Abstract
Introduction: Ambient air pollution is associated with adverse cardiovascular events. This meta-analysis aimed to investigate the short-term association between air pollution and cardiovascular effects on healthy volunteers.
Methods: We searched databases to identify randomized trials with controlled human exposures to either of two models for studying ambient particulate matter: diesel-exhaust or concentrated ambient particles. Estimates of size effect were performed using standardized mean difference (SMD). Heterogeneity was assessed with I2 statistics. Outcomes were vascular function estimated by forearm blood flow (FBF), blood pressure, heart rate, and blood analysis.
Results: Database searches yielded 17 articles (n = 342) with sufficient information for meta-analyses. High levels of heterogeneity for the some outcomes were analyzed using random-effects model. The pooled effect estimate showed that short-term exposure to air pollution impaired FBF response from 2.7 to 2.5 mL/100 mL tissue/min (SMD 0.404; p = .006). There was an increase in 5000 platelet/mm3 following pollution exposure (SMD 0.390; p = .050) but no significant differences for other outcomes.
Conclusion: Controlled human exposures to air pollution are associated with the surrogates of vascular dysfunction and increase in platelet count, which might be related to adverse cardiovascular events. Given the worldwide prevalence of exposure to air pollution, these findings are relevant for public health.
Controlled exposure to air pollution impairs vasomotor response, which is a surrogate for adverse cardiovascular events.
This is the first meta-analysis from randomized clinical trials showing short-term association between air pollution and cardiovascular effects on healthy volunteers.
Given the worldwide prevalence of exposure to air pollution, this finding is important for public health.
KEY MESSAGES
Introduction
The WHO estimates that outdoor air pollution is responsible for over three million deaths each year (Citation1). The epidemiologic association between air pollution exposures and exacerbation of cardiovascular disease is well established, yet the mechanisms underlying the increased risk of cardiovascular events are incompletely understood (Citation2).
Given the widespread prevalence of exposure to traffic-related air pollution, modest contributions to cardiovascular risk can have a substantial effect on population health (Citation3,Citation4). Brief exposures have been associated with coronary artery disease, heart failure, arrhythmias, cerebrovascular disease, and thromboembolic events (Citation5–9). These associations could be mediated through direct and indirect effects on vascular dysfunction, autonomic control, systemic inflammation, and coagulability abnormalities (Citation5,Citation8,Citation10).
Adverse cardiovascular events have been associated with ambient air particulate matter (PM), of which the combustion-derived particulate in diesel exhaust is the principal source when considering urban air pollution or traffic-related air pollution (Citation11,Citation12). It is estimated an excess risk of 11% for cardiovascular mortality per 10 μg/m3 increase in fine PM exposure (Citation13,Citation14).
Epidemiologic and observational clinical studies are limited by imprecise measurements of pollution exposure, the lack of mechanistic data and potential environmental and social factors confounders. Controlled exposure to diesel exhaust particles (DEP) and concentrated ambient particles (CAP) are common models for studying the effects of PM, providing a precisely defined concentration in a regulated environment that can overcome many of the potential biases and confounders inherent to observational studies (Citation15). Controlled exposure studies facilitate the investigation with validated measures of cardiovascular health as vascular function, autonomic control and systemic inflammation. However, to our knowledge, there is no published meta-analysis of randomized controlled trials (RCT) from controlled human exposures to air pollutants. We therefore reviewed the evidence from these RCT for the short-term association between air pollutants and cardiovascular effects on healthy volunteers.
Methods
Protocol and registration
The review protocol was registered with PROSPERO (International database of prospectively registered systematic reviews in health and social care, http://www.crd.york.ac.uk/prospero): CRD42016035275.
Data sources and searches
We searched the Cochrane Central Register of Controlled Trials, PubMed and EMBASE to identify the terms: “Air Pollution” AND “Particulate” AND “Cardiovascular”, with limits “Human” and “Clinical Trial” and published in the English literature until December 2015. We also hand searched the bibliographies of all the included studies and relevant review articles to identify any remaining studies.
