Abstract
Background: Heart rate (HR), heart rate variability (HRV), and inflammation are all associated with cardiovascular morbidity and mortality. The aim of this study was to assess potential interrelationships between these parameters in a young and healthy population.
Methods: Healthy individuals aged 25–41 years were included in a prospective population-based study. All participants underwent 24-h electrocardiography using a validated device. The standard deviation of all normal RR intervals (SDNN) was pre-defined as the main HRV outcome variable. High-sensitivity C-reactive protein (hs-CRP), total leukocyte (LC) count and LC subtypes were obtained from venous blood samples.
Results: A total of 2064 participants (47% men, 37 years) were included in this analysis. In multivariable linear regression analyses using SDNN as the outcome variable, β-coefficients (95% confidence intervals) per 1 standard deviation (SD) increase on the log-scale were −0.11 (−0.16; −0.07), p < .0001 for hs-CRP, −0.13 (−0.17; −0.09), p < .0001 for total LC count, −0.12 (−0.16; −0.08), p < .0001 for neutrophils, −0.04 (−0.09; 0.00), p = .05 for lymphocytes and −0.08 (−0.09; −0.02), p = .005 for monocytes. There were positive relationships between resting and ambulatory HR and inflammatory biomarkers, except for lymphocytes.
Conclusion: In this large cohort of young and healthy adults, inflammatory parameters were strongly associated with increased HR and decreased HRV, suggesting an important interaction between inflammatory pathways and the autonomic nervous system.
Inflammatory biomarkers, such as high-sensitivity C-reactive protein and leukocyte cell count with its subtypes were inversely associated with HRV and positively associated with HR.
Our findings suggest important interrelationships between inflammatory pathways and the ANS.
Key message
Introduction
Inflammation plays a key role in the pathogenesis and progression of atherosclerosis (Citation1). Accordingly, several inflammatory biomarkers have been prospectively associated with all-cause mortality (Citation2–4) and a broad set of cardiovascular outcomes including atrial fibrillation, stroke, and myocardial infarction (Citation5–9).
The autonomic nervous system (ANS) is strongly involved in different mechanisms of the cardiovascular system. Heart rate variability (HRV) has become a validated marker of the autonomic function (Citation10,Citation11). A reduced HRV is associated with an increased risk for cardiovascular and all-cause mortality (Citation12,Citation13). Interestingly, experimental studies have suggested direct relationships between inflammation and the ANS (Citation14,Citation15). Thus, autonomic dysfunction might be one mechanism why individuals with elevated inflammatory biomarkers have an increased cardiovascular risk. Up to now, some studies in rather small populations have evaluated the relationship between inflammation and HRV, and mainly inverse relationships have been described (Citation16–20). However, information on inflammatory biomarkers other than high-sensitivity C-reactive protein (hs-CRP) is scarce (Citation21). In addition, inflammatory biomarkers have recently been associated with heart rate (HR) (Citation22), which by itself is an independent risk factor for cardiovascular outcomes (Citation23). Given the tight link between HRV and HR (Citation24), it is currently unclear whether HRV contains any additional information over and above HR.
In order to get a deeper understanding of these interrelationships between inflammation and the ANS, we assessed the relationships of 24-h HR and HRV with several inflammatory biomarkers in a large population-based cohort of young and healthy adults from the general population.
Methods
All inhabitants of the Principality of Liechtenstein aged between 25 and 41 years, namely 5898 individuals, were invited to participate in the Genotypic and Phenotypic Determinants of Blood Pressure and other Cardiovascular Risk Factors (GAPP) study, a prospective population-based cohort study. Of them, 61% (3605) refused study participation, 66 (1%) women were pregnant and 57 (1%) individuals had at least one exclusion criteria, leaving 2170 (37%) participants that were enrolled in this study. Study design and methodology have been published previously (Citation25). Main exclusion criteria of the GAPP-study were established cardiovascular disease, arrhythmias, known renal failure, a body mass index (BMI) > 35kg/m2, current intake of insulin or antidiabetic drugs, daily intake of nonsteroidal anti-inflammatory drugs, regular intake of steroids, or other severe diseases. For the present study, we excluded 106 participants for the following reasons: missing 24-h ECG or recording time <80% of the target time (n = 39), regular intake of beta blockers (n = 2), missing laboratory values (n = 10), and other missing covariates (n = 55), leaving 2064 participants for the current analysis. The study protocol was approved by the local ethics committee and all study participants have signed a written informed consent.
