Abstract
Background: Chronic lymphocytic leukemia (CLL) is characterized by a heterogeneous clinical course, ranging from stable to more aggressive disease. Herein, we determined the prognostic significance of serum C-reactive protein (CRP) levels in patients with CLL
Methods: A retrospective cohort study reviewing the records of 107 consecutive treatment naïve patients with CLL and a control group comprised of apparently healthy individuals attending for periodic health examinations.
Results: The median CRP level of patients with CLL was 0.19 mg/dL (0–2.9). In univariate analysis, high-CRP levels (≥0.4 mg/dL) were significantly associated with an increased risk of mortality (HR = 3.97, 95%CI 1.64–9.62, p = .002) and development of second solid cancers (HR = 4.54, 95%CI 1.57–13.11, p = .005), compared to low-CRP values (<0.4 mg/dL). In multivariate analysis, high-CRP retained statistical significance for all-cause mortality (HR = 2.81, 95%CI 1.04–7.57, p = .04) and the development of second solid malignancies (HR = 4.54, 95%CI 1.57–13.11, p = .005). Moreover, when compared to an apparently healthy population, CLL patients with high CRP levels had more than an eightfold risk of cancer.
Conclusions: Elevated baseline CRP levels are associated with shorter survival and development of second cancers in patients with CLL. We suggest that increased CRP in patients with CLL may justify a more rigorous search for second cancers.
Elevated CRP levels are associated with a shorter overall survival in CLL.
Elevated CRP levels are associated with an increased risk of second cancers in CLL.
Increased CRP in patients with CLL may justify a more rigorous search for second cancers.
KEY MESSAGES
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world, affecting mostly elderly patients with a median age of 72 years (Citation1). It is characterized by progressive accumulation of mature B-cells in the peripheral blood, bone marrow, lymph nodes, and spleen (Citation1). CLL generally has a relatively benign clinical course and treatment is only indicated when patients are symptomatic or have rapid progression of the disease (Citation2).
C-reactive protein (CRP) is a classical acute phase protein, produced by hepatocytes in response to inflammatory cytokines, particularly interleukin-6 (IL6) (Citation3). Plasma CRP is a sensitive, inexpensive and routinely available biomarker used for clinical diagnosis of acute and chronic inflammation. Over the years, its clinical use has been broadened to include prediction and risk stratification of cardiovascular disorders and monitoring response to treatment of infection and inflammation. Circulating CRP levels are often elevated in patients with cancer (Citation4), and appear to be associated with an increased risk of developing cancer of any type (Citation5). However, the exact causal role of CRP in neoplasia has not been clearly defined (Citation6). Serum CRP levels are also commonly elevated in a variety of lymphoproliferative disorders, including non-Hodgkin’s and Hodgkin’s lymphoma (Citation7–16). Higher levels are common in more aggressive histological subtypes of lymphoma, in patients with B-symptoms, advanced stage, bulky disease and high International Prognostic Index scores and have been shown to correlate with survival (Citation9–16).
In this study, we compared the predictive value of CRP relative to other readily available “bedside” clinical and laboratory parameters and show that in CLL, high levels are associated with shorter survival and an increased risk for developing future solid cancers.
Patients and methods
Patients and study design
In this retrospective cohort study, we reviewed the records of consecutive patients with treatment naïve CLL that have been followed in our hematology institute from July 2007 to May 2015. The Tel Aviv Sourasky Medical Center is a busy tertiary, university hospital in Tel Aviv, Israel. All the patients were diagnosed according to the 2008 International Workshop on Chronic lymphocytic Leukemia (IWCLL) criteria (Citation2). Patients who received any prior anti-leukemic therapy or had an infection at the time of CRP measurement, were excluded from this study.
Data collection
Clinical and laboratory data was collected from medical records after approval by the local institutional Helsinki ethics committee. The data included; age, gender, date of CLL diagnosis, complete blood count including the absolute lymphocyte count (ALC), hemoglobin and platelet count (the latter two were incorporated into the disease staging), Binet clinical stage, date of first treatment, diagnosis of second malignant neoplasms, and date and cause of death. Within 3 months of the CLL diagnosis, serum CRP was quantitatively determined by means of particle-enhanced immunonephelometry using BN II System (Siemens, Marburg, Germany).