Eligibility
We only included peer-reviewed original data from RCT that reported short-term exposure of healthy volunteers to different sessions of DEP/CAP versus clean air and of any range of PM size. We did not include abstracts or studies of long-term exposure to air pollution. Studies were excluded from analysis if they had inadequate methodology and did not provide statistical analyses of their results or only described the relative pollutant-attributable changes from baseline. Data were restricted to healthy volunteers with similar baseline characteristics to avoid conditions that could influence the analysis of outcomes. RCTs that enrolled participants with comorbidities were excluded because vascular dysfunction has been observed in patients with established heart failure and coronary artery disease or coronary risk factors, such as diabetes and metabolic syndrome.
Study selection
Two investigators independently screened titles and abstracts for includable articles. Full-text versions were then screened for final inclusion. Disagreement was resolved by debate.
Data extraction
One investigator extracted data from included trials. We collected data on trial characteristics (corresponding author, collaborative group, publication year, journal, study design, inclusion and exclusion criteria, and funding source), participants (number, age, gender, comorbidities), interventions (length and type of exposure), and outcomes (timing of measurement). We restricted our analysis to published data.
Risk of bias assessment
Two review authors independently assessed each trial and outcome for risk of bias: allocation, blinding of participants and personnel (when appropriate), incomplete outcome data, and selective outcome reporting. Disagreements between the review authors were resolved by a third review author.
Outcomes
We accessed vascular function, blood pressure (BP, mmHg), heart rate (HR, beats per minute), white blood cell, platelets and serum C-reaction protein (CRP, mg/L) concentration. Several techniques have been described for the evaluation of vascular function, and venous occlusion plethysmography (VOP) is the most used for blood flow assessment in experimental studies with DEP/CAP in humans (Citation15–17). We searched vascular function estimated via forearm blood flow (FBF, mL/min/100 mL tissue) by VOP. The main principle of VOP is based on the measurement of tissue blood flow by the assessment of the tissue volume change, which is induced by the inflation of a cuff proximally to the under evaluation tissue. The cuff is inflated up to that pressure which occludes venous return but allows arterial inflow. The FBF technique requires brachial artery cannulation for intra-arterial infusion of vasomotor drugs (in most cases acetylcholine, bradykinin and sodium nitroprusside) at 2 or 6 h after the air pollution exposure (Citation17). To avoid the bias of combining data from different vasodilators, we restricted our analyses to the “baseline vascular assessment” reports, determined immediately after the air pollution exposure but before the drug infusions. We aimed at a sufficiently homogeneous group of outcomes to provide a meaningful summary. Therefore, this meta-analysis was performed with outcomes that were measured at the same time point in each trial. All reported values for BP and HR were assessed immediately at the end of the protocol, while the blood sampling should have been performed within 120 min following the controlled exposure. Originally, this meta-analysis was designed to include further cardiovascular outcomes, such as heart rate variability time and frequency domain measures (SDNN, RMSSD, pNN50, HF, LF, HF/LF) and other cytokines, as TNF alpha, IL-6, PAI-1 and tPA. However, there was insufficient uniformity across studies to perform a meta-analysis on those variables.
Statistical analysis
Estimates of size effect were performed using MedCalc 12.7.0 software (Ostend, Belgium) and standardized mean difference (SMD). Different weights are assigned to the different studies for calculating the summary or pooled effect. Each article was weighted according to the number of patients treated. Studies with smaller standard error and larger sample size are given more weight in the calculation of the pooled effect size. Both the fixed and the random-effects model were used in this study. High levels of heterogeneity were analyzed using random-effects model. Heterogeneity among studies was tested using the Cochran's Q-statistic test with a p value of <.1 for statistical significance. The degree of heterogeneity between studies was assessed using the I2 statistic, which estimates the proportion of the variability in effect that is due to heterogeneity rather than chance. An I2 of less than 25% represent low heterogeneity, 25–50% moderate heterogeneity, and greater than 50% high heterogeneity.
