Abstract
Introduction: Low adiponectin levels may predict the development of atherosclerosis. We examined the association of childhood adiponectin with preclinical carotid atherosclerosis that is defined as plaque and/or high (≥95th percentile) intima-media thickness (IMT) at the carotid bifurcation in adulthood.
Methods: The Cardiovascular Risk in Young Finns Study is a cohort study on cardiovascular risk factors. We used risk factor data from the baseline study (1980) and ultrasound findings from the follow-ups (2001 and 2007). The study population included 1708 participants, aged 3–18 years at baseline.
Results: In multivariate analysis, childhood adiponectin was inversely associated with preclinical carotid atherosclerosis: odds ratio 0.68, 95% confidence interval (CI) 0.53–0.86, p = .001, for 1-SD increase in childhood adiponectin after adjusting for childhood non-high-density lipoprotein cholesterol, body mass index, and blood pressure. When examining the incremental predictive ability, we observed that compared to an approach utilizing only conventional risk factors, the model additionally including adiponectin levels improved c-statistics area under curve from 0.733 (95% Cl 0.694–0.771) to 0.748 (95% Cl 0.710–0.786), p = .02.
Conclusions: Childhood adiponectin levels improve the prediction of carotid atherosclerosis in adulthood over conventional risk factors. This supports the idea that low adiponectin levels may have a role in the development of preclinical atherosclerosis.
Childhood adiponectin levels improve the prediction of increased carotid intima-media thickness in adulthood over conventional cardiovascular risk factors.
These results suggest that adiponectin levels measured in childhood may have a role in the atherosclerotic process.
Key messages
Introduction
Adipose tissue dysregulation in obesity is thought in part to explain the connection between obesity and cardiovascular diseases (Citation1). Adipose tissue derived adipokines affect the development of atherosclerosis both directly by influencing endothelial function, vascular homeostasis, and atherogenesis and indirectly via insulin resistance (Citation2–4). Whether adipokines have importance in the prediction of cardiovascular events or in pharmacological treatment of cardiovascular diseases is controversial (Citation5,Citation6).
Adiponectin is involved in the inflammatory process and has been recognized as a marker of insulin sensitivity and glucose metabolism (Citation2). In contrast to most other adipokines, circulating adiponectin levels are negatively correlated with body mass index (BMI) (Citation7).
Experimental studies indicate that adiponectin may possess atheroprotective properties. In vitro, treatment of human monocyte-derived macrophages with adiponectin suppresses macrophage-to-foam cell transformation (Citation8). In apolipoprotein E-deficient mice, treatment with recombinant adenovirus expressing adiponectin has been shown to attenuate the progression of atherosclerotic lesions (Citation9). In humans, low adiponectin levels have been associated with increased risk of myocardial infarction (Citation10,Citation11).
Clinical complications of atherosclerotic diseases, such as coronary artery disease, stroke, and peripheral artery disease, occur mainly among elderly people. However, there is evidence indicating that the atherosclerotic process begins in childhood. Several studies have shown that conventional risk factors, such as serum lipids, blood pressure, cigarette smoking, and obesity identified in childhood track from childhood to adulthood (Citation12,Citation13). They are related with atherosclerotic lesions such as fatty streaks and fibrous plaques in young people (Citation14,Citation15) and predict increased carotid artery intima-media thickness (Citation16,Citation17) and decreased carotid artery elasticity in adulthood (Citation18). Nevertheless, conventional childhood risk factors explain subsequent cardiovascular risk incompletely. Therefore, identification of novel childhood risk factors would be helpful to better recognize young individuals at high risk of future atherosclerosis. These children might benefit from life-style counseling leading to improved cardiovascular health (Citation19,Citation20).
In our previous study, we found in cross-sectional analyses that low serum adiponectin levels were independently related with increased carotid intima-media thickness (IMT) and attenuated brachial flow-mediated dilatation in young adults (Citation21). Already in childhood, low-adiponectin levels are related with increased IMT, both in obese (Citation22,Citation23) and normal weight children (Citation24). To our knowledge, there are no prior publications concerning the longitudinal effects of childhood adiponectin levels on future atherosclerosis risk in addition to more established childhood risk factors.
Therefore, the main objective of the present study was to assess the role of childhood adiponectin as a novel pediatric risk factor for adult subclinical atherosclerosis. The analyses were based on Cardiovascular Risk in Young Finns Study including data on 1708 subjects aged 3–18 years at baseline (in 1980) followed-up for 27 years.