24-h electrocardiography
Participants underwent 24-h Holter ECG monitoring using a validated three-channel device (Schiller AG, Baar, Switzerland). The 24-h ECG was attached by trained study nurses and started in the morning immediately after the study examination. Participants were advised to perform their normal daily activities. Recordings with a duration of less than 80% of the target time (i.e., <19.2 h) or of low quality were repeated whenever possible. Every 24-h ECG study was post-processed using a dedicated software to remove artefacts and redefine premature ventricular and atrial beats (Medilog Darwin, Schiller AG, Baar, Switzerland). Mean 24-h ambulatory HR was automatically calculated by the software. All heart beats defined as normal were used to calculate HRV. We used the standard deviation of all normal RR intervals (SDNN), total power (TP), low frequency (LF), and high frequency (HF) to quantify HRV. The selection of these HRV variables is based on (Citation1) their strong association with cardiovascular outcomes and (Citation2) their physiologic correlate (Citation12,Citation26). SDNN is measured in milliseconds (ms) whereas TP, LF and HF are measured in ms2. The low- and high-frequency band is between 0.04–0.15 and 0.15–0.40Hz, respectively. LF and HF were normalized by calculating LF/(TP-VLF) × 100 and HF/(TP-VLF) × 100, respectively, to receive values independent of TP. SDNN was pre-defined as the main HRV outcome variable.
Blood sampling
Fasting venous blood samples were collected of every participant and immediately processed. Total number of leukocytes (LC), neutrophils, lymphocytes and monocytes were quantified using a validated method (Sysmex XE 5000, Kobe, Japan). Hs-CRP, creatinine, triglycerides, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were assayed on a Roche Cobas 6000 analyzer (Roche, Basel, Switzerland). Glycated hemoglobin A1c (HbA1c) was measured using high-performance liquid chromatography (Bio-Rad D-10, Bio-Rad Laboratories AG, Reinach, Switzerland). Endothelin-1 (ET-1) was measured using a frozen EDTA plasma sample (Singulex Inc., Alameda, CA). Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) formula. Prediabetes was defined as an HbA1c between 5.6 and 6.4% (Citation27).
Assessment of other study variables
Information about personal, medical, lifestyle and nutritional factors were self-assessed using standardized questionnaires (Citation25). Smoking status was classified as never, former or current smoking. Highest educational status achieved was divided into three categories: high school, college or university degree. Nutritional factors and dietary habits were assessed using the Swiss health survey questionnaire from 2007, where people have to report the frequency of their fruit and vegetable consumption. Fruit and vegetable consumption was dichotomized into ≥5 and <5 servings per day. Alcohol consumption was dichotomized into drinkers and non-drinkers, while non-drinkers were defined as individuals never drinking alcohol or drinking less than once a month. Moderate and vigorous physical activity was estimated using the International Physical Activity Questionnaire (IPAQ) (Citation28). Individuals reported the frequency and duration of moderate and vigorous activity performed per week. Regular physical activity was defined as moderate activity ≥150 or vigorous activity ≥75 min per week, respectively. Height and weight were directly measured in a standardized way. BMI was calculated as weight in kilograms divided by height in meters squared. Body fat was assessed by bioelectrical impedance analysis using a validated device (BIA egofit, 2010, Eggstätt, Germany). Conventional blood pressure was measured three times in a sitting position after 5 min of rest. The mean of the second and third measurement was used for the current analysis. Resting heart rate (HR) was obtained using a 12-channel resting ECG (Schiller AG, Baar, Switzerland).