Results
Search results
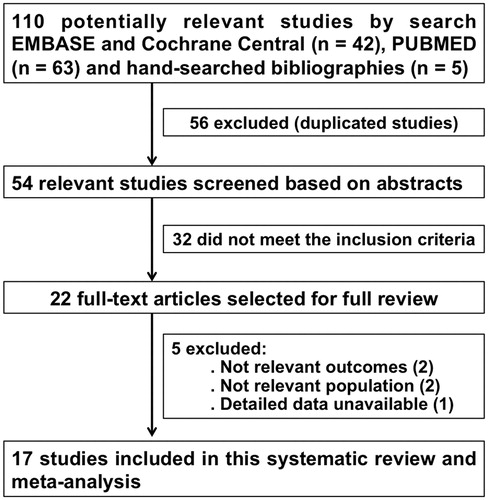
Our initial search yielded 110 potentially relevant studies (). After excluding duplicate publications, we assessed the abstracts of 54 articles and reviewed 22 relevant studies in depth. Of these, we identified 17 RCT (Citation18–34) that fulfilled the eligibility criteria, comprising 342 participants (). The sample sizes in each of these studies ranged from 8 to 32 participants, mainly male (80%) with age ranging from 18 to 60 years. These studies were performed in the United States, Belgium, Sweden, Canada, the United Kingdom, and Brazil. Exposures length ranged from 21 to 120 min.
Table 1. Randomized clinical trials.
Heterogeneity
Except for FBF/VOP (I2 = 0%), there was substantial heterogeneity between trials as seen by I2 values >50% for most outcomes.
Meta-analyses
Vascular function
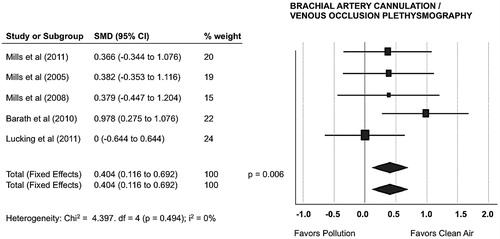
Five studies reported vascular function estimated via FBF/VOP during DEP/CAP-exposure (Citation20,Citation21,Citation26,Citation28,Citation32). The pooled effect estimate from fixed and random-effects model showed that short-term exposure to air pollutants significantly impaired vascular function with a decrease in FBF from 2.7 to 2.5/100 mL tissue/min (SMD 0.404; 95% CI, 0.116–0.692; p = .006), as showed in .
Hemodynamic parameters
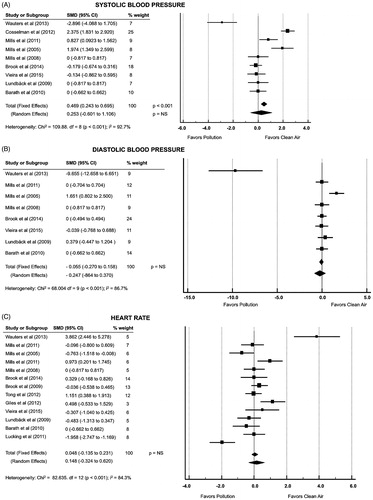
show forest plots for the pooled analysis of the DE effect on systolic blood pressure (9 studies), diastolic blood pressure (8 studies) and heart rate (13 studies), respectively (Citation18–22,Citation25–32,Citation34,Citation35). The pooled effect estimate from fixed-effects model showed an increase in systolic blood pressure during DEP/CAP-exposure (p < .001). However, when accepting the high level of heterogeneity (I2 = 92.7%) by using a random-effects model, that difference was not significant. There were also no differences in heart rate or diastolic blood pressure between exposures.
