coeliac disease is a condition where mucosal recovery is absent and symptoms persist even when the patient adheres to a strict GFD. In other words, there is no clinical or histological response to the diet (Citation36). The condition is relatively rare; a nationwide study in Finland showed that only 0.3% of coeliac patients had refractory disease (Citation37). The management of refractory coeliac disease consists of immune suppressive therapy, but it still carries an increased risk of small-intestinal lymphoma (Citation36,Citation37), which has a poor prognosis. We recently analyzed whether cases refractory to GFD do indeed exist in DH. In seven (1.7%) out of the 403 patients the skin symptoms were non-responsive to a strict GFD and IgA deposits were present in the skin; the mean time for adhering to the diet was 16 years (Citation38). However, small bowel mucosal healing was evident in these cases, and the patients had no abdominal complaints, malabsorption or lymphoma, suggesting that the refractory condition in DH differs from that in coeliac disease.
Dermatitis herpetiformis and coeliac disease: some differences exist
Small bowel enteropathy
The differences in small bowel histology have been discussed earlier. To summarize, three out of four patients with DH have villous atrophy and crypt hyperplasia similar to that occurring in coeliac disease, and the remaining minor mucosal changes consistent with incipient or latent coeliac disease. A recent long-term study on 393 patients with DH documented that the proportion of patients having severe villous atrophy was decreasing (Citation39).
Occurrence
An epidemiologic survey conducted in Finland showed that in the early 1980s, the annual incidence of adult patients with DH almost equaled the number of new patients with coeliac disease (Citation40). Since then there has been a four-fold increase of new cases of coeliac disease, whereas the incidence of DH has decreased significantly (Citation41). Today, the prevalence of DH in the Tampere area in Finland is 75.3 per 100,000, one-eighth of the whole coeliac entity (Citation42). A decreasing incidence of DH has also been reported in the United Kingdom (Citation43). There, the ratio between the frequency of coeliac disease and DH was similarly eight to one, but the prevalence of DH was lower than in the Finnish study, 30 per 100,000. This means that the detection rate of coeliac disease was also lower than in Finland. An earlier study performed in the USA showed that DH was a relatively common disorder in that population with a prevalence of 11.2 per 100,000 (Citation44). A questionnaire study covering the whole USA and including 1138 biopsy-proven patients with coeliac disease showed that 9.8% of them had DH (Citation45). Similarly, a large study of hospitalized patients with coeliac disease and DH from Sweden found a percentage of 10.9% of DH (Citation46). Overall, the ratio between coeliac disease and DH in different populations has been 8–10 to 1, obviously indicating that DH is the commonest manifestation of coeliac disease outside the small bowel (Citation47).
Age at onset and gender
Coeliac disease can be diagnosed at any age, the peak incidence being between early childhood and the age of 40–60 (Citation48). By contrast, DH in childhood seems to be rare, to be found in only 4% of 476 Finnish patients (Citation49). However, local differences exist: in an Italian series comprising 159 DH patients, 36% were below the age of 20 years (Citation50). In children, the diagnosis can be challenging because clinical picture may be atypical and masked by simultaneous atopic dermatitis (Citation50). As to adults, no basic differences exist: the mean age at diagnosis of DH in our register study in Finland was 39 (range 11–80) years and that of coeliac disease 44 (range 1–85) years (Citation42). Despite better diagnostics in coeliac disease, a continuous increase in the mean age at diagnosis occurred from the 1970s to the 2000s. Similar to coeliac disease, DH likewise seems today to manifest on average at an older age than before. The reasons for these changes in the age at diagnosis remain obscure. One explanation may be that people live longer, and coeliac disease may manifest itself and also develop in advanced age (Citation51).
A preponderance of females is evident in adult coeliac disease, female to male ratios ranging up to 3:1 (Citation52). Studies on DH have yielded opposite results, the male to female ratio ranging from 1.5:1 to 2:1 (Citation2). Two recent large studies on adults with DH found the ratio of males to females be close to 1 (Citation41,Citation43). These recent findings suggest that although clinical series still show a female predominance in adults with coeliac disease, gender differences are perhaps not as evident as was earlier thought.