Statistical analysis
Baseline characteristics were stratified by sex. Data are presented as medians (interquartile ranges) for continuous variables and numbers (percentages) for dichotomous variables. Group comparisons were done using Wilcoxon’s rank sum tests or Chi-square tests as appropriate. Distribution of continuous variables was checked using skewness, kurtosis, and visual inspection of the histogram.
In order to assess the linearity of the relationships between HRV and inflammatory biomarkers, we evaluated SDNN, TP, HF, and LF measures across quartiles of individual inflammatory biomarkers in separate multivariable models. p Values for trend were calculated using quartile-specific medians. After confirming approximately linear relationships, we entered inflammatory biomarkers as continuous parameters in the multivariable models. Due to the skewed distribution, inflammatory variables were log-transformed for all analyses. To improve comparability across biomarkers, we calculated β-coefficients (95% confidence interval (CI)) per one standard deviation (SD) increase. In order to have a better comparability of the effect sizes, all HRV variables were transformed into z-scores. The multivariable models were adjusted for age, sex, BMI, smoking status, educational status, alcohol consumption, systolic blood pressure, prediabetes, fruit, and vegetable consumption, fish consumption, physical activity, LDL-C, HDL-C, triglycerides, ET-1, eGFR, family history of cardiovascular disease, and body fat. To assess whether HRV has an incremental effect beyond HR, we additionally adjusted all models for either resting or ambulatory HR. In a separate step, hs-CRP and LC were included in the same multivariable adjusted model, in order to assess whether they remain independently associated with HRV. Finally, we used resting and 24-h HR instead of HRV as the outcome variables to assess its relationship with inflammatory biomarkers.
Subgroup analyses included multivariable linear regression analyses stratified for sex, prediabetes, and BMI (<25, 25–29.9 and ≥30kg/m2). p Value for interaction was calculated using a multiplicative interaction term. Other sex-stratified analyses were performed for the relationships between resting and 24-h HR and inflammatory biomarkers. A p value < .05 was pre-defined to indicate statistical significance. Statistical analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline characteristics stratified by sex are presented in . The median age of the population was 36.9 years and 46.7% were men. Compared to women, men had a significantly higher BMI (median 25.6 versus 22.5 kg/m2, p < .0001), were more often current smokers (25.1 versus 19.1%, p < .0001), had higher systolic and diastolic blood pressure values, and a worse cholesterol profile (all p values <.0001). There were no significant differences for hs-CRP (0.9 versus 0.9 mg/l, p = .39) and total LC count (5.3 and 5.3 G/l, p = .38). Men had a significantly higher lymphocyte (1.8 versus 1.7 G/l, p = .004) and monocyte count (0.5 versus 0.4 G/l, p < .0001) and women had a higher neutrophil count (2.9 versus 2.8 G/l, p = .02). The SDNN, TP, and normalized LF were significantly higher in men than in women (156 versus 145 ms, 4096 versus 3060 ms2, and 57 versus 51 ms2, respectively; all p values < .0001). Normalized HF was significantly higher among women compared to men (16 versus 13 ms2, p < .0001).
Table 1. Baseline characteristics stratified by sex.
Relationships between heart rate variability and inflammatory biomarkers
Results of the multivariable linear regression analyses comparing SDNN values across quartiles of inflammatory parameters are shown in . There were significant inverse and linear associations of SDNN with hs-CRP, LC, neutrophils, lymphocytes, and monocytes after multivariable adjustment. Additional adjustment for either resting or ambulatory HR strongly attenuated these effect sizes, but all associations remained statistically significant (). The association between TP and inflammatory biomarkers was similar, except the not significant relationship with lymphocytes (Table S1). Normalized HF was inversely related to neutrophils and monocytes (Table S2). There was no relationship with hs-CRP and lymphocytes, and the association with total LC count was of borderline significance (p = .08). There were positive linear relationships of normalized LF with total LC count and neutrophils as well as an inverse relationship with hs-CRP (Table S3). The relationships of both LF and HF with inflammatory biomarkers were strongly attenuated after the additional adjustment for resting HR and mainly 24-h HR and most became non-significant (Tables S2 and S3).