Controls
The control cohort was chosen from the data collected at the Tel-Aviv Medical Center Inflammation Survey (TAMCIS), a registered data bank of the Israeli Ministry of Justice (Citation17,Citation18). This is a relatively large survey comprising of apparently healthy individuals attending a center for periodic health examinations. Subjects attending the center for a routine health examination between September 2002 and June 2014, were invited to participate in the TAMCIS. All the individuals who were enrolled were recruited during their routine annual health check-up and gave their written consent in accordance with the guidelines of the institutional ethics committee. A total of 19,385 subjects gave their informed consent (12,272 males, 7113 females). Subjects who did not have a CRP measurement (n = 204) or had a previous history of cancer (n = 564) were excluded from the analysis. Overall, the control cohort consisted of 18,617 subjects. CRP was determined by the Behring BN II Nephelometer (DADE Behring, Marburg, Germany). Data on cancer diagnosis was retrieved from The Israel National Cancer Registry (INCR).
Outcome assessment
Overall survival (OS) was calculated from the date of CLL diagnosis to the date of death from any cause or last follow-up. Time to second cancer was calculated from the date of CLL diagnosis to the diagnosis of a new non-hematological malignancy. Time to treatment was calculated from the date of CLL diagnosis to the date of the first anti-leukemic therapy.
Statistics
Categorical variables are reported as frequency and percentages. Continuous variables were tested for normal distribution using Histogram and Q-Q plot. Normally distributed continuous variables are reported as means and standard deviations (SD) and non-normally distributed continuous variables are reported as medians and ranges. The correlation between continuous variables at baseline was evaluated using Spearman’s correlation coefficient and Mann–Whitney test was used to compare continuous variables between categories. Linear mixed model was employed to evaluate differences in the CRP level over time. Chi-squared automatic interaction detection (CHAID) analysis was used to determine a threshold value for CRP and ALC, according to the mortality status. A Kaplan–Meier plot was used to describe the mortality between categories and the log-rank test was applied to compare between them. Median follow-up time was obtained using the reverse censoring method. Univariate and multivariate Cox regression were used to evaluate the association between mortality and potential predictors. Backward stepwise likelihood ratio method was used for variables selection. The same methods were applied for analyzing new solid malignancy and starting treatment. A two-tailed p < .05 were considered statistically significant. Analyses were performed with SPSS version 22 (Armonk, NY).
Results
Patient characteristics
A total of 107 CLL patients with CLL were reviewed (). More than half of the patients were males (n = 56, 52.5%), with a mean age of 71.8 years. Ninety-three (84.1%) had Binet stage A disease, 6 (5.6%) were stage B, and 8 (7.5%) had Binet stage C. The median ALC and the median CRP levels were 10.4 × 109/L (5.1–200) and 0.19 mg/dL (0–2.86), respectively. During the study follow-up period, serial CRP measurements were available for 77 patients, with a total of 427 cumulative measures. There was no statistically significant difference in the CRP level over time (p = .14). The median follow-up for the entire cohort was 47.6 months (range, 1–93 months). Neither of the above baseline parameters including age, gender, ALC, and Binet stage had a statistically significant correlation with the CRP at the time of diagnosis.
Table 1. Patient characteristics.
Mortality
Overall, there were 20 deaths during the study period. The median OS for the entire cohort was not reached. The most common causes of death were second malignancies (n = 6, 30.0%) and infectious complications (n = 6, 30.0%). Two patients (10%) died due to progressive CLL, while another six deaths were related to a variety of causes, including two sudden deaths, one case of acute renal failure, one therapy-related complication, and two remained unknown.
In univariate analysis, the baseline parameters of age, ALC (≥15 × 109/μL), Binet stage, and CRP levels, were all significantly associated with OS (). The median baseline CRP level in patients who died was 0.43 mg/dL (0–2.86) vs. 0.19 mg/dL (0–0.32) in those alive. When analyzed as a continuous variable, increasing CRP levels predicted for a greater risk of death (HR = 2.42, 95%CI 1.13–5.18, p = .02). By categorizing CRP into two subgroups, according to the model detailed in the material and methods section, CRP levels greater than or equal to 0.4 mg/dL best correlated with a decreased median OS (60.7 months vs. not reached for CRP ≥0.4 mg/dL and <0.4 mg/dL, respectively, p = .001, ). As expected, the median OS was reduced in patients with advanced Binet stages C and B compared to early Binet stage A patients (44 months vs. not reached, respectively, p = .007).