Materials and methods
Subjects and study design
Cardiovascular Risk in Young Finns Study included 3596 children and adolescents aged 3–18 years at original baseline in 1980 (Citation25). Five university hospital regions in Finland (Turku, Tampere, Helsinki, Kuopio and Oulu) participate in the study. The original cohort has been followed-up in repeated examinations between 3 and 6 year intervals. The present study included those participants who had adiponectin measurements done from the stored baseline serum samples (1980) and participated in the carotid ultrasound studies in the 2001 and 2007 follow-ups. A total of 1708 males and females were included in the present analyses. Subjects gave written informed consent and the study was approved by local ethics committees.
Biochemical assays
Venous blood samples were drawn after a 12-h fast. In adulthood (study years 2001 and 2007), serum total adiponectin was measured from serum stored in −70 °C. In 2013, childhood total adiponectin was measured from the serum samples taken in 1980 and stored in −20 °C. Serum total adiponectin concentrations were analyzed with a radioimmunoassay (Human Adiponectin RIA kit, Linco Research Inc, MO). The interassay coefficient of variation was 5.5–11.9%. Total cholesterol, high-density lipoprotein cholesterol (HDL cholesterol) and serum triglycerides were measured as described previously (Citation12). Low-density lipoprotein cholesterol (LDL cholesterol) was calculated using the Friedewald formula for participants with triglycerides <4 mmol/l. Non-HDL cholesterol was calculated as total cholesterol – HDL cholesterol. Childhood serum insulin was measured with immunoassay (Citation26).
Clinical measurements and questionnaires
Height, weight and waist circumference were measured (Citation25). Body mass index was calculated using the formula: weight [kg]/(height [m])2. Blood pressure was measured with a standard mercury sphygmomanometer in 1980 and with a random-zero sphygmomanometer (Hawksley & Sons Ltd, Lancin, UK) in 2001 and 2007. In 1980, blood pressure from 3-year-old children was measured with an ultrasound scanning device (Arteriosonde 1020, Roche, Branchburg, NJ). The average of three measurements was used in statistical analysis. Participants were also asked to complete questionnaires that included questions on smoking habits from the age of 12 years. Daily smokers were classified as smokers.
Carotid IMT
Ultrasound studies were performed using Sequoia 512 ultrasound mainframes (Acuson, CA) with 13.0 MHz linear array transducers (Citation17). Carotid IMT was measured from the carotid artery bifurcation (bulb region) and evident plaque lesions were documented (defined as distinct area of the carotid vessel wall protruding into the lumen >50% of the adjacent intima-media layer). All plaques were observed in the carotid bulb. As a marker of pre-clinical atherosclerosis, we defined a binary outcome variable as carotid IMT ≥95th percentile at the bifurcation (bulb) and/or plaque lesion evident in carotid scans either in 2001 or 2007. In addition to the binary outcome variable, a continuous variable carotid IMT was measured on the posterior (far) wall of the left common carotid artery. Three measurements were performed to derive maximal common carotid IMT. The digitally stored scans were manually analyzed by one reader blinded to subjects’ details. The between-visit coefficient of variation of IMT measurements was 6.4% and the intra-observer coefficient of variation was 3.4% in our laboratory. In statistical analyses, the mean value of maximal carotid IMT levels in 2001 and 2007 was used as a continuous outcome variable.
Statistical methods
The tracking of adiponectin from childhood to adulthood was estimated by calculating Spearman’s rank order correlation coefficients. Bivariate correlations between childhood adiponectin levels with BMI were examined with Spearman’s correlation analysis. To study whether childhood adiponectin level is an independent determinant of adult IMT, we used multivariate modeling. The effect of adiponectin was adjusted for childhood risk factors that have been shown to predict IMT in this cohort: sex, age, BMI, non-HDL cholesterol, and systolic blood pressure (Citation17). With the binary outcome variable, we used logistic regression modeling. With the continuous IMT outcome variable, a linear regression analysis was used. The incremental value of adding risk variables to predict the binary outcome (plaque and/or high bulb IMT) was examined based on multivariate logistic regression models. The ability of several models to predict binary IMT risk was estimated using C statistics by calculating the area under the receiver operating characteristic curve (AUC) and the integrated discrimination index. Model calibration was tested by the Hosmer–Lemeshow X2 test (Citation27). We also divided subjects in subgroups by having adiponectin levels below or above sex and age-specific median in childhood and adulthood and compared percentage of subjects with the binary outcome variable with logistic regression. All analyses were performed using Statistical Analysis System, SAS (version 9.4) (SAS Institute Inc., Cary, NC) and statistical significance was inferred at a 2-tailed p value <.05.