Gastrointestinal symptoms, body mass index (BMI), and bone mineral density
The classic presentation in adults with coeliac disease is diarrhoea, which may be accompanied by weight loss and malabsorption (Citation52). However, diarrhoea has been the main presenting symptom in less than 50% of cases in the past decade. Malaise, occasional loose stools, and subclinical or manifest iron deficiency are the most common clinical symptoms today. A wide variety of other symptoms may appear and serologic screening studies have demonstrated that coeliac disease is often totally asymptomatic (Citation45,Citation48,Citation52). Adult patients with DH rarely suffer from abrupt gastrointestinal symptoms or have signs of malabsorption (Citation2,Citation53). Occasional loose stools and minor gastrointestinal complaints are the most common findings, even though 75% of patients have villous atrophy in the small bowel. When we examined 57 children with DH in the 1980s, 16% presented with chronic diarrhea and only one had a history of failure to thrive (Citation54). In another study, the nutritional status of 86 adult patients with DH was carefully analyzed, and nutritional deficiency was found in only six (7%) patients, and only two had anaemia attributable to malabsorption (Citation55).
BMI was analyzed recently in 698 untreated Finnish adults with coeliac disease (Citation56). The average BMI was lower than in general population, but still 28% of the patients were overweight and 11% obese. A study on 679 patients with coeliac disease from the USA showed similar findings; 21% were overweight and 12% obese (Citation57). An Italian study suggested that BMI was even higher in patients with DH than in those with coeliac disease (odds ratio 1.46) (Citation58).
There is a risk of osteopenia and osteoporosis in untreated coeliac disease (Citation59). Di Stefano et al. (Citation60) reported that the bone mineral density was lower in 16 patients with DH and in 16 with coeliac disease than in healthy controls. Lewis et al. (Citation61) reported that there were no increased risks for fractures in 846 patients with treated DH when they were compared with 4225 controls. Overall, the low rate of gastrointestinal symptoms remains the basic issue distinguishing DH from coeliac disease, whereas the phenotypes do not clearly differ in terms of malabsorption or BMI.
Associated autoimmune diseases
Coeliac disease is known to occur concomitantly with several autoimmune diseases. Type I diabetes mellitus, autoimmune thyroid diseases and Sjögren’s syndrome being the best documented; at least 4–5% of patients suffering from these conditions have symptomatic or asymptomatic coeliac disease (Citation52,Citation62). When we examined a cohort of 305 patients with DH followed up for a mean of 10 years, we found concomitant autoimmune thyroid disease in 4.3%, type 1 diabetes mellitus in 1%, and Sjögren’s syndrome in 1% of the patients (Citation63). In a further study consisting 104 patients with DH, 2.3% had type 1 diabetes (Citation64). Most of the associated autoimmune diseases developed before the diagnosis of DH, and the DH patients with type 1 diabetes responded to GFD in a way similar to that seen in patients with isolated DH (Citation63,Citation64). Altogether, the occurrence of autoimmune conditions seems by and large to be similar in coeliac disease and DH, and is at least partly explained by the association with HLA DQ2. Comparisons between DH and coeliac disease are summarized in .
Table 1. Comparison between dermatitis herpetiformis and coeliac disease.
Malignancy and mortality
As in coeliac disease, the risk of non-Hodgkin’s lymphoma was significantly increased in DH, and in one study, the standardized incidence ratio was 5.14 (Citation65). A strict GFD for more than 5 years seems to protect against lymphoma in DH (Citation66). In agreement with this, our DH patients with lymphoma had not adhered as strictly to the GFD as those without lymphoma (Citation67). Moreover, our large series of DH, where almost all patients adhered to a GFD, showed a significantly increased lymphoma mortality rate during the first 5 years of follow-up, but not thereafter (Citation32). As to the risk of lymphoma, there seems to be one conspicuous difference between DH and coeliac disease. In coeliac disease, the lymphoma risk is limited to enteropathy associated T-cell lymphoma in small bowel (Citation68), whereas B-cell lymphomas predominate in DH (Citation67).