Figure 1. (A) Kaplan–Meier curves of OS, (B) Cumulative incidence risk of developing solid malignancies, and (C) Kaplan–Meier curves of time to anti-leukemic treatment; subgrouped according to baseline CRP levels of <0.4 mg/dL and ≥0.4 mg/dL.
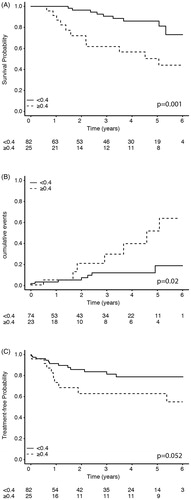
Figure 2. The cumulative incidence risk of developing non-hematological cancers in patients with CLL and apparently healthy individuals, stratified by serum CRP levels (<0.4 mg/dL and ≥0.4 mg/dL).
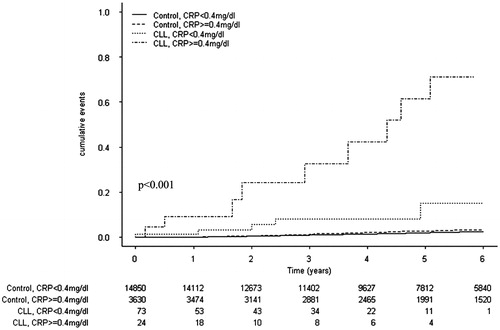
Table 2. Univariate and multivariate analyses of OS, second cancers, and treatment in patients with CLL.
In multivariate analysis, only age, ALC, Binet stage, and CRP levels retained statistical significance for OS (). CRP levels were associated with almost a threefold increase in HR for all-cause mortality (HR = 2.81 95%CI 1.04–7.57, p = .041), while age had an HR of 1.11 per year (95%CI 1.04–1.17, p = .001), ALC (≥15 × 109/μL) had an HR of 2.95 (95%CI 1.01–8.68, p = .049), while the HR for Binet stages B + C was 3.54 (95%CI 1.30–10.22, p = .019).
Non-hematological cancers
Of the entire cohort, 10 patients with CLL (9.3%) had a diagnosis of a solid malignancy (excluding cutaneous basal carcinoma), before the beginning of the study follow-up. In the remaining patients (n = 97), a new solid malignancy (excluding cutaneous basal carcinoma) developed in 15 patients (14.0%) including; 3 squamous cell carcinomas of the skin (one was metastatic), 2 prostate cancers, 2 melanomas, 1 lung cancer, 1 metastatic transitional cell carcinoma of the urinary bladder, 1 metastatic breast cancer, 1 metastatic pancreas carcinoma, 1 renal cell carcinoma, 1 rectal carcinoma, 1 metastatic endometrial cancer and 1 malignant glioma of the brain. The median baseline CRP level of patients without second malignancy was 0.16md/dL (0–2.86) compared to 0.28 mg/dL (0–1.04) in those with malignancy before the diagnosis of CLL and 0.44 mg/dL (0–1.9) in with second cancers occurring after initial diagnosis of CLL (p = .02).
We also focused further on the latter group of patients without malignancy at the time of initial CRP measurement. In univariate analysis, the baseline parameters of age, gender, ALC (≥15 × 109/μL), and Binet stage were not associated with the future development of a new solid tumor (). However, serum CRP concentrations 0.4 mg/dL or more were associated with an increased risk of developing a second cancer compared to lower levels (). Increased serum CRP levels retained statistical significance in multivariate analysis, having more than a fourfold increase in HR for the development of a new solid tumor (HR = 4.54, 95%CI 1.57–13.11, p = .005) and were also associated with a shorter median time to diagnosis of the second tumor (55 vs. 73 months, for CRP ≥0.4 mg/dL and <0.4 mg/dL, respectively and p = .02, ). Furthermore, reanalysis of the entire cohort revealed similar findings, namely that CRP ≥0.4 mg/dL was associated with an increased risk of developing a second cancer (HR = 3.64, 95%CI 1.31–10.03, p = .008).