Table 2. SDNN across quartiles of inflammatory biomarkers.
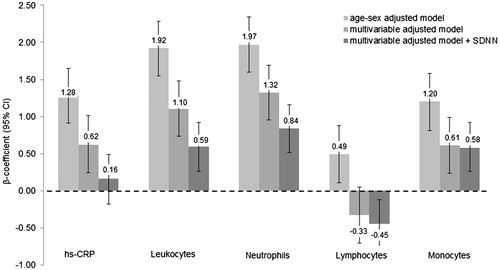
Results of the relationships between HRV variables and continuous inflammatory biomarkers were similar to these results and are presented in and S1. Per 1 SD increase in hs-CRP or LC, the SDNN (z-score) is decreasing by 0.11 (95% CI −0.14; −0.06), p < .0001 and 0.12 (−0.16; −0.08), p < .0001, respectively. After the adjustment for resting HR, the relationships with SDNN attenuated by 36% for hs-CRP, 25% for LC, 36% for neutrophils, 13% for monocytes. The additional adjustment for 24-h HR instead of resting HR attenuated these relationships even stronger (−36% for hs-CRP, −50% for LC, −63% for neutrophils, and −50% for monocytes), although all of them remained significant. The attenuation effect of the correlation between TP and inflammatory biomarkers was even stronger, such that only the relationship with hs-CRP remained significant (Figure S1). The multivariable adjusted β-coefficients for normalized HF and LF are shown in and . Again, all relationships were strongly attenuated after the adjustment for 24-h HR and nearly all of them lost level of significance. Including hs-CRP and LC in the same multivariable adjusted model, both of them remain inversely associated with HRV (Table S4).
Figure 1. Relationship between SDNN and inflammatory biomarkers. Data are presented as β-coefficients (95% confidence intervals) per 1 standard deviation increase. SDNN: standard deviation of all normal RR intervals; Hs-CRP: high-sensitivity C-reactive protein. Model 1 was adjusted for age, sex. Model 2 was additionally adjusted for body mass index, smoking status, educational status, alcohol consumption, fruit and vegetable consumption, fish consumption, systolic blood pressure, prediabetes, physical activity, low- and high-density lipoprotein cholesterol, triglycerides, endothelin-1, estimated glomerular filtration rate, family history of cardiovascular disease and body fat. Model 3 was additionally adjusted for resting heart rate. Model 4 was additionally adjusted for 24-h heart rate instead of resting HR. n = 2096.
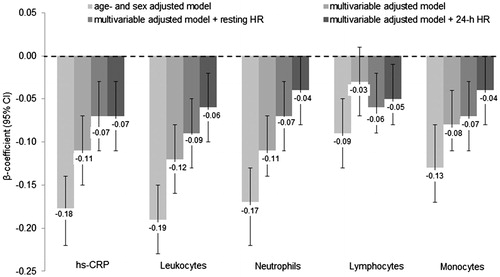
Figure 2. Relationship between normalized HF and inflammatory biomarkers. Data are presented as β-coefficients (95% confidence intervals) per 1 standard deviation increase. HF: high frequency; Hs-CRP: high-sensitivity C-reactive protein. Model 1 was adjusted for age, sex. Model 2 was additionally adjusted for body mass index, smoking status, educational status, alcohol consumption, fruit and vegetable consumption, fish consumption, systolic blood pressure, prediabetes, physical activity, low- and high-density lipoprotein cholesterol, triglycerides, endothelin-1, estimated glomerular filtration rate, family history of cardiovascular disease and body fat. Model 3 was additionally adjusted for resting heart rate. Model 4 was additionally adjusted for 24-h heart rate instead of resting HR. n = 2096.