A meta-analysis of prospective studies in coeliac disease found a significantly increased risk for all-cause (odds ratio 1.24) and non-Hodgkin’s lymphoma (odds ratio 2.61) mortality (Citation69). A recent large register study from the UK could confirm the excess risk of deaths from non-Hodgkin’s lymphoma only (Citation70). Unexpectedly, we found in a prospective follow-up study of 476 Finnish patients with DH (comprising 9079 person-years) that the standardized mortality rate for all-cause mortality was significantly reduced, being 0.70 (95% CI 0.55–0.87) (Citation32). As many as 98% of these patients adhered to a GFD. A study on 846 patients with DH in the UK, in which the adherence to a GFD was only partially known, found a slightly, but non-significantly reduced mortality rate (hazard ratio 0.93) (Citation61). In coeliac disease mortality studies, the adherence to a GFD was not analyzed or was only partially known (Citation69–71) and, therefore, there is a special need to examine the relationship between mortality rate and dietary compliance.
How does dermatitis herpetiformis develop?
The breakthrough in coeliac disease research was the finding that TG2 enzyme was the autoantigen and target for IgA class autoantibody deposition in the small bowel mucosa (Citation72). As a result, the antibody test against tissue transglutaminase was soon developed (Citation73). IgA deposits against tissue transglutaminase (TG2) are highly specific for coeliac disease, and evidence shows that these antibodies are gluten dependent. The deposits can also be seen in the small bowel mucosa of DH patients, even those with apparently normal mucosal architecture (Citation15).
In DH, pathognomonic granular IgA deposits can be detected by direct immunofluorescence (IF) in the papillary dermis (Citation3,Citation4), and it has long been suspected that these IgA deposits derive from the gut. In 2002, Sárdy et al. (Citation74) demonstrated that the autoantigen for deposited cutaneous IgA is epidermal transglutaminase (TG3), which is an enzyme typically expressed in the epidermis and is closely related, but not identical, to TG2 (Citation75). Sardy et al. (Citation74) further showed that both DH and coeliac disease patients also presented with circulating IgA class TG3 antibodies, but that the antibodies of DH patients only recognized TG3 selectively and with high avidity. Since then TG3 autoantibodies have been identified in the serum in untreated DH in higher frequency than in coeliac disease patients (Citation76–78), but their exact role is yet to be elucidated. The ability of TG2 to deamidate and crosslink gluten peptides is essential for the production of TG2 autoantibodies in coeliac disease (Citation79). TG3 forms gluten peptide complexes less efficiently, which could be one reason for the different autoantibody response in DH (Citation80).
It is not known whether there are cases where the individual has granular IgA skin deposits but no clinical manifestation of DH. Suggesting that this is at least uncommon, skin immunofluorescence biopsies performed on the asymptomatic relatives of patients with DH failed to show IgA deposits in any of the subjects (Citation81). In one study, some weak IgA stainings were present in coeliac patients without any skin symptoms (Citation82).
At present, an immunopathogenesis of DH starting from hidden coeliac disease in the gut with TG2 antibody response, eventually evolving to immune complex deposition of high avidity IgA antibodies together with TG3 enzyme in the papillary dermis seems a valid hypothesis (Citation83,Citation84). Further support for this comes from GFD treatment results in DH:TG3 antibodies in the blood disappear along with the dietary treatment and, at the same time, the healing of skin and small bowel villous damage occurs (Citation85). By contrast, the IgA-TG3 aggregates in the skin disappear very slowly with GFD treatment. This seems to be due to active TG3 enzyme in the aggregates resulting in covalent cross-linking of the complex to dermal structures (Citation74,Citation86). An open question is why the blistering papulovesicles in DH have a predilection for areas in the knees, elbows, and buttocks, although IgA-TG3 aggregates are also deposited at sites never involved in the formation of skin lesions (Citation87). The most likely explanation for this unique distribution of the skin lesions involves the influence of local factors, such as pressure and stretching, and warm weather is also known to aggravate the symptoms (Citation2,Citation84).
Conclusions and future aspects
Our knowledge of DH has increased rapidly in the past 50 years. A simple blistering skin disease has turned out to be an autoimmune disorder having its origin in hidden coeliac disease in the gut. Physicians should learn to recognize DH because it is a fairly common extraintestinal manifestation of coeliac disease. The itchy, blistering papulovesicles, and excoriations mostly on the elbows, knees, and buttocks, are typical. The diagnosis is easily confirmed by detecting granular IgA deposits in skin immunofluorescence biopsy (Citation2). Routine small bowel biopsy is not necessary in the diagnosis of DH (Citation88), but we recommend taking a biopsy when gastrointestinal symptoms or malabsorption is present.