We also evaluated the risk of developing cancer in patients with CLL who had high CRP levels compared to the control group. The serum CRP levels of CLL patients were similar to those seen in controls [median and the range was 0.19 (0–2.86) and 1.44 (0.7–3.27)], respectively, p = .598). During a median follow-up period of 63 months, 406 (2.0%) individuals in the control group developed cancer, which was less than that evident in the CLL group (HR 17.13, 95%CI 10.02–28.77). In univariate analysis, the risk for the development of cancer was increased in the controls with CRP ≥0.4 mg/dL (HR 1.33, 95%CI 1.06–1.68, p = .015), in CLL patients with CRP <0.4 mg/dL (HR 9.34, 95%CI 4.15–20.99, p < .001) and CLL patients with CRP ≥0.4 mg/dL (HR 50.55, 95%CI 25.99–98.33, p < .001), compared to the controls with CRP <0.4 mg/dL (). Adjustment for age performed using Cox-regression, showed that age increased the risk of future cancer (HR = 1.073, 95%CI 1.062–1.083, p < .001), while the association for CLL and CRP ≥0.4 mg/dL remained statistically significant (HR = 8.62, 95%CI 4.63–17.46, p < .001) ().
Table 3. Multivariate Cox regression analysis, time to second cancer diagnosis.
Taking into consideration the known link between immunosuppression induced by anti-CLL therapies, particularly fludarabine, and the development of second cancers, we further examined the possibility of such an association in regard to baseline CRP levels. Of the 15 patients who developed second cancers, six had received treatment for CLL. In one case a second cancer was diagnosed before the initiation of therapy while in five patients this was evident after anti-leukemic therapy. The latter included three patients who had received fludarabine, cyclophosphamide, and rituximab (FCR) and two treated with chlorambucil. None of the fludarabine-treated patients had a baseline CRP level ≥0.4 mg/dL.
Treatment for CLL
During the follow-up period, CLL progressed in 21 patients (19.6%), and treatment had to be initiated; 11 patients with chlorambucil, 9 patients with FCR, and a single patient with benadamustine and rituximab. The median time to first treatment for the entire cohort was not reached during the study follow-up period. Patients with higher ALC (≥15 × 109/μL) had a greater risk for subsequent treatment (HR = 3.60, 95%CI 1.47–8.61, p = .005) and those with advanced Binet stages (B and C) had more than a sixfold increase in HR for requiring future treatment (HR = 6.73, 95% CI 2.74–16.55, p < .001). The median baseline CRP level of patients not needing therapy was 0.19 mg/dL (0–1.9) compared to 0.25 mg/dL (0–2.86) in those who eventually required treatment. Overtime, the CRP level increased by 0.1mg/dL per year in patients eventually requiring therapy (p = .007). Serum CRP levels equal to or more than 0.4 mg/dL showed only a trend with for the need of treatment compared to lower CRP values (p = .052, ). Both age and gender were not associated with the need for initiation of treatment ().
In a multivariate analysis, including age, gender, ALC, Binet stage, and CRP (), only ALC and Binet retained statistical significance (HR = 2.75, 95%CI 1.11–6.81, p = .028 for ALC ≥15 × 109/μL and HR = 5.33, 95%CI 2.11–13.43, p < .001 for Binet stages B and C).
Discussion
In this study, we demonstrate that baseline CRP levels at the time of diagnosis of CLL are independently associated with an increased risk of death. CRP remained a significant predictor of early mortality even after adjustment for multiple, “bedside” risk factors, including age, gender, ALC, and Binet stage. In our CLL cohort, the major causes of death were due to malignancies and infections. Generally, it is well recognized that the most common causes of death in CLL include progressive disease, infectious complications and malignancies (Citation19). CRP is an inflammatory acute phase protein produced by hepatocytes, and mostly under transcriptional control by the cytokine IL6 (Citation3). In accordance with our results, serum IL6 has previously been shown to be an independent factor in predicting survival in CLL (Citation20,Citation21).
The median serum CRP level in CLL was relatively low compared to values recorded in other malignancies, and is in fact not different than the value recorded for our controls of apparently healthy population. In CLL, serum CRP concentration did not correlate with other disease baseline parameters and was not an independent risk factor for future anti-leukemic therapy. Unlike lymphomas, especially the more aggressive subtypes, where CRP is markedly increased and linked to poor prognostic parameters (like advanced stage, bulky disease, and presence of B-symptoms), elevated CRP in CLL appears to be less related to the malignancy itself and more obviously associated with other findings, such as the risk of developing second cancers.