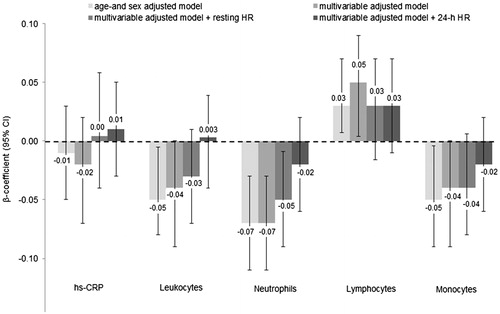
Figure 3. Relationship between normalized LF and inflammatory biomarkers. Data are presented as β-coefficients (95% confidence intervals) per 1 standard deviation increase. LF: low frequency; Hs-CRP: high-sensitivity C-reactive protein. Model 1 was adjusted for age, sex. Model 2 was additionally adjusted for body mass index, smoking status, educational status, alcohol consumption, fruit and vegetable consumption, fish consumption, systolic blood pressure, prediabetes, physical activity, low- and high-density lipoprotein cholesterol, triglycerides, endothelin-1, estimated glomerular filtration rate, family history of cardiovascular disease and body fat. Model 3 was additionally adjusted for resting heart rate. Model 4 was additionally adjusted for 24-h heart rate instead of resting HR. n = 2096.
Subgroup analyses were performed for sex, prediabetes and BMI (Table S5 and S6). A significant interaction was found for the relationship between SDNN, TP and neutrophil cell count, showing a stronger inverse association among men compared to women (both p for interaction = .002). None of the other subgroup analyses were significant.
Relationships between heart rate and inflammatory biomarkers
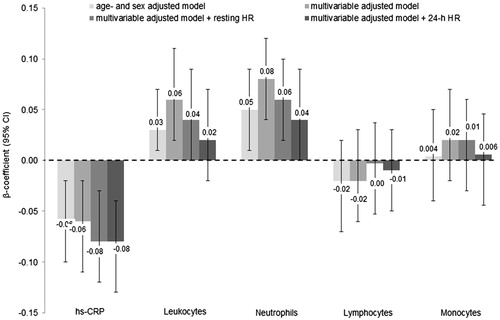
A positive and linear association was found for the association of ambulatory HR with hs-CRP, LC, neutrophils and monocytes (Table S7). Results of the relationships between resting and ambulatory HR with continuous inflammatory biomarkers are presented in and S2. Per one SD increase on the log-scale the multivariable adjusted β-coefficients (95%CI) for ambulatory HR were 0.62 (0.24; 1.01), p = .002 for hs-CRP, 1.10 (0.73; 1.48), p < .0001 for LC, 1.32 (0.95; 1.69), p < .0001 for neutrophils and 0.61 (0.23; 0.99), p = .002 for monocytes (). Additional adjustment for SDNN lowered the effect sizes of these relationships by 74% for hs-CRP, 47% for LC, 38% for neutrophils and 4.5% for monocytes, however with the exception of hs-CRP, all of them remained statistically significant. Lymphocyte count was not significantly associated with HR after multivariable adjustment. A significant sex-interaction was found for the associations of resting and ambulatory HR with neutrophils (p for interaction .02 and <.0001, respectively) with a stronger relationship among men compared to women (Table S8).
Figure 4. Relationship between ambulatory heart rate and inflammatory biomarkers. Data are presented as β-coefficients (95% confidence intervals) per 1 standard deviation increase. Hs-CRP: high-sensitivity C-reactive protein. Model 1 was adjusted for age, sex. Model 2 was additionally adjusted for body mass index, smoking status, educational status, alcohol consumption, fruit and vegetable consumption, fish consumption, systolic blood pressure, prediabetes, physical activity, low- and high-density lipoprotein cholesterol, triglycerides, endothelin-1, estimated glomerular filtration rate, family history of cardiovascular disease and body fat. Model 3 was additionally adjusted for the standard deviation of all normal RR intervals. n = 2096.