Results
Levels and tracking of childhood adiponectin levels
As shown in , girls had higher adiponectin levels than boys in all age groups except in 3-year-olds. In both sexes, there was an inverse age trend in adiponectin levels. Childhood adiponectin levels correlated with adiponectin levels in adulthood (1980 vs 2001, r = .53, p < .0001, 1980 vs 2007, r = .51, p < .0001). The 6-year tracking correlation for adiponectin levels between 2001 and 2007 was r = .77, p < .0001.
Table 1. Mean adiponectin levels (μg/mL) according to study year, age and sex.
The cross-sectional correlation of adiponectin with BMI was similar in childhood (in 1980, r = −.38, p < .0001) and adulthood (in 2007, r = −.35, p < .0001). The correlation of childhood adiponectin with adult BMI was significant, but weaker (r = −.12, p < .0001).
Childhood adiponectin and adult carotid IMT
Adiponectin levels in childhood were inversely associated with the binary IMT variable (n = 160). The age and sex adjusted odds ratio was 0.70 (CI 0.55–0.86) for one SD increase in childhood adiponectin level. This relationship remained similar (odds ratio 0.68, CI 0.53–0.86) after adjustments for conventional childhood risk factors (). The result remained similar when additionally adjusted for insulin levels in childhood, early life smoking and adiponectin levels in adulthood. We found no evidence for either age or sex interactions (interaction term p values > .6) suggesting that the association between adiponectin and adult IMT is not modified by age or sex. The result remained similar also when sex and age-specific adiponectin quartiles were used in the analysis instead of adiponectin level.
Table 2. Multivariable associations between childhood risk factors (in 1980) and adult carotid plaque.
When examining the incremental predictive ability, we observed that compared to an approach utilizing only conventional risk factors, the model additionally including childhood adiponectin levels improved AUC significantly from 0.733 (95% Cl 0.694–0.771) to 0.748 (95% Cl 0.710–0.786), p = .02. Similarly, the integrated discrimination index improved significantly (p < .0001). The Hosmer–Lemeshow goodness of fit test p values were non-significant for both models (p > .4) indicating that there was no evidence for poor fit.
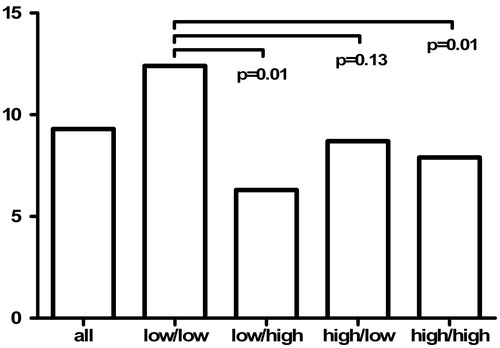
In subgroups, according to adiponectin level below or above sex and age specific median in childhood and/or adulthood, the percentage of subjects with plaque and/or high bulb IMT was highest in those subjects with adiponectin level below median in both childhood and adulthood ().
Figure 1. Subjects (%) with carotid IMT ≥95th percentile at the bifurcation (bulb) and/or plaque lesion evident in carotid scans in subgroups according to serum adiponectin level below (low) or above (high) sex and age-specific median in childhood/adulthood.
In bivariate analyses childhood adiponectin levels were significantly associated with the continuous adult IMT (β = −3.9, p < .0001). The association diluted, but remained statistically significant after adjustment for age and sex (β = −1.1, p = .009). In multivariate analyses adjusted for age, sex and conventional childhood risk factors, the effect of childhood adiponectin was not further diluted and remained statistically significant (). The result remained similar when sex and age specific adiponectin quartiles were used in the analysis instead of adiponectin level. The association became non-significant when additionally adjusted for adiponectin levels in adulthood (adiponectin 2001 in the model, β = −0.19 for childhood adiponectin, p = .70, β = −1.98 for adulthood adiponectin, p = .0009; adiponectin 2007 in the model, β = −.40 for childhood adiponectin, p = .41, β = −1.26 for adulthood adiponectin, p = .01).