GFD is the treatment of choice for all patients with DH irrespective of small intestinal mucosal structure. The knowledge of the risk of lymphoma in patients not adhering to a GFD (Citation66,Citation67) and the excellent prognosis in those adhering (Citation32) are today well documented, and this should be impressed upon the patients. Adherence to a strict GFD is not necessarily easy, and there is a need for dietary education and support from local coeliac society people (Citation34). There is also one important point which physicians taking care of patients with DH and coeliac disease should take into account. There is an increased risk of first-degree relatives suffering from or subsequently developing either DH or coeliac disease (Citation24,Citation27). DH can be easily noticed in family members by enquiring about the occurrence of itchy lesions on the elbows, knees and buttocks. Coeliac disease is by contrast often asymptomatic, and, therefore, we advocate serologic screening of the family members of index cases with either DH or coeliac disease.
The concept of early incipient or potential coeliac disease in patients having coeliac autoantibodies but no villous atrophy has widened the spectrum of coeliac disease (Citation16,Citation17,Citation48). In DH, it has been known for years that one-fourth of patients have no villous atrophy, although they show coeliac-type inflammatory changes in the small bowel mucosa (Citation14,Citation39). These patients with DH respond to a GFD similarly to those with villous atrophy (Citation9), and recent studies on patients with incipient coeliac disease have also shown favorable response to treatment (Citation89).
Recently, the concept of non-coeliac gluten sensitivity has obtained much interest (Citation16). Dermatologists have also found patients with variable skin symptoms who seem to respond to GFD treatment (Citation90). These patients have often granular complement deposits in the skin but no IgA like the patients with DH.
We need to know more to understand why IgA-TG3 aggregates are also deposited in sites never involved in blister formation, and why they disappear from the skin after several years on GFD treatment (Citation74,Citation87). It also remains obscure whether coeliac patients with circulating TG3 antibodies at diagnosis would be at risk of contracting DH if they had continued on a normal gluten containing diet (Citation91).
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Duhring LA. Landmark article, Aug 30, 1884: dermatitis herpetiformis. By Louis A. Duhring. JAMA 1983;250:212–16.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. Part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017–24.
- van der Meer JB. Granular deposits of immunoglobulins in the skin of patients with dermatitis herpetiformis. An immunofluorescent study. Br J Dermatol. 1969;81:493–503.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912–18.
- Losowsky MS. A history of coeliac disease. Dig Dis. 2008;26:112–20.
- Marks J, Shuster S, Watson AJ. Small-bowel changes in dermatitis herpetiformis. Lancet 1966;2:1280–2.
- Fry L, Keir P, McMinn RM, Cowan JD, Hoffbrand AV. Small-intestinal structure and function and haematological changes in dermatitis herpetiformis. Lancet 1967;2:729–33.
- Fry L, Seah PP, Riches DJ, Riches DJ, Hoffbrand AV. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet 1973;1:288–91.
- Reunala T, Blomqvist K, Tarpila S, Halme H, Kangas K. Gluten-free diet in dermatitis herpetiformis. I. Clinical response of skin lesions in 81 patients. Br J Dermatol. 1977;97:473–80.
- Leonard J, Haffenden G, Tucker W, Unsworth J, Swain F, McMinn R, et al. Gluten challenge in dermatitis herpetiformis. N Engl J Med. 1983;308:816–19.
- Dieterich W, Laag E, Bruckner-Tuderman L, Reunala T, Kárpáti S, Zágoni T, et al. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Invest Dermatol. 1999;113:133–6.
- Spencer J, Isaacson PG, MacDonald TT, Thomas AJ, Walker-Smith JA. Gamma/delta T cells and the diagnosis of coeliac disease. Clin Exp Immunol. 1991;85:109–13.
- Järvinen TT, Kaukinen K, Laurila K, Kyrönpalo S, Rasmussen M, Mäki M, et al. Intraepithelial lymphocytes in celiac disease. Am J Gastroenterol. 2003;98:1332–7.
- Savilahti E, Reunala T, Mäki M. Increase of lymphocytes bearing the gamma/delta T cell receptor in the jejunum of patients with dermatitis herpetiformis. Gut 1992;33:206–11.