Blood analysis
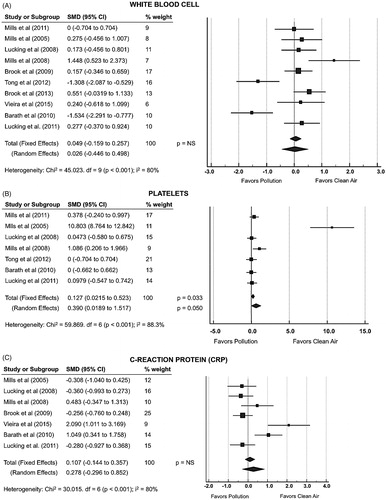
show the forest plots of 10 studies reporting assessment of white blood cell, platelets and CRP during DEP/CAP-exposure (Citation20,Citation21,Citation24,Citation26,Citation28,Citation30–34). The analysis showed no changes on white blood cell or serum CRP between exposures. However, there was a significant increase in 5000 platelet/mm3 following pollution exposure (SMD 0.390; 95% CI 0.019–1.517; p = .050), even accepting the high heterogeneity for the analysis (I2 = 88.3%).
Discussion
There are consistent temporal associations between exposure to air pollutants and cardiovascular disease, (Citation2,Citation7,Citation36) and is fairly well accepted that diesel exhaust induces cardiovascular effects in a wide range of experimental models. Controlled human exposures studies have contributed to elucidate the cardiovascular effects of air pollution, overcoming the imprecise measurements inherent to observational studies. However, some disagreement emerged from different RCTs (Citation18,Citation19,Citation26,Citation32), indicating the need for a meta-analysis to contrast results and identify patterns in the context of multiple studies. To our knowledge, this is the first meta-analysis reporting overall evidence from RCTs for the short-term association between air pollutants and acute cardiovascular effects.
The FBF/VOP analysis had no inconsistency (I2 = 0%) and can hold the homogeneity hypothesis with a narrow uncertainty interval. The reasonably explanation for this finding is that all of the RCTs that assessed FBF were from the same collaborative group and thus followed a similar protocol (Citation20,Citation21,Citation26,Citation28,Citation32). One cited RCT (Citation26) included older volunteers, which could be associated with vascular dysfunction. Nevertheless, we found no differences among studies and the original authors guaranteed that every subject would act as their own control, avoiding problems with matching age and gender, therefore reducing the chance of confounding.
The pooled effect estimate for FBF analysis strengthens the hypothesis that air pollutants from DEP/CAP impairs vasomotor responses, (Citation9,Citation37–41) although it is unclear whether the impairment is primarily mediated by the endothelium or is a result of smooth muscle dysfunction. This is consistent with experimental rat models, (Citation42) and similar results were reported in studies that used different methods to assess vascular impairment (Citation23,Citation25,Citation29,Citation30,Citation34). This is important because the relationship of air pollutants with vascular dysfunction can be associated with an increased risk of cardiovascular events, including cardiac death (Citation43,Citation44). Second, clinical studies of vasomotor function should adjust their results on the level of gaseous air pollutants (Citation45). Third, it was previously showed that respiratory masks, (Citation46) retrofit trap (Citation32), and indoor particle filters (Citation47,Citation48) could prevent the vascular dysfunction associated with short-term exposure to air pollution. However, masks and filters are intended to protect industrial and health workers against occupational risks and should not be recommended as a medium- or long-term solution to the air pollution problem. Instead, attempts to reduce vehicular emissions, smoke from open burning and generator sets, firecrackers and industries could decrease the burden of cardiovascular diseases. Alternatives such as electric vehicles, compressed natural gas and electronic cigarettes, among other interventions, could be tested in future trials.