Table 3. Multivariable associations between childhood risk factors (in 1980) and adult carotid IMT.
Discussion
Previous reports from The Cardiovascular Risk in Young Finns Study and other longitudinal cohorts have shown that several childhood risk factors are predictive of preclinical markers of atherosclerosis in adulthood (Citation16,Citation17,Citation28–30). Of different childhood variables, elevated blood pressure levels, overweight/obesity and dyslipidemia have been most consistently associated with subclinical atherosclerosis. In the present study, we observed that adiponectin levels measured in childhood were related with high carotid IMT in adulthood independent of these conventional risk factors. The effect of adiponectin was approximately comparable to that of non-HDL cholesterol, an established causal risk factor for atherosclerosis. When analyzing risk factors’ association with the binary adult outcome carotid plaque and/or abnormal bulb IMT, the addition of adiponectin data to prediction analyses improved the model performance statistically significantly.
We used two statistical measures, AUC and integrated discrimination index, to assess the performance of the risk prediction models. The AUC describes the overall performance of the model in discriminating individuals with and without the outcome. Pencina et al. (Citation31) have shown that the improvement in the AUC depends strongly on the baseline model. They found that when started with a poor model that had an AUC of 0.60, a new predictor with a strong effect (using effect sizes classified as weak-medium-strong), could raise the AUC by 0.13. The same predictor added to a very good baseline model with an AUC of 0.85 would raise that AUC by only 0.03. In our data, adding adiponectin levels to the baseline model increased AUC from 0.733 to 0.748 (dAUC = 0.015). This suggests that the effect size of adiponectin levels in our data could be considered as medium. Consistently, we found significant improvement in the integrated discrimination index indicating that the difference in average predicted risks between the individuals with and without the outcome increased significantly when adiponectin levels were included in the prediction model. A relatively small, statistically significant increase in AUC or improvement in the integrated discrimination index does not necessarily mean that childhood adiponectin level would prove to be a clinically significant tool for cardiovascular risk assessment. However, together they imply that including adiponectin level in the prediction model with established risk factors merits further research.
In the present analyses, we used two carotid IMT variables as markers of pre-clinical atherosclerosis. A binary variable was defined as a local plaque and/or increased (≥95%) IMT in the carotid bifurcation (bulb). In addition, we used common carotid IMT as a continuous variable. Adiponectin levels in childhood were significantly associated with both outcomes, and these associations were not diluted after adjustments for conventional risk factors. When adult adiponectin levels were taken into account, we observed that childhood adiponectin still had a residual independent effect on adult binary IMT outcome, whereas the association with the continuous outcome was diluted to non-significant. These results are open to two interpretations, not necessarily mutually exclusive: (Citation1) that exposure to low levels of adiponectin in childhood has a role in the initiation of atherosclerosis or (Citation2) that childhood adiponectin levels are strongly associated with adult adiponectin levels that have a more important role in the pathophysiology. Tracking of adiponectin from childhood to adulthood was similar to previously reported tracking of total, HDL and LDL cholesterol (Citation12,Citation32,Citation33), and higher than previously reported tracking of blood pressure and triglycerides (Citation32). Such high correlation between childhood and adulthood values may lead to multicollinearity that hampers the interpretation of statistical models. It is noteworthy, that the multivariable model explaining adult common carotid IMT only explained 18% of the variance in IMT in concordance with previous studies (Citation34). Thus, there evidently are other unknown factors influencing adult IMT. Nevertheless, the results were consistent and robust with both outcomes showing that adiponectin levels measured in childhood may have a role in the pathophysiology of atherosclerosis that is independent of conventional risk markers, including excess adiposity, adverse lipids, elevated blood pressure and high insulin.
The role of adiponectin in the pathophysiology of cardiovascular diseases is not fully established. Evidence from preclinical studies suggests that adiponectin is involved in many of the key events in the development of atherosclerosis. In vitro, adiponectin treatment increases nitric oxide production in bovine endothelial cells (Citation35). Preincubation with adiponectin inhibits tumor necrosis factor α induced monocyte adhesion to human aortic endothelial cells and adhesion molecule expression (Citation36). Furthermore, adiponectin suppresses the transformation of human monocyte-derived macrophages to foam cells (Citation8). In vivo, overexpression of adiponectin attenuates the progression of atherosclerotic lesion in apolipoprotein E (ApoE) knockout (KO) mice (Citation9), and compared to ApoE KO mice, ApoE/adiponectin double-KO mice show accelerated atherogenesis (Citation37).