In our cohort, approximately 9% of the patients had a solid cancer prior to CLL diagnosis, while an additional 14% developed a solid malignancy during follow-up. Baseline circulating CRP levels equal to or greater than 0.4 mg/dL increased the risk of developing future solid tumors by more than fourfold. Importantly, CRP ≥0.4 mg/dL increased the risk of cancer in CLL patients by more than six to eightfold compared to the control group of apparently healthy individuals with either increased or low CRP levels. Previous large prospective studies in the general population showed that an elevated CRP serves as a risk factor for developing a malignancy, especially lung and colorectal cancers (Citation5,Citation22,Citation23), and the risk of developing a second cancer in patients with CLL is higher than that of the general population (Citation24,Citation25). In large-scale studies, development of another cancer after the diagnosis of CLL occurred in 14.8–23% of the patients during a follow-up period of 5.3–6.4 years (Citation26,Citation27). This increased incidence of second cancers may partly be explained by the immune dysfunction accompanying the CLL itself or to the immunosuppression induced by anti-CLL therapies and in particularly purine analogs used for treatment, particularly fludarabine (Citation28,Citation29).
In our series, the treatment rate for CLL stratified according to CRP levels, only differed marginally and was mostly between the first and second years after CLL diagnosis (), while the incidence rate of new solid malignancies diverged later after two years from CLL diagnosis (). Although the numbers are small, our data in patients with high CRP do not appear to support a specific causal role of anti-leukemic therapies in the development of second malignancies in this group. In this respect, only two patients with high CRP developed second malignancies after treatment of CLL These two patients had been treated with chlorambucil, which is only weakly immunosuppressive compared to fludarabine (Citation30). Among the patients treated with fludarabine-based regimens and developed a second solid cancer, none had an increased baseline CRP level prior to the treatment.
The predictive value of CRP for second cancer appears not to be specific for cancer subtypes and may perhaps just reflect the general host inflammatory responses accompanying malignancy. The tumor microenvironment frequently contains an immune infiltrate reflecting a background of low-grade inflammation. The cancer cells themselves may be able to shape their own microenvironment through activation and secretion of pro-inflammatory mediators, including IL6, and this response could well promote the development of cancer and further tumor progression (Citation31–34).
CRP has a variety of advantages in terms of clinical utility. The protein tends to have a stable characteristic level for each individual, and only increases occasionally in response to infections, inflammation, or trauma (Citation35). It is also an exceptionally stable particle in the serum and immunoassays for its measurement are robust, well standardized, reproducible, inexpensive, and readily available in routine laboratories (Citation35).
Our study has several limitations derived mainly from its retrospective nature. First, our patients were managed according to the IWCLL 2008 guideline for general practice (Citation2). Namely, fluorescence in situ hybridization (FISH) analysis was performed only before therapy and immunoglobulin variable heavy-chain (IGHV) mutational status was determined only for those patients who wanted a better prediction of the rate at which their disease might progress. Consequently, data on cytogenetics aberrations and IGHV mutational were available for a relatively small number of patients and was not included in the analysis. Secondly, documentation of malignancies may have been missed implying some biases. In the future, our results should be validated in prospective trials. These studies should also explore the causal role of CRP in developing second cancers in CLL and a possible interaction effect with anti-leukemic therapy.
Taken together, elevated CRP is an independent predictive factor for decreased OS and an increased risk of developing a second solid malignancy in patients with CLL. We propose that increased CRP in patients with CLL is a potential marker for early cancer detection and may well justify a more rigorous search for second cancers in these cases. However, more research is needed to better define these risk stratifications for second cancers in CLL patients and the clinical benefit of early detection.
Disclosure statement
The authors have declared no conflict of interests
References
- Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17:399–408.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56.
- Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–11.
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–70.
- Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–24.
- Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61:824–33.
- Wieland A, Kerbl R, Berghold A, Schwinger W, Mann G, Urban C. C-reactive protein (CRP) as tumor marker in pediatric and adolescent patients with Hodgkin disease. Med Pediatr Oncol. 2003;41:21–5.