In the present meta-analysis, there was a high degree of dissimilarity in the results of BP, HR, and blood analysis. The existence of high levels of heterogeneity suggests that there may exist clinical or methodological differences between studies. In any case, as the level of heterogeneity rises, the explanation for an integrated outcome becomes more difficult. We found no single factor that could justify the high levels of heterogeneity, and the use of a random-effects model was our best approach to summarize data. There was a non-significant increase in systolic blood pressure that supports earlier epidemiological research linking air pollution, especially traffic-related, with increased blood pressure (Citation49,Citation50). Furthermore, there is some evidence to support a direct short-term effect of air pollution on blood pressure, (Citation51,Citation52) and it is well accepted that both air pollution and increased blood pressure contribute to an elevated risk of cardio and cerebrovascular diseases (Citation53). Although the present pooled effect estimate from random-effects model for BP and HR was neutral, we believe it is plausible that exposure to air pollutants could cause systemic effects on vascular resistance and noradrenergic receptors to stimulate the sympathetic nervous system. However, controlled human exposures studies have produced somewhat inconsistent results in terms of BP outcomes. The lack of significance, probably due to high levels of heterogeneity, requests additional RCTs to explore the different mechanisms or components of the pollution exhaust that could mediate the vasomotor and pressor effects. The strength of repeated RCTs with similar protocols was demonstrated by the homogeneous consistency of the FBF outcomes. This should be stressed, especially in light of current efforts in the US to prevent the Environmental Protection Agency (EPA) for conducting controlled human exposures studies (Citation54,Citation55). We believe this type of research could provide valuable knowledge about the underlying mechanisms that associates air pollution with adverse systemic events, assuming their compliance with ethical standards of the committee on human experimentation and the Helsinki Declaration.
Previous observational and experimental studies have explored the association between PM inhalation, vascular dysfunction, and pulmonary inflammation “spill-over”. The evidence is not entirely consistent, but it suggests mild systemic inflammatory responses following exposure to PM (Citation12,Citation48,Citation56–58). Against this hypothesis, our pooled effect estimate was aligned with previous studies that observed no systemic inflammation (Citation58,Citation59) during short-term exposure to PM. Surprisingly, there was a significant increase in platelet count even accepting the high heterogeneity for the analysis. Numerous studies have shown the association between elevated platelet counts and cardiovascular risk (Citation60–62). It is not possible from the present meta-analysis to determine the mechanism of platelet activation, but we propose it is reasonable that free radicals may activate platelets by reducing endothelial and platelet-derived nitric oxide and antioxidants (Citation24,Citation63). The cellular mechanisms of atherosclerosis are complex, but adhesion of platelets to the damaged arterial wall occurs early in response to vascular injury.
Limitations
Studies were carefully selected according to a rigorous search. However, in addition to the classic limitations for meta-analysis, several others should be considered. First, we found high heterogeneity across studies, which could indicate methodological differences. Controlled human exposures studies often focus on a “pollutant of interest”, generally PM, selecting DEP/CAP as the main exposure metrics. Given the way studies were conducted, we have extended our search to both types of exposures. To allow comparison with previously published studies, most authors aimed to deliver a specifically range of PM mass, size, and concentration, continuously monitored through DataRam nephelometers and gravimetric analysis. We extended the eligibility to the short-term effects of coarse, fine and ultrafine particulate. It is noteworthy that, although DEP/CAP might have similar cardiovascular effects, (Citation24) they are not the same exposure and the composition of CAPs could vary daily. These differences are a potential explanation for the heterogeneity in some outcome measures. We observed a surprising homogeneity (I2 = 0%) for FBF, which may lead to a spurious certainty about the similarity of the study results. The clinical implications of this must be examined. Second, the underlying mechanisms behind some outcomes could involve other copollutants, unrelated to PM. Third, we did not have access to primary data and were not able to determine whether participants were included across multiple studies from the same research group. Although most studies came from a single collaborative group, estimates of size effect were performed using SMD and different weights were assigned according to the number of volunteers included. Thirty-seven percent of the participants (n = 127) came from the aforesaid group, while 35% (n = 120) came from a different collaborative group and 28% (n = 95) from five single center trials. RCTs with tight control of confounders contribute more to estimates of overall effect than less well-conducted studies. Finally, according to Valentine et al. (Citation64) just two studies could suffice for a meta-analysis. As this is the first meta-analysis reporting the evidence from RCTs for the short-term association between controlled exposure to air pollution and cardiovascular effects, we believe that even with a small number of studies it provides the best evidence at the time. However, it must be updated as new RCTs become available.