In humans, the association between adiponectin and carotid IMT has been well established in all age groups. In pediatric populations, inverse correlation between adiponectin and IMT has previously been reported for obese juveniles (Citation22,Citation23). Recently, Jaakkola et al. reported a similar association in a cohort of >500 healthy adolescents (Citation24). In young adults, we previously reported a cross-sectional inverse association between adiponectin and IMT(21), and Lo et al. found a similar association in a population of 100 healthy women aged 24–59 years (Citation38). In middle-aged populations, there are several publications reporting association between low adiponectin levels and increased IMT (Citation39–41). In non-diabetic postmenopausal women, Störk et al. found an inverse association with baseline adiponectin level and IMT and age-adjusted adiponectin in the lowest quartile to be related to progression of IMT after 12 months (Citation42). In our previous study on young adults, 6-year change in adiponectin levels was inversely correlated with IMT progression (Citation43). Recent results from the longitudinal international multicenter IMPROVE study showed an independent association with adiponectin levels and baseline mean bifurcation IMT and progression of mean carotid IMT in men but not in women. Moreover, a gene score of adiponectin-raising alleles was inversely associated with baseline mean bifurcation IMT in men (Citation44).
More controversial results have been seen in studies relating adiponectin levels with clinical end-points. There are studies suggesting low adiponectin levels to be associated with increased risk of coronary artery disease prevalence (Citation45) and myocardial infarction in men (Citation11,Citation46), as well as studies reporting no associations (Citation47,Citation48). In 2013, three meta-analysis on adiponectin’s connection with cardiovascular events were published. Hao et al. (Citation49) analyzed 17 prospective studies on association of adiponectin levels with coronary heart disease and stroke. They found increased adiponectin levels to be related with an elevated risk of ischemic stroke, but no clear relationship between adiponectin levels and the risk of coronary heart disease. On the other hand, Zhang et al. (Citation50) included 12 prospective studies and found higher levels of adiponectin to associate with lower risk of coronary heart disease. The meta-analysis by Kanhai et al. (Citation51) included 14 studies on coronary heart disease and 2 studies on stroke. In contrast to the previous meta-analyses, Kanhai et al. found no association between adiponectin levels and risk for future coronary heart disease or stroke events. Thus, although it has been consistently reported that low-adiponectin level is a risk factor for preclinical atherosclerosis in young populations, the connection between adiponectin levels and cardiovascular events in middle-aged and elderly populations remains controversial.
Limitations
Adipose tissue secretes hundreds of proteins affecting inflammation, immune system, satiety, and energy expenditure, glucose metabolism, and insulin sensitivity as well as heart and vasculature (Citation6). In these analyses, we used only adiponectin levels to represent adipose tissue secretion. It is clear, that actions of adiponectin on vasculature are modified by proinflammatory adipokines, of which we have no data. Moreover, there are other adipokines thought to have positive effects on vasculature, such as adipolin and omentin-1 (Citation52). The relationship between adiponectin levels and pre-clinical atherosclerosis is only a part of the complex interaction between adipose tissue and vasculature (Citation1).
We analyzed childhood total adiponectin levels from serum samples that had been collected in 1980 and stored for 32–33 years in −20 °C. During long-term storage, protein levels may be reduced as a result of proteolysis and aggregation. However, the adiponectin levels from 1980 in the older age groups are very similar to those of the younger age groups in 2001, suggesting no major inaccuracy in childhood adiponectin measurements. Higher adiponectin levels for boys in the youngest age group are in concordance with previous data from Chile describing adiponectin levels in 1- to 2-year-old children (Citation53). The inverse age trend and lower adiponectin levels in older boys compared to girls are also similar to previous reports of adiponectin levels according to pubertal development in German (Citation54) and Danish populations (Citation55). In this study population, 90% of the children were prepubertal at the age of 9 years. The difference in adiponectin levels during and after puberty was related to serum androgen levels in previous studies (Citation54,Citation55). Similar correlation of adiponectin and BMI in childhood and adulthood, as well as strong tracking correlations between childhood and adulthood adiponectin values also support the validity of our data. A Norwegian group studying colorectal cancer found no influence by prolonged sample storage in −25 °C on the association of adiponectin with colorectal cancer, based on similar mean adiponectin concentrations and risk estimates in analyses stratified by median storage time of 28.7 years (Citation56). Thus, although the stability of adiponectin in serum samples stored long-term is unknown, we consider it to be unlikely that our results were substantially influenced by sample degradation.