Discussion
In this large population-based study, we found strong and independent relationships of HR and HRV as measured by SDNN with a large set of inflammatory biomarkers, including hs-CRP, total LC count and several LC subpopulations. These relationships persisted after comprehensive multivariable adjustment but were strongly attenuated after additional adjustment for HRV or HR, while ambulatory HR has more strongly attenuated the relationships compared to resting HR. Although these results suggest that HR and HRV carry similar information with regard to their association with inflammatory biomarkers (Citation24), it is important to emphasize that most relationships remained statistically significant after mutual adjustment, suggesting that both parameters carry some incremental information over each other. Up to now, few if any studies have additionally adjusted the relationships for HR, even though it is suggested to take HR into account when investigating HRV (Citation24). Finally, TP and normalized HF and LF did not provide additional information over and above ambulatory HR alone. Therefore, further studies are needed to more accurately assess the additional information of individual HRV variables over and above resting and ambulatory HR.
Our findings confirm and expand prior studies showing negative associations of HRV with mainly hs-CRP (Citation16–19). Importantly, our results were additionally adjusted for HR, providing incremental information. The consistent results across various inflammatory biomarkers found in our study may also indicate a broad interplay between different inflammatory pathways and the ANS. These data are in line with experimental studies showing direct relationships between of the ANS and inflammatory cells (Citation14,Citation15). To our best knowledge this is one of the first studies from the general population showing the relationships of 24-h HRV and HR with not only hs-CRP but also total LC count and its subtypes. Thus, a link between HRV and inflammation may constitute one potential factor that might explain the increased cardiovascular risk previously shown for both entities (Citation2,Citation12). Inflammation is based on complex processes and various pathways are involved. Our findings showed that several inflammatory pathways seem to be involved in the relationship with HRV. Potential underlying mechanisms for these relationships are multifaceted. Norepinephrine, as the main sympathetic neurotransmitter, has been negatively associated with HRV and positively related to inflammatory parameters (Citation17). Experimental studies have shown expression of adrenoceptors on immune cells, suggesting a direct influence of the immune system by adrenoceptor agonists (Citation29–31). Additionally, other studies showed a reduced inflammatory reaction as a consequence of medical sympathectomy (Citation32,Citation33). Vagal activity seems to have an anti-inflammatory effect via a cholinergic pathway, which results in a down-regulation of pro-inflammatory cytokines (Citation14).
Some of the described mechanisms partly explain and support our findings. HF is mainly modulated by parasympathetic activity (Citation10,Citation34). An inverse relationship of HF with neutrophils and monocytes was found, assuming either an anti-inflammatory effect of vagal activity for those immune cells or an increased vagal activity as a consequence of low neutrophil and monocyte cell counts. In contrast, lymphocytes were positively related to HF. This finding could be supported by the results of a previous study showing a protective effect of lymphocytes and an adverse effect for neutrophils for the occurrence of cardiovascular events (Citation35,Citation36). Others have showed that inflammation could increase lymphocyte apoptosis, which may contribute to unfavorable outcomes (Citation37). SDNN was negatively associated with all inflammatory biomarkers, although the relationship with the lymphocyte cell count was weakest and reached only borderline significance. The association of lymphocyte cell count with different HRV variables should further be investigated to understand the underlying mechanisms of the relationship between lymphocytes and autonomic function.