Conclusions
This is the first meta-analysis reporting overall evidence from RCTs for the short-term association between air pollution and acute cardiovascular effects. Controlled human exposures to DEP and CAP are associated with surrogates of vascular dysfunction and platelet activation, which might be related to adverse cardiovascular events. Given the worldwide prevalence of exposure to traffic-related air pollution, these findings are relevant for public health.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- World Health Organization (WHO) air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide global assessment 2005: summary of risk assessment [Text PDF format]. Geneva, Switzerland: World Health Organization; 2006; [cited 2013 Oct 8]. Available from: http://www.who.int/phe/health%5Ftopics/outdoorair%5Faqg/en/index.html.
- Chin MT. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart. 2015;101:253–6.
- Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12:627–42.
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b.
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, et al. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009;6:36–44.
- Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–21.
- Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–48.
- Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295.
- Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond). 2008;115:175–87.
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78.
- Cassee FR, Héroux ME, Gerlofs-Nijland ME, Kelly FJ. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal Toxicol. 2013;25:802–12.
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–42.
- Neophytou AM, Hart JE, Cavallari JM, Smith TJ, Dockery DW, Coull BA, et al. Traffic-related exposures and biomarkers of systemic inflammation, endothelial activation and oxidative stress: a panel study in the US trucking industry. Environ Health. 2013;12:105. [E-pub ahead of print]. doi: 101186/1476-069X-12-105.
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. [E-pub ahead of print]. doi:101186/1476-069X-12-43.
- Ghio AJ, Sobus JR, Pleil JD, Madden MC. Controlled human exposures to diesel exhaust. Swiss Med Wkly. 2012;142:w13597. [E-pub ahead of print]. doi:104414/smw201213597.
- Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–89.
- Higashi Y. Assessment of endothelial function. History, methodological aspects, and clinical perspectives. Int Heart J. 2015;56:125–34.
- Wauters A, Dreyfuss C, Pochet S, Hendrick P, Berkenboom G, van de Borne P, et al. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension. 2013;62:352–8.
- Cosselman KE, Krishnan RM, Oron AP, Jansen K, Peretz A, Sullivan JH, et al. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension. 2012;59:943–8.
- Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32:2660–71.
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–6.
- Mills NL, Finlayson AE, Gonzalez MC, Törnqvist H, Barath S, Vink E, et al. Diesel exhaust inhalation does not affect heart rhythm or heart rate variability. Heart. 2011;97:544–50.
- Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–42.
- Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–51.
- Lundbäck M, Mills NL, Lucking A, Barath S, Donaldson K, Newby DE, et al. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol. 2009;6:7. [E-pub ahead of print]. doi: 101186/1743-8977-6-7.
- Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, et al. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect. 2008;116:709–15.
- Brook RD, Bard RL, Morishita M, Dvonch JT, Wang L, Yang HY, et al. Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location. Environ Health Perspect. 2014;122:624–30.
- Barath S, Mills NL, Lundbäck M, Törnqvist H, Lucking AJ, Langrish JP, et al. Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol. 2010;7:19. [E-pub ahead of print]. doi: 101186/1743-8977-7-19.
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–6.
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–67.
- Tong H, Rappold AG, Diaz-Sanchez D, Steck SE, Berntsen J, Cascio WE, et al. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect. 2012;120:952–7.
- Lucking AJ, Lundbäck M, Barath SL, Mills NL, Sidhu MK, Langrish JP, et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123:1721–8.
- Brook RD, Bard RL, Kaplan MJ, Yalavarthi S, Morishita M, Dvonch JT, et al. The effect of acute exposure to coarse particulate matter air pollution in a rural location on circulating endothelial progenitor cells: results from a randomized controlled study. Inhal Toxicol. 2013;25:587–92.
- Vieira JL, Guimarães GV, André PAd, Cruz F, Saldiva PHN, Bocchi EA. Respiratory filter reduces the cardiovascular effects associated with diesel exhaust exposure: a randomized, prospective, double-blind, controlled study of heart failure (FILTER-HF). J Am Coll Cardiol HF. 2016;4:55–64.