Adiponectin circulates in three different isoforms: low molecular weight (trimer), medium molecular weight (hexamer), and high molecular weight (multimeric) form (Citation57). Total adiponectin levels and high molecular weight form levels have both been used in epidemiological studies. The three isoforms may have different biological effects due to differences in binding to their receptors (Citation57) and some studies have suggested low levels of especially high molecular weight adiponectin to be associated with coronary artery disease (Citation58). Since, we only measured total adiponectin levels, our results do not provide any information on the effect of the different isoforms on pre-clinical atherosclerosis.
Our study cohort is comprised of racially homogenous young adults. Therefore, the generalizability of our results is limited to white European populations. Our cohort is without clinical manifestations of cardiovascular disease and therefore we are not able to study associations between risk factors and cardiovascular events. However, because this is a well-established ongoing cohort study, in the future we will have the potential to evaluate whether subjects with low-adiponectin levels subsequently develop premature cardiovascular events. The strength of this study is the large randomly selected cohort of young men and women with comprehensive cardiovascular risk factor data from childhood to adulthood.
There is an evident need to identify individuals at high cardiovascular risk early in childhood in order to reduce cardiovascular morbidity. Childhood adiponectin improves prediction of high adult carotid IMT over childhood conventional risk factors. Measuring adiponectin might help in identifying young individuals at high risk for later atherosclerosis – these results should be replicated in other populations.
Disclosure statement
The authors report no conflicts of interest.
References
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
- Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16.
- Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. 2013;227:216–21.
- Carbone F, Mach F, Montecucco F. The role of adipocytokines in atherogenesis and atheroprogression. Curr Drug Targets. 2015;16:295–320.
- Ouchi N. Adipocytokines in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2016;23:645–54.
- Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metab Clin Exp 2015;64:131–45.
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83.
- Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–63.
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70.
- Persson J, Lindberg K, Gustafsson TP, Eriksson P, Paulsson-Berne G, Lundman P. Low plasma adiponectin concentration is associated with myocardial infarction in young individuals. J Intern Med. 2010;268:194–205.
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7.
- Porkka KV, Viikari JS, Taimela S, Dahl M, Åkerblom HK. Tracking and predictiveness of serum lipid and lipoprotein measurements in childhood: a 12-year follow-up. The Cardiovascular Risk in Young Finns study. Am J Epidemiol. 1994;140:1096–110.
- Clarke WR, Schrott HG, Leaverton PE, Connor WE, Lauer RM. Tracking of blood lipids and blood pressures in school age children: the Muscatine study. Circulation. 1978;58:626–34.
- Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. New Engl J Med. 1998;338:1650–6.
- McGill HC, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, et al. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The pathobiological determinants of atherosclerosis in youth (PDAY) research group. Arterioscler Thromb Vasc Biol. 2000;20:1998–2004.
- Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–19.
- Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83.
- Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–93.
- Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Rönnemaa T, Niinikoski H, et al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the special Turku coronary risk factor intervention project for children [STRIP] study). Circulation. 2013;127:2088–96.
- Nupponen M, Pahkala K, Juonala M, Magnussen CG, Niinikoski H, Rönnemaa T, et al. Metabolic syndrome from adolescence to early adulthood: effect of infancy-onset dietary counseling of low saturated fat: the special Turku coronary risk factor intervention project (STRIP). Circulation. 2015;131:605–13.
- Saarikoski LA, Huupponen RK, Viikari JS, Marniemi J, Juonala M, Kähönen M, et al. Adiponectin is related with carotid artery intima-media thickness and brachial flow-mediated dilatation in young adults-the cardiovascular risk in Young Finns Study. Ann Med. 2010;42:603–11.
- Pilz S, Horejsi R, Moller R, Almer G, Scharnagl H, Stojakovic T, et al. Early atherosclerosis in obese juveniles is associated with low-serum levels of adiponectin. J Clin Endocrinol Metab. 2005;90:4792–6.
- Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92:3025–32.
- Jaakkola JM, Pahkala K, Viitala M, Rönnemaa T, Viikari J, Niinikoski H, et al. Association of adiponectin with adolescent cardiovascular health in a dietary intervention study. J Pediatr. 2015;167:353–60 e1.