- Blackman JD, Cabana VG, Mazzone T. The acute-phase response and associated lipoprotein abnormalities accompanying lymphoma. J Intern Med. 1993;233:201–4.
- Legouffe E, Rodriguez C, Picot MC, Richard B, Klein B, Rossi JF, et al. C-reactive protein serum level is a valuable and simple prognostic marker in non Hodgkin's lymphoma. Leuk Lymphoma. 1998;31:351–7.
- Li YJ, Li ZM, Xia Y, Huang JJ, Huang HQ, Xia ZJ, et al. Serum C-reactive protein (CRP) as a simple and independent prognostic factor in extranodal natural killer/T-cell lymphoma, nasal type. PLoS One. 2013;8:e64158.
- Cao Y, Shi YX, Chen JO, Tan YT, Cai YC, Luo HY, et al. Serum C-reactive protein as an important prognostic variable in patients with diffuse large B cell lymphoma. Tumour Biol. 2012;33:1039–44.
- Haase R, Vilser C, Mauz-Korholz C, Hasenclever D, Kluge R, Ruschke K, et al. Evaluation of the prognostic meaning of C-reactive protein (CRP) in children and adolescents with classical Hodgkin's lymphoma (HL). Klin Padiatr. 2012;224:377–81.
- Herishanu Y, Perry C, Braunstein R, Metser U, Goor O, Rogowski O, et al. Early-mid treatment C-reactive protein level is a prognostic factor in aggressive non-Hodgkin's lymphoma. Eur J Haematol. 2007;79:150–4.
- Leonard RC, Cuzick J, MacLennan IC, Vanhegan RI, Mackie PH, McCormick CV. Prognostic factors in non-Hodgkin's lymphoma: the importance of symptomatic stage as an adjunct to the Kiel histopathological classification. Br J Cancer. 1983;47:91–102.
- Troppan KT, Schlick K, Deutsch A, Melchardt T, Egle A, Stojakovic T, et al. C-reactive protein level is a prognostic indicator for survival and improves the predictive ability of the R-IPI score in diffuse large B-cell lymphoma patients. Br J Cancer. 2014;111:55–60.
- Wood HF, Diamond RD, Craver LF, Pader E, Eister SK. Determination of C-reactive protein in the blood of patients with Hodgkin's disease. Ann Intern Med. 1958;48:823–33.
- Shenhar-Tsarfaty S, Yayon N, Waiskopf N, Shapira I, Toker S, Zaltser D, et al. Fear and C-reactive protein cosynergize annual pulse increases in healthy adults. Proc Natl Acad Sci USA. 2015;112:E467–71.
- Rogowski O, Shapira I, Shirom A, Melamed S, Toker S, Berliner S. Heart rate and microinflammation in men: a relevant atherothrombotic link. Heart. 2007;93:940–4.
- Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–4.
- Fayad L, Keating MJ, Reuben JM, O'Brien S, Lee BN, Lerner S, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97:256–63.
- Lai R, O'Brien S, Maushouri T, Rogers A, Kantarjian H, Keating M, et al. Prognostic value of plasma interleukin-6 levels in patients with chronic lymphocytic leukemia. Cancer. 2002;95:1071–5.
- Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–90.
- Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719–26.
- Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105:1076–81.
- Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–9.
- Tsimberidou AM, Wen S, McLaughlin P, O'Brien S, Wierda WG, Lerner S, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–10.
- Beiggi S, Johnston JB, Seftel MD, Pitz MW, Kumar R, Banerji V, et al. Increased risk of second malignancies in chronic lymphocytic leukaemia patients as compared with follicular lymphoma patients: a Canadian population-based study. Br J Cancer. 2013;109:1287–90.
- Molica S. Second neoplasms in chronic lymphocytic leukemia: incidence and pathogenesis with emphasis on the role of different therapies. Leuk Lymphoma. 2005;46:49–54.
- Lam CJ, Curtis RE, Dores GM, Engels EA, Caporaso NE, Polliack A, et al. Risk factors for melanoma among survivors of Non-Hodgkin lymphoma. J Clin Oncol. 2015;33:3096–104.
- Cheson BD. Infectious and immunosuppressive complications of purine analog therapy. J Clin Oncol. 1995;13:2431–48.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow?. Lancet. 2001;357:539–45.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12.