- Salmi TT, Hervonen K, Laurila K, Collin P, Mäki M, Koskinen O, et al. Small bowel transglutaminase 2-specific IgA deposits in dermatitis herpetiformis. Acta Derm Venereol. 2014;94:393–7.
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
- Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, et al. Diagnosis and management of adult coeliac disease: guidelines from the British society of gastroenterology. Gut 2014;63:1210–28.
- Kurppa K, Räsänen T, Collin P, Iltanen S, Huhtala H, Ashorn M, et al. Endomysial antibodies predict celiac disease irrespective of the titers or clinical presentation. World J Gastroenterol. 2012;18:2511–16.
- Keuning JJ, Peña AS, van Leeuwen A, van Hooff JP, van Rood JJ. HLA-DW3 associated with coeliac disease. Lancet 1976;1:506–8.
- Solheim BG, Albrechtsen D, Thorsby E, Thune P. Strong association between an HLA-Dw3 associated B cell alloantigen and dermatitis herpetiformis. Tissue Antigens 1977;10:114–18.
- Balas A, Vicario JL, Zambrano A, Acuña D, García-Novo D. Absolute linkage of celiac disease and dermatitis herpetiformis to HLA-DQ. Tissue Antigens 1997;50:52–6.
- Karell K, Korponay-Szabo I, Szalai Z, Holopainen P, Mustalahti K, Collin P, et al. Genetic dissection between coeliac disease and dermatitis herpetiformis in sib pairs. Ann Hum Genet. 2002;66:387–92.
- Kaukinen K, Partanen J, Maki M, Collin P. HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol. 2002;97:695–9.
- Singh P, Arora S, Lal S, Strand TA, Makharia GK. Risk of celiac disease in the first- and second-degree relatives of patients with celiac disease: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:1539–48.
- Meyer LJ, Zone JJ. Familial incidence of dermatitis herpetiformis. J Am Acad Dermatol. 1987;17:643–7.
- Reunala T. Incidence of familial dermatitis herpetiformis. Br J Dermatol. 1996;134:394–8.
- Hervonen K, Hakanen M, Kaukinen K, Collin P, Reunala T. First-degree relatives are frequently affected in coeliac disease and dermatitis herpetiformis. Scand J Gastroenterol. 2002;37:51–5.
- Nistico L, Fagnani C, Coto I, Percopo S, Cotichini R, Limongelli MG, et al. Concordance, disease progression, and heritability of coeliac disease in Italian twins. Gut 2006;55:803–8.
- Hervonen K, Karell K, Holopainen P, Collin P, Partanen J, Reunala T. Concordance of dermatitis herpetiformis and celiac disease in monozygous twins. J Invest Dermatol. 2000;115:990–3.
- Kurppa K, Koskinen O, Collin P, Mäki M, Reunala T, Kaukinen K. Changing phenotype of celiac disease after long-term gluten exposure. J Pediatr Gastroenterol Nutr. 2008;47:500–3.
- Reunala T. Dermatitis herpetiformis: coeliac disease of the skin. Ann Med. 1998;30:416–18.
- Hervonen K, Alakoski A, Salmi TT, Helakorpi S, Kautiainen H, Kaukinen K, et al. Reduced mortality in dermatitis herpetiformis: a population-based study of 476 patients. Br J Dermatol. 2012;167:1331–7.
- See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA. Practical insights into gluten-free diets. Nat Rev Gastroenterol Hepatol. 2015;12:580–91.
- Ciacci C, Ciclitira P, Hadjivassiliou M, Kaukinen K, Ludvigsson JF, McGough N, et al. The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis. United European Gastroenterol J. 2015;3:121–35.
- Herman ML, Rubio-Tapia A, Lahr BD, Larson JJ, Van Dyke CT, Murray JA. Patients with celiac disease are not followed up adequately. Clin Gastroenterol Hepatol. 2012;10:893–9.
- Rubio-Tabia A, Murray JA. Classification and management of refractory coeliac disease. Gut 2010;59:547–57.
- Ilus T, Kaukinen K, Virta LJ, Huhtala H, Mäki M, Kurppa K, et al. Refractory coeliac disease in a country with a high prevalence of clinically-diagnosed coeliac disease. Aliment Pharmacol Ther. 2014;39:418–25.