- Åkerblom HK, Viikari J, Uhari M, Räsänen L, Byckling T, Louhivuori K, et al. Atherosclerosis precursors in Finnish children and adolescents. I. General description of the cross-sectional study of 1980, and an account of the children’s and families’ state of health. Acta Paed Scand Suppl. 1985;318:49–63.
- Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–84.
- Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. discussion 207-12.
- Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–6.
- Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122:2514–20.
- Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–85.
- Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–81.
- Juhola J, Magnussen CG, Viikari JS, Kähönen M, Hutri-Kähönen N, Jula A, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90.
- Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133:884–99.
- Rundek T, Blanton SH, Bartels S, Dong C, Raval A, Demmer RT, et al. Traditional risk factors are not major contributors to the variance in carotid intima-media thickness. Stroke. 2013;44:2101–8.
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6.
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6.
- Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–25.
- Lo J, Dolan SE, Kanter JR, Hemphill LC, Connelly JM, Lees RS, et al. Effects of obesity, body composition, and adiponectin on carotid intima-media thickness in healthy women. J Clin Endocrinol Metab. 2006;91:1677–82.
- Behre CJ, Brohall G, Hulthe J, Wikstrand J, Fagerberg B. Are serum adiponectin concentrations in a population sample of 64-year-old Caucasian women with varying glucose tolerance associated with ultrasound-assessed atherosclerosis? J Intern Med. 2006;260:238–44.
- Dullaart RP, de Vries R, van Tol A, Sluiter WJ. Lower plasma adiponectin is a marker of increased intima-media thickness associated with type 2 diabetes mellitus and with male gender. Eur J Endocrinol. 2007;156:387–94.
- Gardener H, Sjoberg C, Crisby M, Goldberg R, Mendez A, Wright CB, et al. Adiponectin and carotid intima-media thickness in the northern Manhattan study. Stroke. 2012;43:1123–5.
- Störk S, Bots ML, Angerer P, von Schacky C, Grobbee DE, Angermann CE, et al. Low levels of adiponectin predict worsening of arterial morphology and function. Atherosclerosis. 2007;194:e147–53.
- Juonala M, Saarikoski LA, Viikari JS, Oikonen M, Lehtimäki T, Lyytikäinen LP, et al. A longitudinal analysis on associations of adiponectin levels with metabolic syndrome and carotid artery intima-media thickness. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2011;217:234–9.
- Persson J, Strawbridge RJ, McLeod O, Gertow K, Silveira A, Baldassarre D, et al. Sex-Specific Effects of Adiponectin on Carotid Intima-Media Thickness and Incident Cardiovascular Disease. J Am Heart Assoc. 2015;4:e001853
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9.
- Ai M, Otokozawa S, Asztalos BF, White CC, Cupples LA, Nakajima K, et al. Adiponectin: an independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis. 2011;217:543–8.
- Lindsay RS, Resnick HE, Zhu J, Tun ML, Howard BV, Zhang Y, et al. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–16.
- Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–9.
- Hao G, Li W, Guo R, Yang JG, Wang Y, Tian Y, et al. Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis. 2013;228:29–35.
- Zhang H, Mo X, Hao Y, Huang J, Lu X, Cao J, et al. Adiponectin levels and risk of coronary heart disease: a meta-analysis of prospective studies. Am J Med Sci. 2013;345:455–61.
- Kanhai DA, Kranendonk ME, Uiterwaal CS, van der Graaf Y, Kappelle LJ, Visseren FL. Adiponectin and incident coronary heart disease and stroke. A systematic review and meta-analysis of prospective studies. Obes Rev. 2013;14:555–67.
- Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25:348–55.
- Iniguez G, Soto N, Avila A, Salazar T, Ong K, Dunger D, et al. Adiponectin levels in the first two years of life in a prospective cohort: relations with weight gain, leptin levels and insulin sensitivity. J Clin Endocrinol Metab. 2004;89:5500–3.
- Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–61.
- Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007;92:1857–62.
- Lukanova A, Söderberg S, Kaaks R, Jellum E, Stattin P. Serum adiponectin is not associated with risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:401–2.
- Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100.
- Empana JP. Adiponectin isoforms and cardiovascular disease: the epidemiological evidence has just begun. Eur Heart J. 2008;29:1221–3.