- Giles LV, Carlsten C, Koehle MS. The effect of pre-exercise diesel exhaust exposure on cycling performance and cardio-respiratory variables. Inhal Toxicol. 2012;24:783–9.
- Martinelli N, Olivieri O, Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med. 2013;24:295–302.
- Grunig G, Marsh LM, Esmaeil N, Jackson K, Gordon T, Reibman J, et al. Perspective: ambient air pollution: inflammatory response and effects on the lung's vasculature. Pulm Circ. 2014;4:25–35.
- Saber AT, Jacobsen NR, Jackson P, Poulsen SS, Kyjovska ZO, Halappanavar S, et al. Particle-induced pulmonary acute phase response may be the causal link between particle inhalation and cardiovascular disease. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:517–31.
- Davel AP, Lemos M, Pastro LM, Pedro SC, de André PA, Hebeda C, et al. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology. 2012;295:39–46.
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 2008;295:L79–85.
- Rajagopalan S, Sun Q, Chen LC. Particulate pollution and endothelial function: déjà vu all over again in the air. Circulation. 2005;111:2869–71.
- Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, et al. Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ Health Perspect. 2009;117:611–16.
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8.
- Poursafa P, Kelishadi R, Lahijanzadeh A, Modaresi M, Javanmard SH, Assari R, et al. The relationship of air pollution and surrogate markers of endothelial dysfunction in a population-based sample of children. BMC Public Health. 2011;11:115.
- Briet M, Collin C, Laurent S, Tan A, Azizi M, Agharazii M, et al. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension. 2007;50:970–6.
- Vieira JL, Guimarães GV, André PAd, Cruz F, Saldiva PHN, Bocchi EA. Respiratory filter reduces the cardiovascular effects associated with diesel exhaust exposure: a randomized, prospective, double-blind, controlled study of heart failure (FILTER-HF). JACC Heart Fail. 2016;4:55–64.
- Bräuner EV, Forchhammer L, Møller P, Barregard L, Gunnarsen L, Afshari A, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177:419–25.
- Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, et al. An air filter intervention study of endothelial function among healthy adults in a wood smoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–30.
- Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53:853–9.
- Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, et al. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404.
- Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–5.
- Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–6.
- Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014;30:71–5.
- Brander R, Peterka A, Environment & Energy Publishing: EPA - Scientists defend human testing program as critics lay siege, 2015; [cited 2016 Sep 07]. [serial online]. Available from: http://www.eenews.net/stories/1060013033%3E.
- Bell L, Forbes: EPA charged with lethal experiments on hundreds of unsuspecting subjects, 2012; [cited 2016 Sep 07]. [serial online]. Available from: http://www.forbes.com/sites/larrybell/2012/11/13/epa-charged-with-lethal-experiments-on-hundreds-of-unsuspecting-subjects/-4fc683275130.
- Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–41.
- Ostro B, Malig B, Broadwin R, Basu R, Gold EB, Bromberger JT, et al. Chronic PM2.5 exposure and inflammation: determining sensitive subgroups in mid-life women. Environ Res. 2014;132:168–75.
- Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, Graff D, et al. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci. 2014;140:61–72.
- Krishnan RM, Sullivan JH, Carlsten C, Wilkerson HW, Beyer RP, Bammler T, et al. A randomized cross-over study of inhalation of diesel exhaust, hematological indices, and endothelial markers in humans. Part Fibre Toxicol. 2013;10:7. [E-pub ahead of print]. doi: 101186/1743-8977-10-7.
- Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schömig A, et al. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007;98:852–7.
- Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial). Am J Cardiol. 2007;99:1055–61.
- Kaplan S, Kaplan ST, Kiris A, Gedikli O. Impact of initial platelet count on baseline angiographic finding and end-points in ST-elevation myocardial infarction referred for primary percutaneous coronary intervention. Int J Clin Exp Med. 2014;7:1064–70.
- Törnqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400.
- Valentine JC, Pigott TD, Rothstein HR. Tutorial: how many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35:215–47. Available from: http://www.jstor.org/stable/40785162.