Based on previous studies, resting and 24-h HR are known to be positively associated with hs-CRP, fibrinogen and interleukin, which is in line with our findings (Citation17–19,Citation22). Additionally, our data showed a positive association with total LC count, neutrophils and monocytes, but similar to HRV, no significant relationship with lymphocytes. Physical activity and other healthy lifestyle habits reduce HR (Citation38) and improve autonomic function, which may in consequence lower inflammatory biomarker levels. Others suggest that the protective effect of physical activity on inflammation is mediated by autonomic function (Citation39). This cross-sectional study cannot prove the above mentioned pathways, but nevertheless highlights the need for future studies to better understand the relationship between inflammation and the ANS.
A significant sex-interaction for the relationship between HRV, HR and neutrophil cell count was found, with stronger inverse associations among men compared to women. This sex-interaction might in part be reasoned by an influence of ovarian hormones on the immune system (Citation40). However, additional studies are needed to better investigate these sex differences. Although autonomic dysbalances might play an important role in the development of type 2 diabetes mellitus (Citation41), prediabetes was not a significant effect modifier in the relationship between HRV and inflammatory biomarkers.
Strengths and limitations
Major strengths of the present study are the population-based study design, the well-characterized study population, the enrollment of young and healthy participants with a relatively short exposure history to environmental factors and the availability of 24-h ECG data to quantify HRV over an individual’s normal day. However there are several potential limitations, which should be taken into account for the interpretation of our results. First, this is a cross-sectional analysis such that causality of the relationship between HRV and inflammatory biomarkers cannot be addressed. Second, we enrolled mainly Caucasians in our study and the generalizability of our results to other populations is uncertain. Third, as in any observational study, residual confounding could be an issue despite our comprehensive multivariable adjustment. Fourth, it was aimed to get a deeper understanding of the interrelationship between HRV and inflammatory biomarkers. Based on our analyses, risk prediction for future events is not possible. Fifth, it is unclear if variables, such as body fat mass or BMI are confounders or mediators of the observed relationships. If some mediators should have been included in our models, we would expect that the true relationships between inflammation, HR and HRV would be somewhat stronger. Sixth, since regular intake of statins might have an anti-inflammatory effect and few individuals of our study population are currently treated with statins, our results might be minimally influenced.
Conclusion
In this large population of young and healthy adults from the general population, we found strong and independent relationships of HR and HRV with a broad set of inflammatory biomarkers. Relationships between SDNN and inflammatory biomarkers were weakened but remained significant after additional adjustment for ambulatory HR, suggesting incremental information of HRV over HR. Thus, our findings suggest important interrelationships between inflammatory pathways and the ANS.
Rev_Ann_Med_Supplement_HRV_and_InflammationV1.docx
Download MS Word (40.8 KB)FigureS2.tif
Download TIFF Image (228.2 KB)FigureS1.tif
Download TIFF Image (264.1 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
The GAPP study was supported by the Liechtenstein Government, the Swiss Heart Foundation, the Swiss Society of Hypertension, the University of Basel, the University Hospital Basel, the Hanela Foundation, Schiller AG and Novartis. David Conen was supported by grants of the Swiss National Science Foundation [PP00P3_133681 and PP00P3_159322].
References
- Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38.
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40.
- Ahmadi-Abhari S, Luben RN, Wareham NJ, Khaw K-T. Seventeen year risk of all-cause and cause-specific mortality associated with C-reactive protein, fibrinogen and leukocyte count in men and women: the EPIC-Norfolk study. Eur J Epidemiol. 2013;28:541–50.
- O Hartaigh B, Bosch JA, Carroll D, Hemming K, Pilz S, Loerbroks A, et al. Evidence of a synergistic association between heart rate, inflammation, and cardiovascular mortality in patients undergoing coronary angiography. Eur Heart J. 2013;34:932–41.
- Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–6.
- Mora S, Rifai N, Buring JE, Ridker PM. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114:381–7.
- Boekholdt SM, Hack CE, Sandhu MS, Luben R, Bingham SA, Wareham NJ, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187:415–22.
- Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–9.
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72.
- Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65.
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–2.
- Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5.