- Hervonen K, Salmi TT, Ilus T, Paasikivi K, Vornanen M, Laurila K, et al. Dermatitis herpetiformis refractory to gluten-free dietary treatment. Acta Derm Venereol. 2016;96:82–6.
- Mansikka E, Hervonen K, Salmi TT, Kautiainen H, Kaukinen K, Collin P, et al. The decreasing prevalence of severe villous atrophy in Dermatitis herpetiformis: a 45-year experience in 393 patients. J Clin Gastroenterol. In press. PMID: 27136959; doi:10.1097/MCG.0000000000000533.
- Collin P, Huhtala H, Virta L, Kekkonen L, Reunala T. Diagnosis of celiac disease in clinical practice: physician's alertness to the condition essential. J Clin Gastroenterol. 2007;41:152–6.
- Salmi TT, Hervonen K, Kautiainen H, Collin P, Reunala T. Prevalence and incidence of dermatitis herpetiformis: a 40-year prospective study from Finland. Br J Dermatol. 2011;165:354–9.
- Virta LJ, Kaukinen K, Collin P. Incidence and prevalence of diagnosed coeliac disease in Finland: results of effective case finding in adults. Scand J Gastroenterol. 2009;44:933–8.
- West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109:757–68.
- Smith JB, Tulloch JE, Meyer LJ, Zone JJ. The incidence and prevalence of dermatitis herpetiformis in Utah. Arch Dermatol. 1992;128:1608–10.
- Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, Mcmahon DJ, Absan H, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–31.
- Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002;123:1428–35.
- Leffler DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561–71.
- Tack GJ, Verbeek WH, Schreurs MW, Mulder CJ. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204–13.
- Hervonen K, Salmi TT, Kurppa K, Kaukinen K, Collin P, Reunala T. Dermatitis herpetiformis in children: a long-term follow-up study. Br J Dermatol. 2014;171:1242–3.
- Antiga E, Verdelli A, Calabrò A, Fabbri P, Caproni M. Clinical and immunopathological features of 159 patients with dermatitis herpetiformis: an Italian experience. G Ital Dermatol Venereol. 2013;148:163–9.
- Vilppula A, Kaukinen K, Luostarinen L, Krekelä I, Patrikainen H, Valve R, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol. 2009;9:49.
- Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43.
- Gawkrodger DJ, Blackwell JN, Gilmour HM, Rifkind EA, Heading RC, Barnetson RS. Dermatitis herpetiformis: diagnosis, diet and demography. Gut 1984;25:151–7.
- Reunala T, Kosnai I, Karpati S, Kuitunen P, Török E, Savilahti E. Dermatitis herpetiformis: jejunal findings and skin response to gluten free diet. Arch Dis Child. 1984;59:517–22.
- Gawkrodger DJ, Ferguson A, Barnetson RS. Nutritional status in patients with dermatitis herpetiformis. Am J Clin Nutr. 1988;48:355–60.
- Ukkola A, Mäki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, et al. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Intern Med. 2012;23:384–8.
- Kabbani TA, Goldberg A, Kelly CP, Pallav K, Tariq S, Peer A, et al. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther. 2012;35:723–9.
- Zingone F, Bucci C, Tortora R, Santonicola A, Cappello C, Franzese MD, et al. Body mass index and prevalence of skin diseases in adults with untreated coeliac disease. Digestion 2009;80:18–24.
- Valdimarsson T, Toss G, Ross I, Lofman O, Ström M. Bone mineral density in coeliac disease. Scand J Gastroenterol. 1994;29:457–61.
- Di Stefano M, Jorizzo RA, Veneto G, Cecchetti L, Gasbarrini G, Corazza GR. Bone mass and metabolism in dermatitis herpetiformis. Dig Dis Sci. 1999;44:2139–43.
- Lewis NR, Logan RF, Hubbard RB, West J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2008;27:1140–7.
- Collin P, Reunala T, Pukkala E, Laippala P, Keyriläinen O, Pasternack A. Coeliac disease-associated disorders and survival. Gut 1994;35:1215–18.
- Reunala T, Collin P. Diseases associated with dermatitis herpetiformis. Br J Dermatol. 1997;136:315–18.
- Hervonen K, Viljamaa M, Collin P, Knip M, Reunala T. The occurrence of type 1 diabetes in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2004;150:136–8.
- Grainge MJ, West J, Solaymani-Dodaran M, Card TR, Logan RF. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2012;35:730–9.
- Lewis HM, Reunala TL, Garioch JJ, Leonard JN, Fry JS, Collin P, et al. Protective effect of gluten-free diet against development of lymphoma in dermatitis herpetiformis. Br J Dermatol. 1996;135:363–7.
- Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2005;152:82–6.
- Chandesris MO, Malamut G, Verkarre V, Meresse B, Macintyre E, Delarue R. Enteropathy-associated T-cell lymphoma: a review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol Clin Biol. 2010;34:590–605.
- Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35:540–51.
- Abdul Sultan A, Crooks CJ, Card T, Tata LJ, Fleming KM, West J. Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis. Gut 2015;64:1220–6.
- Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet 2001;358:356–61.
- Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801.
- Sulkanen S, Collin P, Laurila K, Maki M. IgA- and IgG-class antihuman umbilical cord antibody tests in adult coeliac disease. Scand J Gastroenterol. 1998;33:251–4.
- Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747–57.
- Grenard P, Bates MK, Aeschlimann D. Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem. 2001;276:33066–78.
- Heil PM, Volc-Platzer B, Karlhofer F, Gebhart W, Huber WD, Benesch T, et al. Transglutaminases as diagnostically relevant autoantigens in patients with gluten sensitivity. J Dtsch Dermatol Ges. 2005;3:974–8.
- Hull CM, Liddle M, Hansen N, Meyer LJ, Schmidt L, Taylor T, et al. Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol. 2008;159:120–4.
- Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book L, Meyer LJ, et al. IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Invest Dermatol. 2009;129:2728–30.
- Di Sabatino A, Vanoli A, Giuffrida P, Luinetti O, Solcia E, Corazza GR. The function of tissue transglutaminase in celiac disease. Autoimmun Rev. 2012;11:746–53.
- Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM. Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxia. Amino Acids 2010;39:1183–91.
- Reunala T, Salo OP, Tiilikainen A, Selroos O, Kuitunen P. Family studies in dermatitis herpetiformis. Ann Clin Res. 1976;8:254–61.
- Cannistraci C, Lesnoni La Parola I, Cardinali G, Bolasco G, Aspite N, Stigliano V, et al. Co-localization of IgA and TG3 on healthy skin of coeliac patients. J Eur Acad Dermatol Venereol. 2007;21:509–14.
- Kárpáti S. Dermatitis herpetiformis. Clin Dermatol. 2012;30:56–9.
- Reunala T, Salmi TT, Hervonen K. Dermatitis herpetiformis: pathognomonic transglutaminase IgA deposits in the skin and excellent prognosis on a gluten-free diet. Acta Derm Venereol. 2015;95:917–22.
- Reunala T, Salmi TT, Hervonen K, Laurila K, Kautiainen H, Collin P, et al. IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis: a significant but not complete response to a gluten-free diet treatment. Br J Dermatol. 2015;172:1139–41.
- Taylor TB, Schmidt LA, Meyer LJ, Zone JJ. Transglutaminase 3 present in the IgA aggregates in dermatitis herpetiformis skin is enzymatically active and binds soluble fibrinogen. J Invest Dermatol. 2015;135:623–5.
- Donaldson MR, Zone JJ, Schmidt LA, Taylor TB, Neuhausen SL, Hull CM, et al. Epidermal transglutaminase deposits in perilesional and uninvolved skin in patients with dermatitis herpetiformis. J Invest Dermatol. 2007;127:1268–71.
- Caproni M, Antiga E, Melani L, Fabbri P. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633–8.
- Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology 2009;136:816–23.
- Bonciolini V, Bianchi B, Del Bianco E, Verdelli A, Caproni M. Cutaneous manifestations of non-celiac gluten sensitivity: clinical histological and immunopathological features. Nutrients 2015;7:7798–805.
- Salmi TT, Kurppa K, Hervonen K, Laurila K, Collin P, Huhtala H, et al. Serum transglutaminase 3 antibodies correlate with age at celiac disease diagnosis. Dig Liver Dis. 2016;48:632–7.