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. Frequency domain measures of heart period variability to assess risk late after myocardial infarction. J Am Coll Cardiol. 1993;21:729–36.
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62.
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–4.
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–70.
- Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, et al. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156:759 e1–7.
- von Känel R, Carney RM, Zhao S, Whooley MA. Heart rate variability and biomarkers of systemic inflammation in patients with stable coronary heart disease: findings from the Heart and Soul Study. Clin Res Cardiol. 2011;100:241–7.
- Haarala A, Kähönen M, Eklund C, Jylhävä J, Koskinen T, Taittonen L, et al. Heart rate variability is independently associated with C-reactive protein but not with Serum amyloid A. The Cardiovascular Risk in Young Finns Study. Eur J Clin Invest. 2011;41:951–7.
- Saito I, Hitsumoto S, Maruyama K, Eguchi E, Kato T, Okamoto A, et al. Impact of heart rate variability on C-reactive protein concentrations in Japanese adult nonsmokers: the Toon Health Study. Atherosclerosis. 2016;244:79–85.
- Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med. 2009;265:439–47.
- Whelton SP, Narla V, Blaha MJ, Nasir K, Blumenthal RS, Jenny NS, et al. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;113:644–9.
- Johansen CD, Olsen RH, Pedersen LR, Kumarathurai P, Mouridsen MR, Binici Z, et al. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J. 2013;34:1732–9.
- Monfredi O, Lyashkov AE, Johnsen A-B, Inada S, Schneider H, Wang R, et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64:1334–43.
- Conen D, Schön T, Aeschbacher S, Paré G, Frehner W, Risch M, et al. Genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP). Swiss Med Wkly. 2013;143:w13728.
- Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–83.
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8–16.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
- Bellinger DL, Lorton D. Autonomic regulation of cellular immune function. Auton Neurosci. 2014;182:15–41.
- Ricci A, Bronzetti E, Conterno A, Greco S, Mulatero P, Schena M, et al. Alpha1-adrenergic receptor subtypes in human peripheral blood lymphocytes. Hypertension. 1999;33:708–12.
- Jänig W. Sympathetic nervous system and inflammation: a conceptual view. Auton Neurosci. 2014;182:4–14.
- Kasahara K, Tanaka S, Hamashima Y. Suppressed immune response to T-cell dependent antigen in chemically sympathectomized mice. Res Commun Chem Pathol Pharmacol. 1977;18:533–42.
- Xu L, Yu W-K, Lin Z-L, Tan S-J, Bai X-W, Ding K, et al. Chemical sympathectomy attenuates inflammation, glycocalyx shedding and coagulation disorders in rats with acute traumatic coagulopathy. Blood Coagul Fibrinolysis. 2015;26:152–60.
- Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–3.
- Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–43.
- Ó Hartaigh B, Bosch JA, Thomas GN, Lord JM, Pilz S, Loerbroks A, et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis. 2012;224:161–9.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
- Aeschbacher S, Bossard M, Ruperti Repilado FJ, Good N, Schoen T, Zimny M, et al. Healthy lifestyle and heart rate variability in young adults. Eur J Prev Cardiol. 2016;23:1037–44.
- Jae SY, Heffernan KS, Yoon ES, Lee M-K, Fernhall B, Park WH. The inverse association between cardiorespiratory fitness and C-reactive protein is mediated by autonomic function: a possible role of the cholinergic antiinflammatory pathway. Mol Med. 2009;15:291–6.
- Smith JM, Shen Z, Wira CR, Fanger MW, Shen L. Effects of menstrual cycle status and gender on human neutrophil phenotype. Am J Reprod Immunol. 2007;58:111–19.
- Jae SY, Kurl S, Laukkanen JA, Zaccardi F, Choi Y-H, Fernhall B, et al. Exercise heart rate reserve and recovery as predictors of incident type 2 diabetes. Am J Med. 2016;129:536.e7–e12.
